Abstract
Preface
Our title appears to be an oxymoron – surely “silenced” chromatin should not be transcribed! But there have been frequent reports of low level transcription in heterochromatic domains, and in Drosophila several hundred genes are found within heterochromatic regions1. Most striking, recent investigations implicate RNAi (in the broadest sense) in targeting and maintaining heterochromatin, and RNAi-based mechanisms are inherently dependent on transcription. While this might involve trans-acting sources of the critical small RNAs, in some cases transcription of the domain to be silenced appears to be required at least initially, a seeming contradiction.
At the first level of organization, the chromatin fiber is composed of generic nucleosome arrays, built using a small number of histones. However, great variety is achieved by a complex system of accessory proteins which modify, bind and reorganize histone complexes to produce different functional domains within the eukaryotic nucleus. Heterochromatin and euchromatin domains reflect different patterns of histone modification and are associated with different modes of nucleosome packaging2; presumably this is reflected in differences in higher order packaging3, 4 and nuclear organization (see Fraser and Bickmore, this issue).
Heterochromatin was initially defined as that portion of the genome that retains deep staining with DNA-specific dyes as the cell returns to interphase from metaphase. Subsequent investigation suggested a constellation of properties for heterochromatin (see Box 1). A link between heterochromatin formation and gene silencing has been inferred from the loss of most gene activity on the inactive X chromosome (the visibly condensed Barr body) in female mammals, and the loss of gene expression (correlated with condensed packaging) in position effect variegation (PEV) in Drosophila and other organisms. PEV results from the juxtaposition of a normally euchromatic gene with heterochromatin through rearrangement or transposition; the resulting variegating phenotype indicates that the gene has been silenced in a fraction of the cells in which it is normally active2. Reporters displaying PEV have a more regular nucleosome array, and (perhaps as a consequence) suffer a loss of the nuclease hypersensitive sites, presumed nucleosome-free regions generally associated with regulatory sequences of active or readily induced genes3, 5. Loss of hypersensitive sites is dependent on Heterochromatin Protein 1 (HP1)6. Studies in Schizosaccharomyces pombe (S. pombe) have shown that HP1 family proteins mediate recruitment/spreading of chromatin modifying factors, such as a histone deacetylase (HDAC)- and Snf2 ATPase-containing complex, SHREC. Such complexes presumably facilitate the nucleosome modification and positioning needed to organize the higher-order chromatin structures essential for heterochromatin functions, including transcriptional silencing, suppression of recombination, maintenance of long-range chromatin interactions, and successful chromosome segregation4, 7.
Box.
Properties of euchromatic and heterochromatic domains. Trying to define heterochromatin is like trying to define life itself: one can specify a cluster of important properties, but there are exceptions in every instance. For example, while centromeres are often associated with blocks of flanking heterochromatin, in Drosophila the inner centromere contains blocks of nucleosomes made with CENP-A, a histone H3 variant, interspersed with blocks of H3 nucleosomes showing a different modification pattern78. The Drosophila telomere elements are non-LTR retrotransposons, which are transcribed79, in contrast to the proximal TAS (telomere-associated sequences), which show more properties of heterochromatin. The characteristics given here are most consistently observed in pericentric heterochromatin, regions rich in remnants of transposable elements that flank the centromeres in the chromosomes of many eukaryotes. Note that we know very little about either the stoichiometry of HP1 or the folding of the chromatin fiber in heterochromatin; the sketch is meant to convey only HP1 association and condensation of the chromatin fiber.
An important characteristic of heterochromatin is the ability of this form of packaging to spread, as evidenced by the occurrence of PEV, and further demonstrated in S. pombe8 (see below). Once established, heterochromatin structures can be stably maintained through mitosis, and even meiosis, as exemplified by the clonal nature of the calico cat, where coat color reflects the choice of X chromosome for inactivation. The general properties of euchromatin, the gene-rich portions of the genome, are antagonistic to those of heterochromatin (see Box 1), although one anticipates much more variation in the histone modification state and disposition of the nucleosome array, depending on the transcription status of a given gene (see Berger, this issue). Indeed, increased expression of a variegating gene (embedded in heterochromatin) in Drosophila has been reported in response to increased amounts of a positive (GAL4) transcription factor, suggesting constant competition in establishing these alternative states9. In this review we will focus on recent findings on how heterochromatin formation is targeted and maintained in specific regions of the genome, examining in particular the potential role of transcription related to the RNAi system. We will draw primarily on results in fungi and animals; very interesting results in plants are reported by Henderson and Jacobson (this issue).
Heterochromatin assembly: Drosophila as a model system
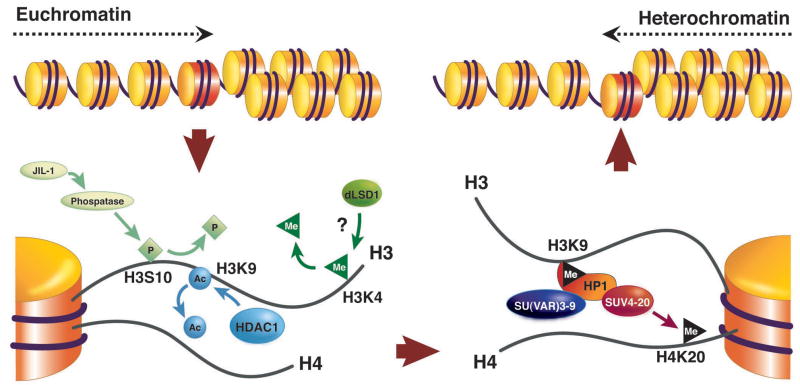
A key tool in investigating heterochromatin has been the ability to screen for suppressors of PEV, second site mutations that lead to loss of silencing at a variegating locus. About 15 such loci have been characterized in Drosophila melanogaster, and many more candidates have been identified10. The Su(var) genes typically code for either proteins that participate directly in the structure of heterochromatin, or enzymes that control changes in histone modification. To a first approximation, a transition between euchromatin and heterochromatin (as might occur in PEV) can be viewed as a series of reactions where the histone modifications and proteins associated with the active state are removed, and the histone modifications and proteins associated with the inactive state are added (Figure 1). A sequential set of reactions is required: e.g., lysine 9 of histone H3 (H3K9) cannot be methylated until it is de-acetylated; binding of SUV4-20 occurs through interaction with HP1 and requires SU(VAR)3-9 activity11. This no doubt contributes to the relative stability of the alternative packaging states. While the heterochromatic state can be inherited through mitotic and even meiotic cell divisions, a given site will switch from a repressed to an active chromatin state and vice versa at a low frequency. Of course, PEV cannot be scored as such in single cell organisms such as yeast, but this switching can be observed in the phenotype of sectors of a growing colony (Figure 2).
