Abstract
Exposure of esophageal mucosa to hydrochloric acid (HCl) is a crucial factor in the pathogenesis of reflux disease. We examined supernatant of HCl-exposed rabbit mucosa for inflammatory mediators enhancing migration of leukocytes and production of H2O2 as an indicator of leukocyte activation. A tubular segment of rabbit esophageal mucosa was tied at both ends to form a sac, which was filled with HCl-acidified Krebs buffer at pH 5 (or plain Krebs buffer as control) and kept oxygenated at 37°C. The medium around the sac (supernatant) was collected after 3 h. Rabbit peripheral blood leukocytes (PBL) were isolated, and sac supernatant was used to investigate PBL migration and H2O2 production. HCl-exposed esophageal mucosa released substance P (SP), CGRP, platelet-activating factor (PAF), and IL-8 into the supernatant. PBL migration increased in response to IL-8 or to supernatant of the HCl-filled mucosal sac. Supernatant-induced PBL migration was inhibited by IL-8 antibodies and by antagonists for PAF (CV3988) or neurokinin 1 (i.e., SP), but not by a CGRP antagonist. Supernatant of the HCl-filled mucosal sac increased H2O2 release by PBL that was significantly reduced by CV3988 and by a SP antagonist but was not affected by IL-8 antibodies or by a CGRP antagonist. We conclude that IL-8, PAF, and SP are important inflammatory mediators released by esophageal mucosa in response to acid that promote PBL migration. In addition, PAF and SP induce production of H2O2 by PBL. These findings provide a direct link between acid exposure and recruitment and activation of immune cells in esophageal mucosa.
Keywords: PAF, substance P, IL-8, gastroesophageal reflux
gastroesophageal reflux disease (GERD) is a common clinical condition, with ∼20% of the adult Western population complaining of symptoms at least once per week (15, 34, 39). The pathophysiology of mucosal injury in GERD remains to be elucidated, but prolonged contact of mucosa with acid contributes to the reflux injury. It was thought that esophagitis may develop from a chemical injury starting at the luminal surface of the squamous epithelium, progressing through epithelium and lamina propria into the submucosa, and resulting in acid-induced death of surface cells and stimulation of a proliferative response in the basal cells (24). This view has recently been challenged; a description of the pathogenetic process has been provided (62), proposing that refluxed gastric juice does not directly damage the esophageal mucosa, but rather stimulates esophageal epithelial cells to secrete chemokines that attract and activate immune cells, causing damage to the esophageal squamous epithelial cells (62). In the present work we provide a functional study of the mediators involved.
Several studies have reported that in experimental and human reflux esophagitis proinflammatory cytokine production may underlie the development of erosive esophagitis (5, 11, 16, 27, 65), which is defined by the infiltration of neutrophils and eosinophils into the submucosa. In an animal model of acute esophagitis, Paterson et al. (49) showed that acid perfusion caused release of platelet-activating factor (PAF) from esophageal mucosa into the lumen and induced significant epithelial injury, prevented by a PAF antagonist.
Using an in vitro model of esophagitis, we have recently demonstrated that acid-induced inflammation of the esophagus begins with activation of acid-sensitive vanilloid receptors (TRPV1) in the mucosa and synthesis of the sensory neurotransmitters substance P (SP) and CGRP, and of the lipid inflammatory mediator PAF by epithelial cells (13). Release of PAF by the mucosa induces production of inflammatory mediators in the circular muscle layer, such as interleukin-6 (IL-6), H2O2, interleukin-1β (IL-1β), and PAF, all of which decrease muscle contraction and, possibly, initiate a self-sustaining cycle (9, 10) of motility abnormalities, leading to enhanced exposure of the mucosa to acid, with further increases in inflammation.
However, the mechanisms whereby acid exposure and PAF lead to the recruitment of immune cells in the esophageal mucosa are not known. Several factors have been suggested as being chemotactic for neutrophils and eosinophils. Expression of interleukin-8 (IL-8), a CXC chemokine with potent chemotactic activity for neutrophils, is elevated in the esophageal mucosa of esophagitis patients (26, 27), and IL-8 expression levels are decreased after lansoprazole treatment (25), suggesting a possible role of IL-8 in the pathogenesis of erosive esophagitis induced by acid reflux. SP is known to be a chemoattractant and activator of lymphocytes, monocytes, mast cells, and, importantly, neutrophils and eosinophils (46).
In addition, PAF may be important for the transmigration of peripheral blood leukocytes (PBL) across endothelial cells (29, 47, 64).
We propose that acid in the lumen of the esophagus activates TRPV1 receptors, causing the production and release of PAF, SP, and CGRP that attract and activate immune cells contributing to inflammation and injury of the esophageal mucosa. To examine the effect of inflammatory mediators released by the mucosa on leukocytes we used an experimental model (11) of acid-induced inflammation that we developed, in which a tubular segment of normal esophageal mucosa is removed from the esophagus, and tied at both ends to form a mucosal sac. The sac is filled with Krebs buffer equilibrated with HCl to pH 5.0 (or with Krebs solution for control) and kept in oxygenated Krebs solution at 37°C. The medium around the HCl-filled sac (supernatant) is collected after 3-h incubation and applied to PBL to examine its effect on PBL migration and activation.
We show that the supernatant of the acid-filled mucosal sac acts as a chemoattractant for rabbit PBL and causes H2O2 release. Leukocyte migration was reduced by IL-8 antibodies, by the PAF receptor antagonist CV3988 or by an SP antagonist. H2O2 release was significantly reduced by CV3988 or by an SP antagonist, but not by IL-8 antibodies or by a CGRP antagonist. These data confirm that IL-8 is involved in GERD pathogenesis by promoting PBL migration and suggest that PAF and SP are important inflammatory mediators released from esophageal mucosa in response to acid that attract and activate PBL and, perhaps, contribute to the onset of erosive esophagitis.
METHODS
Tissue preparation.
