Abstract
Seasonal variation in the volume of various song control nuclei in many passerine species remains one of the best examples of naturally occurring adult neuroplasticity among vertebrates. The lateral portion of the magnocellular nucleus of the anterior nidopallium (lMAN) is a song nucleus that is important for song learning and seems to be critical for inducing variability in the song structure that is later pruned via a feedback process to produce adult crystallized song. To date, lMAN has not been shown to exhibit seasonal changes in volume, probably because it is difficult to resolve the boundaries of lMAN when employing histological methods based on Nissl staining. Here, lMANcore volumes were examined in intact photostimulated (i.e. breeding), castrated photostimulated and photorefractory (i.e. non-breeding) male starlings (Sturnus vulgaris) to investigate the degree of seasonal variation in brain morphology. We present data demonstrating that the volumes of the total MAN and lMANcore delineated by enkephalin immunoreactivity are greater in photostimulated male starlings as compared to photorefractory males. Moreover, two other regions associated with the song system that have not been investigated previously in the context of seasonal plasticity namely i) the medial portion of MAN (mMAN), and ii) the nucleus interfacialis (NIf) did not display significant volumetric variation. We propose that greater lMANcore volumes are associated with the increase in vocal plasticity which is generally observed prior to production of stereotyped song.
Keywords: songbird, neuropeptide, reproduction, neuroplasticity, learning
INTRODUCTION
Seasonally breeding songbirds in the temperate zone display one of the most dramatic examples of naturally-occurring neuroplasticity and behavior known in extant vertebrate species. In particular, many songbird species exhibit profound seasonal changes in singing behavior. This seasonal variation in singing behavior correlates positively with seasonal changes in testosterone concentrations and the volumes of brain nuclei responsible for song learning and production (Nottebohm, 1981; Brenowitz, 2008; Ball et al., 2008; Tramontin and Brenowitz 2000; Ball, 1999). There is a substantial amount of data consistent with the notion that the vernal increase in photoperiod stimulates gonadal growth and the release of gonadal sex steroid hormones such as testosterone that in turn facilitates the volumetric growth of the song system and singing behavior (Ball, 1999; Tramontin and Brenowitz 2000). However, several studies have demonstrated gonadal or testosterone-independent effects on the volumes of several song nuclei (Bernard et al., 1997; Smith et al., 1997; Gulledge and Deviche, 1998; Tramontin et al., 1999; Dloniak and Deviche, 2001; Sartor and Ball, 2005; Boseret et al., 2006). These findings suggest that factors other than steroids can regulate song nucleus volumes (Ball et al., 2004; Ball et al., 2002).
The song control system that governs singing behavior consists of a series of discrete, interconnected nuclei. One part, the anterior forebrain pathway (AFP) in particular is essential for successful song development and stereotype in adulthood (Bottjer et al., 1984, Doupe, 1997; Brainard and Doupe, 2002). The AFP circuitry originates in HVC (acronym used as a proper name; which projects to Area X; that in turn projects to the dorsolateral nucleus of the anterior thalamus (DLM); that connects to the lateral nucleus of the magnocellular nidopallium (lMAN) that projects onto the motor output pathway via a connection to the robust nucleus of the archipallium (RA). Recent studies have proposed that lMAN plays a critical role for inducing vocal plasticity in song structure during development and in adulthood (Olveczky et al., 2005; Thompson and Johnson, 2007). In male zebra finches, (Taeniopygia guttata) bilateral lesions to lMAN resulted in songs with notes that lacked the normal frequency modulations and produced extremely long bouts of songs deficient in typical phrase types (Bottjer et al., 1984; Scharff and Nottebohm, 1991). In zebra finches, lMAN has been shown to have a core and shell architecture (Johnson and Bottjer, 1992; Johnson et al., 1995). Interestingly, during song development in male zebra finches the volume of lMANshell undergoes a striking increase in overall volume during early stages of vocal learning followed by an equally substantial decrease by adulthood when birds have acquired stable song patterns (Johnson and Bottjer, 1992; Iyengar and Bottjer, 2002). Moreover, male zebra finches were observed to have increased lMAN cell soma at 50 days compared to female zebra finches (Nixdorf-Bergweiler, 1998). This increase in volume coincides with the sensorimotor phase of song learning when male zebra finches produce highly variable vocal patterns that are gradually modified to match the initially memorized tutor songs (Nixdorf-Bergweiler, 1998). Many seasonally breeding songbirds, in particular European starlings (Sturnus vulgaris) exhibit seasonal and age dependent variation in song structure (see Eens, 1997 for a review). It has been proposed that seasonally breeding birds undergo an annual sensorimotor phase resulting in variation in song structure (see Brainard and Doupe, 2002 for a review). It is possible that the annual sensorimotor phase coincides with changes in lMANshell and/or lMANcore volumes as a result of the yearly induction of variability in song repertoires similar to the sort of structural changes that occur in lMAN during development in association with sensorimotor learning. Traditional Nissl staining methods can define the borders of lMANcore, (Johnson and Bottjer, 1992). However, previous studies attempting to delineate volumetric variation in seasonally breeding birds have produced inconsistent results due to an inability to crisply delineate nucleus boundaries (Airey and DeVoogd, 2000; MacDougall-Shackleton et al., 1998; MacDougall-Shackleton et al., 2005). However, chemical neuroanatomical studies have revealed a variety of neuromodulatory proteins that do clearly delineate the boundaries of lMANcore and in some cases mMAN (Ball et al., 1988; Ball and Balthazart, 2010). By using these alternative approaches to nuclear boundary definition, one can gain insight into the seasonal regulation of lMANcore volume.
The nucleus interface of the nidopallium (NIf) is a song system nucleus that connects to HVC and the auditory system. NIf sends afferent connections to HVC (Nottebohm et al., 1982; Janata and Margoliash, 1996), the main sensorimotor nucleus necessary for song learning and production. NIf is also reciprocally connected with the caudal lateral mesopallium (CLM), a secondary auditory processing area (Vates et al., 1996). Cells located in NIf exhibit strong selectivity to the bird’s own song (BOS) and the response is dependent on behavioral state. Moreover, NIf cell show strong premotor activity that is time-locked to the production of song syllables (Hahnloser and Fee, 2003; Cardin and Schmidt, 2003, 2004). Bilateral lesions of NIf result in significant reductions in spontaneous and auditory activity in HVC, however, song production was not impaired (Cardin et al., 2005). These data suggest then that NIf is a major source of auditory input to HVC, but is not necessary for motor production.