Figure 1.
Changes in histone modification implicated in the switch from a euchromatic to a heterochromatic state. Active genes are frequently marked by H3K4me (but see Berger, this issue); this mark must presumably be removed by LSD1 (not yet characterized in Drosophila). H3K9 is normally acetylated in euchromatin, and this mark must be removed by a histone deacetylase, typically HDAC1. Phosphorylation of H3S10 can interfere with methylation of H3K9; dephosphorylation may involved a phosphatase targeted through the carboxyl terminus of the JIL1 kinase10. These transitions set the stage for acquisition of the modifications associated with silencing, including methylation of H3K9 by SU(VAR)3-9 or another HMT, binding of HP1, and subsequent methylation of H4K20 by SUV4-20, an enzyme recruited by HP1. Other silencing marks, such as methylation of H3K27 by E(Z) (not shown), appear to be relevant in some domains, although this mark is more prominently used by the Polycomb system. Supporting data comes from genetic identification of modifiers of PEV, biochemical characterization of the activities of such modifiers, and tests of protein-protein interactions 9. (Adapted from10)
Figure 2.
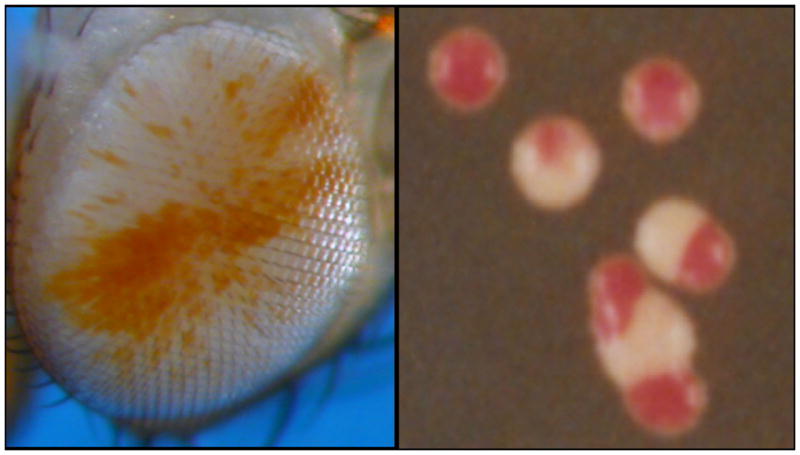
Variegating phenotypes. While alternative chromatin packaging states can be inherited, they do switch at a low frequency, resulting in a variegating phenotype within a clone of cells. Picture: on the left, a fly eye; on the right a sectored colony of yeast S. pombe
A small group of proteins are considered likely structural components of pericentric heterochromatin in flies because of the observed dosage response: while one copy of the encoding gene results in loss of silencing, three copies results in increased silencing, presumably as a consequence of mass action12. This set of proteins includes HP113, the initial chromo domain protein; HP2, a large protein with no conserved structural motifs14; SU(VAR)3-7, a zinc finger protein15; and SU(VAR)3-9, an H3K9-specific histone methyl transferase (HMT)16. However, no well-defined complex of heterochromatin proteins has been isolated (despite interactions of HP1 with all of these proteins, and others17), suggesting that an organized assembly exists only on the chromatin fiber. The small HP1 protein (206 amino acids in D. melanogaster) has two conserved domains, an N-terminal chromo domain and a C-terminal chromo shadow domain (CSD), separated by a short hinge (Figure 3). HP1 dimerizes through the CSD, forming a peptide-binding surface. HP1 interacts stably with SU(VAR)3-9 via its chromo shadow and hinge domains, and with the H3 N-terminal tail marked by di- or tri-methylated K9 (K9me2/3) through its chromo domain2. By interacting with both the modified histone and the modifying enzyme, HP1 can provide a foundation for a self-assembly and spreading mechanism, anticipated from observations of PEV (Figure 3) (see ref18 for review of possible spreading mechanisms). This core assembly appears to be conserved in animals and fungi19, 20 (see Table 1). We note that in many organisms there are multiple homologues of HP1, and multiple H3K9 HMT’s, suggesting the possibility of alternative assemblies19. However, in flies only HP1a (referred to simply as HP1 in this review) appears to be generally associated with known heterochromatic domains, and the ability of the other homologues to mimic HP1 in establishing heterochromatic packaging remains to be established.
Figure 3.
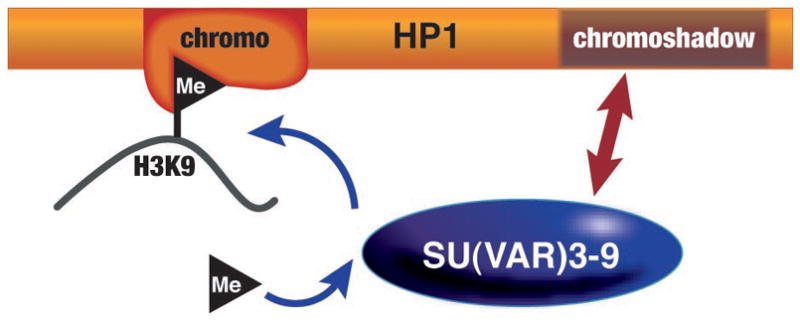
HP1 interacts with H3K9me2/3 through its chromo domain, and with SU(VAR)3-9 through its chromoshadow domain. (Adapted from ref 9.)
Table 1.
