Abstract
Endogenous reproductive hormones and oxidative stress have been independently linked to risk of chronic disease but mostly in postmenopausal women. The interplay between endogenous reproductive hormones and oxidative stress among premenopausal women, however, has yet to be clearly elucidated. The objective of this study was to investigate the association between endogenous reproductive hormones and F2-isoprostanes in the BioCycle Study. Women aged 18–44 years from western New York State were followed prospectively for up to 2 menstrual cycles (n = 259) during 2005–2007. Estradiol, progesterone, luteinizing hormone, follicle-stimulating hormone, sex hormone-binding globulin, F2-isoprostanes, and thiobarbituric acid-reactive substances were measured up to 8 times per cycle at clinic visits timed by using fertility monitors. F2-Isoprostane levels had an independent positive association with estradiol (β = 0.02, 95% confidence interval: 0.01, 0.03) and inverse associations with sex hormone-binding globulin and follicle-stimulating hormone (β = −0.04, 95% confidence interval: −0.07, −0.003; β = −0.02, 95% confidence interval: −0.03, −0.002, respectively) after adjustment for age, race, age at menarche, γ-tocopherol, beta-carotene, total cholesterol, and homocysteine by inverse probability weighting. Thiobarbituric acid-reactive substances, a less specific marker of oxidative stress, had similar associations. If F2-isoprostanes are specific markers of oxidative stress, these results call into question the commonly held hypothesis that endogenous estradiol reduces oxidative stress.
Keywords: hormones, menstrual cycle, oxidative stress, women
High levels of endogenous reproductive hormones, especially estrogens, have been linked to elevated risks of many chronic diseases that affect women including cardiovascular disease, cervical cancer, and breast cancer but predominately in postmenopausal women (1). Accumulating evidence suggests that oxidative stress, a disturbance in the balance between the production of free radicals and reactive oxygen species and antioxidant defenses, may be a risk factor for these same conditions (2–4). Studies have shown that environmental factors, such as heavy metals exposure (5) and poor nutrition (i.e., low antioxidant and/or high fat intake) (6), may be associated with increased reactive oxygen species production. However, whether endogenous sex hormones play a role in the production of reactive oxygen species has yet to be elucidated, particularly in premenopausal women.
Both anti- and pro-oxidant capacities have been proposed for estrogens. Animal studies have shown antioxidant activity of estrogens and related compounds (7–10), experimental studies have shown that estrogens scavenge free radicals (11), while in vivo studies have demonstrated that exogenous estrogens decrease lipid peroxidation (12–14). It was therefore hypothesized that circulating estrogen and perhaps other hormones could explain the differential rates or timing of cardiovascular disease between men and women. Conversely, evidence has surfaced suggesting that estrogens may have pro-oxidant activities. Oxidative stress levels increased in a time-dependent manner following estrogen exposure in rats (15) and, in a cohort of older premenopausal women, higher estrone metabolite levels were correlated with higher oxidative stress levels (16).
The discrepancy of these findings demonstrates that the association between measures of oxidative stress and estrogen has yet to be fully understood. To date, there has been little research on the interplay between oxidative stress and endogenous reproductive hormones in women of reproductive age despite both being prominent biomarkers of disease in postmenopausal women. Currently, F2-isoprostanes, free radical-mediated oxidation products from arachidonic acid and higher polyunsaturated fatty acids, are considered the prevailing “gold standard” biologic markers of lipid peroxidation and oxidative stress because of their excellent stability in their biologic surroundings and their lack of generation by enzymatic processes (17, 18). Given that most previous research has proposed estrogen as an antioxidant, we hypothesized that we would observe an association between estrogen and oxidative stress. The purpose of our research was to investigate the influence of endogenous reproductive hormone levels and related factors on plasma free F2-isoprostane concentrations during 2 menstrual cycles among healthy premenopausal women.
MATERIALS AND METHODS
The BioCycle Study was a prospective cohort study of menstrual cycle function among 259 regularly menstruating, healthy, premenopausal volunteers aged 18–44 years from western New York State. Women were followed for up to 2 menstrual cycles. Details of the study are described elsewhere (19). Exclusion criteria included current use of oral contraceptives, vitamin and mineral supplements, or prescription medications; pregnancy or breastfeeding in the past 6 months; and recent history of infections or diagnosis of chronic conditions, including menstrual or ovulatory disorders. In addition, women with a self-reported body mass index of less than 18 or greater than 35 kg/m2 were excluded. The University at Buffalo Health Sciences Institutional Review Board approved the study, and all participants provided written informed consent.
Participants were followed for 1 (n = 9) or 2 (n = 250) menstrual cycles with blood samples collected in each cycle during early menstruation, mid- and late-follicular phase, 2 days around expected ovulation, and early, mid-, and late-luteal phase. Collection dates were adjusted for cycle length by utilizing fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, Massachusetts) to assist in the timing of specimen collection. Monitor indications of low, high, and peak fertility were used to time midcycle visits, with peak day and the following 2 days being those that would approximate late-follicular, luteinizing hormone (LH) surge, and ovulation dates. The utility of the fertility monitor for scheduling cycle visits has been demonstrated (20). Women were highly adherent to the study protocol, with 94% completing at least 7 clinic visits per cycle and 100% completing at least 5 visits.
Biologic specimens
Fasting blood and urine specimens were collected between 7:00 and 8:30 AM on appointed days (8 visits per cycle for 2 cycles). Collection and handling protocols were designed to minimize variability in preanalytical factors and have been previously described (21). All samples were frozen at −80°C within 90 minutes of phlebotomy and shipped on dry ice to analytical laboratories as a complete cycle (8 samples) from each participant. Samples were measured consecutively, within a single run, to limit analytical variability.