Immunohistochemical studies have demonstrated that NIf is a site for neuromodulation by a number of different transmitter systems. Specifically, neuromodulatory inputs to NIf include catecholaminergic (Soha et al., 1996; Harding et al., 1998; Mello et al., 1998); cholinergic (Ryan and Arnold, 1981a); as well as peptide systems such as vasoactive intestinal polypeptide (VIP) and enkephalin (ENK; Ryan et al., 1981b; Ball et al., 1995). Interestingly, both VIP and ENK clearly delineate the boundaries of the nucleus and reveal a sex difference in the volume of NIf with greater volumes found in male compared to female zebra finches (Ball et al., 1995). Given the importance of auditory feedback for song learning and maintenance of stereotype (Leonardo, 2004, Brainard and Doupe, 2001), and the intimate association of NIf to the song system, this nucleus is a candidate to undergo seasonal variation in volume.
In the present study, we compared the volumes of total MAN, lMANcore, mMAN and NIf based on enkephalin immunoreactivity from male starlings that were either photostimulated intact, photostimulated castrate or photorefractory. We found that lMANcore volumes were significantly greater in photostimulated birds independent of gonadal state and suggest that the variation in lMANcore volume is associated with changes in vocal plasticity in a seasonally breeding songbird.
METHODS
Twenty four wild caught adult male European starlings were caught using a drop down V-trap in early March 2008 in Conneautville, PA (41° 45′ N lat., 80° 22′ W long). Birds were transported to the Johns Hopkins University and group housed (4 per cage). All birds were placed on the natural photoperiod experienced on the date of capture (11L:13D) in order to maintain photosensitivity. Birds were treated and handled in accordance with all appropriate animal care guidelines and permits.
Treatment Groups
Seven days after acclimation to laboratory conditions, all starlings were laparotomized under isoflurane (3–4% induction, then 1–2% maintenance) and the dimensions of the left testis were measured with calipers. Testis volume was determined using the equation V = 4/3πa2b, where a is half the width and b is half the length. All birds were observed to have undeveloped testis indicative of a photosensitive state and suggest that the birds were not currently exhibiting gonadal recrudescence (Mean ± SEM = 58 ± 4 mm3). These data indicate that starlings had not experienced elevated levels of gonadal steroids prior to the present experiment. Eight adult males were selected for castration, starlings were anesthetized and a small incision was made in the lower abdomen and the testes removed with forceps. For the sham surgeries eight adult males were anesthetized and a small incision was made in the lower abdomen and the testes were touched with forceps but not removed. After sacrifice the body cavity was inspected for testicular fragments and none were found in castrates. The eight castrated and intact males were housed individually on short daylengths to maintain sensitivity. Two weeks prior to the termination of the experiment, all males were transferred to long daylengths (16L:8D) to induce gonadal growth and a photostimulated state. Another group of eight males were placed on the same long photoperiod for nine weeks to induce photorefractoriness. The photoperiods utilized in this experiment have previously shown to be effective to induce photostimulation and subsequent photorefractory states (Dawson et al., 2001). The photoperiod treatment conditions were arranged such that all birds were in the required photoperiodic condition so that their brains were collected on the same calendar day. To determine male reproductive condition, beak coloration was assessed throughout the experiment; and blood was collected for testosterone determination and testis volume was measured at the time of brain collection. The color of the beak is a reliable indicator of whether testosterone is present or absent in the circulation in European starlings. Yellow means that testosterone is detectable by standard assay methods such as radioimmunoassay while black means that testosterone is undetectable (Ball and Wingfield, 1987; Gwinner, 1975; Dawson, 1983). Because a yellow beak is a reliable indicator of the presence of testosterone, measuring beak color provides a reliable indicator of the transition across reproductive states (Ball and Wingfield, 1987; Gwinner, 1975; Bernard and Ball, 1995; Falk and Gwinner, 1988). Beak scores were measured on a four point scale with a score of 0 equaling completely black; 1 equals black base with a little yellow tip; 2 equals a yellow beak with a slightly black coloration; and 3 is a yellow beak with blue base. At the termination of the experiment, eight intact males had bright yellow beaks, whereas the castrates and photorefractory males had black beaks indicative of no detectable testosterone being present in the circulation.
Hormone Assay
Samples for hormone analysis were taken via puncturing the alar (wing) vein with a 25-gauge needle and 300–500 μl of blood was collected into heparinized tubes. The blood samples were transferred into microfuge tubes and centrifuged at 8900 rpm for 10 minutes. The supernatant was removed with a Pasteur pipette and stored in Eppendorf vials at −20° until assayed for testosterone. The serum was analyzed in a single run of duplicates (50μl) using a commercially available 125I Coat-A-Count kit for total testosterone (Siemens Medical Solutions Diagnostics, Los Angeles, CA). This kit consistently provides reliable hormone concentrations and has been previously used in a number of avian species including starlings (Stevenson et al., 2008; Stevenson and Ball, 2009). The antiserum is highly sensitive for testosterone (i.e. 100 pg/ml) and shows negligible cross reactivity with other steroids including dihydrotestosterone (<3.5%); 17β-estradiol (< 0.01%); corticosterone (< 0.01%). The intra-assay coefficient of variation averaged 11%.
Perfusion
At the end of the experiment, birds were sacrificed and brains removed. Birds were deeply anaesthetized with secobarbital (50mg/ml im, Sigma). Then, the birds were transcardially perfused with 0.1M phosphate buffered saline (PBS) pH 7.5, followed by 4% paraformaldehyde. Then the brains were dissected out and placed in 4% paraformaldehyde and left overnight at 4°C. The following morning, the brains were transferred to a sucrose solution (30% sucrose in 0.1 M PBS) and left overnight at 4°C. The brains were then frozen with powdered dry ice for five min., and left in the freezer (−70°C) until sectioning. Brains were sectioned coronally (40 μm) using a cryostat, every fourth section was collected and placed in tissue wells containing 0.1M PBS.
Antibody Specificity
Immunocytochemistry was carried out on all brains in random order such that the time from tissue collection to processing was equivalent for all groups. Control tests were conducted to validate the primary antibody used in this protocol. Tissue sections were either incubated in the absence of primary/secondary antibody or presaturated with enkephalin antigen (Bachem). The absence of the primary and secondary antibody eliminated enkephalin immunoreactivity. Furthermore, preabsorption of the enkephalin antibody (1:2000 methionine enkephalin; ENK Immunostar Inc.) with 100 μg of enkephalin (Bachem) significantly reduced enkephalin immunoreactivity.
