Abstract
This review focuses on molecular, cellular, and functional changes that occur in the vasculature during aging; explores the links between mitochondrial oxidative stress, inflammation, and development of vascular disease in the elderly patients; and provides a landscape of molecular mechanisms involved in cellular oxidative stress resistance, which could be targeted for the prevention or amelioration of unsuccessful vascular aging. Practical interventions for prevention of age-associated vascular dysfunction and disease in old age are considered here based on emerging knowledge of the effects of anti-inflammatory treatments, regular exercise, dietary interventions, and caloric restriction mimetics.
Keywords: Vascular aging, Oxidative stress, Endothelial dysfunction, Atherosclerosis, Stroke, Myocardial infarction
CARDIOVASCULAR diseases are the most common cause of death among the elderly patients in the Western world. Age-specific mortality rates from heart disease and stroke increase exponentially with age throughout the later years of life, accounting for more than 40% of all deaths among people aged 65–74 years and almost 60% at age 85 years and older. It is becoming evident that aging results in well-defined phenotypic changes, which render the cardiovascular system prone to disease even in the absence of traditional risk factors (eg, hypertension, diabetes, and smoking). Moreover, age-related alterations in cellular homeostatic mechanisms also render the aged vasculature more susceptible to the damaging effects of the aforementioned pathophysiological conditions. Understanding the mechanisms underlying the age-induced vascular pathophysiological alterations holds promise for reducing cardiovascular mortality in an aging population. In this review, the effect of aging on the vascular system is considered in terms of potential mechanisms involved in vascular dysfunction and age-related atherosclerosis. The possible benefits of emerging therapeutic strategies that have the potential to promote cardiovascular health in the elderly patients are also discussed.
MECHANISMS OF VASCULAR AGING
Oxidative Stress and Endothelial Dysfunction in Aging
Considerable evidence has been published that increased production of reactive oxygen species [ROS; at least, in part, due to an increased activity of NAD(P)H oxidases (1–5)] leads to endothelial dysfunction in aging both in laboratory animals (1,2,6–10) and in humans (4,11) and that oxidative stress promotes the development of coronary artery disease and stroke in the elderly patients. It is well established that nitric oxide (NO) is a crucial factor for the health and function of endothelial cells. One of the consequences of increased oxidative stress in aging is a functional inactivation of NO by high concentrations of O2·− (1,3,6,8) resulting in significant vasomotor dysfunction [recently reviewed elsewhere (12)]. In particular, impaired bioavailability of NO due to age-related oxidative stress in the coronary circulation (1) and other vascular beds (6) results in a severe impairment of flow/shear stress–induced vasodilation compromising minute-to-minute adjustments of blood flow in response to tissue oxygen demand. In addition to maintenance of normal organ blood flow, endothelium-derived NO confers significant vasoprotective and cardioprotective effects, including inhibition of both platelet aggregation and inflammatory cell adhesion to endothelial cells, disruption of proinflammatory cytokine–induced signaling pathways, inhibition of apoptosis, preservation of endothelial progenitor cell (EPC) function, and regulation of tissue energy metabolism. Thus, the severe impairment of NO bioavailability in aging (13), also aggravated by an age-related decline in endothelial nitric oxide synthase (eNOS) expression (1,14–17), reduced availability of tetrahydrobiopterin (18), and/or a decreased intracellular L-arginine availability (19), is likely to promote vascular inflammation and atherogenesis and lead to cellular energetic imbalance. Impaired bioavailability of NO was also shown to account for dysregulation of myocardial O2 consumption in aged rats (3). The key role of endothelium-derived NO in protecting the cardiovascular system during aging is underscored by the findings that eNOS knockout mice exhibit a premature cardiac aging phenotype associated with early mortality (20). Recent studies also suggest that decreased endothelial NO production in aging promotes apoptosis of endothelial cells (17,21) and leads to microvascular rarefaction. There is also an emerging view that ROS, in addition to inactivating NO and causing oxidative macromolecular damage, play important signaling roles in the vascular endothelial and smooth muscle cells as well. In particular, oxidative stress and the consequent activation of redox-sensitive cellular signaling pathways are thought to be implicated in the inflammatory process in the aged vasculature (22). Many of the adverse consequences of oxidative stress are mediated via production of the highly reactive oxidant peroxynitrite, the reaction product of NO and superoxide (23,24). There is convincing data showing a substantially enhanced cardiovascular ONOO− formation in aging (1,3,6,8). The downstream targets of peroxynitrite-induced cytotoxicity are likely multiple [for a comprehensive review, see reference (23)].
In recent years, a number of longevity genes affecting life span and the rate of aging have been identified. In the cases where it was studied, life span extension and attenuation of cellular ROS production in models of successful aging were paralleled by significant improvement of vascular function. In contrast, accelerated vascular aging is generally associated with vascular oxidative stress and endothelial dysfunction. For example, defects in the Klotho gene in the mouse, which is associated with endothelial dysfunction and premature development of atherosclerosis (25,26), result in an accelerated aging phenotype and a short life span.
Recent studies suggest that mitochondrial oxidative stress has an important role in aging-induced vascular dysfunction (10,27). Importantly, mitochondria-derived H2O2 is thought to contribute to the activation of nuclear factor-κB, resulting in a proinflammatory shift in endothelial gene expression profile (10) (Figure 1). Another important link between mitochondrial ROS production and vascular aging is the induction of apoptosis. The underlying mechanisms for mitochondrial oxidative stress in aged endothelial cells are likely multifaceted and may include peroxynitrite-mediated nitration and inhibition of MnSOD (2), decline in reduced glutathione content (28), and a dysfunctional electron transport chain (27). There is increasing evidence that with age, mitochondrial biogenesis is impaired in endothelial cells both in conduit arteries (27) and in the capillaries (29,30), which is likely to increase mitochondrial ROS production. Recent studies demonstrated that the mitochondrial enzyme p66Shc has an important role in regulation of mitochondrial ROS generation, linking oxidative stress to apoptosis (31). Mice lacking p66Shc exhibit reduced production of ROS, which is associated with a 30% increase in life span and improved endothelial function (8,32,33). Although recent large randomized clinical trials have shown no significant benefit when antioxidants such as vitamin E were given to patients with a high-risk coronary arterial disease profile, at present, it is unknown whether systemic administration of mitochondria-targeted antioxidants would affect progression of cardiovascular diseases in elderly patients.
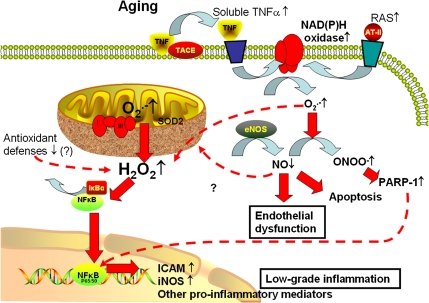
Figure 1.
Proposed scheme for pathways contributing to cellular oxidative stress and NF-κB activation in aged endothelial cells. In aged endothelial cells, increased levels of O2·− generated by the electron transport chain are dismutated to H2O2, which can penetrate the mitochondrial membrane increasing cytoplasmic H2O2 levels. H2O2 contributes to the activation of NF-κB, resulting in a proinflammatory shift in endothelial gene expression profile. Aging is also associated with upregulated expression of membrane-bound tumor necrosis factor-alpha (TNFα), which increases soluble TNFα levels in the vascular wall due to the action of TNFα-converting enzyme (TACE). In aged endothelial cells, increased levels of O2·− generated by NAD(P)H oxidases (stimulated by elevated TNFα levels and/or by the activated local renin–angiotensin system [RAS] in the vascular wall) decrease the bioavailability of NO by forming ONOO −. Lack of NO leads to vasodilator dysfunction and promotes endothelial apoptosis, whereas nitrative stress and increased H2O2 levels lead to poly(ADP-ribose) polymerase (PARP)-1 activation, which contributes to NF-κB-dependent gene transcription. Increased oxidative stress and chronic low-grade vascular inflammation increase the risk for the development of vascular diseases in the elderly patients.
