Abstract
Objective To design and evaluate a camp-based intervention, the goal of which was to increase independence among children, adolescents, and adults with spina bifida. Methods An intervention targeting independence was embedded within a typical week long camp experience. The intervention consisted of the following: collaborative (i.e., parent and camper) goal identification, group sessions consisting of psycho-education and cognitive tools, and goal monitoring by camp counselors. Camper and parent report of demographic variables, goal attainment, spina bifida knowledge, and independence were gathered. Interventionist report of adherence to the treatment manual was also collected. Results Campers made significant gains in individual goals, management of spina bifida responsibilities, and independence with general spina bifida tasks, with medium effect sizes observed in goal attainment. Conclusions Results indicated that significant progress was made on individually oriented goals from pre- to post-camp. Design issues are discussed.
Keywords: camp, cognitive deficits, independence, intervention, spina bifida
Intervention research for chronically ill children within school, home, and medical settings has yielded promising results (Spirito & Kazak, 2005). In contrast, camp-based interventions for many chronic conditions have not received nearly as much empirical attention. Although there are some exceptions, (e.g., ADHD; Pelham & Fabiano, 2008), the limited research in the camp context is surprising given that there are hundreds of camps in the United States for chronically ill children (American Camping Association, 2008). In light of the numerous camps in existence, this setting is an important context for pediatric psychologists to implement intervention and research efforts.
There are other reasons to focus empirical attention on the camp context. Camps provide an unusual opportunity to access groups of children with similar conditions. In addition, the fact that campers usually spend the time apart from families may facilitate greater independence in the area of illness management. Camp settings may also provide campers with opportunities to develop social skills with peers. This outcome is particularly important given that social isolation is common among chronically ill children (Kazak, 1992). Finally, interventions within a camp context—a setting which characteristically emphasizes positive aspects of development over psychopathology—may inspire a higher level of engagement in the intervention because camp programs typically use a more developmentally appropriate, and appealing, approach (i.e., emphasis on fun and prosocial interactions with peers) than other interventions (Commission on Positive Youth Development, 2005).
Among chronic conditions, people with spina bifida are in need of effective interventions. Spina bifida is the second most common birth defect; in 2005, the rate was 17.96 per 100,000 live births (Centers for Disease Control and Prevention, 2008). It occurs when the neural tube fails to close within the first 20 days of pregnancy (McLone & Ito, 1998). Considerable variability in illness severity exists, but there is often a range of secondary physical, cognitive, and psychosocial correlates of this condition (Fletcher, Dennis, & Northrup, 2000; Hommeyer, Holmbeck, Wills, & Coers, 1999; Wills, 1993). Many individuals with spina bifida are required to adhere to several daily self-care tasks such as self-catheterization, bowel programs, skin checks for pressure sores, and proper nutrition. Such self-care is necessary to maintain health and is also an important aspect of independence. Unfortunately, dependence on family and social isolation have been identified as particularly problematic among individuals with spina bifida (Holmbeck, Johnson, et al., 2002; Loomis, Jarvornisky, Monahan, Burke, & Lindsay, 1997).
Despite the great need, intervention research targeting this group is virtually nonexistent (Holmbeck, Greenley, Coakley, Greco, & Hagstrom, 2006). The majority of intervention efforts to date have focused mainly on medical needs such as catheterization, bowel programs, ambulation, and fine motor dexterity (King, Currie, & Wright, 1994; Nergardh, von Hedenberg, Hellstrom, & Ericsson, 1974; Rudeberg, Donati, & Kaiser, 1995; Watson, 1991). In contrast, there has been limited attention to psychosocial issues, despite the well-documented deficits in this area (Appleton et al., 1997; Holmbeck et al., 2003). The handful of interventions that do target psychosocial concerns have demonstrated mixed results (Briery & Rabian, 1999; Engelman, Loomis, & Kleiback, 1994; King et al., 1997). Some showed improvements in self-care but not in social functioning or self-concept (Engelman et al., 1994), while others have yielded improvements in these latter areas (Briery & Rabian, 1999; King et al., 1997). Only one intervention that specifically targeted independence issues was identified (Sherman, Berling, & Oppenheimer, 1985). In addressing goal attainment and parent facilitation of independence, this intervention was deemed effective, but no statistical comparisons were employed to compare outcomes before and after exposure to the program.
Previous intervention work focused on individuals with spina bifida also lacks sensitivity to cognitive abilities (e.g., use of scaffolding tools, simplified language, or repetition). This is surprising given the well-documented array of neurocognitive challenges in this population (Dennis, Landry, Barnes, & Fletcher, 2006). Such deficits may impact treatment efficacy insofar as cognitive deficits relate to one’s ability to learn, retain, and make use of an intervention.
