Abstract
Background and Purpose
Erythropoietin (EPO), a hematopoietic cytokine, exerts neuroprotective effects in experimental stroke. In the present study, we investigated the effect of recombinant human EPO (rhEPO) in combination with tissue plasminogen activator (tPA) on embolic stroke.
Methods
Rats subjected to embolic middle cerebral artery occlusion (MCAo) were treated with rhEPO (5,000 units/kg) in combination with tPA (10 mg/kg) at 2 or 6 h after MCAo. Control groups consisted of ischemic rats treated with rhEPO (5,000 units/kg) alone, tPA (10 mg/kg) alone, or saline at 2 or 6h after MCAo.
Results
The combination therapy of rhEPO and tPA initiated 6h after MCAo did not reduce the ischemic lesion volume and significantly (p<0.05) increased the incidence of brain hemorrhage measured by frequency of gross hemorrhage and a quantitative spectrophotometric hemoglobin assay compared with rats treated with rhEPO alone and tPA alone. However, when the combination therapy was initiated 2h after MCAo, the treatment significantly (p<0.05) reduced the lesion volume and did not substantially increase the incidence of hemorrhagic transformation compared with saline-treated rats. Immunostaining analysis revealed that the combination therapy of rhEPO and tPA at 6h significantly (p<0.05) increased MMP9, NF-κB, and interleukin-1 receptor-associated kinase-1 (IRAK-1) immunoreactive cerebral vessels compared with rats treated with rhEPO alone and saline.
Conclusions
EPO exacerbates tPA-induced brain hemorrhage without reduction of ischemic brain damage when administered 6h after stroke in a rat model of embolic MCAo and that MMP9, NF-κB, and IRAK-1 upregulated by the delayed combination therapy may contribute to augmentation of brain hemorrhage.
Introduction
Recombinant human tissue-type plasminogen activator (tPA) is an effective treatment for acute ischemic stroke only when given within 4.5h of stroke onset 1. However, tPA treatment increases the incidence of hemorrhagic transformation 2, 3. Experimental studies show that neuroprotective or anti-thrombotic agents used in conjunction with tPA increases safety and efficacy of thrombolytic therapy in experimental stroke4–6.
Erythropoietin (EPO) is a naturally occurring cytokine and has been used for treatment of anemia for more than two decades 7. Administration of exogenous recombinant human EPO (rhEPO) after focal or global cerebral ischemia augments the cytoprotective and restorative EPO response pathway leading to a substantial improvement in neurobehavioral outcome 8–10. However, a recent report on the Phase III clinical trial for the treatment of acute ischemic stroke with EPO demonstrated increased mortality and hemorrhage in EPO treated patients compared with control 11. The reasons for the apparent adverse effects of the EPO treatment stand in contrast to the robust preclinical data indicating a therapeutic benefit of EPO treatment 12, 13. Careful review of the trial shows that 63% of patients enrolled in this trial also received tPA, and that many of these patients were treated at or beyond the therapeutic window for tPA 11. Data from the clinical trial also indicate that it was these patients that drove the adverse response to EPO 11. Consistent with the imperative to test preclinically the interaction between tPA and EPO and as a part of our ongoing studies on the neuroprotective effects of EPO treatment, we investigated the effects of combination treatment with EPO and tPA in a model of embolic stroke in the rat. We demonstrate that the combination treatment performed early after stroke (i.e. 2 hours) is neuroprotective; however, outside the therapeutic window (i.e. 6 hours), combination tPA and EPO significantly exacerbates hemorrhagic transformation and negates any benefit of EPO therapy
Methods
All experimental procedures were approved by the Henry Ford Hospital Committee forn the Care of Experimental Animals.
Animal model
Male Wistar rats weighing 350 to 450 g (n=194) were subjected to embolic middle cerebral artery occlusion (MCAo) by placement of an embolus at the origin of the MCA, as described previously14.