Vascular Inflammation in Aging
Abundant experimental and clinical data show that aging is associated with chronic low-grade inflammation (34), which predisposes the vasculature to the development of atherosclerosis [recently reviewed elsewhere (22)]. Recent studies have uncovered an important cross talk between inflammatory processes, generation of ROS, and endothelial dysfunction in the pathogenesis of cardiovascular aging (22). First, ROS per se can act as signaling molecules activating pathways regulating inflammatory processes (10), including endothelial activation and secretion of inflammatory mediators. Second, oxidatively damaged molecules can induce inflammatory processes. Third, inflammation itself promotes cellular oxidative stress (eg, by cytokine-mediated activation of NAD(P)H oxidases). Fourth, metabolic status may influence both aging-related vascular oxidative stress and inflammation (35). In addition, environmental inflammatory factors [eg, infections and environmental inflammogens such as particulate exposure (36)] can also affect vascular aging by promoting vascular oxidative stress and inflammation.
There is growing evidence that age-associated low-grade inflammation accelerates the incidence of chronic diseases, including atherosclerosis. Even in normal healthy aging, there is a proinflammatory shift in vascular gene expression profile, including an upregulation of inflammatory cytokines, chemokines, adhesion molecules, and inducible nitric oxide synthase both in laboratory rodents and in primates (1,10,21,35,37–39). In humans, plasma concentrations of several inflammatory markers (eg, tumor necrosis factor-alpha [TNFα], sVCAM-1, sE-selectin, interleukin [IL]-6, IL-18, and MCP-1) are positively correlated with age, independent of other cardiovascular risk factors (40,41). High levels of inflammatory cytokines contribute to a proinflammatory microenvironment that facilitates both the development of vascular dysfunction (42,43) and promotes endothelial apoptosis in aging (21,42).
Previous studies have shown that endothelial activation and proinflammatory gene expression in aging is promoted, at least in part, by an increased NF-κB activation (4,10,44,45). Chronic activation of NF-κB and endothelial activation is known to predispose arteries to atherosclerosis (46). Increased NF-κB binding in aging is likely responsible for the increased expression of adhesion inducible nitric oxide synthase found in aged coronary vessels (1), carotid arteries, and aortas (10,38), which is a major source of vascular peroxynitrite production. The finding that scavenging of mitochondria-derived H2O2 attenuates NF-κB activation in aged vessels (10) suggests a role for mitochondrial oxidative stress in regulation of endothelial NF-κB activity in aging. These observations suggest that the age-related decline in mitochondrial function is, at least in part, responsible for vascular inflammation in aging (10). Recent studies have shown that in aging mice, overexpression of human catalase in the mitochondria (MCAT) delays cardiac pathology and attenuates age-related oxidative stress (47). Future studies should determine whether inflammatory gene expression is also attenuated in the cardiovascular system of MCAT mice.
Increased Arterial Stiffness in Aging
In addition to impaired endothelial function and chronic low-grade vascular inflammation, increased arterial stiffness is also a clinically important phenotype associated with vascular aging in humans. As the large conduit arteries stiffen, aortic pulse wave velocity and pulse pressure also increase. Increased aortic pulse wave velocity results in early return of the reflected pressure wave, which produces significant systolic pressure augmentation and a decrease in diastolic pressure. Decreased diastolic pressure results in decreased coronary artery blood flow. The sequelae of increases in systolic hypertension result in left ventricular remodeling, diastolic dysfunction, and accelerated development of atherosclerotic lesions, all of which constitute a potential risk factor for increased cardiovascular mortality in the elderly patients. Because the aorta and major elastic arteries also tend to dilate with age, vascular wall tension significantly increases. The resulting alterations in mechanosensitive gene expression are likely to contribute to age-related vascular remodeling, oxidative stress, and proatherogenic phenotypic changes in the vascular wall.
Recent studies called attention to the association of an upregulated tissue renin–angiotensin system with intimal thickening and remodeling in large arteries of aged animals and humans (39,48–50). Angiotensin II signaling, including activation of calpain-1 and matrix metalloproteinase type II, has been linked to an age-associated increase in migration capacity of vascular smooth muscle cells (51), which is central to the arterial remodeling that accompanies advancing age. Accordingly, there is evidence demonstrating that inhibition of ACE activity can reduce arterial stiffness in aged animals and elderly humans independent of changes in blood pressure (52,53). In that regard, it is significant that infusion of angiotensin II into young rats increases carotid arterial MMP2 activation resulting in carotid media thickening and intima infiltration by vascular smooth muscle cells, mimicking the vascular aging phenotype (50). Upregulation of renin–angiotensin system may also contribute to chronic low-grade vascular inflammation and oxidative stress, enhancing the vascular response to injury and rendering the aged vascular wall susceptible to the development of atherosclerosis.
Recent population-based studies suggest that a relationship exists between elevated levels of advanced glycation end products and increased arterial stiffness in elderly humans (54). These findings are consistent with the results of experimental studies, showing that treatment with aminoguanidine (an inhibitor of advanced glycation end product production) attenuates age-related arterial stiffening in laboratory rodents (55). Aminoguandinin was effective in prevention of arterial stiffening without altering collagen and elastin content in the vascular wall, suggesting that its effects are related to a decrease in the advanced glycation end product–mediated cross-linking of the extracellular matrix (55). Interventions to lower levels of advanced glycation end products, in combination with inhibitors of renin–angiotensin system, warrant further studies as putative novel strategies to lower arterial stiffness in elderly humans as well (56).
Role of Endothelial Replicative Senescence in Vascular Aging
Mitotically competent mammalian cells, including endothelial cells, can react to diverse endogenous and exogenous stressors (including oxidative stress, dysfunctional telomeres, DNA damage, paracrine signals) by permanently withdrawing from the cell cycle, a response termed cellular senescence [for an excellent recent review, see reference (57)]. The relationship between aging and cellular senescence is currently hotly debated. The view has emerged that the senescence response may be antagonistically pleiotropic, promoting early-life survival by curtailing the development of cancer in mitotic tissues but eventually contributing to development of age-related diseases as dysfunctional senescent cells accumulate (58). Apart from the alterations related to the block in cell replication, senescent cells acquire distinct phenotypic changes, termed the senescence-associated secretory phenotype (59), which were suggested to contribute to aging and age-related diseases, by impairing the function of neighboring cells via the secretion of paracrine mediators and altering the extracellular matrix environment. Some of these phenotypic changes may be potentially important in affecting the regenerative and angiogenic capacity of the vascular endothelium and the development of atherosclerosis during aging (57). Studies on cultured endothelial cells suggest that oxidative stress is a major stimulus for the induction of senescence (57). Nevertheless, a controversy exists regarding the exact pathophysiological role and the significance of senescence in vascular aging. For example, the number of senescent endothelial cells appears to be low in aged laboratory rodents (10). It is quite possible that the biological function of senescence may differ in cells of short-lived laboratory rodents and long-lived primates (60), despite the many similarities in their vascular aging phenotypes. Future studies using a combination of novel markers of senescence and a detailed analysis of the cellular secretome are needed to better understand the role of endothelial senescence in vascular aging.
Role of Endothelial Apoptosis and Microvascular Rarefaction in Aging
Apoptosis is an attractive hypothesis to account for aging of specific organs (61) and the genesis of cardiovascular pathologies. Yet, the relationship between vascular aging and apoptosis remains unclear. In laboratory rodents, the percentage of apoptotic endothelial cells significantly increases with age (21,35,42). The available data suggest that impaired bioavailability of NO, upregulation of TNFα, and/or mitochondrial oxidative stress are likely to contribute to this phenomenon (21,42). Aging is also associated with enhanced endothelial apoptosis in peripheral arteries of nonhuman primates (62). Yet, in humans, no significant correlation was found between age and number of apoptotic cells in the coronary arteries (63).
