Abstract
BACKGROUND
Isoflavonoids (IFLs) may protect against chronic diseases including cancer. IFL exposure is traditionally measured from plasma but the reliability of urine is uncertain. We assessed whether IFL excretion in overnight urine (OU) or spot urine (SU) reliably reflects IFLs in plasma (PL) and the usefulness of the three matrices to determine soy intake compliance.
METHODS
In a randomized, double-blind, placebo-controlled soy intervention trial with 350 postmenopausal women, IFLs (daidzein, genistein, glycitein, equol, O-desmethylangolensin, dihydrodaidzein, dihydrogenistein) were analyzed by LCMS in OU, SU, and PL collected at baseline and every 6 months over 2.5 years.
RESULTS
High between-subjects intraclass correlations between all three matrices (median 0.94) and high between-subjects Pearson correlations (median rOU-PL=0.80; median rSU-PL=0.80; median rOU-SU=0.92) allowed the development of equations to predict IFL values from any of the three matrices. Equations developed from a randomly selected 87% of all available data were valid as high correlations were found on the residual 13% of data between equation-generated and measured IFL values (median rOU-PL=0.86; median rSU-PL=0.78; median rOU-SU=0.84); median absolute IFL differences for OU-PL, SU-PL, and OU-SU were 8.8 nM, 10.3 nM and 0.28 nmol/mg, respectively. All three matrices showed highly significant IFL differences between the placebo and soy intervention group at study end (P<0.0001) and highly significant correlations between IFL values and counted soy doses in the intervention group.
CONCLUSIONS
OU and SU IFL excretion reflect circulating PL IFL levels in healthy postmenopausal women accurately.
IMPACT
Noninvasively-collected urine can be used to reliably determine systemic IFL exposure and soy intake compliance.
Keywords: isoflavonoids, urine, plasma, compliance, soy, intervention, biomarker
Introduction
IFL exposure occurs predominantly through consumption of soy products that typically contain 0.01–0.3% IFLs composed mainly of glycosides of genistein (GE), daidzein (DE) and glycitein (GLYE) which are associated with the protein fraction of soy foods (1, 2). Isoflavonoids (IFLs) are suggested to protect against many chronic diseases including cancer, osteoporosis and cardiovascular disorders, as well as ameliorate menopausal symptoms (1, 2). The protective effect against breast cancer is particularly strong when soy consumption occurs at an early age (3–6). IFLs might play a role in this context because they have been found to be negatively correlated with breast (7–9) and prostate (10) cancer and can block pathways during carcinogenesis. Several studies have found urinary or plasma (PL) IFLs to be reliable biomarkers of soy consumption (1, 2). In biological fluids IFLs occur >80% as glucuronide and sulfate conjugates and their concentrations change markedly over time (11). Although urinary IFLs have been reported in a limited number of studies comprised of a few participants, they seem to accurately reflect circulating PL levels when timing of urine collection is considered accurately (12–15).
Given the invasiveness of venipuncture, our goal was to conclusively assess whether IFL excretion of overnight urine (OU) or spot urine (SU) reliably reflects IFLs in PL using repeated specimen collections in a large randomized, double-blind, placebo-controlled, soy intervention trial with 350 postmenopausal women. We also evaluated the usefulness of IFLs from SU and OU versus PL as compliance markers for soy consumption.
Materials and Methods
Study products
consisted of 18.3 gram beverage powder packets (providing 12.5 g of soy or 12.8 g of milk protein) and 66 gram bars (providing 13.6 g of soy or 14.6 g of milk protein). The soy products were made with isolated soy protein (partially hydrolyzed) that was selected and processed to maintain a high level of the naturally occurring isoflavones. The soy protein beverage powder and protein bar, respectively, contained the following isoflavone concentrations in mg/g soy protein including aglycons, glycosides, and glycoside esters (expressed as aglycon equivalents ; aglycons relative to total respective isoflavone based on weight in aglycon units): genistein, 3.43 and 3.28 (2.01 and 1.97; 11% for both ); daidzein, 2.48 and 2.22 (1.44 and 1.33; 19% and 18%); glycitein, 0.24 and 0.17 (0.14 and 0.11; 14% for both); total 6.15 and 5.67 (3.59 and 3.41; 14% for both), respectively. This isoflavone profile is very similar to soy foods habitually consumed by Asians (16). The placebo products were made with milk protein isolate (powder) or a combination of calcium sodium caseinate and whey protein concentrate (bars) and contained no isoflavones. Other macronutrients, minerals and vitamins were also kept at similar levels in all soy and milk protein products. Protein, isoflavone (soy products) and select vitamin and mineral content was determined by analytical testing in all production lots before release to assure that the products met the target levels for macro- and micro-nutrients. Products additionally met microbiological and sensory specifications during release testing.
Study Population
Eligible subjects were postmenopausal women with serum estradiol levels < 20 pg/ml participating in a single-center, randomized, double-blind, placebo-controlled trial (Women's Isoflavone Soy Health –WISH). 175 subjects were randomized to the soy protein and 175 to the milk protein matched placebo group (Table 1). One beverage or one bar was consumed in the morning and also in the evening daily during the 30 month study period.
