Abstract
PepT1 is a di/tripeptide transporter highly expressed in the small intestine, but poorly or not expressed in the colon. However, during chronic inflammation, such as inflammatory bowel disease, PepT1 expression is induced in the colon. Commensal bacteria that colonize the human colon produce a large amount of di/tripeptides. To date, two bacterial peptides (N-formylmethionyl-leucyl-phenylalanine and muramyl dipeptide) have been identified as substrates of PepT1. We hypothesized that the proinflammatory tripeptide l-Ala-γ-d-Glu-meso-DAP (Tri-DAP), a breakdown product of bacterial peptidoglycan, is transported into intestinal epithelial cells via PepT1. We found that uptake of glycine-sarcosine, a specific substrate of PepT1, in intestinal epithelial Caco2-BBE cells was inhibited by Tri-DAP in a dose-dependent manner. Tri-DAP induced activation of NF-κB and MAP kinases, consequently leading to production of the proinflammatory cytokine interleukin-8. Tri-DAP-induced inflammatory response in Caco2-BBE cells was significantly suppressed by silencing of PepT1 expression by using PepT1-shRNAs in a tetracycline-regulated expression (Tet-off) system. Colonic epithelial HT29-Cl.19A cells, which do not express PepT1 under basal condition, were mostly insensitive to Tri-DAP-induced inflammation. However, HT29-Cl.19A cells exhibited proinflammatory response to Tri-DAP upon stable transfection with a plasmid encoding PepT1. Accordingly, Tri-DAP significantly increased keratinocyte-derived chemokine production in colonic tissues from transgenic mice expressing PepT1 in intestinal epithelial cells. Finally, Tri-DAP induced a significant drop in intracellular pH in intestinal epithelial cells expressing PepT1, but not in cells that did not express PepT1. Our data collectively support the classification of Tri-DAP as a novel substrate of PepT1. Given that PepT1 is highly expressed in the colon during inflammation, PepT1-mediated Tri-DAP transport may occur more effectively during such conditions, further contributing to intestinal inflammation.
Keywords: inflammation, inflammatory bowel diseases
members of the proton-oligopeptide cotransporter family, SLC15, mediate di- and tripeptide transport that is prominent in a number of tissues, notably the gut epithelium and the kidney (8). The intestinal isoform, designated PepT1 (SLC15A1), is primarily expressed in the brush border membranes of enterocytes in the small intestine, the bile duct epithelial cells, the proximal tubular cells of the kidney, and the immune cells (4). The closely related family member PepT2 (SLC15A2) is the renal isoform (8). Recently, we found that PepT1 is mainly located in the lipid raft membrane microdomains of immune cells and intestinal epithelial cells (IECs) (22). Under normal conditions, PepT1 is not detectable or poorly expressed in the colon (9, 25, 26). However, as we have previously reported, PepT1 is expressed in epithelial cells of the chronically inflamed human colon (19), suggesting that PepT1 expression can be induced in colonic cells under inflammatory conditions and may be involved in intestinal inflammation. In these colonic epithelial cells, PepT1 is mainly expressed at the apical membrane, as is the case in normal epithelial cells of the small intestine (19). A recent clinical study has confirmed our initial observation, showing that colonic PepT1 is upregulated in inflammatory bowel disease (IBD) (34). Following our studies showing that PepT1 may play a role in intestinal inflammation, Zucchelli and colleagues demonstrated that a PepT1 gene polymorphism is associated with IBD (35). Studies by our group and others suggest that the transcription factor Cdx2 plays a crucial role in PepT1 expression (6, 21, 27). Recently, we reported that pathogenic bacteria induced the expression of PepT1 in colonocytes via Cdx2 (23); however, the mechanisms underlying colonic PepT1 expression remain largely unclear.
The colon is normally colonized by commensal bacteria, which produce peptides that are potential substrates of PepT1. To date, two bacterial peptides have been reported to be transported by PepT1 and both of them possess proinflammatory effects. The first is muramyl dipeptide (MDP) (32, 35), a motif present in the cell wall of both gram-positive and gram-negative bacteria, the second is N-formylmethionyl-leucyl-phenylalanine (fMLP) (20), a chemoattractant tripeptide produced by Escherichia coli. Peptidoglycan is a major constituent of the bacterial wall, and MDP is the minimal motif found in all peptidoglycans. l-Ala-γ-d-Glu-meso-diaminopimelic acid (Tri-DAP) is a natural peptide released during peptidoglycan degradation that is mostly found in the peptidoglycans from gram-negative bacteria. It has been shown that Tri-DAP is recognized by intracellular nucleotide-binding oligomerization domain 1 (NOD1) proteins; once inside the cells, Tri-DAP induces inflammatory responses (2, 12). A major question raised by these observations is how Tri-DAP penetrates into the cells. A recent study demonstrated that, in human lung epithelial cells, this uptake is PepT2 mediated (30). Since PepT1 is closely related to PepT2 and is the representative di/tripeptide transporter in the gut, we hypothesized that PepT1 can transport Tri-DAP into IECs. We further speculated that, since colonic PepT1 expression is induced during intestinal inflammation, PepT1-mediated Tri-DAP transport might contribute to intestinal inflammation. To test these hypotheses, we evaluated PepT1-mediated transport of Tri-DAP and assessed its consequent inflammatory effects in two IEC models: the human small intestinal Caco2-BBE cell line, which expresses PepT1, and the human colonic epithelial HT29-Cl.19A cell line, which does not express PepT1. These were validated by Tri-DAP stimulation of colonic tissues from wild-type and PepT1 transgenic mice expressing PepT1 only in IECs.
MATERIALS AND METHODS
Cell culture and transfection.
The human small intestinal epithelial Caco2-BBE cells (passages 20–30) and human colonic epithelial HT29-Cl.19A cells (passages 20–30) were grown in DMEM supplemented with 10% fetal bovine serum (FBS) and 1.5 mg/ml plasmocin (Invivogen). HT29-Cl.19A cells were transfected with the PepT1/pEGFP-C3 plasmid (Clontech) constructed as previously described (19) or the empty vector by using Lipofectamine 2000 (Invitrogen) in Opti-MEM I serum-reduced medium (Invitrogen). Transfected cells were then stably selected in culture medium supplemented with 1.2 mg/ml geneticin (Invitrogen).
Generation of TetR Caco2-BBE cell line containing hPepT1-shRNA constructs.
Caco2-BBE cells were first transfected with the pcDNA4TO/myc-His/LacZ vector (Invitrogen), which encodes the tetracycline repressor (TetR). This TetR Caco2-BBE cell line was stably selected in DMEM supplemented with 10% Tet system-FBS (Clontech) and 10 μg/ml blasticidin (Invivogen).