It is thought that increased apoptotic cell death contributes to the age-related microvascular rarefaction that has been observed in multiple organ systems, including the heart (64), kidney (65), and skin (66). Importantly, as the nervous system ages, there is also a rarefaction of the microvasculature in certain regions of the brain, including the hippocampus, as well as alterations in the structure of the remaining vessels, which may contribute to cognitive dysfunction in the absence of or preceding neurodegeneration in the elderly patients (67–69). Another mechanism that likely contributes to microvascular rarefaction is an age-related impairment of angiogenesis (70). Age-related microvascular rarefaction contributes to a decline in cerebral blood flow that reduces metabolic support for neural signaling, especially when neuronal activity is high. In addition, aging reduces microvascular plasticity and the ability of the cerebral circulation to respond appropriately to changes in metabolic demand. The age-related loss of microvascular plasticity has significance beyond metabolic support for neuronal signaling because neurogenesis in the adult brain is regulated coordinately with capillary growth (69). Recent studies demonstrate that growth hormone (GH) supplementation substantially increases cortical vascular density in older rats (67), which was accompanied by a significant improvement of cognitive function (68,71–75). Further studies are needed to demonstrate that similar beneficial effects can be reached by GH supplementation or by other pharmacological treatments targeting the microcirculation in elderly humans.
Impaired Endothelial Progenitor Cell Function in Aging
The link between mammalian aging and a decline in the replicative function of somatic stem cells is controversial. From recent studies, the general theme emerges that, although overt stem cell failure does not frequently occur in self-renewing organs, the cardiovascular repair system in particular exhibits age-related functional impairment (76). Importantly, advanced age is known to impair neovascularization, a process known to depend on the function of highly proliferative EPCs. Although there are contradicting data whether aging affects total EPC number in humans and laboratory animals (77–79), previous studies clearly demonstrated that aging impairs the function of circulating EPCs (78,80). Recent studies identified a pool of resident coronary vascular progenitor cells in human and canine hearts, which appear to be able to regenerate large, intermediate, and small coronary arteries and capillaries (81). Additional studies are needed to determine how aging affects the function of these cell populations.
The age-related loss of EPC function is likely mediated partly by an imbalance between factors promoting growth, migration, and survival and factors enhancing oxidative stress and promoting senescence. For example, EPC trafficking to sites of ischemia is thought to be impaired in aging due to the failure of aged tissues to activate the HIF-1α-mediated hypoxia response (77). Chronic low-grade inflammation in aging is also likely to contribute to impaired EPC function. Vascular aging is associated with upregulation of TNFα (1,21,37,42), which can induce premature senescence in highly proliferative EPCs (82). Angiotensin II was also shown to accelerate EPC senescence (83), and aging is associated with upregulation of tissue renin–angiotensin system (49,50). Inhibition of angiotensin II function significantly decreases cardiovascular mortality in humans, but the role of EPCs in this protective effect remains to be determined. The insulin-like growth factor-1 (IGF-1)/IGF-1R system is thought to exert beneficial effects on the function of progenitor cells in the cardiovascular system, including antioxidant effects, upregulation of telomerase activity, delaying replicative senescence, and increasing the pool of functionally competent progenitor cells (84). The synthesis of IGF-1 is attenuated in aging, likely diminishing the ability of IGF-1 to activate cell growth and promote the survival of EPCs (85).
Recent advances suggest that an intrinsic timekeeping system, located within the hypothalamic suprachiasmatic nuclei, plays a fundamental role in synchronizing various biological processes within an organism. Studies using mice deficient in components of the circadian system suggest that in addition to their role in timing of a wide variety of circadian processes, some of the components of the circadian system are involved in modulation of cellular stress responses and the aging process itself. Recent findings provide a mechanistic link between dysregulation of the circadian system and age-related vascular pathologies. Accordingly, mutation of Per2, a circadian gene, was shown to cause Akt-dependent senescence and impair ischemia-induced revascularization through the alteration of EPC function (86). Bmal1-knockout and Clock mutant mice, which also exhibit aberrant circadian rhythms, are also characterized by endothelial dysfunction, increased pathological remodeling, and vascular injury (87). Taken together, these findings raise the interesting possibility that an ineluctable genetic clock directly modulates EPC function and thereby cardiovascular decline during aging.
Whether age-related EPC dysfunction is reversible is a matter of current debate. In that regard, it is significant that regular aerobic exercise was recently reported to increase both the number and the migratory activity of EPCs in previously sedentary older men (88). Recent studies also showed that the presence of sera from young rats in the culture medium improves the function of EPCs isolated from aged rats (89). These findings raise the possibility that age-related alterations in endocrine or neuroendocrine factors adversely affect the function of EPCs and that normalization of these alterations would exert beneficial effects on the regenerative capacity of the cardiovascular system in the elderly patients. Indeed, recent studies demonstrated that in humans, an increase in IGF-1 in response to GH treatment reverses age-related EPC dysfunction (90). These findings are consistent with previous observations that the IGF-1/IGF-1R pathway preserves telomere length and promotes cardiac progenitor cell growth and survival (84) and that injection of IGF-1 in the damaged heart promotes the migration and homing of cardiac stem cells and facilitates neovascularization (91). Other pharmacological strategies to interfere with age-related EPC dysfunction may include statins and peroxisome proliferator-activated receptor gamma agonists, which act, at least in part, via increasing bioavailability of NO in EPCs (92).
THERAPEUTIC STRATEGIES TO DELAY VASCULAR AGING
Novel Anti-inflammatory Treatments
As noted previously, vascular aging is characterized by low-grade chronic vascular inflammation. In particular, it is well established that vascular aging is associated with dysregulation of TNFα expression (1,21,37,42,93). With its implication as the master regulator of other inflammatory cytokines, chemokines, adhesion molecules, and redox regulation in the vascular wall, TNFα emerged as a key player in the pathophysiology of atherosclerosis. Plasma levels of TNFα (41) increase in aging and correlate with morbidity and mortality in the elderly patients (94). It is significant that chronic anti-TNFα treatment (eg, etanercept, which binds and inactivates TNFα) exerts multifaceted vasculoprotective effects, including a significant reduction in endothelial apoptosis, downregulation of NAD(P)H oxidases, and an improvement in endothelial function in aged rats (42,43,95). Whether anti-TNFα treatments can confer similar vasoprotective effects in “healthy” aging in humans is still unclear.
Inhibition of the endocannabinoid anandamide metabolizing enzyme, a fatty acid amide hydrolase (FAAH), is also emerging as a promising novel approach for the treatment of various inflammatory disorders. It is significant that the age-associated decline in cardiac function and increased myocardial gene expression of TNFα, inducible nitric oxide synthase, and gp91phox; increased nitrotyrosine formation; and enhanced apoptotic cell death observed in aged FAAH+/+ mice are largely attenuated in FAAH−/− mice (93). In addition, targeting of cannabinoid-2 receptors with selective agonists in vitro was shown to disrupt TNFα-induced proinflammatory signaling pathways in endothelial cells (96). Thus, cannabinoid-2 receptor antagonists may also offer a novel therapeutic target to inhibit TNFα-induced cardiovascular inflammation and to convey vasoprotection in aging.
Pharmacological inhibition of the poly(ADP-ribose) polymerase (PARP) pathway also represents a novel therapeutic target to improve aging-associated cardiovascular dysfunction. PARP-1 belongs to the DNA damage surveillance network, and its catalytic activity is likely increased in aging due to an increased presence of peroxynitrite-mediated DNA strand interruptions (97–99). Upon activation, PARP-1 transfers 50–200 molecules of ADP-ribose to various nuclear proteins, including transcription factors and histones. As a result, PARP-1 activation has been shown to modulate the transcriptional regulation of various inflammatory genes (100,101). Important for vascular aging are the findings that PARP-1 can regulate NF-κB activation (102–104). PARP-1, similar to SIRT1, is an NAD+ utilizing enzyme. Thus, an increased PARP-1 activity can negatively affect substrate availability for SIRT1 decreasing its activity, which may represent an additional mechanism by which PARP-1 overactivation can promote vascular dysfunction in aging. The available evidence suggests that inhibition of PARP-1 activation may confer cardiovascular protective effects in aging (98,99,105–107). Future studies should elucidate the exact molecular mechanisms by which PARP-1 inhibition attenuates vascular inflammation in aging and determine whether the aforementioned experimental findings can be reciprocated in elderly humans.