Oxidative stress.
Plasma free F2-isoprostanes were measured with a gas chromatography-mass spectrometry–based method by the Molecular Epidemiology and Biomarker Research Laboratory (University of Minnesota, Minneapolis, Minnesota) (9.4% coefficient of variation (CV)). The method used an internal standard, 2H4-labeled 8-iso-prostaglandin F2α (>98% pure; Caymen Chemical Company, Ann Arbor, Michigan), wherein the deuterium atoms were located at the nonexchangeable positions 3 and 4 of the molecule. Plasma thiobarbituric acid-reactive substances (TBARS) were measured at the University at Buffalo by using OxiTech reagent kits (ZeptoMetrix Corporation, Buffalo, New York) and expressed in nmol/mL of malondialdehyde equivalents (22). TBARS pigment was measured at excitation of 535 nm and emission of 552 nm on a RF-5000U spectrofluorometer (Shimadzu Scientific Instruments, Inc., Columbia, Maryland). Its interassay CV was 8.3%.
Antioxidants.
Vitamin A (retinol; 6.1% CV), vitamin E (α-, δ-, and γ-tocopherols; 2.3%, 2.2%, and 21.2% CV, respectively), and beta-carotene (7.5% CV) were measured simultaneously in serum by using high-performance liquid chromatography (23). Total ascorbic acid (vitamin C) was determined by the dinitrophenylhydrazine method (24) in heparin plasma stabilized in 6% metaphosphoric acid. The absorbance of each dinitrophenylhydrazine-derivatized sample was determined at 520 nm on a Shimadzu model 160U spectrophotometer (Shimadzu Scientific Instruments, Inc.) (9.6% CV).
Cholesterol.
A complete lipid profile (total cholesterol, high-density lipoprotein cholesterol, and triglycerides) was performed for each visit by an autochemistry analyzer at the Kaleida Center for Laboratory Medicine (Buffalo, New York). Low-density lipoprotein cholesterol was determined by using the Friedewald formula (25). The analytical imprecision across the study period was less than 5% CV for all lipid and lipoprotein assays.
Homocysteine.
Serum homocysteine was measured at 3 cycle visits (midfollicular, ovulation, and midluteal). Samples were analyzed at the Kaleida Laboratory by using an Immulite 2000 homocysteine competitive immunoassay (CV < 10.4% at all levels).
Reproductive hormones.
Reproductive hormone levels were measured in serum collected at each visit and included estradiol, progesterone, LH, follicle-stimulating hormone (FSH), and sex hormone-binding globulin (SHBG). Estradiol was measured by radioimmunoassay. FSH, LH, progesterone, and SHBG were measured by Specialty Laboratories, Inc. (Valencia, California) by using solid-phase competitive chemiluminescent enzymatic immunoassays on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, Illinois). The CV was less than 5% for LH and FSH, less than 10% for estradiol and SHBG, and less than 14% for progesterone.
Covariate assessment
At baseline, participants were asked to complete questionnaires regarding lifestyle, physical activity (International Physical Activity Questionnaire, long form 2002), and reproductive health history (26). High, moderate, and low physical activity categories were formed on the basis of the standard International Physical Activity Questionnaire cutpoints. Additionally, height and weight were measured by standardized protocol and used to calculate body mass index. Cycle length was defined as the number of days between the first day of menstrual bleeding and the last day before the next onset of at least 2 consecutive days of bleeding. All covariates had less than 5% total missing data.
Statistical analysis
The median and 25th and 75th percentile (quartiles 1 and 3) levels of F2-isoprostane, TBARS, and antioxidant vitamins were calculated for each visit. Repeated-measures analysis of variance was used to evaluate the association between cycle visit and oxidative stress and vitamins on the log scale, with Bonferroni-adjusted P values. For descriptive purposes, cycles were placed into quartiles on the basis of the average F2-isoprostane concentration across the cycle. Descriptive statistics were calculated for demographic characteristics, hormone levels, and measures of oxidative stress according to these quartiles. Chi-square tests and analysis of variance were used to test for associations between demographic variables and quartiles of F2-isoprostane. Because of the difference in F2-isoprostane concentrations by cycle, quartiles were assigned using cycle-specific cutpoints.
Linear mixed models on the log scale of the hormones were used to evaluate the unadjusted association between hormone levels and F2-isoprostane concentration. Random intercepts were specified to account for the variation in levels of F2-isoprostanes between women and the correlation between cycles of the same woman. For estradiol, LH, FSH, and SHBG, models included concentrations measured at all times throughout the cycle as separate observations, including up to 8 measurements per cycle. For progesterone, only concentrations during the luteal phase were included because of minimal biologic variation during the follicular phase.
For the adjusted models, we used marginal structural models to appropriately adjust for time-dependent confounders (27). Exposure weights were created by inverse probability weighting. In order to estimate the stabilized weights, the conditional density of estradiol levels at each cycle visit while adjusting for other factors was obtained by ordinary least-squares regression and estimated by the normal distribution (27). The choice of covariates in the weighted models was determined by a review of the prior literature and based on hypothesized relations between the factors included in a directed acyclic graph. The a priori confounders (based on the directed acyclic graph) that we considered were age, body mass index, race, physical activity, smoking status, and several serum antioxidants, in particular vitamin C. However, as including all potential confounders may reduce the precision of our estimates, only those minimal sets of a priori confounders that control for confounding which also changed the exposure coefficient by more than 15% were included in the final adjusted models. In the fully adjusted models (model 3), the weights were estimated while also adjusting for levels of progesterone, LH, and FSH at each cycle visit. The possibility of collinearity and interaction between variables of interest was evaluated and found not to be a factor (data not shown). Analyses were conducted by using SAS, version 9.1, software (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Demographics
Overall, women were young (mean age, 27.5 years) and of healthy weight (mean body mass index, 24.1), had moderate to high physical activity (90.5%), and were mostly nonsmokers (82%) (Table 1). Noticeably, women with the highest average F2-isoprostane concentrations over the cycle were more likely to be younger in age, of higher current body mass index, and Caucasian, to have an earlier onset of menarche, and to be nulliparous or nulligravida.