Table 1.
Baseline demographics by treatment, WISH trial
| Variable | Placebo (n = 175) | Soy (n = 175) | P* |
|---|---|---|---|
| Age (n) | |||
| 40 – 54 | 32 (18%) | 35 (20%) | 0.95 |
| 55 – 59 | 47 (27%) | 41 (23%) | |
| 60 – 64 | 44 (25%) | 44 (25%) | |
| 65 – 69 | 32 (18%) | 35 (20%) | |
| ≥ 70 | 20 (12%) | 20 (12%) | |
| Age (y) | 60.9 ± 6.8 | 61.0 ± 7.4 | 0.93 |
| Race (n) | |||
| White (non-Hispanic) | 118 (67%) | 105 (60%) | 0.60 |
| Black (non-Hispanic) | 9 (5%) | 12 (7%) | |
| Hispanic | 24 (14%) | 31 (18%) | |
| Asian | 19 (11%) | 19 (11%) | |
| Other | 5 (3%) | 8 (5%) | |
| Education (n) | |||
| ≤ High school | 5 (3%) | 14 (8%) | 0.03 |
| > High school | 170 (97%) | 161 (92%) | |
| Smoking history (n) | |||
| Current | 5 (3%) | 3 (2%) | 0.51 |
| Former | 63 (36%) | 72 (41%) | |
| Never smoked | 107 (61%) | 100 (57%) | |
| Years smoked | |||
| among current or former smokers (n) | 16.7 ± 12.2 | 16.9 ± 11.9 | 0.85 |
| Weight (lbs) | 153 ± 32 | 152 ± 30 | 0.82 |
| Body mass index (kg/m2) | 26.7 ± 5.4 | 26.5 ± 5.0 | 0.76 |
| Pulse rate (b/min) | 66 ± 7 | 66 ± 7 | 0.38 |
| Systolic blood pressure (mm Hg) | 119 ± 14 | 117 ± 14 | 0.45 |
| Diastolic blood pressure (mm Hg) | 75 ± 9 | 75 ± 9 | 0.55 |
| Cholesterol (mg/dL) | 222 ± 31 | 219 ± 29 | 0.31 |
| Triglycerides (mg/dL) | 109 ± 56 | 112 ± 64 | 0.89 |
| HDL-cholesterol (mg/dL) | 63 ± 17 | 62 ± 16 | 0.57 |
| LDL-cholesterol (mg/dL) | 137 ± 30 | 134 ± 26 | 0.32 |
Mean ± SD, or n (%)
t-test was used for all comparisons, except for years smoked, triglycerides and plasma isoflavone, where Wilcoxon rank sum test was used.
Subject Follow-up and Sample Collection
Clinic visits occurred in the morning and OU collection began the previous night. Subjects emptied their bladders immediately before retiring and recorded the time of void, but did not collect the urine. Urine was collected and refrigerated throughout the night. Upon arising, the first-morning urine was collected and the time was recorded. SU was collected at the clinic visit at the time of the blood collection. The SU was typically the next urinary void following the first-morning urine collection. All specimens, obtained during a fasting state, were immediately processed and stored at − 80° C.
Follow-up clinical evaluations were conducted every month for the first 6 months following randomization and then every other month thereafter for a period of 2.5 years. At every clinic visit, data regarding dietary intake, product compliance, non-study and nutritional medications, clinical adverse events as well as vital signs were ascertained. Number of consumed soy protein packs and soy bars were counted at each visit when specimens were collected. EDTA blood and urine samples were collected every 6 months for 2.5 – 3 years.
Product Compliance
Number of protein powder packets and protein bars consumed was calculated at each visit by subtracting the numbers of packets and bars returned from the number distributed at the prior visit. Percent compliance at each visit was calculated as the numbers of packets and bars consumed since the last visit, divided by the number that should have been consumed.
Laboratory Assays
DE, GE, glycitein (GLYE), equol (EQ), dihydrodaidzein (DHDE), dihydrogenistein (DHGE), and O-desmethylangolensin (DMA) were analyzed from PL and urine by HPLC with isotope dilution electrospray ionization (negative mode) tandem mass spectrometry as described in detail (1, 2). Samples from each individual were run in one batch to limit variability. Between-day coefficients of variation ranged 4–18% for all analytes, while intra-day variation was half or less of that. All urinary IFL concentrations were adjusted for urinary creatinine concentrations and expressed in nanomoles per mg creatinine units. Urinary creatinine concentrations were measured with a Roche-Cobas MiraPlus chemistry autoanalyzer using a kit from Randox Laboratories (Crumlin, UK) that is based on a kinetic modification of the Jaffé reaction with a limit of quantitation of < 15 μM (1.7 mg/L) and mean inter-assay CV of 0.8% at 187 μM (21.1 mg/L). Creatinine-based urinary excretion was converted to time-based units using established conversion factors taking into account gender, body weight and age (17–19).
