Abstract
Intermediate conductance K+ (Kcnn4) channels are present in both mucosal and serosal membranes of colon. However, only serosal Kcnn4 channels have been shown to be essential for agonist-induced (cAMP and Ca2+) anion secretion. The present study sought to determine whether mucosal Kcnn4 channels also play a role in colonic anion secretion. Mucosal-to-serosal and serosal-to-mucosal unidirectional 86Rb (K+ surrogate) fluxes as well as short-circuit current (Isc; a measure of anion secretion) were measured under voltage-clamp conditions in distal colon from rats fed either a standard or K+-free diet. 5,6-Dichloro-1-ethyl-1,3-dihydro-2H-benzimidazole-2-one (DC-EBIO) was used to activate Kcnn4 channels. Mucosal DC-EBIO both induced K+ secretion and enhanced anion secretion in normal rat distal colon. The DC-EBIO-induced K+ secretion was completely blocked by nonspecific (Ba2+) and Kcnn4-specific (TRAM-34) inhibitors, but was not blocked by the large-conductance K+ (iberiotoxin), small-conductance K+ (apamin), or KCNQ1 (chromanol 293B) specific blockers. Ba2+ and TRAM-34 also inhibited DC-EBIO-enhanced anion secretion. The DC-EBIO-enhanced anion secretion was completely inhibited by the nonspecific anion channel blocker 5-nitro-2-(3-phenylpropyl-amino)benzoic acid, whereas it was only partially inhibited by CFTR [CFTRinh-172, glibenclamide]- and CaCC (niflumic acid)-specific Cl− channel blockers. In contrast, mucosal DC-EBIO-enhanced K+ and anion secretion was not present in distal colon of dietary K-depleted rats, indicating absence of mucosal Kcnn4 channels. These observations indicate that mucosal Kcnn4 channels are capable of driving agonist-induced anion secretion mediated via CFTR and CaCC and likely contribute to stool K+ losses that accompany diarrheal illnesses.
Keywords: short-circuit current, stripped mucosa, unidirectional fluxes, K+ channels, diarrhea
fluid secretion results from active anion (Cl− and HCO3−) secretion mediated by ion channels that reside within epithelial cell membranes (3). A component of active anion secretion contributes to virtually every diarrheal illness of inflammatory, neuroendocrine, and/or infectious etiology (23). Continuous active anion secretion requires a mechanism for maintaining cell hyperpolarization (3). In human, as well as in rat colon, agonist-induced (cAMP and Ca2+) anion secretion appears to be energized by intermediate conductance Ca2+-activated K+ channels (8, 21, 26). Molecular studies have identified the intermediate-conductance K+ channels as Kcnn4 channels (also known as rSK4, rIK1, MIK1, SMIK1, and KCa3.1) (5, 16, 20, 32, 34). Electrophysiological studies have characterized serosal Kcnn4 channels, whereas ion flux studies have provided evidence for mucosal Kcnn4 channels in rat colon (16, 34). Immunological studies have localized Kcnn4-like proteins to both mucosal and serosal membrane domains in intestine and colon (12, 15, 16). In recent studies, we have cloned three distinct Kcnn4 transcripts (Kcnn4a, Kcnn4b, and Kcnn4c) that exhibit tissue- and membrane-specific expression patterns in the colon (2). The Kcnn4a transcripts have been shown to be expressed in colonic smooth muscle, whereas Kcnn4b and Kcnn4c proteins have been localized to the basolateral and mucosal membranes of colonic epithelial cells, respectively (2).
Whereas Kcnn4 channels reside on both mucosal and serosal membranes, only serosal Kcnn4 channels have been hypothesized to hyperpolarize cells and provide the driving force for agonist-induced anion secretion (18, 34). This conclusion is based on observations that clotrimazole (CLT; an antifungal antimicrobial compound) inhibited intracellular free Ca2+-induced anion secretion (26, 34). In these studies, intracellular Ca2+ levels were enhanced either by carbachol (a cholinergic agonist) or by thapsigargin (an inhibitor of the Ca2+-ATPase of the endoplasmic reticulum). However, on the basis of these data, it remains unclear whether mucosal or serosal Kcnn4 channels provide the driving force for anion secretion because increasing intracellular free Ca2+ concentration would have activated, whereas lipophilic CLT would have inhibited, Kcnn4 channels within both domains. Thus the present study was initiated to identify whether the driving force for anion secretion in rat distal colon is mediated by activation of serosal Kcnn4 channels alone and/or by concurrent activation of mucosal Kcnn4 channels. In this study, membrane domain-specific Kcnn4 channels were activated by either mucosal or serosal addition of the Kcnn4 channel opener DC-EBIO (5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazole-2-one), which has been shown to activate mucosal cystic fibrosis transmembrane conductance (CFTR) Cl− channels and serosal Kcnn4 channels in the human colonic tumor cell line T84 (29). Unidirectional 86Rb+ fluxes (a K+ surrogate) and short-circuit currents (Isc; a measurement of anion secretion) were measured in epithelial sheets from rat distal colon under voltage-clamp conditions. Addition of mucosal DC-EBIO enhanced both K+ and anion secretion in normal rat distal colon. The mucosal DC-EBIO-induced K+ and anion secretion was completely inhibited by the nonspecific K+ channel blocker (Ba2+), as well as the Kcnn4 channel-specific blocker (TRAM-34). Mucosal application of the nonspecific anion channel blocker NPPB [5-nitro-2-(3-phenylpropyl-amino)benzoic acid] completely inhibited DC-EBIO-enhanced anion secretion but only partially inhibited the DC-EBIO-enhanced K+ secretion in normal rat distal colon. DC-EBIO-induced K+ and anion secretion were present in the distal colon of the dietary K-depleted rats. These results demonstrate that mucosal Kcnn4 channels provide a component of the driving force for Cl− secretion in secretory diarrhea and likely contribute to stool K+ loss.