Table 1.
Characteristics of the 259 Study Participants According to F2-Isoprostane Quartile in the BioCycle Study, Buffalo, New York, 2005–2007
| Total Cohort |
Log(F2-Isoprostane) Quartiles |
||||||||||||||
| 1 |
2 |
3 |
4 |
||||||||||||
| No. (%) | Mean (SD) | Median (IQR, Q1–Q3) | No. (%) | Mean (SD) | Median (IQR, Q1–Q3) | No. (%) | Mean (SD) | Median (IQR, Q1–Q3) | No. (%) | Mean (SD) | Median (IQR, Q1–Q3) | No. (%) | Mean (SD) | Median (IQR, Q1–Q3) | |
| No. of cyclesa | 509 | 127 | 129 | 127 | 126 | ||||||||||
| Demographics | |||||||||||||||
| Age, years* | 27.5 (8.2) | 28.6 (8.0) | 27.9 (8.2) | 27.5 (8.6) | 25.5 (7.8) | ||||||||||
| Body mass index, kg/m2** | 24.1 (3.9) | 23.1 (3.3) | 23.4 (3.6) | 24.8 (4.2) | 25.2 (3.9) | ||||||||||
| Physical activityb | |||||||||||||||
| Low | 48 (9.4) | 14 (11.0) | 16 (12.4) | 9 (7.1) | 9 (7.1) | ||||||||||
| Moderate | 182 (35.8) | 55 (43.3) | 39 (30.2) | 50 (39.4) | 38 (30.2) | ||||||||||
| High | 278 (54.7) | 58 (45.7) | 73 (56.6) | 68 (53.5) | 79 (62.7) | ||||||||||
| Race** | |||||||||||||||
| Caucasian | 302 (59.3) | 58 (45.7) | 73 (56.6) | 77 (60.6) | 94 (74.6) | ||||||||||
| African American | 101 (19.8) | 28 (22.0) | 25 (19.4) | 28 (22.0) | 20 (15.9) | ||||||||||
| Asian | 76 (14.9) | 32 (25.2) | 19 (14.7) | 16 (12.6) | 9 (7.1) | ||||||||||
| Other | 30 (5.9) | 9 (7.1) | 12 (9.3) | 6 (4.7) | 3 (2.4) | ||||||||||
| Years of education* | |||||||||||||||
| High school or less | 64 (12.6) | 10 (7.9) | 17 (13.2) | 25 (19.7) | 12 (9.5) | ||||||||||
| History of smoking | |||||||||||||||
| No | 415 (81.5) | 105 (82.7) | 103 (79.8) | 109 (85.8) | 98 (77.8) | ||||||||||
| Yes, previously | 72 (14.1) | 16 (12.6) | 22 (17.1) | 13 (10.2) | 21 (16.7) | ||||||||||
| Yes, currently | 22 (4.3) | 6 (4.7) | 4 (3.1) | 5 (3.9) | 7 (5.6) | ||||||||||
| Past oral contraceptive use | |||||||||||||||
| Yes | 276 (54.2) | 70 (55.1) | 71 (55.0) | 65 (51.2) | 70 (55.6) | ||||||||||
| Cycle length, days | 28.8 (4.1) | 29.1 (4.2) | 28.2 (3.5) | 28.5 (4.0) | 29.5 (4.6) | ||||||||||
| Age at menarche, years | 12.4 (1.2) | 12.6 (1.2) | 12.5 (1.3) | 12.4 (1.2) | 12.2 (1.3) | ||||||||||
| Parity* | |||||||||||||||
| ≥1 | 134 (26.3) | 42 (33.1) | 37 (28.7) | 33 (26.0) | 22 (17.5) | ||||||||||
| Gravidity* | |||||||||||||||
| ≥1 | 156 (30.6) | 50 (39.4) | 42 (32.6) | 35 (27.6) | 29 (23.0) | ||||||||||
| Menstrual hormones | |||||||||||||||
| Estradiol, pg/mL* | 82.0 (46.0–152.0) | 77.0 (45.0–143.0) | 87.0 (48.0–160.0) | 83.0 (45.0–149.5) | 82.0 (46.0–148.0) | ||||||||||
| Luteal progesterone, ng/mL | 7.0 (2.0–11.3) | 7.1 (2.1–12.4) | 7.0 (2.1–11.3) | 6.8 (1.9–10.3) | 7.1 (1.7–11.1) | ||||||||||
| Luteinizing hormone, ng/mL | 5.7 (3.7–9.7) | 5.4 (3.6–9.2) | 5.7 (3.8–9.4) | 5.8 (3.7–10.6) | 5.8 (3.8–9.8) | ||||||||||
| Follicle-stimulating hormone, mIU/mL** | 5.6 (3.7–7.7) | 5.6 (3.6–7.7) | 5.9 (3.9–8.1) | 5.6 (3.9–7.7) | 5.3 (3.5–7.6) | ||||||||||
| Sex hormone-binding globulin, nmol/L** | 44.7 (31.9–60.9) | 45.8 (34.8–59.6) | 47.9 (33.4–63.1) | 44.7 (31.1–62.8) | 40.1 (28.7–57.0) | ||||||||||
| Oxidative stress markers | |||||||||||||||
| F2-Isoprostane, pg/mL** | 47.2 (38.2–60.2) | 34.0 (30.7–36.2) | 42.6 (40.4–45.2) | 52.7 (49.9–55.6) | 71.2 (64.3–83.5) | ||||||||||
| Thiobarbituric acid-reactive substances, nmol/mL | 0.85 (0.75–0.99) | 0.85 (0.74–1.02) | 0.83 (0.74–0.97) | 0.87 (0.76–1.00) | 0.84 (0.75–0.96) | ||||||||||
Abbreviations: IQR, interquartile range; Q1, 25th percentile; Q3, 75th percentile; SD, standard deviation.
