Abstract
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency of premature infants. Previously, we showed that luminal bile acids (BAs) are increased and correlated with disease development and that the apical sodium-dependent BA transporter (ASBT), which transports BAs from the ileal lumen into enterocytes, is upregulated in rats with NEC. We hypothesized that intraenterocyte, rather than luminal, BAs are associated with NEC and that upregulation of ASBT may be a mechanism by which this occurs. Neonatal rats with or without the ASBT inhibitor SC-435, mice in which ASBT was knocked out, and mice that overproduce BAs were subjected to the NEC protocol. Disease development, ASBT, and the farnesoid X receptor protein, along with luminal and intraenterocyte BA levels, were assessed. In addition, ileal sections from premature infants with and without NEC were examined for ASBT via immunohistology and real-time PCR. When BAs were not transported into enterocytes (rats given SC-435 and ASBT knockout mice), severity and incidence of NEC were reduced. In contrast, in mice that overproduce BAs, ASBT was elevated, intraenterocyte BAs were increased, and disease development was increased. ASBT staining was more intense on the apical membrane of ileal enterocytes from premature infants with NEC than premature infants with non-NEC diagnoses. In addition, ASBT mRNA levels were significantly higher in infants with NEC. These data show that accumulation of intraenterocyte BAs contributes to disease development, elevated ASBT increases disease severity in experimental models of NEC, and ASBT is elevated in human NEC. These data confirm that BAs and upregulation of ASBT play a crucial role in NEC pathogenesis and suggest that inhibition of ASBT could be utilized as a therapeutic modality against this disease.
Keywords: epithelial transport, SC-435, bile salt export pump
necrotizing enterocolitis (NEC) affects thousands of newborns each year in the United States alone, and with mortality rates of 10–50%, this disease remains a major cause of morbidity and mortality in prematurely born infants (8, 29). Severe NEC is characterized by an extensive hemorrhagic inflammatory necrosis of the distal ileum and proximal colon (27). The disease can be mild to severe, with clinical presentation ranging from abdominal distension, pneumatosis intestinalis, occult or frank blood in stools, intestinal gangrene, bowel perforation, sepsis, and shock (4, 36). Survivors of a severe episode of NEC frequently suffer the sequelae of short bowel syndrome (8, 30), resulting in long-term medical expenses and chronic gastrointestinal difficulties (44). The pathophysiology of this disease remains poorly understood; however, prematurity, enteral feeding, and bacterial colonization are considered major risk factors. The incidence of NEC varies with gestational age, is highest among smaller, more premature neonates, and is rare in full-term neonates or older children (7, 43).
Bile acids (BAs) are physiological compounds that facilitate emulsification, absorption, and transport of fats and sterols in the intestine and liver. We previously showed that ileal luminal BA accumulation contributes to ileal damage in a neonatal rat model of NEC (17). It has been proposed that breast-fed newborns may not have the same need for BAs as formula-fed newborns, because digestion of human milk fat is different from digestion of solid foods or cow's milk-based formula (1, 20–22, 26, 32). Diet can also affect levels and composition of BAs; levels of fecal deoxycholic acid (DCA) and lithocholic acid (LCA) and the percentage of secondary BAs excreted in feces are significantly lower in breast-fed than formula-fed infants (19). Importantly, the incidence of NEC is 6–10 times higher in formula-fed than breast-fed infants (33).
Enterohepatic circulation of BAs is an essential process involving coordinated regulation of BA synthesis in the liver, transport of BAs from the liver to the intestine, and transport of BAs back to the liver. Ileal luminal BAs are transported across the apical membrane of enterocytes via the apical sodium-dependent BA transporter (ASBT). While we have shown that ASBT is significantly elevated in neonatal rats with NEC (17), the rat ASBT gene is not regulated via a negative-feedback mechanism by BAs, as is the mouse (or human) ASBT gene (2, 35). In addition, the contribution of luminal vs. intraenterocyte BAs has not been evaluated in experimental NEC. We hypothesized that ASBT plays a crucial role in disease development and that when BA transport from the lumen into enterocytes is blocked, development of experimental NEC will decrease.
To more comprehensively examine ASBT in NEC, we utilized rat and mouse NEC models in all experiments. SC-435, a competitive inhibitor of ASBT, inhibits only intestinal ASBT when administered orally (6, 31) and was used to inhibit ileal uptake of BAs during the development of experimental NEC in neonatal rats. Mice in which the ASBT gene has been knocked out were also used in these studies. Additionally, we utilized mice that overexpress the bile salt export pump (BSEP), the transporter responsible for secretion of BAs from the liver into bile. In these mice, biliary secretion of bile and hydrophobicity of the bile salt pool are increased. Finally, we examined ASBT in tissue samples taken from human infants with surgical NEC.
MATERIALS AND METHODS
Animal models.
The protocols were approved by the Animal Care and Use Committee of the University of Arizona (A-324801-95081). Sprague-Dawley rats (Charles River Labs, San Diego, CA) were collected by cesarean section 1 day prior to scheduled birth. Three studies using 12 separate litters were utilized. Pups were randomly assigned to three experimental groups: fed by a foster mother (DF, n = 15), hand-fed a cow's milk-based formula (NEC, n = 34), and hand-fed a cow's milk-based formula supplemented with SC-435 (5 μg·g−1·day−1; Pfizer, Groton, CT; NEC + SC-435, n = 29). All pups were subjected to asphyxia and cold stress (A/C stress) twice daily (11, 14, 16). After 96 h, all surviving animals were terminated via decapitation.