Experimental Protocols
Recombinant human EPO (rhEPO, epoietin α, AMGEN) was given (i.p) at a dose of 5,000 units/kg 2 or 6h after embolic MCAo and was followed by a second and third dose of 5,000 units/kg 24 and 48h after the first dose. Recombinant human tPA (Genentech) was infused intravenously at a dose of 10 mg/kg (10% bolus 2 or 6h after MCAo, and the remainder at a continuous infusion over a 30 min interval using a syringe infusion pump, (Harvard Apparatus). After MCAo, animals were randomly assigned to monotherapy with rhEPO at 2h (n=27) or 6h (n=24), tPA at 2h (n=26) or 6h (n=26), combination therapy with rhEPO and tPA at 2h (n=39), 6h (n=36), or saline (n=26). Rats were killed at 1 or 7 days after MCAo.
Measurements of infarct volume and hemorrhage
Seven days after MCAo, infarct volume and gross hemorrhage were measured as previously described 14 (see Supplemental Methods for more details).
Spectrophotometric measurement of intracerebral hemorrhage
The hemorrhage volume wasquantified with a spectrophotometric hemoglobin assay 24h after stroke onset (see Supplemental Methods)15.
Immunohistochemistry and Weestern blots
Immunohistochemistry and Western blots were performed on brain tissue from rats sacrificed 24 h after ischemia 6. The following antibodies were used in the present study: a mouse anti-MMP9 (1:100, Chemicon), a mouse anti-fibrin/fibrinogen antibody (1:1000, Accurate Chemical& Scientific), a mouse anti-endothelial barrier antigen (EBA, 1:1000, SMI 71, Sternberger Monoclonals Inc), a mouse anti-NF-κB (P65, 1:150, Chemicon), which is specific for the detection of activated NF-κB 16, and a mouse anti-interleukin-1 receptor-associated kinase-1 (IRAK-1) (1:50, Santa Cruz). (see Supplemental Methods).
Statistics
Data were evaluated for normality. Ranked data were used in the analysis when they were not normally distributed. The incidence of hemorrhage was compared using Chi-square analysis. The 2X2 factorial design and 2-way ANOVA were considered for each rhEPO and tPA combination at each administration time. The analysis started testing for overall group effect/treatment interactions, followed by the pair-wise group comparisons if the overall group effect/treatment interaction was detected at the 0.05 level; otherwise, the pair-wise group comparisons would be considered as exploratory analysis. P<0.05 was considered a significant difference. All values are presented as mean ± SE.
Results
Mortality
The mortality rates were 15, 20, 17, 30, 33, and 38% for rats treated with saline, EPO at 2h, EPO at 6h, tPA at 6h, the combination of EPO and tPA at 2 and 6h, respectively. No significant differences were detected among the groups. The majority of animals died within 24h of onset of MCAo. Rats that died were excluded from further evaluation.
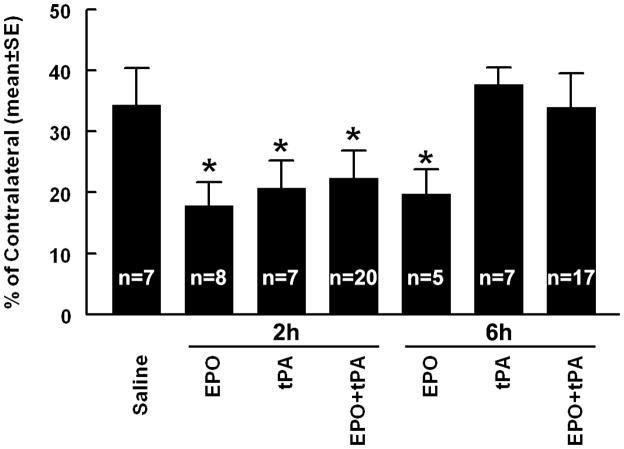
The effect of rhEPO in combination with tPA on ischemic lesion volume
To examine whether a combination therapy of rhEPO and tPA has a neuroprotective effect, ischemic rats were treated 2 or 6h after MCAo and killed 7 days after MCAo. When the treatment initiated at 2h of onset of stroke, monotherapy of rhEPO or tPA significantly (p<0.05) reduced ischemic lesion volume (Figure 1), which is consistent with published studies 13, 18. The combination therapy with rhEPO and tPA also significantly (p<0.05) reduced the lesion volume compared with rats treated with saline (Figure 1), but no treatment interaction (synergistic effect) or additive effect on the lesion volume was detected compared with the monotherapy. However, when the combination of rhEPO with tPA was administered 6h after stroke, the combination did not reduce the lesion volume compared with the saline group. As expected in this model 13, monotherapy of rhEPO but not tPA given 6h after stroke substantially reduced the lesion volume (Figure 1). These data indicate that the efficacy of 6h treatment of stroke with rhEPO is negated by tPA.
