Abstract
Background
Ketamine may be effective in blocking central sensitization of pain transmission neurons through its effect on NMDA receptors in refractory CRPS patients. At higher doses ketamine infusions can be associated with significant risks, while outpatient therapy requires return visits for a 10-day period with variable efficacy and duration.
Objective
This study determined the efficacy of a 5-day moderate dose, continuous racemic ketamine infusion. The pharmacodynamic responses to racemic ketamine and norketamine were examined.
Design
Observational study
Methods
In this, ketamine was titrated from 10-40 mg/hr in 16 CRPS patients, and maintained for 5 days. Pain was assessed daily and ketamine and norketamine concentrations were obtained on Day 1 before starting the infusion, and at 60-90 min, 120-150 min, 180-210 min, 240-300 min after the initiation of the infusion, on Days 2, 3, 4, 5, and on Day 5 at 60 min after the conclusion of the infusion. The plasma concentrations of (R)-ketamine, (S)-ketamine, (R)-norketamine and (S)-norketamine were determined using an enantioselective liquid chromatography – mass spectrometry method. The pain evaluations were repeated at 3 and 6 months.
Results
Ketamine and norketamine infusion rates stabilized 5 hr after the start of the infusion. The subjects showed no evidence of significant tachycardia, arterial oxygen desaturation, or hallucinatory responses. Subjects generally experienced minimal pain relief on day 1 followed by significant relief by day 3. Mean pain scores decreased from the 8-9 to 3-5 ranges however, the analgesic response to ketamine infusion was not uniform. On day 5, there was little or no change in the pain measure assessed as the worst pain experienced over the last 24 hr in 37% of the subjects. (R)- and (S)-ketamine concentrations peaked at 240-300 min. (R)- and (S)-norketamine concentrations were lower and peaked on day 2 of the infusion, as opposed to day 1 for R and S ketamine. Significant pain relief was achieved by the second day of infusion and correlated with the maximum plasma levels of ketamine and norketamine. The pain relief continued to significantly improve over the 5 day infusion at concentrations of 200-225 ng/ml for R and S ketamine, and 90-120 ng/ml for R and S norketamine. The 3 and 6 months evaluations showed pain relief for 60% and 40% of the subjects, respectively.
Conclusions
A 5 day ketamine infusion was generally effective (60% of subjects) for the treatment of severe CRPS for 3 months. The pain relief experienced on day two of the infusion continued to improve over the 5 day infusion period and correlated with the maximum plasma levels of ketamine and norketamine. We speculate that downstream metabolites of ketamine and norketamine may play a role in its therapeutic efficacy.
Keywords: ketamine, norketamine, CRPS, pharmacodynamics, chronic pain, enantiomers
INTRODUCTION
Complex regional pain syndrome (CRPS) is associated with pain that is out of proportion to the inciting injury, is neuropathic in nature and is regional in distribution1. This pain most commonly follows an injury, is often unilateral in its onset, and can spread to the contra lateral side at times being generalized.2,3 Factor analysis of the signs and symptoms of CRPS patients revealed four distinct clusters: 1) abnormalities in pain processing; 2) autonomic dysregulation ; 3) neurogenic edema; 4) a motor dysfunction associated with atrophy and dystrophy. 4,5
There is abundant evidence supporting a dynamic change in the physiology and structure of central pain projecting neurons mediated through the N-methyl-D-aspartate (NMDA) receptor.6,7,8,9,10,11 A nociceptive barrage initiates and maintains a state of central sensitization in the central pain projecting pathways7,8. This central sensitization results in a lower threshold to fire pain transmission neurons (PTNs), increases their receptive fields and is associated with thermal and mechanical allodynia and spontaneous pain.9 A significant percentage of CRPS patients who do not respond to conventional treatment have disease recurrence12 along with spread of illness from the area of original injury13. A critical factor in the initiation of central sensitization is the release of the magnesium block of the NMDA receptor that results in calcium influx and the initiation of intracellular enzymatic cascades, thus increasing the excitability of pain transmission neurons10,11. This observation led to the use of ketamine to block the NMDA receptor in neuropathic pain states.
