Introduction
Within the past decade, a number of significant advancements have occurred in our knowledge of benign prostatic hyperplasia (BPH) resulting in new approaches to both the diagnosis and treatment of this common and potentially progressive condition of aging men. The current document attempts to summarize the state-of-the-art knowledge regarding BPH and to highlight the essential diagnostic and therapeutic information in a Canadian context. The information included in this document was obtained from a MEDLINE search of the English language literature. Although references of historical importance are included, management recommendations are based on literature published between 2000 and 2009.
These guidelines are directed toward the typical male patient over 50 years of age, presenting with lower urinary tract symptoms (LUTS) believed to be associated with benign prostatic obstruction (BPO). Men with LUTS associated with non-BPO causes will require more extensive diagnostic workup, different treatment considerations and their management will not be covered in this document.
In this document we will address both diagnostic and treatment issues. Diagnostic guidelines are described in the following terms as: mandatory, recommended, optional or not recommended. Guidelines for treatment are described using the terminology: standard of care (evidence-based, whenever possible), optional (insufficient evidence or patient preference) or not recommended (based on the best available evidence). Whenever possible, levels of evidence and grades of recommendation will be provided to support guideline statements.
Diagnostic guidelines
Mandatory:
In the initial evaluation of a man presenting with LUTS, the evaluation of symptom severity and bother is essential. Medical history should include relevant prior and current illnesses as well as prior surgery and trauma. Current medication, including over-the-counter drugs and phytotherapeutic agents, must be reviewed. A focused physical examination, including a digital rectal exam (DRE), is also mandatory. Urinalysis is required to rule out diagnoses other than BPH that may cause LUTS and may require additional diagnostic tests.1–9
History
Physical examination including DRE
Urinalysis (routine and microscopic, culture and sensitivity)
Recommended:
A formal symptom inventory (e.g., International Prostate Symptom Score (IPSS) or AUA Symptom Score) is recommended for an objective assessment of symptoms at initial contact, for follow-up of symptom evolution for those on watchful waiting and for evaluation of response to treatment.10–17 (Level 2 Evidence, Grade C Recommendation).
Testing of prostate-specific antigen (PSA) should be offered to patients who have at least a 10-year life expectancy and for whom knowledge of the presence of prostate cancer would change management, as well as those for whom PSA measurement may change the management of their voiding symptoms (estimate for prostate volume). Among patients without prostate cancer, serum PSA may also be a useful surrogate marker of prostate size and may also predict risk of BPH progression.18
Symptom inventory (should include bother assessment)
PSA (selected patients)
Optional:
In cases where the physician feels it is indicated, it is reasonable to proceed with one or more of the following:
Serum creatinine
Urine cytology (if irritative symptoms are significant component of LUTS)3
Uroflow
Voiding diary
Post-void residual
Sexual function questionnaire
Not Recommended:
The following diagnostic modalities are not recommended in the routine initial evaluation of a typical patient with BPH-associated LUTS.
These investigations may be required in patients with a definite indication, such as hematuria, uncertain diagnosis, DRE abnormalities, poor response to medical therapy or for surgical planning.5
Cystoscopy
Cytology
Urodynamics
Radiological evaluation of upper urinary tract
Prostate ultrasound
Prostate biopsy
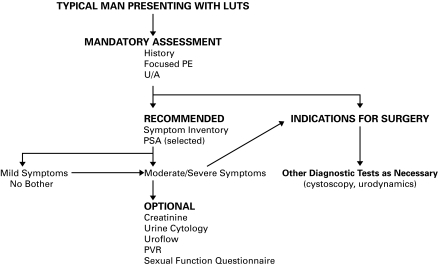
An algorithm summarizing the appropriate diagnostic steps in the workup of a man with LUTS is shown in Figure 1.
Fig. 1.
Diagnostic algorithm. LUTS = lower urinary tract symptoms; PE = physical examination; U/A = urinalysis; PSA = prostate-specific antigen; PVR = post-void residual.
Treatment guidelines
Principles of treatment
Therapeutic decision-making should be guided by the severity of the symptoms, the degree of bother and patient preference. Information on the risks and benefits of BPH treatment options should be explained to all patients who are bothered enough to consider therapy. Patients should be invited to participate as much as possible in the treatment selection.
Standard of Care:
Patients with mild symptoms (e.g., IPSS <7) should be counselled about a combination of lifestyle modification and watchful waiting. Patients with mild symptoms and severe bother should undergo further assessment.
