Abstract
Recent applications of micro-reactor (microfluidics) technology to radiofluorination chemistry within our laboratory are presented, based on use of either a simple T-shaped glass micro-reactor or a more advanced microfluidics instrument. The topics include reaction optimization and radioligand production, in particular the study of the radiofluorination of diaryliodonium salts, [18F]fluoride ion exchange with xenon difluoride, esterification with [18F]2-fluoroethyl tosylate, and the syntheses of [18F]fallypride, [18F]FBR and [18F]SL702 from [18F]fluoride ion.
Keywords: micro-reactor, microfluidics, fluorine-18, fluorination, PET
Introduction
It is increasingly recognized that micro-reactor (or microfluidics) technology can provide considerable advantages in radiochemistry with short-lived positron-emitting fluorine-18 (t1/2 = 109.7 min).1–5 Possible benefits to be derived from this technology include: (1) the use of smaller amounts of materials, especially non-radioactive precursor, which may be precious or difficult to obtain; (2) easier and more efficient radioactive product purification; (3) reduced radiation exposure to radiochemists by allowing more efficient radiosynthesis with less radioactivity; and (4) potential for scale-up and a high degree of automation.
We have used micro-reactor technology to optimize radiofluorination procedures and to produce radioligands reliably and reproducibly for molecular imaging in rodents with positron emission tomography (PET).6–15 Other groups are also exploring microfluidics in PET chemistry.16–20 This presentation tracks the growth of our research in this area covering the early development of a simple glass T-shaped micro-reactor to recent progress in several areas of fluorine-18 chemistry in a more advanced microfluidics instrument. Our examples show-case some of the various benefits of using microfluidics in fluorine-18 chemistry.
Results and Discussion
[18F]3-(3-Pyridinyl)propionic-2′-fluoroethyl ester by esterification
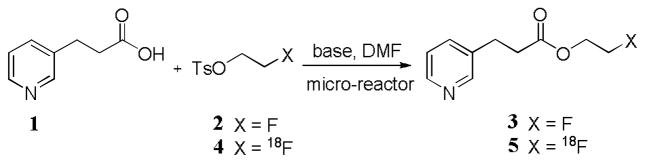
As a proof-of-principle study, our first examples of radiosyntheses with a simple hydrodynamically-driven glass micro-reactor were those of 18F-labeled esters (Figure 1).6 2-[18F]Fluoroethyl esters are sometimes proposed as PET radiotracers.21 Such esters may be prepared by reactions of carboxylic acid salts with [18F]2-fluoroethyl tosylate (4). The synthesis of the model 2-fluoroethyl ester 3 in moderate yield from the reaction of 1 with 2-fluoroethyl tosylate (2) required warming the micro-reactor to 80 °C. Yields were dependent on reactant concentration and flow rate. At lower flow rate, a higher concentration of reactant 1 gave more ester 3. The reaction could be performed with as low as 0.75 μg (5 nmol) of 1 in 10 μL of solution. Reactions of 1 with the labeling agent 4 at 80 °C, at infusion rates of 1 μL/min, gave the corresponding 18F-labeled ester 5 in 10% decay-corrected radiochemical yield (RCY). This device had however limited scope for control of temperature and reaction stoichiometry. Radiosyntheses were necessarily carried out at < 2 mCi level because the procedure was not highly automated.
Figure 1.
Labeling of 5 with [18F]2-fluoroethyl tosylate in a simple micro-reactor.
[18F]Fallypride by aliphatic nucleophilic substitution
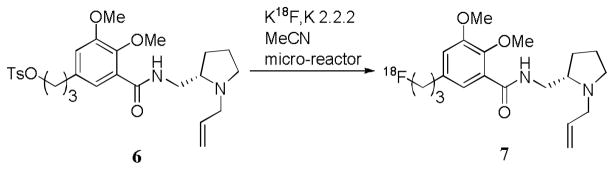
A commercial coiled-tube micro-reactor (NanoTek; Advion) subsequently became a convenient platform for the study of 18F-labeling with microfluidics in our laboratory. Radiosynthesis of the brain dopamine D2 receptor radioligand, [18F]fallypride (7)22 from the tosylate-precursor 6 was rapidly optimized in this apparatus, with respect to the effects of precursor amount, reaction temperature, flow rate and [18F]fluoride ion to precursor concentration ratio (Figure 2).7 Each radiosynthesis used low amounts (20–40 μg; 39–77 nmol) of 6 and [18F]fluoride ion/K 2.2.2 (0.5–2.5 mCi). RCYs of 7 (up to 88%) were reproducible. The low amounts of material used in each radiosynthesis allowed crude 7 to be purified rapidly on an analytical-size reversed phase HPLC column, preceding formulation for intravenous injection. Scale-up of the reaction was achieved by continuously infusing precursor and [18F]fluoride ion solutions into the reactor to obtain 7 in much greater radioactivity (> 10 mCi). In this instrument, 7 was conveniently synthesized in small doses (0.3–1.5 mCi) for micro-PET studies in rodents.