Factors implicated in heterochromatin formation: commonalities and differences in different systems. (Adapted from ref. 16.)
| Component | Organism | ||||
|---|---|---|---|---|---|
| S. pombe | Neurospora | Drosophila | Mouse | Arabidopsis | |
| Repetitive DNA | YES | YES | YES | YES | YES |
| DNA methylation | NO | YES | NO* | YES | YES |
| H3K9 methylation | YES | YES | YES | YES | YES |
| HP1a | YES | YES | YES | YES | NO* |
| Small RNAs | YES | NO* | YES | YES | YES |
| RNA Pol II | YES | N/D | N/D | N/D | N/D |
| RNA-dependent | |||||
| RNA polymerase | YES | NO* | NO | NO | YES |
YES indicates that the factor has been implicated in playing a role in heterochromatin formation in the given organism. NO indicates that the factor is not present in the organism. NO* indicates that the organism has the given component but that it does not appear to play a role in heterochromatin formation. N/D means that the organism has the component but a role for it in heterochromatin formation has not been demonstrated.
The role of RNAi: S. pombe as a model system
Genetic and biochemical studies using S. pombe as a model system have provided important insights into mechanisms of heterochromatin assembly. Many factors involved in heterochromatin formation in Drosophila and mammals are conserved in S. pombe19, 20. In particular, the evolutionarily conserved Clr4 protein – the fission yeast homolog of Drosophila SU(VAR)3-9 – present in a Cullin 4-containing E3 ubiquitin ligase complex, has been shown to specifically methylate H3K921–26. Methylated H3K9 serves as a binding site for recruitment of chromodomain proteins, including Chp1, Chp2 and Swi6 (the latter a homologue of Drosophila HP1) to heterochromatic loci22, 27–29. Mapping of heterochromatin factors, including H3 methylated at K9 and Swi6, has identified extended chromosomal domains coated with heterochromatin complexes at centromeres, telomeres and the mating type locus30. Interestingly, all three heterochromatic regions share a common feature – each of these domains contains dg/dh repeat elements that are preferential targets of heterochromatin formation8, 31–33. Recent investigations into mechanisms by which these repeat elements might trigger heterochromatin formation have led to the surprising discovery that the RNA interference (RNAi) system is involved in the nucleation and assembly of heterochromatin8, 33.
RNAi was first described as a posttranscriptional silencing mechanism in which double-stranded RNA (dsRNA) triggers destruction of cognate RNAs34. Subsequent studies have implicated RNAi-related mechanisms in diverse cellular functions. In S. pombe, mutations in factors involved in RNAi such as Dicer (Dcr1, an enzyme that cuts double-stranded RNA), Argonaute (Ago1, a PAZ/PIWI domain protein, capable of binding small RNAs), and Rdp1 (an RNA-dependent RNA polymerase) result in defects in heterochromatin assembly, as shown by loss of silencing at reporter loci8, 33. An “RNAi-induced initiation of transcriptional silencing” (RITS) complex, containing both a chromatin-associated protein and an RNAi-associated protein, has been identified35. RITS contains Chp1 (a chromo domain protein), Ago1, and a protein of unknown function, Tas3. In addition, RITS also contains siRNAs (small interfering RNAs) derived from the dg/dh repeats present at the different heterochromatic loci30, 35. Genome mapping analyses have shown that components of RITS and Rdp1 are distributed throughout heterochromatic domains in a pattern almost identical to the H3K9 methylation and Swi6 distributions30. Stable binding of RITS to chromatin depends at least in part on the binding of the Chp1 chromodomain to methylated H3K936. Deletion of Clr4, as well as a mutation in the Chp1 chromodomain, results in delocalization of RITS from heterochromatic loci. Interestingly, one sees concurrent defects in the processing of repeat transcripts into siRNAs 30, 36, suggesting that siRNAs are produced within a heterochromatin environment. RITS also recruits an RNA-directed RNA polymerase complex (RDRC) containing Rdp1; this RdRP activity is essential for siRNA production and heterochromatin assembly37, 38. The generation of siRNAs also requires an RNaseH-like RNA cleavage activity (Slicer) known to be associated with Argonaute family proteins, such as Ago1, found in RITS; mutations in conserved Ago1 residues that abolish this activity severely affect processing of repeat transcripts, and result in defects in heterochromatin assembly39, 40. The slicer function of Ago1 has been suggested to be important for spreading of heterochromatin39. It is also possible that siRNAs generated by Ago1-mediated processing of transcripts have a direct structural role in the assembly of higher-order structures, that in addition to mediating silencing facilitates local heterochromatin spreading. These mechanisms, however, cannot by themselves account for spreading of heterochromatin across large domains, as this requires HP1 proteins to serve as a platform for recruiting chromatin-modifying activities involved in heterochromatin assembly2, 8.
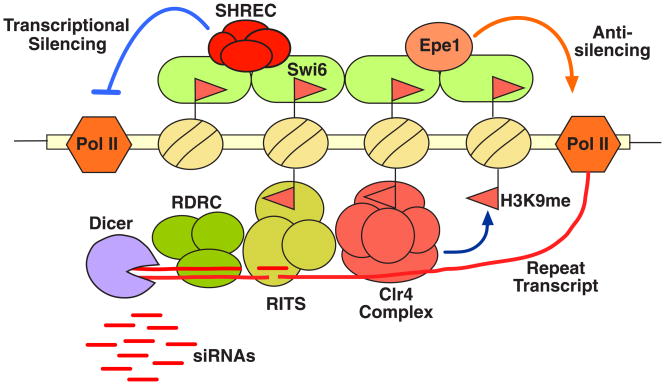
These findings suggest that RNAi-mediated heterochromatin assembly in S. pombe might occur via a self-reinforcing loop mechanism36, 37. In this model, siRNAs (possibly generated elsewhere) and /or DNA binding proteins mediate initial targeting of heterochromatin factors, leading to establishment of H3K9 methylation marks. The presence of methylated H3K9 and associated silencing factors in turn allows stable binding of RITS across heterochromatin domains (Figure 4). RITS presumably serves as a core for binding other RNAi factors, such as RDRC, essential for processing any transcripts of the dg/dh repeats. The siRNA-guided cleavage of nascent repeat transcripts by Ago1 (a component of RITS) is believed to be an important step in producing additional siRNAs. It is possible that cleaved transcripts serve as preferential targets for Rdp1, which generates double stranded RNAs, necessary for generation of short siRNAs by Dicer. siRNAs produced in cis can feed back to target additional heterochromatin complexes, but might also have additional functions (see below).
Figure 4.