Experimental procedures were approved by the Animal Welfare Committee of Rhode Island Hospital. Adult rabbits weighing between 3.0 and 4.5 kg were used in this study. Animals were initially anesthetized with ketamine (Aveco, Fort Dodge, IA), then euthanized with an overdose of phenobarbital (Schering, Kennilworth, NJ). The chest and abdomen were opened with a midline incision exposing the esophagus and stomach. The esophagus and stomach were removed together and separated immediately above the lower esophageal sphincter (LES). The esophagus was pinned on a wax block and the smooth muscle layer was opened along the long axis and removed by sharp microdissection at the level of the submucosa, leaving the mucosa intact as a tube, and taking care to keep the submucosa in its entirety with the mucosa preparation. This procedure has been previously described in detail (11). The esophageal mucosal tube consisted of epithelial cells, lamina propria, muscularis mucosae, and submucosa, including submucosal and lamina propria neurons (13), with the epithelial layer on the inside. The separation between mucosal sac and circular muscle strips was as close to the inner layer of the circular muscle as could be surgically achieved under dissecting microscope. The esophageal mucosa tube was divided in two parts and each part was tied at both ends. One was filled with Krebs buffer (0.5 ml/cm of tube) and used as a control; the other one was filled with the same volume of Krebs buffer equilibrated with HCl to pH 4.8–5.0. In previous work (11) we assessed epithelial cell viability after exposure of the mucosal sac preparation to acidic solutions of different pH, by examining the percentage of cells excluding Trypan blue and by measuring lactate dehydrogenase released in the supernatant as an index of cell death. Production of cytokines was highest at pH between 5.8 and 4.8 and declined when the pH was lowered to 4, most likely reflecting tissue damage or necrosis. Thus in this rabbit model we used pH 5.
Both tubes were kept in Krebs buffer with 95% O2, 5% CO2, at 37°C for 3 h, using 1 ml of Krebs buffer per 100 mg of mucosa. The pH of the supernatant remained at 7.0–7.4 and needed no adjustment. After 3 h, the supernatant surrounding the tubes was collected and analyzed. This experimental preparation has been described in detail (11).
Blood collection and isolation of leukocytes.
The rabbit was tranquilized by acepromazine maleate injection (1 mg/kg weight subcutaneously, Phoenix Pharmaceutical, St. Joseph, MD). Ten ml of whole blood was collected from the central ear artery and placed in heparin-treated tubes. The heparinized whole blood was used to isolate leukocytes by density centrifugation using Percoll (Sigma-Aldrich, St. Louis, MO) according to a modification of the method developed by Harbeck (22).
Percoll was first diluted with 10× PBS (Percoll-10× PBS, 9:1 vol/vol) and then diluted again with PBS (Percoll-PBS, 4:1 vol/vol). This final Percoll solution at a ratio of 1:1 with whole blood was used to separate PBL from whole blood by centrifugation at 1,800 g for 20 min.
After centrifugation, the top layer, which consisted of serum and leukocytes, was transferred to a new tube containing two volumes of PBS. The contents were mixed, then centrifuged at 400 g for 10 min. The supernatant was discarded and the cells were resuspended and washed twice with medium. Cells used in H2O2 assay were washed with Krebs-Ringer phosphate buffer (145 mM NaCl, 5.7 mM sodium phosphate, 4.86 mM KCl, 0.54 mM CaCl2, 1.22 mM MgSO4, 5.5 mM glucose, pH 7.35), for cell migration assay the cells were washed with RPMI 1640 medium. When necessary, a red blood cell lysis buffer was used to eliminate red blood cells (eBioscience, San Diego, CA). The PBL were resuspended in their wash medium and counted.
RT-PCR.
Total RNA from esophageal mucosa was isolated by RNeasy Mini Kit (Qiagen, Valencia, CA). To eliminate DNA contamination, 1 μg of total RNA was treated by DNase I according to the product manual. RNA was reversely transcribed and subjected to PCR by using GeneAmp Gold RNA PCR reagent kit (Applied Biosystems, Foster City, CA).
Primers for TRPV1 mRNA were sense 5′-ATGGGCGACCTGGAGTTCAC-3′ and antisense 5′-TTGATGATGCCCACGTTGGT-3′. The primers were derived from the published cDNA sequences of rabbit as described by Zhang et al. (67). We confirmed, through the BLAST database, that the primers were specific for TRPV1. Reactions were carried out in a PTC-100 Programmable Thermal Controller (MJ Research, Waltham, MA) for 1 cycle at 95°C for 10 min, followed by 40 cycles at 94°C for 20 s, 58°C for 20 s, and 72°C for 30 s; the last step was 72°C for 7 min.
Western blot analysis.
Rabbit mucosa was homogenized with lysis buffer containing 50 mM Tris·HCl, pH 7.5, 100 mM NaCl, 50 mM NaF, 5 mM EDTA, 1% (vol/vol) Triton X-100, 40 mM β-glycerol phosphate, 40 mM p-nitrophenyl phosphate, 200 μM sodium orthovanadate, 100 μM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, and 1 μg/ml aprotinin. The homogenate was centrifuged at 10,000 g for 5 min, and the protein concentration in the supernatant was determined by using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA), based on the Bradford dye-binding method (4). The supernatant containing 80 μg protein was used for Western blot assay. The primary antibody, goat anti-TRPV1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), was diluted 1:1,000. The secondary antibody, horseradish peroxidase-conjugated donkey anti-goat antibody (Santa Cruz Biotechnology), was diluted 1:2,000. Detection was achieved with Western Lightning ECL agent (PerkinElmer, Waltham, MA). Molecular weight was estimated by comparison of sample bands with prestained molecular weight marker (Bio-Rad, Melville, NY).
Lyso-PAF acetyltransferase activity.
Mucosal samples were homogenized in 200 μl of ice-cold homogenization buffer containing 0.25 M sucrose, 10 mM EDTA, 5 mM mercaptoethanol, 50 mM NaF, 10−5 M phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, and 50 mM Tris·HCl (pH 7.4) and were homogenized by sonication (4×20 s at 1-min intervals). The homogenates were centrifuged at 4°C, 600 g for 10 min. The supernatants were collected for the lyso-PAF acetyl-CoA transferase (lyso-PAF acetyltransferase) activity assay and the protein concentration in the supernatants was measured by the Bradford method (4). The activity of the lyso-PAF acetyltransferase was measured by a method described by Nomikos et al. (44). Briefly, supernatants containing 10 μg of protein were incubated for 30 min at 37°C with 4 nmol of lyso-PAF and 40 nmol of [3H]acetyl-CoA (100 Bq/nmol) in a final volume of 200 μl of 50 mM Tris·HCl buffer (pH 7.4) containing 0.25 mg/ml BSA and 1 mM dithiothreitol. After incubation, 4 μl of BSA 100 mg/ml were added and the reaction was stopped by addition of 64 μl of 40% cold trichloroacetic acid solution. The reaction mixtures were kept in ice for 30 min and centrifuged at 10,000 g for 2 min. The supernatants were discarded and the pellets containing the [3H]PAF bound to the denaturated BSA were dissolved in EcoLume scintillation cocktail (MP Biomedicals, Solon, OH), and the radioactivity was determined by liquid scintillation counting. Matching controls were run in the absence of lyso-PAF to subtract the radioactivity of the endogenously produced [3H]PAF.