129S1/SvImJ (ASBT WT), 129-Slc10a2 −/− (ASBT KO), and C57BL/6J (BSEP WT) mice were purchased from Jackson Laboratory (Bar Harbor, ME). BSEP-overexpressing mice (C57BL/6 TTR-Abcb11; BSEP OE) were kindly provided by Dr. Richard M. Green (Northwestern University, Evanston, IL) (13, 23). Each strain was divided into two groups: one containing pups that were dam-fed [ASBT WT DF (n = 10), ASBT KO DF (n = 10), BSEP WT DF (n = 10), and BSEP OE DF (n = 10)] and the other consisting of pups subjected to the NEC protocol, i.e., newborn mice collected immediately after birth to prevent suckling of maternal milk and hand-fed a cow's milk-based formula using the Hoshiba nipple [Yajima style (25, 45); Meiji Dairies, Tokyo, Japan] every 2–3 h for up to 3 days [ASBT WT NEC (n = 16), ASBT KO NEC (n = 24), BSEP WT NEC (n = 25), and BSEP OE NEC (n = 21)]. All pups from the DF and NEC groups were subjected to A/C stress twice daily (18). Three separate studies, using a minimum of three litters per strain per study, were utilized for ASBT WT and ASBT KO studies; and four separate studies, using a minimum of four litters per strain, were utilized for BSEP WT and BSEP OE studies.
NEC evaluation.
Pathological changes in intestinal architecture were evaluated using our previously published NEC scoring system (11, 17, 18). Histological changes were scored by a blinded evaluator and graded as follows: 0 (normal; i.e., no damage), 1 (mild; i.e., slight submucosal and/or lamina propria separation), 2 (moderate; i.e., moderate separation of submucosa and/or lamina propria and/or edema in submucosal and muscular layers), 3 (severe; i.e., severe separation of submucosa and/or lamina propria and/or severe edema in submucosa and muscular layers, regional villous sloughing), and 4 (necrosis; i.e., loss of villi and necrosis). Intermediate scores of 0.5, 1.5, 2.5, and 3.5 were utilized to more accurately assess levels of ileal damage when necessary (10, 11, 14–16, 18). To determine the incidence of NEC, only animals with histological scores ≥2 were considered to have developed experimental NEC (10, 11, 14–16, 18).
Human ileal sections.
Deidentified, archived human ileal sections from preterm infants with NEC (n = 5; median gestational age 27 wk, median age at tissue collection 14 days), non-NEC diagnoses [NND (n = 5): congenital atresia (n = 3) and spontaneous ileal perforation (n = 2); median gestational age 34 wk, median age at tissue collection 10 days], or ileostomy take-downs after NEC diagnosis [ITD after NEC (n = 4); median gestational age 27 wk, median age at tissue collection 78 days] were obtained under appropriate oversight by the Institutional Review Board of Vanderbilt University. Sections were stained with anti-human ASBT antibody (P. A. Dawson, Wake Forest University) and then with Alexa Fluor 488 secondary antibody (Invitrogen, Eugene, OR). Control slides were stained with secondary antibody alone. Stained slides were mounted with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) to stain nuclei. Sections were photographed using an Olympus IX-70 inverted fluorescence microscope equipped with a ×40 objective.
BA levels and composition.
In rats, ileal luminal BA levels were determined by flushing a 3-cm section of distal ileum with sterile, cold PBS (17). After it was flushed with additional PBS, the same section of ileum was weighed and then homogenized in fresh PBS for determination of intraenterocyte BA levels. In mice, a 1-cm section of distal ileum was flushed and then homogenized as described above. Samples were frozen at −70°C until assayed. Total BA levels were determined using the Total Bile Acids Assay Kit (Diazyme, San Diego, CA) according to the manufacturer's protocol. BA composition was determined from intraenterocyte samples by the Arizona Cancer Center Analytical Core Shared Service (Tucson, AZ) using a TSQ Quantum triple-quadrupole LC/MS/MS system with a Surveyor MS pump and auto sampler (Thermo Finnigan, San Jose, CA). The mass spectrometer was run in negative-ion mode utilizing electrospray ionization. Selective ion monitoring was used for detection. Chromatographic separation was accomplished using a Beckman Ultrasphere-XL C-18 column. Cholic acid (CA), DCA, LCA, chenodeoxycholic acid (CDCA), and ursodeoxycholic acid levels were evaluated.
RNA preparation.
RNA from fixed, paraffin-embedded human tissue was extracted using the FFPE RNA extraction kit (SA Biosciences, Fredrick, MD). RNA concentration was quantified by UV spectrophotometry at 260 nm, and the purity was determined by the ratio of absorbance at 260 nm to absorbance at 280 nm (A260/A280; SPECTRAmax PLUS, Molecular Devices, Sunnyvale, CA). The integrity of RNA was verified by electrophoresis on a formaldehyde agarose gel. Only tissue from animals without full necrosis was extracted and analyzed.
Reverse transcription and real-time PCR.
Single-stranded cDNA was reverse-transcribed as previously described in detail (12). The amounts of total RNA used in the RT reactions were calculated from the absorbance at 260 nm and verified by densitometry of the 28S rRNA band separated on denaturing agarose gels (by Gel Doc 1000 Documentation System with Molecular Analyst/PC software, Bio-Rad, Hercules, CA). Real-time PCR amplification was performed using Assay By Design (Applied Biosystems, Foster City, CA) sequenced primers and probes for human ASBT (premade primer and probes). Samples were analyzed as previously described (15–17).
Protein preparation.
Individual frozen ileum samples were homogenized in a 5× volume of ice-cold homogenization buffer [50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% sodium DCA, 1% Triton X-100, 50 mM DTT, 50 μg/ml aprotinin, 50 μg/ml leupeptin, and 5 mM PMSF]. The homogenates were centrifuged at 10,000 rpm for 5 min at 4°C, and the supernatant was collected. Total protein concentration was quantified using the Bradford protein assay.
Western blotting.