Model showing RNAi-mediated heterochromatin assembly and silencing in S. pombe. Centromeric repeat (dg and dh) transcripts produced by RNA polymerase II are processed by the RNAi machinery, including RITS and RDRC effector complexes that interact with each other and localize across heterochromatic domains. The slicer activity of Ago1 (a component of RITS) and the RNA-dependent RNA polymerase activity of Rdp1 (a subunit of RDRC) are required for processing the repeat transcripts into siRNAs. siRNA-guided cleavage of nascent transcripts by Ago1 might make them preferential substrates for Rdp1 to generate dsRNA, which in turn is processed into siRNAs by Dicer. siRNAs are believed to mediate targeting of histone modifying activities including the Clr4 complex. This process most likely involves siRNA base pairing with nascent transcripts, but the precise mechanism remains undefined. siRNAs produced by heterochromatin-bound RNAi “factories” might also prime assembly of RISC-like complexes capable of mounting a classic RNAi response. Methylation of H3K9 by Clr4 is necessary for stable association of RITS with heterochromatic loci, apparently via binding of the Chp1 chromodomain. This methylation event also recruits Swi6, which along with other factors mediates spreading of various effectors such as SHREC. SHREC may facilitate proper positioning of nucleosomes to organize the higher-order chromatin structure essential for diverse heterochromatin functions, including transcriptional gene silencing. Swi6 also recruits an antisilencing protein, Epe1, that modulates heterochromatin to facilitate repeat transcription in addition to other functions. The system as a whole maintains a balance that results in loss of expression from reporter genes inserted within the domain.
The exact mechanism by which siRNAs target histone modifications is not clear. Binding of RITS to heterochromatic domains requires dg/dh siRNAs as part of the complex. It has been suggested that RITS, tethered to nascent transcripts via siRNAs, might mediate recruitment of histone methyltransferases such as Clr435, or that siRNAs directly facilitate recruitment of chromatin-modifying activities, such as a Clr4 complex, to heterochromatic repeats8, 23. It is certainly possible that siRNAs target heterochromatin by base pairing with nascent transcripts41; subunits of the RITS and RDRC complexes can be cross linked to noncoding centromeric repeat transcripts38. However, it is not known whether this binding simply reflects the roles of these factors in processing repeat transcripts, or whether it indicates an additional function in recruiting heterochromatin proteins. Artificial tethering of RITS to nascent transcripts has recently been shown to induce local heterochromatin assembly42. However, this process requires Dicer, presumably for production of siRNAs. Hence besides the targeting of RITS, additional siRNA-dependent steps are necessary for stable RNAi-mediated heterochromatin nucleation. The emerging view is that RNAi machinery, tethered to specific loci, though associations with heterochromatin components, helps process transcripts generated from these loci into siRNA, thus effectively causing posttranscriptional silencing in cis; but in addition, the siRNAs produced in this process facilitate additional targeting of heterochromatin modifications, such as H3K9 methylation. H3K9 methylation allows HP1 family proteins such as Swi6 to localize across heterochromatin domains; these factors in turn facilitate localization of diverse cellular activities such as SHREC. The HDAC and SNF2 ATPase activities of SHREC are critical for proper positioning of nucleosomes to achieve transcriptional gene silencing4. But how can transcription to produce siRNAs occur within a silenced domain?
Transcription of heterochromatic repeats
The results described above argue that heterochromatic repeat elements need to be transcribed to produce the siRNAs that target heterochromatin formation- indeed, a circular process! In support, recent studies have shown that heterochromatic repeats present in the S. pombe genome are transcribed by RNA polymerase II30, 43, and that mutations in Pol II impair RNAi-mediated heterochromatin assembly43, 44. An apparent paradox arises, however, in that heterochromatin in general is believed to be relatively inaccessible to factors involved in different aspects of DNA metabolism, including the transcriptional machinery2. How does Pol II gain access to sequences packaged as heterochromatin? Since heterochromatic silencing is thought to be plastic and can be overcome by increased concentration of transcription factors9, it can be argued that promoters driving the transcription of repeats, unlike promoters of euchromatic genes, have evolved to be somewhat impervious to heterochromatic repression. Indeed, one strand of centromeric repeats in S. pombe is always transcribed at a low level33 but is silenced posttranscriptionally via RNAi-mediated processing of transcripts33.
Transcription of repeats might be facilitated by a specialized mechanism(s) that modulates heterochromatin to provide access for factors involved in chromosomal transactions. Swi6/HP1 is believed to serve as an oscillator of heterochromatin transcription by directing recruitment of both silencing and anti-silencing factors20. In addition to factors such as SHREC that repress Pol II transcription4, Swi6/HP1 also recruits a JmjC domain-containing protein Epe140, which was identified in a screen for factors that negatively regulate heterochromatic silencing45. Epe1 facilitates Pol II transcription of repeats specifically in a heterochromatin context but does not appear to have an obligatory role in transcription per se; it is dispensable for transcription when heterochromatin is disrupted40. The mechanism by which Epe1 counteracts heterochromatic silencing is not known. Since several JmjC domain proteins have been shown to catalyze histone demethylation46, it is formally possible that Epe1 affects heterochromatin stability through removal of repressive lysine methylation marks. However, no such activity has been detected for Epe147. Epe1 may modulate chromatin via a yet undefined mechanism. Additional factors targeted to heterochromatic loci via Swi6/HP1 or other mechanisms are also likely important for heterochromatic transcription.
Apart from heterochromatin assembly, the transcription of repeat elements embedded within heterochromatin domains likely has other biological implications. It has been suggested that transcription of heterochromatic repeats may be necessary for continuous production of siRNAs to prime RISC-like complexes required to neutralize future invasions by similar sequences20. Heterochromatin-bound RNAi factors might be components of a memory mechanism that selectively generates a reservoir of siRNAs aimed against parasitic DNA elements. It should be noted that in S. pombe, RNAi machinery targeted to specific elements can spread to surrounding sequences, including nearby genes, via a process dependent on H3K9 methylation and Swi636. This might also allow the RNAi machinery to exert heritable control over expression of sequences located adjacent to repeat elements. Finally, the role of RNAi in destroying viral or transposable element transcripts is conserved in other systems, including Teterahymena and Drosophila48, 49.
