Abstract
The CANadian Network and Centre for Trials INternationally (CANNeCTIN) was jointly funded by the Canadian Institutes of Health Research and the Canadian Foundation for Innovation in April 2008 to provide infrastructure for clinical studies of cardiovascular diseases and diabetes mellitus. Its functional components include a national coordinating centre at the Population Health Research Institute (PHRI) in Hamilton (Ontario), a collaborative Canadian network and an affiliated international network of hospitals and clinics. The rationales for CANNeCTIN include the global health burden of cardiovascular diseases and diabetes, the strengths of randomized controlled trials – particularly large, multicentre and international – and the track record of success of the PHRI. CANNeCTIN will provide investigators from across Canada with opportunities to become the principal investigators of national and international trials coordinated by the PHRI. CANNeCTIN will support priority pilot studies, and successful investigators will be encouraged and assisted to apply for peer review and industrial funding for full studies to be conducted within the network and coordinated by the PHRI. An extensive education program offers hands-on experience in organizing and leading large national/international clinical trials led by accomplished researchers, distance learning courses in clinical research methodology, biostatistics and study coordination, and ‘cutting-edge’ workshops. A knowledge translation program seeks opportunities arising from clinical trials and encourages research into this paradigm for understanding how best to close the gaps between knowledge and effective practice. The five-year goals are to enhance the capacity of Canadian investigators to lead major clinical studies, facilitate knowledge translation and exchange, and augment Canada’s capacity to train the next generation of leaders in cardiovascular and diabetes clinical research.
Keywords: Cardiovascular diseases, Clinical research, Diabetes mellitus, Research infrastructure, Research networks
Abstract
Le CANadian Network and Centre for Trials INternationally (réseau et centre canadien pour les essais internationaux, ou CANNeCTIN) a été fondé conjointement par les Instituts de recherche en santé du Canada et la Fondation canadienne pour l’innovation en avril 2008 afin de fournir une infrastructure aux études cliniques sur les maladies cardiovasculaires et le diabète. Ses éléments fonctionnels incluent un centre de coordination national au Population Health Research Institute (PHRI) de Hamilton, en Ontario, un réseau coopératif canadien et un réseau international d’hôpitaux et de cliniques affiliés. La raison d’être du CANNeCTIN inclut le fardeau de santé mondial des maladies cardiovasculaires et du diabète, la solidité des essais aléatoires et contrôlés, notamment de grands essais multicentriques et internationaux, et le dossier de réussites du PHRI. Le CANNeCTIN soutiendra les études pilotes prioritaires, et les chercheurs retenus seront encouragés et aidés pour profiter d’examens par les pairs et faire des demandes de financement industriel afin de mener de vastes études au sein du réseau, coordonnées par le PHRI. Un programme de formation complet propose de l’expérience pratique d’organisation et de direction de grands essais cliniques nationaux et internationaux dirigés par des chercheurs accomplis, des cours d’apprentissage à distance en méthodologie de recherche clinique, en biostatistique et en coordination d’études et des ateliers de pointe. Un programme de transmission du savoir tire profit des possibilités soulevées par les essais cliniques et favorise la recherche sur ce paradigme afin de découvrir le meilleur moyen de corriger les lacunes entre les connaissances et une pratique efficace. Les objectifs quinquennaux consistent à accroître la capacité des chercheurs canadiens à mener d’importantes recherches cliniques, à faciliter la transmission et l’échange du savoir et à accroître la capacité canadienne à former la prochaine génération de chefs de file en recherche clinique sur les maladies cardiovasculaires et le diabète.
In the 1990s, national concern about the support of health research in Canada led to substantial increases in federal government funding. However, increases in the funding of clinical research have lagged behind those of biomedical, health services and population health research (1,2). Whereas the National Institutes of Health (NIH; USA) allocates 32% to 38% of its budget to clinical research, the Canadian Institutes of Health Research (CIHR) allocates only 22% (defining clinical research using NIH’s broad definition) (2). The NIH devotes approximately 12% of its budget to clinical trials, while the CIHR allocates approximately 4%. In 2004, the CIHR launched its clinical research initiative (3) in response to a clear need and opportunity to strengthen clinical research in Canada. In April 2007, the CIHR and the Canadian Foundation for Innovation (CFI) jointly invited proposals for substantial strategic initiatives in support of clinical research programs, teams and networked infrastructure that focused on high-impact, clinically relevant health problems. The CANadian Network and Centre for Trials INternationally (CANNeCTIN) was one of five successful applications awarded funding in April 2008. CANNeCTIN offers the promise of a new era of Canadian leadership of high-impact clinical trials, registries and epidemiological studies of cardiovascular diseases and diabetes.