Ketamine is a chiral compound that is administered as a racemic (50:50) mixture of its enantiomers (R)-ketamine and (S)-ketamine. It has been previously demonstrated that (R)-ketamine and (S)-ketamine have significantly different pharmacodynamic activities as the (S)-ketamine is a more potent analgesic agent than (R)-ketamine.14 The post-hypnotic stimulatory properties and agitated behavior observed with ketamine have been associated with (R)-ketamine15. In addition, ketamine is extensively metabolized by N-demethylation producing norketamine, a non-competitive NMDA receptor antagonist which may also exhibit enantioselective pharmacological activity, e.g. (S)-norketamine has an 8-fold higher affinity than (R)-norketamine in a rat cortical wedge preparation16.
Clinically, ketamine has shown efficacy in the treatment of neuropathic pain17, in the reduction of chronic orofacial pain18, and in acute visceral and cutaneous pain19. Previous studies also support the effectiveness of ketamine in blocking central sensitization through its effect on the NMDA receptor, and therefore as an effective approach to the treatment of CRPS20-25. A 5-day low dose ketamine infusion administered in an inpatient setting was shown to be effective in patients with less severe distal disease of one extremity10. A 5-day continuous infusion of ketamine and midazolam, at coma inducing doses administered in an ICU setting, was also effective in the treatment of patients with intractable pain24. In addition, outpatient protocols utilizing low dose (40 to maximum of 80 mg) ketamine infusions administered over several days were effective in treating CRPS23,25. In a recent double blind, randomized, placebo controlled study by Sigtermans and associates, those authors administered the (S) form of ketamine (max dose 22 mg/h) in patients with CRPS. Although they demonstrated a significant improvement in pain, there was no evidence of a functional improvement, and pain relief was no longer significant by week 12 post (S) ketamine infusion22.
While these treatments appeared to be efficacious, they are not optimal. In the high dose setting, the infusion can be associated with significant risks including chronic catheterization with the need for parenteral nutrition, and endotracheal intubation. The outpatient therapy requires the patient to return for a 10-day period of time and, though efficacious, generally showed loss of effectiveness after 6-12 weeks.
The current study was designed as an observational study to determine the efficacy and pharmacodynamics of a moderate dose of racemic ketamine administered as an inpatient 5-day continuous infusion.
METHODS
Subjects
After approval from the Cooper University Institutional Review Board, 16 American Society of Anesthesiologists Physical Status Classification I or II patients with a primary diagnosis of CRPS gave written informed consent to participate in this prospective study.
All 16 subjects fulfilled the 1993 IASP-CRPS diagnostic clinical and proposed research modifications4, as well as the diagnostic criteria proposed at the 2005 Budapest Consensus Conference on CRPS. The patients were recruited from the CRPS clinic at the Department of Neurology of Drexel University College of Medicine by a neurologist (RJS). Cluster analysis of these patients placed them in the subgroup of a florid CRPS syndrome5. No distinction was made between CRPS I and II patients.
The average daily pain intensity was required to be 7 or greater on a numeric rating scale (NRS) in which the endpoints were 0: no pain, and 10 the worse pain imaginable for at least 6 months while on standard therapy. Patients had to have failed standard pharmacologic therapy (NSAIDs, AEDs, narcotics, antidepressants), interventional (sympathetic nerve blocks or, in some patients, dorsal column stimulators or morphine pumps), as well as physical therapy and psychiatric care. Failure was defined as: 1) no benefit from treatment; or 2) pain relief lasting no longer than 6 weeks; 3) recurring, persisting or progression of disease.
Exclusion criteria included allergies to ketamine, clonidine, midazolam, or known contraindications to ketamine use which included severe arterial hypertension, hyperthyroidism, ischemic heart disease or heart failure. Patients were excluded who had a history of substance or drug abuse or suspected somatoform pain disorder. All patients had psychiatric and cardiac clearance which included echocardiography, ECG, Holter monitoring studies, and a detailed neuropsychological battery prior to treatment. Inclusion and exclusion criteria were validated by a neurologist (RJS) and an anesthesiologist (MEG) prior to entry into the study.
Treatment Protocol
Patients were admitted to a monitored telemetry unit (continuous ECG and pulse oximetry) and maintained on their usual pain medications during the infusion period. The patient demographic data are presented in table 1.
Table 1.