Immunocytochemistry
The immunocytochemistry protocol commenced with the sections washed in 0.1 M phosphate buffer saline (PBS) three times, once in 0.5% H2O2 for 15 min., then washed three times in 0.1 M PBS and left overnight in tyramide blocking reagent solution (TNB; Perkin Elmer, TSA Biotin System) at 4°C. The following day sections were incubated in TNB and primary antibody (1:2000 methionine enkephalin; ENK Immunostar Inc.) for 1 hr at room temperature and then place at 4°C overnight with agitation. The sections were then washed three times in 0.1M PBS with 1% Triton X (PBS/T; Fisher Scientific Laboatories), then incubated in biotinylated secondary antibody (goat anti rabbit IgG, 1:250) for 1 h, washed three times in 0.1% PBS/T, incubated in avidin biotin horseradish-peroxidase complex (Vectastain ABC, Elite Kit 1:200) for 1 h and then washed again three times in 0.1% PBS/T. Sections were then incubated in biotinylated tryamine (1:150; Perkin Elmer) and then washed three times in 0.1% PBS/T. Followed by one hour incubation in horseradish peroxidase (Perkin Elmer, TSA Biotin System, 1:200) and subsequently washed three times in 0.1% PBS/T. Antibodies were visualized by incubating the sections with diaminobenzidine (Sigma Fast DAB) for 4 minutes. Finally, sections were washed three times with 0.1 M PBS and mounted onto gelatin coated microscope slides. Sections were then serially dehydrated in ethanol and then placed in xylene for ten minutes. The slides were coverslipped using Permount (Fisher).
Song Nucleus Volume Reconstruction and Statistical Analysis
We measured the volume of HVC, Area X, and RA using thionin, a dye that stains for Nissl bodies. We collected 40 μm sections from regions of interest and placed in .1 M PBS solution, then mounted onto gelatin-coated microscope slides. The slides were dried, stained with thionin for two minutes, serially dehydrated in ethanol at 50%, 75%, 95%, 100% for one minute and a final step in 100% ethanol for five minutes. The slides were then cleared in xylene (Fisher Scientific) then coverslipped with Permount (Fisher Scientific). The boundaries of lMANcore, mMAN, and NIF were defined by enkephalin immunoreactivity. In brief, brain regions of interest were digitized using a bright field light microscope (Zeiss Axioskop, Carl Zeiss, Thornwood NY) with a CCD camera connected to a MacIntosh computer. For each image, the area of the brain region was measured using Openlab 5.0.2 (Improvision, Lexington, MA). The volume of each region was then reconstructed combining the areas of subsequent sections with the sampling interval (160 μm) using the formula for a truncated cone (developed by Smith et al., 1995) as used previously in starlings (Bernard and Ball, 1995; Bentley et al., 1999; Bernard and Ball, 1997). For each bird we used the average volume of the left and right hemispheres, summed the values across sections and then multiplied by the width of the interval. The volumes of all nuclei examined could be successfully delineated in all males.
One-way ANOVAs were conducted for beak scores, plasma testosterone concentrations, and brain nucleus volume. For testis volume, the castrates were removed from the statistical analysis and a t-test was conducted between intact photostimulated and photorefractory starlings. Tukey’s post hoc tests were conducted to evaluate pair-wise comparisons. Pearson’s correlation tests were conducted to evaluate inter-hemispheric differences in song nucleus volumes lMANcore, mMAN and NIf that were obtained using enkephalin immunoreactivity.
RESULTS
Assessment of Reproductive State
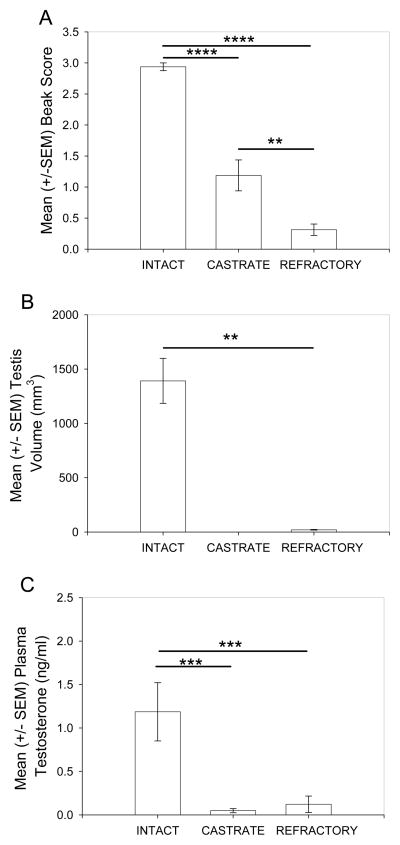
A one-way ANOVA revealed a significant difference in beak scores across treatment groups (F(2,23) = 72.21, P < 0.001; Fig. 1A). Tukey’s post-hoc analysis demonstrated that intact males had significantly higher beak scores compared to castrate (p < 0.001) and photorefractory males (p < 0.001). Furthermore, castrated males had higher beak scores compared to photorefractory males (p < 0.01). A t-test revealed a significant difference in testis volume between photostimulated and photorefractory starlings (t(14) = 12.18, p < 0.001). A one-way ANOVA for plasma testosterone concentrations showed a significant difference across treatment groups (F(2,23) = 9.97, P < 0.001; Fig 1C). Tukey’s post-hoc analysis revealed a significant difference with intacts having greater amounts of testosterone compared to castrates (p < 0.005) and photorefractory birds (p < 0.005). However, there was no significant difference between castrated and photorefractory males (p = 0.96 ns).
Figure 1.
The reproductive state in male European starlings. A) Mean (+/− SEM) beak score; B) Mean (+/− SEM) testis volume; C) Mean (+/− SEM) plasma testosterone concentrations at sacrifice. Significance was determined at * P < 0.05; ** P < 0.01; *** P < 0.005; **** P < 0.001 and asterisks indicate significant differences.
Song Nucleus Volumes
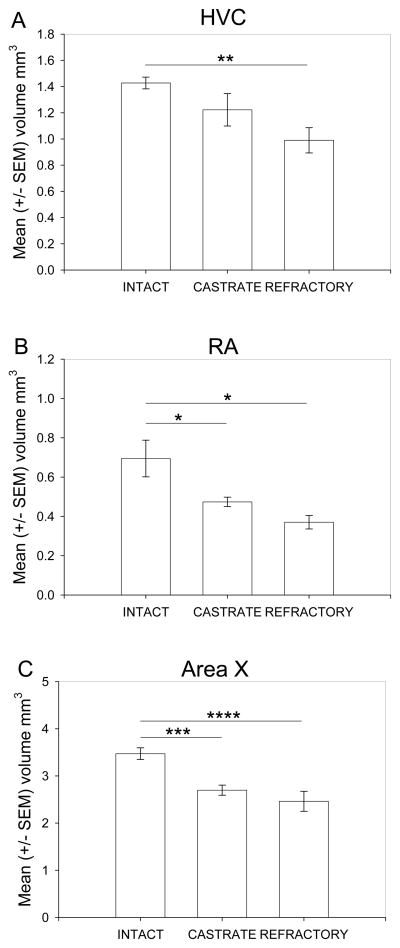
A one-way ANOVA revealed a significant difference in HVC volume across treatment groups (F(2,23) = 5.38, P < 0.05; Fig. 2A). Tukey’s post-hoc analysis demonstrated that intact males had significantly larger HVC volumes compared to photorefractory birds (p < 0.01). However, there were no significant differences between intact and castrate (p = 0.29), and castrate and photorefractory males (p = 0.21). There was a significant difference in the volumes of Area X across treatment groups F(2,23) = 11.53, P < 0.001; Fig. 2B). Intact starlings had significantly greater Area X volumes compared to castrates (p < 0.005) and photorefractory starlings (p < 0.001). There was no significant difference between castrated and photorefractory starlings (p = 0.61). There was a significant difference in RA volume across treatment groups (F(2,23) = 8.86, P < 0.005; Fig. 2C). Intact starlings had significantly greater RA volumes compared to castrates (p < 0.05) and photorefractory starlings (p < 0.001). There was no significant difference between castrated and photorefractory starlings (p = 0.35).