METHODS
Animals.
Nonfasting normal male Sprague-Dawley rats (200–250 g) were maintained on either standard or a potassium-deficient rat chow (MP Biochemicals, Solon, OH). All animals had access to tap water ad libitum. All the experimental protocols used in this study were approved by the West Virginia University Institutional Animal Care and Use Committee.
Ussing chamber studies.
Isc and 86Rb+ (K+ surrogate; PerkinElmer, Billerica, MA) fluxes were measured in distal colon mounted under voltage-clamp conditions, as described previously (17, 33). In brief, colon excised from euthanized rats was flushed with ice-cold saline. Mucosal layers were gently separated from serosal muscular layers opened along the mesenteric border. Two distal (1 cm proximal to rectum) segments obtained from each animal were mounted in Lucite chambers (with an opening of 1.12 cm2), and both sides were bathed with equal volume of Ringer solution (in mM: 115 NaCl, 25 NaHCO3, 2.4 K2HPO4, 0.4 KH2PO4, 1.2 CaCl2, 1.2 MgCl2, and 10 glucose; pH 7.4). The bathing solutions were maintained at 37°C and gassed continuously with 5% CO2-95% O2. Isc and conductance were recorded every 20 s using an automated multichannel voltage/current clamp instrument (Physiological Instruments, San Diego, CA). For flux studies, 1 μCi 86RbCl was added to either serosal or mucosal bath solution. After a 45-min equilibration period, serosal-to-mucosal (s-m) and mucosal-to-serosal (m-s) 86Rb+ fluxes were measured on different tissues under voltage-clamp conditions. Net fluxes were calculated from the difference between m-s and s-m fluxes across tissue pairs that were matched on the basis of differences in basal conductance of <10%. Positive and negative values represent active absorption and active secretion, respectively. In all experiments, three 15-min flux periods were combined to obtain a single flux value for each tissue pair. The Isc and 86Rb fluxes values are expressed as microequivalents per hour per square centimeter, whereas conductance is expressed as millisiemens per square centimeter.
In additional experiments, following basal measurements, conductance, Isc and 86Rb+ fluxes were also measured in the presence of 100 μM mucosal and serosal DC-EBIO. Inhibitors were added to mucosal and/or serosal bath solution at the end of the DC-EBIO period: 3 mM Ba2+, 50 μM CLT, 50 μM TRAM-34, 100 nM iberiotoxin (IbTX), 10 nM apamin (APA), 30 μM chromanol 293B, 100 μM NPPB, 100 μM niflumic acid, or 300 μM glibenclamide was added to the mucosal and/or serosal bath solution. Na+ concentration was adjusted to maintain isosmolarity when Ba2+ was used.
Statistics.
Results are presented as means ± SE. Statistical analyses were performed by a paired t-test and Bonferroni's one-way ANOVA post hoc test. P < 0.05 was considered to be statistically significant.
RESULTS
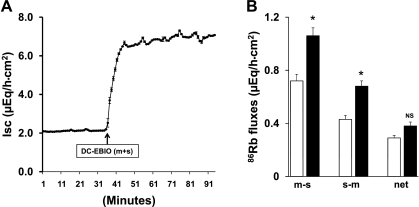
Initial studies were designed to identify whether the Kcnn4 channel opener DC-EBIO would enhance Isc (as a measure of anion secretion) and 86Rb (K+ surrogate) fluxes in normal rat distal colon. In these studies, the effect of DC-EBIO added simultaneously to both mucosal and serosal bathing solutions was examined. As shown in Fig. 1A, simultaneous addition of DC-EBIO to mucosal and serosal bathing solutions enhanced Isc threefold (basal vs. DC-EBIO: 2.1 ± 0.4 vs. 7.0 ± 0.7 μeq·h−1·cm−2; P < 0.001). The DC-EBIO activation of mucosal Kcnn4 channels also significantly enhanced the transepithelial tissue conductance from 8.3 ± 0.3 to 11.7 ± 0.6 mS/cm2 (P < 0.002). The DC-EBIO-enhanced Isc and conductance were stable for up to 60 s (Figs. 1A). In parallel, we also examined 86Rb fluxes in the same tissues. As shown in Fig. 1B, similar to earlier demonstration by Sweiry and Binder (30), under basal conditions, net 86Rb absorption occurs in normal rat distal colon. Mucosal and serosal addition of DC-EBIO did not alter the net 86Rb flux (Fig. 1B). However, DC-EBIO significantly enhanced m-s and s-m fluxes (Fig. 1B). These observations indicate that 1) DC-EBIO-enhanced Isc occurred as a result of anion secretion since net 86Rb flux was not altered; and 2) DC-EBIO-activated K+ efflux/exit occurs in both mucosal and serosal membranes of normal rat distal colon.
Fig. 1.
Effect of mucosal (m) and serosal (s) presence of 5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazole-2-one (DC-EBIO) on short-circuit current (Isc) and 86Rb fluxes in normal rat distal colon. Both time courses of Isc (A) and 86Rb fluxes (B) were measured as described in methods. A: simultaneous addition of DC-EBIO (100 μM) to both mucosal and serosal medium enhanced Isc. The DC-EBIO-enhanced Isc was stable for up to 60 min. B: mucosal to serosal (m-s) and serosal to mucosal (s-m) 86Rb fluxes in the absence (open bars), and in the presence of both mucosal and serosal DC-EBIO (solid bars). DC-EBIO significantly enhanced both m-s and s-m but did not alter the net 86Rb fluxes. The basal and post-DC-EBIO tissue conductance were 8.2 ± 0.3 and 11.7 ± 0.6 mS/cm2 (P < 0.002), respectively. Results presented represent means ± SE from 5 tissue pairs for 86Rb fluxes. *P < 0.05, compared with basal fluxes; NS, not significant.