The demographic data are presented for the 16 subjects with the baseline pain scores in the right column. The means and standard deviations are below the table.
| Subject number | Age (years) | Height (cm) | Weight (kg) | 0-10 Pain level pre dosing | 0-10 Pain level Day 5 | % Change Pain level Day 1 -5 |
|---|---|---|---|---|---|---|
| 1 | 26 | 182.9 | 81.8 | 4 | 1 | 75 |
| 2 | 25 | 157.5 | 51.8 | 8.5 | 7.5 | 13 |
| 3 | 41 | 156.2 | 118.2 | 8 | 0 | 100 |
| 4 | 18 | 170.2 | 61.4 | 8 | 8 | 0 |
| 5 | 20 | 160.0 | 53.1 | 10 | 5 | 50 |
| 6 | 40 | 167.6 | 52.3 | 8 | 7 | 10 |
| 7 | 44 | 170.2 | 62.0 | 8 | 10 | 0 |
| 8 | 25 | 160.0 | 50.0 | 7 | 4 | 43 |
| 9 | 17 | 157.5 | 72.7 | 7 | 2 | 71 |
| 10 | 42 | 175.3 | 56.8 | 10 | 6 | 40 |
| 11 | 38 | 165.1 | 77.3 | 10 | 10 | 0 |
| 12 | 47 | 167.6 | 77.7 | 10 | 10 | 0 |
| 13 | 36 | 162.6 | 73.2 | 10 | 2 | 80 |
| 14 | 45 | 177.8 | 93.6 | 10 | 4 | 60 |
| 15 | 37 | 165.1 | 62.3 | 10 | 7 | 30 |
| 16 | 27 | 162.6 | 41.5 | 8 | 3 | 63 |
| Means | 33.0 | 166.1 | 67.9 | 8.5 | 5.4 | |
| SD | 10.2 | 7.7 | 19.3 | 0.4 | 0.8 | |
Ketamine was mixed in a 500 ml bag of normal saline and started at an infusion rate of 10 mg/hr and titrated to a maximum of 40 mg/hr to achieve comfort without evidence of significant side effects or oxygen desaturation (< 92%). Transdermal clonidine 0.1 mg/day was administered to block sympathomimetic, psychomimetic and potential neurotoxic ketamine side effects26. Midazolam was administered using a 2 to 4 mg dose every 4 hours if patients were unduly restless, dysphoric, or hallucinating. The infusion was maintained for 5 days with 24 hr monitoring of the subject. During the titration period, an advanced practice nurse and a research assistant collected the study data and blood samples.
Pain assessments
The subjects rated their pain intensity daily using a 0-10 numerical scale (0 no pain, 10 worst pain imaginable). The subjects were asked to provide a measure of their overall pain relief using 0 percent as no relief, and 100 percent as complete pain relief, compared to their pain prior to the start of the infusion. These measurements were selected to provide assessments of not only pain intensity, but also about the subject's perceived treatment effectiveness by quantifying the relief they experienced after treatment. The pain assessments were obtained by an experienced pain nurse on the morning of each day at approximately 10 AM, and on the last day within 1 hour after termination of the infusion. At three and six months post infusion, the subjects were re-evaluated to determine if they were still experiencing “meaningful pain relief” from the end of their treatment. These last two evaluations were performed over the telephone using a written question with a yes/no answer to obtain an overall impression of the subjects’ assessments of the long term effects of the therapy. Knowing the importance of maximizing the accuracy of this measurement, this same evaluator was required to only obtain a yes/no response without influencing the subject's answer. The evaluator used slow and clear wording and did not answer any questions related to the subject's assessment of pain until a yes/no answer was provided by the subject. None of the subjects had difficulty providing a yes/no answer to their subjective assessment of “meaningful pain relief”.
Ketamine Blood Levels
Blood samples (7 ml) were obtained on Day 1 before starting the infusion, and at 60-90 min, 120-150 min, 180-210 min, 240-300 min after the initiation of the infusion, on Days 2, 3, 4, 5 (morning collection), and on Day 5 at 60 min after the conclusion of the infusion. The samples were centrifuged and the plasma collected and frozen at -80°C until analysis. The plasma concentrations of (R)-ketamine, (S)-ketamine, (R)-norketamine and (S)-norketamine were determined using a previously reported enantioselective liquid chromatography – mass spectrometry method27.
Statistical Analyses
Sample size was determined using a power analysis for paired t-test based on an achieved pain reduction of 0.5 (SD=0.4) from baseline to the end of treatment on day 5. This level of pain reduction was based on prior experience, as well as others having achieved similar levels of analgesia in a comparable patient population. With 16 data pairs the study had a power of 80.1% to yield a statistically significant result. Pain and blood level data measured across time were analyzed using ANOVA with repeated measures. Post hoc analysis was used to compare differences between means after confirming significant main effects, and a p<0.05 was considered statistically significant. Demographic data are presented as means ± standard deviation while pain and drug blood levels data as means ± standard error of the mean. The analyses were performed using Systat Software version 11.00.01 (Systat Software Inc., Chicago, IL).