Three human (h) PepT1-small interfering RNA (siRNA) candidates were siRNA1, 1,399*-76 bp; siRNA2, 346*-76 bp; siRNA3, 1,706*-76 bp (*position in the PepT1 sequence, NCBI accession no. NM_005073.3.) Short hairpin (sh) constructs were designed, generated and introduced into the pRNATin-H1.2/Hygro vector (Genscript). The TetR Caco2-BBE cells were transfected with the pRNATin-H1.2/Hygro empty vector or hPepT1-shRNA constructs and stably selected in DMEM supplemented with 10% Tet system-FBS, 10 μg/ml blasticidin, and 500 μg/ml hygromycin (Invitrogen). Expression of siRNAs was induced by treating cells with 2.5 μg/ml tetracycline for 2 days.
Transport experiments.
Transport experiments were performed as we previously described (5, 6, 22). Briefly, Caco2-BBE cells (passages 20–30) grown on 12-well plates for 8 days were washed twice with Hanks' balanced salt solution (HBSS; Sigma) supplemented with 10 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.2) and stabilized in the same buffer for 15 min at 37°C. Cells were then incubated with HBSS-10 mM MES (pH 6.2) containing 20 μM [14C]glycine-sarcosine ([14C]Gly-Sar; specific activity of 50 mCi/mM; American Radiolabeled Chemicals) in the presence or absence of 20 mM glycine-leucine (Gly-Leu, Sigma) or different concentrations of Tri-DAP (Anaspec) for 15 min at room temperature. Wells were washed three times with ice-cold PBS. Radioactivity was determined by using a β-counter (1219 Rackbeta). Specific uptake of [14C]Gly-Sar mediated by hPepT1 was calculated as follow: (uptake of [14C]Gly-Sar) − (uptake of [14C]Gly-Sar + Tri-DAP). The Ki value, which provides a measure of the affinity of Tri-DAP to PepT1, was determined from the Dixon plots (competitive inhibition) by using the SigmaPlot 8.0 software.
Butyrate uptake cells mediated by the monocarboxylate transporter 1 (MCT-1) in Caco2-BBE was performed as described previously (22). Briefly, cells were washed and then incubated with HBSS-10 mM MES (pH 6.2) containing 20 μM [14C]butyrate (specific activity of 16 mCi/mmol, Sigma) in the presence or absence of 1 mM α-cyano-4-hydroxycinnamate (CHC) for 1 h at room temperature. Specific uptake of [14C]butyrate mediated by MCT-1 was calculated as follows: (uptake of [14C]butyrate) − (uptake of [14C]butyrate + CHC).
Intracellular pH measurements.
Intracellular pH of cells was measured as previously described (3, 31) by ratiometric measurement of fluorescence of the pH sensitive dye 2′,7′-bis-(2-carboxy-ethyl)-5 (and-6) carboxyfluorescein, acetoxymethyl ester (BCECF-AM; Molecular Probe). Briefly, cells (passages 20–30) were grown on glass-bottom microwell dishes (35 mm petri dishes, 14 mm microwell; MatTek) for 8 days to reach confluency. Cells were then loaded with 10 μM BCECF-AM for 1 h at 37°C. Cells were washed with HBSS supplemented with 10 mM HEPES pH 7.4 to remove the excess of dye. Ratiometric measurement of fluorescence in cells were performed by using the Intracellular Imaging apparatus (Intracellular Imaging) consisting of a Nikon TSE 100 Ellipse inverted microscope with epifluorescence attachments. BCECF was successively excited at 440 and 490 nm, and light emitted at 535 nm was detected by a cooled charge-coupled device imaging camera. An electronic shutter (Sutter Instruments) was used to minimize photobleaching of dye. Data were collected in real time by use of the InCyt Im2 software (Intracellular Imaging). Forty cells per monolayer were selected for each measurement.
Western blot.
Total proteins were extracted from the cells (passages 20–30, cultured on plastic plates for 8 days) by use of RIPA buffer [150 mM NaCl, 0.5% sodium deoxycholate, 50 mM Tris·HCl (pH 8.0), 0.1% SDS, 0.1% Nonidet P-40, 2 mM Na3VO4, 10 mM NaF supplemented with protease inhibitor cocktail (Roche)], resolved on polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked and then probed overnight at 4°C with relevant antibodies: anti-IκB-α (dilution 1:1,000; Santa Cruz no. sc-371), anti-phospho-ERK½ (dilution 1:1,000, Cell Signaling no. 4,370), anti-phospho-p38 (dilution 1:1,000, Cell Signaling no. 9216), anti-ERK½ (dilution 1:1,000, Cell Signaling no. 4695), anti-p38 (dilution 1:1,000, Cell Signaling no. 9212), anti-GAPDH (dilution 1:1,000, Ambion no. AM4300), anti-human MCT-1 (dilution 1:1,000, Alpha Diagnostic no. MCT-12A), and anti-PepT1 [generated as previously described (19)]. After washes, membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (anti-mouse, dilution 1:1,000, Amersham Biosciences no. NA931V; anti-rabbit, dilution 1:5,000, Amersham Biosciences no. NA934V). Immunoreactive bands were detected using the enhanced chemiluminescence detection system (Amersham Biosciences). Films were analyzed by densitometry, and signal intensity was quantified using a Gel documentation system (Alpha Innotech).
Real-time RT-PCR.
Caco2-BBE cells (passages 20–30) were cultured on plastic plates for 8 days and stimulated or not with Tri-DAP. Total RNA was isolated from the cells with TRIzol reagent (Invitrogen) followed by reverse transcription using cDNA first-strand synthesis kit (Fermentas). Real-time RT-PCR was performed by using the iQ SYBR Green Supermix (Bio-Rad) and the real-time iCycler sequence detection system (Bio-Rad). cDNA was amplified at 95°C for 3 min followed by 45 cycles of 95°C for 30 sec and 60°C for 1 min with 1 μM of specific primers: IL-8 sense 5′-GTG CAG TTT TGC CAA GGA GT-3′; IL-8 antisense 5′-AAA TTT GGG GTG GAA AGG TT-3′; 18S sense, 5′-CCC CTC GAT GCT CTT AGC TGA GTG T-3′; 18S antisense, 5′-CGC CGG TCC AAG AAT TTC ACC TCT-3′. 18S was used as a housekeeping gene, and fold induction was calculated by using the cycle threshold method (Ct) method as follows: ΔΔCt = (Cttarget − Cthousekeeping) treatment − (Cttarget − Cthousekeeping) nontreatment, and the final data were derived from 2−ΔΔCt.