Figure 2.
Nissl defined boundaries for HVC, RA and Area X volumes. A) Mean (+/− SEM) HVC volumes; B) Mean (+/− SEM) RA; and C) Mean (+/− SEM) Area X volumes. Significance was determined at * P < 0.05; ** P < 0.01; *** P < 0.005; **** P < 0.001 and asterisks indicate significant differences.
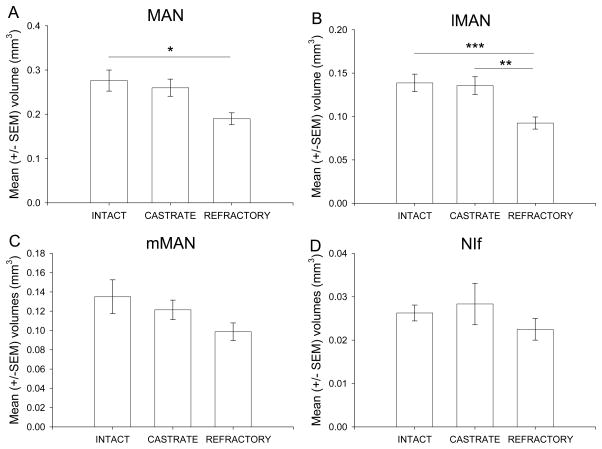
A one-way ANOVA revealed a significant difference in total MAN volumes across the treatment groups (F(2,23) = 5.29, P < 0.05; Fig. 3A, Fig. 4). Tukey’s post-hoc test showed that intact males were significantly greater MAN volumes compared to photorefractory males (p < 0.05). However, there was no significant difference between intacts versus castrates (p = 0.86, ns) and castrates versus photorefractory males (p = 0.06). A one-way ANOVA demonstrated a significant difference in lMANcore volumes across treatment groups (F(2,23) = 8.66, P < 0.005; Fig. 3B). Intact males had significantly greater lMANcore volumes compared to photorefractory males (p < 0.005). Furthermore, castrated males had significantly greater volumes compared to photorefractory males (p < 0.01). However, there was no significant difference between intact and castrated males (p = 0.97, ns). There was no significant difference across treatment groups on measures of mMAN volumes (F(2,23) = 2.16, P = 0.14, ns; Fig. 3C). There was no significant difference in NIf volumes across treatment groups (F(2,23) = 0.97, P = 0.39, ns; Fig. 3D). Pearson’s correlations were conducted for lMANcore, mMAN and NIf volumes to evaluate the relationship between the measurements collected from the left and right hemispheres. All song nucleus volumes had significantly positive inter-hemisphere correlations (lMANcore: r = 0.77; P < 0.0001; mMAN: r = 0.80; P < 0.0001; NIf: r = 0.53; P < 0.0001).
Figure 3.
Enkephalin immunoreactivity defined variation in song nuclei lMAN and auditory nuclei NIf volumes. A) Mean (+/− SEM) total MAN volumes; B) Mean (+/− SEM) lMAN volume; C) Mean (+/− SEM) mMAN volumes; D) Mean (+/− SEM) NIf volume. Significance was determined at * P < 0.05; ** P < 0.01; *** P < 0.005; **** P < 0.001 and asterisks indicate significant differences.
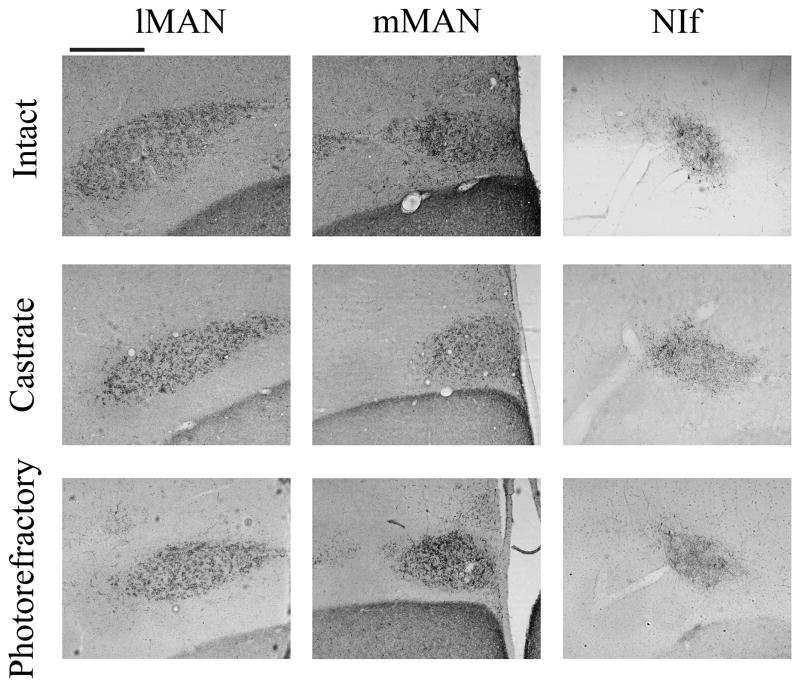
Figure 4.
Photomicrographs of representative sections for lMAN, mMAN and NIf across treatment conditions. The columns represent the song nuclei lMAN, mMAN and NIf. The rows represent the treatment groups: photostimulated intact, photostimulated castrate and photorefractory male European starlings. Scale bar represents 130 μm.
DISCUSSION
This study provides evidence for the occurrence of photoperiodic changes in the volume of lMANcore, but not the closely associated mMAN or NIf in male European starlings. The song nucleus volumes of both intact and castrated photostimulated males had significantly greater lMANcore volumes compared to photorefractory males. These data suggest that the increase in photoperiod can regulate lMANcore volume in male European starlings independently of gonadal state. We also found that the volume of mMAN and NIf were relatively constant across the treatment conditions. These findings provide novel insight into the photoperiodic regulation of brain regions that are necessary for song learning and maintenance. We also replicated previous studies in starlings indicating that photoperiod and gonadal testosterone can regulate the volume of song nuclei such as HVC, RA and Area X (e.g., Bernard and Ball, 1995; Riters et al. 2002) as has been shown in a variety of other songbird species (Tramontin and Brenowitz, 2000).