RNAi in silencing of repetitious sequences in metazoans
While heterochromatin, built from repetitious DNA, has become an essential part of the eukaryotic chromosome, maintaining these repetitious sequences in a stable, silent form (repressing both transposition and recombination) is clearly a challenge and a necessity. Heterochromatin packaging once initiated is self re-enforcing through multiple feedback loops50. The RNAi machinery appears to be able to detect and respond to repetitious DNA in a variety of ways. To what extent might RNAi components be utilized in metazoans, either to initially target or to maintain silent domains, and to what extent might silencing of repetitious DNA be dependent on transcription in cis? The system described above is unlikely to be universally applicable, as many metazoans, including Drosophila and mammals, appear to lack canonical RNA-dependent RNA polymerase19.
Post-transcriptional gene silencing (PTGS) using RNAi, degrading or blocking translation of mRNA, is well established in all metazoans examined to date. The first suggestions of RNAi-based transcriptional gene silencing (TGS) in Drosophila came from work showing a loss of expression when multiple copies of a transgene are present51. Subsequent analysis showed suppression of PEV (a loss of silencing, monitored using tandem mini-white arrays and white transgenes in heterochromatin) by mutations in factors involved in the RNAi pathway, with decreased levels of H3K9me251–53. As in other organisms (notably plants; see review by Henderson and Jacobson, this issue), the RNAi system in D. melanogaster may have originated as a viral defense mechanism54, 55. About one-third of the genome is considered heterochromatic, and much of that DNA is made up of remnants of transposable elements, both DNA transposons and retroviruses. Drosophila has five PAZ/PIWI domain proteins, thought to bind small RNAs: PIWI, AUBERGINE (AUB), AGO-1, AGO-2, and AGO-3. PIWI is required for germ stem cell self-renewal, apparently playing a key role in silencing retrotransposons and blocking their mobilization in the germ line56. Both PIWI and AUB are found with siRNAs of 24–29 nts derived from repetitious sequences in the germline49, 57. In vitro PIWI exhibits RNA cleavage activity57, and it has been suggested that germline siRNA may be produced by a unique processing mechanism, dependent on cleavage from long single strand transcripts rather than dsRNA49. How this silencing activity might impact heterochromatin formation in somatic chromosomes (if at all) is not clear at present.
The impacts of mutations in ago-2 are clearly seen in the early Drosophila embryo as defects in chromosome condensation, nuclear kinesis, and spindle assembly, all potentially correlated with defects in the formation of centric heterochromatin58. Similar defects are observed on failure of heterochromatin formation in S. pombe and other systems59–61. In flies with mutations in the genes for SU(VAR)3-9 (one of three known H3K9 methyltransferases), HP1, or Dicer 2, cells display disorganized nucleoli as well as disorganized centric heterochromatin. Under these circumstances, there is a substantial increase in extrachromosomal repetitious DNA in mutant tissues62. Similarly, mutations in the RNAi machinery in S. pombe have also been found to result in defects in maintaining chromosome integrity, including high rates of recombination at rDNA loci30. Thus while repetitious DNA now contributes to essential chromosome structures, it is critical to maintain that DNA specifically in a heterochromatic form, and genetic analysis indicates that the RNAi system plays a role. In the absence of any recognizable RdRP activity, this might be accomplished by targeting heterochromatin formation to specific sites either by DNA-protein interactions or by an RNAi-based recognition system, followed by spreading of the heterochromatin modifications and structure. As in S. pombe, spreading of heterochromatin in D. melanogaster (as monitored by PEV) is dependent on HP1 and on SU(VAR)3-9.
Targeting heterochromatin formation
While much of our discussion focuses on RNAi-based mechanisms, it is important to note that heterochromatin proteins can be recruited to specific sites (‘silencers’) by DNA binding factors. For example, in addition to the RNAi-mediated targeting of heterochromatin to a dg/dh-like repeat element located in the silent mating-type region of S. pombe, the DNA binding proteins Atf1/Pcr1 (belonging to the ATF/CREB family) have been shown to cooperate with components of SHREC to independently nucleate heterochromatin assembly in this domain7, 63, 64. Similarly, redundant mechanisms of heterochromatin nucleation also operate at telomeres in S. pombe, where the TRF family DNA binding protein Taz1, in conjunction with Ccq1, acts in parallel to the RNAi machinery to nucleate heterochromatin4, 32. Regardless of nucleation mechanism, however, heterochromatin targeted to specific sites is able to spread and provides a sequence-independent platform for recruitment of appropriate cellular activities (such as SHREC, the RNAi machinery and cohesin) across large domains20.
In several cases documented in mammalian cells, HP1 can be targeted to specific promoters by interaction with DNA binding complexes, and apparently contributes to silencing at these loci (see ref.65 for an example). However, in these cases a different HMT appears to be responsible for the accompanying H3K9 methylation, and spreading is generally not observed. This suggests that the interactions of HP1 with both the modified histone (H3K9me3) and the modifying enzyme [usually SU(VAR)3-9] are critical for heterochromatin spreading (Fig. 3). Repetitious DNA is a hallmark of heterochromatin. In the case of satellite DNA (simple sequence tandem repeats), one might suggest that a specific DNA binding protein could recognize that particular DNA sequence to trigger heterochromatin assembly. In Drosophila the D1 protein, which has a mutant Su(var) phenotype, binds AT-rich satellite III preferentially66, 67. Similarly, the heterochromatin-associated protein DDP1 binds to a conserved dodeca-satellite sequence; however, this protein, with 15 tandemly organized KH domains, binds strongly to single-stranded nucleic acids with this sequence, including RNA68. Recent work has shown that DDP1, which also has a mutant Su(var) phenotype, plays a critical role in HP1 and methylated H3K9 deposition at chromocenter heterochromatin68. Given the ability of DDP1 (and its mammalian homologues, the vigilins) to bind RNA, it is possible that RNA mediates this interaction. Aside from the blocks of satellite DNA, repetitious sequences in Drosophila (primarily remnants of transposable elements and DNA transposons), are very diverse. Consequently, a recognition process based on RNA (rather than specific protein binding) appears most parsimonious, and this sugestion has been supported by studies on the heterochromatic D. melanogaster fourth chromosome.