Measurement of PAF.
PAF was extracted from esophageal sac supernatant by a modification of the method of Bligh and Dyer (3). Briefly, 1.6 ml of supernatant was transferred to a tube containing 2 ml of chloroform, 4 ml methanol for a final ratio of 0.8:1:2 H2O-chloroform-methanol (vol/vol/vol). The mixture was vortexed, then centrifuged (2,000 g, 10 min). The upper phase was discarded, keeping the lower phase. A mixture of 6.4 ml of H2O-chloroform-methanol (0.8:1:2, vol/vol/vol) was vortexed, then centrifuged (2,000 g, 10 min), and its upper phase was used to wash the lower phase of the supernatant mixture. Samples of this washed chloroform phase were dried under nitrogen and stored at −80°C. Measurement of PAF was performed within 72 h of extraction. PAF was measured by a radio-receptor binding assay method developed by Aoki and colleagues (1, 57) using PAF receptor-enriched membranes from the heart and skeletal muscle of PAF receptor transgenic mice. Dried sample residues were reconstituted in binding buffer (25 mM HEPES-NaOH, pH 7.4, 10 mM MgCl2, 0.1% BSA). Cell membranes containing PAF receptor were adjusted to 80 μg in 100 μl binding buffer. Reconstituted samples in 50 μl binding buffer together with 50 μl 40 nM [3H]WEB 2086 were added to a 96-well microplate and incubated at 25°C for 90 min. At the end of the incubation, the [3H]WEB 2086 bound to the receptor was separated from the unbound [3H]WEB 2086 by filtration through a UniFilter-96 GF/C (PerkinElmer, Shelton, CT) using a Filtermate Harvester (Packard, Meriden, CT). The filters were washed eight times with the binding buffer. The UniFilter plate was dried at 50°C. MicroScint-O scintillation cocktail (25 μl) (PerkinElmer, Shelton, CT) was added to each well and radioactivity was measured by using TopCount-NXT scintillation counter (Packard). The binding in the presence of 2 μM PAF was used as nonspecific binding and was subtracted from the values for statistical analysis.
Measurement of CGRP and SP.
The mucosal sac supernatant was frozen at −80°C for later use. The concentration of CGRP and SP present in the mucosal sac supernatant was measured by use of enzyme immunoassay kits from Cayman Chemical (Ann Arbor, MI). Before measurement of CGRP and SP, the mucosal sac supernatant was diluted to 1:4 with EIA buffer provided by the immunoassay kits.
Measurement of IL-8.
IL-8 levels in sac supernatant and in mucosal tissue, were measured using the KPL ELISA kit anti-mouse ABTS system (KPL, Gaithersburg, MD). Primary mouse anti-rabbit IL-8 was purchased from Abcam (Cambridge, MA). To measure tissue IL-8, esophageal mucosa (50 mg) was homogenized in 0.25 ml cell lysis buffer from the human IL-8 ELISA kit (RayBiotech, Norcross, GA) and centrifuged at 10,000 g, 4°C, for 10 min. An aliquot of the supernatant was used to quantify the protein concentration by use of a Bio-Rad protein assay kit (Bio-Rad), based on the Bradford dye-binding method (4). The supernatant was then adjusted to 10 μg/ml using the coating solution provided by the KPL ELISA kit.
To measure IL-8 levels in sac supernatant, the supernatant was diluted 1:1 using the coating solution provided by the KPL ELISA kit. Mouse anti-rabbit IL-8 monoclonal antibody (Abcam) diluted at 1:1,500 was used as primary antibody. The other steps of ELISA were performed as instructed by the product manual.
Measurement of H2O2.
To examine the effect of mucosal supernatant on H2O2 production, PBL were incubated for 20 min at 37°C in mucosal supernatant from sac incubated in Krebs buffer alone (control) or in Krebs buffer at pH 5. When using antagonists or antibodies, cells were pretreated with the antagonist or antibody for 30 min before exposure to mucosal supernatant. The antagonists used were CV-3988 (10−5 M) for PAF, CGRP8–37 (10−6 M) for CGRP, the neurokinin-1 receptor antagonist (10−5 M) for substance P. IL-8 antibodies (1:200) were used to immunoneutralize IL-8. H2O2 levels released from isolated leukocytes were measured using the Amplex Red hydrogen peroxide assay kit (Invitrogen, Eugene OR), using 0.5–1 × 106 cells in 20 μl of Krebs-Ringer phosphate buffer (40).
Peripheral blood leukocyte migration.
Peripheral blood leukocyte migration was measured by the ATP luminescence-based motility-invasion assay developed by de la Monte et al. (14). Briefly, 100,000 cells in 100 μl RPMI-1640 cell culture medium (Invitrogen, Grand Island, NY) were seeded into the upper chamber of a dual-chamber motility system (Neuro Probe, Gaithersburg, MD) and separated from the lower chamber by a polycarbonate track-etch (PCTE) membrane with 3-μm pores (Neuro Probe). Cell migration was allowed to proceed from the upper to the lower chamber for 60 min at 37°C in a CO2 incubator. The lower chamber contained materials to be tested for their effect on PBL motility. The materials tested were IL-8 (0.5 × 10−9 M), acid-treated sac supernatant alone, or acid-treated sac supernatant with antagonists to PAF (CV-3988, 10−5 M), CGRP (CGRP8–37, 10−6 M), or SP (neurokinin-1 receptor antagonist, 10−5 M). Sac supernatant was also tested in the presence of IL-8 antibodies (1:200). When using antagonists or antibodies, cells were pretreated with the antagonist or antibody for 30 min before being placed in the upper chamber. Cells were collected from the upper chambers (nonmigrated cells), as well as under the surface of the membranes and at the bottom of the wells (migrated cells). Cells were quantified with use of an ATPLite kit (PerkinElmer, Waltham, MA). The percentages of nonmigrated and migrated cells were calculated and used for statistical analysis.
Materials and reagents.