For protein analysis, samples were run on a 10–20% gradient polyacrylamide gel and transferred to Immuno-Blot polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked with 5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (Sigma, St. Louis, MO) and then incubated with anti-ASBT (kindly provided by P. A. Dawson, Wake Forest University) or anti-farnesoid X receptor (FXR; Santa Cruz Biotechnologies, Santa Cruz, CA) antibody overnight at 4°C. After they were washed, the membranes were incubated at room temperature with the appropriate horseradish peroxidase-conjugated secondary antibody. Proteins were visualized with a chemiluminescent system (Pierce, Rockford, IL) and exposed to X-ray film. Membranes were stripped and probed with anti-β-actin (Sigma-Aldrich, St. Louis, MO) to determine equal loading of protein. Densitometry was performed to compare protein expression between groups using Bio-Rad QuantityOne software, and data were normalized on the basis of densitometry readings for β-actin.
Statistics.
Statistical analyses between groups were performed using ANOVA followed by Fisher's protected least significant difference test. Analysis of NEC score was accomplished using the Mann-Whitney test for nonparametric values, and the χ2 test was utilized to analyze differences in incidence of disease. Correlation analyses were performed using Spearman's rank correlation. All statistical analyses were determined using the statistical program StatView (Abacus Concepts, Berkeley, CA).
RESULTS
Development of experimental NEC.
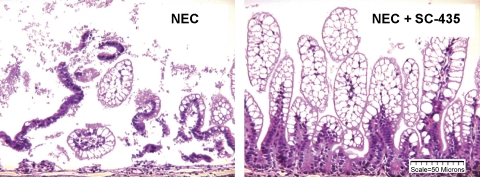
Histological examination of the distal ileum showed significantly higher median ileal damage scores in the NEC than the DF rats and significantly less damage in the NEC + SC-435 than the NEC animals (Table 1). Damage to villi and submucosa was evident in the NEC group, with significantly less ileal damage in the NEC + SC-435 group (Fig. 1). In addition, the incidence of NEC was significantly lower in neonatal rats given the ASBT inhibitor, where 3 of 29 animals developed disease compared with 20 of 32 animals in the NEC group. As expected, the incidence of NEC in the DF rats was 0% (Table 1).
Table 1.
Incidence and severity of NEC in the neonatal rat model
| Group | n | Incidence, % | Median Ileal Damage, interquartile range |
|---|---|---|---|
| DF | 15 | 0.0* | 0.0 (0.00)* |
| NEC | 32 | 62.5 | 2.0 (1.25) |
| NEC + SC-435 | 29 | 10.3* | 0.5* (1.00) |
Histological ileal damage was scored on a scale of 0 (normal) to 4 (necrosis). Animals with an ileal damage score ≥2 were considered to have developed necrotizing enterocolitis (NEC). DF, dam-fed animals; NEC, animals hand-fed with a cow's milk-based formula; NEC + SC-435, NEC animals given SC-435.
P ≤ 0.05 vs. NEC. Incidence of NEC was determined using the χ2 test; analysis of median NEC score was determined using the Mann-Whitney test.
Fig. 1.
Ileal structure in neonatal rat model of necrotizing enterocolitis (NEC). Representative histology shows areas of necrosis and damage to ileal architecture in the NEC group compared with NEC animals given SC-435 (NEC + SC-435).
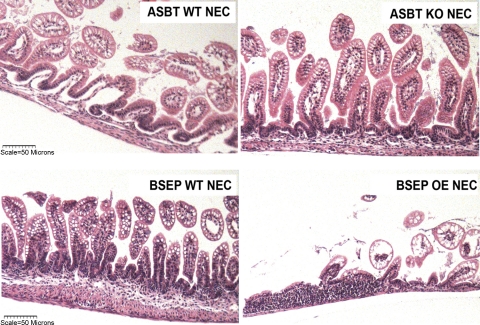
Severity and incidence of disease were greater in ASBT WT NEC and ASBT KO NEC than DF mice (Table 2). However, ileal damage was significantly less in ASBT KO NEC than ASBT WT NEC mice. Histological evaluation of distal ileum revealed significant damage to the villi and submucosa in ASBT WT NEC mice that was not observed in the ASBT KO NEC group (Fig. 2, top). The incidence of NEC was also significantly lower in ASBT KO NEC than ASBT WT NEC mice: 6 of 22 ASBT KO NEC mice developed NEC compared with 13 of 18 mice with functional ASBT (Table 2).
Table 2.
Incidence and severity of NEC in neonatal mouse models
| Group | n | Incidence, % | Median Ileal Damage, interquartile range |
|---|---|---|---|
| ASBT WT DF | 10 | 0.0 | 0.5 (0.50) |
| ASBT WT NEC | 18 | 72.2a,b | 2.5a,b (0.75) |
| ASBT KO NEC | 22 | 27.3c | 1.5 (1.00) |
| ASBT KO DF | 10 | 0.0 | 0.5 (0.50) |
| BSEP WT DF | 10 | 0.0 | 0.0 (0.0) |
| BSEP WT NEC | 24 | 12.5d | 0.5d (0.75) |
| BSEP OE NEC | 14 | 71.4e | 2.5e (1.50) |
| BSEP OE DF | 10 | 0.0 | 0.5 (0.50) |
Histological ileal damage was determined on a scale of 0 (normal) to 4 (necrosis). Animals with an ileal damage score ≥2 were considered to have developed NEC. ASBT, apical sodium-dependent bile acid transporter; WT, wild-type; KO, knockout; BSEP, bile salt export pump; OE, overexpressing. Incidence of NEC was determined using the χ2 test. Analysis of median NEC score was determined using the Mann-Whitney test.
P ≤ 0.01 vs. ASBT WT DF.
P ≤ 0.01 vs. ASBT KO NEC.
P ≤ 0.05 vs. ASBT KO DF.
P ≤ 0.01 vs. BSEP OE NEC.
P ≤ 0.01 vs. BSEP OE DF.
Fig. 2.