RESULTS
Patient characteristics
The subjects in this study included 15 females and one male ages 17-47 years (mean 33±10.2), height 156.2-182.9 cm (mean 166.1 ± 7.7) and weight 41.5-118.2 kg (mean 67.9 ± 19.3, see Table 1). The mean baseline pain level was 8.5 ± 1.6 assessed as the worst pain experienced over the last 24 hours prior to dosing using a 0-10 categorical pain scale (Table 1).
Clinical Response
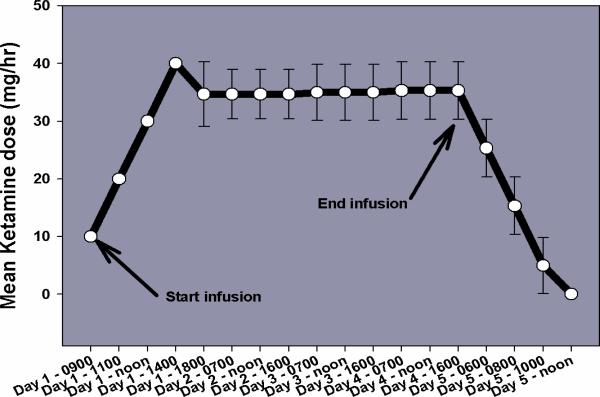
All of the subjects tolerated the infusions without difficulty. The ketamine infusion rate was gradually increased from 10 to 40 mg/hr for all subjects after which a stable ketamine infusion rate was achieved approximately 5 hr after the start of infusion. (Figure 1). The subjects showed no evidence of clinically significant tachycardia (HR >100 bpm), arterial oxygen desaturation (SpO2 < 92%), or hallucinatory responses. Each subject received at least 2 mg of midazolam during the initial titration phase (day 1), and 13/16 (81%) subjects had a clonidine patch (0.1 mg dose) applied for the duration of their 5-day hospital stay. None of the subjects required removal of the clonidine patch subsequent to hypotension. The subjects were maintained on their outpatient medications with no changes in dosing except for opioid medications that were titrated to lower doses by day five.
Figure 1.
This figure illustrates the mean ± SEM infusion rates for each day over the 5 day treatment period.
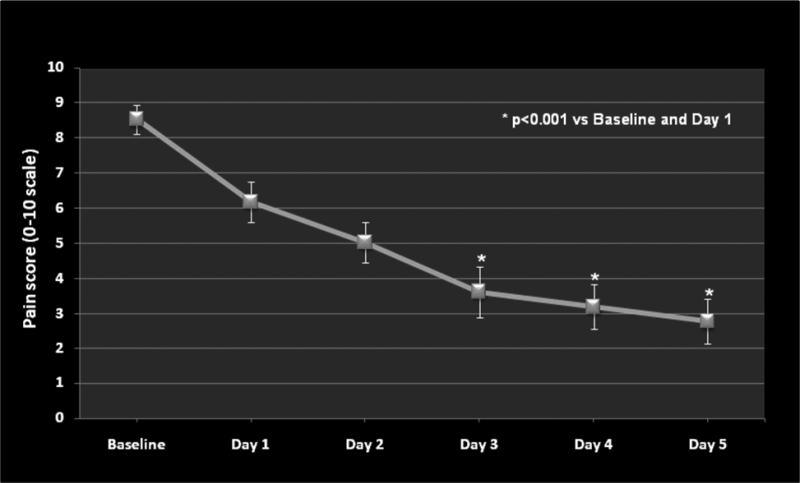
In general, the patients experienced minimal pain relief on day 1 followed by significant relief by day 3 (Figure. 2). Mean pain intensity gradually decreased from 8.5 ± 0.4 (day 1) to 5.4 ± 0.8 (day 5), p<0.001 over the 5-day period (Figure. 2). The decreased pain levels corresponded to a gradual increase in overall daily pain relief which reached statistical significance (p<0.05) on day 4 (Figure. 3). However, the response to the ketamine infusion was not uniform. On day 5, there was little or no change in the pain measure, assessed as the worst pain experienced over the last 24 hours, in 6 of the 16 subjects (37.5%) (Table 1). Follow up evaluations demonstrated meaningful pain relief lasting approximately 3 months for 60% (9/15) of the study subjects. Forty percent (6/15) of the subjects reported treatment effectiveness up to 6 months following their hospital discharge. One study subject could not be contacted for completion of the 3 and 6 months follow up pain assessments.