Bio-Rad protein assay kit was purchased from Bio-Rad. SP and CGRP EIA kits, PAF C-16, and lyso-PAF were obtained from Cayman Chemical. KPL ELISA kit anti-mouse ABTS system was purchased from KPL. Amplex Red hydrogen peroxide assay kit was obtained from Invitrogen. Primary mouse anti-rabbit IL-8 was purchased from Abcam. IL-8 was purchased from R&D Systems (Minneapolis, MN). CV-3988 was from Biomol International (Plymouth Meeting, PA). Neurokinin-1 receptor antagonist was purchased from Calbiochem (La Jolla, CA), and SP, CGRP, and CGRP antagonists (CGRP8–37) were purchased from AnaSpec (Fremont, CA). The dual-chamber motility system and PCTE membrane were from Neuro Probe. The ATPLite kit was purchased from PerkinElmer (Waltham, MA). MicroScint-O scintillation cocktail, UniFilter-96 GF/C plates, [3H]acetyl-CoA, and [3H]WEB 2086 were purchased from PerkinElmer (Shelton, CT). EcoLume scintillation cocktail was from MP Biomedicals. The other reagents were obtained from Sigma.
RESULTS
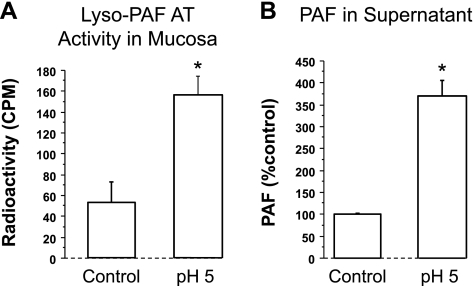
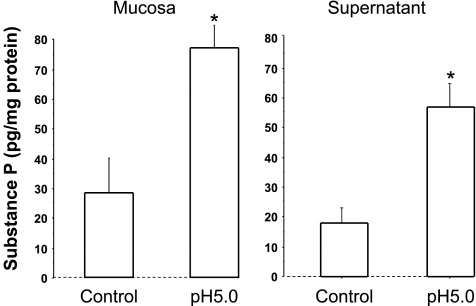
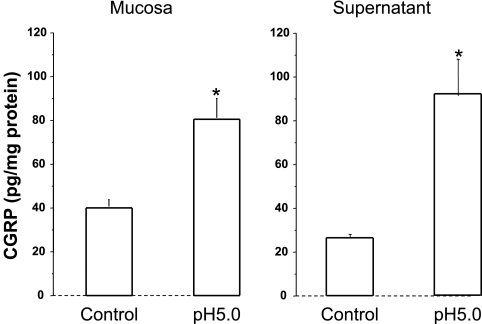
We demonstrate that, similarly to cat esophageal mucosa (13), rabbit mucosa contains TRPV1 receptors and responds to HCl exposure (pH 5) by releasing PAF, CGRP, and SP, in addition to IL-8, into the surrounding supernatant (Figs. 1–5). First we show via Western blot and RT-PCR data supporting the presence of TRPV1 receptors in the mucosal sac (Fig. 1). Second, 3-h exposure to pH 5.0 results in increased mucosal activity of the enzyme responsible for the production of PAF through the remodeling pathway (52, 56) (Fig. 2A). Third, PAF release into the supernatant increases in response to exposure to HCl (Fig. 2B). Taken together, Fig. 2, A and B, indicates that HCl causes increased lyso-PAF acetyltransferase activity in the sac tissue resulting in release of PAF into the supernatant. In addition, HCl causes increased SP and CGRP production in the mucosa and increased SP and CGRP release into the sac supernatant, similar to the cat esophageal mucosa (13) (Figs. 3 and 4).
Fig. 1.
RT-PCR and Western blot for transient receptor potential channel, vanilloid subfamily member 1 (TRPV1) in rabbit esophageal mucosa. The primers for RT-PCR were derived from the published cDNA sequences of rabbit as described by Zhang et al. (67). We confirmed, through the BLAST database, that the primers were specific for TRPV1. RT-PCR indicates the presence of TRPV1 receptor mRNA in rabbit esophageal mucosa. A 320-bp band was recognized as expected. Data are shown for 2 animals. Western blot analysis was performed with a TRPV1 antibody. A 100-kDa band was immunoblotted, confirming the presence of TRPV1 receptors in rabbit esophageal mucosa. Western blots from 2 separate animals are shown.
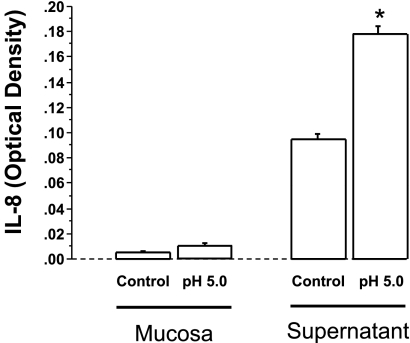
Fig. 5.
When the mucosal sac was filled with HCl (pH 5) for 3 h, IL-8 levels approximately doubled in the tissue and in the supernatant compared with control (pH 7.4) (*P < 0.05 ANOVA). Data represent means + SE of mucosa and supernatant from 3 animals.
Fig. 2.
A: the mucosa sac was filled with HCl (pH 5) for 3 h, then the activity of the lyso-platelet-activating factor (PAF) acetyl-CoA transferase (lyso-PAF acetyltransferase) was measured by a method described by Nomikos et al. (44). Lyso-PAF acetyltransferase activity in the mucosa increased 3 times compared with control (pH 7.4) (*P < 0.05 ANOVA). Data represent means + SE of mucosal tissue from 3 animals. B: similarly, PAF levels in the supernatant increased 3.7 times compared with control (*P < 0.01 ANOVA). Data represent means + SE of supernatant from 4 animals.
Fig. 3.
When mucosa sac was filled with HCl (pH 5) for 3 h, substance P levels doubled in the mucosa and increased 3 times in the supernatant, compared with control (pH 7.4) (*P < 0.05 ANOVA). Data represent means + SE of mucosal tissue from 3 animals.
Fig. 4.
When mucosa sac was filled with HCl (pH 5) for 3 h, CGRP levels doubled in the mucosa and in the supernatant compared with control (pH 7.4) (*P < 0.05 ANOVA). Data represent means + SE of mucosal tissue from 3 animals.