Optional:
Treatment options for patients with bothersome moderate (e.g., IPSS 8 – 18) and severe (e.g., IPSS 19 – 35) symptoms of BPH include watchful waiting/lifestyle modification, as well as medical, minimally invasive or surgical therapies.
Lifestyle modifications with watchful waiting
Standard of Care:
Patients on watchful waiting should have periodic physician-monitored visits.
Optional:
Physicians can use baseline age, LUTS severity, prostate volume and/or serum PSA to advise patients of their individual risk of symptom progression, acute urinary retention or future need for BPH-related surgery (these risk factors identify patients at risk for progression).
Optional:
A variety of lifestyle changes may be suggested for patients with nonbothersome symptoms. These can include the following:
Fluid restriction particularly prior to bedtime
Avoidance of caffeinated beverages, spicy foods
Avoidance/monitoring of some drugs (e.g., diuretics, decongestants, antihistamines, antidepressants)
Timed or organized voiding (bladder retraining)
Pelvic floor exercises
Avoidance or treatment of constipation
Medical treatment
Alpha-blockers
Optional:
Alpha-blockers are an excellent first-line therapeutic option for men with symptomatic bother who desire treatment.19–27 (Level 1 Evidence, Grade A Recommendation).
Alfuzosin, doxazosin, tamsulosin and terazosin are appropriate treatment options for LUTS secondary to BPH. They do not alter the natural progression of the disease.
Recommendation:
Although there are differences in the adverse-event profiles of these agents, we believe that all 4 agents have equal clinical effectiveness. The choice of agent should depend on the patient’s comorbidities, side effect profiles and tolerance.
5 alpha-reductase inhibitors
Optional:
The 5 alpha-reductase inhibitors (dutasteride and finasteride) are appropriate and effective treatments for patients with LUTS associated with demonstrable prostatic enlargement.28 Several studies have demonstrated that in addition to improving symptoms, the natural history of BPH can be altered through a reduction in the risk of acute urinary retention (AUR) and the need for surgical intervention.28–31 (Level 1 Evidence, Grade A Recommendation).
Prognostic factors suggesting the potential for BPH risk progression32–34 include:
Serum PSA >1.4 ng/mL
Age >50
Gland volume >30 cc
Combination therapy (alpha-blocker and 5 alpha-reductase inhibitor)
Optional:
The combination of an alpha-adrenergic receptor blocker and a 5 alpha-reductase inhibitor is an appropriate and effective treatment strategy for patients with LUTS associated with prostatic enlargement. Clinical trial results have shown that combination therapy significantly improves in symptom score and peak urinary flow compared with either of the monotherapy options. Combination medical therapy can effectively delay symptomatic disease progression, while combination therapy and/or 5 alpha-reductase monotherapy is associated with decreased risk of urinary retention and/or prostate surgery.29–30,35 (Level 1 Evidence, Grade A Recommendation).
Patients successfully treated with combination therapy may be given the option of discontinuing the alpha-blocker after 6 to 9 months of therapy.36,37 If symptoms recur, the alpha-blocker should be restarted.
Role of anticholinergics medications
Level 1 Evidence would suggest that for selected patients with bladder outlet obstruction due to BPH and concomitant detrusor overactivity, combination therapy with an alpha-receptor antagonist and anticholinergic can be helpful.38 (Level 1 Evidence, Grade A Recommendation) Caution is recommended, however, when considering these agents in men with an elevated residual urine volume or a history of spontaneous urinary retention.
Role of phosphodiesterase inhibitors
The phosphodiesterase (PDE) isoenzymes 4 and 5 are present in the prostate and regulate smooth muscle tone. Subsequent isoenzyme inhibition with medications, such as sildenafil and tadalafil, have shown improvement in symptoms and quality-of-life in men with LUTS.39 At the present time, however, these agents are not recommended for men with symptomatic BPH-related LUTS.
Phytotherapies
Optional:
If patients are interested in complementary approaches (phytotherapeutic or other supplements) for LUTS secondary to BPH, they may be counselled that some plant extracts, such as Serenoa repens (saw palmetto berry extract) and Pygeum africanum (African Plum), have shown some efficacy in several small clinical series. (Level 3 Evidence, Grade B Recommendation).
Saw palmetto has been studied most rigorously, including a published randomized controlled double-blind trial which failed to show any significant difference over placebo in symptom score, maximum flow rate, prostate size, residual urine volume, PSA levels or quality of life over a 1-year period.40,41 (Level 2 Evidence, Grade B Recommendation)
Not Recommended:
Phytotherapeutic agents and other dietary supplements cannot be recommended as the standard treatment of BPH at this time.