Histochemical delineation of song nucleus volume
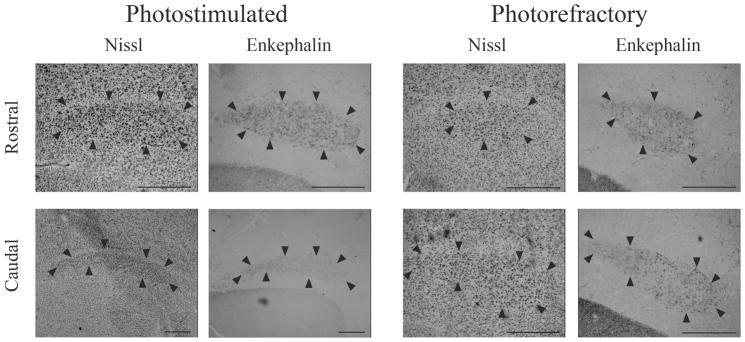
Nissl staining methods have traditionally been employed to delineate song nucleus boundaries for volumetric analyses. However, Nissl stains are limited in the information they provide. Nissl stains label more prominently components of a cell that are highly basophilic (i.e. ribosomes, Raine, 1989). Since ribosomes are essential organelles involved in protein synthesis; this method provides an indication of relative cellular activity with darker stains associated with more active cells (Raine, 1989). Nissl staining in general works extremely well for delineating the boundaries of many song nuclei (i.e. HVC, RA and Area X), however, other methods work better in the case of other nuclei (e.g., lMAN, NIf). Other histological markers have been demonstrated to provide an alternative method for measuring the volumes of song control nucleus by clearly delineating the boundaries of individual nuclei (Bernard and Ball, 1995; Gahr, 1990). Specifically, the Nissl stain is based on a grade of intensity and in some brain regions the borders are challenging to identify whereas the pattern of enkephalin immunoreactivity in the case of lMAN, for example, is more of a binary decision (i.e. present versus absent). It has been argued that a comprehensive analysis of song nucleus neuroplasticity requires the combination of cytoarchitectural, cytochemical and hodological experiments (Ball et al., 1994; Gahr, 1997; also see Vogels, 1997; Ball and Balthazart, 1997; Bottjer and Johnson, 1997; Brenowitz and Smith, 1997). The advantage of using enkephalin as a histological marker is that it provides a clear distinction of lMANcore from other surrounding brain areas (see Fig. 4; Fig. 5). Taken together, the use of ENK immunoreactivity in the current experiment provides a direct means to determine volumetric changes in a telencephalic song nucleus, lMANcore.
Figure 5.
Photomicrographs of lMANcore from intact photostimulated and photorefractory starlings defined by Nissl stain and enkephalin immunoreactivity. The columns are sequential sections that compare enkephalin immunoreactivity and Nissl stain for the two treatment groups. The rows indicate sections taken from the rostral and caudal regions of lMANcore. Arrows indicate boundaries used to delineate the volume using enkephalin immunoreactivity and approximated boundaries in Nissl stained sections. Scale bar represents 130 μm.
Functional Significance of Seasonal Changes in lMAN Volume
The seasonal variation in the song control system was initially observed for three song nucleus, HVC, RA and Area X based on studies in canaries (Nottebohm, 1981). The initial hypothesis for the functional significance of seasonal variation in song control nucleus proposed that the greater volumes of HVC and RA nuclei were necessary for song learning (Nottebohm, 1981; Nottebohm et al., 1981) and was consequently associated with an increase in song repertoire sizes (DeVoogd et al., 1993). However, the validity of this claim has been questioned by the fact that species that exhibit no seasonal change in song repertoire do exhibit marked seasonal changes in volume (e.g., Brenowitz et al., 1991) and by a study that demonstrated that population differences in repertoire size is positively correlated with large volumes of HVC and RA observed in marsh wrens (Cistothorus palustris) developed independently of song learning (Brenowitz et al., 1995). Another set of hypotheses proposed to explain seasonal variation in song nucleus volume attributes this variation to seasonal variation in song performance (e.g., Smith et al., 1995; 1997). According to these hypotheses the quality of song performance (e.g., degree of song stereotypy) (Smith et al., 1995; 1997) and/or the rate at which birds engage in singing behavior (Sartor and Ball, 2005) are associated with greater HVC volumes. There is substantial support from a variety of species for these types of explanations of the function of song system plasticity (Brenowitz, 2008).
In starlings, HVC and RA do exhibit variation in song nucleus volume as a function of photoperiodic state (Bernard and Ball, 1995; Bernard et al., 1996; Bentley et al., 1999) or season (Riters et al., 2000). The variation in song nucleus volumes are positively correlated with song bout length (Bernard et al., 1996) and singing rate (Sartor and Ball, 2005). Unlike HVC, lMAN is not required for song production in adult birds, but it is necessary for normal song learning in juveniles (based on studies in zebra finches; Bottjer et al., 1984) and plays a role in producing song variability in adult and juvenile zebra finches (Olveczky et al., 2005, Thompson et al., 2007; Aronov et al., 2008). We propose that the photoperiodic variation in lMANcore volumes is associated with increased singing performance as a result of the song remodeling that is associated with the yearly plasticity in stereotypy and other measures of song performance. Support for this hypothesis requires comparing the amount of variability in song structure with lMANcore volumes during the annual sensorimotor phase of song learning that occurs in male starlings (Eens, 1997). In male zebra finches, lMANshell exhibits a marked increase in overall volume during the early stages of vocal development followed by a dramatic retraction by adulthood when birds have acquired stable song patterns (Johnson and Bottjer, 1992). The decrease in lMAN volume is paralleled by a substantial decline in the numbers of lMAN neurons (Bottjer and Sengelaub, 1989). Moreover, the total number of DLM neurons remains stable throughout this period and the extensive changes in DLM to lMAN circuit are presumably attributable to the dynamic rearrangements at the level of individual DLM axon arbors over the period of song development (Iyengar and Bottjer, 2002). These findings indeed suggest that the volumetric changes in lMAN during song development in juvenile finches are associated with changes in vocal plasticity.
European starlings exhibit seasonal variation in song bout length with longer bouts associated with the breeding period (Riters et al., 2000; Eens et al., 1994; Eens, 1997). The annual change in song behavior is associated with the seasonal variation in the volumes of HVC, Area X and RA with larger volumes occurring in association with breeding conditions (Bernard et al., 1996; Riters et al., 2000). Here, we also observed photoperiodic-induced volumetric changes in HVC, RA and Area X. Moreover, HVC volumes in photostimulated castrates were intermediate to intact and photorefractory starlings. Whereas nucleus RA and Area X were both significantly smaller in photostimulated castrates compared to intacts. These findings are reminiscent of those previously reported in white-crowned sparrows in which the growth of HVC precedes RA and Area X (Tramontin et al., 2000). Specifically, we observed a slight increase in HVC volume in castrates without an increase in Area X and RA. Since photostimulated castrates did not have gonadal steroids, HVC may have been unable to cause the full trans-synaptic effects on RA and Area X. Thus, these findings appear to provide further support for the hypothesis that there is sequential growth of the song system (i.e. RA and Area X after HVC) and that these volume increases in RA and Area X are dependent on the initial increase in HVC volume. Interestingly, the significant increase in lMANcore volume observed in photostimulated castrates suggests that gonadal steroids are not necessary for regulating the seasonal change in lMANcore volume. It is tempting to speculate that lMANcore growth may actually precede the volumetric changes in HVC, RA, and Area X; in which the former is essential for inducing vocal plasticity while the later is critical for the development of stereotyped song.