Ileal structure in neonatal mouse models of NEC. Top: representative histology showing severe separation of lamina propria and moderate separation of submucosa in the apical sodium-dependent bile acid transporter (ASBT) wild-type (WT) NEC (ASBT WT NEC) group compared with the ASBT knockout (KO) NEC (ABST KO NEC) group. Bottom: representative histology showing areas of complete villous loss in the bile salt export pump (BSEP)-overexpressing (OE) group compared with the BSEP WT NEC group.
In contrast, the severity and incidence of NEC were significantly higher in the BSEP OE NEC than BSEP WT NEC mice (Table 2, Fig. 2, bottom). Because of unusually high morbidity in the BSEP OE NEC group, both groups were subjected to formula feeding and A/C for only 48 h. The low incidence of NEC in the BSEP WT NEC group, where only 3 of 24 mice developed signs of NEC, is likely due to the shortened protocol [NEC incidence at 72 h for the BSEP WT NEC strain is traditionally 62% (18)].
ASBT protein in rat and mouse models of NEC.
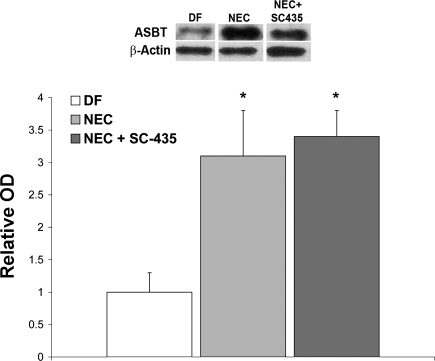
We previously showed that ASBT expression is increased in neonatal rats with NEC compared with control littermates (17); however, it is unclear whether this increase is associated with disease development. Thus we examined ASBT expression in our rat and mouse models. Because SC-435 is a competitive inhibitor of ASBT, it was not surprising that ASBT protein levels in the NEC + SC-435 group were not significantly different from ASBT protein levels in the NEC group that was not given the inhibitor (Fig. 3).
Fig. 3.
Representative Western blots and densitometry data from the rat NEC model. After normalization with β-actin, mean optical density (OD) for the dam-fed (DF) group (n = 5) was assigned a value of 1.0, and mean ODs for groups hand-fed with a cow's milk-based formula (NEC, n = 9) and NEC + SC-435 animals (n = 9) were determined relative to this number. ASBT was significantly elevated in the NEC and NEC + SC-435 groups compared with the DF group (*P ≤ 0.05 by ANOVA).
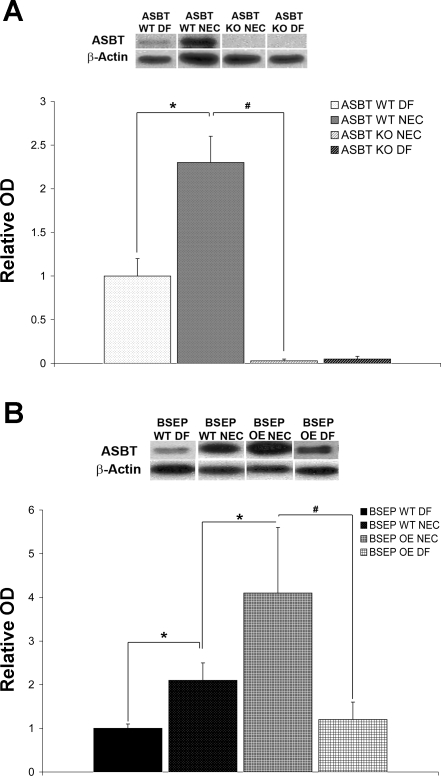
We have shown that subjecting mice to the NEC protocol (formula-fed, A/C-stressed) results in development of NEC compared with DF, A/C-stressed littermates (18). We confirmed that ASBT protein is increased during the development of experimental NEC in ASBT WT NEC mice compared with ASBT WT DF mice. As expected, ASBT protein was significantly higher in ASBT WT NEC than ASBT KO NEC mice (Fig. 4A). It has been previously shown that ileal ASBT mRNA is significantly reduced in adult mice that overexpress BSEP (13). Interestingly, we found that ASBT protein was significantly increased in the BSEP OE NEC group compared with the BSEP WT NEC pups (Fig. 4B). Consistent with ASBT protein levels in the ASBT WT mice, BSEP WT DF and BSEP OE DF mice had significantly less ASBT than littermates subjected to the NEC protocol (BSEP WT NEC and BSEP OE NEC, respectively). There was no statistical difference between the BSEP WT DF and the BSEP OE DF group, however (Fig. 4B).
Fig. 4.
ASBT protein in neonatal mouse models of NEC. A: representative Western blots and densitometry data from ASBT WT DF (n = 5), ASBT WT NEC (n = 8), ASBT KO NEC (n = 8), and ASBT KO DF (n = 5) mice. After normalization with actin, mean OD for the ASBT WT DF group was assigned a value of 1.0, and mean ODs for all other groups were determined relative to this number. ASBT was significantly increased in the ASBT WT NEC group compared with ASBT WT DF and ASBT KO NEC groups (*P ≤ 0.05, #P ≤ 0.01). B: representative Western blots and densitometry data from BSEP WT DF (n = 5), BSEP WT NEC (n = 10), BSEP OE NEC (n = 10), and BSEP OE DF (n = 5) mice. After normalization with actin, mean OD for the BSEP WT DF group was assigned a value of 1.0, and mean ODs for all other groups were determined relative to this number. ASBT was significantly increased in BSEP WT NEC compared with BSEP WT DF animals (*P ≤ 0.05), BSEP OE NEC compared with BSEP WT NEC animals (*P ≤ 0.05), and BSEP OE NEC compared with BSEP OE DF animals (#P ≤ 0.01). Densitometry data are expressed as means ± SD. Statistical analyses were performed using ANOVA.
FXR expression in experimental NEC.
