Abstract
Recently, we described a 3.4-kb mitochondrial genome deletion having significance for identifying malignant and benign prostate tissues (p < 0.001). This biomarker was also present in normal appearing tissue, in close proximity to a tumour indicating a “field effect.” In the present study, we report 4 cases (3 malignant, 1 benign) which suggest that this field effect may occur before tumourigenesis; this effect may also identify the presence of a small tumour focus/foci, which are difficult to detect with single or multiple biopsy procedures.
Introduction
The role of mitochondria from intracellular energy generation to apoptosis indicates that this organelle is an important part of oncogenesis.1–4 Additionally, there are several specific biological features of the mitochondrial genome (mtgenome) that identify this molecule as a valuable biomarker. Mitochondria are maternally inherited which precludes recombination, with the result that the sequence of the mtgenome is generally stable through generations.5,6 Compared to the nuclear component of genetic material, the mtgenome has a massive copy number advantage. There are thousands of mtgenomes in a cell as opposed to the diploid nuclear genome content. These copies are clonal or identical under normal cellular conditions. The number and clonal nature of this molecule enables relatively easy scoring of biomarkers, even at low frequencies.7 Importantly, the mtgenome has a rapid somatic mutation rate,6 especially in the presence of certain tissue pathologies, such as prostate cancer.8 It is well known that somatic mitochondrial genome mutations, from SNPs to large genomic deletions, are common in many solid tumours and are also found in many neuropathologies.9–11
The mutations in the mtgenome often occur before well-characterized morphological changes.12 Such molecular findings in histologically normal prostatic tissues close to tumour foci are also reported using nuclear genome targets, such as promoter methylation of glutathione S-transferase pi (GSTPi).13 These molecular changes are consistent with Slaughter’s concept of field effect (i.e., in the presence of a tumour, the histologically normal appearing cells in the proximity to the tumour harbor identical genetic changes to the tumour).14 This characteristic suggests that mtgenome mutations may be used as a “biosensor,” particularly for early prostate cancer detection. Specific markers can be quantified with real-time quantitative polymerase chain reaction (RT-PCR).15
It is important to note that the cases described here suggest that a molecular transition occurs early in normal appearing tissue (field effect); this molecular transition enables, rather than precludes, potential tumour growth.16 The behaviour of a 3.4-kb mtgenome deletion (3.4 mtΔ), as further described in this study, seems to identify the occurrence of this prostate cancer genetic field.
Methods
Ethics
All patients were recruited and prostate samples from existing biopsies were requested in accordance with the ethics guidelines of the Thunder Bay Regional Hospital Ethics Board and the Trafalgar Ethics Board (Oakville, Ontario). Both boards operate in accordance with the Tri-Council Policy Statement on Ethical Conduct for Research Involving Humans (Ottawa, Ontario).
Case histories
Case histories were drawn from the results of a large study reported elsewhere.17 The histories were selected based on the following criteria:
Deletion data analysis must be available from at least 1 needle biopsy core from the initial biopsy procedure. For histories 1 and 4, data was available on a representative core from each region of the prostate, with 6 obtained from each subject. Histories 2 and 3 only had data from 1 core each.
There must be at least 1 follow-up biopsy procedure.
A malignant diagnosis must be followed by a radical prostatectomy (RP).
All procedures must have a corresponding pathology report.
RT-PCR for the 3.4 mtΔ
The RT-PCR for the 3.4 mtΔ has been described previously.17 Briefly, DNA was extracted from 20 micron formalin-fixed paraffin embedded (FFPE) cross sections of prostate needle core biopsies using a QIAamp DNA Mini Kit (Qiagen Inc., Mississauga, Ontario). A qualified pathologist had previously graded biopsy tissues and cases were chosen with reference to the associated pathology report. The PCR reactions were seeded with 20-ng total DNA extracts. Each extract was amplified for the deletion, total mitochondrial DNA (mtDNA) and tumour necrosis factor (TNF), a single copy nuclear locus using 1X iQ Syber Green Supermix (Bio-Rad Laboratories, Mississauga, Ontario).