* P < 0.05; **P < 0.001.
Cycle 1 cutpoints: total cohort, 3.08–5.17 pg/mL2; quartile 1, 3.08–3.68; quartile 2, 3.69–3.90; quartile 3, 3.91–4.10; quartile 4, 4.11–5.17. Cycle 2 cutpoints: total cohort, 3.11–4.91 pg/mL2; quartile 1, 3.11–3.58; quartile 2, 3.59–3.77; quartile 3, 3.78–4.04; quartile 4, 4.05–4.91.
One missing value.
Of the hormone-related factors presented in Table 1, age at menarche is considered to be a possible confounder because it is hypothesized to be a predictor of F2-isoprostane levels and lifetime exposure to estrogen. Age at menarche was observed to have a strong association with F2-isoprostane levels, independent of reproductive hormone levels and after adjustment for race and age (β = −0.04; P = 0.01). Current body mass index, a measure of body fat strongly related to estrogen levels, was significantly associated with F2-isoprostane levels after adjustment for race and age (β = 0.03; P ≤ 0.001). Current body mass index was not included in the model to avoid overadjustment bias (28), as it was hypothesized to be a descendent of a causal intermediate in the pathway between age at menarche and F2-isoprostanes.
Oxidative stress variation over the cycle
Table 2 displays the median (quartiles 1 and 3) levels of oxidative stress and other markers of antioxidant activity over the menstrual cycle in our cohort of 259 women. Measures of both oxidative stress (F2-isoprostane and TBARS) and antioxidant vitamins (α-tocopherol, vitamin A, and vitamin C) significantly varied across the menstrual cycle (P < 0.05). F2-isoprostane levels were highest in the days before ovulation and then rose again in the early luteal phase. On average, isoprostane concentrations were the lowest on the day of ovulation. After ovulation, F2-isoprostane levels increased 6.1%, on average, by the early luteal phase.
Table 2.
F2-Isoprostane, Thiobarbituric Acid-reactive Substances, Vitamin E, Vitamin A, and Vitamin C Concentrations by Menstrual Cycle Phase Among the 259 Women (n = 509 Cycles) in the BioCycle Study, Buffalo, New York, 2005–2007
| Median (Q1–Q3) Concentrations by Phase of Menstrual Cycle |
||||||||
| Menses | Mid Follicular | Late Follicular | LH/FSH Surge | Ovulation | Early Luteal | Mid Luteal | Late Luteal | |
| F2-Isoprostane, pg/mL* | 45.8 (36.2–57.9) | 46.3 (36.9–59.0) | 47.4 (37.4–60.7) | 46.8 (38.1–60.1) | 44.2 (35.7–57.4) | 46.9 (37.5–58.6) | 45.5 (36.9–58.9) | 45.9 (36.8–59.0) |
| TBARS, nmol/mL* | 0.86 (0.73–1.01) | 0.86 (0.75–1.01) | 0.86 (0.73–1.02) | 0.85 (0.74–1.01) | 0.85 (0.71–1.00) | 0.85 (0.72–1.01) | 0.82 (0.70–0.98) | 0.81 (0.70–0.94) |
| γ-Vitamin E, μg/mL | 1.71 (1.37–2.16) | 1.75 (1.37–2.19) | 1.74 (1.34–2.16) | 1.67 (1.35–2.15) | 1.70 (1.31–2.11) | 1.78 (1.40–2.24) | 1.74 (1.37–2.19) | 1.72 (1.32–2.13) |
| α-Vitamin E, μg/mL* | 7.88 (6.65–9.23) | 8.14 (6.98–9.53) | 8.09 (6.91–9.34) | 7.99 (6.83–9.33) | 7.94 (6.83–9.10) | 7.90 (6.82–9.22) | 7.96 (6.72–9.17) | 7.70 (6.54–9.22) |
| δ-Vitamin E, μg/mL | 0.146 (0.11–0.19) | 0.146 (0.11–0.19) | 0.146 (0.11–0.19) | 0.146 (0.11–0.19) | 0.145 (0.11–0.19) | 0.148 (0.11–0.20) | 0.145 (0.11–0.19) | 0.143 (0.11–0.19) |
| Vitamin A, μg/mL* | 0.355 (0.31–0.41) | 0.376 (0.33–0.43) | 0.371 (0.32–0.40) | 0.373 (0.32–0.42) | 0.364 (0.32–0.43) | 0.372 (0.32–0.43) | 0.368 (0.32–0.42) | 0.359 (0.31–0.40) |
| Vitamin C, mg/dL* | 1.64 (1.37–1.98) | 1.70 (1.42–2.04) | 1.69 (1.42–2.02) | 1.70 (1.42–2.01) | 1.69 (1.40–2.04) | 1.70 (1.43–2.03) | 1.70 (1.44–2.04) | 1.66 (1.41–2.01) |
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; Q1, 25th percentile; Q3, 75th percentile; TBARS, thiobarbituric acid-reactive substances.
P < 0.05.