HCl-induced IL-8 production in the mucosa and supernatant is shown in Fig. 5. The figure indicates that in the presence of HCl (pH 5) rabbit mucosa significantly increased IL-8 production and release into the supernatant. This confirms that the rabbit mucosal response to acid is comparable to our previously described cat model.
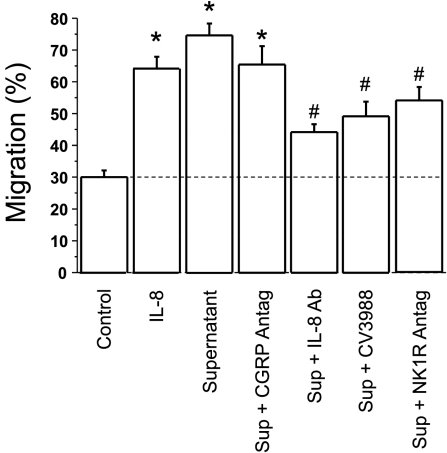
IL-8 is a powerful chemokine (2, 33). We therefore examined PBL migration in response to IL-8 and to mucosal supernatant by using a migration chamber. Figure 6 shows that IL-8 (4.0 ng/ml) induced PBL migration. Supernatant of HCl-treated mucosa induced PBL migration that was comparable to that induced by IL-8. Selective antagonists were used to assess the individual contribution of inflammatory mediators present in the supernatant. The figure shows that a selective CGRP antagonist did not affect PBL migration. In contrast, a selective IL-8 antibody, a PAF receptor antagonist, and an NK-1 receptor antagonist significantly reduced PBL migration, supporting a role of IL-8, PAF, and SP as relevant chemokines.
Fig. 6.
Peripheral blood leukocyte (PBL) migration was examined by using a dual-chamber motility system, in which the upper chamber was separated from the lower chamber by a polycarbonate track-etch membrane with 3-μm pores. Cell migration was allowed to proceed from the upper to the lower chamber for 60 min at 37°C in a CO2 incubator. The lower chamber contained materials to be tested for their effect on PBL motility. PBL motility was measured with an ATP luminescence-based motility-invasion assay. PBL migration (60 min at 37°C) was significantly increased by IL-8 (0.5 × 10–9 M), as expected, and by the supernatant (Sup) of the HCl (pH 5, 3 h)-filled mucosal sac (*P < 0.05 ANOVA). When using antagonists (Antag) or antibodies, cells were pretreated with the antagonist or antibody for 30 min before being placed in the upper chamber. The lower chamber contained the same concentration of antagonists or antibodies as the upper chamber. The increased PBL migration was significantly reduced by IL-8 immunoneutralization by an IL-8 antibody (1:200), by a PAF receptor antagonist (CV3988, 10−5 M), and by an NK-1 receptor (NK1R) antagonist (10−5 M) (*P < 0.05 ANOVA). The increased PBL migration was not affected by a CGRP antagonist (CGRP8–37, 10−6 M). Data represent means + SE of 3 experiments.
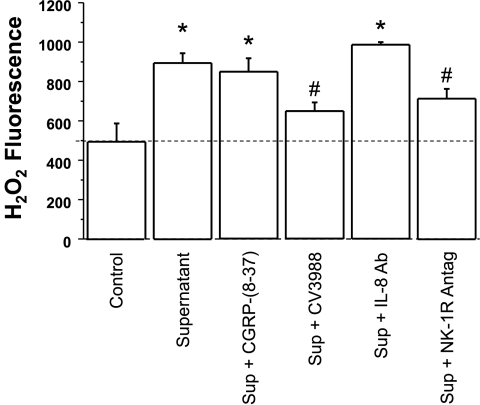
PAF is known to be a powerful activator of PBL, particularly eosinophils (64), evoking the release of reactive oxygen species (58, 71). The contribution of the individual mediators to PBL activation was assessed by using the appropriate antagonists. H2O2 levels, taken as measure of PBL activation, increased when PBL were exposed to sac supernatant at pH 5 (Fig. 7). The increase was not affected by a CGRP antagonist or by IL-8 neutralization by IL-8 antibodies. These data suggest that neither CGRP nor IL-8 at the concentrations present in the supernatant play a role in inducing H2O2 production by PBL. In contrast, a PAF antagonist and a neurokinin-1 (NK-1) receptor antagonist (i.e., SP antagonist) significantly reduced supernatant-induced H2O2 production..
Fig. 7.
To examine the effect of mucosal supernatant on H2O2 production, PBL were incubated for 20 min at 37°C in mucosal supernatant from sacs incubated in Krebs buffer alone (control) or in acidified Krebs buffer (pH 5, 3 h) (supernatant). The supernatant of the acidified mucosal sac caused a 2-fold increase in H2O2 production by PBL. When using antagonists or antibodies, PBL were pretreated with the antagonist or antibody for 30 min before exposure to the supernatant. The increase in H2O2 levels was not affected by a CGRP antagonist (CGRP8–37, 10−6 M) or by IL-8 immunoneutralization by an IL-8 antibody (1:200). The increased H2O2 levels, however, were significantly reduced by a PAF receptor antagonist (CV3988, 10−5 M) and by an NK-1 receptor antagonist (10−5 M) (*P < 0.05 ANOVA). Data represent means + SE of 8 experiments.
DISCUSSION
We have studied inflammation-induced changes in esophageal/LES circular muscle in the cat (8, 10, 12) because its muscle is smooth as it is in humans. For inflammation-induced changes in esophageal mucosa, however, the rabbit has been used extensively (6, 32, 43, 48, 63) and is thought to constitute a suitable model for the study of the human esophageal mucosa. We therefore first confirmed that rabbit esophageal mucosa, similarly to cat (13), contains TRPV1 receptors and responds to HCl by producing SP, CGRP, and PAF. In addition, the rabbit esophageal mucosa produces IL-8, a powerful chemokine that is known to be elevated in GERD and to attract leukocytes, particularly neutrophils (2, 20, 41, 66, 72).
Immune cells may be activated by numerous agents, particularly when used at pharmacological concentrations. It is therefore important to establish which inflammatory mediators are released by HCl-stimulated esophageal mucosa at in vivo concentrations sufficient to attract and activate peripheral blood leukocytes. To study the interaction of mucosa and leukocytes we adopted a relevant ex vivo sac model (11) that allows to examine the inflammatory mediators released by the tissue in a system mimicking the in vivo situation.