The small fourth chromosome of D. melanogaster is considered entirely heterochromatic by the criteria cited above (see Box), but has 88 genes in the distal 1.2 Mb. Mapping with a white reporter transgene has shown interspersed heterochromatic domains (inducing a variegating phenotype) and euchromatic domains (allowing expression giving a full red eye). Detailed examination of the region around the Hcf gene identified the 1360 element, remnants of a DNA transposon, as a potential site for heterochromatin initiation; reporters lying within 10 kb of a 1360 element showed a variegating phenotype, while those further away showed a red eye69. A direct test, using a P transposon carrying one copy of 1360 upstream of a white reporter, demonstrated that the 1360 element contributes to silencing, as silencing of the reporter is largely lost when the adjacent 1360 is deleted. However, stable heterochromatin (resulting in a variegating phenotype) is only observed when that P element is located in a region close to the centromere, indicating a requirement for a high local repeat density and/or proximity to the pericentric heterochromatin, where HP1 is most abundant. Genetic analysis indicates that this silencing is dependent not only on HP1 and SU(VAR)3-9, but also on RNAi components, notably AUB53. Whether or not transcription occurs at this 1360 element is unknown. Small RNAs have been recovered from 1360 and from about 40 other Drosophila transposable elements70. 1360 is probably not the only TE targeted for heterochromatin formation. However, it appears unlikely that all transposable element remnants are targets, given the mapping results obtained on chromosome four with the white reporter69. The critical characteristics of targets are as yet unknown, but could include the presence of start sites for transcription53. Many 1360 remnants have a sequence known to function as a promoter in the multi-copy Su[Ste] locus, where they generate inverse transcripts used in suppression of the multi-copy Stellate gene71. These results suggest that remnants of trasposable elements could be targeted for silencing by a mechanism using a small RNA, and that transcription of some such elements might be involved.
Concluding remarks
Eukaryotes that tolerate high levels of repetitious sequences in their genomes generally have both the RNAi machinery and the enzymes and structural proteins necessary to generate a heterochromatin structure based on H3K9 methylation. While some features of the RNAi system (such as RdRP) and some features of the heterochromatin structure (such as DNA methylation) are used in only a subset of the metazoans, this key shift in histone modification from euchromatin to heterochromatin appears to be universal (see Table 1). The RNAi system per se can limit gene expression through post transcriptional gene silencing, and hence eliminate some sources of damage from invading repetitious elements, but by itself cannot generate the compact chromatin structures required to maintain chromosome integrity and mitotic chromosome function. Hence the suggestion that PTGS is sufficient to explain the silencing of repetitious elements seems quite unlikely. A model in which RNAi components play a key role in generating siRNAs involved in targeting chromatin components (H3K9 HMTs and HP1) to silence repetitious DNA remains most attractive, given the additional understanding of a delicate balance between the need for expression and the need for silencing described above.
While an assembly of heterochromatin structure based on binding of HP1 proteins to methylated H3K9 marks provides a foundation for spreading, the molecular mechanisms by which heterochromatin exerts long-range repressive effects are not fully understood. The oligomerization of chromatin-bound Swi6/HP1 via the chromoshadow domains might mediate condensation. However, recent evidence suggests that Swi6/HP1 binding is dynamic72, 73. An alternative emerging view is that HP1 proteins facilitate recruitment of other heterochromatin components responsible for silencing. Indeed, as described above, HP1 family proteins mediate preferential binding of SHREC, which includes HDAC activity4. Deacetylation of histones, which is a universal property of heterochromatic domains, may result in lower affinity of transcription factors for target loci or could be critical for higher-order packaging of nucleosomes, both contributing to silencing. The HP1/H3K9 methylation system may use several routes to minimize H3K9 acetylation, a key characteristic of the active state.
Evidence from different systems suggests that once triggered, a repressive chromatin structure can be sustained over many generations. In fission yeast, heterochromatin structures established by RNAi and/or DNA binding factors are inherited in cis for many generations in a manner dependent upon Swi6/HP1 and histone modifying activities8, 74. Moreover, a recent study in C. elegans, an organism known to silence repetitious DNA using RNAi and chromatin factors75, 76, showed that a single exposure to RNAi resulted in dominant silencing of a reporter gene in about 30% of the progeny for many generations77. A screen for mutations that affected the maintenance of silencing identified four essential genes: hda-4 (a histone deacetylase), K03D10.3 (a histone acetyltransferase), isw-1 (a homologue of the chromatin-remodeling ISW1), and mrg-1 (a chromo-domain protein). Coupled with the observation that trichostatin A, a histone deacetylase inhibitor, relieves silencing, the results argue that maintenance of silencing is a consequence of heterochromatin formation, heritable even in the absence of the initial RNAi stimulus77. While much remains to be learned about the mechanisms involved, it is clear that the proper interplay of the RNAi and heterochromatin systems is critical for the maintenance and function of our genomes.
Supplementary Material
Acknowledgments
We thank members of our laboratory groups for critical reading of the manuscript, and Gabriella Farkas for design of figures 1 and 3. Work in the authors laboratories is supported by grants from the NIH (SCRE) or NIH intramural support (SISG).
Contributor Information
Shiv S. Grewal, National Cancer Institute, NIH
Sarah C R Elgin, Department of Biology, Washington University in St. Louis.