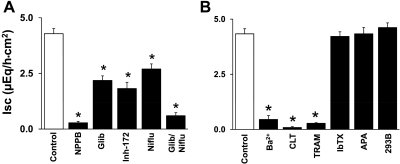
Although the benzimidazolone derivative DC-EBIO has been shown to activate serosal Kcnn4 channels and mucosal Cl− channels in T84 cells (a human colon cancer cell line), the benzimidazolone derivative NS004 has been shown to activate large-conductance K+ (BK) and CFTR Cl− channels (14, 22). Therefore, to establish that DC-EBIO-enhanced Isc occurred as a result of activation of CFTR and that the driving force for the anion secretion is provided by DC-EBIO-activated K+ channels, the effect of anion and K+ channel blockers (added to both mucosal and serosal bath) were examined on DC-EBIO-enhanced Isc (Fig. 2). The DC-EBIO-enhanced Isc was almost completely (93%) inhibited by the nonspecific anion channel blocker NPPB, whereas the CFTR Cl− channel blockers glibenclamide (50%) and CFTRinh-172 (54%), as well as the CaCC (Ca2+-activated Cl− channel) blocker niflumic acid (37%) only partially inhibited the DC-EBIO-enhanced Isc (Fig. 2A). Simultaneous addition of glibenclamide and niflumic acid exhibited additive (86%) inhibition of the DC-EBIO-enhanced Isc (Fig. 2A). Similar to anion channel blockers, K+ channel blockers also exhibited varying effects on inhibition of DC-EBIO-enhanced Isc (Fig. 2B). The nonspecific K+ channel blocker Ba2+ inhibited DC-EBIO-enhanced Isc by 97%, whereas the Kcnn4 channel blockers CLT and TRAM-34 inhibited DC-EBIO-enhanced Isc by 98 and 94%, respectively (Fig. 2B). The BK channel blocker IbTX, the small-conductance K+ (SK) channel blocker APA, and the KCNQ1 channel blocker chromanol 293B did not inhibit DC-EBIO-enhanced Isc (Fig. 2B). Both anion channel and K+ channel blockers also significantly inhibited DC-EBIO-enhanced conductance (Fig. 2, legend). These observations establish that DC-EBIO-enhanced Isc occurred as a result of anion secretion mediated by mucosal CFTR and CaCC anion channels. These results also indicate that Kcnn4 channels provided the driving force for DC-EBIO-enhanced anion secretion.
Fig. 2.
Effect of anion channel and K+ channel blockers on mucosal and serosal DC-EBIO-enhanced Isc in normal rat distal colon. Basal Isc was measured as described in methods. A: DC-EBIO-enhanced Isc was measured in the absence (Control) and in the presence of 100 μM 5-nitro-2-(3-phenylpropyl-amino)benzoic acid (NPPB), 300 μM glibenclamide (Glib), 100 μM niflumic acid (Niflu), 20 μM CFTRinh-172 (Inh-172), and Glib + Niflu. B: DC-EBIO-enhanced Isc was measured in the absence (Control) and after the addition of 3 mM Ba2+, 50 μM clotramizole (CLT), 50 μM TRAM-34, 100 nM iberiotoxin (IbTX), 10 nM apamin (APA), and 30 μM chromanol 293B (293B). Absolute DC-EBIO-enhanced Isc presented was calculated by subtracting basal Isc obtained in absence of DC-EBIO from that in the presence of DC-EBIO. The control and post-anion channel blocker tissue conductances were 12.1 ± 0.8 (control), 8.2 ± 0.6 (P < 0.001; NPPB), 11.7 ± 1.1 (NS; Glib), 11.2 ± 0.9 (NS; CFTRinh-172), 10.9 ± 0.8 (NS; niflumic acid), and 8.7 ± 0.6 (P < 0.05; Glib/Niflu). The control and post-K+ channel blocker tissue conductances were 11.1 ± 0.7 (Control), 7.8 ± 0.6 (P < 0.001; Ba2+), 7.6 ± 0.6 (P < 0.001; CLT), 7.6 ± 0.8 (P < 0.001; TRAM-34), 10.4 ± 1.3 (NS; IbTX), 10.9 ± 0.9 (NS; APA), and 10.9 ± 1.0 (NS; chromanol 293B). DC-EBIO and inhibitors were added to both mucosal and serosal baths. Results presented represent means ± SE of 10 tissues. *P < 0.001, compared with respective control.
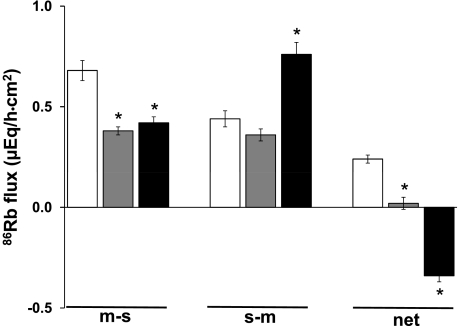
Although DC-EBIO significantly enhanced m-s and s-m fluxes, the net 86Rb flux was not altered in normal distal colon (Fig. 1B). It is possible that the DC-EBIO-induced 86Rb secretion mediated by mucosal Kcnn4 channels is masked by 86Rb absorption via mucosal H-K-ATPase. Thus 86Rb fluxes were measured after the addition of mucosal orthovanadate (VO4; H-K-ATPase inhibitor). As shown in Fig. 3, under basal conditions, mucosal VO4 completely inhibited the net 86Rb absorption by reducing m-s fluxes, whereas, in contrast, it did not inhibit s-m fluxes. In the presence of mucosal VO4, serosal and mucosal addition of DC-EBIO-induced 86Rb secretion (Fig. 3). This DC-EBIO-induced 86Rb secretion occurred as a result of enhanced s-m fluxes (Fig. 3). Thus to characterize mucosal Kcnn4 channels, all further studies were performed in the presence 1 mM mucosal VO4.