Figure 2.
This figure illustrates the mean ± SEM pain scores (0-10 scale) for the 5 day treatment period. The * symbol indicates statistical significance versus baseline and day one pain scores.
Figure 3.
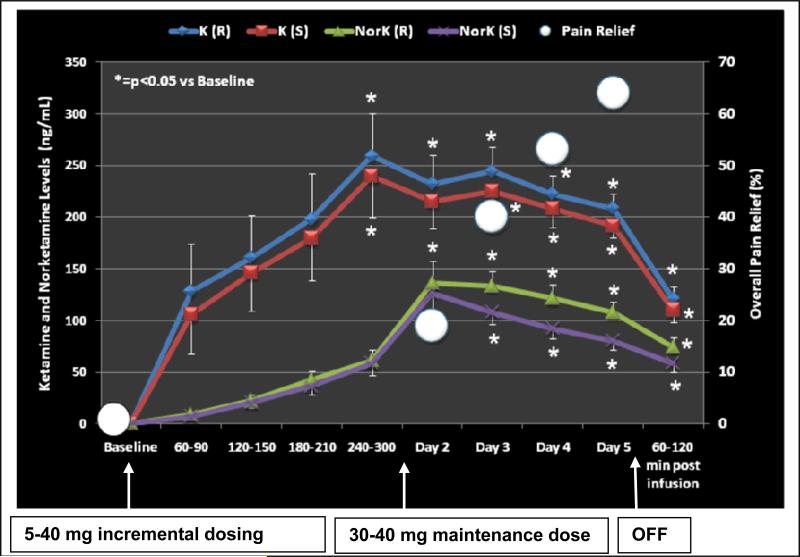
This figure illustrates the mean ± SEM Ketamine (K) levels for the R (solid blue line and diamond symbols) ) and S enantiomers (solid red line and square symbols). Norketamine levels are similarly illustrated with a solid green line and triangles and a solid purple line and x symbols. The X axis shows the baseline and infusion period with the corresponding Ketamine and Norketamine blood levels during the 4-5 hour incremental dosing period. The subjects’ pain relief scores are illustrated by white circles in the figure to provide a reference to the achieved level of analgesia. Blood levels for both Ketamine and Norketamine were significantly (p<0.05) increased from baseline at all time points with a corresponding increase in pain relief.
Ketamine Blood Concentrations
Serial blood serial samples were obtained from all of the subjects in the study and complete profiles were obtained for 13/16 subjects. The samples were analyzed using a validated assay and (R)-ketamine, (S)-ketamine and their respective N-demethylated metabolites, i.e. norketamine, were detected and quantified. The average (R)- and (S)-ketamine plasma concentrations peaked at 240-300 min after the start of the infusion and were significantly (p<0.05) increased from baseline at all time points (Figure. 4). A similar trend was noted for the average (R)- and (S)-norketamine plasma concentrations, although those were lower and peak concentrations were only reached on day 2 of the infusion, as opposed to day 1 for R and S ketamine (Figure. 4).
Significant pain relief was achieved by the second day of infusion and correlated with the maximum plasma levels of ketamine and norketamine. The pain relief continued to be significant and improve over the 5 day infusion at concentrations of 200-225 ng/ml for R and S ketamine, and 90-120 ng/ml for R and S norketamine. Both norketamine plasma levels peaked approximately 24 hours later than the ketamine plasma levels and were near the same concentration at the termination of the infusion (Figure 4). It appears that once stable blood levels of ketamine and norketamine are achieved, pain relief rapidly ensues and continues during the course of the infusion. This relief lasted in some cases for 6 months after termination of the infusion.