Figure 1. Infarct volume.
Bar graph shows the infarct volume assessed 7 days after MCAo. Values are mean ± SE. *P < 0.05 as compared with the saline-treated group.
The effect of rhEPO in combination with tPA on hemorrhage
A major concern of thrombolysis is the incidence of hemorrhagic transformation 3. To examine whether the combination of rhEPO with tPA affects the incidence of brain hemorrhage, we measured frequency of the gross hemorrhage and quantified brain hemorrhagic volume with a spectrophotometric hemoglobin assay 24h after stroke 19. Neither the combination therapy of rhEPO with tPA (35%) nor monotherapy of rhEPO (25%) or tPA (28%) initiated 2h after stroke significantly increased the incidence of hemorrhagic transformation compared with saline treated rats (14%). In contrast, the combination of rhEPO and tPA at 6h significantly increased (p<0.05) the incidence of gross hemorrhage from 14% in the saline group to 59% in the combination-treated group. The EPO monotherapy at 6h did not significantly increase the incidence of gross hemorrhage (25%) compared saline treated rats. The monotherapy of tPA at 6h resulted in a trend toward increase in the incidence of gross hemorrhage (43%), which did not reach statistical significance. However, quantitative data analysis of the brain hemorrhage revealed that the tPA monotherapy at 6h significantly (p<0.05) increased the hemorrhagic volume (4.4 μl, Figure 2) compared with the volume in the saline group (1.4 μl, Figure 2). Moreover, the combination of rhEPO and tPA further augmented (p<0.05) the hemorrhage volume (9 μl, Figure 2) compared with the tPA alone group (Figure 2). The monotherapy of rhEPO did not significantly increase the hemorrhage volume compared with the volume in the saline group (Figure 2).
Figure 2. Effects of EPO and tPA on hemorrhage.
Panel A shows the hemorrhage volume assessed with the spectrophotometric assay 24h after stroke onset. Panels B and C show intracerebral hemorrhage in H&E-stained coronal brain section from representative rats treated saline (B) and the combination of rhEPO and tPA (C) 6h after stroke onset. Values are mean ± SE. *P < 0.05 as compared with the saline-treated group. +P < 0.05 as compared with tPA-treated at 6h group. Bar=100μm.
Brain parenchymal deposition of the large plasma protein fibrin/fibrinogen is an indicator of BBB leakage 20. To further examine vascular leakage, we measured fibrin/fibrinogen deposition in ischemic brain. The combination of rhEPO and tPA at 6h substantially increased the number of cerebral vessels with extravascular fibrin deposition compared with ischemic rats treated with saline or the combination therapy at 2h (Figure 3), suggesting that the delayed combination therapy exacerbates BBB disruption.
Figure 3. Expression of MMP9, NF-κB, IRAK1, fibrin(ogen) and eNOS.
Panel A shows the MMP9 immunoreactivity measured 24h after MCAo. Panels B and C show MMP9 positive vessels in representative rats treated with saline (B) and the combination of rhEPO and tPA initiated at 6h (C). Panels D to F show the double immunofluorescent staining of NF-κB (green, D and E) with EBA (red, D) and fibrin(ogen) (red, E), and IRAK1 (red, F) with fibrin(ogen) (green, F) from a representative rat treated with rhEPO in combination with tPA at 6h. An arrow in panels indicates NF-κB (E) and IRAK1 positive vessels with appearances of extravascular fibrin deposition 24h after stroke onset. Quantitative data show that the combination treatment with rhEPO and tPA at 6h after stroke onset significantly increases the number of vessels with NF-κB (G), extravascular fibrin (H), and IRAK1 (I) immunoreactivity compared with the combination therapy at 2h or saline treated rats. Western blots show phospho-eNOS (J) and nuclear NF-κB (K) levels. Values are mean ± SE. *P < 0.05 as compared with the saline-treated group. Bars in panels B through F=50μm.