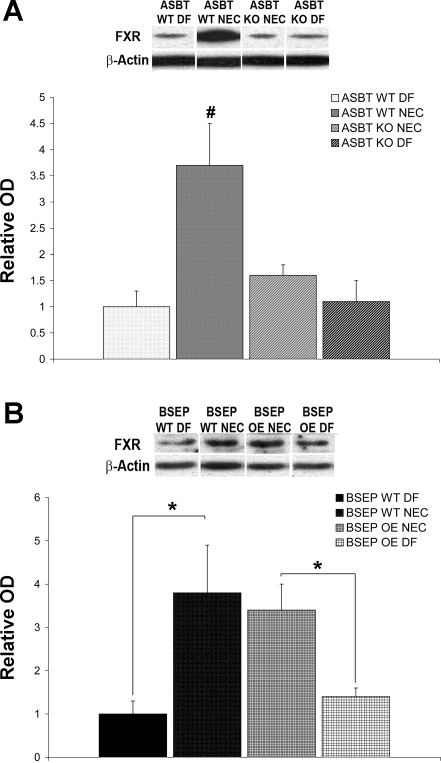
In mice and humans, ASBT expression is controlled by a negative-feedback regulatory mechanism, where hydrophobic BAs such as CDCA and CA can transcriptionally regulate their own biosynthesis and enterohepatic transport through activation of FXR (34). To examine possible mechanisms for increased ASBT during experimental NEC, we used Western blotting to measure FXR in our mouse models. FXR protein was significantly higher only in the ASBT WT NEC group than the ASBT KO NEC and DF groups (Fig. 5A). However, FXR protein was elevated in BSEP WT NEC and BSEP OE NEC mice compared with their DF counterparts (Fig. 5B).
Fig. 5.
Farsenoid X receptor (FXR) protein in neonatal mouse models of NEC. A: representative Western blots and densitometry data from ASBT WT DF (n = 5), ASBT WT NEC (n = 8), ASBT KO NEC (n = 8), and ASBT KO DF (n = 5) mice. After normalization with actin, mean OD for the ASBT WT DF group was assigned a value of 1.0, and mean ODs for all other groups were determined relative to this number. FXR was significantly increased in ASBT WT NEC compared with all other groups (#P ≤ 0.01). B: representative Western blots and densitometry data from BSEP WT DF (n = 5), BSEP WT NEC (n = 10), BSEP OE NEC (n = 10), and BSEP OE DF (n = 5) mice. After normalization with actin, mean OD for the BSEP WT DF group was assigned a value of 1.0, and mean ODs for all other groups were determined relative to this number. FXR was significantly increased in the BSEP WT NEC compared with the BSEP WT DF group and in the BSEP OE NEC compared with the BSEP OE DF group (*P ≤ 0.05). Densitometry data are expressed as means ± SD. Statistical analyses were performed using ANOVA.
BA levels and composition during development of NEC.
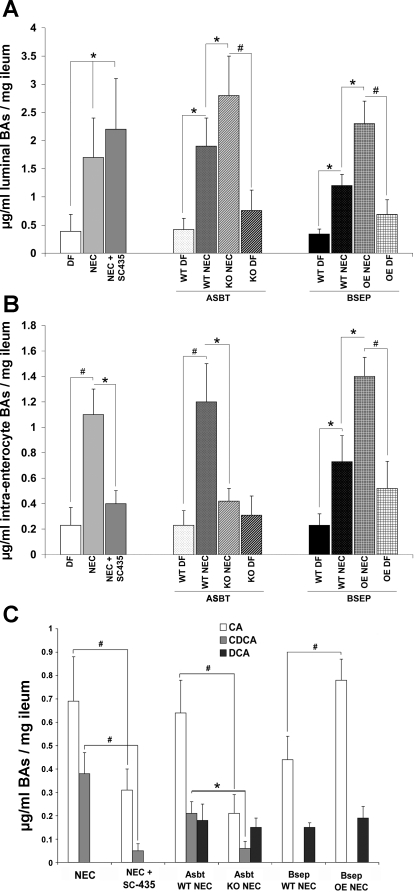
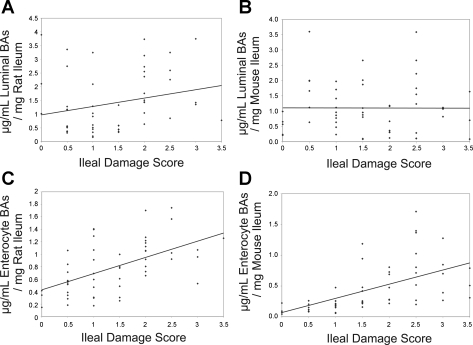
Total ileal luminal (Fig. 6A) and intraenterocyte (Fig. 6B) BA levels were examined in all groups. There was no significant difference between luminal BA levels in rats in the NEC group and luminal BA levels in the NEC + SC-435 ASBT inhibitor group. However, intraenterocyte BA levels were significantly decreased in the animals where ASBT was inhibited. Similar to our previously reported data in rats (17), luminal BA levels were significantly lower in all DF mice than in their littermates subjected to the NEC protocol (Fig. 6A). In contrast to rats in which ASBT was inhibited, total luminal BA levels were higher in ASBT KO NEC than ASBT WT NEC pups (Fig. 6A). However, total intraenterocyte BA levels were lower in the ASBT KO NEC than the ASBT WT NEC group (Fig. 6B). Total luminal and intraenterocyte BAs were significantly increased in the BSEP OE NEC group compared with the BSEP WT NEC group (Fig. 6, A and B). In addition, the correlation between total luminal or intraenterocyte BA levels and ileal damage scores in rat and mouse models of NEC were analyzed (Fig. 7). Ileal luminal BA levels in rat and mouse and intraenterocyte levels in rat and mouse models were compared with histological ileal damage scores. Rat luminal BAs (Fig. 7A) and rat and mouse intraenterocyte BAs (Fig. 7, C and D, respectively) were positively correlated with the ileal damage.
Fig. 6.