Functional Significance of mMAN and NIf Volume
The functional significance of mMAN for song learning and production has been studied to a lesser extent compared to lMAN in all songbird species. mMAN sends projections to HVC (Nottebohm et al., 1982; Bottjer et al., 1989) and receives input from the DLM and RA (Foster et al., 1997; Vates et al., 1997). The transient inactivation of mMAN using TTX does not significantly affect song structure in zebra finches, suggesting that an intact mMAN is not necessary for either song production or vocal plasticity (Olveczky et al., 2005). The lack of seasonal variation in nucleus volume provides further evidence suggesting that mMAN is not necessarily involved with changes in song production and/or song quality. It remains to be determined what the functional significant of mMAN is in relation to song. Given its connections with other brain nucleus involved in the regulation of song development and production it is certainly reasonable to postulate that it does play a role.
NIf receives afferent connections from the thalamic nucleus Uva and sends efferent connections directly into HVC (Nottebohm et al., 1982). It has been postulated that NIf provides key auditory input to the song system (Janata and Margoliash, 1999) for the sort of sensory-motor integration that is essential for song development. However, lesions of NIf do not prevent successful song learning in male zebra finch (Gardner and Fee, 2007), suggesting that auditory input to HVC also originates from auditory processing areas other than NIf. Uva also receives auditory input and can gate auditory responses to song in HVC and Nif (Coleman et al., 2007) so it may play an important role in sensory-motor integration needed for song learning and maintenance. Here we present evidence that NIf does not undergo photoperiodic variation in nucleus volume when the boundaries of the nucleus are clearly defined by enkephalin immunoreactivity. However, the delineation of NIf by enkephalin immunoreactivity does reveal a sex difference in volume based on studies in zebra finches (Ball et al., 1995). Thus this delineation of NIf has functional significance.
Neuromodulatory role of Enkephalin in lMAN, mMAN and NIf
Many neuromodulators have been identified in lMANcore; mMAN and NIf (Soha et al., 1996; Harding et al., 1998; Mello et al., 1998; Ryan and Arnold, 1981a; Ryan et al., 1981b; Ball et al., 1995). In HVC, RA, and NIf catecholamines and cholinergic activity modulate auditory processing and/or motor production (Shea and Margoliash, 2003; Cardin and Schmidt, 2003, 2004). Few studies have investigated the functional significance of enkephalin in songbird species. Pharmacological studies have suggested that peripheral enkephalin administration can influence the frequency of song behavior (Riters, 2010). Enkephalin antagonist increased singing behavior (Riters et al., 2005) whereas agonist decreased singing behavior in male starlings (Schroeder et al., 2006). These findings suggest that opioids may regulate the motivation to engage in song production and not necessarily vocal plasticity or song quality. Further experiments determining how the opioid system modulates the electrochemical properties during song perception or song production are needed to formulate hypotheses regarding neuromodulatory control during song learning and maintenance.
Testosterone Independent Regulation of Song Control System
A number of studies have demonstrated that a change in photoperiod can have profound effects on the volume of song control nuclei independent of testosterone and/or gonad state (Bernard et al., 1997; Smith et al., 1997; Gulledge and Deviche, 1998; Tramontin et al., 1999; Bentley et al. 1999; Dloniak and Deviche, 2001; Boseret et al., 2006). Here, we provide further support for testis independent regulation of a nucleus within the song system. The neural changes associated with the reduction in lMAN during development include a decline in neuron number (Bottjer and Sengelaub, 1989). However, the neural attributes associated with the photoperiodic increase in lMANcore volume are not currently known in part because seasonal changes in lMANcore volume have not been previously reported. Several neural attributes have been observed to account for the neuroplasticity in the song system (see Tramontin and Brenowitz, 2000; Brenowitz, 2004 for a review). One plausible explanation for the photoperiodic regulation of lMANcore observed in this study stems from evidence for an increase in axonal arboration from DLM efferent projections during development (Johnson and Bottjer, 1992). This hypothesis would predict that DLM efferent connections would vary considerably across reproductive states. A second hypothesis for the observed increase in lMANcore volume is the result of greater neuronal soma sizes (Nixdorf-Bergweiler, 1998). Male zebra finches exhibit larger soma sizes during the sensorimotor phase of song learning and this may contribute to the increase in lMANcore reported in the present study. How the change in photoperiod results in the recruitment of genes involved in these types of neuroplasticity requires further exploration.
However, even though the gonads of the starlings at capture had not undergone recrudescence, it remains possible that the lMANcore volume had already started to increase. Previous studies have shown that several song nuclei are close to fully recrudesced prior to the vernal increase in plasma testosterone concentrations (Tramontin et al., 2001; Riters et al., 2002; Caro et al., 2005; Caro et al., 2006). An alternative explanation for the observed data is therefore that there was substantial growth in the lMANcore by the time of capture even though the birds had relatively small gonads. As a result, lMANcore volumes could have increased in intacts and castrates prior to photostimulation and the effect of gonadectomy may not have resulted in the regression of the nucleus volume before the end of the experiment. Nevertheless, the data presented here provide support for marked changes in lMANcore volume that is dependent on the reproductive state.
Summary and Conclusions
The prevailing hypothesis in the study of neuroplasticity in songbirds is that the vernal increase in testosterone concentrations drives the seasonal change in song nucleus volume (Brenowitz, 2008; Ball et al., 2008; Tramontin and Brenowitz 2000; Ball, 1999). Here, we provide the first report of a photoperiodic increase in lMANcore volume, a nucleus that is critical for inducing vocal plasticity in song structure (Olveczky et al., 2005; Thompson and Johnson, 2007). It remains to be clarified how seasonal changes in the volume of song nuclei and song performance can be enhanced by environmental factors such as long photoperiods prior to the bird experiencing high testosterone concentrations or even in the absence of significant testosterone as was shown in this study. One hypothesis that emerges from the current findings is that variation in the neural attributes of lMANcore may underlie the induction of vocal plasticity during song development and the annual sensorimotor integration of new song types in adulthood.
Acknowledgments
We would like to thank Adam Troyer for his assistance trapping starlings and Marc Calabrese for his technical assistance. This research was supported by NIH/NINDS RO1 35467 to GFB; TJS was supported by an NSERC PGS-D 334570.