OVERVIEW OF CANNeCTIN
CANNeCTIN has engaged more than 115 clinical researchers from 19 Canadian universities, including all 17 medical schools (Figure 1). Building on the internationally recognized achievements of Canadian investigators in cardiovascular diseases and diabetes, CANNeCTIN brings together accomplished clinical researchers from across the country to achieve major discoveries not possible for a single centre. Its functional components include a national coordinating centre based within the Population Health Research Institute (PHRI) of Hamilton Health Sciences and McMaster University (Hamilton, Ontario), a collaborative network of Canadian hospitals and clinics active in clinical research, and an affiliated international network of more than 1500 hospitals and clinics from 80 countries. CANNeCTIN provides organizational capacity and infrastructure to enhance the capacity of Canadian investigators to lead major clinical studies of important questions, facilitate knowledge translation and exchange, and augment Canada’s capacity to train the next generation of leaders in cardiovascular and diabetes clinical research.
Figure 1).
Locations of the 19 CANadian Network and Centre for Trials INternationally (CANNeCTIN) collaborating universities. Newfoundland and Labrador: Memorial University of Newfoundland, St John’s (1). Nova Scotia: Dalhousie University, Halifax (2). Quebec: Université Laval, Quebec City (3); Université de Sherbrooke, Sherbrooke (4); McGill University, Montreal (5); Université de Montreal, Montreal (6). Ontario: University of Ottawa, Ottawa (7); Queen’s University, Kingston (8); University of Toronto, Toronto (9); McMaster University, Hamilton (10); University of Waterloo, Waterloo (11); University of Western Ontario, London (12); Northern Ontario Medical School, Sudbury and Thunder Bay (13). Manitoba: University of Manitoba, Winnipeg (14). Saskatchewan: University of Saskatchewan, Saskatoon (upper circle) and Regina (lower circle) (15). Alberta: University of Alberta, Edmonton (16); University of Calgary, Calgary (17). British Columbia: Simon Fraser University, Vancouver (18); University of British Columbia, Vancouver (19)
RATIONALE FOR CANNeCTIN
Cardiovascular diseases are collectively responsible for the largest health burden worldwide (as well as within Canada), accounting annually for 18 million deaths (4–6). Although the past four decades have witnessed a decline in the age-specific rates of cardiovascular mortality in many Western countries (7), an aging population will ensure that cardiovascular diseases remain their number one health burden for decades to come. The problem is worse in low- and middle-income countries, which are increasingly the source of most of Canada’s immigrants. Indeed, 80% of all cardiovascular diseases occur in these countries, and rates there are projected to increase due to urbanization, changes in lifestyles and population aging, coupled with suboptimal access to high-quality health care. By 1971, diabetes mellitus had become an epidemic – affecting more than 170 million persons globally. Its prevalence is estimated to increase by nearly 50% by 2010 (8). The growing diabetes burden is associated with obesity and is highly prevalent in lower socioeconomic groups and regions in Canada and worldwide.
Randomized controlled trials (RCTs) are essential to identify interventions that are effective, ineffective or even harmful. Hypotheses generated from basic science research, clinical observations and epidemiological research may suggest potentially useful interventions, but data from such sources may mislead clinicians. The results of RCTs have transformed the prevention and treatment of most diseases. The widespread application of the positive results of RCTs of acetylsalicylic acid (9), statins (10), modulators of the renin-angiotensin-aldosterone system (11) and fibrinolytic agents (12) in atherosclerotic diseases has contributed to marked reductions in morbidity and mortality. Indeed, approximately one-half of the decline in coronary disease mortality observed in the United States from 1980 to 2000 is estimated to be due to the use of therapies whose efficacy was proven by carefully designed large RCTs (13). On the other hand, several seemingly innocuous but promising therapies such as vitamin supplementation (eg, vitamin E [14], beta-carotene [15], and folate plus vitamin B12 [16]) have been found through RCTs to be ineffective or harmful, and formerly widely used therapies such as hormone replacement (17) and antiarrhythmic drugs (18) were discovered to be harmful and, appropriately, there has been a sharp reduction in their use.