BAs in experimental NEC models. A: total luminal BAs. B: total intraenterocyte BAs. C: composition of intraenterocyte BAs. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid. Values are means ± SD. *P ≤ 0.05, #P ≤ 0.01. Statistical analyses were performed using ANOVA.
Fig. 7.
Correlation between total BA levels and ileal damage scores in rat (NEC and NEC + SC-435) and mouse (ASBT WT NEC, ASBT KO NEC, BSEP WT NEC, and BSEP OE NEC) models of NEC. Ileal luminal BA levels in rat (A) and mouse (B) models and intraenterocyte levels in rat (C) and mouse (D) models were compared with histological ileal damage scores. Rat luminal BAs (r = 0.320, P ≤ 0.05) and rat and mouse intraenterocyte BAs (r = 0.579 and 0.726, respectively; P ≤ 0.0001) are positively correlated with progression of ileal damage. Statistical analyses were performed using Spearman's rank correlation.
Because these results indicate that intraenterocyte, rather than luminal, BAs play a role in experimental NEC, BA composition was determined in intraenterocyte BAs and between NEC groups only (Fig. 6C). In rats subjected to the NEC protocol, CA and CDCA were the only BAs detected in either group. CA and CDCA were significantly lower in the NEC + SC-435 than the NEC group. CA and CDCA were significantly decreased in ASBT KO NEC mice compared with ASBT WT NEC mice; CDCA was detected at equal levels in both groups. In BSEP OE NEC and BSEP WT NEC mice, CA and CDCA were detectable, but a statistically significantly increase was observed only for CA.
ASBT in human preterm infants with NEC.
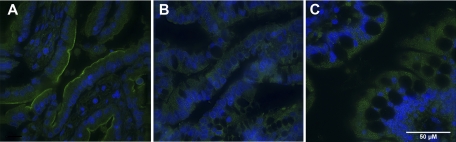
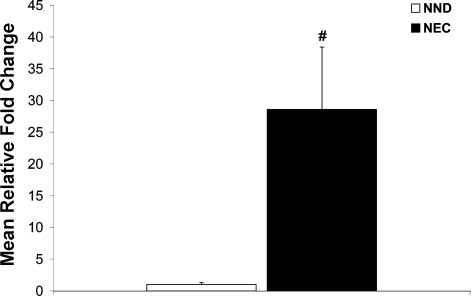
ASBT staining in ileal surgical samples from preterm infants diagnosed with NEC was compared with that in ileal surgical samples from preterm infants with non-NEC diagnoses (NND) and ITD after NEC diagnosis. ASBT was found at higher levels on the apical membrane of ileal enterocytes in tissue samples from human preterm infants diagnosed with NEC (Fig. 8A) than in preterm infants with NND (Fig. 8B) or ITD after NEC (Fig. 8C). Although there was staining in NND and ITD after NEC tissues, it was not localized on the apical membrane (where ASBT is found) but was dispersed throughout the cytoplasm of the enterocytes. In addition, ASBT mRNA was significantly higher in the NEC than the NND group (Fig. 9).
Fig. 8.
Representative ileal sections stained with anti-ASBT from premature infants diagnosed with NEC (A), non-NEC diagnoses (NND; B), and ileostomy-take down after NEC diagnosis (ITD after NEC; C). Note higher levels of ASBT on the apical membrane of ileal enterocytes from surgical specimens from NEC patients.
Fig. 9.
ASBT mRNA levels in NEC and NND infants. Mean steady-state mRNA levels for the NND group was assigned a value of 1.0, and mean mRNA levels for the NEC group were determined relative to this number. ASBT mRNA was significantly higher in the NEC than the NND group (#P ≤ 0.01). Values are means ± SD. Statistical analyses were performed using ANOVA.
DISCUSSION
Previously, we showed that BAs accumulate in the ileal lumen and are positively correlated with disease development in an experimental rat model of NEC. In these studies, ASBT protein was significantly increased in pups subjected to the NEC protocol (17). We hypothesized that elevated ASBT leads to increased accumulation of intraenterocyte BA levels, which, in turn, account for the increased incidence and severity of disease. Data presented here show that intraenterocyte, but not luminal, BA levels are important in experimental NEC development and that upregulation of ASBT contributes to disease by facilitating increased intraenterocyte levels. Importantly, our data from human tissue samples suggest that ASBT plays a role in the development of human NEC.
ASBT is the transporter responsible for most of the active transport of BAs from the lumen of the intestine into ileal enterocytes. There are fundamental differences between regulation of the rat and mouse/human ASBT gene by BAs, however. When rats are fed BAs, ileal ASBT expression can increase (39) or remain unchanged (2). In our original NEC studies using neonatal rat pups, ASBT was upregulated as BA levels increased as a result of disease development (17). In healthy mice and humans, however, increased BAs result in decreased expression of ASBT (9, 35). The negative-feedback regulation in mice and humans is mediated by the liver receptor homolog 1 (LRH-1), which activates the mouse and human ASBT promoter. Negative feedback by BAs is not seen in rats, which lack a functional LRH-1 cis-acting element (9). BAs such as CDCA and CA can activate FXR (34) and repress mouse and human ASBT through the interaction between activated FXR, the short heterodimer partner, and LRH-1.