Peripheral blood leukocytes were used either to examine cell migration or production of H2O2 in response to the inflammatory mediators or chemokines present in the supernatant of the HCl-stimulated mucosal sac. Activated polymorphonuclear leukocytes (including neutrophils, basophils, and eosinophils) have been used as the prototype of cells that vigorously produce superoxide anions (28), which, in turn, generate other reactive oxygen species (ROS) together with microbiocidal peptides and proteases. ROS comprise species such as superoxide, hydrogen peroxide (H2O2), nitric oxide (NO), and hydroxyl radicals (19). These highly reactive molecules are known to regulate many important cellular events, including gene expression (45), transcription factor activation (54), DNA synthesis (30), and cellular proliferation (42). The enzymes NADPH oxidase and dual oxidase generate ROS in a regulated manner, producing reactive oxygen in cells and tissues in response to growth factors, cytokines, and calcium signals (31).
Although activated PBL produce inflammatory products other than H2O2, we have used H2O2 as a model for PBL-derived ROS because H2O2 is physiologically produced in large amounts by cells such as granulocytes, is relatively stable, and has been widely used to assess the effects of ROS (19).
Similarly to cat esophageal mucosa (13) and the human esophageal epithelial cell line HET-1A (35), rabbit esophageal mucosa contains TRPV1 receptors, which are activated by several stimuli, including acid (7). Exposure of rabbit mucosa to HCl causes activation of lyso-PAF acetyltransferase and production of PAF, similarly to cat and human epithelial cells, where signaling for production of PAF has been described in detail (35).
PAF and lyso-PAF acetyltransferase.
We have shown that esophageal mucosa releases PAF when exposed to acid (9, 10, 13, 35) and demonstrated that epithelial cells are the site of PAF production (13, 35). PAF is an important inflammatory mediator that acts as a chemoattractant and activator of immune cells. PAF induces production of H2O2 in leukocytes (58, 71) and in esophageal circular muscle (9, 10). Other than for one experimental study in the opossum (49), and work from our laboratory (9, 10, 12, 13, 35), a role of PAF in esophageal inflammation has not been investigated to any significant extent. Because PAF is a potent phospholipid mediator of many leukocyte functions, however, its activation in esophageal epithelial cells may play an important role in the pathophysiology of inflammatory disorders in the esophagus.
The enzymatic synthesis of PAF has been characterized in endothelial cells (68, 70), in inflammatory and vascular cells (69) and, recently, in esophageal epithelial cells (35). This pathway is highly regulated and most commonly involves a two-step mechanism. Namely, the precursor of PAF, lyso-PAF, is synthesized by the action of phospholipase A2 (51, 55, 61), removing arachidonic acid (AA) from a membrane phospholipid, and resulting in AA and 1-alkyl-phosphatidylcholine (lysoPAF). Lyso-PAF is converted to PAF by lyso-PAF acetyltransferase, which has been recently cloned by Shindou and coworkers (21, 56).
In HCl-stimulated rabbit esophageal mucosa PAF is produced at concentration sufficient to induce production of H2O2 and migration of PBL. The role of PAF in these PBL functions is demonstrated by the significant reduction in H2O2 production and PBL migration by the selective PAF receptor antagonist CV3988. These data support a role of PAF as a potentially important inflammatory mediator and chemokine in HCl-induced esophageal injury.
SP and GGRP.
Similarly to the cat, 3-h exposure of rabbit esophageal mucosa to a moderately low pH (pH 5) results in increased levels of SP and CGRP in the mucosa and increased SP and CGRP release into the mucosa supernatant. Increased levels of SP and CGRP in the mucosal tissue suggest synthesis of these neurotransmitters and the presence of neural cells in this mucosal sac preparation. SP- and CGRP-containing neural cells have been demonstrated in the human esophageal submucous plexus (59, 60) and epithelium (59) and in cat submucosa (13). We have recently demonstrated SP and CGRP immunoreactivity localized in ganglion cells in the myenteric and submucosal plexus of the cat esophagus (13). Increased levels of SP and CGRP may produce symptoms of neurogenic inflammation by interacting with endothelial cells, mast cells, immune cells, and arterioles (36). Similar findings were reproduced by administration of SP or CGRP agonists and attenuated by administration of antibodies directed against these peptides or by their receptor antagonists (36).
Most of the published research on neurogenic inflammation has focused on release of SP through neural mechanisms (17, 37, 38). Our data indicate that both CGRP and SP are released by esophageal mucosa and submucosa in response to HCl, but, at the concentration present in the supernatant, CGRP does not contribute to attracting PBL or inducing H2O2 production. In contrast, SP is present at concentrations sufficient to act as a chemokine and as an activator of PBL, consistent with a role of SP in acid-induced inflammation (46).
SP induces release of inflammatory mediators such as cytokines, oxygen radicals, arachidonic acid derivatives, and histamine; potentiates tissue injury; and stimulates further leukocyte recruitment (46), amplifying the inflammatory response (23). SP can specifically stimulate chemotaxis of lymphocytes, monocytes, neutrophils, and fibroblasts (18, 53). In general, SP potently affects the migratory and cytotoxic functions of human leukocytes, suggesting that neurogenic stimuli may prime neutrophils for an increased inflammatory response to other mediators (50).
In the rabbit, supernatant of HCl-stimulated esophageal mucosa induces H2O2 production in PBL and PBL migration, and both responses are significantly inhibited by an NK1 receptor antagonist. The inhibition was comparable to that caused by a PAF antagonist, supporting a role of SP as an activator and chemokine in rabbit esophageal mucosa.
IL-8.
IL-8 has long been established as a chemokine, with preference for neutrophils (2, 20, 72). IL-8 has been extensively studied in GERD and is expressed in high amounts in the affected mucosa of GERD patients (16, 27, 65) and in experimental models of reflux-induced inflammation (62). IL-8 levels in the esophagus correlate and increase with both endoscopic and histological disease severity (27, 65). Our data suggest that 3-h incubation at pH 5 significantly increases IL-8 release in the tissue supernatant. IL-8 (4.0 ng/ml)-induced PBL migration, as shown in Fig. 6, was of a magnitude comparable to that induced by the supernatant. Immunoneutralization of IL-8 in the supernatant by IL-8 antiserum (1:200) significantly reduced supernatant-induced migration, indicating the presence of an effective IL-8 concentration in the supernatant. The same immunoneutralization, however, had no effect on H2O2 production (Fig. 6), suggesting that supernatant IL-8 levels may be selectively effective in attracting PBL, but not in inducing H2O2 production.