The effect of rhEPO in combination with tPA on expression of MMP9, NF-κB, and IRAK1
Upregulation of MMP9 contributes to hemorrhagic transformation after stroke 21, 22. To examine whether the delayed (6h) combination therapy of rhEPO and tPA affects MMP9 expression, immunohistochemical staining with antibodies against MMP9 was performed on coronal brain sections obtained from rats sacrificed 24h after stroke. Combination treatment with rhEPO and tPA at 6h significantly increased the MMP9 immunoreactive area compared with saline treated rats (Figure 3), suggesting that the increased MMP9 may contribute to exacerbation of hemorrhagic transformation.
In addition to its many functions, activation of NF-κB triggers upregulation of MMP9 and acts as a primary transactivator for tPA-induced BBB breakdown and hemorrhage transformation23, 24. We therefore measured NF-κB protein nuclear translocation at 2 and 24h after the treatments using Western blots. The combination treatment with EPO and tPA at 2 or 6h did not significantly alter the nuclear NF-κB protein levels compared with that in the saline, rhEPO alone and tPA alone groups (Figure 3). However, immunostaining analysis revealed that the delayed combination rhEPO and tPA significantly increased the number of NF-κB immunoreactive vessels compared with the combination therapy at 2h (Figure 3). Concurrently, the delayed combination treatment robustly increased the number of IRAK1 immunoreactive vessels compared with the number in rats treated with the combination therapy at 2h. IRAK1 triggers activation of NF-κB 25. In addition, double immunostaining revealed that some of NF-κB immunoreactive vessels exhibited parenchymal fibrin/fibrinogen deposition (Figure 3). The anti-NF-κB antibody used in the present study is specific for the detection of activated NF-κB 16. Thus, our data suggest that the delayed combination treatment upregulates IRAK1 and activates NF-κB, which may trigger MMP9 expression, leading to brain hemorrhage.
Activation of eNOS enhances thrombolysis and reduces brain hemorrhage in the ischemic brain 26. EPO elevates levels of phosphorylated eNOS protein in cerebral vessels 27. To examine whether the combination of rhEPO and tPA affects eNOS levels, Western blot analysis was performed. The combination treatment with EPO and tPA at 2 or 6h had no effects on eNOS protein levels (Figure 3).
Discussion
The present study demonstrated that the combination of rhEPO and tPA initiated 6h after embolic MCAo failed to reduce ischemic lesion volume and exacerbated hemorrhagic transformation, whereas the combination therapy started 2h after stroke significantly reduced ischemic damage without increasing the hemorrhagic transformation. These data suggest that rhEPO exacerbates tPA-induced brain hemorrhage when the combination treatment starts 6h after stroke in a rat model of embolic MCA occlusion.
Previous experimental studies in the treatment of acute ischemic stroke demonstrated that tPA in combination with neuroprotective agents such as neuroserpin, minocycline, and atorvastatin given 4 to 6h after embolic stroke enhances the neuroprotective effect by attenuating the side effects of thrombolysis 28–30. Since the efficacy of EPO in experimental stroke has been well demonstrated, we hypothesized that the combination treatment of rhEPO with tPA extends the therapeutic window for tPA. However, to our surprise, the present study showed that the combination of rhEPO and tPA initiated 6h after stroke substantially augmented the incidence of brain hemorrhage compared with monotherapy of rhEPO or tPA given at the same time point. The efficacy of the monotherapy of rhEPO at 2 and 6h observed in the present study are consistent with published reports 13. In this model of embolic stroke, we have demonstrated that the therapeutic window for tPA is less than 4h after stroke onset, and delayed tPA treatment increases the incidence of hemorrhagic transformation 28, 31. The present study shows that monotherapy of tPA at 6h augmented the incidence of brain hemorrhage. However, rhEPO in combination with tPA at 6h aggravated the hemorrhage, suggesting that EPO likely exacerbates tPA-induced hemorrhage. These experimental results are consistent with observations made from the recent double-blind, placebo-controlled, randomized German Multicenter EPO Stroke Trial 11, reporting that with a high proportion of tPA treated patients enrollment (63%), there was a significant increase in death rate when stroke patients received EPO within 6h of the onset of stroke compared with the death rate in the placebo group 11. The elevated tPA protocol violation rate (50%) including treatment beyond the 3h time window may have contributed to the high death rate in the EPO treated patients.