Figure 2.
Synthesis of 7 in a micro-reactor.
[18F]FBR by aliphatic nucleophilic substitution
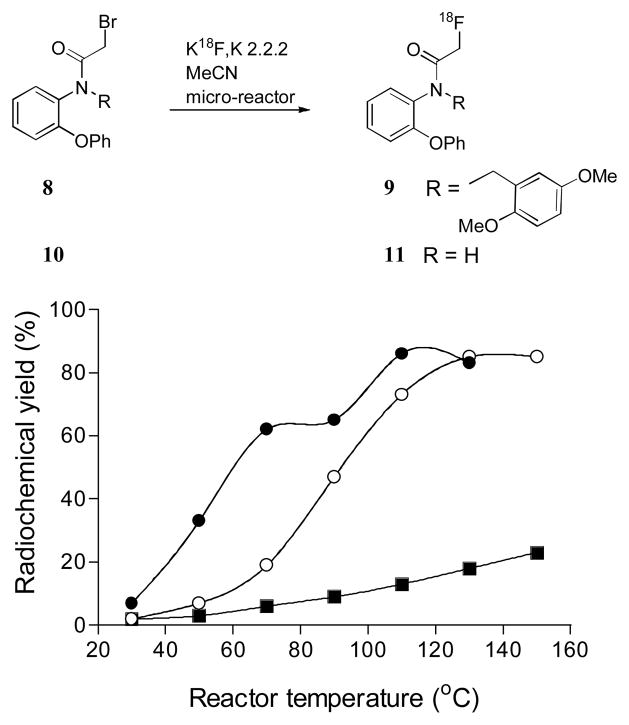
[18F]FBR (9) is an effective TSPO radioligand8 which is now being used to study inflammatory conditions in human subjects.23,24 We have shown that 9 can be produced in high RCY in the Advion microfluidic apparaus (Figure 3). Below 90 °C the RCY of 9 was lower in slightly wet acetonitrile than in anhydrous acetonitrile. However, this difference in RCYs disappeared when the reactor temperature was raised above 110 °C. RCYs reached 85% at 110 °C. Further temperature increase gave no improvement in RCY. The synthesis of the main metabolite (11) of 9 was also investigated in the microfluidic apparatus under similar conditions. The RCY was substantially lower than for 9 over the temperature range 30–150 °C. 9 and 11 were each prepared with this microfluidic apparatus in sufficient amounts for intravenous injection into rodents.
Figure 3.
Synthesis of 9 and 11 in a micro-reactor and temperature dependence of RCYs of 9 in anhydrous acetonitrile (●) or acetonitrile with 0.3% v/v water (○), and of 11 in anhydrous acetonitrile (■).
[18F]SL702 synthesis by flow or stopped-flow mode
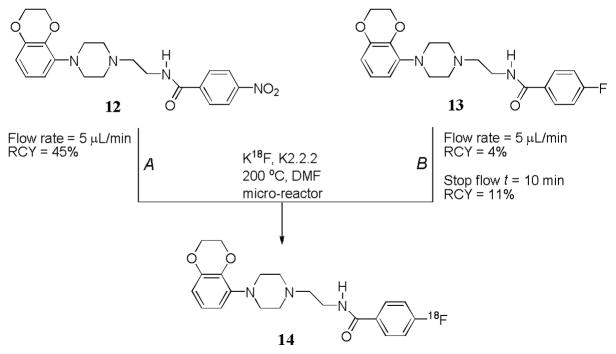
[18F]SL702 (14), a new potential agonist radioligand for brain 5-HT1A receptors, was successfully prepared in the Advion micro-reactor from a nitro-precursor (12) in a moderate RCY, similar to that obtained in a conventional microwave procedure (Route A, Figure 4).
Figure 4.
Synthesis of 14 in a micro-reactor by aromatic nucleophilic substitution in a nitro-precursor (Route A) or from 18F for 19F exchange (Route B).
Direct 18F for 19F exchange could be a useful route for labeling with fluorine-18 where the target radiotracer is not required to have high specific radioactivity. Although, high specific activity would be required for applications of [18F]SL702, we wished to test the feasibility of performing 18F for 19F exchange for this type of structure. We first investigated the exchange reaction using a low concentration of 13 (Route B, Figure 4). When the flow rate was 5 μL/min, the RCY was about 4%. A ‘stopped flow’ method was introduced to allow longer reaction time in the micro-reactor. For 10 min reaction time the RCY increased to 11%. Further optimization for this and other targets is under investigation.