DISCUSSION
The data from this study indicate that a 5 day continuous infusion of moderate (subanesthetic) dose ketamine is effective in the treatment of CRPS pain symptoms for up to 3 months. This reduction in VAS scores is consistent with our previous findings using a 10 day low dose outpatient ketamine infusion25. The data from the present study however point to more profound pain relief with the 5 day infusion compared to our low dose 10 day infusion (mean VAS at end of infusion 2.8 ± 0.65 vs. 5.44 ± 0.91, respectively). The 5 day moderate dose ketamine therapy used in this study provided significant reduction (≥ 30%) in the perceived pain level in 10/16 patients compared to baseline day 1 pain scores, although 6/16 patients reported no significant pain reduction (≤ 15%) (Table 1). In addition, we noted large inter-patient variability in pain responses in the patients who could be considered to have benefited from the treatment (Table 1). This inter-patient variability in the antinociceptive response produced by ketamine has been previously reported by Rabben, et al. in patients with trigeminal neuropathic pain18. In their study18, 26 patients were evaluated after receiving a single intramuscular injection of ketamine (0.4 mg/kg) and 3 different response patterns were observed: 1) long-term (6 – 24 h) pain relief in 8/26 of the patients, 2) short term (<2 h) pain relief in 9/26 of the patients, and 3) no pain relief in 9/26 patients. The relative pain relief provided in the three sub-populations was determined as a percentage of the baseline pain score measured by a VAS which also demonstrated that the degree of pain relief varied between the three groups.
The observation of three qualitatively different response groups led Rabben, et al. to postulate that the data did not support the paradigm of NMDA receptor-mediated sensitization as a universal mechanism of neuropathic pain18. Other studies have suggested that the beneficial effects on inflammatory pain are NMDA mediated while those on acute, non-inflammatory pain involve a PAG descending inhibitory system35 and also differ based upon the source of the pain19. In addition to the NMDA receptor blockade, ketamine also interacts with a number of other receptors including opioidergic, cholinergic and monoaminergic pathways, all of which could play a role in the analgesic response19. In this study, the exact role of NMDA receptors in the observed responses could not be determined, nor was the role that other families of receptors may play. The presence and therapeutic significance of single nucleotide polymorphisms (SNPs) of the NMDA receptor cannot be overlooked and opens new grounds for research.
This study also reports the blood levels of (R)-ketamine, (S)-ketamine, (R)-norketamine and (S)-norketamine following an extended infusion. Our data are consistent with the results of previous studies involving single administrations of the racemic drug28,29.
In a recent study22, the (S) enantiomer of ketamine was utilized in a placebo controlled, double blind protocol to treat patients with chronic CRPS who had refractory pain. The infusion rate was somewhat lower (22mg/h/70kg vs 40 mg/h independent of weight) and the plasma concentrations of (S) ketamine and (S) norketamine achieved were similar22. (peaked at 250ng/ml and 225 ng/ml, respectively ). Our study utilized the racemic mixture which is commonly used in the United States. The plasma concentrations of (R) and (S) ketamine and (R) and (S) norketamine did not differ significantly from one another (Figure 4). Similarly to Sigtermans group, we found the effect to wane by approximately 12 weeks post infusion although in some cases pain relief lased up to 6 months.
Our study did not include a placebo group because of the ethical dilemma concerning withheld treatment in this population. Those patients have typically exhausted other therapies and would most likely be able to identify placebo versus active treatment due to the psychomimmetic effects of ketamine. To construct a sham group with a drug such as midazolam, would be easier in the low dose setting where the effects of ketamine are not as pronounced23. In the in- patient setting with a moderate ketamine dose, the midazolam dosing, or that of other medications to mimic this effect, would expose the subjects to significant risk. It is interesting to note, that despite the lack of a control group, our results are quite similar to those of Sigtermans et al.22
At the present time, ketamine and norketamine are considered to be the active agents responsible for the antinociceptive response produced by the administration of racemic-ketamine, with the activity primarily residing in the (S)-enantiomers of these compounds. This assumption is based upon the observations that (S)-ketamine is a more potent analgesic agent than (R)-ketamine14, that (S)-norketamine has an 8-fold higher affinity than (R)-norketamine in a rat cortical wedge preparation16, and the recent data from a study utilizing a rat model of peripheral neuropathy which demonstrated that the antinociceptive properties of norketamine are due to (S)-norketamine30.
Although the current study involved a limited number of patients, we cannot discount the possibility that the systemic administration of ketamine and norketamine may not be responsible for all of this drug's antinociceptive properties. The varied responses to treatment observed in this study, and in the study of Rabben et al.,18 may not reflect different mechanism of pain, but rather inter-individual differences in the ability to metabolize ketamine, i.e. pharmacogenic differences, as was observed between European and Japanese subjects31. Thus downstream metabolites of ketamine and norketamine may play a role in its therapeutic efficacy.