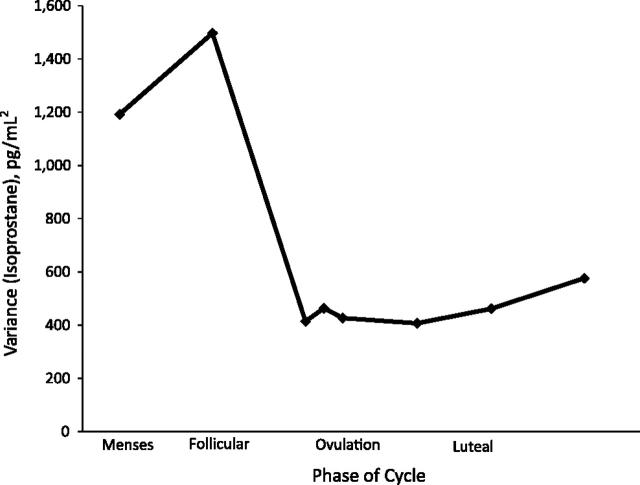
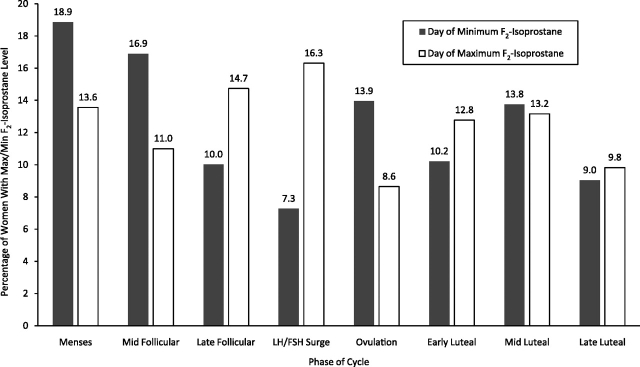
The variability in oxidative stress between women, as represented by daily variances of F2-isoprostane concentrations over the menstrual cycle, can be seen in Figure 1. The variability of F2-isoprostanes decreased, on average, 66% from menses to early follicular phase with the lowest levels of variability occurring in early luteal phase. Figure 2 shows the percentage of visits that recorded either the maximum or minimum F2-isoprostane concentrations across the cycle. Combined, menses and mid-follicular phase (typically periods of low hormone variation) had 36% of the minimum F2-isoprostane concentrations, while the days before ovulation (late-follicular and LH/FSH surge) had 31% of the maximum F2-isoprostane concentrations.
Figure 1.
Between-women variance of F2-isoprostanes across the menstrual cycle in the BioCycle Study, Buffalo, New York, 2005–2007. Each data point represents the variance of F2-isoprostane measurements at each clinic visit between women at each phase of the menstrual cycle among 259 women (n = 509 cycles).
Figure 2.
Percentage of women with maximum or minimum F2-isoprostane concentrations across the menstrual cycle at each clinic visit among 259 women (n = 509 cycles) in the BioCycle Study, Buffalo, New York, 2005–2007. FSH, follicle-stimulating hormone; LH, luteinizing hormone; Max, maximum; Min, minimum.
Menstrual hormones
After mixed-model analysis weighted for age, race, age at menarche, γ-tocopherol, beta-carotene, total cholesterol, and homocysteine, the estradiol concentration was positively associated with F2-isoprostane concentrations across the menstrual cycle (β = 0.02; P ≤ 0.001) (Table 3). This significant positive association held after additionally adjusting for progesterone, LH, and FSH concentrations throughout the menstrual cycle (β = 0.01; P = 0.03). SHBG concentrations across the menstrual cycle had a significant inverse association with F2-isoprostane concentrations (β = −0.04; P = 0.03), which was strengthened after weighting for the reproductive hormones (β = −0.04; P = 0.01). Progesterone in the luteal phase had a significant positive association with F2-isoprostane in the unadjusted model (β = 0.01; P ≤ 0.001) but became not significant (P < 0.10) after further adjustment for other covariates. FSH showed a highly significant inverse association with F2-isoprostane concentration in all models (β = −0.04; P ≤ 0.001 in fully adjusted). LH showed no significant trends with F2-isoprostane concentrations. TBARS had consistent findings to F2-isoprostane regarding the direction of association with estrogen, progesterone, and SHBG, although none of the models yielded significant findings (data not shown).
Table 3.
Marginal Structural Models of the Association Among Estradiol, Progesterone, Luteinizing Hormone, Follicle-stimulating Hormone, Sex Hormone-binding Globulin, and F2-Isoprostanes in the BioCycle Study, Buffalo, New York, 2005–2007
| Model 1a |
Model 2b |
Model 3c |
||||
| β | 95% CI | β | 95% CI | β | 95% CI | |
| Estradiol, pg/mL | 0.019 | 0.010, 0.029** | 0.020 | 0.010, 0.029** | 0.011 | 0.001, 0.022** |
| Progesterone, ng/mL | 0.011 | 0.000, 0.022* | 0.009 | −0.002, 0.020 | 0.010 | −0.001, 0.021 |
| Luteinizing hormone, ng/mL | 0.005 | −0.003, 0.013 | 0.002 | −0.007, 0.010 | −0.0001 | −0.011, 0.011 |
| Follicle-stimulating hormone, mIU/mL | −0.021 | −0.034, −0.008* | −0.016 | −0.029, −0.002* | −0.035 | −0.051, −0.019* |
| Sex hormone-binding globulin, nmol/L | −0.084 | −0.120, −0.048* | −0.035 | −0.066, −0.003* | −0.043 | −0.076, −0.009** |
Abbreviations: β, effect estimate; CI, confidence interval.
* P < 0.05; **P < 0.001.
Model 1: unadjusted.