Statistical analyses
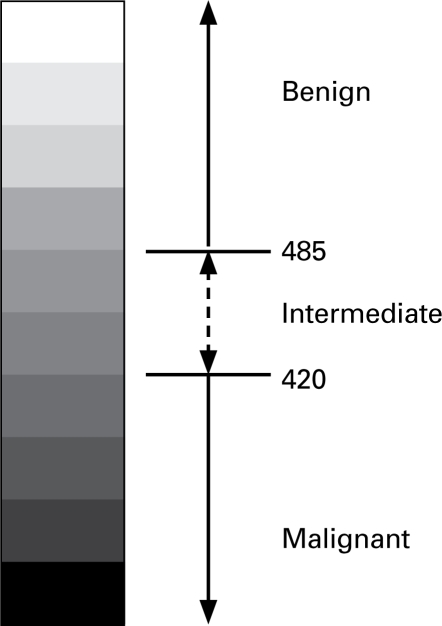
The cycle threshold (Ct) of the 3.4 mtΔ was compared with the Ct of 2 additional targets designed to capture the total amount of mtDNA (12SrRNA gene), and the number of cells contributing DNA to the reaction (single copy TNF). Cycle thresholds of all 3 targets were tested in a simple formula [(Ctdel-Cttot)Cttnf], which provides a score for each sample. This score is hereafter referred to as “residual mtDNA score or rate of mutation (RM) score.”17 Serial dilutions of each template (previously amplified and purified) were diluted through 6 concentrations and were used to generate standard curves for data analyses. Based on previous pilot and validation studies, a scale was constructed based on RM scores for determining the malignant, benign or intermediate status of a particular core sample (Fig. 1). This progressive scale implements the 99% confidence intervals (CI) of the means of both studies. The benign group consisted of 131 samples with a lower CI of 485, while the malignant group included 106 samples with an upper CI of 420.17 The intermediate region is the interval between these respective confidence bounds.
Fig. 1.
RM scores (in brackets) and biopsy results for each procedure are shown in their respective boxes. “B” indicates a biopsy followed by the procedure number. A biopsy followed by another B indicates a benign result. A malignant result is denoted by the Gleason score. A core suspicious for malignancy is indicated by PCa? Finally, the temporal relationships of the procedures are shown in the right column, the RM scale is shown at right.
Results
We present 4 cases of men who had multiple prostate biopsy procedures for the diagnosis of prostate cancer. Three of the men were subsequently diagnosed with prostate cancer, and one remained benign. Available negative biopsy samples from the initial biopsy procedure were assayed for the frequency of the 3.4 mtΔ deletion as a measure of prostate cancer “field effect.”
Case 1
A 58-year-old male was referred to a urologist for suspicion of prostate cancer due to an elevated prostate-specific antigen (PSA) of 10.3 ng/mL. A transrectal ultrasound (TRUS) guided biopsy revealed an enlarged prostate of 80 g (the average is 30 g). Of the 8 biopsy cores taken, only the core from the left base was suspicious for prostate cancer. In addition, basal cell staining with 34BE12 was suggestive of cancer in this area; however, the patient was untreated and still followed-up for a definitive diagnosis of prostate cancer. Eight months after the initial biopsy, an 8-core biopsy procedure revealed a Gleason score of 6 in 1 of the 2 cores taken from the left mid zone. The remaining cores, including the left basewhich was initially suspicious for prostate cancer, were found to be benign. A prostatectomy 3 months later revealed tumour (GS6) involvement of both right and left lobes of the prostate.
Six biopsies from the initial biopsy of the right and left apex and the mid and base of the prostate gland were analyzed for the deletion. The RM scores from the 6 cores indicated the presence of malignant foci in all biopsies from the left lobe and right mid zone (RM scores <420). The RM values for the right base and apex suggested that these areas were intermediate and well below the benign threshold of 485. These results raised concern since the values indicated a non-benign status and, in the right apex, just exceed the malignant RM threshold (420). In contrast, a histopathology analysis identified a suspicion of prostate cancer in the left base only. A secondary biopsy 8 months later indicated a GS6 tumour focus in the left mid zone. It is interesting to note that this area had the highest correlation with malignancy with respect to RM scores. A RP demonstrated a tumour in both the right and left lobes. Importantly, the frequency of the 3.4 mtΔ predicted this outcome 11 months prior to surgery (Fig. 1).
Case 2
A 65-year-old man was referred to a urologist for suspicion of prostate cancer following an elevated PSA of 8.9 ng/mL. A TRUS guided biopsy procedure returned normal results in all 9 cores, as did a follow-up 10-core biopsy procedure 7 months later. A third 11-core biopsy procedure 1 year after the second procedure found GS7 prostate cancer only in the left base and prostate intraepithelial neoplasia (PIN) in the right base. Nearly 2 months later, prostatectomy results verify tumour involvement of the right and left lobes, with the largest mass found in the left mid zone.