References
- 1.Yasuhara JC, Wakimoto BT. Oxymoron no more: the expanding world of heterochromatic genes. Trends Genet. 2006;22:330–8. doi: 10.1016/j.tig.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Grewal SI, Elgin SC. Heterochromatin: new possibilities for the inheritance of structure. Curr Opin Genet Dev. 2002;12:178–87. doi: 10.1016/s0959-437x(02)00284-8. [DOI] [PubMed] [Google Scholar]
- 3.Sun FL, Cuaycong MH, Elgin SC. Long-range nucleosome ordering is associated with gene silencing in Drosophila melanogaster pericentric heterochromatin. Mol Cell Biol. 2001;21:2867–79. doi: 10.1128/MCB.21.8.2867-2879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugiyama T, et al. SHREC, an Effector Complex for Heterochromatic Transcriptional Silencing. Cell. 2007;128:491–504. doi: 10.1016/j.cell.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Wallrath LL, Elgin SC. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 1995;9:1263–77. doi: 10.1101/gad.9.10.1263. [DOI] [PubMed] [Google Scholar]
- 6.Cryderman DE, Tang H, Bell C, Gilmour DS, Wallrath LL. Heterochromatic silencing of Drosophila heat shock genes acts at the level of promoter potentiation. Nucleic Acids Res. 1999;27:3364–70. doi: 10.1093/nar/27.16.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada T, Fischle W, Sugiyama T, Allis CD, Grewal SI. The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol Cell. 2005;20:173–85. doi: 10.1016/j.molcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Hall IM, et al. Establishment and maintenance of a heterochromatin domain. Science. 2002;297:2232–7. doi: 10.1126/science.1076466. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad K, Henikoff S. Modulation of a transcription factor counteracts heterochromatic gene silencing in Drosophila. Cell. 2001;104:839–47. doi: 10.1016/s0092-8674(01)00281-1. [DOI] [PubMed] [Google Scholar]
- 10.Elgin SCR, Reuter G. In: Epigenetics. Allis CD, Jenuwein T, Reinberg R, editors. Cold Spring Harbor Laboratory Press; New York: 2006. in press. [Google Scholar]
- 11.Schotta G, et al. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18:1251–62. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke J, Kotarski MA, Tartof KD. Dosage-dependent modifiers of position effect variegation in Drosophila and a mass action model that explains their effect. Genetics. 1988;120:181–98. doi: 10.1093/genetics/120.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eissenberg JC, et al. Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1990;87:9923–7. doi: 10.1073/pnas.87.24.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaffer CD, et al. Heterochromatin protein 2 (HP2), a partner of HP1 in Drosophila heterochromatin. Proc Natl Acad Sci U S A. 2002;99:14332–7. doi: 10.1073/pnas.212458899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuter G, et al. Dependence of position-effect variegation in Drosophila on dose of a gene encoding an unusual zinc-finger protein. Nature. 1990;344:219–23. doi: 10.1038/344219a0. [DOI] [PubMed] [Google Scholar]
- 16.Tschiersch B, et al. The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3–9 combines domains of antagonistic regulators of homeotic gene complexes. Embo J. 1994;13:3822–31. doi: 10.1002/j.1460-2075.1994.tb06693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greil F, de Wit E, Bussemaker HJ, van Steensel B. HP1 controls genomic targeting of four novel heterochromatin proteins in Drosophila. Embo J. 2007;26:741–51. doi: 10.1038/sj.emboj.7601527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talbert PB, Henikoff S. Spreading of silent chromatin: inaction at a distance. Nat Rev Genet. 2006;7:793–803. doi: 10.1038/nrg1920. [DOI] [PubMed] [Google Scholar]
- 19.Huisinga KL, Brower-Toland B, Elgin SC. The contradictory definitions of heterochromatin: transcription and silencing. Chromosoma. 2006;115:110–22. doi: 10.1007/s00412-006-0052-x. [DOI] [PubMed] [Google Scholar]
- 20.Grewal SI, Jia S. Heterochromatin revisited. Nat Rev Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- 21.Rea S, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–9. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–3. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 23.Jia S, Kobayashi R, Grewal SI. Ubiquitin ligase component Cul4 associates with Clr4 histone methyltransferase to assemble heterochromatin. Nat Cell Biol. 2005;7:1007–13. doi: 10.1038/ncb1300. [DOI] [PubMed] [Google Scholar]
- 24.Hong EJE, Villen J, Gerace EL, Gygi S, Moazed D. A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biology. 2005;2:106–111. doi: 10.4161/rna.2.3.2131. [DOI] [PubMed] [Google Scholar]
- 25.Horn PJ, Bastie JN, Peterson CL. A Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev. 2005;19:1705–14. doi: 10.1101/gad.1328005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thon G, et al. The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe. Genetics. 2005;171:1583–95. doi: 10.1534/genetics.105.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannister AJ, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–4. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 28.Partridge JF, Scott KS, Bannister AJ, Kouzarides T, Allshire RC. cis-acting DNA from fission yeast centromeres mediates histone H3 methylation and recruitment of silencing factors and cohesin to an ectopic site. Curr Biol. 2002;12:1652–60. doi: 10.1016/s0960-9822(02)01177-6. [DOI] [PubMed] [Google Scholar]
- 29.Sadaie M, Iida T, Urano T, Nakayama J. A chromodomain protein, Chp1, is required for the establishment of heterochromatin in fission yeast. Embo J. 2004;23:3825–35. doi: 10.1038/sj.emboj.7600401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cam HP, et al. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat Genet. 2005;37:809–19. doi: 10.1038/ng1602. [DOI] [PubMed] [Google Scholar]
- 31.Grewal SI, Klar AJ. A recombinationally repressed region between mat2 and mat3 loci shares homology to centromeric repeats and regulates directionality of mating-type switching in fission yeast. Genetics. 1997;146:1221–38. doi: 10.1093/genetics/146.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanoh J, Sadaie M, Urano T, Ishikawa F. Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr Biol. 2005;15:1808–19. doi: 10.1016/j.cub.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 33.Volpe TA, et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–7. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 34.Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 35.Verdel A, et al. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–6. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noma K, et al. RITS acts in cis to promote RNA interference-mediated transcriptional and post-transcriptional silencing. Nat Genet. 2004;36:1174–80. doi: 10.1038/ng1452. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama T, Cam H, Verdel A, Moazed D, Grewal SI. RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc Natl Acad Sci U S A. 2005;102:152–7. doi: 10.1073/pnas.0407641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motamedi MR, et al. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell. 2004;119:789–802. doi: 10.1016/j.cell.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 39.Irvine DV, et al. Argonaute slicing is required for heterochromatic silencing and spreading. Science. 2006;313:1134–7. doi: 10.1126/science.1128813. [DOI] [PubMed] [Google Scholar]