This study evaluated a camp-based intervention targeting independence among children, adolescents, and adults with spina bifida. It was anticipated that campers would make progress on their individually oriented spina bifida and social goals during their week at camp and that these gains would be maintained one month after camp ended. In addition, it was expected that campers would demonstrate improvement in responsibility for spina bifida tasks, general independence related to spina bifida, and knowledge about spina bifida from pre- to post-camp.
Methods
Participants
Participants consisted of campers aged 7–37 years attending three separate week long overnight summer camp programs at Camp Ability located in northern Illinois, funded by the Spina Bifida Association of Illinois (SBAIL). Programming included typical camp experiences (e.g., horseback riding, swimming) with accommodations. No campers were excluded from participation in the research study. Camp sessions were conducted separately and consecutively for three age groups: Ability A (children, 7–12 years; M = 10.68, SD = 1.13), Ability B (adolescents, 13–17 years; M = 14.88, SD = 1.77), and Ability C (adults, 18–37 years; M = 23.35, SD = 4.72).
Initial research participation rates for the three age groups were as follows: Ability A = 81% (22 of 27 campers); Ability B = 90% (27 of 29 campers); and Ability C = 82% (27 of 33 campers) for a total of 76 campers at Time 1. Common stated reasons for non-participation included a lack of time or lack of interest. The retention rate at Time 2 was 100% of those who participated at Time 1 (N = 76). Retention rate at Time 3 was as follows: Ability A = 55% (12 out of 22); Ability B = 63% (17 out of 27); Ability C = 78% (21 out of 27) for a total of 50 participants at Time 3 (i.e., a total of 66% retained). Campers who declined participation at Time 3 did not differ from those who were retained at Time 2 on measures of socioeconomic status, ethnicity, camper age, number of shunt surgeries, and camper gender. Available information about demographic and condition information is summarized in Table I.
Table I.
Camper Demographic Information
| Camper age (N = 74) | M = 16.61 years (SD = 6.07); range = 8–37 |
| Camper gender (N = 74) | 43% = Male |
| Camper ethnicity (N = 74) | 84% = Caucasian |
| 5% = African American | |
| 10% = Hispanic | |
| Socioeconomic status (N = 70) | M = 41.04 (SD = 14.52); range = 17–66 |
| Type of spina bifida (N = 40) | 98% = Myelomeningocele |
| 2% = Lipomeningocele | |
| Lesion level (N = 40) | 13% = Sacral |
| 78% = Lumbar | |
| 10% = Thoracic | |
| Number of shunt surgeries (N = 63) | M = 5.78 (SD = 8.72); range = 0–40 |
| Type of ambulation (N = 60) | 23% = Knee–ankle–foot orthoses |
| 8% = Hip–knee–ankle–foot orthoses | |
| 68% = Wheelchair |
Note. Variability in N values is due to parents not providing this information.
Procedure
In collaboration with the SBAIL, this study was conducted by researchers at Loyola University Chicago and was approved by the university’s Institutional Review Board. Upon enrollment in camp, a letter explaining the intervention and the research component was mailed to families. Then, approximately 5 weeks prior to the start of camp, Time 1 questionnaires and informed consent forms were mailed. Follow-up phone calls were made to ensure receipt of materials, review the consent form, and to answer any questions. A self-addressed stamped envelope was included so that the questionnaires could be returned prior to camp. Additionally, campers and parents had the option of turning in or completing questionnaires at orientation. Therefore, completion of Time 1 data ranged from 1 month to 1 day before camp. Parents and campers were each compensated $5 at Time 1.
Time 2 data collection occurred on the last day of the intervention (i.e., Friday for each age group). Campers received $5 for completing the questionnaires at Time 2. Time 3 data collection took place approximately 1 month after the end of camp. Questionnaires were sent via mail 3 weeks after camp ended and follow-up phone calls were made to ensure receipt of materials and to answer questions. At Time 3, parents and campers each received $10.
Intervention
The intervention targeted independence, was designed to address some of the unique needs of this group, and consisted of three main components. First, there was collaborative goal identification; parents and campers jointly identified goals for the camper to work on during camp. Parent involvement was important for transitioning towards increased independence because of the high degree of familial dependence and the cognitive limitations among people with spina bifida (Loomis et al., 1997; Wills, 1993). In addition, individualized goal setting addressed the variability in illness severity (McLone & Ito, 1998). Finally, both self-care and social goals were targeted given the physical correlates and social isolation commonly found in this group (Holmbeck, Johnson et al., 2002).