It is however encouraging to note that our findings are similar to the previously described placebo controlled trial22 where pain relief is not dependent on the dose administered. The search to identify the active metabolite(s) should continue, as well as what effect a longer infusion might have on pain relief in these patients.
Acknowledgments
This work was supported in part by funds from the Intramural Research Program of the National Institute on Aging/NIH. This work was presented in part at the 2007 ASA Annual Meeting, October 2007, San Francisco, CA
REFERENCES
- 1.Schwartzman RJ, Alexander GM, Grothusen J. Pathophysiology of complex regional pain syndrome. Expert Rev Neurother. 2006;6(5):669–81. doi: 10.1586/14737175.6.5.669. [DOI] [PubMed] [Google Scholar]
- 2.Schwartzman RJ, McLellan TL. Reflex sympathetic dystrophy. A review. Archives of Neurology. 1987;44(5):555–61. doi: 10.1001/archneur.1987.00520170081028. [DOI] [PubMed] [Google Scholar]
- 3.Schwartzman RJ, Popescu A. A reflex sympathetic dystrophy. Curr Rheumatol Rep. 2002;4(2):165–9. doi: 10.1007/s11926-002-0012-2. [DOI] [PubMed] [Google Scholar]
- 4.Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M, Gayles R, Rudin N, Bhugra MK. Stanton-Hicks M. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81(1-2):147–54. doi: 10.1016/s0304-3959(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 5.Burton AW, Bruehl S, Harden RN. Current diagnosis and therapy of complex regional pain syndrome: refining diagnostic criteria and therapeutic options. Expert Review of Neurotherapeutics. 2005;5(5):643–51. doi: 10.1586/14737175.5.5.643. [DOI] [PubMed] [Google Scholar]
- 6.Bell RF. Low-dose subcutaneous ketamine infusion and morphine tolerance. Pain. 1999;83(1):101–3. doi: 10.1016/s0304-3959(99)00096-2. [DOI] [PubMed] [Google Scholar]
- 7.Clark JL, Kalan GE. Effective treatment of severe cancer pain of the head using low-dose ketamine in an opioid-tolerant patient. J Pain Symptom Manage. 1995;10(4):310–4. doi: 10.1016/0885-3924(95)00010-V. [DOI] [PubMed] [Google Scholar]
- 8.Eide PK, Jorum E, Stubhaug A, Bremnes J, Breivik H. Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind, crossover comparison with morphine and placebo. Pain. 1994;58(3):347–54. doi: 10.1016/0304-3959(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 9.Harbut RE, Correll GE. Successful treatment of a nine-year case of complex regional pain syndrome type 1 (reflex sympathetic dystrophy) with intravenous ketamine-infusion therapy in a warfarin-anticoagulated adult female patient. Am Acad Pain Med. 2002;3:147–155. doi: 10.1046/j.1526-4637.2002.02029.x. [DOI] [PubMed] [Google Scholar]
- 10.Correll GE, Maleki J, Gracely EJ, Muir JJ, Harbut RE. Subanesthetic ketamine infusion therapy: a retrospective analysis of a novel therapeutic approach to complex regional pain syndrome. Pain Med. 2004;5(3):263–75. doi: 10.1111/j.1526-4637.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 11.Kvarnstrom A, Karlsten R, Quiding H, Emanuelsson BM, Gordh T. The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol Scand. 2003;47(7):868–77. doi: 10.1034/j.1399-6576.2003.00187.x. [DOI] [PubMed] [Google Scholar]
- 12.Veldman PH, Goris RJ. Multiple reflex sympathetic dystrophy. Which patients are at risk for developing a recurrence of reflex sympathetic dystrophy in the same or another limb. Pain. 1996;64(3):463–6. doi: 10.1016/0304-3959(95)00160-3. [DOI] [PubMed] [Google Scholar]
- 13.Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome type I (reflex sympathetic dystrophy). Pain. 2000;88:259–266. doi: 10.1016/S0304-3959(00)00332-8. [DOI] [PubMed] [Google Scholar]
- 14.Øye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- 15.White PF, Schuettler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Br. J. Anaesthesia. 1985;57:197–203. doi: 10.1093/bja/57.2.197. [DOI] [PubMed] [Google Scholar]