Only the core biopsy from the left base of the original biopsy was available for analysis. The RM score of 273 for this biopsy clearly indicated the presence of concurrent tumour; however, all needle cores were negative in histopathological analyses. Similar negative histological findings were reported from a subsequent biopsy at 7 months. A third procedure 12 months later indicated malignancy in the left base (GS7) with PIN apparent in the right base. Results from a RP demonstrated the presence of a tumour in both lobes, with the highest concentration in the left mid zone. Left base involvement was consistent with the original RM value and may reflect the major mass of tumour in the left mid zone observed after the RP (Fig. 1). The RM score predicted this outcome 21 months prior to this final procedure. Interestingly, a total of 3 cores, which were all negative, were taken from this zone during the initial and secondary biopsies.
Case 3
A 66-year-old male was referred to a urologist for suspicion of prostate cancer due to an elevated PSA (8.4 ng/mL). A TRUS guided 8-core biopsy of the patient’s prostate demonstrated micro foci of tumour (GS6) in the right mid zone and right base. A second procedure 13 months later was negative for prostate cancer in all 11 cores taken. A third biopsy procedure identified GS7 tumour in biopsies from the base, mid and apex of the right lobe in all 5 cores taken from these areas. The left mid zone was suspicious for cancer; however, the rest of the lobe was negative. Two months later, prostatectomy results revealed tumour (GS7) in the right and left lobes.
This case was also limited to one sample from the left apex for analysis. The RM score of 348 was strongly indicative of prostate cancer. In this initial biopsy, GS6 tumour was seen in the right base and mid zone, but not in the left apex. Thirteen months later, an additional procedure was negative. A third biopsy, 15 months after the previous procedure, revealed a GS7 tumour in the right lobe, but was again absent in the left apex. Importantly, the histopathological results from surgery not only show the presence of a tumour in the right and left lobes, but indicate the prominence of the mass in the left lobe, which further demonstrates the often significant discord between needle biopsy results and the histology of the RP.18 This report is consistent with the RM value of the left apex from the original procedure (Fig. 1). The RM score suggested this outcome 31 months prior to the RRP.
Case 4
In 2000, a 71-year-old male was referred to a urologist for a prostate needle biopsy as a result of a high PSA value (19.3 ng/mL). An 8-core biopsy procedure recorded normal histology with the exception of a moderate reduction in the number of glands and chronic diffuse inflammation in the stroma. Five years later, a follow-up 11-core biopsy procedure again confirmed the absence of malignant lesions; any presence of chronic inflammation is absent in this pathology report.
This benign case covers a period of 5 years during which the benign status of the prostate gland was mirrored by the RM scores of the initial procedure and again confirmed by a follow-up biopsy procedure 5 years later. This suggests that the original RM values (>550), taken from all zones of the prostate, successfully predicted the negative status of this prostate gland and agreed with the histology (Fig. 1). The RM scores were all well above the 485 lower RM threshold for benign status.
Discussion
An ongoing and confounding issue in urology is the male patient with negative prostate biopsy results who presents with elevated PSA. This outcome is frequent. For example, a comprehensive study of 319 men reported a positive biopsy rate of 39%, even though 10 needle cores were sampled from 292 of these patients.19 Of these men, 78% were African-American, who are known to have a higher incidence of prostate cancer,20 which could account for the high positive rate in this series. In many instances, there are potentially high false-negative outcomes, and the urologist usually continues to monitor PSA levels, including a supplemental periodic biopsy, when indicated. Another management strategy for clinically insignificant or indolent prostate cancer termed “active surveillance,” requires a PSA doubling time of 3 years or less and a biopsy demonstrating an increase in Gleason grade to qualify for radical intervention.21,22 This strategy works well, but only for those with “favourable risk prostate cancer,” attested by patient survival rate of 99% within 8 years after initiation of this study. A clinical difficulty with prostate cancer diagnosis is the limitation of the volume of tissue that is sampled and analyzed by the biopsy procedure. Currently, biopsy procedures assess about 1/2500 of the prostate depending on size. Common practice requires 10 to 12 cores taken per biopsy procedure; however, small tumour foci are consistently missed.