A second component of the intervention was daily group sessions. These meetings involved psycho-education and cognitive strategies that were intended to address cognitive deficits believed to impede independence. Two interventionists led 1½ hr group sessions. Each day, different psycho-education topics were emphasized based on common self-care and social goals identified by campers in a pilot study conducted at the same camp in 2005. After an introductory first day, Day 2 focused on catheterization and initiating a social conversation, Day 3 involved organizational skills and talking about spina bifida, Day 4 emphasized hygiene and expressing needs, and Day 5 concentrated on healthy habits and confronting someone. To maximize individual attention and participation during group sessions, the approximately 30 campers in each age group were divided into two groups that met at different times of the day, each containing approximately 12–15 campers. Campers remained with the same group throughout the entire week.
The group sessions included memory, problem-solving, and communication strategies in the service of independence goals. For memory, campers were provided with diaries that they kept throughout the entire week at camp and took home with them after camp ended. Counselors provided assistance with writing in diaries for younger campers and those with fine motor deficits. For problem-solving, campers were taught a problem-solving acronym [i.e., I CAN; (I = Identify the problem, C = Choices available, A = Any barriers?, N = Need to check it out)]. For communication, three skills (i.e., initiating a conversation, staying on topic, and asking questions) were discussed and practiced in role-plays.
The following provides an illustration of the format of the group sessions. On Day 2, the focus was on catheterization and initiating a conversation. During the first half of the session, information about proper catheterization was provided and campers learned how the memory diary could serve as a reminder of when and how to properly self-catheterize. The problem-solving strategy was then taught in the context of obstacles to proper catheterization (e.g., finding a bathroom when it is time to self-catheterize). During the second half of the group session, a role-play was conducted about initiating a conversation and asking appropriate questions when it was necessary to communicate with other people about catheterization (e.g., being in an unfamiliar place and needing to ask where the bathroom is).
A third component of the intervention was counselor monitoring of camper goals. Similar to the cognitive tools, this was intended to address cognitive limitations (Fletcher et al., 2000). There was approximately one counselor for every two campers. Counselors were provided with a copy of camper goals and a checklist of six tasks to include in their daily, individual conversations with campers: (a) review goals, (b) revise goals as necessary, (c) review steps to achieve goals, (d) revise steps to achieve goals if necessary, (e) discuss barriers to goals, and (f) discuss how the memory diary can be used to reach one’s goals.
Several strategies were utilized to evaluate the intervention: a checklist (see above) to guide counselor interactions with campers, an intervention manual, and treatment integrity checks (Kendall & Morris, 1991). The manual used for this study (O’Mahar, Jandasek, Zukerman, & Holmbeck, 2006) was a revised version of one created for a pilot intervention study (Jandasek, O’Mahar, Zukerman, & Holmbeck, 2005). There were three versions of the manual for the three age groups; variations in language as a function of age were necessary to make the intervention developmentally appropriate. In addition, the effect size of change over time was evaluated. Finally, both camper and parent report were used to decrease the likelihood that findings were due to bias in camper reports (Holmbeck, Li, Schurman, Friedman, & Coakley, 2002).
Measures
All measures were collected at Time 1 to obtain both camper and parent report of camper pre-intervention functioning. Only camper data were collected at Time 2 because parents had not observed camper behavior since the intervention and could therefore not report on camper functioning at that time. In addition, the number of measures was reduced at Time 2 due to time constraints for data collection; individual goals were expected to change the most within one week and were therefore assessed. Finally, all measures, except demographic information, were collected at Time 3 to obtain camper and parent report of camper post-intervention functioning.
Medical and Demographic Information (T1)
Parent report of the following was obtained: lesion level (sacral, lumbar, thoracic), type of spina bifida (myelomeningocele, lipomeningocele, other), total number of shunt surgeries, and ambulation method (ankle–foot orthoses, knee–ankle–foot orthoses, hip–knee–ankle–foot orthoses, and wheelchair); combinations of such variable are frequently used to assess severity (Holmbeck & Faier-Routman, 1995). The Hollingshead (1975) scoring system was used for socioeconomic information.
Individual Spina Bifida and Social Goals (T1, T2, & T3)
A new measure was developed for this study based on goal attainment scaling to quantify goals (Joyce, Rockwood, & Mate-Kole, 1994). At Time 1, campers and parents were instructed to discuss and agree upon spina bifida-related and social goals that the camper would work on during camp. A minimum of one and a maximum of three goals, within each category, could be identified. If more than one goal was identified within a category, a mean score across the goals within that category was calculated. Spina bifida and social goals were analyzed separately. Parents (T1 and T3) and campers (T1, T2, and T3) independently rated the degree to which each goal was currently being attained on a Likert scale ranging from 1 to 5. Higher scores indicated greater goal attainment. The validity of goal attainment scaling has been documented (Malec, 1999).