Fig. 3.
Effect of DC-EBIO on 86Rb fluxes in the presence of orthovanadate (VO4) in normal rat distal colon. Basal 86Rb fluxes (open bars) were measured as described in methods. 86Rb flux was also measured after the addition of VO4 (shaded bars) and VO4 + DC-EBIO (solid bars); 1 mM VO4 was present only in the mucosal bath, whereas DC-EBIO was present in both mucosal and serosal baths. The basal, post-VO4, and post-VO4 plus DC-EBIO tissue conductances were 7.2 ± 0.6, 7.3 ± 0.7 (NS), and 10.1 ± 0.6 (P < 0.004), respectively. Results presented represent means ± SE of 4 tissue pairs. *P < 0.001, compared with respective basal values.
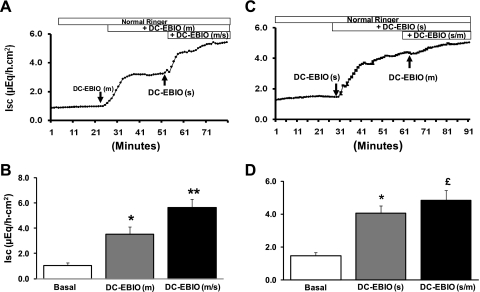
The effect on Isc of sequential addition of mucosal-followed-by-serosal and serosal-followed-by-mucosal DC-EBIO was examined to identify whether mucosal and/or serosal Kcnn4 channels provide the driving force for anion secretion. Addition of mucosal DC-EBIO alone significantly enhanced Isc (Fig. 4, A and C). The mucosal DC-EBIO-enhanced Isc was further enhanced by the subsequent addition of serosal DC-EBIO [i.e., DC-EBIO (m/s)] (Fig. 4, A and C). An almost similar rate of enhanced Isc by mucosal DC-EBIO alone (ΔIsc 2.3 μeq·h−1·cm−2) and serosal followed by mucosal DC-EBIO [DC-EBIO (m) vs. DC-EBIO (m/s): ΔIsc 1.9 μeq·h−1·cm−2] suggests that the activation of both mucosal and serosal Kcnn4 channels contributes equally to optimal anion secretion (Fig. 4, A and C). In reverse order of DC-EBIO addition (serosal followed by mucosal), serosal DC-EBIO alone substantially enhanced Isc (ΔIsc 2.6 μeq·h−1·cm−2) (Fig. 4, B and D). Subsequent mucosal addition of DC-EBIO in the continued presence of serosal DC-EBIO minimally (ΔIsc 0.8 μeq·h−1·cm−2) but significantly enhanced Isc (Fig. 4, B and D). The low rate of Isc enhanced by mucosal-followed-by-serosal DC-EBIO (ΔIsc 0.8 μeq·h−1·cm−2) compared with serosal-followed-by-mucosal DC-EBIO (ΔIsc 1.9 μeq·h−1·cm−2) suggests that serosal membranes are more permeable to DC-EBIO, thus partially activating mucosal Kcnn4 channels. Despite significantly enhancing Isc, mucosal DC-EBIO did not alter the conductance [basal vs. mucosal DC-EBIO: 7.2 ± 0.6 vs. 7.3 ± 0.7 mS/cm2; not significant (NS)]. However, subsequent addition of serosal-followed-by-mucosal DC-EBIO significantly enhanced the conductance [basal vs. DC-EBIO (m/s): 7.2 ± 0.6 vs. 10.1 ± 0.6 mS/cm2; P < 0.004]. In contrast, serosal addition of DC-EBIO alone significantly enhanced the Isc (basal vs. serosal DC-EBIO: 6.5 ± 0.3 vs. 9.6 ± 0.4 μeq·h−1·cm−2; P < 0.002). Subsequent mucosal addition of DC-EBIO did not alter the conductance [DC-EBIO (s) vs. DC-EBIO (s/m): 9.6 ± 0.4 vs. 9.8 ± 0.5 mS/cm2 (NS), respectively]. These observations suggest that both mucosal and serosal Kcnn4 channels may, in part, provide a driving force for agonist induced anion secretion.
Fig. 4.
Effect of sequential addition of mucosal-to-serosal and serosal-to-mucosal DC-EBIO on Isc in normal rat distal colon. Time course of Isc was measured as described in methods. A: time course of mucosal [DC-EBIO (m)] and subsequent serosal [DC-EBIO (s)] addition of DC-EBIO in the continued presence of mucosal DC-EBIO [DC-EBIO (m/s)]. B: basal and DC-EBIO-enhanced rate of Isc were derived from A. The basal and post-DC-EBIO (m) and post-DC-EBIO (m/s) tissue conductances were 7.2 ± 0.3, 8.1 ± 0.2 (P < 0.02) and 10.0 ± 0.6 (P < 0.002), respectively. C: time course of serosal [DC-EBIO (s)] and subsequent mucosal [DC-EBIO (m)] addition of DC-EBIO in the continued presence of serosal DC-EBIO [DC-EBIO (s/m)]. D: basal and DC-EBIO-enhanced rate of Isc were derived from B. The basal and post-DC-EBIO (s) and post-DC-EBIO (s/m) tissue conductances were 6.5 ± 0.3, 9.6 ± 0.4 (P < 0.002), and 9.8 ± 0.5 (NS), respectively. Results presented represent means ± SE of 10 tissues from 5 different rats. *P < 0.001 compared with basal; **P < 0.001 compared with DC-EBIO (m); £P < 0.05 compared with DC-EBIO (s).