GH/IGF-1 Supplementation
Increasing evidence suggests the existence of a relationship between declining levels of GH and IGF-1 (the synthesis of which is regulated by GH) and the age-related functional decline in many organ systems (68,72). In particular, there is strong evidence that IGF-1 is an important protective factor in the cardiovascular system (Figure 2). Accordingly, epidemiological studies clearly indicate that in humans, GH and IGF-I deficiency is associated with premature atherosclerosis and elevated cardiovascular disease mortality (108). Cardiovascular disease risk is even elevated among apparently healthy individuals who have serum IGF-1 levels in the low normal range (109).
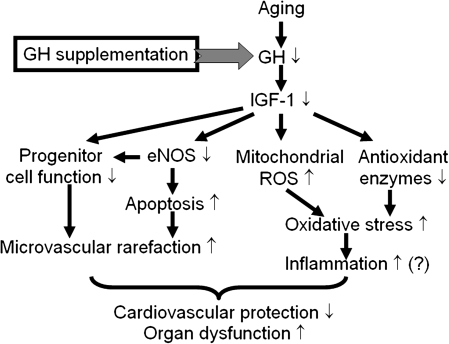
Figure 2.
Proposed scheme for the mechanisms by which insulin-like growth factor-1 (IGF-1) confers antioxidative and anti-inflammatory vasoprotective effects in aging. During aging, increased mitochondria-derived reactive oxygen species (ROS) production enhances NF-κB activation, which promotes inflammatory cytokine and chemokine expression, microvascular endothelial activation, leukocyte adhesion, and extravasation. The ensuing inflammatory response contributes to the age-related decline of organ function (eg, heart failure and cognitive decline). The model predicts that IGF-1, via upregulating antioxidant enzymes and exerting mitochondrial protective effects, significantly attenuates mitochondrial oxidative stress in aging, resulting in inhibition of endothelial activation and vascular inflammation. IGF-1 also promotes progenitor cell function, improves NO bioavailability, and limits apoptotic cell death, which contributes to its microvascular protective effects. GH = growth hormone.
Despite the compelling human data on the cardiovascular protective role of IGF-1 during aging, previous studies on model organisms created significant controversy regarding the role of IGF-1 signaling in determination of life span and healthspan. Experimental disruption of IGF-1 signaling in Caenorhabditis elegans results in a quiescent state of diapause (dauer form), which is associated with extended longevity (110). Yet, because of the inherent limitations of these model systems (eg, the lack of a cardiovascular system) and the interspecies differences in the main cause-of-death (bacterial invasion in the gut in C elegans, whereas cardiovascular diseases and cancer are prevalent in humans) the conclusions drawn from these studies regarding the role of IGF-1 in regulation of life span and healthspan cannot be generalized. There have been several attempts to reconcile the invertebrate data and the human observations using various mammalian models of lifelong deficiency of GH and/or IGF-1 [reviewed in reference (111)]. From these studies, the view has emerged that low IGF-1 levels significantly decrease cancer risk, and because the main cause of death in laboratory rodents is cancer, the net result of lowering IGF-1 levels is a tendency for increased average life span in many [eg, Ames (112) and Snell dwarf mice (111,113)], but not all, rodent models of defective IGF-1 signaling [male mice heterozygous for the deletion of the IGF-1 receptor (114), genetically GH/IGF-1-deficient Lewis dwarf rats (115)]. Meta-analysis of multiple human studies indicates that there is also a significant association between the concentration of IGF-I and development of premenopausal breast, prostatic, and colon cancer in humans as well (116). Experimental findings obtained in laboratory rodents regarding the role of IGF-1 on cardiovascular health also accord with the human observations. For example, mice with hypopituitary dwarfism (Ames dwarf) have low plasma IGF-1 levels, and their aortas exhibit increased endothelial ROS generation, mitochondrial oxidative stress, and downregulation of major antioxidant enzymes as compared with vessels from wild-type mice (117). The available data support the conclusion that supplementation of IGF-1 may exert vasculoprotective effects in aging (118,119), improving cardiac diastolic function (120), and preventing hippocampal microvascular rarefaction (67,68,121). IGF-1 was also shown to protect cardiac myocytes from apoptotic cell death (122–124) and to promote cardiac stem cell survival and proliferation (84,91). Moreover, in cultured coronary arterial endothelial cells, administration of recombinant IGF-1 significantly attenuates cellular O2·− and H2O2 production and ROS generation by mitochondria and upregulates expression of antioxidant enzymes and eNOS (117). The finding that cardiac overexpression of IGF-1 significantly improves cardiomyocyte contractile function in old mice (125) supports the view that IGF-1 signaling exerts a protective role in the cardiovascular system and that loss of IGF-1 contributes to the development of the cardiovascular aging phenotype. In that regard, it should also be noted that the physiological effects of circulating IGF-1 and the local IGF-1 system may not be identical. For example, previous studies by Delaughter et al. (126) suggest that long-term overexpression of local IGF-1 in the myocardium may have unwanted side effects, promoting cardiac hypertrophy. Taken together, modulation of the circulating GH/IGF-1 axis by therapeutic interventions may represent a potential novel strategy to delay the onset of age-related decline of cardiovascular function.
In the aging brain upregulation of proinflammatory cytokines is known to impair behavioral and neural processes and promote neuroinflammation. Age-associated microvascular inflammation is thought to contribute to this increase in neuroinflammation, which is likely to be a key pathophysiological factor in the development of Alzheimer’s disease in the elderly patients. In cultured endothelial cells, IGF-1 attenuates mitochondrial oxidative stress, upregulates key components of cellular antioxidant systems, and exerts anti-inflammatory effects (117). Recent studies also have demonstrated that GH/IGF-1-deficient dwarf mice exhibit a pro-oxidative vascular phenotype that resembles accelerated vascular aging (117). However, studies on the links between GH/IGF-1, cerebrovascular oxidative stress, and neuroinflammation in aging have received little attention. Future studies should determine whether treatment with GH/IGF-1 or GH secretagogues, in addition to the prevention of microvascular rarefaction and exerting beneficial neuronal and cardiac effects (67,71,127–130), protects the cerebrovasculature from the deleterious effects of oxidative stress and inflammation associated with aging.
Vasoprotection by Regular Exercise in Aging
Habitual physical activity is proving to strongly benefit health and longevity in humans, including a reduced risk of cardiovascular disease, likely due, at least in part, to its direct vasoprotective effects. For example, in the Harvard Alumni Health Study, men who participated in some form of regular moderate physical activity had about one-third lower mortality risk and a significantly decreased incidence of stroke (131). The mechanisms of vasoprotection conferred by exercise are likely complex but includes a significant improvement of endothelial function, possibly by augmenting NO bioavailability and attenuating oxidative stress (18,132–136). The mechanisms by which exercise exerts its beneficial endothelial effects include temporary increases in shear stress, which are known to modulate gene expression in endothelial cells. Other vasoprotective actions of regular aerobic exercise include beneficial effects of increased systemic metabolism, for example, increased insulin sensitivity, decreased fat content, and attenuation of hyperlipidemia. Furthermore, exercise confers anti-inflammatory actions, such as suppression of TNFα, and thereby may offer protection against TNFα-induced vascular impairment. Regular exercise also promotes mitochondrial health, induces mitochondrial biogenesis, and upregulates mitochondrial antioxidant systems, which also may contribute to its vasoprotective properties during aging. Finally, there is evidence that exercise exerts a positive influence on the number and/or function of EPCs.