Sharing of Spina Bifida Management Responsibilities (T1 & T3)
The Sharing of Spina Bifida Management Responsibilities (SOSBMR) measure was adapted from the Diabetes Family Responsibility Questionnaire (DFRQ; Anderson, Auslander, Jung, Miller, & Santiago, 1990) and used to assess changes in responsibility for illness-related tasks. Campers and parents indicated who has responsibility, with higher scores indicating greater camper responsibility (i.e., camper = 3, equal = 2, parent = 1, or N/A if the task was not part of the camper’s care), across 34 spina bifida-related tasks (e.g., Getting in and out of my wheelchair). The mean score was used in analyses and higher scores indicated greater camper responsibility. The DFRQ has demonstrated adequate internal consistency (α = 0.85; Anderson et al., 1990).
Spina Bifida Independence Survey (T1 & T3)
The Spina Bifida Independence Survey (SBIS) was adapted from the Diabetes Independence Survey (DIS; Wysocki et al., 1996) and used as a general measure of independence in spina-bifida related tasks. It included 48-items covering a range of self-care tasks such as medication, catheterization, and requests for assistance. Choosing from “yes,” “no,” “not sure,” and “n/a,” parents of campers in the two youngest groups indicated whether specific skills were mastered. For the adult group, a self-report version (as opposed to a parent report version) of this measure was used. For analyses, a ratio score was calculated based on the total number of “yes” responses to the total number of “yes” and “no” responses. This ratio provided information regarding the degree to which a camper had mastered spina bifida-related tasks. “Not sure” responses were not included in the ratio because such answers indicated ambiguity as to whether the camper could manage the task. “N/A” responses were also not included because such tasks were not relevant for that camper. Scores ranged from 0 to 1, with a 1 indicating that the camper had mastered all spina bifida-related tasks relevant for that camper. For statistical analyses, the ratio was multiplied by 100 so that data transformations could be completed (Tabachnick & Fidell, 2001). The DIS has demonstrated adequate internal consistency (α = 0.91) and validity (Wysocki et al., 1996).
Knowledge of Spina Bifida (T1 & T3)
The child-report version of the Knowledge of Spina Bifida measure (KOSB; Greenley, Coakley, Holmbeck, Jandasek, & Wills, 2006) was used for the child and adolescent groups; questions were altered slightly to be age appropriate for the adult group. Campers answered eighteen questions about spina bifida (e.g., How old do you have to be to get spina bifida?). An expert coder from the Greenley et al. (2006) study scored these responses as correct (2 points), inconclusive (1 point), and incorrect (0 points). A mean of the scores on these 18 items was calculated and used in analyses. Higher scores indicated greater knowledge. Scale α’s were adequate and this measure has demonstrated adequate validity (Greenley et al., 2006).
Treatment Integrity
A measure to monitor interventionists’ adherence to the treatment manual was created for this study. The two interventionists independently completed a checklist of areas to be covered each day. Space was provided on the form for documentation of deviations from the manual. Reports from both interventionists indicated that on two occasions materials could not be covered on the assigned day due to time constraints. The material was covered on the subsequent day and, as a result, 100% of the manual was presented.
Results
Preliminary Analyses
Scale α’s were computed for measures with three or more items. Because several participants identified the minimum number of goals (i.e., one spina bifida related and one social goal), α’s could not be calculated for goals. In addition, α’s for the SBIS and the SOSBMR could not be computed owing to our use of “not sure” and N/A response options. Scale α’s were calculated for the KOSB; all were adequate (α’s: Time 1, Ability A = 0.89, Ability B = 0.93, Ability C = 0.91; Time 3, Ability A = 0.94, Ability B = 0.96, Ability C = 0.95).
For measures with more than one reporter, correlations were employed to determine whether reports could be collapsed. The criterion for collapsing across reporters (i.e., r ≥.40; Holmbeck, Li et al., 2002) was met only for the SOSBMR; therefore camper and parent report were collapsed. In contrast, correlations between parent and camper report for goal attainment varied across age groups and at different time periods, therefore analyses for this scale were conducted separately for parent and camper report. Lastly, for the SBIS, either parent or camper data were collected for each age group and only one reporter was used at any one time point.
Skewness analyses were conducted for all variables. Z-scores were calculated from the skewness values using the formula [S− 0/ss; where S = value reported for skewness, and ss = the standard error for skewness (Tabachnick & Fidell, 2001)]. Variables that were negatively skewed were first reflected so that subsequent data transformations could be conducted (i.e., SBIS and KOSB); therefore, the direction of these variables was reversed such that high scores indicated poorer functioning. Those variables with skewness z-score values of 3.3 or higher were transformed using a square-root transformation. This value was chosen because it was a conservative cut-off value (p < .001) for skewness (Tabachnick & Fidell, 2001). The KOSB and SBIS measures were transformed using a square root transformation.
Two participants were dropped from the analyses due to incomplete questionnaires. These individuals did not differ significantly from the sample on measures of socioeconomic status, ethnicity, camper age, or number of shunt surgeries. Finally, complete information on medical severity was not available as many parents stated they did not know the correct answers about their camper’s illness status and history.