We conclude that rabbit esophageal mucosa responds to HCl by producing several inflammatory mediators, including IL-8, CGRP, SP, and PAF. At the concentrations released into the sac supernatant, IL-8 is an effective chemokine promoting migration of PBL, but it does not induce production of H2O2. CGRP is a sensory neurotransmitter, perhaps mediating esophageal sensation, but ineffective in promoting PBL migration or H2O2 production. In contrast, SP and PAF are effective in promoting PBL migration and production of H2O2 and may be important mediators to induce the onset of esophageal inflammation. Thus the inflammatory mediators produced by HCl exposure may act as chemokines, as activators, or both. In our system, IL-8 acts only as a chemokine, substance P and PAF act as both chemokines and activators, and CGRP does neither. The data suggest that PBL migration and H2O2 production may be different functions, possibly depending on different signal transduction pathways.
These results, derived from an ex vivo model mimicking reflux-induced events in vivo, identify the type and quantity of inflammatory mediators likely produced in GERD, which may become future targets for novel therapeutic approaches. Since cellular immune infiltrate is a prerequisite for severe inflammation and tissue damage, inhibition of IL-8, SP, and PAF-induced PBL migration and activation may provide potential targets for therapy.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 57030.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors express thanks to Takao Shimizu for contribution to this investigation.
REFERENCES
- 1.Aoki Y, Nakamura M, Kodama H, Matsumoto T, Shimizu T, Noma M. A radioreceptor binding assay for platelet-activating factor (PAF) using membranes from CHO cells expressing human PAF receptor. J Immunol Methods 186: 225–231, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett 307: 97–101, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Cao W, Cheng L, Behar J, Fiocchi C, Biancani P, Harnett KM. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol 287: G1131–G1139, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Carney CN, Orlando RC, Powell DW, Dotson MM. Morphologic alterations in early acid-induced epithelial injury of the rabbit esophagus. Lab Invest 45: 198–208, 1981 [PubMed] [Google Scholar]
- 7.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Cao W, Behar J, Biancani P, Harnett KM. Inflammation induced changes in arachidonic acid metabolism in cat LES circular muscle. Am J Physiol Gastrointest Liver Physiol 288: G787–G797, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Cheng L, Cao W, Behar J, Fiocchi C, Biancani P, Harnett KM. Acid-induced release of platelet-activating factor by human esophageal mucosa induces inflammatory mediators in circular smooth muscle. J Pharmacol Exp Ther 319: 117–126, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. HCl-induced inflammatory mediators in cat esophageal mucosa and inflammatory mediators in esophageal circular muscle in an in vitro model of esophagitis. Am J Physiol Gastrointest Liver Physiol 290: G1307–G1317, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. In vitro model of acute esophagitis in the cat. Am J Physiol Gastrointest Liver Physiol 289: G860–G869, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. Platelet-activating factor and prostaglandin E2 impair esophageal ACh release in experimental esophagitis. Am J Physiol Gastrointest Liver Physiol 289: G418–G428, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Cheng L, de la Monte S, Ma J, Hong J, Tong M, Cao W, Behar J, Biancani P, Harnett KM. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 297: G135–G143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De la Monte SM, Lahousse SA, Carter J, Wands JR. ATP luminescence-based motility-invasion assay. Biotechniques 33: 98–100, 102, 104 passim, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol 41: 131–137, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 50: 451–459, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujino K, de la Fuente SG, Takami Y, Takahashi T, Mantyh CR. Attenuation of acid induced oesophagitis in VR-1 deficient mice. Gut 55: 34–40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haines KA, Kolasinski SL, Cronstein BN, Reibman J, Gold LI, Weissmann G. Chemoattraction of neutrophils by substance P and transforming growth factor-beta 1 is inadequately explained by current models of lipid remodeling. J Immunol 151: 1491–1499, 1993 [PubMed] [Google Scholar]
- 19.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186: 1–85, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 56: 559–564, 1994 [PubMed] [Google Scholar]
- 21.Harayama T, Shindou H, Ogasawara R, Suwabe A, Shimizu T. Identification of a novel noninflammatory biosynthetic pathway of platelet-activating factor. J Biol Chem 283: 11097–11106, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Harbeck RJ, Hoffman AA, Redecker S, Biundo T, Kurnick J. The isolation and functional activity of polymorphonuclear leukocytes and lymphocytes separated from whole blood on a single percoll density gradient. Clin Immunol Immunopathol 23: 682–690, 1982 [DOI] [PubMed] [Google Scholar]
- 23.Holzer P, Holzer-Petsche U. Tachykinins in the gut part II roles in neural excitation, secretion and inflammation. Pharmacol Ther 73: 219–263, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Ismail-Beigi F, Horton PF, Pope CE., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology 58: 163–174, 1970 [PubMed] [Google Scholar]
- 25.Isomoto H, Nishi Y, Kanazawa Y, Shikuwa S, Mizuta Y, Inoue K, Kohno S. Immune and inflammatory responses in GERD and lansoprazole. J Clin Biochem Nutr 41: 84–91, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isomoto H, Saenko VA, Kanazawa Y, Nishi Y, Ohtsuru A, Inoue K, Akazawa Y, Takeshima F, Omagari K, Miyazaki M, Mizuta Y, Murata I, Yamashita S, Kohno S. Enhanced expression of interleukin-8 and activation of nuclear factor kappa-B in endoscopy-negative gastroesophageal reflux disease. Am J Gastroenterol 99: 589–597, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K, Murata I, Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol 98: 551–556, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Karnovsky MJ. Robert Feulgen Lecture 1994. Cytochemistry and reactive oxygen species: a retrospective. Histochemistry 102: 15–27, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Kimani G, Tonnesen MG, Henson PM. Stimulation of eosinophil adherence to human vascular endothelial cells in vitro by platelet-activating factor. J Immunol 140: 3161–3166, 1988 [PubMed] [Google Scholar]