Caloric Restriction
Caloric restriction (CR) is a dietary regimen, which improves health and slows the aging process in evolutionarily distant organisms by limiting dietary energy intake (137–140). There is increasing epidemiological and experimental evidence that CR confers vasoprotection in aging and in pathological conditions associated with accelerated vascular aging [recently reviewed elsewhere (141); Figure 3]. The mechanisms underlying the beneficial cardiovascular effects of CR are undoubtedly multifaceted and may include improvement of systemic risk factors for atherosclerosis, such as decreases in serum cholesterol, triglycerides, fasting glucose and fasting insulin levels, and reduction of systolic and diastolic blood pressure as well as direct antiaging effects exerted on the vasculature per se (141). In this overview, we will focus on cell-autonomous effects (eg, upregulation of NO, changes in mitochondrial function), changes in paracrine regulation (altered cytokine microenvironment, low-grade inflammation), and the effects mediated by neuroendocrine factors.
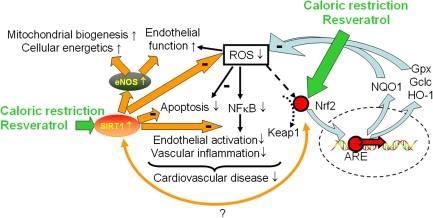
Figure 3.
Proposed scheme for the mechanisms by which caloric restriction and the caloric restriction mimetic resveratrol confers vasoprotection. In aging, calorie restriction and resveratrol induce/activate SIRT1 and upregulate eNOS in the endothelial cells promoting mitochondrial biogenesis, restoring cellular energetics, attenuating mitochondrial oxidative stress, improving endothelial function, attenuating apoptotic cell death, and inhibiting NF-κB. Caloric restriction and/or resveratrol may also activate Nrf2 (NF-E2-related factor 2). Nrf2 is translocated to the nucleus and binds to the antioxidant response element, which upregulates antioxidant enzymes, increases glutathione synthesis (upregulating γ-glutamylcysteine synthetase), and induces the NQO1-dependent transplasma membrane–associated redox system. The model predicts that there is an interaction between SIRT1 and activation of Nrf2-dependent ROS detoxification pathways.
The molecular mechanism by which CR promotes a youthful endothelial phenotype includes upregulation and activation of eNOS, which results in increased bioavailability of NO and improves endothelial function in aged animals (141–144) and perhaps humans (145–148). Because endothelium-derived NO plays a fundamental role in regulation of tissue metabolism [eg, by inducing mitochondrial biogenesis (143)], CR-induced improvement of endothelial health and the resulting increased NO bioavailability may be directly involved in the extension of life span in mammals. This idea is supported by the findings that eNOS−/−-deficient mice exhibit an accelerated aging phenotype associated with premature cardiac failure, mitochondrial dysfunction, and increased mortality (20,149). Finally, because mitochondriopathy in the vasculature per se represents an early manifestation of endothelial dysfunction (150), improved NO bioavailability by CR may prevent vascular energetic dysfunction (151), which is likely to contribute to vascular functional alterations in aging (152).
Attenuation of the age-associated increase in oxidative stress also is thought to contribute to the antiaging action of CR (153,154). Indeed, recent data clearly demonstrate that CR effectively decreases vascular production of ROS in aged laboratory rodents (141). This finding is consistent with the observations that endothelial cells obtained from CR mice exhibit decreased ROS production compared with those obtained from mice fed ad libitum (155). Previous studies also demonstrated that CR significantly attenuates oxidative DNA damage (156) and normalizes markers of lipid peroxidation in aortas of aged rats (157).
As noted previously, vascular aging is characterized by chronic low-grade inflammation. In this regard, it is significant that CR appears to attenuate vascular NF-κB induction and endothelial activation in aged rats (44,158). CR can also disrupt other proinflammatory signaling pathways as well, including JNK and P38 activation and AP-1 DNA-binding activity (157). It is significant that even short-term CR may confer anti-inflammatory effects (159), suggesting that some form of adult-onset CR may play a role in the therapeutic paradigm for cardiovascular diseases. The observation that CR in humans decreases circulating CRP and TNFα (145) provides preliminary evidence that CR may also reduce chronic low-grade inflammation in humans.
A key role of circulating factors in phenotypic responses due to CR was first indicated by the observations that in vitro treatment of cultured hepatocytes with sera from CR animals mimics phenotypic effects observed in vivo during CR (160). Neuroendocrine mediators present in the circulation reach endothelial cells and elicit a variety of responses, and we have good reason to believe that these circulating factors confer vasoprotective effects during CR. In support of this point of view, recent data show that sera of CR animals convey significant anti-inflammatory effects in cultured endothelial cells, including inhibition of TNFα-induced NF-κB activation, which mimic the vascular phenotypic changes induced in animals by CR in vivo (28). The actual circulating factor(s) by which the vasoprotective effects of CR are mediated are presently unknown. One potential candidate is adiponectin, whose serum level is known to be increased by CR, both in laboratory animals (161–163) and in humans (147,164). Recent evidence suggests that CR promotes skeletal muscle revascularization in response to ischemia via an AMPK-eNOS-dependent mechanism that is mediated by adiponectin (144). Adiponectin in vitro also can inhibit NF-κB activation (165) and induce mitochondrial biogenesis (166), mimicking the effects of CR. Thus, future studies are needed to further elucidate the role of adiponectin in the antiaging vascular effects of CR in aging.
There is increasing evidence that the evolutionarily highly conserved NAD+-dependent protein deacetylase SIRT1 contributes to the protective effects of CR (167,168). SIRT1 is abundantly expressed in the cardiovascular system (142,161,169) and is induced by CR (161). It is significant that sera from both CR animals and humans induce SIRT1 in cultured cells (168,170), including cultured human coronary arterial endothelial cells (28), suggesting that SIRT1 activation contributes to the vasoprotective effects conveyed by circulating factors. This concept is further supported by the results of recent studies indicating that pharmacological SIRT1 activation or SIRT1 overexpression confers significant antioxidative and anti-inflammatory effects in cultured endothelial cells (169). Moreover, endothelium-specific overexpression of SIRT1 effectively attenuates development of atherosclerosis in ApoE-deficient mice (171).
More recent studies provided convincing evidence that genes regulated by Nrf2 (NF-E2-related factor 2) are involved in the regulation of the aging process in model organisms (172) and may contribute to the protective effects of CR in mammals (173). Nrf2 is a transcription factor that binds to the antioxidant response element of target genes and increases the transcription of a variety of proteins involved in oxidative stress resistance and detoxification of free radicals (such as glutathione S-transferase, NQO1, and the antioxidant enzymes glutathione reductase, glutathione peroxidase, and catalase). The aforementioned Nrf2-dependent ROS detoxification systems are expressed and functional in the vasculature. Previous studies demonstrate that atheroprotective laminar flow regulates antioxidant response element-mediated genes through an Nrf2-dependent mechanism (174–177). Induction of Nrf2 was also shown to suppress endothelial activation (176). In aged rats, there is a significant decline in transcriptional activity of Nrf2, which causes age-related loss of glutathione synthesis (178), promoting cellular oxidative stress. Our recent studies demonstrate that CR is associated with induction of Nrf2-regulated genes, which contributes to the anticarcinogenic effects of CR (173). Thus, further studies are warranted to understand how aging and CR affect Nrf2 transcriptional activity in the vascular endothelial and smooth muscle cells.