Statistical Analyses
All analyses used SAS 9.2 software (SAS Institute, Cary, NC). Because of non-normal distributions, all urine and PL IFL values were log-transformed. We assumed that the distributions of the 3 bivariate pairs (OU-PL, SU-PL, and OU-SU) were bivariate lognormal. Linear relations (reliabilities) of log-transformed OU and SU IFL values were assessed by correlating them with PL measures using between-subjects (20) Pearson correlations (association between two variables) and between-subjects intraclass correlations (ICC) (estimate of reliability across three or more variables) using means across time for each person (21). IFL values at baseline (prior to randomization) and end of the study in each matrix were also compared between active soy and placebo groups using Wilcoxon rank sum tests; this non-parametric approach was chosen for these comparisons due to their more conservative nature and their standard application for reporting of clinical trial data. To assess the association of IFL levels with product compliance, IFL values in each matrix were correlated with measures of percentage product compliance; these between-subjects correlations were computed in each treatment group separately.
We developed equations for predicting PL from urine values using linear regression analysis with the PL measure regressed onto a urine measure. Development of these models used a random 87% of available data. After back-transformation, these equations were of the form PLASMA [nM] = a * (URINE[nmol/mg])b (Table 2), where a = slope and b = exponent. Validity of the equations was evaluated by correlating and computing mean differences in estimated versus measured values of the residual 13% of data.
Table 2.
Isoflavonoid correlations and equation parameters for converting plasma, overnight urine, and spot urine values
| ICC* | OU vs. PL | SU vs. PL | OU vs. SU | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r† (MAD°) | a‡ (95% CI) | b‡ (95% CI) | rtest¶ | r† (MAD°) | a‡ (95% CI) | b‡ (95% CI) | rtest¶ | r† (MAD°) | a§ (95% CI) | b§ (95% CI) | rtest¶ | ||
| DE | 0.95 | 0.83 (16.4) | 9.33 (8.62–10.10) | 1.08 (1.04–1.12) | 0.87 | 0.83 (17.7) | 11.43 (10.56–12.37) | 1.15 (1.11–1.20) | 0.78 | 0.93 (0.81) | 0.97 (0.91–1.02) | 0.82 (0.79–0.84) | 0.84 |
| GE | 0.97 | 0.91 (27.8) | 9.49 (8.94–10.08) | 1.51 (1.47–1.56) | 0.93 | 0.89 (4.4) | 12.14 (11.33–13.03) | 1.54 (1.49–1.60) | 0.82 | 0.92 (0.39) | 0.93 (0.89–0.97) | 0.83 (0.80–0.85) | 0.81 |
| GLYE | 0.86 | 0.60 (2.6) | 4.85 (4.67–5.05) | 0.97 (0.90.–1.05) | 0.66 | 0.61 (2.0) | 6.44 (6.25–6.64) | 1.11 (1.03–1.20) | 0.67 | 0.82 (0.11) | 0.65 (0.62–0.67) | 0.79 (0.76–0.82) | 0.70 |
| EQ | 0.94 | 0.80 (4.4) | 5.97 (5.66–6.30) | 1.21 (1.16–1.26) | 0.86 | 0.79 (4.4) | 5.62 (5.29–5.96) | 1.16 (1.11–1.22) | 0.85 | 0.94 (0.04) | 1.02 (0.99–1.05) | 0.94 (0.92–0.97) | 0.93 |
| DMA | 0.95 | 0.83 (6.3) | 5.96 (5.53–6.42) | 1.31 (1.26–1.36) | 0.86 | 0.81 (8.1) | 6.63 (6.17–7.12) | 1.25 (1.20–1.31) | 0.82 | 0.93 (0.36) | 1.12 (1.07–1.17) | 0.88 (0.85–0.90) | 0.90 |
| DHDE | 0.93 | 0.76 (3.2) | 5.81 (5.41–6.23) | 1.07 (1.01–1.12) | 0.82 | 0.80 (3.5) | 5.81 (5.42–6.23) | 1.12 (1.07–1.17) | 0.78 | 0.92 (0.23) | 1.03 (0.98–1.08) | 0.85 (0.82–0.87) | 0.85 |
| DHGE | 0.93 | 0.78 (0.8) | 2.6 (2.47–2.73) | 1.26 (1.20–1.31) | 0.79 | 0.79 (1.0) | 2.69 (2.56–2.84) | 1.24 (1.18–1.30) | 0.77 | 0.90 (0.05) | 0.92 (0.88–0.95) | 0.85 (0.82–0.88) | 0.77 |
Based on log- transformed data comparing between subjects ; except for rtest all values are based on 87% of randomly selected data (1459 OU-PL, 1536 SU-PL, 1444 OU-SU pairs)
Intraclass correlation as reliability estimate across OU, SU, and PL (between subjects)
Pearson correlation coefficients between OU, SU, and PL pairs (between subjects)
Parameters are for the equation to convert urine values (nmol/mg) into plasma levels (nM) using 87% of available data; plasma = a * (urine)b
rtest = Pearson correlation coefficients of residual 13% of available data between predicted plasma levels obtained from converting urine values with developed equations and measured plasma levels
Parameters are for the equation to interconvert urine values (nmol/mg) using 87% of available data; spot urine = a * (overnight urine)b
Median absolute difference (OU-PL and SU-PL in nM; OU-SU in nmol/mg) of predicted versus measured IFL levels
Abbreviations: DE = daidzein, GE = genistein, GLYE = glycitein, EQ = equol, DMA = O-desmethylangolensin, DHDE = dihydrodaidzein, DHGE = dihydrogenistein ; OU = overnight urine (nmol/mg creatinine), PL = plasma (nM), SU = spot urine (nmol/mg creatinine); CI=confidence interval
Results
Demographic characteristics of the WISH cohort are summarized in Table 1. Collections from all three matrices totaled 5223 samples (1677 OU, 1763 SU, 1783 PL) providing 1677 OU-PL, 1763 SU-PL, and 1661 OU-SU and OU-SU-PL matched sets which were used for between-subjects correlation calculations after adjustment for within-person variation. Of 350 participants, 302 provided two or more samples.