Analyses
Repeated measures analyses of variance (ANOVA) determined whether significant change in individually oriented goals occurred from Time 1 to Time 2 to Time 3. It was hypothesized that campers would improve in individual spina bifida-related goals and individual social goals from Time 1 to Time 2. We expected gains would be maintained but not further improved, from Time 2 to Time 3. For management of spina bifida responsibilities, independence with general spina bifida-related tasks, and knowledge of spina bifida, improvement was expected from Time 1 to Time 3 (no Time 2 data were collected on these variables). Results of the tests for main effects are presented in Table II.
Table II.
Repeated Measures Analyses of Variance for Main Effects
| T1 | T2 | T3 | |||
|---|---|---|---|---|---|
| Variable | M (SD) | M (SD) | M (SD) | F | d |
| Individual SB goals (c)a,b,cn = 39 | 2.44 (0.13) | 3.55 (0.15) | 3.27 (0.17) | 36.21*** | .662 |
| Individual social goals (c)a,b,cn = 35 | 2.38 (0.17) | 3.62 (0.18) | 3.55 (0.18) | 12.33*** | .428 |
| Individual SB goals (p)a,b,cn = 45 | 2.30 (0.11) | N/A | 2.98 (0.14) | 29.51*** | .401 |
| Individual social goals (p)a,b,cn = 37 | 2.24 (0.15) | N/A | 2.93 (0.15) | 24.64*** | .406 |
| SOSBMRa,b,cn = 54 | 2.04 (0.06) | N/A | 2.12 (0.07) | 18.41*** | .258 |
| ϕSBIS (c)cn = 17 | 2.68 (0.57) | N/A | 2.22 (0.60) | 2.28 | .125 |
| ϕSBIS (p)a,bn = 33 | 4.00 (0.37) | N/A | 3.44 (0.41) | 6.90* | .177 |
| ϕKOSB (c)a,b,cn = 67 | 0.71 (0.04) | N/A | 0.88 (0.05) | 10.20** | .141 |
Note. SB, spina bifida; (c), camper report; (p), parent report.
aAbility A (ages 7–12).
bAbility B (ages 13–17).
cAbility C (ages 18–37).
ϕThese variables were reflected and transformed, therefore the direction of values shown is opposite to the original.
*p < .05; **p < .01; ***p < .001.
Significant results were found for the following outcomes: camper report of individual spina bifida goals (F = 36.21; p = .000; ES = .662), camper report of individual social goals (F = 12.33; p = .000; ES = .428), parent report of individual spina bifida goals (F = 29.51; p = .000; ES = .401); parent report of individual social goals (F = 24.64; p = .000; ES = .406), management of spina bifida responsibilities (F = 18.41; p = .000; ES = .258), parent report (i.e., Ability A and B only) of independence with general spina bifida tasks (F = 6.90; p < .05; ES = .177), and camper knowledge of spina bifida (F = 10.20; p < .01; ES = .141). For all outcomes except knowledge of spina bifida, significant change was in the direction of improved functioning; camper knowledge about spina bifida significantly decreased.
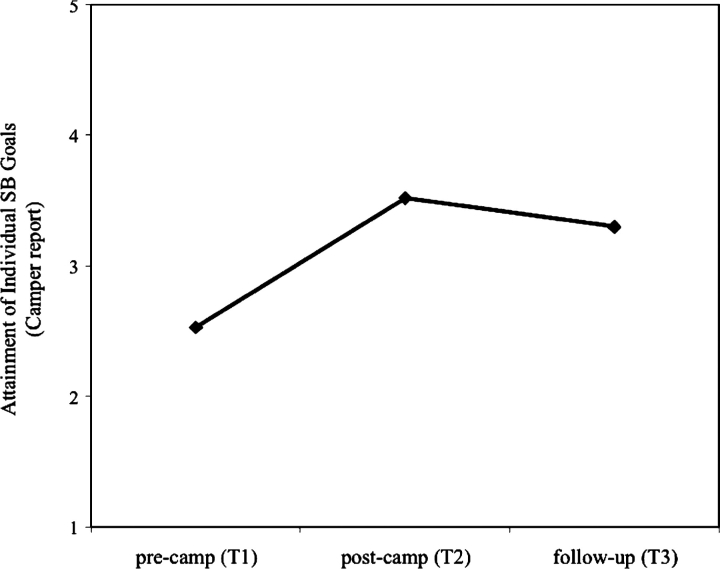
For those ANOVA results that yielded significant findings and included more than two time points (i.e., camper report of individual spina bifida goals and camper report of individual social goals), post hoc analyses were necessary to determine when the change occurred. Hypotheses were supported in that significant change was observed from Time 1 to Time 2, and not from Time 2 to Time 3. Specifically, for camper report of individual spina bifida goals and individual social goals, there was significant improvement from Time 1 to Time 2 (t = −8.02, p = .000; t = −6.78, p = .000, respectively). As predicted, there was no significant change from Time 2 to Time 3, indicating that there was not significant decay or improvement in goal attainment. Lastly, comparison of Time 1 to Time 3 indicated that gains in goals were maintained (t = −6.80, p = .000; t = −4.33, p = .000, respectively; see Figure 1).