All available cores for each patient described here were analyzed for RM values. These 4 cases suggest that the RM value may be a sensitive indicator of tumour “field effect.” Most importantly, this value may also identify patients who are truly benign, in contrast to other clinical indicators, such as PSA, which is known to have marginal specificity. The RM score predicted outcome of case 1 indicates that RM scores from the complete suite of biopsies may be informative from a clinical perspective, enabling detection of potential tumour foci in advance of histopathological changes and highlighting the areas and extent of disease. Although, the “field effect”23 demonstrated by the mitochondrial genome marker is interesting in urology and may have important utility in early prostate cancer detection, the concept requires clinical validation. Moreover, the “field effect,” if proven to be an indication of early preconditioned prostate epithelia, should have the potential for intervention with chemoprevention agents, such as finasteride.
Conclusion
The encouraging published results from studies of this marker in helping to resolve the status of a prostate with negative biopsies17 has led to planned clinical validation studies, which involve several urologists and centres across Canada. The outcome of this study should form a strong basis for adoption of this ancillary test by urologists in making repeat biopsy decisions.
Table 1.
| Case 1 | |||
| Right lobe | Left lobe | Time (mos) | |
| Base | B1:B(440) B2:B RP:GS6 |
B1: PCa? (270) B2: B RP:GS6 |
t = 0 t = 8m t = 12m |
| Mid | B1: B(289) B2:B RP:GS6 |
B1:B(269) B2:GS6 RP:GS6 |
t = 0 t = 8m t = 12m |
| Apex | B1:B(423) B2:B RP:GS6 |
B1:B(326) B2:B RP:GS6 |
t = 0 t = 8m t = 12m |
| Case 2 | |||
| Right lobe | Left lobe | Time (mos) | |
| Base | B1:B B2:B B3:PIN RP:GS7 |
B1:B(273) B2:B B3:GS7 RP:GS7 |
t = 0 t = 7m t = 19m t = 21m |
| Mid | B1:B B2:B B3:B RP:GS7 |
B1:B B2:B B3:B RP:GS7 |
t = 0 t = 7m t = 19m t = 21m |
| Apex | B1:B B2:B B3:B RP:GS7 |
B1:B B2:B B3:B RP:GS7 |
t = 0 t = 7m t = 19m t = 21m |
| Case 3 | |||
| Right lobe | Left lobe | Time (mos) | |
| Base | B1:GS6 B2:B B3:GS7 RP:GS7 |
B1:B B2:B B3:B RP:GS7 |
t = 0 t = 13m t = 29m t = 31m |
| Mid | B1:GS6 B2:B B3:GS7 RP:GS7 |
B1:B B2:B B3:PCa? RP:GS7 |
t = 0 t = 13m t = 29m t = 31m |
| Apex | B1:B B2:B B3:GS7 RP:GS7 |
B1:B (348) B2:B B3:B RP:GS7 |
t = 0 t = 13m t = 29m t = 31m |
| Case 4 | |||
| Right lobe | Left lobe | Time (mos) | |
| Base | B1:B(658) B2:B |
B1:B(600) B2: B |
t = 0 t = 60m |
| Mid | B1: B(571) B2:B |
B1:B(552) B2:B |
t = 0 t = 60m |
| Apex | B1:B(689) B2:B |
B1:B(559) B2:B |
t = 0 t = 60m |
Footnotes
Competing interests: Parr and Reguly work for and are stock owners of Genesis Genomics, Inc. Certain commercial equipment, instruments, materials or companies are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation nor endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are the best available for the purpose.
This paper has been peer-reviewed.