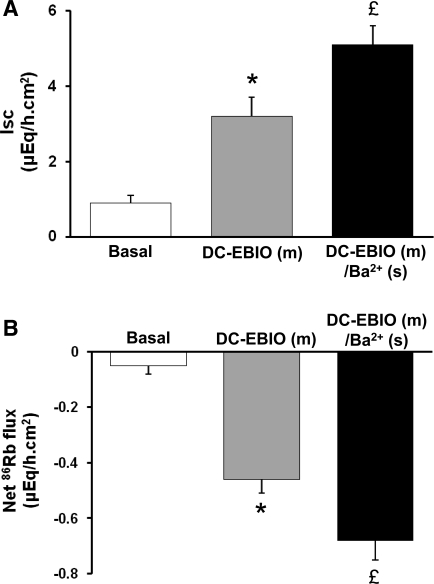
It is also possible that DC-EBIO might permeate through mucosal membranes to activate serosal Kcnn4 channels, thus underestimating the contribution of mucosal Kcnn4 channels to anion secretion (Isc). Therefore, the effect of serosal Ba2+ was examined on mucosal DC-EBIO-enhanced Isc and 86Rb secretion. Serosal Ba2+ did not inhibit mucosal DC-EBIO-enhanced Isc (Fig. 5A). In contrast, serosal Ba2+ significantly enhanced 86Rb secretion in normal rat distal colon (Fig. 5B). It is to be noted here that in theory blocking the K+ exits via serosal K+ channels would enhance the K+ secretion by 3–4 μeq·h−1·cm−2, since both Na-K-pump and Na-K-2Cl cotransporter actively bring K+ into cells. However, serosal Ba2+ only enhanced the K+ secretion by only 0.2 μeq·h−1·cm−2 in rat distal colon (Fig. 5A). It is likely that the rest of the K+ might exit through KCl cotransport localized on the serosal membranes of rat distal colon (27). These observations suggest that, in the absence of functional serosal Kcnn4 channels, enhanced K+ secretion via mucosal Kcnn4 channels compensates to achieve an optimal driving force for anion secretion.
Fig. 5.
Effect of serosal Ba2+ on mucosal DC-EBIO-enhanced Isc and net 86Rb fluxes in normal rat distal colon. Both basal Isc and 86Rb fluxes were measured as described in methods (open bars). Isc and net 86Rb fluxes were also measured in presence of mucosal DC-EBIO alone (shaded bars) and in the presence of mucosal DC-EBIO and 3 mM serosal Ba2+ (solid bars). Mucosal medium contained 1 mM VO4. The basal, post-VO4, and post-DC-EBIO (m)/Ba2+ (s) tissue conductances were 7.3 ± 0.5, 7.8 ± 0.6 (NS), and 8.1 ± 1.0 (NS), respectively. Results presented represent means ± SE of 6 tissue pairs. *P < 0.001 compared with respective basal values; £P < 0.05 compared with DC-EBIO (m).
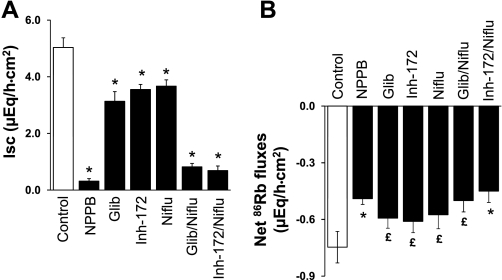
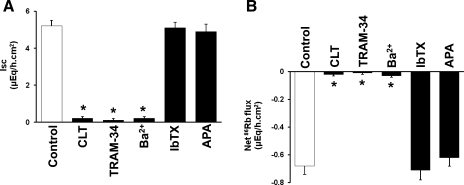
To establish the relationship between mucosal anion channels and Kcnn4 channels, we examined the effects of anion channel blockers and K+ channel blockers on mucosal DC-EBIO-enhanced Isc and 86Rb secretion (Figs. 6 and 7). Mucosal NPPB, glibenclamide, and niflumic acid inhibited DC-EBIO-enhanced Isc by 94, 38, and 27%, respectively (Fig. 6A). Simultaneous presence of glibenclamide and niflumic acid inhibited DC-EBIO enhance Isc by 85% (Fig. 6A). Mucosal NPPB, glibenclamide, and niflumic acid also significantly inhibited the DC-EBIO-induced 86Rb secretion (Fig. 6B). Simultaneous addition of both glibenclamide and niflumic acid inhibited the DC-EBIO-induced 86Rb secretion similar to that of NPPB (Fig. 6B). Nonspecific K+ channel blocker Ba2+ and Kcnn4 channel-specific blockers CLT and TRAM-34 inhibited both the mucosal DC-EBIO-enhanced Isc and DC-EBIO-induced 86Rb secretion (Fig. 7, A and 7B). The observations that mucosal anion channel blockers partially inhibited the DC-EBIO-enhanced 86Rb secretion indicate that part of the driving force for K+ secretion is provided by anion secretion and that the mucosal Kcnn4 channels and anion channels are coupled to regulate anion secretion.
Fig. 6.
Effect of anion channel blockers on mucosal DC-EBIO-enhanced Isc and net 86Rb fluxes in normal rat distal colon. Both basal Isc (A) and net 86Rb flux (B) were measured as described in methods. Isc and net 86Rb fluxes were also measured in presence of mucosal DC-EBIO (control) and mucosal DC-EBIO + mucosal inhibitors. The inhibitors used were 100 μM NPPB, 300 μM glibenclamide, 100 μM CFTRinh-172, 100 μM niflumic acid, Glib/Niflu, and Glib/Inh-172/Niflu. The anion channel blockers did not significantly alter the control conductance (7.9 ± 0.7). Absolute DC-EBIO-enhanced Isc and net 86Rb fluxes presented were calculated by subtracting Isc and 86Rb fluxes obtained in the absence of DC-EBIO from that in presence of DC-EBIO, respectively. Mucosal medium always contained 1 mM VO4, whereas serosal medium contained 3 mM Ba2+. Results presented represent means ± SE of 6 tissue pairs. *P < 0.001 compared with respective control; £P < 0.05 compared with respective control.