Vasoprotection by Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene), a diet-derived polyphenol, is a prototype of a new class of drugs referred to as CR mimetics (179), which are being developed to reverse organ pathologies associated with aging and metabolic diseases. Resveratrol was reported to mimic many aspects of CR (35,180–182), and recent studies have provided evidence that resveratrol treatment exerts vasoprotective effects in aged mice (35) and rats (10), attenuating oxidative stress, improving endothelial function, inhibiting vascular inflammation, and decreasing the rate of endothelial apoptosis (Figure 3). In vitro studies in endothelial cells suggest that the molecular mechanisms of resveratrol-mediated vasoprotection involve a direct inhibition of NF-κB, upregulation of eNOS and antioxidant enzymes, induction of mitochondrial biogenesis, and prevention of oxidative stress–induced apoptosis (169,183–185). Epidemiological studies indicate that Mediterranean diets are rich in resveratrol and are associated with significantly reduced risk of cardiovascular disease in humans (186,187). These studies raise the possibility that resveratrol supplementation may confer significant vasoprotection in elderly humans.
Pathways that regulate mitochondrial biogenesis have recently emerged as potential therapeutic targets for the amelioration of endothelial and vascular dysfunction observed in metabolic diseases (188). Our recent studies showed that resveratrol induces mitochondrial biogenesis both in cultured endothelial cells and in endothelia of mice with accelerated vascular aging (183). Formation of new mitochondria was associated with activation of SIRT1, upregulation of eNOS, and induction of specific mitochondrial biogenesis factors (183). Induction of mitochondrial biogenesis by resveratrol also significantly attenuates both basal and stimulated mitochondrial O2·− generation in endothelial cells (169). Thus, further studies are evidently needed to determine whether induction of mitochondrial biogenesis contributes to the vasoprotective action of CR mimetics in the elderly patients.
FUTURE DIRECTIONS
Although significant progress has been achieved in describing age-related alterations in vascular function and phenotype, the specific roles for cell-autonomous (eg, mitochondrial alterations) and noncell-autonomous mechanisms (eg, hormonal effects) need to be elucidated further. There is reasonable consensus that oxidative stress has a key role in the pathogenesis of atherosclerosis and redox-sensitive molecular pathways (eg, NF-κB-mediated inflammatory processes), and these pathways are under intense investigation as the common denominators of the pathophysiology of several cardiovascular risk factors. In this view, the concept that evolutionarily conserved pathways (such as SIRT, Nrf2/antioxidant response element) control the aging process in mammals, regulating ROS production, and determining cellular and organismal sensitivity to oxidative stress raises the question of whether pharmacological or nutritional modulation of these pathways may be effective in delaying the onset of age-dependent vascular disease. Thus, research efforts should persist in these directions to fully elucidate the specific relationship between age-related alterations in ROS production and the pathways involved in cellular oxidative stress resistance and their interaction with other risk factors, which lead to the increased cardiovascular morbidity and mortality in the elderly patients.
FUNDING
This work was supported by grants from the American Diabetes Association, the American Federation for Aging Research, and the National Institutes of Health (NIH) (HL077256, HL43023, AG11370) and the Intramural Research Program of NIH (to R.d.C.).
References
- 1.Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 2.van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler A, Messina E, Sherman B, et al. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2003;285(3):H1015–H1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 4.Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson A, Yan C, Gao Q, et al. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol. 2007;293(3):H1344–H1350. doi: 10.1152/ajpheart.00413.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun D, Huang A, Yan EH, et al. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286(6):H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37(2):529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 8.Francia P, delli Gatti C, Bachschmid M, et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110(18):2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole-rat. Am J Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 10.Ungvari ZI, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293(1):H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 11.Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol. 2007;103(5):1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci. 2008;13:5056–5070. doi: 10.2741/3064. [DOI] [PubMed] [Google Scholar]
- 13.Tschudi MR, Barton M, Bersinger NA, et al. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest. 1996;98(4):899–905. doi: 10.1172/JCI118872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanabe T, Maeda S, Miyauchi T, et al. Exercise training improves ageing-induced decrease in eNOS expression of the aorta. Acta Physiol Scand. 2003;178(1):3–10. doi: 10.1046/j.1365-201X.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 15.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93(5):1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. Circ Res. 2001;89(9):793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann J, Haendeler J, Aicher A, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89(8):709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 18.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of aging and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkowitz DE, White R, Li D, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108(16):2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Mital S, Ojaimi C, Csiszar A, Kaley G, Hintze TH. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. J Mol Cell Cardiol. 2004;37(3):671–680. doi: 10.1016/j.yjmcc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17(1):21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 22.Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-{kappa}B. J Appl Physiol. 2008;105(4):1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csiszar A, Podlutsky A, Wolin MS, Losonczy G, Pacher P, Ungvari Z. Oxidative stress and accelerated vascular aging: implications for cigarette smoking. Front Biosci. 2009;14:3128–3144. doi: 10.2741/3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Saito Y, Ohyama Y, et al. Production of nitric oxide, but not prostacyclin, is reduced in klotho mice. Jpn J Pharmacol. 2002;89(2):149–156. doi: 10.1254/jjp.89.149. [DOI] [PubMed] [Google Scholar]
- 27.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294(5):H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 28.Csiszar A, Labinskyy N, Jimenez R, et al. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130(8):518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns EM, Kruckeberg TW, Comerford LE, Buschmann MT. Thinning of capillary walls and declining numbers of endothelial mitochondria in the cerebral cortex of the aging primate, Macaca nemestrina. J Gerontol. 1979;34(5):642–650. doi: 10.1093/geronj/34.5.642. [DOI] [PubMed] [Google Scholar]
- 30.Burns EM, Kruckeberg TW, Gaetano PK. Changes with age in cerebral capillary morphology. Neurobiol Aging. 1981;2(4):283–291. doi: 10.1016/0197-4580(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 31.Napoli C, Martin-Padura I, de Nigris F, et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci U S A. 2003;100(4):2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camici GG, Cosentino F, Tanner FC, Luscher TF. The role of p66Shc deletion in age-associated arterial dysfunction and disease states. J Appl Physiol. 2008;105(5):1628–1631. doi: 10.1152/japplphysiol.90579.2008. [DOI] [PubMed] [Google Scholar]
- 33.Yamamori T, White AR, Mattagajasingh I, et al. P66shc regulates endothelial NO production and endothelium-dependent vasorelaxation: implications for age-associated vascular dysfunction. J Mol Cell Cardiol. 2005;39(6):992–995. doi: 10.1016/j.yjmcc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 35.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orosz Z, Csiszar A, Labinskyy N, et al. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol. 2007;292(1):H130–H139. doi: 10.1152/ajpheart.00599.2006. [DOI] [PubMed] [Google Scholar]
- 37.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J. 2003;17(9):1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 38.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, et al. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83(3):279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50(1):219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 40.Miles EA, Rees D, Banerjee T, et al. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196(1):298–305. doi: 10.1016/j.atherosclerosis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Bruunsgaard H, Skinhoj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121(2):255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am J Pathol. 2007;170(1):388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-{alpha} antagonism. Am J Physiol Heart Circ Physiol. 2006;290(3):H1259–H1263. doi: 10.1152/ajpheart.00990.2005. [DOI] [PubMed] [Google Scholar]
- 44.Zou Y, Yoon S, Jung KJ, et al. Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci. 2006;61(3):232–244. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]
- 45.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7(6):805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97(16):9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308(5730):1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 48.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 49.Wang M, Takagi G, Asai K, et al. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41(6):1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 50.Wang M, Zhang J, Spinetti G, et al. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol. 2005;167(5):1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang L, Wang M, Zhang J, et al. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS ONE. 2008;3(5):e2231. doi: 10.1371/journal.pone.0002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayashi K, Miyagawa K, Sato K, Ueda R, Dohi Y. Temocapril, an angiotensin converting enzyme inhibitor, ameliorates age-related increase in carotid arterial stiffness in normotensive subjects. Cardiology. 2006;106(3):190–194. doi: 10.1159/000093024. [DOI] [PubMed] [Google Scholar]