Results for the overall log-transformed data showed excellent reliability between IFL measurements of the three matrices since most Pearson correlations were > 0.8 (range of correlations 0.60– 0.94, Table 2). The ICCs, as reliability estimates across all three measures (OU, SU, and PL), were even higher for all IFLs with a maximum observed for GE (ICC=0.97) and GE or DMA (ICC=0.95) and a minimum ICC for GLYE (ICC=0.86) using all data or a random 87%-fraction thereof (Table 2). This indicates that all three matrices (OU, SU, PL) can be utilized to assess the comparative IFL level. In comparison with all data combined the soy group showed very similar values which was true also for the placebo group however, the latter showed somewhat smaller (on average 0.18) PL-OU and PL-SU Pearson correlations but all relationships remained to be highly significant.
Because of these strong correlations, we determined equations for directly converting between any of the 3 matrices applying nmol/mg creatinine units for urine and nM values for PL using the log-transformed data. The equation (y=axb) developed for the conversions used a randomly selected 87% of all available data (1459 OU-PL, 1536 SU-PL, 1444 OU-SU pairs). Testing this model used the residual 13% of the data (Table 2). Overall results from the 87% dataset (median ICC=0.93; median rOU-PL = 0.78; median rSU-PL = 0.76) were almost unchanged relative to all data (Figure 1) Equations showed excellent validity since correlations between equation-based and measured values using the residual 13% of data showed high correlations (median rOU-PL = 0.86; median rSU-PL = 0.78). All correlations were highly significant (P < 0.00001) and on average median absolute IFL differences between predicted and measured values were small (OU-PL=8.8 nM, SU-PL=10.3 nM, OU-SU=0.28 nmol/mg) (Table 2).
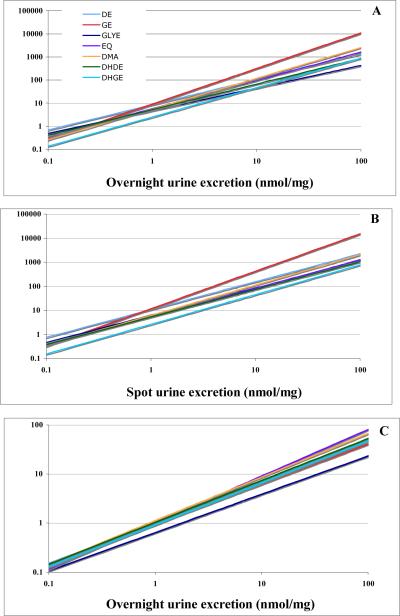
Figure 1. Relationship of urine excretion and plasma levels of isoflavonoids.
Log transformed data were used to establish conversions from OU into plasma (A) or from SU into plasma (B) based on the exponential equation Plasma = a * (Urine)t, and from OU into SU (C) based on the equation SU = a * (OU)t. Details of equations are listed in Table 2.
Creatinine-based urine excretion (nmol/mg) was converted into a time based unit (nmol/hour) by adjusting for body weight and age according to established procedures (17–19). The overall results provided similar and highly significant correlations (for example for GE rOU-PL = 0.80 and rSU-PL = 0.72; P <0.00001).
As further tests of reliability we stratified the data by visit and found again very similar reliability indices (DE, and GE showed standard deviations for ICCs, rOU-PL, and rSU-PL between 0.02 and 0.06 when each 6-month visit was evaluated; other results were similar but are not shown) suggesting that the time of observation did not have a substantial impact on these correlations.
The difference in IFL measurements between the placebo and soy intervention group at study end was highly significant (P < 0.001) in all three matrices except for EQ measured in OU (Table 3). Median IFLs were 5–9 fold higher in the intervention compared to the placebo group when determined in OU, SU, or PL, except for EQ (13–19 fold higher) and GLYE (3–4 fold higher). Medians of 93% and 94% of the instructed amounts were consumed in the placebo and intervention group, respectively, according to dose counts (see Methods). After adjustment for within-person variation between-subjects correlations between log IFL values and percentage of study product doses ideally consumed as determined from each specimen collection visit were again similar in all three matrices (Table 3). Correlation between IFL values and percentage of study product doses were of small to moderate magnitude and statistically significant for the intervention group but were not correlated in the placebo group.