Fig. 7.
Effect of mucosal K+ channel blockers on mucosal DC-EBIO-enhanced Isc and net 86Rb fluxes in normal rat distal colon. Both basal Isc (A) and net 86Rb fluxes (B) were measured as described in methods. Isc and net 86Rb fluxes were also measured in presence of mucosal DC-EBIO (control) and mucosal DC-EBIO + inhibitors. The inhibitors used were 3 mM Ba2+, 50 μM clotrimazole, 50 μM TRAM-34, 100 nM iberiotoxin, and 10 nM apamin. Absolute DC-EBIO-enhanced rate of Isc and net 86Rb secretion presented were calculated by subtracting basal rate of Isc and net 86Rb fluxes obtained in the absence of DC-EBIO from that in the presence of DC-EBIO, respectively. Mucosal medium always contained 1 mM VO4, whereas serosal medium contained 3 mM Ba2+. The K+ channel blockers did not significantly alter the control conductance (8.2 ± 0.6) (data not shown). Results presented represent means ± SE of 6 tissue pairs. *P < 0.001 compared with control.
In previous studies, we have shown downregulation of Kcnn4 specific mRNA abundance and Ca2+-activated K+ secretion as a mechanism for K+ conservation in dietary K-depleted rat proximal colon (16). Therefore, to establish that mucosal Kcnn4 channels both mediate K+ secretion and provide driving force for anion secretion, we also examined the effect mucosal DC-EBIO in dietary K-depleted rat distal colon. Under basal condition, net 86Rb absorption in dietary K-depleted rat colon is significantly higher than that in normal rat colon (Fig. 8B). Mucosal VO4 completely inhibited the net K+ absorption in both normal and dietary K-depleted rat distal colon (Fig. 8B). Similar to earlier observations, in the presence of mucosal VO4 and serosal Ba2+, mucosal addition of DC-EBIO enhanced Isc and induced 86Rb secretion in normal colon. In contrast, neither enhanced Isc nor 86Rb secretion is present in K-depleted rat colon (Fig. 8, A and B). This observation establishes the conclusion that mucosal Kcnn4 channels provide driving force for anion secretion in normal rat distal colon.
Fig. 8.
Effect of mucosal DC-EBIO on Isc and 86Rb secretion in dietary K-depleted rat distal colon. Both basal Isc (A) and net 86Rb fluxes (B) were measured in normal and dietary K-depleted rat distal colon as described in methods. Isc and net 86Rb fluxes were also measured in the presence of mucosal VO4 (1 mM) and mucosal VO4 + DC-EBIO. Serosal medium always contained 3 mM Ba2+. The basal conductance for normal and dietary K-depleted animal was 7.6 ± 0.6 and 8.1 ± 0.6 (NS), respectively. The mucosal addition of VO4 and DC-EBIO did not significantly alter the conductance either in normal and dietary K-depleted diet-fed rat colon (data not shown). Results presented represent means ± SE of 4 tissue pairs. *P < 0.001 compared with respective basal value; **P < 0.05 compared with respective basal value; £P < 0.001 compared with normal basal flux.
DISCUSSION
Although both inhibition of active Na+ absorption and stimulation of active anion (Cl− and HCO3−) secretion contribute to isotonic fluid loses, anion secretion mediated via activated anion channels (CFTR and CaCC) localized on mucosal membranes of intestine and colon is the major driving force for fluid secretion in diarrhea (3, 10). Intracellular levels of second messengers like cAMP and Ca2+ that activate anion channels are elevated during chronic inflammation in inflammatory bowel diseases such as ulcerative colitis and infectious diarrhea such as cholera (1, 28, 35). Agonist-induced anion secretion that depolarizes cells requires compensatory K+ efflux to maintain continuous anion secretion (3). Although large- (BK), intermediate- (Kcnn4), and small-conductance (SK) Ca2+-activated K+ channels and cAMP-activated K+ channels (KCNQ1) are expressed, only CLT-sensitive Kcnn4 channels have been shown to provide a driving force for agonist-induced anion secretion in both human and rat colon (13, 16, 25, 26, 34). Despite the functional demonstration and immunofluorescence localization of Kcnn4 channels on both mucosal and serosal membranes, only serosal Kcnn4 channels have been hypothesized to provide the driving force for agonist-induced Cl− secretion (13, 34). Since this conclusion was arrived at on the basis of lipophilic CLT inhibition of Cl− secretion, it is not known whether mucosal and/or serosal Kcnn4 channels drive agonist-induced anion secretion (26, 34). Thus the present study was designed to identify the role of mucosal Kcnn4 channels on anion secretion in rat distal colon. In this study, mucosal Kcnn4 channels were activated by use of DC-EBIO, whereas serosal K+ channels were blocked by use of the nonspecific K+ channel blocker Ba2+.