There is enormous potential to increase the scope and impact of the health benefits arising from RCTs because of the following:
Even proven therapies are only partially effective and there are many cardiovascular diseases for which we have few proven therapies.
Uncertainty persists regarding the applicability of the evidence from many RCTs to particular patient subgroups, because insufficient numbers of individuals of different ethnicities and cultural backgrounds were included. Given the wide diversity of Canada’s population and the reality that the greatest prevalence of cardiovascular diseases and diabetes is in nonwhite populations, future RCTs must generate knowledge of the effects of drugs in multiple ethnic groups.
RCTs offer an underutilized opportunity to study health services and knowledge translation.
- The substantial costs of large trials and limited public funding means that the research agenda is excessively driven by commercial interests. Accordingly, there is a need to facilitate the evaluation of the following:
- interventions for neglected diseases and conditions (eg, diseases that are uncommon in Canada but are common elsewhere, particularly in poor countries);
- neglected treatments (eg, drugs and devices beyond their patent life, and lifestyle and behavioural interventions);
- neglected people (eg, those often excluded from RCTs and yet who might benefit from [or be harmed by] established therapies); and
- neglected outcomes (eg, newly recognized consequences of cardiovascular diseases such as chronic renal disease and vascular cognitive impairment).
Reliable answers to important questions require large, multicentre and, often, international trials. Whereas RCTs were initially undertaken in the 1950s to detect significant benefits (ie, 50% risk reductions) such as those seen in infectious diseases, current interventions in cardiovascular diseases are likely to produce only moderate (yet important) RR reductions of 10% to 30% in outcomes such as death, myocardial infarction or stroke. Therefore, very large trials involving several thousands of patients are needed to detect these differences reliably (19). Conducting such trials requires a large, ideally international network of participating sites coordinated by a centre with clinical, scientific, methodological and logistical expertise in research ethics and regulatory affairs, drug importation and packaging, advanced biostatistics, computing, communications and organizational management, as well as familiarity with the legal and cultural challenges posed by working in different countries. Large RCTs (even when efficient) are expensive, and the recruitment, follow-up and analysis must be achieved in a limited timeframe requiring well-organized teamwork.
There are very few academic groups in Canada, and indeed the world, that can conduct the large RCTs required to answer important clinical questions reliably. Over the past 16 years, the PHRI has built global networks and a high-quality expert team with the requisite advanced knowledge and experience to conduct large international clinical trials. At the heart of the CIHR/CFI application lies a request to provide the PHRI with funds to upgrade and standardize the physical and computing infrastructure necessary to meet evolving modern standards, core funding for key staff to stabilize and sustain its expertise beyond a single granting cycle, and resources to sustain and strengthen a national network of centres capable of collaborating in large RCTs addressing important questions, including those involving generic therapies and projects initiated by academic investigators as well as by industry.
CANNeCTIN RESOURCES AND ORGANIZATION
CANNeCTIN is funded by the CFI ($10 million [M] in capital funding, matched by $15M from other sources and supplemented by $1M in capital maintenance funding from the CFI) and the CIHR ($9.6M in operating funds over five years). The CFI award has been deployed to upgrade and consolidate the operations of the PHRI. As of March 2009, they are located within the David Braley Cardiac, Vascular and Stroke Research Institute, a six-storey building at Hamilton General Hospital in Hamilton that is funded by the CFI awards and other contributions in excess of $90M. The CIHR award provides core support of dedicated staff, enabling a substantial number of pilot studies to be conducted and the personnel infrastructure for multicentre trials, epidemiological studies and national registries to be stabilized. Resources are allocated to support communications, meetings and programs of education and knowledge translation. In addition, the network has raised $200,000 per year for five years in the form of unconditional grants from two companies (AstraZeneca Canada Inc and Boehringer Ingelheim [Canada] Ltd) to initiate pilot studies.