- 53.Michel JB, Heudes D, Michel O, et al. Effect of chronic ANG I-converting enzyme inhibition on aging processes. II. Large arteries. Am J Physiol. 1994;267(1, pt 2):R124–R135. doi: 10.1152/ajpregu.1994.267.1.R124. [DOI] [PubMed] [Google Scholar]
- 54.Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens. 2009;22(1):74–79. doi: 10.1038/ajh.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corman B, Duriez M, Poitevin P, et al. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc Natl Acad Sci U S A. 1998;95(3):1301–1306. doi: 10.1073/pnas.95.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kass DA, Shapiro EP, Kawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104(13):1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 57.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106(1):326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Coppe JP, Patil CK, Rodier F, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5(8):741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kajstura J, Cheng W, Sarangarajan R, et al. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol. 1996;271(3, pt 2):H1215–H1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- 62.Asai K, Kudej RK, Shen YT, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20(6):1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 63.Boddaert J, Mallat Z, Fornes P, et al. Age and gender effects on apoptosis in the human coronary arterial wall. Mech Ageing Dev. 2005;126(6–7):678–684. doi: 10.1016/j.mad.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Anversa P, Li P, Sonnenblick EH, Olivetti G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am J Physiol. 1994;267(3, pt 2):H1062–H1073. doi: 10.1152/ajpheart.1994.267.3.H1062. [DOI] [PubMed] [Google Scholar]
- 65.Kang DH, Anderson S, Kim YG, et al. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37(3):601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 66.Montagna W, Carlisle K. Structural changes in aging human skin. J Invest Dermatol. 1979;73(1):47–53. doi: 10.1111/1523-1747.ep12532761. [DOI] [PubMed] [Google Scholar]
- 67.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138(8):3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 68.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54(1):25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 69.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2(2):149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- 70.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99(1):111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 71.Hua K, Forbes ME, Lichtenwalner RJ, Sonntag WE, Riddle DR. Adult-onset deficiency in growth hormone and insulin-like growth factor-I alters oligodendrocyte turnover in the corpus callosum. Glia. 2008;57(10):1062–1071. doi: 10.1002/glia.20829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4(2):195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Ramsey MM, Weiner JL, Moore TP, Carter CS, Sonntag WE. Growth hormone treatment attenuates age-related changes in hippocampal short-term plasticity and spatial learning. Neuroscience. 2004;129(1):119–127. doi: 10.1016/j.neuroscience.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area CA3 of hippocampus. Neuroscience. 2001;107(2):231–238. doi: 10.1016/s0306-4522(01)00341-4. [DOI] [PubMed] [Google Scholar]
- 75.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 76.Gonzalez A, Rota M, Nurzynska D, et al. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102(5):597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 77.Chang EI, Loh SA, Ceradini DJ, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116(24):2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 78.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45(9):1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 79.Thijssen DH, Vos JB, Verseyden C, et al. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell. 2006;5(6):495–503. doi: 10.1111/j.1474-9726.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 80.Keymel S, Kalka C, Rassaf T, Yeghiazarians Y, Kelm M, Heiss C. Impaired endothelial progenitor cell function predicts age-dependent carotid intimal thickening. Basic Res Cardiol. 2008;103(6):582–586. doi: 10.1007/s00395-008-0742-z. [DOI] [PubMed] [Google Scholar]
- 81.Bearzi C, Leri A, Lo Monaco F, et al. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Zhang Y, Herbert BS, Rajashekhar G, et al. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23(5):1358–1365. doi: 10.1096/fj.08-110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imanishi T, Hano T, Nishio I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens. 2005;23(1):97–104. doi: 10.1097/00004872-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 84.Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94(4):514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 85.Humpert PM, Djuric Z, Zeuge U, et al. Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol Med. 2008;14(5-6):301–308. doi: 10.2119/2007-00052.Humpert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang CY, Wen MS, Wang HW, et al. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation. 2008;118(21):2166–2173. doi: 10.1161/CIRCULATIONAHA.108.790469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anea CB, Zhang M, Stepp DW, et al. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119(11):1510–1517. doi: 10.1161/CIRCULATIONAHA.108.827477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol. 2007;102(3):847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 89.Zhu G, Song M, Wang H, et al. Young environment reverses the declined activity of aged rat-derived endothelial progenitor cells: involvement of the phosphatidylinositol 3-kinase/Akt signaling pathway. Ann Vasc Surg. 2009;23(4):519–534. doi: 10.1016/j.avsg.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Thum T, Hoeber S, Froese S, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100(3):434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 91.Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97(7):663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 92.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102(11):1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Batkai S, Rajesh M, Mukhopadhyay P, et al. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol. 2007;293(2):H909–918. doi: 10.1152/ajpheart.00373.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 95.Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic tumor necrosis factor-alpha inhibition enhances NO modulation of vascular function in estrogen-deficient rats. Hypertension. 2005;46(1):76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- 96.Rajesh M, Mukhopadhyay P, Batkai S, et al. Cannabinoid-2 receptor stimulation attenuates TNF{alpha}-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol Heart Circ Physiol. 2007;293(4):H2210–8. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burkle A, Beneke S, Muiras ML. Poly(ADP-ribosyl)ation and aging. Exp Gerontol. 2004;39(11–12):1599–1601. doi: 10.1016/j.exger.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 98.Pacher P, Vaslin A, Benko R, et al. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311(2):485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135(6):1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci U S A. 2002;99(5):3270–3275. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carrillo A, Monreal Y, Ramirez P, et al. Transcription regulation of TNF-alpha-early response genes by poly(ADP-ribose) polymerase-1 in murine heart endothelial cells. Nucleic Acids Res. 2004;32(2):757–766. doi: 10.1093/nar/gkh239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andreone TL, O’Connor M, Denenberg A, Hake PW, Zingarelli B. Poly(ADP-ribose) polymerase-1 regulates activation of activator protein-1 in murine fibroblasts. J Immunol. 2003;170(4):2113–2120. doi: 10.4049/jimmunol.170.4.2113. [DOI] [PubMed] [Google Scholar]
- 103.Zingarelli B, Hake PW, O’Connor M, Denenberg A, Kong S, Aronow BJ. Absence of poly(ADP-ribose)polymerase-1 alters nuclear factor-kappa B activation and gene expression of apoptosis regulators after reperfusion injury. Mol Med. 2003;9(5–8):143–153. doi: 10.2119/2003-00011.zingarelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol Chem. 1999;380(7–8):953–959. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- 105.Radovits T, Seres L, Gero D, et al. Single dose treatment with PARP-inhibitor INO-1001 improves aging-associated cardiac and vascular dysfunction. Exp Gerontol. 2007;42(7):676–685. doi: 10.1016/j.exger.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Radovits T, Seres L, Gero D, et al. The peroxynitrite decomposition catalyst FP15 improves ageing-associated cardiac and vascular dysfunction. Mech Ageing Dev. 2007;128(2):173–181. doi: 10.1016/j.mad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 107.Pacher P, Mabley JG, Soriano FG, Liaudet L, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med. 2002;9(6):659–664. [PubMed] [Google Scholar]
- 108.Elhadd TA, Abdu TA, Oxtoby J, et al. Biochemical and biophysical markers of endothelial dysfunction in adults with hypopituitarism and severe GH deficiency. J Clin Endocrinol Metab. 2001;86(9):4223–4232. doi: 10.1210/jcem.86.9.7813. [DOI] [PubMed] [Google Scholar]
- 109.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115(6):429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277(5328):942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 111.Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and lifespan. Trends Genet. 2002;18(6):295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- 112.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 113.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98(12):6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 115.Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146(7):2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 116.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 117.Csiszar A, Labinskyy N, Perez V, et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295(5):H1882–1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8(3):247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 119.Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer's disease. J Neurosci. 2007;27(4):824–831. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Groban L, Pailes NA, Bennett CD, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61(1):28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 121.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197(pt 4):575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Q, Li B, Wang X, Leri A, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100(8):1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leri A, Liu Y, Wang X, et al. Overexpression of insulin-like growth factor-1 attenuates the myocyte renin-angiotensin system in transgenic mice. Circ Res. 1999;84(7):752–762. doi: 10.1161/01.res.84.7.752. [DOI] [PubMed] [Google Scholar]