- 30.Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, Stevenson DE, Walborg EF., Jr The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect 106, Suppl 1: 289–295, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Lanas A, Royo Y, Ortego J, Molina M, Sainz R. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterology 116: 97–107, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Leonard EJ. Nap-1 (Il-8). Immunol Today 11: 223–224, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 112: 1448–1456, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Harnett KM, Behar J, Biancani P, Cao W. Signaling in TRPV1-induced platelet activating factor (PAF) in human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol 298: G233–G240, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol 45: 1–98, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Mantyh CR, Pappas TN, Lapp JA, Washington MK, Neville LM, Ghilardi JR, Rogers SD, Mantyh PW, Vigna SR. Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology 111: 1272–1280, 1996 [DOI] [PubMed] [Google Scholar]
- 38.McVey DC, Vigna SR. The capsaicin VR1 receptor mediates substance P release in toxin A-induced enteritis in rats. Peptides 22: 1439–1446, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet 367: 2086–2100, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Mohanty JG, Jaffe JS, Schulman ES, Raible DG. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods 202: 133–141, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Mukaida N. Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol 72: 391–398, 2000 [PubMed] [Google Scholar]
- 42.Murrell GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J 265: 659–665, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naya MJ, Pereboom D, Ortego J, Alda JO, Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut 40: 175–181, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nomikos TN, Iatrou C, Demopoulos CA. Acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine acetyltransferase (lyso-PAF AT) activity in cortical and medullary human renal tissue. Eur J Biochem 270: 2992–3000, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Nose K, Shibanuma M, Kikuchi K, Kageyama H, Sakiyama S, Kuroki T. Transcriptional activation of early-response genes by hydrogen peroxide in a mouse osteoblastic cell line. Eur J Biochem 201: 99–106, 1991 [DOI] [PubMed] [Google Scholar]
- 46.O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol 201: 167–180, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Transmigration of eosinophils through basement membrane components in vitro: synergistic effects of platelet-activating factor and eosinophil-active cytokines. Am J Respir Cell Mol Biol 16: 455–463, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Orlando RC, Powell DW, Carney CN. Pathophysiology of acute acid injury in rabbit esophageal epithelium. J Clin Invest 68: 286–293, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paterson WG, Kieffer CA, Feldman MJ, Miller DV, Morris GP. Role of platelet-activating factor in acid-induced esophageal mucosal injury. Dig Dis Sci 52: 1861–1866, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Perianin A, Snyderman R, Malfroy B. Substance P primes human neutrophil activation: a mechanism for neurological regulation of inflammation. Biochem Biophys Res Commun 161: 520–524, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Prescott SM, Zimmerman GA, McIntyre TM. Platelet-activating factor. J Biol Chem 265: 17381–17384, 1990 [PubMed] [Google Scholar]
- 52.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem 69: 419–445, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Schratzberger P, Reinisch N, Prodinger WM, Kahler CM, Sitte BA, Bellmann R, Fischer-Colbrie R, Winkler H, Wiedermann CJ. Differential chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. J Immunol 158: 3895–3901, 1997 [PubMed] [Google Scholar]
- 54.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 10: 2247–2258, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu T, Ohto T, Kita Y. Cytosolic phospholipase A2: biochemical properties and physiological roles. IUBMB Life 58: 328–333, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J Biol Chem 282: 6532–6539, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Shindou H, Ishii S, Uozumi N, Shimizu T. Roles of cytosolic phospholipase A(2) and platelet-activating factor receptor in the Ca-induced biosynthesis of PAF. Biochem Biophys Res Commun 271: 812–817, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Shute JK, Rimmer SJ, Akerman CL, Church MK, Holgate ST. Studies of cellular mechanisms for the generation of superoxide by guinea-pig eosinophils and its dissociation from granule peroxidase release. Biochem Pharmacol 40: 2013–2021, 1990 [DOI] [PubMed] [Google Scholar]
- 59.Singaram C, Sengupta A, Stevens C, Spechler SJ, Goyal RK. Localization of calcitonin gene-related peptide in human esophageal Langerhans cells. Gastroenterology 100: 560–563, 1991 [DOI] [PubMed] [Google Scholar]
- 60.Singaram C, Sengupta A, Sugarbaker DJ, Goyal RK. Peptidergic innervation of the human esophageal smooth muscle. Gastroenterology 101: 1256–1263, 1991 [DOI] [PubMed] [Google Scholar]
- 61.Snyder F. Platelet-activating factor and its analogs: metabolic pathways and related intracellular processes. Biochim Biophys Acta 1254: 231–249, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, Zhang X, Yu C, Hormi-Carver K, Genta RM, Spechler SJ. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 137: 1776–1784, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Tobey NA, Orlando RC. Mechanisms of acid injury to rabbit esophageal epithelium. Role of basolateral cell membrane acidification. Gastroenterology 101: 1220–1228, 1991 [DOI] [PubMed] [Google Scholar]
- 64.Wardlaw AJ, Moqbel R, Cromwell O, Kay AB. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest 78: 1701–1706, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida N, Uchiyama K, Kuroda M, Sakuma K, Kokura S, Ichikawa H, Naito Y, Takemura T, Yoshikawa T, Okanoue T. Interleukin-8 expression in the esophageal mucosa of patients with gastroesophageal reflux disease. Scand J Gastroenterol 39: 816–822, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Zeilhofer HU, Schorr W. Role of interleukin-8 in neutrophil signaling. Curr Opin Hematol 7: 178–182, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Xiang B, Li YM, Wang Y, Wang X, Wang YN, Wu LL, Yu GY. Expression and characteristics of vanilloid receptor 1 in the rabbit submandibular gland. Biochem Biophys Res Commun 345: 467–473, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Zimmerman GA, McIntyre TM, Mehra M, Prescott SM. Endothelial cell-associated platelet-activating factor: a novel mechanism for signaling intercellular adhesion. J Cell Biol 110: 529–540, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med 30: S294–301, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Zimmerman GA, Whatley RE, McIntyre TM, Benson DM, Prescott SM. Endothelial cells for studies of platelet-activating factor and arachidonate metabolites. Methods Enzymol 187: 520–535, 1990 [DOI] [PubMed] [Google Scholar]
- 71.Zoratti EM, Sedgwick JB, Vrtis RR, Busse WW. The effect of platelet-activating factor on the generation of superoxide anion in human eosinophils and neutrophils. J Allergy Clin Immunol 88: 749–758, 1991 [DOI] [PubMed] [Google Scholar]
- 72.Zwahlen R, Walz A, Rot A. In vitro and in vivo activity and pathophysiology of human interleukin-8 and related peptides. Int Rev Exp Pathol 34: 27–42, 1993 [DOI] [PubMed] [Google Scholar]