In the present studies, formula-fed, A/C-stressed WT mice had elevated luminal and intraenterocyte BA levels with increased ASBT compared with WT mice that were DF and A/C-stressed. It is still unclear why ASBT is increased in rats (17) and mice subjected to the NEC protocol. In mice, one would expect elevated BAs to activate FXR and inhibit ASBT. Our data show increased FXR protein in mice subjected to the NEC protocol compared with DF littermates, except for ASBT KO NEC vs. ASBT KO DF mice. FXR was also increased in ASBT WT NEC compared with ASBT KO NEC mice. These increases cannot be explained simply by the increased ASBT in the groups with elevated FXR, because BSEP OE NEC mice have elevated ASBT compared with BSEP WT NEC mice without a corresponding elevation in FXR. The level of ileal FXR is significantly lower in neonatal animals than in weanlings or adults and might account for these discrepancies, as the increases in FXR in neonates may not reach physiologically significant levels to adequately regulate ASBT. It is also surprising that ASBT protein was similar in NEC rats compared with NEC rats given a competitive inhibiter of ASBT, SC-435, when previous studies in non-NEC and nonrat models showed that administration of SC-435 increased ASBT mRNA (6, 31). These differences may be due to the lack of a negative-feedback mechanism for ASBT in rats. Alternatively, the overall increase of ASBT expression when rats are subjected to the NEC protocol may override any additional increases after SC-435 administration.
The majority of premature infants that develop NEC are fed enterally with formula prior to disease onset (7, 8), and a protective role of maternal milk has been suggested (5, 11, 33, 37). The incidence of NEC in exclusively breast-fed low-birth-weight infants is 1% compared with an incidence of 7% in formula-fed infants in the same birth-weight group (44). Studies in animals have shown that many of the key processes involved in BA homeostasis are immature in newborns (3, 24, 26, 38, 40–42) and reach maturity at weaning. In these studies, fecal BAs were significantly lower in breast-fed than formula-fed infants, and formula-fed infants had more toxic, hydrophobic BAs than breast-fed infants (19). Thus there is evidence to suggest that BA levels and composition are altered in premature and formula-fed infants, who are at the highest risk for NEC. Prematurity, enteral feeding, and bacterial colonization are considered major risk factors for development of NEC. The role of BAs in disease development satisfies the majority of NEC-associated findings: the majority of normal BA reclamation occurs in the ileum, and thus altered BA recirculation would manifest mainly at the site of NEC injury, bacterial colonization is required for formation of more hydrophobic (and more toxic) BAs, and formula feeding induces higher BA levels. Our data clearly show that elevated ASBT contributes to disease development and that formula-fed animals have higher and more hydrophobic intraenterocyte BA levels than DF pups.
Surgical intervention is often necessary in the most severe cases of NEC and leaves few intact villi. For this reason, comprehensive evaluation of ASBT in resected ileum from infants with NEC can be problematic. However, ASBT staining on the apical membrane of ileal enterocytes was more intense and ASBT mRNA levels were greater in ileal sections from preterm infants in which NEC was diagnosed and villous structure was intact than in sections from infants with NND. ASBT was also seen at higher levels on the apical membrane from an NEC patient at diagnosis than later, when the ileum healed (data not shown). These data strongly suggest that our findings in rodent models of experimental NEC correspond to the human disease and point to dysregulation of BA transport as a significant contributor to disease pathogenesis. While samples from NND and ITD after NEC displayed fluorescent staining throughout the enterocytes, this staining was not found where ASBT should be localized and, therefore, may not represent the actual transporter.
It is known that accumulation of BA in the intestine can result in damage to the intestinal epithelium (28). Our previously published data strongly suggest that accumulation of BA in the ileum during experimental NEC is a critical component of disease development, a novel concept in experimental NEC pathogenesis (17). In the studies presented here, our data show that when BAs are not transported into ileal enterocytes, the incidence and severity of NEC are significantly decreased. Indeed, while luminal BA levels were elevated and incidence of disease was low in ASBT KO NEC mice, their intraenterocyte BA levels were quite low. Furthermore, overproduction of ASBT in BSEP OE NEC mice leads to more severe disease. We speculate that, in experimental NEC, BA levels increase in response to formula feeding. Formula feeding also leads to altered bacterial colonization of the gut, which may promote production of more toxic BAs. Immature ileal enterocytes, which are developmentally unprepared for the increased BA load, upregulate ASBT. As more BAs enter enterocytes, accumulation of BAs results in damage to the ileal architecture, which promotes a cascade of events required to develop NEC.
In summary, these data show that accumulation of intraenterocyte BAs contributes to the development of experimental NEC via upregulation of ileal ASBT. Furthermore, increased ASBT in human ileal samples from infants with NEC strongly suggests that the dysregulation of apical BA transport also occurs in human disease. Thus we provide additional evidence that BAs play a critical role in the pathogenesis of NEC. In addition, regulation of BAs via inhibition of ASBT may be important in future prevention or therapeutic interventions for this often devastating gastrointestinal emergency.