Model 2: adjusted for age (continuous), race (Caucasian, African American, Asian, other), age at menarche (continuous), γ-vitamin E (continuous), beta-carotene (continuous), total cholesterol (continuous), and homocysteine (continuous), using inverse probability of exposure weights.
Model 3: adjusted for the variables in model 2 and other reproductive hormones (continuous), using inverse probability of exposure weights.
DISCUSSION
Markers of oxidative stress, particularly F2-isoprostane concentrations, vary (showing a significant, positive association with estradiol and inverse associations with SHBG and FSH) throughout the menstrual cycle. These associations persisted after controlling for demographic (i.e., age, race, and age at menarche) and time-dependent confounders (i.e., serum γ-tocopherol and beta-carotene levels, total cholesterol, and homocysteine). The direction of the association was also consistent with TBARS, another measure of lipid peroxidation, and several other markers of estrogen exposure such as age at menarche and body mass index. The observed associations between endogenous hormones and F2-isoprostane add critical understanding to the interplay of oxidative stress and endogenous hormones in women of reproductive age. Furthermore, if F2-isoprostanes are a specific marker of oxidative stress, this calls into question the commonly held hypothesis that endogenous estradiol acts as an antioxidant, protecting premenopausal women from risk of chronic disease.
Studies on the associations between reproductive hormone levels, in particular estrogens, and rigorously validated biomarkers of oxidative stress such as F2-isoprostanes, among premenopausal women, are sparse. The observed positive association between F2-isoprostane and estradiol is in contradiction to findings from in vitro and animal studies. However, caution should be taken when inferences are made from these previous studies, as they often rely on estrogenic compounds with widely different biologic (and potentially antioxidant) activity and concentrations of hormones that are significantly higher than the physiologic ranges (8–10, 12–14). The findings from observational studies are too sparse to clarify the role of estrogens as pro- or anti-oxidants. Investigations among postmenopausal women cannot be used to infer the relation between physiologic levels of natural hormones and oxidative status. A prior study focusing on older premenopausal women found a positive association between estrone metabolites and F2-isoprostanes (16). Of noticeable difference to our study, the median age of their premenopausal group of women (50 years compared with 27 years in our study) could affect the range of estradiol and estrone concentrations and the collection of data only in the early follicular phase when estrogen levels are low (compared with our 8 measurements across the menstrual cycle). Despite the differences, the conclusions are consistent: Neither study supports the commonly held hypothesis that levels of endogenous estradiol or its estrone metabolites favorably modify oxidative stress by decreasing F2-isoprostane levels.
The significant inverse association between SHBG and F2-isoprostane levels is biologically plausible given that SHBG binds to both estradiol and testosterone, mediating their effects (29). The antiandrogenic effects of SHBG have been hypothesized to partially explain previous observations that SHBG is inversely associated with risk of breast cancer (30) and type 2 diabetes (31). Its antiandrogenic effects could also partly explain its inverse association with oxidative stress in the present study. Another potential mediator is through insulin resistance, which has been shown to be negatively associated with SHBG levels and positively associated with oxidative stress levels (32).
The inverse association between F2-isoprostane levels and FSH was not unexpected on the basis of the current knowledge regarding the inhibitory effect of estrogen on gonadotropin-releasing hormone production in the hypothalamus (33) and the inverse relation with body mass index and age. However, the significant association between FSH and F2-isoprostanes remained strong, even after adjustment for these factors, which suggests that FSH could act independently on F2-isoprostane levels, although more research is needed to identify the exact mechanism.
Our finding that F2-isoprostane levels vary during the menstrual cycle in concordance with estradiol concentrations uncovers limitations of previous research on F2-isoprostane levels and clinical outcomes in premenopausal women; study designs need to take into account hormone levels or cycle variability and standardize the timing of the specimen collection. This important source of variability, specific to women, could help to explain the inconsistencies of findings between genders in previous studies that have attempted to link markers of oxidation to a number of clinical outcomes. For example, the Coronary Artery Risk Development in Young Adults (CARDIA) Study evaluated the relation between plasma F2-isoprostanes and coronary artery calcification (34). The mean and standard deviation (SD) of F2-isoprostanes was 140.4 (SD, 55.6) pmol/L in men (n = 1,302) and 190 (SD, 108.9) pmol/L in women (n = 1,548). Subsequently, the adjusted odds ratios for any calcification in men versus women were 1.19 (95% confidence interval: 1.01, 1.40) and 1.13 (95% confidence interval: 0.89, 1.44), respectively (34). Although the point estimates are similar across genders, the variability in women is larger than that in men, which could have reduced the precision and possibly result in the differences and conclusions. As demonstrated in Figure 1, the variability of F2-isoprostanes differs significantly by phase of the menstrual cycle. Thus, a standardized sample collection should take place during the early luteal phase when variability is minimized.
This study had a number of important strengths including the intensive monitoring of a large number of young and ethnically diverse women throughout 2 menstrual cycles. No previous study of premenopausal women had multiple longitudinal measures of F2-isoprostane concentrations and reproductive hormone levels throughout the menstrual cycle. Moreover, multiple clinic visits timed with fertility monitors were a significant improvement over previous studies. Advantages of F2-isoprostanes include their stability in their biologic surroundings, their lack of generation by enzymatic processes, and their availability in the urine as well as plasma, providing a noninvasive source of specimen for clinical analysis (18). The prospective design and exclusion criteria of the BioCycle Study strengthen the ability to draw inference, having reduced the potential for bias from known risk factors for menstrual/hormonal disorders. In addition, standardized assessment of a wide variety of participant characteristics increased the ability to adjust for confounding.