NF-κB activation study.
Caco2-BBE cells (passages 20–30) grown on plastic plates for 4 days were transfected with 2 μg of the pNF-κB-luc vector (BD Biosciences) and 5 ng of a construct encoding Renilla luciferase (Promega) by using 10 μg/ml lipofectin (Invitrogen). Cells were washed, serum starved overnight, and then stimulated with different concentrations of Tri-DAP for the indicated times. After stimulation, cells were lysed, and luciferase activity was detected with a luminometer (Luminoskan; Thermo LabSystems) and normalized to Renilla luciferase activity.
Tri-DAP stimulation of cultured mouse colon.
Colons were taken from 8-wk-old, sex-matched FVB wild-type and PepT1 transgenic mice that we previously generated (7). Mouse colonic tissues were cultured as previously described (28). Briefly, colonic samples were removed, cut open longitudinally, and washed in HBSS supplemented with penicillin and streptomycin (Cellgro). The colons were then further cut into segments of ∼1 cm2 and placed into 24 flat-bottom-well culture plates containing fresh RPMI 1640 medium (Invitrogen) supplemented with penicillin and streptomycin. The colon segments were cultured at 37°C in a 5% CO2 atmosphere and 90% humidity for 12 h in the presence of 5 mM Tri-DAP or vehicle. Culture supernatants were collected, centrifuged at 13,000 g for 10 min, and stored at −20°C until used. All procedures using mice were approved by the Institutional Animal Care and Use Committee at Emory University.
ELISA.
IL-8 levels secreted into cell culture media were quantified by using the IL-8 enzyme-linked immunosorbent assay (ELISA) kit (eBioscience). Keratinocyte-derived chemokine (KC) levels secreted into colon culture supernatant were quantified by the mouse ELISA CXCL1/KC kit (R&D Systems).
Statistical analysis.
Values were expressed as means ± SE or means ± SD. Statistical analysis was performed using unpaired two-tailed Student's t-test by InStat v3.06 (GraphPad) software. P < 0.05 was considered statistically significant.
RESULTS
Tri-DAP inhibits PepT1-mediated transport of glycine-sarcosine in intestinal epithelial cells.
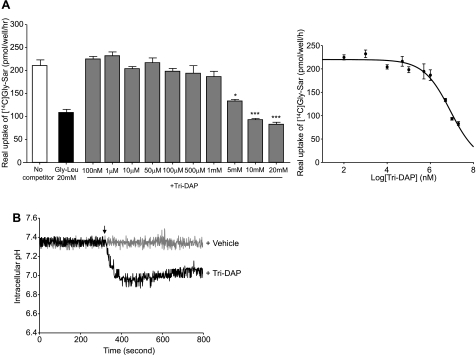
Intestinal epithelial Caco2-BBE cells were grown on 12-well plastic plates until they reached confluence, after which PepT1-mediated specific uptake of [14C]Gly-Sar was measured in the presence of cold 20 mM Gly-Leu, a substrate of PepT1 (5, 20, 22), or different concentrations of Tri-DAP. As expected, 20 mM Gly-Leu significantly suppressed PepT1-mediated uptake of [14C]Gly-Sar (Fig. 1A, left). Interestingly, Tri-DAP at final concentration of ≥5 mM significantly decreased [14C]Gly-Sar uptake. A subsequent analysis of the concentration dependence of this inhibitory effect showed that Tri-DAP at 5, 10, and 20 mM inhibited PepT1-mediated [14C]Gly-Sar by up to ∼40, 55, and 60%, respectively (Fig. 1A). The level of inhibition by Tri-DAP reached ∼60% of uptake. This could be due to the low affinity of Tri-DAP for PepT1. The Ki value of Tri-DAP for PepT1 was then determined from the Dixon plot (competitive inhibition) by using the SigmaPlot 8.0 software. The Ki value of Tri-DAP was 4.78 ± 0.24 mM. Therefore Tri-DAP could be classified as a substrate of PepT1 with medium-low affinity according to the evaluation of Brandsch et al. (1).
Fig. 1.
l-Ala-γ-d-Glu-meso-DAP (Tri-DAP) inhibits PepT1-mediated transport of [14C]glycine-sarcosine ([14C]Gly-Sar) into Caco2-BBE cells. Caco2-BBE cells (passages 20–30) were grown on plastic plates for 8 days. A: uptake of 20 μM [14C]Gly-Sar in the absence or presence of 20 mM glycine-leucine (Gly-Leu) or the indicated concentrations of Tri-DAP was determined as described undermaterials and methods. Results were expressed as real uptake of [14C]Gly-Sar. The Ki value, for Tri-DAP to PepT1, was determined from Dixon plot (competitive inhibition) by use of the SigmaPlot 8.0 software. Values represent means ± SE of n = 6 wells/condition. Data are representative of 3 determinations (*P < 0.05; ***P < 0.001) vs. no competitor. B: intracellular pH of Caco2-BBE cells in response to Tri-DAP stimulation. Caco2-BBE cells (passages 20–30) grown on glass-bottom microwell dishes for 8 days were loaded with the fluorescent dye BCECF-AM, and intracellular pH before and after addition of 5 mM Tri-DAP or vehicle (arrow) was measured in real time as described in materials and methods. For each condition, 3 independent experiments were performed. Graphs represent the mean of intracellular pH of 40 selected cells from 1 assay.
Since it is known that PepT1 cotransports peptides with H+ (29), we measured the intracellular pH of Caco2-BBE cells before and after Tri-DAP stimulation. As shown in Fig. 1B, 5 mM Tri-DAP induced a significant drop of the intracellular pH, indicating an increase of the intracellular proton concentration. In contrast, the intracellular pH of vehicle-treated cells was not changed during measurement. These results demonstrate that the uptake of Tri-DAP into Caco2-BBE cells was coupled with that of H+, suggesting the transport of Tri-DAP by PepT1 into Caco2-BBE cells.