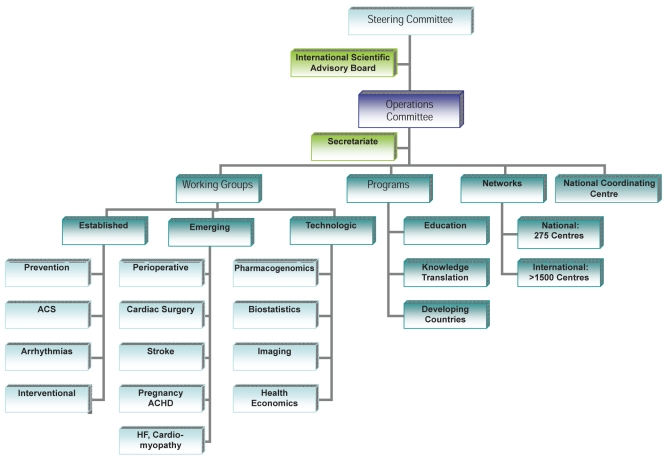
The organizational framework (Figure 2) includes a 30-member Steering Committee with representation from every Canadian medical school, which meets twice yearly to set priorities and provide broad oversight of CANNeCTIN. An 11-member (one-half from McMaster and one-half from other Canadian universities) Operations Committee, co-chaired by Salim Yusuf of McMaster University (Hamilton) and John Cairns of the University of British Columbia (Vancouver, British Columbia), meets monthly by conference call to develop policies and make operational decisions. Located at the David Braley Cardiac, Vascular and Stroke Research Institute and managed by a program director, the Secretariate provides network administrative services while the National Coordinating Centre provides administrative and research personnel who assist network investigators in the design, organization, conduct, analysis and reporting of CANNeCTIN studies, and facilitate global linkages. An International Scientific Advisory Board, comprised of six renowned scientists from the United States, United Kingdom, India and Canada, meets once yearly to provide direction and guidance from an international perspective. Working Groups in disease/discipline fields that support technologies and programs are the key functional components of CANNeCTIN, and are composed of expert investigators from across the country (Table 1).
Figure 2).
The CANadian Network and Centre for Trials INternationally (CANNeCTIN) organization chart. ACHD Adult congenital heart disease; ACS Acute coronary syndrome; HF Heart failure
TABLE 1.
CANadian Network and Centre for Trials INternationally (CANNeCTIN) Working Groups
| Working Group | Chair(s) | PHRI Facilitators (McMaster University, Hamilton, Ontario) |
|---|---|---|
| Disease/Discipline Groups | ||
| Acute Coronary Syndrome | Pierre Theroux (Université de Montréal, Montreal, Quebec), John Cairns (UBC, Vancouver, British Columbia) | Shamir Mehta |
| Adult Congenital Heart Disease and Pregnancy | Samuel Siu (UWO, London, Ontario) | Omid Salehian |
| Arrhythmias | Paul Dorian (University of Toronto, Toronto, Ontario) | Stuart Connolly |
| Cardiac Surgery | Stephen Fremes (Sunnybrook Health Sciences Centre, Toronto), Richard Novick (UWO) | Andre Lamy, Kevin Teoh |
| Heart Failure/LV Dysfunction | Malcolm Arnold (UWO) | Robert McKelvie |
| Intervention | Vlad Dzavik (University of Toronto) | Madhu Natarajan |
| Perioperative Medicine | Thomas Schricker (McGill University, Montreal) | PJ Devereaux |
| Prevention | Gilles Dagenais (Université Laval, Quebec City, Quebec), Bernie Zinman (University of Toronto) | Hertzel Gerstein, Eva Lonn |
| Stroke | Ashfaq Shuaib (University of Alberta, Edmonton, Alberta) | Martin O’Donnell |
| Technology Groups and Programs | ||
| Biostatistics and Methodological Innovation | Richard Cook (University of Waterloo, Waterloo, Ontario) | Janice Pogue |
| Developing Countries | Koon Teo (McMaster University) | |
| Education | PJ Devereaux (McMaster University) | |
| Knowledge Translation | Jafna Cox (Dalhousie University, Halifax, Nova Scotia), Jack Tu (University of Toronto) | Stuart Connolly |
| Pharmacogenomics | Robert Roberts (University of Ottawa, Ottawa, Ontario) | Sonia Anand |
LV Left ventricle; PHRI Population Health Research Institute; UBC University of British Columbia; UWO University of Western Ontario
SUPPORTING CLINICAL RESEARCH STUDIES
Under the guidance of their chairs (generally from outside McMaster University) and facilitators (associated with the PHRI), the CANNeCTIN Working Groups evaluate ideas for potential RCTs, registries and epidemiological studies proposed by their members. The Working Groups identify principal investigators and prioritize pilot study proposals to compete once or twice annually for support and potential funding from CANNeCTIN. Each proposal is submitted on a detailed application form modelled on the CIHR clinical trials application process, and is reviewed and rated using formal criteria by a member of the Steering Committee and an external expert. Final decisions regarding support are made by the Operations Committee based on scientific excellence, feasibility and strategic considerations with regard to engagement of all Working Groups and PHRI coordination capacity. Investigators with proposals that are scientifically exciting, address important questions and demonstrate feasibility during their pilot phases, will be assisted by the relevant Working Group and the National Coordinating Centre to apply for funding from peer-review agencies, industry or both. The PHRI will coordinate funded studies that will be conducted within the CANNeCTIN network of Canadian centres. When large enrollments and ethnic/geographical diversity require international participation, the network of affiliated international clinical research sites will be engaged to create the necessary capacity.