Mechanisms underlying the exacerbation and the increased incidence of hemorrhage are currently unknown when the combination therapy of rhEPO and tPA was initiated 6h after stroke. Thrombolysis with tPA upregulates MMP9, a protein-digesting enzyme which degrades the major extracellularmatrix components, and thereby exacerbates BBB disruption and hemorrhagic transformation 32–34. The present study shows that tPA in combination with rhEPO at 6h but not 2h significantly increased the MMP9 levels, which was concurrent with an increase in the number of leaking vessels, suggesting that upregulation of MMP9 contributes to augmentation of brain hemorrhage. NF-κB triggers upregulation of MMP9 expression 35. EPO regulates the NF-κB signaling pathway that mediates EPO-induced neuroprotection 36. Thus, EPO could activate the NF-κB signaling pathway leading to an upregulation of MMP9 expression when EPO is administered in combination of tPA. Indeed, the delayed (6h) combination treatment substantially activated NF-κB signaling pathway in cerebral vessels, although our Western blot analysis did not reveal significant differences of NF-κB levels among experimental groups either at acute (2h after initiated treatments) or delayed (24h after initiated treatments) time point. Moreover, the delayed combination treatment upregulated IRAK1 in cerebral vessels. IRAK1 plays an important role in signal transduction of Toll-like receptors and interleukin-1 receptors 25. IRAK1 along with other molecules activate NF-κB 25. Collectively, we speculate that the delayed combination therapy may activate the NF-κB signaling pathway and IRAK1, leading to upregulation of MMP9 that mediates augmentation of brain hemorrhage observed in the present study. A possible limitation of the present study is that the dose of tPA employed was 10 times higher than the dose used in humans, and this dose may upregulate IRAK1 and NF-κB and thereby contribute to hemorrhagic transformation. Future studies focusing on investigating the cause-effect of the NF-κB signaling pathway and IRAK1 upregulated by the delayed combination of rhEPO and tPA on exacerbating brain hemorrhage are warranted.
In summary, the present study indicates that the combination treatment with rhEPO and tPA carries a higher risk of intracerebral hemorrhage without reduction of ischemic brain damage when administered 6h after embolic stroke, while the combination therapy initiated 2h after stroke does not exacerbate brain hemorrhage and reduces ischemic cell damage.
Supplementary Material
Acknowledgments
The authors wish to thank Cindi Roberts and Qing-e Lu for technical assistance. This work was supported by NINDS grants PO1 NS23393 and RO1 HL64766.