Table 3.
Isoflavonoids in plasma and urine in the placebo and intervention groups and correlations with compliance to study product
| Isoflavone Values |
Isoflavone Values versus Stud Product Doses¶ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo |
Soy |
P-value* |
Placebo |
Soy |
||||||
| Baseline | End | Baseline | End | Baseline | End | r | P | r | P | |
| PL † | ||||||||||
| DE | 62 ± 131 | 79 ± 249 | 52 ± 113 | 400 ± 510 | 0.78 | <.0001 | 0.11 | 0.21 | 0.40 | <.0001 |
| GE | 63 ± 161 | 64 ± 151 | 53 ± 103 | 517 ± 577 | 0.11 | <.0001 | 0.04 | 0.62 | 0.44 | <.0001 |
| GLYE | 5.2 ± 12 | 8.4 ± 32 | 5.8 ± 11 | 16 ± 29 | 0.93 | <.0001 | 0.15 | 0.07 | 0.37 | <.0001 |
| EQ | 13 ± 55 | 25 ± 72 | 9.8 ± 24 | 129 ± 232 | 0.25 | 0.0008 | 0.03 | 0.70 | 0.17 | 0.03 |
| DMA | 22 ± 59 | 31 ± 64 | 23 ± 70 | 214 ± 283 | 0.70 | <.0001 | 0.13 | 0.11 | 0.31 | .0001 |
| DHDE | 23 ± 75 | 38 ± 184 | 18 ± 43 | 120 ± 224 | 0.76 | <.0001 | 0.10 | 0.23 | 0.30 | .0001 |
| DHGE | 6.8 ± 30 | 14 ± 100 | 5.8 ± 16 | 27 ± 88 | 0.71 | <.0001 | 0.08 | 0.31 | 0.21 | 0.009 |
| OU ‡ | ||||||||||
| DE | 4.2 ± 9.6 | 3.8 ± 9.2 | 3.7 ± 7.7 | 25 ± 25 | 0.45 | <.0001 | −0.03 | 0.74 | 0.46 | <.0001 |
| GE | 1.6 ± 5.7 | 1.5 ± 5.2 | 1.4 ± 3.0 | 13 ± 14 | 0.22 | <.0001 | 0.01 | 0.94 | 0.47 | <.0001 |
| GLYE | 0.6 ± 1.5 | 0.5 ± 1.2 | 0.5 ± 1.0 | 2.3 ± 2.6 | 0.34 | <.0001 | −0.04 | 0.64 | 0.45 | <.0001 |
| EQ | 0.9 ± 5.1 | 1.4 ± 6.0 | 0.5 ± 1.7 | 9.6 ± 18 | 0.77 | 0.21 | −0.12 | 0.13 | 0.16 | 0.05 |
| DMA | 1.4 ± 3.0 | 1.1 ± 3.0 | 1.4 ± 3.4 | 12 ± 13 | 0.57 | <.0001 | 0.00 | 0.96 | 0.34 | <.0001 |
| DHDE | 2.3 ± 6.9 | 1.7 ± 4.9 | 1.8 ± 4.0 | 11 ± 14 | 0.69 | <.0001 | 0.00 | 0.97 | 0.32 | .0001 |
| DHGE | 0.9 ± 4.6 | 0.6 ± 2.4 | 0.8 ± 2.8 | 3.9 ± 9.3 | 0.10 | <.0001 | 0.02 | 0.81 | 0.23 | 0.004 |
| SU § | ||||||||||
| DE | 3.3 ± 9.4 | 5 ± 15 | 3.0 ± 6.7 | 16 ± 20 | 0.36 | <.0001 | −0.10 | 0.22 | 0.47 | <.0001 |
| GE | 1.3 ± 3.8 | 2.7 ± 13 | 1.2 ± 3.0 | 8.0 ± 9.7 | 0.23 | <.0001 | −0.06 | 0.45 | 0.45 | <.0001 |
| GLYE | 0.4 ± 1.3 | 0.4 ± 1.0 | 0.4 ± 1.5 | 1.2 ± 1.6 | 0.51 | <.0001 | −0.05 | 0.52 | 0.40 | <.0001 |
| EQ | 0.6 ± 3.9 | 1.3 ± 6.3 | 0.6 ± 2.4 | 10 ± 21 | 0.25 | 0.02 | −0.11 | 0.19 | 0.17 | 0.03 |
| DMA | 1.5 ± 3.8 | 2.3 ± 6.9 | 1.7 ± 4.0 | 11 ± 12 | 0.61 | <.0001 | −0.03 | 0.69 | 0.35 | <.0001 |
| DHDE | 1.8 ± 4.9 | 3.2 ± 13 | 2.1 ± 4.8 | 9.7 ± 14 | 0.40 | <.0001 | −0.05 | 0.52 | 0.35 | <.0001 |
| DHGE | 0.6 ± 2.2 | 3.2 ± 22 | 0.9 ± 3.1 | 3.6 ± 12 | 0.10 | 0.001 | −0.06 | 0.50 | 0.24 | 0.002 |
Wilcoxon rank sum test comparing placebo to soy group
Plasma mean ± SD in nM; baseline n= 175 placebo+175 soy subjects; Study End n=136 placebo+143 soy subjects
Overnight urine mean ± SD in nmol/mg creatinine; baseline n= 172 placebo+170 soy subjects; Study End n=120 placebo+126 soy subjects
Spot urine mean ± SD in nmol/mg creatinine; baseline n= 174 placebo, 175 active; Study End n = 135 placebo, 143 soy subjects
Comparing inter-individually logged isoflavone values with percentage of ideally consumed soy protein doses (bars or protein packages) determined at each specimen collection visit; r=Pearson correlations; P = t-test value; 653 (PL), 618(OU, and 643 (SU) data pairs in the placebo group from 146 subjects; 721 (PL), 673 (OU) and 714 (SU) data pairs in the soy group from 154 subjects
Abbreviations: PL= plasma, OU=overnight urine, SU= spot urine, DE= daidzein, GE= genistein, GLYE= glycitein, EQ= equol, DMA= O-desmethylangolensin, DHDE= dihydrodaidzein, DHGE= dihydrogenistein