Surgery
Transurethral resection of the prostate (TURP)
Standard of Care:
Monopolar TURP remains the gold standard treatment for patients with bothersome moderate or severe LUTS who request active treatment or who either fail or do not want medical therapy.42–51 (Level 2 Evidence Grade B Recommendation).
Patients should be informed that the procedure may be associated with short- and long-term complications. Recent data suggest that contemporary TURP-related morbidity includes a risk of blood transfusion and TUR syndrome ranging from 2.0% to 4.8% and 0 to 1.1% of cases, respectively,52 while the need for retreatment can be as high as 14.7% during an 8-year follow-up.53
Optional:
Bipolar TURP has evolved as an equivalent alternative to the monopolar technique, (Level 2 Evidence, Grade B Recommendation). Recent reports suggest bipolar resection is associated with a reduction in the risk of dilutional hyponatremia (TUR syndrome), improvements in intraoperative visibility and may result in shorter catheterization times.54–57
Laser prostatectomy
Optional:
Several laser wavelengths (Potassium titanyl phosphate [KTP], Holmium:Yttrium aluminium garnet [Ho:YAG], Thulium) and delivery systems (end-firing; side-firing; interstitial) are available for prostatic tissue coagulation or vaporization/ablation and each has particular characteristics and potential advantages.
Holmium laser enucleation (HoLEP) can be used effectively in larger glands and in patients on anticoagulation with reported reduced hospitalization, bleeding and duration of catheterization. Results both early and long-term are similar to TURP, confirming this modality is a suitable first-line surgical option among urologists skilled with the technique.58 Randomized trials comparing HoLEP to TURP and to open prostatectomy have demonstrated favourable outcomes especially among men with larger prostates.59,60 (Level 1–2 Evidence, Grade B Recommendation).
Greenlight laser or photoselective vaporization prostatectomy (PVP) is a suitable treatment option for most men considering surgical alternatives, particularly for those on anticoagulation.61,62 (Level 2 Evidence, Grade B Recommendation).
Standard of Care:
Absolute indications to recommend TURP include: urinary retention (intractable) and renal insufficiency (caused by BPO). Relative indications to recommend TURP include: failure of medical therapy, recurrent cystitis, bladder calculi and persistent prostatic bleeding.
Transurethral incision of the prostate (TUIP)
Optional:
TUIP is appropriate surgical therapy for men with prostate gland volumes less than 30 grams. These patients should experience symptom improvements similar to TURP with a lower incidence of retrograde ejaculation.63
Open prostatectomy
Optional:
Open prostatectomy remains indicated for men whose prostates, in the view of the treating urologist, are too large for TURP for fear of incomplete resection, significant bleeding or the risk of dilutional hyponatremia (TUR syndrome).
Minimally invasive surgical therapies (MIST)
Transurethral microwave therapy (TUMT)
Optional:
TUMT is a reasonable treatment consideration for the patient who has moderate symptoms, small to moderate gland size and a desire to avoid more invasive therapy for potentially less effective results.64 TUMT may be associated with a higher re-treatment rate over a 5-year follow-up interval than for men receiving TURP.64,65 TUMT is not an insurable service anywhere in Canada at this time; patients are required to pay for this procedure.
Transurethral needle ablation (TUNA)
Optional:
TUNA may be a therapeutic option for the relief of symptoms in the younger, active individual in whom sexual function remains an important quality of life issue (less risk of retrograde ejaculation), however limited data is available on long-term outcomes.66–68 TUNA is not an insurable service anywhere in Canada at this time; patients are required to pay for this procedure.
Stents
Optional:
Temporary and permanent stents may be considered in patients with severe urinary obstruction secondary to BPH who are medically unfit for surgery (or waiting to become medically fit for surgery or MIST).69 Stents are not recommended as standard therapy for LUTS associated with BPH.
Other MIST therapies
Not Recommended:
Although clinical trials have been or are being conducted to assess a number of other novel interventions, the following evolving MIST therapies are not recommended as standard therapy at this time.
Absolute ethanol injection
High intensity focused ultrasound
Water-induced thermotherapy
Intraprostatic botulinum toxin injection
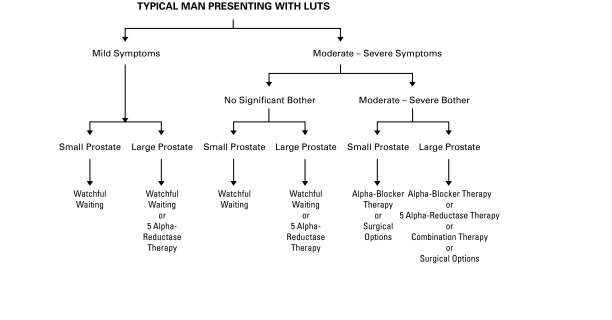
The therapeutic options available to the patient with bothersome LUTS stratified by symptom severity and prostate gland size are displayed in Fig. 2.