A major goal of CANNeCTIN is to increase the number and scope of high-impact cardiovascular and diabetes trials, registries and epidemiological studies developed and led by Canadian investigators. CANNeCTIN is committed to the creation of research leadership opportunities for investigators located across the country, many of whom may be in centres that do not have the infrastructure for coordinating and managing large and international clinical trials. CANNeCTIN will give such researchers the potential to address important questions as the principal investigators of large international multicentre trials supported by a world-class coordinating and data centre.
TRAINING, MENTORSHIP AND CAREER DEVELOPMENT FOR THE NEXT GENERATION OF CARDIOVASCULAR INVESTIGATORS
Although many Canadian universities offer clinical epidemiology courses, few offer programs that provide hands-on experience in organizing and leading large national and international clinical trials. CANNeCTIN’s internships, studentships and research fellowships in RCTs will integrate learners into all aspects of clinical trials led by internationally recognized researchers. For instance, twice yearly, a three-day interactive, small group RCT training workshop will take place for individuals selected in a competitive process from across Canada and internationally. Participants will submit a research protocol, and work through all aspects of study design and implementation planning with guidance from external reviewers and participating methodological experts. CANNeCTIN will build Canadian research capacity through enhanced training of graduate students and postgraduate research fellows in biostatistics. Student exchanges will provide trainees from across the country with opportunities to work in interdisciplinary teams on real problems encountered in ongoing CANNeCTIN trials.
CANNeCTIN distant learning initiatives include the creation of Internet-based courses in clinical research methodology and study coordination, accessible from across Canada and internationally. CANNeCTIN will also host an annual “Cutting-edge Workshop” featuring national and international experts addressing emerging issues related to a specific clinical problem, research methodology or new scientific developments.
KNOWLEDGE TRANSLATION
While clinical research has produced a wealth of knowledge that defines optimal care, relatively less study has been devoted to understanding how best to ensure the application of this knowledge to clinical practice. Thus, there is a substantial care gap that can result in unwanted variations in practice and suboptimal patient outcomes even in cardiovascular diseases and diabetes, despite an especially rich scientific base for many therapeutic interventions that are truly disease modifying. Knowledge translation (KT) has recently been proposed as a paradigm to understand and close the gap between knowledge and effective practice. It involves a systematic and integrated approach to accelerate optimal use of the best available research evidence. There is a major opportunity to serve a KT research agenda within the CANNeCTIN framework.
Clinical trials may be used to obtain insights into KT approaches, for example, when it becomes apparent during the conduct of a clinical trial that certain sites are more effective or efficient at implementing treatment protocols or at achieving study targets. In some instances, this can be traced to local practice patterns, care maps or other implementation tools. Rather than simply disseminating such tools, promising KT approaches can be evaluated in subsequent studies, using appropriate scientific designs. In particular, CANNeCTIN will seek to harness the methodological and analytical expertise acquired through long experience with clinical trials, to conduct specific research into the most effective strategies for dissemination and behaviour change. By harnessing a collaborative network that is national – and even global – in scope, CANNeCTIN can play an important role in exploring KT initiatives focusing on system-wide change aimed at ensuring the optimal use of the best available research evidence, with attendant improvement in the quality of care and patient outcomes. Additionally, there is the potential for research into KT itself. There is a need to develop a better understanding of the dynamics and drivers of behaviour change, and also to develop more insight into how and where best to direct KT efforts so as to derive the greatest benefit in the shortest time. It is likely that the most significant impacts will result from multifaceted interventions aimed at various levels in the system (eg, patient, provider and policy maker), with tailoring of the message according to the end user or target group.