References
- 1.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Intracerebral hemorrhage after intravenous t-pa therapy for ischemic stroke. The ninds t-pa stroke study group. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 3.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Wang CX, Ding X, Shuaib A. Treatment with melagatran alone or in combination with thrombolytic therapy reduced ischemic brain injury. Exp Neurol. 2008;213:171–175. doi: 10.1016/j.expneurol.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: Generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–416. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Intravenous administration of a gpiib/iiia receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke. 2004;35:2890–2895. doi: 10.1161/01.STR.0000147963.68238.da. [DOI] [PubMed] [Google Scholar]
- 7.Jelkmann W. Erythropoietin: Structure, control of production, and function. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 8.Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- 9.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 10.Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jahnig P, Herrmann M, Knauth M, Bahr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 12.Minnerup J, Heidrich J, Rogalewski A, Schabitz WR, Wellmann J. The efficacy of erythropoietin and its analogues in animal stroke models: A meta-analysis. Stroke. 2009;40:3113–3120. doi: 10.1161/STROKEAHA.109.555789. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, Lu M, Pool C, Heavner G, Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain Res. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 15.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- 16.Kaltschmidt C, Kaltschmidt B, Henkel T, Stockinger H, Baeuerle PA. Selective recognition of the activated form of transcription factor nf-kappa b by a monoclonal antibody. Biol Chem Hoppe Seyler. 1995;376:9–16. doi: 10.1515/bchm3.1995.376.1.9. [DOI] [PubMed] [Google Scholar]
- 17.Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappab and cell death after experimental intracerebral hemorrhage in rats. Stroke. 1999;30:2472–2477. doi: 10.1161/01.str.30.11.2472. discussion 2477–2478. [DOI] [PubMed] [Google Scholar]
- 18.Zhang RL, Zhang ZG, Chopp M, Zivin JA. Thrombolysis with tissue plasminogen activator alters adhesion molecule expression in the ischemic rat brain. Stroke. 1999;30:624–629. doi: 10.1161/01.str.30.3.624. [DOI] [PubMed] [Google Scholar]
- 19.Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH. Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke. 2002;33:2100–2104. doi: 10.1161/01.str.0000023534.37670.f7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang ZG, Chopp M, Goussev A, Lu D, Morris D, Tsang W, Powers C, Ho KL. Cerebral microvascular obstruction by fibrin is associated with upregulation of pai-1 acutely after onset of focal embolic ischemia in rats. J Neurosci. 1999;19:10898–10907. doi: 10.1523/JNEUROSCI.19-24-10898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini MgE, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: A possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein c inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 25.Gottipati S, Rao NL, Fung-Leung WP. Irak1: A critical signaling mediator of innate immunity. Cell Signal. 2008;20:269–276. doi: 10.1016/j.cellsig.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Zhang ZG, Liu X, Hozeska A, Stagliano N, Riordan W, Lu M, Chopp M. Treatment of embolic stroke in rats with bortezomib and recombinant human tissue plasminogen activator. Thromb Haemost. 2006;95:166–173. [PubMed] [Google Scholar]
- 27.Santhanam AV, Smith LA, Akiyama M, Rosales AG, Bailey KR, Katusic ZS. Role of endothelial no synthase phosphorylation in cerebrovascular protective effect of recombinant erythropoietin during subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2005;36:2731–2737. doi: 10.1161/01.STR.0000190021.85035.5b. [DOI] [PubMed] [Google Scholar]
- 28.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Zhang L, Yepes M, Jiang Q, Li Q, Arniego P, Coleman TA, Lawrence DA, Chopp M. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation. 2002;106:740–745. doi: 10.1161/01.cir.0000023942.10849.41. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Zhang ZG, Ding GL, Jiang Q, Liu X, Meng H, Hozeska A, Zhang C, Li L, Morris D, Zhang RL, Lu M, Chopp M. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;112:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Zhang ZG, Zhang RL, Lu M, Adams J, Elliott PJ, Chopp M. Postischemic (6-hour) treatment with recombinant human tissue plasminogen activator and proteasome inhibitor ps-519 reduces infarction in a rat model of embolic focal cerebral ischemia. Stroke. 2001;32:2926–2931. doi: 10.1161/hs1201.100207. [DOI] [PubMed] [Google Scholar]
- 32.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 33.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 34.Liu XS, Zhang ZG, Zhang L, Morris DC, Kapke A, Lu M, Chopp M. Atorvastatin downregulates tissue plasminogen activator-aggravated genes mediating coagulation and vascular permeability in single cerebral endothelial cells captured by laser microdissection. J Cereb Blood Flow Metab. 2006;26:787–796. doi: 10.1038/sj.jcbfm.9600227. [DOI] [PubMed] [Google Scholar]
- 35.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: An absolute requirement for transcription factor nf-kappa b. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 36.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between jak2 and nf-kappab signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.