Fig. 2.
Therapeutic algorithm. LUTS = lower urinary tract symptoms.
Special situations
Symptomatic prostatic enlargement but without bothersome symptoms
Optional:
Patients with symptomatic prostatic enlargement in the absence of significant bother may be offered a 5 alpha-reductase inhibitor to prevent progression of the disease. The disadvantages and the need for long-term daily therapy should be discussed with the patient in relation to his risk of progression.
Acute urinary retention
Standard of Care:
Men with AUR due to BPH should be offered a trial of voiding 2 to 7 days after catheterization while receiving an alpha-blocker. Recent data suggest that in patients with AUR, the use alpha-blockers (specifically tamsulosin and alfuzosin) during the period of catheterization will increase the chances of successful voiding after catheter removal and may decrease the risk of future prostate surgery.70,71 (Level 1 Evidence, Grade A Recommendation).
If the trial of voiding fails, the patient should be considered for surgical intervention.
BPH-related bleeding
Standard of Care:
A complete assessment, including history and physical examination, urinalysis (routine microscopy, culture & sensitivity, cytology), upper tract radiologic assessment and cystoscopy, is necessary to exclude other sources of bleeding.
Optional:
In men with BPH-related hematuria, a trial with a 5 alpha-reductase inhibitor is appropriate. If the bleeding persists, TURP is recommended. (Level 3 Evidence, Grade B Recommendation).
BPH patients with prostate cancer concern
Optional:
The BPH patient with an elevated serum PSA and negative prostate biopsy may be counselled on the proven benefits of using finasteride, a Type 2 selective 5 alpha-reductase inhibitor or dutasteride, a dual Type 1 and 2, 5 alpha-reductase inhibitor for prostate cancer risk reduction.72,73 (Level 1 Evidence, Grade A Recommendation).
While both finasteride and dutasteride uses were associated with similar reductions in the overall rate of prostate cancer, there was one observed difference between the trials.72,73 In the PCPT (Prostate Cancer Prevention Trial) study, a slight increase in the risk of high grade (Gleason 8 or greater) prostate cancer was observed among the finasteride cohort compared to the placebo group.72 Most experts believe this phenomenon was due to an artifact of prostate glandular cytoreduction, induced by the 5 alpha-reductase inhibitor, although some controversy exists.74 In the REDUCE (Reduction by Dutasteride of Prostate Cancer Events) trial, the number of patients found to have Gleason 7 or greater prostate cancer was not significantly different between the dutasteride and placebo groups.73
Patients who experience a rising PSA after 6 to 12 months of 5 alpha-reductase inhibitor therapy should be assessed for the possibility of high-grade prostate cancer.75
Summary
BPH is one of the most common age-related disorders afflicting men. As the aging of the Canadian population continues, more men will be seeking advice and looking for guidance from their health care providers on the management of their symptoms. It is hoped the information offered in this guideline document will aid Canadian urologists, as they strive to provide state-of-the-art care to their patients.
Footnotes
Competing interests: Dr. Nickel reports receiving consulting fees from Merck, GlaxoSmithKline, Watson, Genyous Biomed, Pfizer and research support from Merck, GlaxoSmithKline, Allergan, Pfizer and Watson. Drs. Méndez-Probst, Whelan and Paterson report no potential conflicts of interest. Dr. Razvi reports receiving research support from Cook Urological, GlaxoSmithKline and Allergan.
This paper has been peer-reviewed.