THE NEXT FIVE YEARS
We will measure our early success by documenting the numbers and scope of projects at the pilot and full study level conducted under the auspices of CANNeCTIN, and led by both established research leaders and emerging investigators who have not previously led projects of substantial size, complexity and scope. Careful documentation of publications and citation analysis will reflect, in the longer term, the increasing impact of research conducted under the auspices of CANNeCTIN.
Since the notification of funding in April 2008, CANNeCTIN has moved quickly to activate its organizational structure. The Working Groups have been meeting by conference call and in person, and are growing as additional investigators are encouraged to participate. The first national meeting was in June 2008 and, in October 2008, more than 100 investigators from across Canada met in Toronto to launch the collaboration. The third national meeting in May 2009 was held in Hamilton in conjunction with the inauguration of the David Braley Cardiac, Vascular and Stroke Research Institute at the Hamilton General Hospital, and included a symposium on international collaboration in cardiovascular clinical trials and the first cutting-edge symposium on pharmacogenomics. CANNeCTIN’s first large international study is underway, having enrolled more than 8000 patients to evaluate major vascular complications close to the time of noncardiac surgery (Table 2). A second RCT is evaluating steroids for the prevention of vascular complications following cardiac surgery and six more are enrolling. Several full studies to be conducted under the auspices of CANNeCTIN are seeking funding from the CIHR and other agencies. Ten pilot studies are funded (seven of them are enrolling) and a further five are being reviewed. The educational program has held two two-day workshops for emerging clinical trial leaders. Interactive, accredited, online courses in research methodology will be offered during the 2010/2011 academic year through McMaster University. A monthly videoconference on biostatistics and clinical research methodology is offered at five sites. Linkages are being forged with other KT research groups across the country, and the first evaluation of a KT intervention – one that aims to promote better and safer warfarin use in primary care – has begun. The achievements during the first 15 months are key steps toward realizing CANNeCTIN’s vision: investigators from across Canada will increasingly lead major international studies in cardiovascular diseases and diabetes contributing to health improvements worldwide.
TABLE 2.
Studies initiated or conducted under the auspices of the CANadian Network and Centre for Trials INternationally (CANNeCTIN) as of February 2009 (Feb 09)
| Study and design | Principal investigator(s)* | Target enrollment | Status |
|---|---|---|---|
| Vascular events in noncardiac surgery patients cohort evaluation (VISION): A global registry | McMaster University | 40,000 patients in Brazil, China, Canada, Colombia and Italy | CANNeCTIN funding, 8000 patients enrolled; CIHR application Feb 09 |
| Evaluation of a warfarin maintenance dose algorithm on INR control. Cluster RCT | McMaster University | 900 patients at 45 Canadian family practices | CANNeCTIN/AFP funding Feb 09 |
| Perioperative Ischemic Evaluation-2 (POISE-2). RCT | McMaster University and University of Alberta | 90 patients at two Canadian academic hospitals | CANNeCTIN/PSI pilot funding Oct 08; CIHR application Feb 09 |
| Investigation of the Management of Pericarditis (IMPI). RCT | University of Cape Town and McMaster University | 800 patients from 25 centres in Malawi, Mozambique, Nigeria and South Africa | CANNeCTIN pilot funding Oct 08; CIHR application Feb 09 |
| Understanding the Increased Cardiovascular Risk after Preeclampsia/Eclampsia. Data analysis | McMaster University | Data from 4459 patients recruited in global cardiovascular disease study | CANNeCTIN pilot funding Oct 08 |
| Heart Failure & Conditioned Nutritional Deficiency in the Myocardium. RCT | University of Western Ontario | 60 patients from eight Canadian hospitals | CANNeCTIN pilot funding Feb 09 |
| Left Atrial Appendage Occlusion Study I (LAAOS I). RCT | McMaster University | 50 patients from five Canadian academic hospitals | CANNeCTIN pilot funding Feb 09 |
| Prevention of Arrhythmia Device Infection Trial (PADIT). RCT | University of Western Ontario | 500 patients at eight Canadian hospitals | CANNeCTIN pilot funding Feb 09 |