References
- Airey DC, DeVoogd TJ. Greater song complexity is associated with augmented song system anatomy in zebra finches. Neuroreport. 2000;11:2339–2344. doi: 10.1097/00001756-200007140-00054. [DOI] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. Specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Ball GF, Wingfield JC. Changes in plasma levels of luteinizing hormone and sex steroid hormones in relation to multiple-broodiness and nest site density in male starlings. Physiol Zool. 1987;60:191–199. [Google Scholar]
- Ball GF, Casto JM, Bernard DJ. Sex differences in the volume of avian song control nuclei: comparative studies and the issue of brain nucleus delineation. Psychoneuroendocrinol. 1994;19:485–504. doi: 10.1016/0306-4530(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Ball GF, Absil P, Balthazart J. Peptidergic delineations of nucleus interface reveal a sex difference in volume. NeuroReport. 1995;6:957–960. doi: 10.1097/00001756-199505090-00002. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. How should brain nuclei be delineated? They don’t need to be! TINS. 1997;20:344. [PubMed] [Google Scholar]
- Ball GF. The neuroendocrine basis of seasonal changes in vocal behavior among songbirds. In: Hauser MD, Konishi M, editors. The Design of Animal Communication. MIT press; 1999. [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system – Multiple brain sites of steroid hormone action and the importance of variation in song behavior. Annals NY Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Ball GF, Riters LV, MacDougall-Shackleton SA, Balthazart J. Neuroscience of birdsong. Cambridge University Press; Cambridge, UK: 2008. Sex differences in brain and behavior and the neuroendocrine control of the motivation to sing; pp. 320–331. [Google Scholar]
- Ball GF, Balthazart J. Seasonal and hormonal modulation of neurotransmitter systems in the song control circuit. J Chem Neuroanatomy. 2010;39:82–95. doi: 10.1016/j.jchemneu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Van’t Hof TJ, Ball GF. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc Nat Acad Sci USA. 1999;96:4674–4679. doi: 10.1073/pnas.96.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard DJ, Ball GF. Two histological markers reveal a similar photoperiodic difference in the volume of the high vocal center in male European starlings. J Comp Neurol. 1995;260:726–734. doi: 10.1002/cne.903600415. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Eens M, Ball GF. Age- and behavior-related variation in volumes of song control nuclei in male European starlings. J Neurobiol. 1996;30:329–339. doi: 10.1002/(SICI)1097-4695(199607)30:3<329::AID-NEU2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Wilson FE, Ball GF. Testis-dependent and independent effect of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea) Brain Res. 1997;760:163–169. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries. J Neurobiol. 2006;66:1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:3152–3163. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Sengelaub DR. Cell death during development of a forebrain nucleus involved with vocal learning in zebra finches. J Neurobiol. 1989;20:609–618. doi: 10.1002/neu.480200702. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. How should brain nuclei be delineated? They don’t need to be! TINS. 1997;20:344–345. [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Post-learning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions. J Neurosci. 2001;21:2501–2517. doi: 10.1523/JNEUROSCI.21-07-02501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–360. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Nalls B, Wingfield JC, Kroodsma DE. Seasonal-changes in avian song nuclei without seasonal-changes in song repertoire. J Neurosci. 1991;11:1367–1374. doi: 10.1523/JNEUROSCI.11-05-01367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K, Kroodsma DE. Brain space for learned song in birds develops independently of song learning. J Neurosci. 1995;15:6281–6286. doi: 10.1523/JNEUROSCI.15-09-06281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Smith GT. How should brain nuclei be delineated? They don’t need to be! TINS. 1997;20:345. [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the adult avian song control system. Annals NY Acad Sci. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Neuroscience of birdsong. Cambridge University Press; Cambridge, UK: 2008. Plasticity of the song control system in adult birds; pp. 332–349. [Google Scholar]
- Cardin JA, Schmidt MR. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. J Neurophysiol. 2003;90:2884–2899. doi: 10.1152/jn.00391.2003. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MR. Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. J Neurophysiol. 2004;91:2148–2163. doi: 10.1152/jn.00918.2003. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Raskin JN, Schmidt MR. Sensorimotor nucleus NIf is necessary for auditory processing but not vocal motor output in the avian song system. J Neurophysiol. 2005;93:2157–2166. doi: 10.1152/jn.01001.2004. [DOI] [PubMed] [Google Scholar]
- Caro SP, Lambrechts MM, Balthazart JB. Early seasonal development of brain song control nuclei in male blue tits. Neurosci Letters. 2005;386:139–144. doi: 10.1016/j.neulet.2005.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro SP, Lambrechts MM, Chastel O, Sharp PJ, Thomas DW, Balthazart J. Simultaneous pituitary-gonadal recrudescence in two Corsican populations of male blue tits with asynchronous breeding dates. Horm Behav. 2006;50:347–360. doi: 10.1016/j.yhbeh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci. 2007;27:10024–10036. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. Plasma gonadal-steroid levels in wild starlings during the annual cycle and in relation to the stages of breeding. Gen Comp Endocrinol. 1983;209:519–526. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Devoogd TJ, Krebs JR, Healy SD, Purvis A. Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc R Soc Lond Ser B. 1993;254:75–82. doi: 10.1098/rspb.1993.0129. [DOI] [PubMed] [Google Scholar]
- Dloniak SM, Deviche P. Effects of testosterone and photoperiodic condition on song production and vocal control region volumes in adult male dark-eyed juncos (Junco hyemalis) Horm Behav. 2001;39:95–105. doi: 10.1006/hbeh.2000.1621. [DOI] [PubMed] [Google Scholar]
- Doupe AJ. Song and order selective neurons in the songbird anterior forebrain and their emergence during vocal development. J Neurosci. 1997;17:1147–1167. doi: 10.1523/JNEUROSCI.17-03-01147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Variation in singing activity during the breeding cycle of the European starling Sturnus vulgaris. Belg J Zool. 1994;124:167–174. [Google Scholar]
- Eens M. Understanding the complex song of the European starling: an integrated ethological approach. Adv Stud Anim Behav. 1997;26:355–434. [Google Scholar]
- Falk H, Gwinner E. Timing of photorefractoriness in the European starling: significance of photoperiod early and late in the reproductive cycle. Biol Reprod. 1988;39:1004–1008. doi: 10.1095/biolreprod39.5.1004. [DOI] [PubMed] [Google Scholar]
- Foster EF, Mehta RP, Bottjer SW. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. J Comp Neurol. 1997;382:364–381. doi: 10.1002/cne.903820305. [DOI] [PubMed] [Google Scholar]
- Gahr M. Delineation of a brain nucleus: comparison of cytochemical, hodological, and cytoarchitectural views of the song control nucleus HVC of the adult canary. J Comp Neurol. 1990;294:30–36. doi: 10.1002/cne.902940104. [DOI] [PubMed] [Google Scholar]