References
- 1.Emberton M, Andriole GL, de la Rosette J, et al. Benign prostatic hyperplasia: a progressive disease of aging men. Urology. 2003;61:267–73. doi: 10.1016/s0090-4295(02)02371-3. [DOI] [PubMed] [Google Scholar]
- 2.McConnell JD, Barry MJ, Bruskewitz RC, et al. Clinical practice guideline for benign prostatic hyperplasia: diagnosis and treatment. Rockville, Maryland: Agency for Health Care Policy and Research, Public Health Service; 1994. No. 94-0582, [PubMed] [Google Scholar]
- 3.AUA Guidelines on management of benign prostatic hyperplasia (2003) Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–47. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 4.de la Rosette J, Alivizatos G, Madersbacher S, et al. EUA Guidelines on benign prostatic hyperplasia (BPH) Eur Urol. 2001;40:256–63. doi: 10.1159/000049784. [DOI] [PubMed] [Google Scholar]
- 5.Madersbacher S, Alivizatos G, Nordling J, et al. EUA 2004 guidelines on assessment, therapy and follow up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH Guidelines) Eur Urol. 2004;46:547–54. doi: 10.1016/j.eururo.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Denis L, McConnell J, Khoury S, et al. Recommendations of the International Scientific Committee: The evaluation and treatment of lower urinary tract symptoms (LUTS) suggestive of benign prostatic obstruction. In: Denis L, Griffiths K, Khoury S, et al., editors. Proceedings of the 4th International Consultation on Benign Prostatic Hyperplasia; United Kingdom: Plymbridge Distributors Ltd; 1998. pp. 669–84. [Google Scholar]
- 7.Chatelain C, Denis L, Foo JKT, et al. Recommendations of the International Scientific Committee: Evaluation and treatment of lower urinary tract symptoms (LUTS) in older men. In: Chatelain C, Denis L, Foo KT, Khoury S, McConnell J, editors. Proceedings of the 5th International Consultation on Benign Prostatic Hyperplasia. United Kingdom: Health Publications Ltd; 2001. pp. 519–34. [Google Scholar]
- 8.Nickel JC, Saad J. The American Urological Association 2003 guidelines on management of benign prostatic hyperplasia: a Canadian opinion. Can J Urol. 2004;11:2186–93. [PubMed] [Google Scholar]
- 9.Ramsey EW, Elhilali M, Goldenberg GS, et al. for the Canadian Prostate Health Council Practice patterns of Canadian urologists in benign prostatic hyperplasia and prostate cancer. J Urol. 2000;163:499–502. [PubMed] [Google Scholar]
- 10.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 11.Gacci M, Bartoletti R, Figlioli S, et al. Urinary symptoms, quality of life and sexual function in patients with benign prostatic hypertrophy before and after prostatectomy: a prospective study. BJU Int. 2003;91:196–200. doi: 10.1046/j.1464-410x.2003.04072.x. [DOI] [PubMed] [Google Scholar]
- 12.Temml C, Brössner C, Schatzl G, et al. Prostate study group of the Austrian Society of Urology. The natural history of lower urinary tract symptoms over five years. Eur Urol. 2003;43:374–80. doi: 10.1016/s0302-2838(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 13.Bosch JL, Bangma CH, Groeneveld FP, et al. The long-term relationship between a real change in prostate volume and a significant change in lower urinary tract symptom severity in population-based men: the Krimpen study. Eur Urol. 2008;53:819–25. doi: 10.1016/j.eururo.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 14.Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology. 2004;64:1144–8. doi: 10.1016/j.urology.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Boyle P, Robertson C, Mazzetta C, et al. The relationship between lower urinary tract symptoms and health status: the UREPIK study. BJU Int. 2003;92:575–80. doi: 10.1046/j.1464-410x.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 16.Robertson C, Link CL, Onel E, et al. The impact of lower urinary tract symptoms and comorbidities on quality of life: the BACH and UREPIK studies. BJU Int. 2007;99:347–54. doi: 10.1111/j.1464-410X.2007.06609.x. [DOI] [PubMed] [Google Scholar]
- 17.O’Leary MP, Wei JT, Roehrborn CG, et al. BPH registry and patient survey steering committee. Correlation of the international prostate symptom score bother question with the benign prostatic hyperplasia impact index in a clinical practice setting. BJU Int. 2008;101:1531–5. doi: 10.1111/j.1464-410X.2008.07574.x. [DOI] [PubMed] [Google Scholar]
- 18.Levitt JM, Slawin KM. PSA and PSA derivatives as predictors of BPH progression. Curr Urol Rep. 2007;8:269–74. doi: 10.1007/s11934-007-0072-y. [DOI] [PubMed] [Google Scholar]