Discussion
Due to the predominant occurrence of isoflavonoids in biological fluids as conjugates (11) we determined the total of conjugated and unconjugated isoflavone levels in all matrices after enzymatic hydrolysis. In this sample of postmenopausal women, we found remarkably high ICCs (0.86–0.97; Table 2) among isoflavones measured across the three matrices. In addition, very high Pearson correlation coefficients (0.60–0.91; Table 2) between urine and PL values were observed, while the correlations between OU and SU were as expected, even higher an indication for urine values reliably reflecting circulating IFL levels. An equation developed from 87% of all data after log-transformation allowed prediction of PL values from urine values (Table 2). Resulting equations shown in Table 2 (y=axb) proved to be valid since the residual 13% of data showed excellent correlations between equation-based and measured values (mean rOU-PL = 0.83; mean rSU-PL = 0.78; mean rOU-SU=0.83) and low absolute differences (<10.3 nM). The selection of 87% and 13% of all data seemed appropriate to yield sufficient data for determining the algorithms and their evaluation, respectively. All correlations were highly significant (P <0.00001) and all comparable outcome measures similar to those from the entire dataset.
GE is more bioavailable and more concentrated in the circulation at a given urinary excretion than the other IFLs: by a factor of 3 versus DE, EQ, and DMA in contrast to factors of 5 versus DHDE and 7 versus DHGE and GLYE applying the developed algorithms. This is in excellent agreement with previous pharmacokinetic findings (2) as well as previous experimental results when one individual was followed over many time periods with repeated and strictly timed urine and blood collections (12).
Although SU was collected closer to the time of venipuncture, OU correlated on average as well with PL as SU (mean difference in correlations=0.01). Variabilities could have been caused by an inconsistent urine collection period (UCP) which is the period of time used to collect the entire bladder content starting with an empty bladder. or by varying time intervals before or after blood collection although this seems to have played a minor role in this study due to the collection of SU never more than 90–120 minutes before or after the blood draw. These variabilities would lead to inconsistent reflections of IFL exposure relative to the PL based determination. OU was collected over a relatively consistent period (ca. 7 hours) and a relatively consistent period of time between last urine collection and blood draw but did not lead to better OU-PL than SU-PL correlations. Food intake between the urine and blood collections could not have been a factor in this study since all specimens were collected consecutively and during a fasting state.
After converting creatinine-based excretion to time-based excretion, the preferred unit for urine, by adjusting for body weight and age (17–19), we did not find significant changes in our outcome measures. This could be due to the rather homogenous study population or to chance.
Since each IFL acts so differently, particularly GE versus the other IFLs (see above; Table 2), and with DE being quantitatively the predominant IFL in urine thereby masking effects of other IFLs, combining all individual IFLs for a total IFL value is relatively meaningless and was omitted here.