Figure 1.
Mean level of goal attainment for individual spina bifida goals at times 1, 2, and 3
Discussion
The purpose of the present study was to design and evaluate a camp-based intervention targeting independence among children, adolescents, and adults with spina bifida. The intervention addressed some of the unique needs of this group, such as cognitive deficits, complicated daily self-care, and social isolation (Loomis et al., 1997; McLone & Ito, 1998). The intervention had three components, namely collaborative goal identification, group sessions consisting of psycho-education and cognitive tools, and counselor monitoring of goal attainment.
Main effects analyses indicated that both campers and parents observed significant improvement in individually oriented spina bifida and social goals, management of spina bifida responsibilities, and independence with general spina bifida tasks. However, the clinical significance of the latter two measures was questionable because the difference in mean scores was minimal. Moreover, repeated measures analyses include only those participants with data at all time points. Therefore, it is possible that the participants who provided data differed from those that did not (e.g., they were more motivated), thereby biasing the findings. The medium effect sizes observed for individual spina bifida and social goals may suggest that the “goals” aspect of the intervention was effective. This area of strength may be the result of the collaborative approach to goal setting, the cognitive strategies, the group sessions, a combination of such factors, or other aspects of the intervention or camp experience. Future investigations should be designed to tease apart the differing contributing roles of such factors.
We targeted memory, problem-solving, and communication to help campers make gains in independence. However, we did not directly assess these cognitive areas and therefore could not determine whether such domains were in fact problematic for this sample. Moreover, we could not examine interactions between these cognitive areas and independence or between cognitive abilities and the efficacy of the intervention. It is also possible that cognitive functioning played a role in the decline in camper knowledge of spina bifida (i.e., campers with limited cognitive abilities were confused by the information presented). Future studies should examine cognitive functioning as a potential moderator or mediator of associations between independence and intervention efficacy.
There were several other notable design and measurement limitations. A traditional control group was not feasible in this study because the collaborating organization wanted all campers to receive the intervention. Future investigations should work with organizations to develop ways to employ alternative types of control groups. As the intervention was embedded within a larger camp experience, we cannot assume that the intervention was responsible for the changes observed here. For example, it is conceivable that the experience of being away from parents facilitated independence. The drop in retention rate (i.e., one-third of the sample) at follow-up was also concerning. It is possible that those who did not participate differed from those who were retained on variables not assessed in this study.
In addition, the wide age range may have been problematic because the utility of different interventions may vary as a function of age (e.g., greater emphasis on social functioning for the adolescent group). Another limitation was that the interventionists provided the ratings of treatment integrity. These reporters were likely invested in the intervention and possibly biased in their observations. Finally, broad measures of adaptive functioning and comprehensive assessment of self-care skills were not obtained.
Findings from this investigation suggest directions for future camp-based interventions. First, given the medium effects observed, the continued emphasis on individualized goal setting, with parent, camper, and counselor involvement, is recommended. Second, greater emphasis on social skills is encouraged as the camp setting naturally lends itself to such a focus; moreover, campers seemed most engaged in the social aspects of the intervention. Third, more feasible and appealing cognitive tools should be incorporated. It was observed anecdotally that the memory diary was often not integrated into camper routine.
Fourth, we recommend that campers be provided with assistance for transferring independence gains to other settings because it may be difficult to generalize treatment effects beyond the camp context (Pelham & Fabiano, 2008). One way to increase skill generalization is parent involvement, which should vary depending upon the individual child’s ability to appropriately manage their illness (Palmer et al., 2009). It may be helpful for parents, campers, and counselors to discuss the camper’s progress as part of an exit interview. Specifically, strategies to maintain these gains at home, including how much parental assistance is necessary, could be explored. In addition, parents and campers could identify new independence goals for the upcoming year. Maintenance of gains may also be facilitated by booster sessions (Clarke, Rohde, Lewinsohn, Hops, & Seeley, 1999); the interventionist could call families every three months to assess current level of goal attainment, help generate new independence goals, and provide assistance with obstacles. Fifth, and finally, we recommend continuity in camp programming as many campers return to the same camp for several years. Specifically, tracking camper progress from year to year, making improvements in the intervention, and encouraging campers to use previously taught skills would likely strengthen programming and improve camper outcomes.