- 19.Lukacs B, Grange JC, Comet D. One-year follow-up of 2829 patients with moderate to severe lower urinary tract symptoms treated with alfuzosin in general practice according to IPSS and a health-related quality-of-life questionnaire. BPM Group in General Practice. Urology. 2000;55:540–6. doi: 10.1016/s0090-4295(99)00539-7. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto T, Masumori N, Rahman M, et al. Change in international prostate symptom score, prostrate-specific antigen and prostate volume in patients with benign prostatic hyperplasia followed longitudinally. Int J Urol. 2007;14:321–4. doi: 10.1111/j.1442-2042.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 21.Chapple CR. Alpha-adrenoreceptor antagonist in the year 2000: Is there anything new? Curr Opin Urol. 2001;11:9–16. doi: 10.1097/00042307-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Marberger M, Harkawa R, de la Rosette J. Optimizing the medical management of benign prostatic hyperplasia. Eur Urol. 2004;45:411–9. doi: 10.1016/j.eururo.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Bozlu M, Ulusoy E, Cayan S, et al. A comparison of four different alpha 1-blockers in benign prostatic hyperplasia patients with and without diabetes. Scand J Urol Nephrol. 2004;38:391–5. doi: 10.1080/00365590410015678. [DOI] [PubMed] [Google Scholar]
- 24.Kirby RS. A randomized, double-blind crossover study of tamsulosin and controlled-release doxazosin in patients with benign prostatic hyperplasia. BJU Int. 2003;91:41–4. doi: 10.1046/j.1464-410x.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- 25.de Reijke TM, Klarskov P. Comparative efficacy of two alphaadrenoreceptor antagonists, doxazosin and alfuzosin, in patients with lower urinary tract symptoms from benign prostatic enlargement. BJU Int. 2004;93:757–62. doi: 10.1111/j.1464-410X.2003.04720.x. [DOI] [PubMed] [Google Scholar]
- 26.Hutchison A, Farmer R, Verhamme K, et al. The efficacy of drugs for the treatment of LUTS/BPH, a study in 6 European countries. Eur Urol. 2007;51:207–15. doi: 10.1016/j.eururo.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Wilt TJ, Howe RW, Rutks IR, et al. Terazosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002:CD003851. doi: 10.1002/14651858.CD003851. [DOI] [PubMed] [Google Scholar]
- 28.Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–41. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 29.McConnell JD, Roehrborn CG, Oliver OM, et al. for the MTOPS Research Group The long term effect of doxazosin, finasteride and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2385–96. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 30.Roehrborn CG, Siami P, Barkin J, et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostate enlargement: two-year results from the CombAT study. J Urol. 2008;179:616–21. doi: 10.1016/j.juro.2007.09.084. [DOI] [PubMed] [Google Scholar]
- 31.McConnell JD, Bruskewitz R, Walsh P, et al. the PLESS Study Group The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–63. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 32.Roehrborn CG, Siami P, Barkin J, et al. the CombAT Study Group The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: Four-year results from the combination of avodart and tamsulosin (CombaT) study. Presented by Claus Roehrborn, British Association of Urological Surgeons Annual Meeting; Glasgow, Scotland. June 22, 2009. [Google Scholar]
- 33.McConnell JD, Roehrborn CG, Bautista OM, et al. for the Medical Therapy of Prostatic Symptoms (MTOPS) Research Group The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JB, Roehrborn CG, Schalken JA, et al. The progression of benign prostatic hyperplasia: examining the evidence and determining the risk. Eur Urol. 2001;39:390–9. doi: 10.1159/000052475. [DOI] [PubMed] [Google Scholar]
- 35.Marks L, Roehrborn C, Andriole G. Prevention of benign prostatic hyperplasia disease. J Urol. 2006;176:1299–306. doi: 10.1016/j.juro.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Barkin J, Guimares M, Joacobi G, et al. Alpha blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5-alpha reductase inhibitor dutasteride. Eur Urol. 2003;44:461–6. doi: 10.1016/s0302-2838(03)00367-1. [DOI] [PubMed] [Google Scholar]
- 37.Nickel J, Barkin J, Koch C, et al. Finasteride monotherapy maintains stable lower urinary tract symptoms in men with BPH following cessation of alpha blockers. Can Urol Assoc J. 2008;2:16–21. doi: 10.5489/cuaj.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319–28. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 39.Andersson KE, Uckert S, Stief C, et al. Phosphodiesterases (PDEs) and PDE inhibitors for treatment of LUTS. Neurourol Urodyn. 2007;26:928–33. doi: 10.1002/nau.20485. [DOI] [PubMed] [Google Scholar]