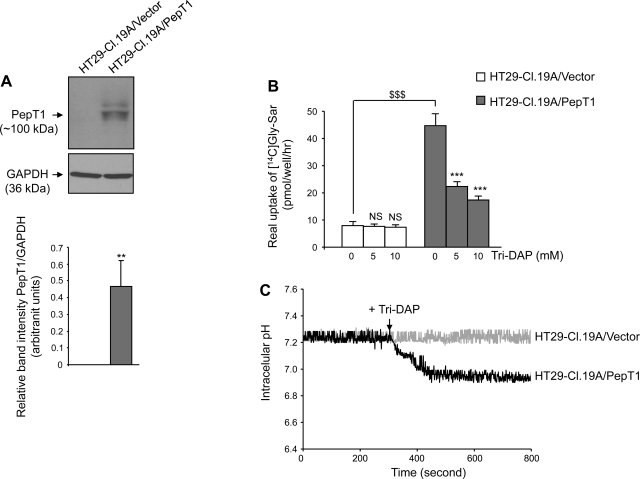
To verify these finding, we assessed the inhibitory effect of Tri-DAP on [14C]Gly-Sar uptake in another IEC model, the colonic epithelial HT29-Cl.19A cells, which normally do not express PepT1 (19, 20). HT29-Cl.19A cells were stably transfected with a plasmid encoding PepT1 (HT29-Cl.19A/PepT1) or with the empty vector (HT29-Cl.19A/vector). As expected, Western blot analysis showed that PepT1 was strongly expressed in HT29-Cl.19A/PepT1 cells but not in HT29-Cl.19A/vector cells (Fig. 2A). [14C]Gly-Sar uptake in the presence or absence of Tri-DAP in these cells was then determined. As shown in Fig. 2B, Tri-DAP at 5 and 10 mM significantly inhibited [14C]Gly-Sar transport in HT29-Cl.19A/PepT1 cells, reducing uptake by more than 50 and 60%, respectively. In contrast, in HT29-Cl.19A/vector, Tri-DAP only very slight inhibited uptake, even at a concentration of 10 mM. The marked increase in the inhibitory effect of Tri-DAP on [14C]Gly-Sar uptake produced by exogenous expression of PepT1 in HT29-Cl.19A cells confirms that PepT1 transports Tri-DAP into IECs. Most importantly, Tri-DAP induced an intracellular acidification in HT29-Cl.19A cells expressing PepT1 but not in cells that did not express PepT1 (Fig. 2C). Collectively, our data demonstrate that PepT1 transports Tri-DAP into IECs.
Fig. 2.
Tri-DAP inhibits the uptake of [14C]Gly-Sar in HT29-Cl.19A cells stably transfected with PepT1. HT29-Cl.19A cells stably transfected with a vector encoding PepT1 (HT29-Cl.19A/PepT1) or an empty vector (HT29-Cl.19A/vector) (passages 20–30) were cultured on plastic plates for 8 days. A: Western blot analysis of PepT1 expression in HT29-Cl.19A/PepT1 and HT29-Cl.19A/vector cells. Blots are representative of 3 similar determinations (top), and bars show means ± SD of relative intensity of the bands obtained from 3 determinations (bottom). **P < 0.005. B: uptake of 20 μM [14C]Gly-Sar in the absence or presence of 5 or 10 mM of Tri-DAP was determined by transport assay, and results were expressed as [14C]Gly-Sar real uptake. Values represent means ± SE of n = 6 wells/condition. Data are representative of 3 determinations. $$$P < 0.001; NS, not statically significant vs. untreated HT29-Cl.19A/vector; ***P < 0.001 vs. untreated HT29-Cl.19A/PepT1. C: HT29-Cl.19A/PepT1 and HT29-Cl.19A/vector cells (passages 20–30) grown on glass-bottom microwell dishes for 8 days were loaded with the fluorescent dye BCECF-AM, and intracellular pH before and after addition of 5 mM Tri-DAP (arrow) was measured in real time as described in materials and methods. For each condition, 3 independent experiments were performed. Graphs represent the mean of intracellular pH of 40 selected cells from 1 assay.
Tri-DAP induces proinflammatory response in Caco2-BBE cells.
Since our results showed that Tri-DAP is transported into IECs that express PepT1 and because Tri-DAP has been reported to exert a proinflammatory effect (12, 30), we assessed Tri-DAP-induced inflammatory responses in IECs.
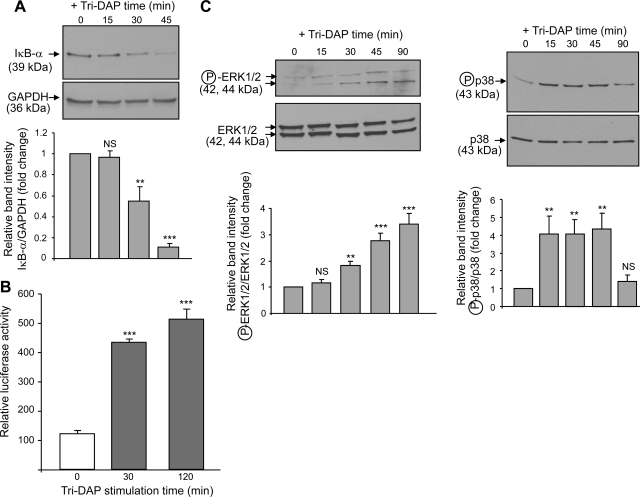
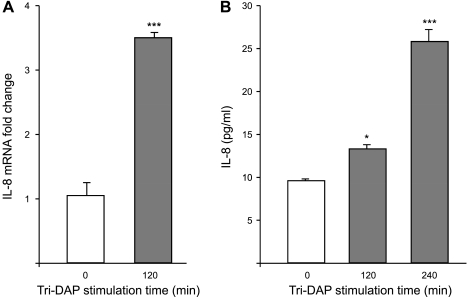
IECs express the nuclear factor-κB (NF-κB), a transcription factor activated in response to immune and proinflammatory signals (15). NF-κB is known to be involved in the upregulation of several immunomodulatory genes, including the proinflammatory cytokine interleukin-8 (IL-8) (13). Given that 5 mM Tri-DAP efficiently competed with Gly-Sar for PepT1-mediated transport into Caco2-BBE cells (Fig. 1A), we used this concentration for subsequent experiments. Caco2-BBE cells were stimulated with Tri-DAP for the indicated times, and IκB-α degradation, which is a prerequisite and an indicator for NF-κB activation, was assessed by Western blotting. We found that IκB-α was rapidly degraded in Caco2-BBE cells upon stimulation with Tri-DAP (Fig. 3A). NF-κB activation was further assessed after stimulation with Tri-DAP for the indicated times by measuring luciferase activity in Caco2-BBE cells transiently transfected with a NF-κB-responsive luciferase reporter plasmid. As shown in Fig. 3B, Tri-DAP treatment resulted in a significant increase in luciferase activity in the cells, indicating activation of NF-κB. Because mitogen-activated protein kinases (MAPKs) are also involved in inflammation (13), activation of MAPKs in Caco2-BBE cells in response to Tri-DAP stimulation was next analyzed by Western blot. We found that Tri-DAP stimulation of Caco2-BBE cells induced a rapid increase in the levels of phospho-ERK½ and phospho-p38, indicating that these MAPKs were activated (Fig. 3C). It has been shown that activation of proinflammatory pathways, such as MAPK and NF-κB pathways, in IECs leads to production of IL-8 (13, 15). Quantitative real-time RT-PCR analysis revealed a significant increase in IL-8 mRNA levels by ∼3.5-fold in Caco2-BBE cells at 2 h after stimulation with Tri-DAP (Fig. 4A). The levels of IL-8 in the culture medium of Caco2-BBE cells treated with Tri-DAP for 2 and 4 h, quantified by ELISA, were also significantly increased (Fig. 4B). Taken together, these results indicate that Tri-DAP induces proinflammatory response in intestinal epithelial Caco2-BBE cells.