Funding
Completion of this article was supported in part by research grants from the Spastic Paralysis Research Foundation: Illinois-Eastern Iowa District of Kiwanis International and the National Institute of Child Health and Human Development (RO1 HD048629).
Conflicts of interest: None declared.
Acknowledgments
The authors wish to thank Camp Ability, the Spina Bifida Association of Illinois, Sara Talbert, and numerous undergraduate and graduate research assistants for help with data collection and data entry. Most importantly, we gratefully acknowledge the contributions to this study by the parents and children who participated in this research.
References
- American Camping Association. 2008. The camp resource for families. Retrieved July 1, 2008 from http://find.acacamps.org/fingin_advanced.php. [Google Scholar]
- Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. Journal of Pediatric Psychology. 1990;15:477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- Appleton PL, Ellis NC, Minchom PE, Lawson V, Boll V, Jones P. Depressive symptoms and self-concept in young people with spina bifida. Journal of Pediatric Psychology. 1997;22:707–722. doi: 10.1093/jpepsy/22.5.707. [DOI] [PubMed] [Google Scholar]
- Briery BG, Rabian B. Psychosocial changes associated with participation in a pediatric summer camp. Journal of Pediatric Psychology. 1999;24:183–190. doi: 10.1093/jpepsy/24.2.183. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2008. Trends in spina bifida and anencephalus in the United States, 1991–2005. Retrieved July 20, 2008 from http://ww.cdc.gov/nchs/products/pubs/pubd/hestats/spine_anen.htm. [Google Scholar]
- Clarke GN, Rohde P, Lewinsohn PM, Hops H, Seeley JR. Cognitive- behavioral treatment of adolescent depression: Efficacy of acute group treatment and booster sessions. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:272–279. doi: 10.1097/00004583-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Evans DW, Foa EB, Gur RE, Hendin H, O’Brian CP, Seligman MEP, et al. Commission on Positive Youth Development. Treating and preventing adolescent mental health disorders: What we know and what we don’t know. 2005. The positive perspective on youth development. (pp. 497–527). New York: Oxford University Press. [Google Scholar]
- Dennis M, Landry SH, Barnes M, Fletcher JM. A model of neurocognitive function in spina bifida over the life span. Journal of the International Neuropsychological Society. 2006;12:285–296. doi: 10.1017/S1355617706060371. [DOI] [PubMed] [Google Scholar]
- Engelman BE, Loomis JW, Kleiback L. A psychoeducational group addressing self-care, self-esteem, and social skills in children with spina bifida. European Journal of Pediatric Surgery. 1994;4:38–39. [PubMed] [Google Scholar]
- Fletcher JM, Dennis M, Northrup H. Hydrocephalus. In: Yeates KO, Ris MD, Taylor HG, editors. Pediatric neuropsychology: Research, theory, and practice. New York: Guilford Press; 2000. pp. 25–46. [Google Scholar]
- Greenley RN, Coakley RM, Holmbeck GN, Jandasek B, Wills K. Condition- related knowledge among children with spina bifida: Longitudinal changes and predictors. Journal of Pediatric Psychology. 2006;31:828–839. doi: 10.1093/jpepsy/jsj097. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four factor index of social status. 1975. Unpublished manuscript, Yale University, New Haven, CT. [Google Scholar]
- Holmbeck GN, Faier-Routman J. Spinal lesion level, shunt status, family relationships, and psychosocial adjustment in children and adolescents with spina bifida myelomeningocele. Journal of Pediatric Psychology. 1995;20:817–832. doi: 10.1093/jpepsy/20.6.817. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN, Greenley RN, Coakley RM, Greco J, Hagstrom J. Family functioning in children and adolescents with spina bifida: An evidence-based review of research and interventions. Journal of Developmental and Behavioral Pediatrics. 2006;27:249–277. doi: 10.1097/00004703-200606000-00012. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN, Johnson SZ, Wills KE, McKernon W, Rose B, Erklin S. Observed and perceived parental overprotection in relation to psychosocial adjustment in preadolescents with a physical disability: The mediational role of behavioral autonomy. Journal of Consulting and Clinical Psychology. 2002;70:96–110. doi: 10.1037//0022-006x.70.1.96. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN, Li ST, Schurman JV, Friedman D, Coakley RM. Collecting and managing multisource and multimethod data in studies of pediatric populations. Journal of Pediatric Psychology. 2002;27:5–18. doi: 10.1093/jpepsy/27.1.5. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN, Westhoven VC, Phillips WS, Bowers R, Gruse C, Nikolopoulos T. A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. Journal of Consulting and Clinical Psychology. 2003;71:782–796. doi: 10.1037/0022-006x.71.4.782. [DOI] [PubMed] [Google Scholar]