Syntheses of [18F]fluoroarenes from diaryliodonium salts
Reactions of diaryliodonium salts with [18F]fluoride ion are increasingly useful for the preparation of [18F]fluoroarenes as radiotracers from NCA [18F]fluoride ion.25,26 The Advion micro-reactor apparatus has enabled us to study the RCYs, product selectivity, kinetics and energetics of these reactions in detail (Figure 5).10–14 This platform was very convenient for running multiple reactions rapidly under well-controlled conditions of reactant concentrations, reaction times and reaction temperatures. High RCYs of a vast array of NCA [18F]fluoroarenes were obtained in short reaction times (< 7 min).
Figure 5.
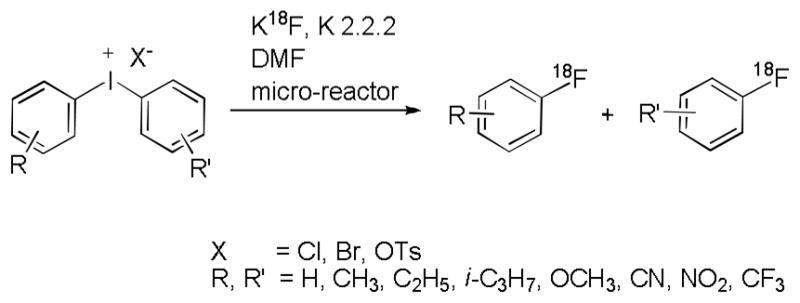
Reactions of diaryliodonium salts with [18F]fluoride ion to produce [18F]fluoroarenes in a micro-reactor
[18F]XeF2 by direct exchange
18F-Labeled xenon difluoride ([18F]XeF2) is a potentially useful ‘electrophilic’ radiofluorination agent. We have previously prepared [18F]xenon difluoride by exchange of xenon difluoride with [18F]fluoride ion (as 18F−-Cs+/K 2.2.2) in dichloromethane at room temperature (Figure 6).27 This process is attractive because of the availability of [18F]fluoride ion in high activity and high specific activity from the 18O(p,n)18F reaction on [18O]water. We exploited a microfluidic device to study further the influence of different conditions on this reaction.15 [18F]Xenon difluoride was obtained in high RCY (50%) by exchange of [18F]fluoride ion with xenon difluoride in either dichloromethane or acetonitrile. In acetonitrile, the reaction may be performed in the presence of Cs+, with or without K 2.2.2, or with K+-K 2.2.2 at elevated temperature. The use of a micro-reactor allowed close monitoring of the progress of the exchange reaction with time and temperature. The procedure has potential to produce [18F]xenon difluoride consistently and rapidly, and at a usefully high specific radioactivity.
Figure 6.
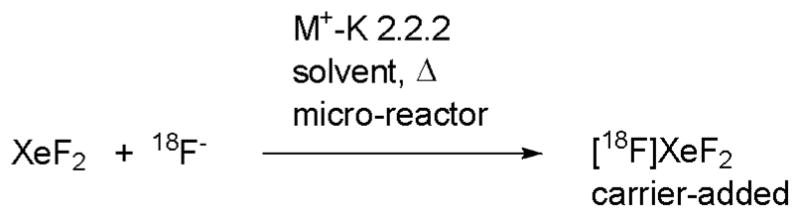
Preparation of [18F]xenon difluoride by exchange with [18F]fluoride ion in a micro-reactor
Conclusions
Research in our laboratory and elsewhere has clearly demonstrated that micro-reactor or microfluidics technology is well suited to PET radiotracer development and production. Our results exemplify some of the potential advantages of this methodology for radiotracer development and synthesis. This technology should be increasingly amenable to greater sophistication to encompass entire radiosyntheses in a versatile high throughput manner and should play a more significant role in advancing PET radiochemistry.
Acknowledgments
This research was supported by the Intramural Research Program (Project No Z01-MH-002793) of the NIH, NIMH. SYL and VWP dedicate this presentation to the memory of Professor John R. Jones for invaluable mentorship and friendship in former times.