Fig. 3.
Tri-DAP induces activation of NF-κB and MAP kinases in Caco2-BBE cells. A: Caco2-BBE cells (passages 20–30) grown on plastic plates for 8 days were stimulated with 5 mM Tri-DAP for the indicated times, and degradation of IκB-α was assessed by Western blot. B: Caco2-BBE cells (passages 20–30) grown on plastic plates for 4 days were transiently transfected with a NF-κB-luciferase reporter construct and then stimulated with 5 mM Tri-DAP for 30 or 120 min. Luciferase activity in cell lysates were quantified by luciferase assay as described in materials and methods. Data were normalized to Renilla activity and expressed as relative luciferase activity. Values represent means ± SE of n = 6 wells/condition. Data are representative of 3 determinations with similar results. ***P < 0.001 vs. control (without Tri-DAP stimulation). C: Caco2-BBE cells (passages 20–30) grown on plastic plates for 8 days were stimulated with 5 mM Tri-DAP for the indicated times, and levels of phospho (circled P)-ERK½ and phospho-p38 were analyzed by Western blot. Blots in A and C are representative of 3 similar determinations (top), and bars show means ± SD of relative intensity of the bands obtained from 3 determinations (bottom). NS, not statically significant; **P < 0.005; ***P < 0.001 vs. control.
Fig. 4.
Tri-DAP induces production of IL-8 in Caco2-BBE cells. Caco2-BBE cells (passages 20–30) grown on plastic plates for 8 days were stimulated with 5 mM Tri-DAP for the indicated times, and levels of IL-8 mRNA (A) and protein (B) were assessed by real-time RT-PCR and ELISA, respectively. Values represent means ± SE of n = 6 wells/condition. Data are representative of 3 determinations. (*P < 0.05; ***P < 0.001) vs. control (without Tri-DAP stimulation).
Tri-DAP-induced proinflammatory response in intestinal epithelial cells is PepT1 dependent.
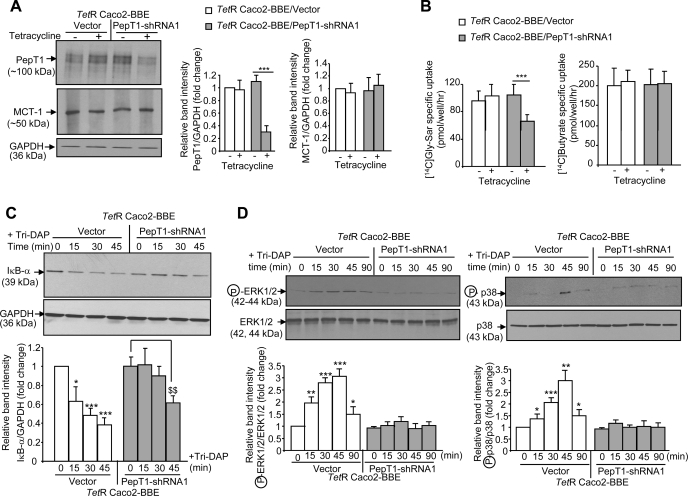
Since we found that Tri-DAP is transported into IECs by PepT1, we next investigated whether PepT1 is involved in Tri-DAP-mediated inflammation. PepT1 expression was modulated in Caco2-BBE cells by using a Tet-off system. TetR Caco2-BBE cells were stably transfected with PepT1-shRNA constructs (TetR Caco2-BBE/PepT1-shRNA) or the empty vector (TetR Caco2-BBE/vector). Western blot analyses revealed that the PepT1-shRNA1 construct (see materials and methods) was effective in knocking down PepT1, with highest repression effect after 2 days of 2.5 μg/ml tetracycline treatment and therefore was used for other studies. As shown in Fig. 5A, tetracycline treatment decreased PepT1 expression in TetR Caco2-BBE/PepT1-shRNA1 cells but had no such effect in TetR Caco2-BBE/vector cells. Expression of the monocarboxylic transporter-1 (MCT-1), used as a control, in TetR Caco2-BBE/PepT1-shRNA1 cells was also not affected by tetracycline treatment (Fig. 5A). Uptake experiments further showed that specific uptake of 20 μM [14C]Gly-Sar in the presence of 5 mM Tri-DAP was significantly reduced in TetR Caco2-BBE/PepT1-shRNA1 cells treated with tetracycline but not in tetracycline-treated TetR Caco2-BBE/vector. In contrast, specific uptake of [14C]butyrate mediated by MCT-1 in TetR Caco2-BBE/PepT1-shRNA1 cells treated with tetracycline were not inhibited (Fig. 5B). Together, these results demonstrate that TetR Caco2-BBE/PepT1-shRNA1 cells exhibited decreases in expression and activity of PepT1, but not those of another transporter MCT-1.
Fig. 5.
Downregulation of PepT1 expression in Caco2-BBE cells decreases Tri-DAP-induced inflammation. Caco2-BBE cells were stably transfected first with the pcDNA4TO/myc-His/LacZ vector, which encodes the tetracycline repressor (TetR), and then with the PepT1-short hairpin RNA (shRNA)1 construct (TetR Caco2-BBE/PepT1-shRNA1) or the empty vector (TetR Caco2-BBE/vector). Cells (passages 25–30) grown on plastic plates for 6 days were treated or not with 2.5 μg/ml tetracycline for 2 days. A: PepT1 and monocarboxylate transporter 1 (MCT-1) expression was analyzed by Western blot. B: uptake of 20 μM [14C]Gly-Sar ± 5 mM Tri-DAP and uptake of 20 μM [14C]butyrate ± 1 mM α-cyano-4-hydroxycinnamate (CHC) in these cells were determined. Data are presented as specific uptake: PepT1-mediated [14C]Gly-Sar specific uptake = (uptake of [14C]Gly-Sar) − (uptake of [14C]Gly-Sar + Tri-DAP); MCT-1-mediated [14C]butyrate specific uptake = (uptake of [14C]butyrate) − (uptake of [14C]butyrate + CHC). Values represent means ± SE of n = 6 wells/condition from 1 determination. ***P < 0.001. C and D: TetR Caco2-BBE/PepT1-shRNA1 and TetR Caco2-BBE/vector cells were treated with 2.5 μg/ml tetracycline for 2 days and then stimulated with 5 mM Tri-DAP for the indicated times. Degradation of IκB-α (C) and activation of the MAPKs ERK½ and p38 (D) were assessed by Western blot. All blots in A, C, and D are representative of 3 similar determinations, and bars show means ± SD of relative intensity of the bands obtained from 3 determinations. ***P < 0.001 (A and B); (*P < 0.05; **P < 0.005; ***P < 0.001) vs. untreated TetR Caco2-BBE/vector (C and D); $$P < 0.005 (C).