- Hommeyer JS, Holmbeck GN, Wills KE, Coers S. Condition severity and psychosocial functioning in pre-adolescents with spina bifida: Disentangling proximal functional status and distal adjustment outcomes. Journal of Pediatric Psychology. 1999;24:499–509. doi: 10.1093/jpepsy/24.6.499. [DOI] [PubMed] [Google Scholar]
- Jandasek B, O’Mahar K, Zukerman J, Holmbeck GN. Camp ability: A camp curriculum addressing independence, social skills, emotional wellness, and self-care. 2005. Unpublished manual. [Google Scholar]
- Joyce BM, Rockwood KJ, Mate-Kole CC. Use of goal attainment scaling in brain injury in a rehabilitation hospital. American Journal of Physical Medicine and Rehabilitation. 1994;73:10–14. [PubMed] [Google Scholar]
- Kazak A. The social context of coping with childhood chronic illness: Family systems and social support. In: La Greca AM, Siegel LJ, Wallander JL, Walker CE, editors. Stress and coping in child health. New York: Guilford Press; 1992. pp. 262–278. [Google Scholar]
- Kendall PC, Morris RJ. Child therapy: Issues and recommendations. Journal of Consulting and Clinical Psychology. 1991;59:777–784. doi: 10.1037//0022-006x.59.6.777. [DOI] [PubMed] [Google Scholar]
- King JC, Currie DM, Wright E. Bowel training in spina bifida: Importance of education, patient compliance, age, and anal reflexes. Archives of Physical Medical Rehabilitation. 1994;75:243–247. doi: 10.1016/0003-9993(94)90022-1. [DOI] [PubMed] [Google Scholar]
- King GA, Specht JA, Schultz I, Warr-Leeper G, Redekop W, Risebrough N. Social skills training for withdrawn unpopular children with physical disabilities: A preliminary evaluation. Rehabilitation Psychology. 1997;42:47–60. [Google Scholar]
- Loomis JW, Javornisky JG, Monahan JJ, Burke G, Lindsay A. Relations between family environment and adjustment outcomes in young adults with spina bifida. Developmental Medicine and Child Neurology. 1997;39:620–627. doi: 10.1111/j.1469-8749.1997.tb07498.x. [DOI] [PubMed] [Google Scholar]
- Malec JF. Goal attainment scaling in rehabilitation. Neuropsychological Rehabilitation. 1999;9:253–275. [Google Scholar]
- McLone DG, Ito J. An introduction to spina bifida. 1998. Unpublished manuscript. Chicago: Children’s Memorial Hospital MM Team. [Google Scholar]
- Nergardh A, von Hedenberg C, Hellstrom B, Ericsson N. Continence training of children with neurogenic bladder dysfunction. Developmental Medicine and Child Neurology. 1974;16:47–52. doi: 10.1111/j.1469-8749.1974.tb02710.x. [DOI] [PubMed] [Google Scholar]
- O’Mahar K, Jandasek B, Zukerman J, Holmbeck GN. Camp ability 2006 independence intervention – cognitive strategies emphasis. 2006. Unpublished manual. [Google Scholar]
- Palmer DL, Berg CA, Butler J, Fortenberry K, Murray M, Lindsay R. Mothers’, fathers’, and children’s perceptions of parental diabetes responsibility in adolescence: Examining the roles of age, pubertal status, and efficacy. Journal of Pediatric Psychology. 2009;34:195–204. doi: 10.1093/jpepsy/jsn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA. Evidence-based psychosocial treatments for attention deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2008;37:184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Rudeberg A, Donati F, Kaiser G. Psychosocial aspects in the treatment of children with myelomeningocele: An assessment after a decade. European Journal of Pediatrics. 1995;154:S85–S89. doi: 10.1007/BF02191514. [DOI] [PubMed] [Google Scholar]
- Sherman RG, Berling BS, Oppenheimer S. Increasing community independence for adolescents with spina bifida. Adolescence. 1985;20:1–13. [PubMed] [Google Scholar]
- Spirito A, Kazak A. Effective and emerging treatments in pediatric psychology. New York: Oxford University Press; 2005. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4th. Needham Heights, MA: Allyn & Bacon; 2001. [Google Scholar]
- Watson D. Occupational therapy intervention guidelines for children and adolescents with spina bifida. Child: Care, Health, and Development. 1991;17:367–380. doi: 10.1111/j.1365-2214.1991.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Wills KE. Neuropsychological functioning in children with spina bifida and/or hydrocephalus. Journal of Clinical Child Psychology. 1993;22:247–265. [Google Scholar]
- Wysocki T, Meinhold PM, Taylor A, Hough BS, Barnard MU, Clark WL. Psychometric properties and normative data for the parent version of the Diabetes Independence Survey. The Diabetes Educator. 1996;22:587–591. doi: 10.1177/014572179602200606. [DOI] [PubMed] [Google Scholar]