- 16.Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur. J. Pharmacol. 1997;333:99–104. doi: 10.1016/s0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- 17.Øye I, Rabben T, Fagerlund TH. Analgesic effect of ketamine, an NMDA receptor antagonist, in a patient with neuropathic pain. J. Norw. Med. Assoc. 1996;116:3130–3131. [PubMed] [Google Scholar]
- 18.Rabben T, Skjelbred P, Øye I. Prolonged Analgesic Effect of Ketamine, an N-Methyl-D- Aspartate Receptor Inhibitor, in Patients with Chronic Pain. Pharmacol. Exp. Ther. 1999;289:1060–1066. [PubMed] [Google Scholar]
- 19.Strigo IA, Duncan GH, Bushnell MC, Boivin M, Wainer I, Rodriguez Rosas ME, Persson J. The effects of racemic ketamine on painful stimulation of skin and viscera in human subjects. Pain. 2005;113:255–264. doi: 10.1016/j.pain.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Baron R, Fields HL, Janig W, Kitt C, Levine JL. National institute of health workshop: reflex sympathetic dystrophy/complex regional pain syndromes: state of sciences. Anesth Analg. 2002;95:1812–1816. doi: 10.1097/00000539-200212000-00064. [DOI] [PubMed] [Google Scholar]
- 21.Kiefer RT, Rohr P, Ploppa A, Dieterich HJ, Grothusen J, Koffler S, Altemeyer KH, Unertl K, Schwartzman RJ. Efficacy of ketamine in anesthetic dosage for the treatment of refractory complex regional pain syndrome: an open-label phase II study. Pain Med. 2008;9:1173–201. doi: 10.1111/j.1526-4637.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- 22.Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–11. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 23.Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: A double-blind placebo controlled study. Pain. 2009 doi: 10.1016/j.pain.2009.08.015. In Press. [DOI] [PubMed] [Google Scholar]
- 24.Koffler SP, Hampstead BM, Irani F, Tinker J, Kiefer RT, Rohr P, Schwartzman RJ. The neurocognitive effects of 5 day anesthetic ketamine for the treatment of refractory complex regional pain syndrome. Arch Clin Neuropsychol. 2007;22(6):719–29. doi: 10.1016/j.acn.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg ME, Domsky R, Scaringe D, Hirsh R, Dotson J, Sharaf I, Torjman MC, Schwartzman RJ. Multi-day low dose ketamine infusion for the treatment of complex regional pain syndrome. Pain Physician. 2005;8(2):175–9. [PubMed] [Google Scholar]
- 26.Jevtovic-Todorovic V, Wozniak DF, Benshoff ND, Olney JW. A comparative evaluation of the neurotoxic properties of ketamine and nitrous oxide. Brain Research. 2001;895(1-2):264–7. doi: 10.1016/s0006-8993(01)02079-0. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez Rosas ME, Patel S, Wainer IW. Determination of the enantiomers of ketamine and norketamine in human plasma by enantioselective liquid chromatography-mass spectrometry. J.Chromatog. B. 2003;794:99–108. doi: 10.1016/s1570-0232(03)00420-3. [DOI] [PubMed] [Google Scholar]
- 28.Geisslinger G, Hering W, Thomann P, Knoll R, Kamp HD. Pharmacokinetics and pharmacodynamics of ketamine enantiomers in surgical patients using stereoselective analytical method. Br. J. Anaesthesia. 1993;70:666–671. doi: 10.1093/bja/70.6.666. [DOI] [PubMed] [Google Scholar]
- 29.Ihmsen H, Geisslinger G, Schuttler J. Stereoselective pharmacokinetics of ketamine: R(-)-ketamine inhibits the elimination of S(+)-ketamine. Clin. Pharmacol Ther. 2001;70:431–8. doi: 10.1067/mcp.2001.119722. [DOI] [PubMed] [Google Scholar]
- 30.Holtman JR, Crooks PA, Johnson-Hardy JK, Hojomat M, Kleven M, Wala EP. Effects of norketamine enantiomers in rodent models of persistent pain. Pharmacol. Biochem. Behavior. 2008;90:676–685. doi: 10.1016/j.pbb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Yanagihara Y, Ohtani M, Kariya S, Uchino K, Hiraishi T, Ashizawa N, Aoyamo T, Yamamura Y, Yamada Y, Iga T. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Bopharmaceutics Drug Disp. 2003;24:37–43. doi: 10.1002/bdd.336. [DOI] [PubMed] [Google Scholar]