References
- 1.Singh KK. Mitochondrial dysfunction is a common phenotype in aging and cancer. Ann N Y Acad Sci. 2004;1019:260–4. doi: 10.1196/annals.1297.043. [DOI] [PubMed] [Google Scholar]
- 2.Modica-Napolitano JS, Singh K. Mitochondria as targets for detection and treatment of cancer. Expert Rev Mol Med. 2002;4:1–19. doi: 10.1017/S1462399402004453. [DOI] [PubMed] [Google Scholar]
- 3.Brenner C, Kroemer G. Apoptosis. Mitochondria--the death signal integrators. Science. 2000;289:1150–1. doi: 10.1126/science.289.5482.1150. [DOI] [PubMed] [Google Scholar]
- 4.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 5.Olivo PD, Van de Walle MJ, Laipis PJ, et al. Nucleotide sequence evidence for rapid genotypic shifts in the bovine mitochondrial DNA D-loop. Nature. 1983;306:400–2. doi: 10.1038/306400a0. [DOI] [PubMed] [Google Scholar]
- 6.Parsons TJ, Muniec DS, Sullivan K, et al. A high observed substitution rate in the human mitochondrial DNA control region. Nat Genet. 1997;15:363–8. doi: 10.1038/ng0497-363. [DOI] [PubMed] [Google Scholar]
- 7.Jones JB, Song JJ, Hempen PM, et al. Detection of mitochondrial DNA mutations in pancreatic cancer offers a “mass”-ive advantage over detection of nuclear DNA mutations. Cancer Res. 2001;61:1299–304. [PubMed] [Google Scholar]
- 8.Chen JZ, Gokden N, Greene GF, et al. Simultaneous generation of multiple mitochondrial DNA mutations in human prostate tumours suggests mitochondrial hyper-mutagenesis. Carcinogenesis. 2003;24:1481–7. doi: 10.1093/carcin/bgg102. [DOI] [PubMed] [Google Scholar]
- 9.Parr RL, Dakubo GD, Thayer RE, et al. Mitochondrial DNA as a potential tool for early cancer detection. Hum Genomics. 2006;2:252–7. doi: 10.1186/1479-7364-2-4-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiMauro S, Schon EA. Mitochondrial DNA mutations in human disease. Am J Med Genet. 2001;106:18–26. doi: 10.1002/ajmg.1392. [DOI] [PubMed] [Google Scholar]
- 11.Jakupciak JP, Wang W, Markowitz ME, et al. Mitochondrial DNA as a cancer biomarker. J Mol Diagn. 2005;7:258–67. doi: 10.1016/S1525-1578(10)60553-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parr RL, Dakubo GD, Crandall KA, et al. Somatic mitochondrial DNA mutations in prostate cancer and normal appearing adjacent glands in comparison to age-matched prostate samples without malignant histology. J Mol Diagn. 2006;8:312–9. doi: 10.2353/jmoldx.2006.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrotra J, Varde S, Wang H, et al. Quantitative, spatial resolution of the epigenetic field effect in prostate cancer. Prostate. 2008;68:152–60. doi: 10.1002/pros.20675. [DOI] [PubMed] [Google Scholar]
- 14.Dakubo GD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakupciak JP, Dakubo GD, Maragh S, et al. Analysis of potential cancer biomarkers in mitochondrial DNA. Curr Opin Mol Ther. 2006;8:500–6. [PubMed] [Google Scholar]
- 16.Malins DC, Gilman NK, Green VM, et al. A cancer DNA phenotype in healthy prostates, conserved in tumours and adjacent normal cells, implies a relationship to carcinogenesis. Proc Natl Acad Sci U S A. 2005;102:19093–6. doi: 10.1073/pnas.0509630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maki J, Robinson K, Reguly B, et al. Mitochondrial genome deletion aids in the identification of false- and true-negative prostate needle core biopsy specimens. Am J Clin Pathol. 2008;129:57–66. doi: 10.1309/UJJTH4HFEPWAQ78Q. [DOI] [PubMed] [Google Scholar]
- 18.Fleshner NE, Cookson MS, Soloway SM, et al. Repeat transrectal ultrasound-guided prostate biopsy: a strategy to improve the reliability of needle biopsy grading in patients with well-differentiated prostate cancer. Urology. 1998;52:659–62. doi: 10.1016/s0090-4295(98)00226-x. [DOI] [PubMed] [Google Scholar]
- 19.Porter CR, O’Donnell C, Crawford ED, et al. Predicting the outcome of prostate biopsy in a racially diverse population: a prospective study. Urology. 2002;60:831–5. doi: 10.1016/s0090-4295(02)01882-4. [DOI] [PubMed] [Google Scholar]
- 20.Takeda N, Ota Y, Tanaka Y, et al. Myocardial adaptive changes and damage in ischemic heart disease. Ann N Y Acad Sci. 1996;793:282–8. doi: 10.1111/j.1749-6632.1996.tb33521.x. [DOI] [PubMed] [Google Scholar]
- 21.Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol. 2005;23:8165–9. doi: 10.1200/JCO.2005.03.3134. [DOI] [PubMed] [Google Scholar]
- 22.Klotz L. Active surveillance with selective delayed intervention for favorable risk prostate cancer. Urol Oncol. 2006;24:46–50. doi: 10.1016/j.urolonc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]