References
- 1.Lu SY, Pike VW. PET Chemistry. In: Schubiger PA, Lehmann L, Friebe M, editors. The Driving Force in Molecular Imaging. Springer-Verlag; Heiderberg: 2007. pp. 271–287. [Google Scholar]
- 2.Lucignani G. Eur J Nucl Med Mol Imaging. 2006;33:849–851. doi: 10.1007/s00259-006-0149-8. [DOI] [PubMed] [Google Scholar]
- 3.Cai LS, Lu SY, Pike VW. Eur J Org Chem. 2008;17:2853–2873. [Google Scholar]
- 4.Miller PW. J Chem Technol Biotechnol. 2009;84:309–315. [Google Scholar]
- 5.Elizarov A. Lab Chip. 2009;9:1326–1333. doi: 10.1039/b820299k. [DOI] [PubMed] [Google Scholar]
- 6.Lu SY, Watts P, Chin FT, Hong J, Musachio JL, Briard E, Pike VW. Lab Chip. 2004;4:523–525. doi: 10.1039/b407938h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu SY, Giamis AM, Pike VW. Curr Radiopharm. 2009;2:49–55. doi: 10.2174/1874471010902010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briard E, Zoghbi SS, Siméon FG, Imaizumi M, Gourley GP, Shetty HU, Lu SY, Fujita M, Innis RB, Pike VW. J Med Chem. 2009;52:688–699. doi: 10.1021/jm8011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu SY, Clements JT, Gilde MJ, Prak A, Watts P, Pike VW. J Labelled Compd Radiopharm. 2007;50:597–599. [Google Scholar]
- 10.Chun J, Lu S, Pike VW. J Labelled Compd Radiopharm. 2009;52(Suppl. 1):S2. [Google Scholar]
- 11.Telu S, Chun J, Siméon FG, Lu SY, Pike VW. J Labelled Compd Radiopharm. 2009;52(Suppl 1):S4. [Google Scholar]
- 12.Chun J, Lu S, Pike VW. J Labelled Compd Radiopharm. 2009;52(Suppl 1):S14. [Google Scholar]
- 13.Chun J, Lu S, Pike VW. J Labelled Compd Radiopharm. 2009;52(Suppl 1):S156. [Google Scholar]
- 14.Chun J, Lu S, Lee YS, Pike VW. J Labelled Compd Radiopharm. 2009;52(Suppl 1):S159. [Google Scholar]
- 15.Lu S, Lee YS, Pike VW. J Labelled Compd Radiopharm. 2009;52(Suppl 1):S496. [Google Scholar]
- 16.J Labelled Compd Radiopharm; Abstracts of Presentations, Session 2: Microfluidics and nanotechnology, 18th International Symposium on Radiopharmaceutical Sciences; 2009. pp. S8–S14. [Google Scholar]
- 17.J Labelled Compd Radiopharm; Abstracts of Presentations, Novel technology: nanotechnology, microreactors and microfluidics posters; 2009. pp. S496–S508. [Google Scholar]
- 18.Lee CC, Sui GD, Elizarov A, Shu CYJ, Shin YS, Dooley AN, Huang J, Daridon A, Wyatt P, Stout D, Kolb HC, Witte ON, Satyamurthy N, Heath JR, Phelps ME, Quake SR, Tseng HR. Science. 2005;310:1793–1796. doi: 10.1126/science.1118919. [DOI] [PubMed] [Google Scholar]
- 19.Gillies JM, Prenant C, Chimon GN, Smethurst GJ, Perrie W, Hamblett I, Dekker B, Zweit J. Appl Radiat Isot. 2006;64:325–332. doi: 10.1016/j.apradiso.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Steel CJ, O’Brien AT, Luthra SK, Brady F. J Labelled Compd Radiopharm. 2007;50:308–311. [Google Scholar]
- 21.Lu SY, Chin FT, McCarron JA, Pike VW. J Labelled Compd Radiopharm. 2004;47:289–297. [Google Scholar]
- 22.Mukherjee J, Yang ZY, Brown T, Lew R, Wernick M, Ouyang XH, Yasillo N, Chen CT, Mintzer R, Cooper M. Nucl Med Biol. 1999;26:519–527. doi: 10.1016/s0969-8051(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 23.Imaizumo M, Briard E, Zoghbi SS, Hong J, Musachio JL, Pike VW, Innis RB, Fujita M. Synapse. 2007;61:595–605. doi: 10.1002/syn.20394. [DOI] [PubMed] [Google Scholar]
- 24.Fujimura Y, Zoghbi SS, Simèon FG, Taku A, Pike VW, Innis RB, Fujita M. J Nucl Med. 2009;50:1047–1053. doi: 10.2967/jnumed.108.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pike VW, Aigbirhio FI. J Chem Soc, Chem Commun. 1995:2215–2216. [Google Scholar]
- 26.Shah A, Pike VW, Widdowson DA. J Chem Soc, Perkin Trans I. 1998:2043–2046. [Google Scholar]
- 27.Constantinou M, Aigbirhio FI, Smith RG, Ramsden CA, Pike VW. J Am Chem Soc. 2001;123:1780–1781. doi: 10.1021/ja003321j. [DOI] [PubMed] [Google Scholar]