Numerous previous studies have investigated the association between urine and PL IFLs but most of these studies had a limited number subjects or repeat collections, and often with little time control. It is important to keep in mind that specimen timing needs to be kept consistent in order to obtain valid results since the UCP can change urinary values distinctly. Assuming an IFL elimination half life of 8 hours (reviewed in (2)) the urinary excretion rate (nmol/hour) decreases approximately by 4% per hour. Accordingly, excretion rates of urine collected over longer UCPs will be markedly smaller versus shorter UCPs. Since SU is lacking the UCP information considerable variability is introduced. Similarly, the period between completion of urine collection and blood draw introduces and error of the same magnitude. Not surprisingly strong PL-urine associations were therefore observed only in those previous studies, that considered timing accurately. A soy intervention among 12 female and 2 male subjects with time-controlled specimen collections reported a PL-24 hour urine correlation of rGE=0.99 (13), similar to rGE=0.91 from our strictly time-controlled, multi-time point intervention with one male subject (12) or our intervention among 8 male and 4 female health professionals (r=0.93, P<0.001) using area under curve values that integrate over the time domain always connected with urine collections (19). We also found rTotal IFLs=0.95 (P<0.001) between PL and SU in an intervention with one female participant whose 9 blood and SU collections were consistently timed (22). Again, excellent correlations between plasma and urine IFL values were reported when timing was considering appropriately (23). In contrast, 2 Japanese cross-sectional studies (n=90 men and women, n=106 women) with only one time point showed weak (albeit significant) correlations ranging 0.22–0.45 for DE and 0.32–0.50 for GE (24, 25). These lower correlations were likely due to PL and urine being collected at widely varying points in time (24), the ambiguous timing (absolute time of day and duration) of urine collections and interval between urine and PL collections (25). A British case control study examining the relationship between serum and SU adjusted for urinary creatinine concentration reported significant correlations (p=<0.001) with coefficients ranging from 0.63 for GLYE to 0.88 for DE (23). Interestingly, these correlations were based on single untimed samples from 284 subjects with low IFL exposure (3% of the population consumed soy foods and the average daily dietary intake for all subjects was 437 μg). However, it is important to note that these urine-PL correlations do not apply to renal failure patients whose main IFL excretion pathway through the kidney is partially or entirely blocked (26, 27).
We found OU, SU, and PL all to be equally suitable as a compliance marker matrix in this large soy intervention trial. All IFLs measured in the three matrices showed very similar quantitative differences: a 3–19 fold difference between placebo and soy group was found at the end of the study and significant correlations between IFL values and percent compliance to the ideal soy doses were found in the soy but as expected not in the placebo group (Table 3). All differences in IFL measurements at the end of the study were highly significant in all matrices except EQ in OU (P = 0.05) despite a 19-fold higher amount found in the soy versus placebo group. The lack of significance for this difference might be due to the variable EQ production inter- and intra-individually leading to large standard deviations resulting in large P-values.
Strengths of this study are foremost the very large number of participants, the long period of the study, the frequent sample collection from the same individuals, the state-of-the-art methodology, and the ability to examine the relationship of PL, OU and SU since all specimens were collected at the same time. However, this study was limited by observations restricted to postmenopausal women. This precludes generalization to other age groups and to men. On the other hand, this led to a very homogenous cohort avoiding many confounders.
Our findings indicate that IFLs in urine reflect circulating IFL levels. Although we investigated in our study exclusively postmenopausal women we believe that the presented PL-urine relationships holds true also for other gender and age groups after adjustment of creatinine concentrations (19) due to the good agreement of our results with previous studies that included men and younger participants. While the determination of blood levels is required to obtain detailed pharmacokinetic parameters urine is superior to PL for compliance measurement purposes during soy intervention studies due to easier collection and handling than PL at equal or superior functionality. Benefits of using urine include its noninvasiveness (particularly important for research in children), compared with venipuncture, as well as the ability to collect a concentrated matrix in large amounts leading to low quantitation limits. Urine can be self-obtained by participants without medical supervision, in privacy without the expense of a trained phlebotomist, and with minimal time, effort and biological hazard for participants and study staff. Most importantly, urine can be accumulated over many hours (even days) reflecting exposures over much longer time periods compared to data from blood, which only reflects one given point in time per collection or requires repeated invasive venipunctures. In addition, the accuracy of urine collection can be examined by comparing the measured urine creatinine amount with established body-weight dependent data for each gender and age group (19). Based on these benefits and our outcome results we believe that urine, including SU due to the logistic ease and overall effortlessness of collection, is a superior alternative to blood, especially as a matrix for compliance testing in soy intervention studies.
Acknowledgements
We thank Laurie Custer and Kerry Kakazu for the skillful performance of the isoflavonoid analyses by tandem LCMS. WISH was funded by the National Center of Complementary and Alternative Medicine and the Office of Dietary Supplements (U01AT001653). The NIH also provided instrumentation support (S10RR020890). Solae LLC (St. Louis, MO) provided the study products.
The National Institutes for Health provided instrumentation support through the National Center for Research Resources (S10-RR020890) and funded the WISH study through the National Center of Complementary and Alternative Medicine, the Office of Dietary Supplements and the Office of Research on Women's Health (U01AT001653).