Tri-DAP-mediated proinflammatory response was then assessed in tetracycline-treated Caco2-BBE/PepT1-shRNA1 and TetR Caco2-BBE/vector cells. As shown in Fig. 5, C and D, Tri-DAP rapidly induced the degradation of IκB-α and activation of the MAPKs ERK½ and p38 in TetR Caco2-BBE/vector cells at 15 min posttreatment. In contrast, these actions were delayed until 45 min after treatment with Tri-DAP in TetR Caco2-BBE/PepT1-shRNA1 cells. The delayed activation of NF-κB and MAPKs caused by PepT1 downregulation in Caco2-BBE cells strongly suggests that PepT1 is functionally involved in Tri-DAP-induced intestinal inflammation.
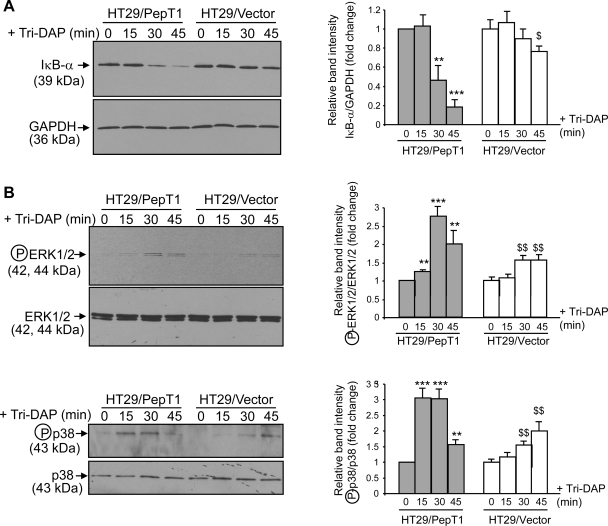
To further confirm that the proinflammatory effects of Tri-DAP are PepT1 dependent, we stimulated HT29-Cl.19A/vector and HT29-Cl.19A/PepT1 cells with Tri-DAP for the indicated times and assessed activation of NF-κB and MAPKs by Western blotting. In HT29-Cl.19A cells expressing PepT1, Tri-DAP induced a rapid and robust degradation of IκB-α, indicating NF-κB activation (Fig. 6A). In contrast, IκB-α was poorly degraded in HT29-Cl.19A/vector cells, which do not express PepT1. Activation of MAPKs upon stimulation with Tri-DAP, reflected by phosphorylation of ERK½ and p38, was also stronger in HT29-Cl.19A/PepT1 cells than in HT29-Cl.19A/vector cells (Fig. 6B).
Fig. 6.
Expression of PepT1 in HT29-Cl.19A increases Tri-DAP-induced proinflammatory response. HT29-Cl.19A stably transfected with a vector encoding PepT1 (HT29/PepT1) or the empty vector (HT29/vector) at passages 25–30 were grown on plastic plates for 8 days. Cells were stimulated with 5 mM Tri-DAP for the indicated times. Levels of IκB-α (A), phospho-ERK½ (B, top), and phospho-p38 (B, bottom) were analyzed by Western blot. All blots are representative of 3 determinations, and bars show means ± SD of relative intensity of the bands obtained from 3 determinations. (**P < 0.005; ***P < 0.001) vs. untreated HT29/PepT1; ($P < 0.05; $$P < 0.005) vs. untreated HT29/vector.
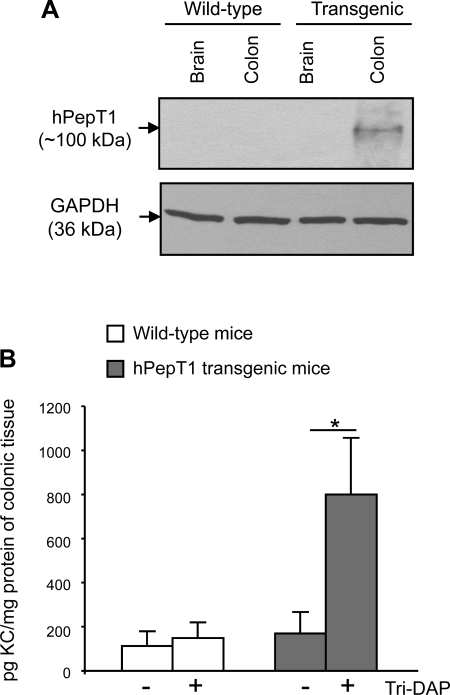
Finally, the role of PepT1 in Tri-DAP-mediated proinflammatory response in IECs was confirmed using a PepT1 transgenic mouse model which we previously generated (7). Transgenic mice expressed human (h) PepT1 specifically in IECs since hPepT1 expression was controlled by a villin promoter. As expected, Western blot analysis showed that hPepT1 is expressed only in colonic epithelial cells of transgenic mice, but not in those from wild-type mice (Fig. 7A). Colonic tissues from wild-type and transgenic mice were cultured and stimulated with 5 mM Tri-DAP or vehicle for 12 h, and the levels of KC, a potent murine neutrophil chemoattractant (16), in the supernatants were measured by ELISA. We found that Tri-DAP induced a significant increase in KC secretion in colonocytes from transgenic mice (Fig. 7B). In contrast, in the colons from wild-type mice, where PepT1 is not or is poorly expressed (19, 34), KC production was not significantly increased in response to Tri-DAP stimulation. These results strongly support the in vitro finding that PepT1 mediates Tri-DAP transport into IECs.
Fig. 7.