- Gahr M. How should brain nuclei be delineated? Consequences for developmental mechanisms and for correlations of area size, neuron numbers and functions of brain nuclei. TINS. 1997;20:58–62. doi: 10.1016/s0166-2236(96)10076-x. [DOI] [PubMed] [Google Scholar]
- Gardner TJ, Fee MS. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2007. Nucleus interfacialis is not necessary for sensory-motor learning in a juvenile songbird. Program No 430.24. [Google Scholar]
- Gulledge CC, Deviche P. Photoperiod and testosterone independently affect vocal control regions volumes in adolescent male songbirds. J Neurobiol. 1998;36:550–558. [PubMed] [Google Scholar]
- Gwinner E. Die circannuale Peroidik der Fortpflanzungsaktivitat beim Star (Sturnus vulgaris) unter dem Einfluβ gleich- und andersgeschlechtiger Artgenossen. Z Tierpsychol. 1975;38:34–43. [PubMed] [Google Scholar]
- Hahnloser RH, Fee MS. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Single neuron recordings in nucleus interface of singing zebra finches. Program No 294.4. [Google Scholar]
- Harding CF, Barclay SR, Waterman SA. Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J Neurobiol. 1998;34:329–346. [PubMed] [Google Scholar]
- Herrmann K, Arnold AP. The development of afferent projections to the robust archistriatal nucleus in the male zebra finches: a quantitative electron microscopic study. J Neurosci. 1991;11:2063–2074. doi: 10.1523/JNEUROSCI.11-07-02063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Bottjer SW. Development of individual axon arbors in a thalamocortical cicuit necessary for song learning in zebra finches. J Neurosci. 2002;22:901–911. doi: 10.1523/JNEUROSCI.22-03-00901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male zebra finch song system. J Neurosci. 1999;19:5108–5188. doi: 10.1523/JNEUROSCI.19-12-05108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Growth and regression of thalamic efferents in the song control system of male zebra finches. J Comp Neurol. 1992;326:442–450. doi: 10.1002/cne.903260309. [DOI] [PubMed] [Google Scholar]
- Johnson F, Sablan MM, Bottjer SW. Topographic organization of a forebrain pathway involved with vocal learning in zebra finches. J Comp Neurol. 1995;358:260–278. doi: 10.1002/cne.903580208. [DOI] [PubMed] [Google Scholar]
- Leonardo A. Experimental test of the birdsong error-correction model. PNAS. 2004;101:16935–16940. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Hulse SH, Ball GF. Neural correlates of singing behavior in male zebra finches (Taeniopygia guttata) J Neurobiol. 1998;36:421–430. [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Ball GF, Edmonds E, Sul R, Hahn TP. Age- and sex- related variation in song-control regions in Cassin’s Finches, Carpodacus cassinii. Brain Behav Evol. 2005;65:262–267. doi: 10.1159/000084644. [DOI] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine beta hydroxylase. J Comp Neurol. 1998;400:207–228. [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE. Enlargement of neuronal somata in the lMAN coincides with the onset of sensorimotor learning for song. Neurobiol Learning Memory. 1998;69:258–273. doi: 10.1006/nlme.1998.3819. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A Brain for All Seasons - Cyclical Anatomical Changes in Song Control. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kasparian S, Pandazis C. Brain space for a learned task. Brain Res. 1981;213:99–109. doi: 10.1016/0006-8993(81)91250-6. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Reassessing the mechanisms and origins of vocal learning in birds. TINS. 1991;14:206–211. doi: 10.1016/0166-2236(91)90107-6. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal Experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS. 2005;3:153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine CS. Neurocellular anatomy. In: Siegel GJ, Agranoff BW, editors. Nasic Neurochemistry. 4. Raven Press; New York: 1989. pp. 3–33lib. [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffer DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha(2)-noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:245–255. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Riters LV. Evidence for opioid involvement in the motivation to sing. J Chem Neuroanatomy. 2010;39:141–150. doi: 10.1016/j.jchemneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Arnold AP. Evidence for cholinergic participation in the control of bird song: acetylcholinesterase distribution and muscarinic receptor autoradiography in the zebra finch brain. J Comp Neurol. 1981a;202:211–219. doi: 10.1002/cne.902020207. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Arnold AP, Elde RP. Enkephalin-like immunoreactivity in vocal control regions of the zebra finch brain. Brain Res. 1981b;229:236–240. doi: 10.1016/0006-8993(81)90763-0. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;199:233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaris) Horm Behav. 2005;47:467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. Comparative study of the behavioral deficits following lesions of various parts of the finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song in male European starlings. Physiol Behav. 2006;88:575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Shea S, Margoliash D. Basal forebrain cholinergic modulation of auditory activity in the zebra finch song system. Neuron. 2003;40:1213–1226. doi: 10.1016/s0896-6273(03)00723-2. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC, Baptista LF. Seasonal changes in song nuclei and song behavior in Gambel’s white-crowned sparrows. J Neurobiol. 1995;28:114–125. doi: 10.1002/neu.480280110. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Wingfield JC. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J Neurobiol. 1997;32:426–442. doi: 10.1002/(sici)1097-4695(199704)32:4<426::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Bentley GE, Ubuka T, Arckens L, Hampson E, MacDougall-Shackleton SA. Effects of social cues on GnRH-I, GnRH-II and reproductive physiology in female house sparrows (Passer domesticus) Gen Comp Endo. 2008;156 :385–394. doi: 10.1016/j.ygcen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Ball GF. Anatomica llocalization of the effects of reproductive state, castration, and social milieu on cells immunoreactive for gonadotropin-releasing hormone-I in male european starlings. J Comp Neurol. 2009;517:146–155. doi: 10.1002/cne.22159. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Johnson F. HVC microlesions do not destabilize the vocal patterns of adult male zebra finches with prior ablation of lMAN. Develop Neurobiol. 2007;67:205–218. doi: 10.1002/dneu.20287. [DOI] [PubMed] [Google Scholar]
- Tramontin AT, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19:476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AT, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tramontin AT, Perfito N, Wingfield JC, Brenowitz EA. Seasonal growth of song control nuclei precedes seasonal reproductive development in wild adult song sparrows. Gen Comp Endocrinol. 2001;122:1–9. doi: 10.1006/gcen.2000.7597. [DOI] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalmo-“cortical” loops in the song system of oscine songbirds. J Comp Neurol. 1997;380:275–290. [PubMed] [Google Scholar]
- Vogels O. How should brain nuclei be delineated? They don’t need to be! TINS. 1997;20:344, 343–344. doi: 10.1016/s0166-2236(97)89929-8. [DOI] [PubMed] [Google Scholar]
- Voigt C, Leitner S, Gahr M. Socially induced brain differentiation in a cooperatively breeding songbird. Proc Royal Soc B. 2007;274:2645–2652. doi: 10.1098/rspb.2007.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]