Footnotes
No author has any conflict of interest
References
- 1.Franke AA, Halm BM, Kakazu K, Li X. Metabolism, Bioavailability, and Analysis of Dietary Isoflavones. In: Fraga C, editor. Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology. Wiley & Sons; 2009. [Google Scholar]
- 2.Franke AA, Halm BM, Kakazu K, Li X, Custer L. Phytoestrogenic isoflavonoids in epidemiologic and clinical research. Drug Testing and Analysis. 2009;1:14–21. doi: 10.1002/dta.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada) Cancer Causes Control. 2006;17:1253–61. doi: 10.1007/s10552-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 4.Korde LA, Wu AH, Fears T, et al. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18:1050–9. doi: 10.1158/1055-9965.EPI-08-0405. [DOI] [PubMed] [Google Scholar]
- 5.Shu XO, Jin F, Dai Q, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–8. [PubMed] [Google Scholar]
- 6.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian- Americans. Carcinogenesis. 2002;23:1491–6. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 7.Ingram D, Sanders K, Kolybaba M, Lopez D. Case-control study of phyto-oestrogens and breast cancer. Lancet. 1997;350:990–7. doi: 10.1016/S0140-6736(97)01339-1. [DOI] [PubMed] [Google Scholar]
- 8.Zheng W, Dai Q, Custer LJ, et al. Urinary excretion of isoflavones and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:35–40. [PubMed] [Google Scholar]
- 9.Dai Q, Franke AA, Jin F, et al. Urinary Excretion of Phytoestrogens and Risk of Breast Cancer among Chinese Women in Shanghai. Cancer Epidemiol Biomarkers Prev. 2002;11:815–21. [PubMed] [Google Scholar]
- 10.Park SY, Wilkens LR, Franke AA, et al. Urinary phytoestrogen excretion and prostate cancer risk: a nested case-control study in the Multiethnic Cohort. Br J Cancer. 2009;101:185–91. doi: 10.1038/sj.bjc.6605137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu L, House SE, Prior RL, et al. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr. 2006;136:1215–21. doi: 10.1093/jn/136.5.1215. [DOI] [PubMed] [Google Scholar]
- 12.Franke AA, Custer LJ, Hundahl SA. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutr Cancer. 2004;50(2):141–54. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
- 13.Ritchie MR, Morton MS, Deighton N, Blake A, Cummings JH. Plasma and urinary phyto-oestrogens as biomarkers of intake: validation by duplicate diet analysis. Br J Nutr. 2004;91:447–57. doi: 10.1079/BJN20031062. [DOI] [PubMed] [Google Scholar]
- 14.Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S. Isoflavones in breastfed infants after mothers consume soy. Am J Clin Nutr. 2006;84:406–13. doi: 10.1093/ajcn/84.1.406. [DOI] [PubMed] [Google Scholar]
- 15.Franke AA, Halm BM, Ashburn LA. Urinary isoflavones are increased in adults, but decreased in children, consuming soy when on oral antibiotic therapy. Nutrition and Cancer. 2008;60:627–35. doi: 10.1080/01635580802065310. [DOI] [PubMed] [Google Scholar]
- 16.Franke AA, Hankin JH, Yu MC, Maskarinec G, Low SH, Custer LJ. Isoflavone levels in soy foods consumed by multiethnic populations in Singapore and Hawaii. J Agric Food Chem. 1999;47:977–86. doi: 10.1021/jf9808832. [DOI] [PubMed] [Google Scholar]
- 17.Fomon SJ. Nutrition of normal infants. St. Louis; Mosby: 1993. [Google Scholar]
- 18.Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75:561–9. doi: 10.1093/ajcn/75.3.561. [DOI] [PubMed] [Google Scholar]
- 19.Franke A, Halm B, Ashburn L. Isoflavones In Children and Adults Consuming Soy. Arch Biochem Biophys. 2008;476:161–70. doi: 10.1016/j.abb.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 2--Correlation between subjects. BMJ. 1995;310:633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Hebshi S, Custer L, Franke A. The relation of soy intake and isoflavone levels in nipple aspirate fluid. European Journal of Cancer Prevention. 2008;17:67–70. doi: 10.1097/CEJ.0b013e3281108101. [DOI] [PubMed] [Google Scholar]
- 23.Grace PB, Taylor JI, Low YL, et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol Biomarkers Prev. 2004;13:698–708. [PubMed] [Google Scholar]
- 24.Yamamoto S, Sobue T, Sasaki S, et al. Validity and reproducibility of a self-administered food-frequency questionnaire to assess the isoflavone intake in a Japanese population in comparison with dietary records and blood and urine isoflavones. JNutr. 2001;131:2741–7. doi: 10.1093/jn/131.10.2741. [DOI] [PubMed] [Google Scholar]
- 25.Arai Y, Uehara M, Sato Y, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10:127–35. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- 26.Fanti P, Sawaya PB, Custer LJ, Franke AA. Serum levels and metabolic clearance of the isoflavones genistein and daidzein in hemodialysis patients. JAmSocNephrol. 1999;40:382–9. doi: 10.1681/ASN.V104864. [DOI] [PubMed] [Google Scholar]
- 27.Fanti P, Stephenson TJ, Kaariainen IM, et al. Soy food intake and serum levels of soy isoflavones in Japanese, Thai and North American end-stage renal failure patients on chronic hemodialyis therapy. Nephrology, Dialysis and Transplantation. 2003;18:1862–8. doi: 10.1093/ndt/gfg229. [DOI] [PubMed] [Google Scholar]