Tri-DAP increases keratinocyte-derived chemokine (KC) production in the colons from PepT1 transgenic mice. A: Western blot analysis of human PepT1 (hPepT1) expression in tissue extracts from wild-type and transgenic mice. B: colonic tissues from 6–8 wk old, sex-matched wild-type and PepT1 transgenic mice were cultured and stimulated with 5 mM Tri-DAP for 12 h. KC levels in the supernatant were determined by ELISA. Data are means ± SE of N = 9 mice per group per condition. Data are representative of 2 determinations with similar results. *P < 0.05.
Collectively, our results suggest that PepT1 can transport Tri-DAP into IECs, thereby increasing intestinal inflammation.
DISCUSSION
Bacteria are normally present in the gastrointestinal tract, where their density increases from very low levels in the stomach, which is nearly sterile, to 1011-1012 count-forming units per gram in the colon (24). It is known that bacteria produce peptides that can be transported into IECs. The intestinal di/tripeptide transporter PepT1 has been shown to transport the proinflammatory bacterial peptides MDP and fMLP into IECs (20, 32). Under normal conditions, PepT1 is not expressed in the colon (19), thereby limiting the access of these peptides into the interior of cells. PepT1 expression is turned on in the colon during chronic inflammation, such as IBD. In the present study, we investigated whether the tripeptide Tri-DAP, a proinflammatory breakdown product of bacterial peptidoglycan, is transported by PepT1.
It has been reported that Tri-DAP is the optimal peptidoglycan motif recognized by NOD1 but not NOD2 (2, 10, 12), whereas the dipeptide γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP) is the minimal NOD1-recognized motif (12). iE-DAP failed to induce production of IL-8 in the colonic epithelial cells LoVo and SW620; however, its proinflammatory properties were restored after chemical modification of its NH2 residues by acylation, which improves its membrane permeability (18). Pathogenic bacteria, such as Shigella flexneri (11) and Helicobacter pylori (33), activate NOD1 signaling in epithelial cells either by cell invasion or via their secretion system, suggesting that peptidoglycan must be internalized into cells to induce downstream signaling events. Collectively, these studies indicate that colonic epithelia only respond to the peptidoglycan motifs, Tri-DAP or iE-DAP, when these products are present in the cytosol. Thus colonocytes fail to transport Tri-DAP or are “inert” to Tri-DAP under normal conditions. A recent study demonstrated that Tri-DAP was transported into lung epithelial cells by PepT2 (30), a transporter closely related to PepT1 (8). Since PepT2, unlike PepT1, is not expressed in the gastrointestinal tract (8), we hypothesized that PepT1 could be responsible for Tri-DAP transport in the gut.
Using various approaches, we show here that PepT1 is capable of mediating the transport of Tri-DAP into human IECs. First, we observed that the transport of [14C]Gly-Sar, a reference substrate of PepT1, was inhibited by Tri-DAP in Caco2-BBE cells, a small intestinal epithelial cell line that express PepT1, indicating that Tri-DAP is transported by PepT1. A Ki value of ∼4.8 mM was determined for Tri-DAP. Several studies have been performed on Caco2 cells to determine the affinity constants for numerous substrates of PepT1 by measuring their ability to inhibit transport of radiolabeled Gly-Sar, and a broad range of Ki between 2 μM-14 mM has been reported (1). The Ki for Tri-DAP found in our study is therefore in the range of inhibitory constants that have been described for PepT1 substrates. Second, addition of Tri-DAP to Caco2-BBE cells led to a drop of the intracellular pH, further demonstrating that Tri-DAP is transported by the peptide/H+ cotransporter PepT1.
We also found that Tri-DAP induced activation of proinflammatory pathways in Caco2-BBE cells, characterized by activation of NF-κB and MAPKs and production of IL-8. Importantly, downregulation of PepT1 expression in Caco2-BBE cells decreased Tri-DAP-induced inflammatory response, whereas exogenous expression of PepT1 in human colonic HT29-Cl.19A cells increased this inflammatory response. A study recently published by Lee et al. (17) reported that Tri-DAP required up to 3–4 h to induce a detectable activation of a NF-κB-luciferase reporter gene in the human epithelial kidney cells HEK293T, which are reported to express very low levels of PepT1. However, when the cells were stimulated with Tri-DAP in the presence of the membrane-permeabilizing agent digitonin, a strong activation of the NF-κB-luciferase reporter gene was detected within 10 min of treatment. In intestinal epithelial Caco2-BBE cells, which highly express PepT1, Tri-DAP induced significant activation of NF-κB within 30 min of stimulation, suggesting that these cells efficiently uptake Tri-DAP and highlighting a potential role for PepT1 in this process. Expression of PepT1 in HT29-Cl.19A cells efficiently increased Tri-DAP-induced activation of NF-κB and MAPK pathways, further confirming the importance of PepT1 for the proinflammatory effects of Tri-DAP in IECs. These were validated by the findings that Tri-DAP significantly increased KC production in colonic tissues from PepT1 transgenic mice. However, in our study, Tri-DAP also stimulated NF-κB and MAPK in HT29-Cl.19A cells lacking PepT1 expression (HT29-Cl.19A/vector), although to a lesser extent than in HT29-Cl.19A/PepT1 cells. Therefore, we cannot rule out the possibility that other transporter(s)/receptor(s) for Tri-DAP may also be present in IECs and make a marginal contribution to the proinflammatory effects of this peptide.
In an elegant study of the inhibitory effects of Tri-DAP on [3H]Gly-Sar uptake in Xenopus laevis oocytes microinjected with a plasmid encoding PepT1, Ismair et al. (14) found that Tri-DAP did not compete with Gly-Sar for PepT1. In this study, the authors did not directly study PepT1-mediated cotransport of Tri-DAP/H+ in oocytes expressing PepT1. Furthermore, PepT1 normally has a low affinity for its substrates (8). In our competition transport study, Tri-DAP significantly inhibited [14C]Gly-Sar uptake in Caco2-BBE cells only at concentrations ≥5 mM. Thus, in the study of Ismair et al., it is likely that the concentration of Tri-DAP used (25 μM) might have been too low to compete out Gly-Sar.
Interestingly, a recent study by Girardin's group (17) identified endocytosis as a new route for NOD1 ligands to enter cells and induce inflammation. This study also demonstrated that the SLC15A4 transporter, an isoform of the proton/oligopeptide cotransporter family (8), is involved in the transport of NOD1 ligands in early endosomes. Expression of SLC15A4 is significantly increased in the colon from patients with IBD (17). Recently, Zucchelli et al. (35) reported that a PepT1 gene polymorphism was associated with IBD and that this mutation increased the transport of MDP mediated by PepT1. It would be therefore interesting to assess the affinity of Tri-DAP for this form of PepT1 during IBD. Together with these studies, our results reported here suggest a role for the SLC15 transporter family during intestinal inflammation.