- 124.Li B, Setoguchi M, Wang X, et al. Insulin-like growth factor-1 attenuates the detrimental impact of nonocclusive coronary artery constriction on the heart. Circ Res. 1999;84(9):1007–1019. doi: 10.1161/01.res.84.9.1007. [DOI] [PubMed] [Google Scholar]
- 125.Li Q, Wu S, Li SY, et al. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol. 2007;292(3):H1398–H1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 126.Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 1999;13(14):1923–1929. doi: 10.1096/fasebj.13.14.1923. [DOI] [PubMed] [Google Scholar]
- 127.Groban L, Jobe H, Lin M, Houle T, Kitzman DA, Sonntag W. Effects of short-term treadmill exercise training or growth hormone supplementation on diastolic function and exercise tolerance in old rats. J Gerontol A Biol Sci Med Sci. 2008;63(9):911–920. doi: 10.1093/gerona/63.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sonntag WE, Bennett C, Ingram R, et al. Growth hormone and IGF-I modulate local cerebral glucose utilization and ATP levels in a model of adult-onset growth hormone deficiency. Am J Physiol Endocrinol Metab. 2006;291(3):E604–E610. doi: 10.1152/ajpendo.00012.2006. [DOI] [PubMed] [Google Scholar]
- 129.Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb Cortex. 2005;15(5):571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- 130.Ramsey MM, Adams MM, Ariwodola OJ, Sonntag WE, Weiner JL. Functional characterization of des-IGF-1 action at excitatory synapses in the CA1 region of rat hippocampus. J Neurophysiol. 2005;94(1):247–254. doi: 10.1152/jn.00768.2004. [DOI] [PubMed] [Google Scholar]
- 131.Lee IM, Paffenbarger RS., Jr. Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke. 1998;29(10):2049–2054. doi: 10.1161/01.str.29.10.2049. [DOI] [PubMed] [Google Scholar]
- 132.Taddei S, Galetta F, Virdis A, et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101(25):2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 133.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 134.Spier SA, Delp MD, Stallone JN, Dominguez JM, II, Muller-Delp JM. Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2007;292(6):H3119–H3127. doi: 10.1152/ajpheart.00588.2006. [DOI] [PubMed] [Google Scholar]
- 135.Trott DW, Gunduz F, Laughlin MH, Woodman CR. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2009;106(6):1925–1934. doi: 10.1152/japplphysiol.91232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Durrant JR, Seals DR, Connell ML, et al. Voluntary wheel running restores endothelial function in conduit arteries of old mice: Direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587(Pt13):3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCay CCM, Crowell MMF, Maynard LLA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 138.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 139.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Eng J Med. 1997;337(14):986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Austad SN. Life extension by dietary restriction in the bowl and doily spider, Frontinella pyramitela. Exp Gerontol. 1989;24(1):83–92. doi: 10.1016/0531-5565(89)90037-5. [DOI] [PubMed] [Google Scholar]
- 141.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102(5):519–528. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mattagajasingh I, Kim CS, Naqvi A, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104(37):14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 144.Kondo M, Shibata R, Miura R, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284(3):1718–1724. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol. 2006;47(2):398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 146.Pierce GL, Beske SD, Lawson BR, et al. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52(1):72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Raitakari M, Ilvonen T, Ahotupa M, et al. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24(1):124–128. doi: 10.1161/01.ATV.0000109749.11042.7c. [DOI] [PubMed] [Google Scholar]
- 148.Sasaki S, Higashi Y, Nakagawa K, et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15(4, pt 1):302–309. doi: 10.1016/s0895-7061(01)02322-6. [DOI] [PubMed] [Google Scholar]
- 149.Ojaimi C, Li W, Kinugawa S, et al. Transcriptional basis for exercise limitation in male eNOS-knockout mice with age: heart failure and the fetal phenotype. Am J Physiol Heart Circ Physiol. 2005;289(4):H1399–H1407. doi: 10.1152/ajpheart.00170.2005. [DOI] [PubMed] [Google Scholar]
- 150.Addabbo F, Ratliff B, Park HC, et al. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol. 2009;174(1):34–43. doi: 10.2353/ajpath.2009.080650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lopez-Lluch G, Hunt N, Jones B, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103(6):1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Csiszar A, Labinskyy N, Orosz Z, Ungvari Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med Hypotheses. 2006;67(4):904–908. doi: 10.1016/j.mehy.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 153.Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Long-term caloric restriction increases UCP3 content but decreases proton leak and reactive oxygen species production in rat skeletal muscle mitochondria. Am J Physiol. 2005;289(3):E429–E438. doi: 10.1152/ajpendo.00435.2004. [DOI] [PubMed] [Google Scholar]
- 154.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang H, Shi M, Story J, Richardson A, Guo Z. Food restriction attenuates age-related increase in the sensitivity of endothelial cells to oxidized lipids. J Gerontol A Biol Sci Med Sci. 2004;59(4):316–323. doi: 10.1093/gerona/59.4.b316. [DOI] [PubMed] [Google Scholar]
- 156.Guo ZM, Yang H, Hamilton ML, VanRemmen H, Richardson A. Effects of age and food restriction on oxidative DNA damage and antioxidant enzyme activities in the mouse aorta. Mech Ageing Dev. 2001;122(15):1771–1786. doi: 10.1016/s0047-6374(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 157.Castello L, Froio T, Cavallini G, et al. Calorie restriction protects against age-related rat aorta sclerosis. FASEB J. 2005;19(13):1863–1865. doi: 10.1096/fj.04-2864fje. [DOI] [PubMed] [Google Scholar]
- 158.Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction 1. FASEB J. 2004;18(2):320–322. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]
- 159.Jung KJ, Lee EK, Kim JY, et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm Res. 2009;58(3):143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- 160.de Cabo R, Furer-Galban S, Anson RM, Gilman C, Gorospe M, Lane MA. An in vitro model of caloric restriction. Exp Gerontol. 2003;38(6):631–639. doi: 10.1016/s0531-5565(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 161.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295(6):H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116(24):2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 163.Niemann B, Silber RE, Rohrbach S. Age-specific effects of short- and long-term caloric restriction on the expression of adiponectin and adiponectin receptors: influence of intensity of food restriction. Exp Gerontol. 2008;43(7):706–713. doi: 10.1016/j.exger.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 164.Jung SH, Park HS, Kim KS, et al. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2008;19(6):371–375. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 165.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 166.Civitarese AE, Ukropcova B, Carling S, et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab. 2006;4(1):75–87. doi: 10.1016/j.cmet.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 168.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 169.Csiszar A, Labinskyy N, Podlutsky A, et al. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294(6):H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Allard JS, Heilbronn LK, Smith C, et al. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS ONE. 2008;3(9):e3211. doi: 10.1371/journal.pone.0003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang QJ, Wang Z, Chen HZ, et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80(2):191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tullet JM, Hertweck M, An JH, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132(6):1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pearson KJ, Lewis KN, Price NL, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105(7):2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101(7):723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 175.Warabi E, Takabe W, Minami T, et al. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42(2):260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 176.Mostoslavsky R, Chua KF, Lombard DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 177.Chen XL, Varner SE, Rao AS, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278(2):703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 178.Suh JH, Shenvi SV, Dixon BM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101(10):3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 180.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26(13):3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 183.Csiszar A, Labinskyy N, Pinto JT, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297(1):H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ungvari Z, Orosz Z, Rivera A, et al. Resveratrol increases vascular oxidative stress resistance. Am J Physiol. 2007;292(5):H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 185.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. Am J Physiol. 2006;291(4):H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 186.Keys A, Menotti A, Karvonen MJ, et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124(6):903–915. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- 187.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 188.Schulz E, Dopheide J, Schuhmacher S, et al. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118(13):1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]