- 40.Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557–66. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 41.Dreikorn K. The role of phytotherapy in treating lower urinary tract symptoms in benign prostatic hyperplasia. World J Urol. 2002;19:426–35. doi: 10.1007/s00345-002-0247-6. [DOI] [PubMed] [Google Scholar]
- 42.Hindley RG, Mostafid AH, Brierly RD, et al. The 2-year symptomatic and urodynamic results of a prospective randomized trial of interstitial radiofrequency therapy vs transurethral resection of the prostate. BJU Int. 2001;88:217–20. doi: 10.1046/j.1464-410x.2001.02232.x. [DOI] [PubMed] [Google Scholar]
- 43.Liedberg F, Adell L, Hagberg G, et al. Interstitial laser coagulation versus transurethral resection of the prostate for benign prostatic enlargement: a prospective randomized study. Scand J Urol Nephrol. 2003;37:494–7. doi: 10.1080/00365590310001773. [DOI] [PubMed] [Google Scholar]
- 44.Schatzl G, Madersbacher S, Djavan B, et al. Two-year results of transurethral resection of the prostate versus four “less invasive” treatment options. Eur Urol. 2000;37:695–701. doi: 10.1159/000020220. [DOI] [PubMed] [Google Scholar]
- 45.Madersbacher S, Schatzl G, Djavan B, et al. Long-term outcome of transrectal high-intensity focused ultrasound therapy for benign prostatic hyperplasia. Eur Urol. 2000;37:687–94. doi: 10.1159/000020219. [DOI] [PubMed] [Google Scholar]
- 46.Francisca EA, d’Ancona FC, Hendriks JC, et al. A randomized study comparing high-energy TUMT to TURP: quality-of-life results. Eur Urol. 2000;38:569–75. doi: 10.1159/000020357. [DOI] [PubMed] [Google Scholar]
- 47.Roehrborn CG, Nuckolls JG, Wei JT, et al. BPH registry and patient survey steering committee. The benign prostatic hyperplasia registry and patient survey: study design, methods and patient baseline characteristics. BJU Int. 2007;100:813–9. doi: 10.1111/j.1464-410X.2007.07061.x. [DOI] [PubMed] [Google Scholar]
- 48.McAllister WJ, Karim O, Plail RO, et al. Transurethral electrovaporization of the prostate: is it any better than conventional transurethral resection of the prostate? BJU Int. 2003;91:211–4. doi: 10.1046/j.1464-410x.2003.04073.x. [DOI] [PubMed] [Google Scholar]
- 49.Vesely S, Knutson T, Damber JE, et al. TURP and low energy TUMT treatment in men with LUTS suggestive of bladder outlet obstruction elected by means of pressure-flow studies: 8-year follow-up. Neurourol Urodyn. 2006;25:770–5. doi: 10.1002/nau.20233. [DOI] [PubMed] [Google Scholar]
- 50.Fowler C, McAllister W, Plail R, et al. Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia. Health Technol Assess. 2005;9:iii–iv. 1–30. doi: 10.3310/hta9040. [DOI] [PubMed] [Google Scholar]
- 51.Schatz LG, Madersbacher S, Lang T, et al. The early post-op morbidity of transurethral resection of the prostate and of 4 minimally invasive treatment alternatives. J Urol. 1997;158:105–11. doi: 10.1097/00005392-199707000-00029. [DOI] [PubMed] [Google Scholar]
- 52.Rassweiler J, Teber D, Kuntz R, et al. Complications of TURP incidence, management and prevention. Eur Urol. 2006;50:969–79. doi: 10.1016/j.eururo.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 53.Madersbacher S, Lackner J, Brössner C, et al. Prostate Study Group of the Austrian Society of Urology Reoperation, myocardial infarction and mortality after transurethral and open prostatectomy: a nation-wide, long-term analysis of 23,123 cases. Eur Urol. 2005;47:499–504. doi: 10.1016/j.eururo.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Patenkar S, Jamkar A, Dobhada S, et al. Plasmakinetic superpulse transurethral resection versus conventional transurethral resection of prostate. J Endourol. 2006;20:215–19. doi: 10.1089/end.2006.20.215. [DOI] [PubMed] [Google Scholar]
- 55.Yang S, Lin WC, Chang HK, et al. Gyrus Plasmasect: is it better than monopolar transurethral resection of prostate? Urol Int. 2004;73:258–61. doi: 10.1159/000080838. [DOI] [PubMed] [Google Scholar]
- 56.Michielsen DP, Debacker T, De Boe V, et al. Bipolar transurethral resection in saline an alternative surgical treatment for bladder outlet obstruction? J Urol. 2007;178:2035–39. doi: 10.1016/j.juro.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 57.Karaman MI, Kaya C, Ozturk M, et al. Comparison of transurethral vaporization using plasmakinetic energy and transurethral resection of prostate: 1-year follow-up. J Endourol. 2005;19:734–7. doi: 10.1089/end.2005.19.734. [DOI] [PubMed] [Google Scholar]