- 40.Zofall M, Grewal SI. Swi6/HP1 recruits a JmjC domain protein to facilitate transcription of heterochromatic repeats. Mol Cell. 2006;22:681–92. doi: 10.1016/j.molcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Grewal SI, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301:798–802. doi: 10.1126/science.1086887. [DOI] [PubMed] [Google Scholar]
- 42.Buhler M, Verdel A, Moazed D. Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell. 2006;125:873–86. doi: 10.1016/j.cell.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 43.Djupedal I, et al. RNA Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes Dev. 2005;19:2301–6. doi: 10.1101/gad.344205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato H, et al. RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science. 2005;309:467–9. doi: 10.1126/science.1114955. [DOI] [PubMed] [Google Scholar]
- 45.Ayoub N, et al. A novel jmjC domain protein modulates heterochromatization in fission yeast. Mol Cell Biol. 2003;23:4356–70. doi: 10.1128/MCB.23.12.4356-4370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–27. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 47.Tsukada Y, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–6. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 48.Mochizuki K, Gorovsky MA. Small RNAs in genome rearrangement in Tetrahymena. Curr Opin Genet Dev. 2004;14:181–7. doi: 10.1016/j.gde.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Vagin VV, et al. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–4. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 50.Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell. 2002;108:489–500. doi: 10.1016/s0092-8674(02)00644-x. [DOI] [PubMed] [Google Scholar]
- 51.Pal-Bhadra M, Bhadra U, Birchler JA. RNAi related mechanisms affect both transcriptional and posttranscriptional transgene silencing in Drosophila. Mol Cell. 2002;9:315–27. doi: 10.1016/s1097-2765(02)00440-9. [DOI] [PubMed] [Google Scholar]
- 52.Pal-Bhadra M, et al. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–72. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- 53.Haynes KA, Caudy AA, Collins L, Elgin SC. Element 1360 and RNAi Components Contribute to HP1-Dependent Silencing of a Pericentric Reporter. Curr Biol. 2006;16:2222–7. doi: 10.1016/j.cub.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Rij RP, et al. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–95. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang XH, et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–4. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalmykova AI, Klenov MS, Gvozdev VA. Argonaute protein PIWI controls mobilization of retrotransposons in the Drosophila male germline. Nucleic Acids Res. 2005;33:2052–9. doi: 10.1093/nar/gki323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito K, et al. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 2006;20:2214–22. doi: 10.1101/gad.1454806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deshpande G, Calhoun G, Schedl P. Drosophila argonaute-2 is required early in embryogenesis for the assembly of centric/centromeric heterochromatin, nuclear division, nuclear migration, and germ-cell formation. Genes Dev. 2005;19:1680–5. doi: 10.1101/gad.1316805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–33. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- 60.Fukagawa T, et al. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat Cell Biol. 2004;6:784–91. doi: 10.1038/ncb1155. [DOI] [PubMed] [Google Scholar]
- 61.Hall IM, Noma K, Grewal SI. RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast. Proc Natl Acad Sci U S A. 2003;100:193–8. doi: 10.1073/pnas.232688099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng JC, Karpen GH. H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat Cell Biol. 2007;9:25–35. doi: 10.1038/ncb1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jia S, Noma K, Grewal SI. RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science. 2004;304:1971–6. doi: 10.1126/science.1099035. [DOI] [PubMed] [Google Scholar]
- 64.Kim HS, Choi ES, Shin JA, Jang YK, Park SD. Regulation of Swi6/HP1-dependent heterochromatin assembly by cooperation of components of the mitogen-activated protein kinase pathway and a histone deacetylase Clr6. J Biol Chem. 2004;279:42850–9. doi: 10.1074/jbc.M407259200. [DOI] [PubMed] [Google Scholar]
- 65.Schultz D, Ayyanathan K, Negorev D, Maul G, Rauscher FR. SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev. 2002;16:1855–69. doi: 10.1101/gad.973302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aulner N, et al. The AT-hook protein D1 is essential for Drosophila melanogaster development and is implicated in position-effect variegation. Mol Cell Biol. 2002;22:1218–32. doi: 10.1128/MCB.22.4.1218-1232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blattes R, et al. Displacement of D1, HP1 and topoisomerase II from satellite heterochromatin by a specific polyamide. Embo J. 2006;25:2397–408. doi: 10.1038/sj.emboj.7601125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huertas D, Cortes A, Casanova J, Azorin F. Drosophila DDP1, a multi-KH-domain protein, contributes to centromeric silencing and chromosome segregation. Curr Biol. 2004;14:1611–20. doi: 10.1016/j.cub.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 69.Sun FL, et al. cis-Acting determinants of heterochromatin formation on Drosophila melanogaster chromosome four. Mol Cell Biol. 2004;24:8210–20. doi: 10.1128/MCB.24.18.8210-8220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aravin AA, et al. The small RNA profile during Drosophila melanogaster development. Dev Cell. 2003;5:337–50. doi: 10.1016/s1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- 71.Aravin AA, et al. Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr Biol. 2001;11:1017–27. doi: 10.1016/s0960-9822(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 72.Cheutin T, et al. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299:721–5. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- 73.Festenstein R, et al. Modulation of heterochromatin protein 1 dynamics in primary Mammalian cells. Science. 2003;299:719–21. doi: 10.1126/science.1078694. [DOI] [PubMed] [Google Scholar]
- 74.Nakayama J, Klar AJ, Grewal SI. A chromodomain protein, Swi6, performs imprinting functions in fission yeast during mitosis and meiosis. Cell. 2000;101:307–17. doi: 10.1016/s0092-8674(00)80840-5. [DOI] [PubMed] [Google Scholar]
- 75.Grishok A, Sinskey JL, Sharp PA. Transcriptional silencing of a transgene by RNAi in the soma of C. elegans. Genes Dev. 2005;19:683–96. doi: 10.1101/gad.1247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robert VJ, Sijen T, van Wolfswinkel J, Plasterk RH. Chromatin and RNAi factors protect the C. elegans germline against repetitive sequences. Genes Dev. 2005;19:782–7. doi: 10.1101/gad.332305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vastenhouw NL, et al. Gene expression: long-term gene silencing by RNAi. Nature. 2006;442:882. doi: 10.1038/442882a. [DOI] [PubMed] [Google Scholar]
- 78.Sullivan BA, Karpen GH. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol. 2004;11:1076–83. doi: 10.1038/nsmb845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.George JA, Pardue ML. The promoter of the heterochromatic Drosophila telomeric retrotransposon, HeT-A, is active when moved into euchromatic locations. Genetics. 2003;163:625–35. doi: 10.1093/genetics/163.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.