This study demonstrates that activation of K+ exit across both mucosal and serosal membranes, in part, provides a driving force for anion secretion mediated by both mucosal CFTR and CaCC anion channels (Fig. 1, A and B). Inhibition of anion secretion by the Kcnn4 channel blocker TRAM-34 established that Kcnn4 channels provided the driving force for DC-EBIO-enhanced anion secretion in normal rat distal colon (Figs. 2B and 7A). Partial inhibition of anion secretion by the individual addition of a mucosal CFTR-inhibitor (glibenclamide) and a CaCC-inhibitor (niflumic acid) invoked complete inhibition, which indicated that DC-EBIO activated K+ channels enhanced anion secretion as a result of activation of both CFTR and CaCC anion channels (Figs. 2A and 6A). Both CFTR and CaCC channels have been shown to be expressed in mucosal membranes of colonic epithelial cells (11, 24). DC-EBIO, which opens Kcnn4 channels, would have also stimulated CFTR Cl− channels (14), whereas CaCC channels might have been activated by indirect cellular mechanisms. DC-EBIO and other benzimidazolone derivatives such as NS004 and 1-EBIO have been shown to activate glibenclamide-sensitive Cl− currents in mucosal membranes of T84 cells and rat colon, whereas NS004 has also been shown to activate CFTR expressed in in vitro (6, 7, 14, 22, 29). Although both CFTR and CaCC anion channels are expressed on mucosal membranes of T84 cells and rat distal colon, only CFTR has been shown to be activated by benzimidazolone derivatives (7). Thus DC-EBIO activation of CaCC anion channels present in rat distal colon might have occurred as a result of enhanced intracellular free Ca2+ levels. The hyperpolarized cells generated by DC-EBIO activated Kcnn4-mediated K+ exit might have enhanced intracellular free Ca2+ levels by mobilizing intracellular stores or activating extracellular Ca2+ influx via mucosal Ca2+ channels. Ca2+ channels have been suggested to localize in mucosal membranes of rat distal colon (9). Although CaCC anion channels are expressed, Devor et al. (7) did not identify DC-EBIO-stimulated mucosal Cl− conductance mediated via CaCC in T84 cells and rat distal colon, since this study utilized serosal membrane permeabilized monolayers and dexamethasone-treated rat distal colon, respectively.
This study hypothesizes that K+ exit mediated via both mucosal and serosal Kcnn4 channels, in part, contributes to the driving force for anion secretion. However, in the absence of serosal K+ exit, the enhanced mucosal K+ efflux compensates to provide the driving force for optimal anion secretion. The conclusion that mucosal K+ efflux provides a driving force for anion secretion is supported by the following observations: 1) Simultaneous addition of both mucosal and serosal DC-EBIO, but not addition of either mucosal or serosal DC-EBIO alone, enhanced the maximal rate of anion secretion (Fig. 4). 2) Cumulative rate of anion secretion is enhanced by the addition of mucosal DC-EBIO alone (55%), and subsequent serosal DC-EBIO addition (45%) (i.e., in the continued presence of mucosal DC-EBIO) is essentially equivalent to the maximal rate of anion secretion seen with DC-EBIO in both mucosal and serosal bath (Fig. 4, A and C). 3) Inhibition of serosal K+ exit enhanced mucosal DC-EBIO-induced K+ efflux (secretion), but serosal K+ channel inhibition did not affect DC-EBIO-enhanced anion secretion (Fig. 5, A and B). 4) Absence of mucosal DC-EBIO-induced K+ secretion failed to enhance anion secretion in dietary K-depleted rat distal colon (Fig. 8, A and B). The absence of DC-EBIO-induced K+ secretion is consistent with our recent report that downregulation of mucosal Kcnn4 channels is a mechanism of K+ conservation in the dietary K-depleted rat colon (16). The absence of mucosal Kcnn4 channels resulted in the inability of mucosal DC-EBIO to activate anion secretion in dietary K-depleted rat distal colon. This observation supports the conclusion that mucosal K+ efflux, in part, provides the driving force for anion secretion in normal rat distal colon.
In theory, since agonist-induced anion secretion is electrogenic, anion channels that mediate anion secretion and hyperpolarizing K+-exit channels should be expressed on mucosal and serosal membranes for a cellular model of anion secretion, respectively (3, 4). In contrast, it might have been presumed, if both anion channels and K+ channels are localized on the same membrane (i.e., mucosal membrane), that the agonist-induced anion secretion would be electroneutral. However, it may not be the case because the rate of K+ efflux is 8- to 10-fold lower than that of anion secretion (Figs. 5–8) and provides the driving force for anion secretion. The rate of serosal K+ exit has been reported to be less than 4% of the rate of Cl− secretion in anion-secreting T84 cells (19). Since negligible amounts of K+ exit are required to maintain cell hyperpolarization to drive agonist-induced anion secretion, it is physiologically feasible for mucosal K+ efflux to subserve this role. In addition to driving anion secretion, mucosal K+ efflux also accounts for the substantially increased level of K+ (i.e., 5- to 10-fold) that prevails in diarrheal stools (31).
In summary, to our knowledge this is the first study to report that K+ efflux mediated via mucosal Kcnn4 channels provides, in part, the driving force for agonist-induced anion secretion. The Kcnn4 channels provide the driving force for anion secretion that is mediated via both CFTR and CaCC Cl− channels. We conclude from these data that mucosal Kcnn4 channels are capable of driving agonist-induced anion secretion mediated via CFTR and CaCC and likely contribute to stool K+ losses that accompany diarrheal illnesses.
GRANTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant (DK-018777).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Al-Awqati Q. Alternative treatment for secretory diarrhea revealed in a new class of CFTR inhibitors. J Clin Invest 110: 1599–1601, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barmeyer C, Rahner C, Yang Y, Sigworth FJ, Binder HJ, Rajendran VM. Cloning and identification of tissue specific expression of kcnn4 splice variants in rat colon. Am J Physiol Cell Physiol. doi:10.1152/ajpcell.00091,.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bleich M, Riedemann N, Warth R, Kerstan D, Leipziger J, Hor M, Driessche WV, Greger R. Ca2+ regulated K+ and non-selective cation channels in the basolateral membrane of rat colonic crypt base cells. Pflügers Arch 432: 1011–1022, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Chou CC, Lunn CA, Murgolo NJ. KCa3.1 target and marker for cancer, autoimmune disorder and vascular inflammation? Expert Rev Mol Diagn 8: 179–187, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Devor DC, Singh AK, Bridges RJ, Frizzell RA. Modulation of Cl− secretion by benzimidazolones. II. Coordinate regulation of apical GCl and basolateral GK. Am J Physiol Lung Cell Mol Physiol 271: L785–L795, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl− secretion by benzimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. Am J Physiol Lung Cell Mol Physiol 271: L775–L784, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Devor DC, Singh AK, Gerlach AC, Frizzell RA, Bridges RJ. Inhibition of intestinal Cl− secretion by clotrimazole: direct effect on basolateral membrane K+ channels. Am J Physiol Cell Physiol 273: C531–C540, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Donowitz M, Levin S, Powers G, Elta G, Cohen P, Cheng H. Ca2+ channel blockers stimulate ileal and colonic water absorption. Gastroenterology 89: 858–866, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Fordtran JS. Speculations on the pathogenesis of diarrhea. Fed Proc 26: 1405–1414, 1967 [PubMed] [Google Scholar]
- 11.Fuller CM, Benos DJ. CFTR! Am J Physiol Cell Physiol 263: C267–C286, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Furness JB, Robbins HL, Selmer IS, Hunne B, Chen MX, Hicks GA, Moore S, Neylon CB. Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract. Cell Tissue Res 314: 179–189, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Greger R, Bleich M, Riedemann N, van Driessche W, Ecke D, Warth R. The role of K+ channels in colonic Cl− secretion. Comp Biochem Physiol A Physiol 118: 271–275, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Gribkoff VK, Champigny G, Barbry P, Dworetzky SI, Meanwell NA, Lazdunski M. The substituted benzimidazolone NS004 is an opener of the cystic fibrosis chloride channel. J Biol Chem 269: 10983–10986, 1994 [PubMed] [Google Scholar]
- 15.Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl− and K+ secretion across distal colonic epithelium. Am J Physiol Cell Physiol 291: C636–C648, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Joiner WJ, Basavappa S, Vidyasagar S, Nehrke K, Krishnan S, Binder HJ, Boulpaep EL, Rajendran VM. Active K+ secretion through multiple KCa-type channels and regulation by IKCa channels in rat proximal colon. Am J Physiol Gastrointest Liver Physiol 285: G185–G196, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Krishnan S, Rajendran VM, Binder HJ. Apical NHE isoforms differentially regulate butyrate-stimulated Na absorption in rat distal colon. Am J Physiol Cell Physiol 285: C1246–C1254, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Mall M, Bleich M, Schurlein M, Kuhr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am J Physiol Gastrointest Liver Physiol 275: G1274–G1281, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Mandel KG, McRoberts JA, Beuerlein G, Foster ES, Dharmsathaphorn K. Ba2+ inhibition of VIP- and A23187-stimulated Cl− secretion by T84 cell monolayers. Am J Physiol Cell Physiol 250: C486–C494, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between KCa channel diversity and smooth muscle cell function. Circ Res 85: e33–e43, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Nielsen MS, Warth R, Bleich M, Weyand B, Greger R. The basolateral Ca2+-dependent K+ channel in rat colonic crypt cells. Pflügers Arch 435: 267–272, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Olesen SP, Munch E, Moldt P, Drejer J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur J Pharmacol 251: 53–59, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Petri WA, Jr, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest 118: 1277–1290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajendran VM, Geibel J, Binder HJ. Role of Cl channels in Cl−dependent Na/H exchange. Am J Physiol Gastrointest Liver Physiol 276: G73–G78, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Rufo PA, Jiang L, Moe SJ, Brugnara C, Alper SL, Lencer WI. The antifungal antibiotic, clotrimazole, inhibits Cl− secretion by polarized monolayers of human colonic epithelial cells. J Clin Invest 98: 2066–2075, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rufo PA, Merlin D, Riegler M, Ferguson-Maltzman MH, Dickinson BL, Brugnara C, Alper SL, Lencer WI. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and in an in vivo mouse model of cholera. J Clin Invest 100: 3111–3120, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sangan P, Brill SR, Sangan S, Forbush B, 3rd, Binder HJ. Basolateral K-Cl cotransporter regulates colonic potassium absorption in potassium depletion. J Biol Chem 275: 30813–30816, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Schmidt C, Kosche E, Baumeister B, Vetter H. Arachidonic acid metabolism and intracellular calcium concentration in inflammatory bowel disease. Eur J Gastroenterol Hepatol 7: 865–869, 1995 [PubMed] [Google Scholar]
- 29.Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther 296: 600–611, 2001 [PubMed] [Google Scholar]
- 30.Sweiry JH, Binder HJ. Active potassium absorption in rat distal colon. J Physiol 423: 155–170, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dinter TG, Jr, Fuerst FC, Richardson CT, Ana CA, Polter DE, Fordtran JS, Binder HJ. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology 129: 1268–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Vandorpe DH, Shmukler BE, Jiang L, Lim B, Maylie J, Adelman JP, de Franceschi L, Cappellini MD, Brugnara C, Alper SL. cDNA cloning and functional characterization of the mouse Ca2+-gated K+ channel, mIK1. Roles in regulatory volume decrease and erythroid differentiation. J Biol Chem 273: 21542–21553, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Vidyasagar S, Rajendran VM, Binder HJ. Three distinct mechanisms of HCO3− secretion in rat distal colon. Am J Physiol Cell Physiol 287: C612–C621, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Warth R, Hamm K, Bleich M, Kunzelmann K, von Hahn T, Schreiber R, Ullrich E, Mengel M, Trautmann N, Kindle P, Schwab A, Greger R. Molecular and functional characterization of the small Ca2+-regulated K+ channel (rSK4) of colonic crypts. Pflügers Arch 438: 437–444, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Zlatkina AR, Belousova EA, Vinitsky LI, Avtandilov GG, Chervonnaya LV. Cyclic nucleotide concentrations of rectal mucosa in ulcerative colitis. Scand J Gastroenterol 25: 341–344, 1990 [DOI] [PubMed] [Google Scholar]