GRANTS
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R01 HD-54485 (M. D. Halpern) and K08 HD-061607 (J. H. Weitkamp) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-058404 (Vanderbilt Digestive Disease Research Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Armand M, Hamosh M, Mehta NR, Angelus PA, Philpott JR, Henderson TR, Dwyer NK, Lairon D, Hamosh P. Effect of human milk or formula on gastric function and fat digestion in the premature infant. Pediatr Res 40: 429–437, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Arrese M, Trauner M, Sacchiero RJ, Crossman MW, Shneider BL. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology 28: 1081–1087, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Balistreri WF, Heubi JE, Suchy FJ. Immaturity of the enterohepatic circulation in early life: factors predisposing to “physiologic” maldigestion and cholestasis. J Pediatr Gastroenterol Nutr 2: 346–354, 1983 [PubMed] [Google Scholar]
- 4.Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr 117: S6–S13, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, Schullinger JN. An experimental study of acute neonatal enterocolitis—the importance of breast milk. J Pediatr Surg 9: 587–595, 1974 [DOI] [PubMed] [Google Scholar]
- 6.Bhat BG, Rapp SR, Beaudry JA, Napawan N, Butteiger DN, Hall KA, Null CL, Luo Y, Keller BT. Inhibition of ileal bile acid transport and reduced atherosclerosis in apoE−/− mice by SC-435. J Lipid Res 44: 1614–1621, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr 13: 111–115, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Caplan MS, MacKendrick W. Necrotizing enterocolitis: a review of pathogenetic mechanisms and implications for prevention. Pediatr Pathol 13: 357–369, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem 278: 19909–19916, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Dvorak B, Halpern MD, Holubec H, Dvorakova K, Dominguez JA, Williams CS, Meza YG, Kozakova H, McCuskey RS. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res 53: 426–433, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Dvorak B, Kolinska J, McWilliam DL, Williams CS, Higdon T, Zakostelecka M, Koldovsky O. The expression of epidermal growth factor and transforming growth factor-α mRNA in the small intestine of suckling rats: organ culture study. FEBS Lett 435: 119–124, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Figge A, Lammert F, Paigen B, Henkel A, Matern S, Korstanje R, Shneider BL, Chen F, Stoltenberg E, Spatz K, Hoda F, Cohen DE, Green RM. Hepatic overexpression of murine Abcb11 increases hepatobiliary lipid secretion and reduces hepatic steatosis. J Biol Chem 279: 2790–2799, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Halpern MD, Dominguez JA, Dvorakova K, Holubec H, Williams CS, Meza YG, Ruth MC, Dvorak B. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr 36: 126–133, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Halpern MD, Holubec H, Dominguez JA, Meza YG, Williams CS, Ruth MC, McCuskey RS, Dvorak B. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 284: G695–G702, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res 51: 733–739, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 130: 359–372, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpern MD, Khailova L, Molla-Hosseini D, Arganbright K, Reynolds C, Yajima M, Hoshiba J, Dvorak B. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol 294: G20–G26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammons JL, Jordan WE, Stewart RL, Taulbee JD, Berg RW. Age and diet effects on fecal bile acids in infants. J Pediatr Gastroenterol Nutr 7: 30–38, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Hamosh M. Digestion in the newborn. Clin Perinatol 23: 191–209, 1996 [PubMed] [Google Scholar]
- 21.Hamosh M. Digestion in the premature infant: the effects of human milk. Semin Perinatol 18: 485–494, 1994 [PubMed] [Google Scholar]
- 22.Hamosh M, Iverson SJ, Kirk CL, Hamosh P. Milk lipids and neonatal fat digestion: relationship between fatty acid composition, endogenous and exogenous digestive enzymes and digestion of milk fat. World Rev Nutr Diet 75: 86–91, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Henkel A, Wei Z, Cohen DE, Green RM. Mice overexpressing hepatic Abcb11 rapidly develop cholesterol gallstones. Mamm Genome 16: 903–908, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Heubi JE, Balistreri WF, Suchy FJ. Bile salt metabolism in the first year of life. J Lab Clin Med 100: 127–136, 1982 [PubMed] [Google Scholar]
- 25.Hoshiba J. Method for hand-feeding mouse pups with nursing bottles. Contemp Top Lab Anim Sci 43: 50–53, 2004 [PubMed] [Google Scholar]
- 26.Hwang ST, Henning SJ. Ontogenic regulation of components of ileal bile acid absorption. Exp Biol Med (Maywood) 226: 674–680, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Israel EJ. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr Suppl 396: 27–32, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Jackson GD, Dai Y, Sewell WA. Bile mediates intestinal pathology in endotoxemia in rats. Infect Immun 68: 4714–4719, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Curr Opin Infect Dis 16: 349–355, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine-year experience. II. Outcome assessment. Am J Dis Child 135: 608–611, 1981 [DOI] [PubMed] [Google Scholar]
- 31.Li H, Xu G, Shang Q, Pan L, Shefer S, Batta AK, Bollineni J, Tint GS, Keller BT, Salen G. Inhibition of ileal bile acid transport lowers plasma cholesterol levels by inactivating hepatic farnesoid X receptor and stimulating cholesterol 7α-hydroxylase. Metabolism 53: 927–932, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Lien EL. The role of fatty acid composition and positional distribution in fat absorption in infants. J Pediatr 125: S62–S68, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 336: 1519–1523, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Neimark E, Chen F, Li X, Shneider BL. Bile acid-induced negative feedback regulation of the human ileal bile acid transporter. Hepatology 40: 149–156, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am 43: 409–432, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics 103: 1150–1157, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Shneider BL, Setchell KD, Crossman MW. Fetal and neonatal expression of the apical sodium-dependent bile acid transporter in the rat ileum and kidney. Pediatr Res 42: 189–194, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Stravitz RT, Sanyal AJ, Pandak WM, Vlahcevic ZR, Beets JW, Dawson PA. Induction of sodium-dependent bile acid transporter messenger RNA, protein, and activity in rat ileum by cholic acid. Gastroenterology 113: 1599–1608, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Suchy FJ, Balistreri WF. Uptake of taurocholate by hepatocytes isolated from developing rats. Pediatr Res 16: 282–285, 1982 [DOI] [PubMed] [Google Scholar]
- 41.Suchy FJ, Balistreri WF, Heubi JE, Searcy JE, Levin RS. Physiologic cholestasis: elevation of the primary serum bile acid concentrations in normal infants. Gastroenterology 80: 1037–1041, 1981 [PubMed] [Google Scholar]
- 42.Suchy FJ, Courchene SM, Balistreri WF. Ontogeny of hepatic bile acid conjugation in the rat. Pediatr Res 19: 97–101, 1985 [DOI] [PubMed] [Google Scholar]
- 43.Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 119: 630–638, 1991 [DOI] [PubMed] [Google Scholar]
- 44.Weimer J. The Economic Benefits of Breastfeeding: A Review and Analysis. Washington, DC: Food and Rural Economics Division, Economic Research Service, US Department of Agriculture, 2001 [Google Scholar]
- 45.Yajima M, Hoshiba J, Terahara M, Yajima T. Diminishing thymic size and delayed appearance of splenic CD4+ and CD8+ cells in artificially reared mouse pups. Biosci Biotechnol Biochem 71: 2420–2427, 2007 [DOI] [PubMed] [Google Scholar]