Our study did, however, have a few potential limitations. Although our study included the use of fertility monitors to time visits, bias could have been introduced through mistimed sample collection; however, various indicators of successfully timed visits were found to be unrelated to estrogen or F2-isoprostane concentrations, and thus any misclassification is likely to be nondifferential. In addition, F2-isoprostane samples were not run in duplicate because of cost, and therefore measurement error was not adjusted for in the analysis. Similarly, although F2-isoprostanes are currently advocated as sensitive and specific biomarkers of oxidative stress, care should be taken to infer an oxidative stress mechanism based solely on one biomarker. F2-isoprostanes are highly specific markers of lipid peroxidation, but their use as a biomarker has some significant drawbacks including their trace levels, complex and expensive sample preparation, and analysis, as well as the fact that they are only minor products generated from arachidonic acid peroxidation, which is, in turn, a relatively minor component of total biologic polyunsaturated fatty acids (17, 35). Furthermore, F2-isoprostanes may not provide sufficient evidence in view of reported intra- and intersubject variability (21, 36) and the fact that F2-isoprostanes have biologic activities outside their role as biomarkers of oxidative stress (37).
It is possible to speculate that, unlike an association with oxidative stress, the association between F2-isoprostanes and estradiol may be a reflection of increased eicosanoid and prostanoid metabolism during the menstrual cycle. It has been shown that eicosanoid and prostaglandin precursors derived from arachidonic acid accumulate in the endometrium toward the time of menstruation (38). Also, phospholipase A2 activity in the human endometrium is related to the stage of the menstrual cycle and suggests that arachidonic acid release may be influenced by estrogen and progesterone (39). F2-isoprostanes are derived solely from free radical attack on arachidonic acid/eicosanoid intermediates, and upregulation of the eicosanoid pathways during the menstrual cycle could simply provide more eicosanoid substrate for free radical attack leading to increased F2-isoprostanes. The fact that TBARS tends to mimic these findings may also be attributable to the fact that TBARS generation is closely correlated with arachidonic levels in experimental lipid peroxidation (40). Despite the above possibilities, our findings clearly suggest an association between endogenous estrogens and oxidative stress biomarkers.
We observed that levels of endogenous estradiol and factors that are positively related to estrogen exposure in premenopausal women are positively associated with F2-isoprostane levels throughout the menstrual cycle. SHBG and FSH were also negatively associated with F2-isoprostane levels. Although the effects of reproductive hormones on levels of F2-isoprostanes were very small in magnitude and might not have clinical importance, these findings help us to understand the biologic mechanisms that occur in vivo between a marker of oxidative stress and hormones. Of interest to future research, because of the significant cyclic variation in F2-isoprostane concentrations that we observed, cycle phase should be carefully accounted for when measuring levels of F2-isoprostanes in future studies of premenopausal women. To date, this study provides the most comprehensive assessment of endogenous hormones and oxidative stress interplay in women of reproductive age. If F2-isoprostanes are a specific marker of oxidative stress, these findings question the hypothesis that endogenous estrogen serves as a means of cardioprotection by inhibiting oxidative stress formation in premenopausal women. More research is warranted to further clarify the role of endogenous hormones on other biomarkers of oxidative stress, including markers of protein and nucleic acid damage as well as other biomarkers of lipid peroxidation in premenopausal women.
Acknowledgments
Author affiliations: Division of Epidemiology, Statistics, and Prevention Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland (Enrique F. Schisterman, Audrey J. Gaskins, Sunni L. Mumford, Edwina Yeung, Mary Hediger, Cuilin Zhang, Neil Perkins); Departments of Biotechnical and Clinical Laboratory Sciences, University at Buffalo, State University of New York, Buffalo, New York (Richard W. Browne); Health Sciences System of the Nevada System of Higher Education, Las Vegas, Nevada (Maurizio Trevisan); and Department of Social and Preventive Medicine, University at Buffalo, State University of New York, Buffalo, New York (Kathleen Hovey, Jean Wactawski-Wende).
The BioCycle Study and its researchers were supported in part or full by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health.
The authors are indebted to all the investigators and staff at the Epidemiology Branch, NICHD, and the University at Buffalo for their respective roles in the study.
Conflict of interest: none declared.