Locations of universities: McMaster University (Hamilton, Ontario); University of Alberta (Edmonton, Alberta); University of Cape Town (Rondebosch, South Africa); University of Western Ontario (London, Ontario). AFP Academic Financial Plan; CIHR Canadian Institutes of Health Research; INR International normalized ratio; Oct 08 October 2008; PSI Physicians’ Services Incorporated; RCT Randomized controlled trial
Footnotes
CANNeCTIN STEERING COMMITTEE: Yusuf S (co-chair; McMaster University, Hamilton, Ontario), Cairns J (co-chair; University of British Columbia, Vancouver, British Columbia), Anand S (McMaster University), Anderson T (University of Calgary, Calgary, Alberta), Arnold M (University of Western Ontario, London, Ontario), Cook R (University of Waterloo, Waterloo, Ontario), Connolly S (McMaster University), Cox J (Dalhousie University, Halifax, Nova Scotia), Dagenais G (Université Laval, Quebec City, Quebec), Devereux P (McMaster University), Dorian P (University of Toronto, Toronto, Ontario), Dzavick V (University of Toronto), Fremes S (University of Toronto), Gerstein H (McMaster University), Lear S (Simon Fraser University, Vancouver), McAlister F (University of Alberta, Edmonton, Alberta), Nguyen M (Université de Sherbrooke, Sherbrooke, Quebec), Novick R (University of Western Ontario), Roberts R (University of Ottawa, Ottawa, Ontario), Schricker T (McGill University, Montreal, Quebec), Shuaib A (University of Alberta), Simpson C (Queen’s University, Kingston, Ontario), Siu S (University of Western Ontario), Sussex B (Memorial University of Newfoundland, St John’s, Newfoundland and Labrador), Tam J (University of Manitoba, Winnipeg, Manitoba), Teo K (McMaster University), Theroux P (Université de Montréal, Montreal, Quebec), Tu J (University of Toronto), Zinman B (University of Toronto) and Coverett K (University of Sakatchewan, Saskatoon, Saskatchewan).
SOURCES OF FUNDING: The CIHR and the CFI.
CONFLICTS: There are no conflicts of interest to report.
REFERENCES
- 1.Raghaven M, Sandham JD. Clinical research lags behind biomedical, population-based health, and health services research at multiple levels. Clin Investig Med. 2007;30:E152–8. doi: 10.25011/cim.v30i4.1776. [DOI] [PubMed] [Google Scholar]
- 2.Raghaven M, Sandham JD. The number, scope and geographic distribution of clinical researchers in Canada. Clin Investig Med. 2008;31:E222–30. doi: 10.25011/cim.v31i5.4867. [DOI] [PubMed] [Google Scholar]
- 3.Cairns JA. The Canadian clinical research initiative: Transforming Canadian clinical research capacity. Clin Investig Med. 2004;27:292–5. [PubMed] [Google Scholar]
- 4.Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: Part II: Variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–64. doi: 10.1161/hc4701.099488. [DOI] [PubMed] [Google Scholar]
- 6.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating Group Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 7.Thom T, Haase N, Rosamond W, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics – 2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 8.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 9.Antithrombotic Trialists Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;371:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 11.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: A combined analysis of three trials. Lancet. 2006;368:581–8. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- 12.Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group Indications for fibrinolytic therapy in suspected acute myocardial infarction: Collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994;343:311–22. [PubMed] [Google Scholar]
- 13.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–98. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 14.Lonn E, Bosch J, Yusuf S, et al. HOPE and HOPE-TOO Investigators. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 15.Hennekens CH, Albert CM, Godfried SL, Gaziano JM, Buring JE. Adjunctive drug therapy of acute myocardial infarction – evidence from clinical trials. N Engl J Med. 1996;335:1660–7. doi: 10.1056/NEJM199611283352207. [DOI] [PubMed] [Google Scholar]
- 16.Bosch J, Lonn E, Pogue J, Arnold JMO, Dagenais GR, Yusuf S, for the HOPE/HOPE-TOO study investigators Long-term effects of ramipril on cardiovascular events and on diabetes: Results of the HOPE study extension. Circulation. 2005;112:1339–46. doi: 10.1161/CIRCULATIONAHA.105.548461. [DOI] [PubMed] [Google Scholar]
- 17.Roussouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 18.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients received encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S. Randomised controlled trials in cardiovascular medicine: Past and future achievements. BMJ. 1999;319:564–8. doi: 10.1136/bmj.319.7209.564. [DOI] [PMC free article] [PubMed] [Google Scholar]