In conclusion, our study demonstrates that, once expressed in human IECs, PepT1 efficiently transports the tripeptide Tri-DAP into the interior of the cells, inducing inflammatory response. We speculate that during IBD the induced expression of PepT1 in the colon could be involved in the transport of Tri-DAP into colonocytes, thereby aggravating inflammation.
GRANTS
This work was supported by grants from the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants R24-DK-064399 (center grant), R56-DK-061941 (to D. Merlin), and RO1-DK55850 (to S. V. Sitaraman). D. Merlin is a recipient of a Senior Research Award from the Crohn's and Colitis Foundation of America. G. Dalmasso is a recipient of a Research Fellowship Award from the Crohn's and Colitis Foundation of America.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address for L. Charrier-Hisamuddin: Division of Gastroenterology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104.
REFERENCES
- 1.Brandsch M, Knutter I, Leibach FH. The intestinal H+/peptide symporter PEPT1: structure-affinity relationships. Eur J Pharm Sci 21: 53–60, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, Valvano MA, Foster SJ, Mak TW, Nunez G, Inohara N. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol 4: 702–707, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Charrier L, Driss A, Yan Y, Nduati V, Klapproth JM, Sitaraman SV, Merlin D. hPepT1 mediates bacterial tripeptide fMLP uptake in human monocytes. Lab Invest 86: 490–503, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Charrier L, Merlin D. The oligopeptide transporter hPepT1: gateway to the innate immune response. Lab Invest 86: 538–546, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Dalmasso G, Charrier-Hisamuddin L, Thu Nguyen HT, Yan Y, Sitaraman S, Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology 134: 166–178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmasso G, Nguyen HT, Yan Y, Charrier-Hisamuddin L, Sitaraman SV, Merlin D. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS One 3: e2476, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalmasso G, Nguyen HT, Yan Y, Rojas M, Sitaraman SV, Merlin D. Expression of PepT1 aggravates intestinal inflammation (Abstract). Gastroenterology 136: A-40, 2009 [Google Scholar]
- 8.Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflügers Arch 447: 610–618, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Freeman TC, Bentsen BS, Thwaites DT, Simmons NL. H+/di-tripeptide transporter (PepT1) expression in the rabbit intestine. Pflügers Arch 430: 394–400, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jehanno M, Viala J, Tedin K, Taha MK, Labigne A, Zahringer U, Coyle AJ, DiStefano PS, Bertin J, Sansonetti PJ, Philpott DJ. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300: 1584–1587, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep 2: 736–742, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem 278: 41702–41708, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol 72: 847–855, 2002 [PubMed] [Google Scholar]
- 14.Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE. hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can J Physiol Pharmacol 84: 1313–1319, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Jobin C, Haskill S, Mayer L, Panja A, Sartor RB. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol 158: 226–234, 1997 [PubMed] [Google Scholar]
- 16.Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol 26: 307–316, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Tattoli I, Wojtal KA, Vavricka SR, Philpott DJ, Girardin SE. pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling. J Biol Chem 284: 23818–23829, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, Fujimoto Y, Kawasaki A, Foster SJ, Horie Y, Mak TW, Nunez G, Chinnaiyan AM, Fukase K, Inohara N. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med 203: 203–213, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merlin D, Si-Tahar M, Sitaraman SV, Eastburn K, Williams I, Liu X, Hediger MA, Madara JL. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology 120: 1666–1679, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Merlin D, Steel A, Gewirtz AT, Si-Tahar M, Hediger MA, Madara JL. hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J Clin Invest 102: 2011–2018, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nduati V, Yan Y, Dalmasso G, Driss A, Sitaraman S, Merlin D. Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors. J Biol Chem 282: 1359–1373, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen HT, Charrier-Hisamuddin L, Dalmasso G, Hiol A, Sitaraman S, Merlin D. Association of PepT1 with lipid rafts differently modulates its transport activity in polarized and nonpolarized cells. Am J Physiol Gastrointest Liver Physiol 293: G1155–G1165, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Nguyen HT, Dalmasso G, Powell KR, Yan Y, Bhatt S, Kalman D, Sitaraman SV, Merlin D. Pathogenic bacteria induce colonic PepT1 expression: an implication in host defense response. Gastroenterology 137: 1435–1447, e1–e2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 7: 688–693, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogihara H, Saito H, Shin BC, Terado T, Takenoshita S, Nagamachi Y, Inui K, Takata K. Immuno-localization of H+/peptide cotransporter in rat digestive tract. Biochem Biophys Res Commun 220: 848–852, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Sai Y, Tamai I, Sumikawa H, Hayashi K, Nakanishi T, Amano O, Numata M, Iseki S, Tsuji A. Immunolocalization and pharmacological relevance of oligopeptide transporter PepT1 in intestinal absorption of beta-lactam antibiotics. FEBS Lett 392: 25–29, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Shimakura J, Terada T, Shimada Y, Katsura T, Inui K. The transcription factor Cdx2 regulates the intestine-specific expression of human peptide transporter 1 through functional interaction with Sp1. Biochem Pharmacol 71: 1581–1588, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA. IL-1 beta-converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci USA 98: 13249–13254, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steel A, Nussberger S, Romero MF, Boron WF, Boyd CA, Hediger MA. Stoichiometry and pH dependence of the rabbit proton-dependent oligopeptide transporter PepT1. J Physiol 498: 563–569, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swaan PW, Bensman T, Bahadduri PM, Hall MW, Sarkar A, Bao S, Khantwal CM, Ekins S, Knoell DL. Bacterial peptide recognition and immune activation facilitated by human peptide transporter PEPT2. Am J Respir Cell Mol Biol 39: 536–542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thwaites DT, Hirst BH, Simmons NL. Direct assessment of dipeptide/H+ symport in intact human intestinal (Caco-2) epithelium: a novel method utilising continuous intracellular pH measurement. Biochem Biophys Res Commun 194: 432–438, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology 127: 1401–1409, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5: 1166–1174, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Wojtal KA, Eloranta JJ, Hruz P, Gutmann H, Drewe J, Staumann A, Beglinger C, Fried M, Kullak-Ublick GA, Vavricka SR. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos 37: 1871–1877, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, Assadi G, Lindgren GB, Svanfeldt M, Janson M, Noble CL, Pettersson S, Lappalainen M, Paavola-Sakki P, Halme L, Farkkila M, Turunen U, Satsangi J, Kontula K, Lofberg R, Kere J, D'Amato M. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis 15: 1562–1569, 2009 [DOI] [PubMed] [Google Scholar]