Glossary
Abbreviations
- CV
coefficient of variation
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- SHBG
sex hormone-binding globulin
- TBARS
thiobarbituric acid-reactive substances
References
- 1.Belchetz PE. Hormonal treatment of postmenopausal women. N Engl J Med. 1994;330(15):1062–1071. doi: 10.1056/NEJM199404143301508. [DOI] [PubMed] [Google Scholar]
- 2.Rossner P, Jr, Gammon MD, Terry MB, et al. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15(4):639–644. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- 3.Castelao JE, Gago-Dominguez M. Risk factors for cardiovascular disease in women: relationship to lipid peroxidation and oxidative stress. Med Hypotheses. 2008;71(1):39–44. doi: 10.1016/j.mehy.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Gonçalves TL, Erthal F, Corte CL, et al. Involvement of oxidative stress in the pre-malignant and malignant states of cervical cancer in women. Clin Biochem. 2005;38(12):1071–1075. doi: 10.1016/j.clinbiochem.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Flora SJ, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res. 2008;128(4):501–523. [PubMed] [Google Scholar]
- 6.Schwedhelm E, Maas R, Troost R, et al. Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. Clin Pharmacokinet. 2003;42(5):437–459. doi: 10.2165/00003088-200342050-00003. [DOI] [PubMed] [Google Scholar]
- 7.Yagi K. Female hormones act as natural antioxidants—a survey of our research. Acta Biochim Pol. 1997;44(4):701–709. [PubMed] [Google Scholar]
- 8.Yoshino K, Komura S, Watanabe I, et al. Effect of estrogens on serum and liver lipid peroxide levels in mice. J Clin Biochem Nutr. 1987;3(3):233–240. [Google Scholar]
- 9.Keaney JF, Jr, Shwaery GT, Xu A, et al. 17 Beta-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation. 1994;89(5):2251–2259. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Larrea MB, Leal AM, Liza M, et al. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59(6):383–388. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Larrea MB, Martín C, Martínez R, et al. Antioxidant activities of estrogens against aqueous and lipophilic radicals; differences between phenol and catechol estrogens. Chem Phys Lipids. 2000;105(2):179–188. doi: 10.1016/s0009-3084(00)00120-1. [DOI] [PubMed] [Google Scholar]
- 12.Huber LA, Scheffler E, Poll T, et al. 17 Beta-estradiol inhibits LDL oxidation and cholesteryl ester formation in cultured macrophages. Free Radic Res Commun. 1990;8(3):167–173. doi: 10.3109/10715769009087990. [DOI] [PubMed] [Google Scholar]
- 13.Subbiah MT, Kessel B, Agrawal M, et al. Antioxidant potential of specific estrogens on lipid peroxidation. J Clin Endocrinol Metab. 1993;77(4):1095–1097. doi: 10.1210/jcem.77.4.8408459. [DOI] [PubMed] [Google Scholar]
- 14.Mazière C, Auclair M, Ronveaux MF, et al. Estrogens inhibit copper and cell-mediated modification of low density lipoprotein. Atherosclerosis. 1991;89(2-3):175–182. doi: 10.1016/0021-9150(91)90058-b. [DOI] [PubMed] [Google Scholar]
- 15.Mense SM, Remotti F, Bhan A, et al. Estrogen-induced breast cancer: alterations in breast morphology and oxidative stress as a function of estrogen exposure. Toxicol Appl Pharmacol. 2008;232(1):78–85. doi: 10.1016/j.taap.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowers M, McConnell D, Jannausch ML, et al. Oestrogen metabolites in relation to isoprostanes as a measure of oxidative stress. Clin Endocrinol (Oxf) 2008;68(5):806–813. doi: 10.1111/j.1365-2265.2007.03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milne GL, Sanchez SC, Musiek ES, et al. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2(1):221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 18.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of Oxidative Stress Study II. Are oxidation products of lipids, proteins, and DNA markers of CCl4poisoning? Free Radic Biol Med. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Wactawski-Wende J, Schisterman EF, Hovey KM, et al. BioCycle Study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23(2):171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howards PP, Schisterman EF, Wactawski-Wende J, et al. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169(1):105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne RW, Bloom MS, Schisterman EF, et al. Analytical and biological variation of F2-isoprostanes during the menstrual cycle. Clin Chem. 2009;55(6):1245–1247. doi: 10.1373/clinchem.2008.122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- 23.Browne RW, Armstrong D. Methods in Molecular Biology. Vol 108. Totowa, NJ: Humana Press; 1998. Simultaneous determination of serum retinol, tocopherols, and carotenoids by HPLC; pp. 269–275. [DOI] [PubMed] [Google Scholar]
- 24.Chalmers AH, McWhinney BC. Two spectrophotometric methods compared for measuring low concentrations of ascorbate in plasma and urine. Clin Chem. 1986;32(7):1412–1413. [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 27.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 1974;3(1):69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaaks R, Rinaldi S, Key TJ, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European Prospective Investigation into Cancer and Nutrition. Endocr Relat Cancer. 2005;12(4):1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 31.Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 32.Park K, Gross M, Lee DH, et al. Oxidative stress and insulin resistance: the Coronary Artery Risk Development in Young Adults Study. Diabetes Care. 2009;32(7):1302–1307. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiPiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach, 2007. 7th ed. Columbus, OH: The McGraw-Hill Companies, Inc; 2007. [Google Scholar]
- 34.Gross M, Steffes M, Jacobs DR, Jr, et al. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clin Chem. 2005;51(1):125–131. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 35.Spiteller G. Linoleic acid peroxidation—the dominant lipid peroxidation process in low density lipoprotein—and its relationship to chronic diseases. Chem Phys Lipids. 1998;95(2):105–162. doi: 10.1016/s0009-3084(98)00091-7. [DOI] [PubMed] [Google Scholar]
- 36.Kato I, Ren J, Heilbrun LK, et al. Intra- and inter-individual variability in measurements of biomarkers for oxidative damage in vivo: Nutrition and Breast Health Study. Biomarkers. 2006;11(2):143–152. doi: 10.1080/13547500600565693. [DOI] [PubMed] [Google Scholar]
- 37.Comporti M, Signorini C, Arezzini B, et al. F2-isoprostanes are not just markers of oxidative stress. Free Radic Biol Med. 2008;44(3):247–256. doi: 10.1016/j.freeradbiomed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Rosing U, Olund A. Endometrial content of prostaglandin precursors during the menstrual cycle. Prostaglandins Leukot Essent Fatty Acids. 1989;36(3):195–198. doi: 10.1016/0952-3278(89)90062-8. [DOI] [PubMed] [Google Scholar]
- 39.Bonney RC. Measurement of phospholipase A2 activity in human endometrium during the menstrual cycle. J Endocrinol. 1985;107(2):183–189. doi: 10.1677/joe.0.1070183. [DOI] [PubMed] [Google Scholar]
- 40.Fraga CG, Tappel AL, Leibovitz BE, et al. Lability of red blood cell membranes to lipid peroxidation: application to humans fed polyunsaturated lipids. Lipids. 1990;25(2):111–114. doi: 10.1007/BF02562214. [DOI] [PubMed] [Google Scholar]