- 58.Tooher R, Sutherland P, Costello A, et al. A systematic review of holmium laser prostatectomy for benign prostatic hyperplasia. J Urol. 2004;171:1773–81. doi: 10.1097/01.ju.0000113494.03668.6d. [DOI] [PubMed] [Google Scholar]
- 59.Kuntz RM, Lehrich K. Transurethral holmium enucleation versus transvesical open enucleation for prostate adenoma greater than 100 gm: a randomized prospective trial of 120 patients. J Urol. 2002;168:1465–9. doi: 10.1016/S0022-5347(05)64475-8. [DOI] [PubMed] [Google Scholar]
- 60.Kuntz RM, Ahyai S, Lehrich K, et al. Transurethral holmium laser enucleation of the prostate versus transurethral electrocautery resection of the prostate: a randomized prospective trial in 200 patients. J Urol. 2004;172:1012–6. doi: 10.1097/01.ju.0000136218.11998.9e. [DOI] [PubMed] [Google Scholar]
- 61.Ruszat R, Wyler S, Forster T, et al. Safety and effectiveness of photoselective vaporization of the prostate (PVP) in patients on ongoing oral anticoagulation. Eur Urol. 2007;51:1031–8. doi: 10.1016/j.eururo.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Hai MA. Photoselective vaporization of prostate: five-year outcomes of entire clinic patient population. Urology. 2009;73:807–10. doi: 10.1016/j.urology.2008.08.502. [DOI] [PubMed] [Google Scholar]
- 63.Yang Q, Peters TJ, Donovan JL, et al. Transurethral incision compared with transurethral resection for the prostate for bladder outlet obstruction: a systematic review and meta-analysis of randomized controlled trials. J Urol. 2001;165:1526–32. [PubMed] [Google Scholar]
- 64.de la Rosette J, Laguna MP, Gravas S, et al. Transurethral microwave thermotherapy: the gold standard for minimally invasive therapies for patients with benign prostatic hyperplasia? J Endourol. 2003;17:245–51. doi: 10.1089/089277903765444393. [DOI] [PubMed] [Google Scholar]
- 65.Mattiasson A, Wagrell L, Schelin S, et al. Five-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: a prospective randomized multicenter study. Urology. 2007;69:91–6. doi: 10.1016/j.urology.2006.08.1115. [DOI] [PubMed] [Google Scholar]
- 66.Hill B, Belville W, Bruskewitz R, et al. Transurethral needle ablation versus transurethral resection of the prostate for the treatment of symptomatic benign prostatic hyperplasia: 5-year results of a prospective, randomized, multicenter clinical trial. J Urol. 2004;171:2336–40. doi: 10.1097/01.ju.0000127761.87421.a0. [DOI] [PubMed] [Google Scholar]
- 67.Zlotta AR, Giannakopoulos X, Machlum O, et al. Long-term evaluation of transurethral needle ablation of the prostate (TUNA) for the treatment of symptomatic benign prostatic hyperplasia: clinical outcome up to 5 years from 3 centers. Eur Urol. 2003;44:89–93. doi: 10.1016/s0302-2838(03)00218-5. [DOI] [PubMed] [Google Scholar]
- 68.Bouza C, López T, Magro A, et al. Systematic review and meta-analysis of transurethral needle ablation in symptomatic benign prostatic hyperplasia. BMC Urol. 2006;21:14. doi: 10.1186/1471-2490-6-14. 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kletscher BA, Oesterling JE. Prostatic Stents. Current perspectives for the management of benign prostatic hyperplasia. Urol Clin North Amer. 1995;22:423–30. [PubMed] [Google Scholar]
- 70.Lucas MG, Stephenson TP, Nargund V. Tamsulosin in the management of patients in acute urinary retention from benign prostatic hyperplasia. BJU Int. 2005;95:354–7. doi: 10.1111/j.1464-410X.2005.05299.x. [DOI] [PubMed] [Google Scholar]
- 71.McNeill SA, Hargreave TB, Roehrborn CG, Alfaur study group Alfuzosin 10 mg once daily in the management of acute urinary retention: Results of a double-blind placebo-controlled study. Urology. 2005;65:83–9. doi: 10.1016/j.urology.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 72.Thompson IN, Goodman PJ, Tangen CM, et al. The influence of finasteride in the development of prostate cancer. N Engl J Med. 2003;349:211–20. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 73.Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 74.Kramer BS, Hagerty KL, Justman S, et al. Use of 5α-reductase inhibitors for prostate cancer chemoprevention: American society of clinical oncology/American urological association 2008 clinical practice guideline. J Urol. 2009;181:1642–57. doi: 10.1016/j.juro.2009.01.071. [DOI] [PubMed] [Google Scholar]
- 75.Andriole GL. Dutasteride shown to reduce prostate cancer in high-risk men. Urology Times. 2009 Jun 1; [Google Scholar]