Abstract
Gender differences in the association of blood and urine cadmium concentrations with peripheral arterial disease (PAD) were evaluated by using data from 6,456 US adults aged ≥40 years who participated in the 1999–2004 National Health and Nutrition Examination Survey. PAD was defined as an ankle-brachial blood pressure index of <0.9 in at least one leg. For men, the adjusted odds ratios for PAD comparing the highest with the lowest quintiles of blood and urine cadmium concentrations were 1.82 (95% confidence interval (CI): 0.82, 4.05) and 4.90 (95% CI: 1.55, 15.54), respectively, with a progressive dose-response relation and no difference by smoking status. For women, the corresponding odds ratios were 1.19 (95% CI: 0.66, 2.16) and 0.56 (95% CI: 0.18, 1.71), but there was evidence of effect modification by smoking: among women ever smokers, there was a positive, progressive dose-response relation; among women never smokers, there was a U-shaped dose-response relation. Higher blood and urine cadmium levels were associated with increased prevalence of PAD, but women never smokers showed a U-shaped relation with increased prevalence of PAD at very low cadmium levels. These findings add to the concern of increased cadmium exposure as a cardiovascular risk factor in the general population.
Keywords: cadmium, health surveys, metals, peripheral vascular diseases, sex characteristics
Cadmium is a highly toxic and carcinogenic metal widely distributed in the environment (1, 2). Experimental models support a role for cadmium in atherosclerosis development (3, 4), and recent epidemiologic studies have evidenced associations between cadmium levels and carotid intima-media thickness (4), peripheral arterial disease (PAD) (5, 6), prevalent myocardial infarction (7), and cardiovascular and coronary mortality (8).
Several lines of evidence suggest that the effects of cadmium may be different in men and women (7–16). At similar exposure levels, women have higher blood or urine cadmium concentrations. In cadmium-polluted areas, cadmium-induced renal and bone disease has been shown to be more common and severe in women than in men (9, 11, 13, 14). In the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994), urine cadmium was associated with prevalent electrocardiogram-diagnosed myocardial infarction in women but not in men (7). Serum cadmium was also associated with increased carotid intima-media thickness in women 18–22 years of age from Austria (men were not included in this study) (4). However, increasing urine cadmium concentrations were prospectively associated with increased cardiovascular and coronary heart disease mortality among men but not women in the NHANES III Mortality Follow-up Study (8). Because of the uncertainty of these differences, we decided to evaluate gender susceptibility to the cardiovascular effects of cadmium.
In NHANES 1999–2000, urine and blood cadmium were associated with PAD (5, 6), an atherosclerosis-related narrowing of the arteries of the legs. Because of a limited sample size, the associations were not reported separately by gender. From 2001 through 2004, NHANES continued measuring the ankle-brachial blood pressure index (ABPI) to assess PAD. In this study, we took advantage of the increased sample size in NHANES 1999–2004 to evaluate gender differences in the cross-sectional association of blood and urine cadmium concentrations with PAD.
MATERIALS AND METHODS
Study population
NHANES uses a complex, multistage sampling design to obtain representative samples of the noninstitutionalized US population (17). For the present analysis, we used data from 9,145 adults, aged 40 years or older, who participated in the NHANES 1999–2004 interviews and physical examinations. The overall participation rate among participants aged 40 years or older was 68%. We further excluded 10 pregnant women, 410 participants missing blood cadmium information, 2 participants with urine cadmium corrected for molybdenum interference equal to zero, 1,383 participants missing ABPI determinations in both legs, 38 participants with an ABPI of >1.4 in at least one leg, and 846 participants missing other variables of interest, leaving 6,456 participants for this study. Participants in the final analyses were similar to the overall NHANES 1999–2004 sample regarding sociodemographic characteristics (age, sex, race/ethnicity, education), body mass index, and smoking status (data not shown).
Blood and urine cadmium
Blood and urine cadmium were measured at the Environmental Health Laboratory of the Centers for Disease Control and Prevention's National Center for Environmental Health (Atlanta, Georgia). Extensive quality control procedures were followed, including confirmation that collection and storage materials were not contaminated with background cadmium (17).
Cadmium and lead were measured simultaneously in whole blood on a Perkin-Elmer Model SIMAA 6000 multielement atomic absorption spectrometer (The Perkin-Elmer Corporation, Norwalk, Connecticut), with Zeeman background correction in 1999–2002 and on an inductively coupled plasma-mass spectrometer in 2003–2004. National Institute of Standards and Technology (Gaithersburg, Maryland) whole-blood standard reference materials were used for external calibration. The interassay coefficients of variation for blood cadmium ranged from 3.2% to 9.4%. The limits of detection were 0.3 μg/L in NHANES 1999–2002 and 0.2 μg/L in NHANES 2003–2004, resulting in 21.7% and 13.5% of observations, respectively, being less than the limit of detection.
Cadmium in urine was measured by using inductively coupled plasma-mass spectrometry (Perkin-Elmer Sciex ELAN 500; The Perkin-Elmer Corporation). The interassay coefficients of variation for urine cadmium ranged from 1.2% to 4.7%. The limit of detection was 0.06 μg/L, resulting in 2% of observations being less than the limit of detection. For observations less than the limit of detection, urine cadmium value was imputed as the limit of detection divided by the square root of 2 (17). National Institute of Standards and Technology urine reference material was used for external calibration. In NHANES 1999–2002, cadmium levels in urine were corrected for interference from molybdenum oxide. Urine cadmium was measured only in a random one-third subsample (n = 2,098) of our study population and was missing completely at random for two-thirds of the sample. For participants with blood cadmium concentrations less than the limit of detection and for participants with urine cadmium concentrations missing completely at random, cadmium concentrations were imputed from a joint prediction model for blood and urine cadmium levels (refer to the “Statistical analysis” portion of this section).
Peripheral arterial disease
ABPI was computed for each leg as the mean systolic blood pressure in each ankle (posterior tibial artery) divided by the mean systolic blood pressure in the right arm (brachial artery). For participants with conditions interfering with readings in the right arm, the left arm was used to calculate the ABPI in both legs. The blood pressure determinations for ABPI estimation were obtained in the horizontal position and separately from the determinations used to define hypertension (17). Blood pressure was measured twice at each site for participants aged 40–59 years and once at each site for those aged 60 years or older by using a Parks Mini-Lab IV Doppler device, model 3100 (Parks Medical Electronics, Inc., Aloha, Oregon). PAD was defined as an ABPI of <0.90 in at least one leg.
Other variables
Information on age; sex; race/ethnicity; education; menopause status; smoking; and medication for treating hypertension, diabetes, and hypercholesterolemia was based on self-report. Body mass index was calculated by dividing measured weight in kilograms by measured height in meters squared. Blood pressure was measured following the American Heart Association guidelines (17). Three and sometimes 4 systolic and diastolic blood pressure determinations were taken on the same day in the sitting position. Hypertension was defined as a mean systolic blood pressure of ≥140 mm Hg, a mean diastolic blood pressure of ≥90 mm Hg, a self-reported physician diagnosis, or medication use.
Diabetes was defined as a fasting glucose concentration of ≥126 mg/dL, a nonfasting glucose concentration of ≥200 mg/dL, a self-reported physician diagnosis, or medication use. C-reactive protein was analyzed in serum by high-sensitivity latex-enhanced nephelometry. Serum total cholesterol was measured enzymatically by using the Cholesterol High Performance reagent (Roche Diagnostics, Basel, Switzerland). High density lipoprotein cholesterol was measured by using a direct high density lipoprotein reagent (Roche Diagnostics). Serum cotinine was measured by an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometric method.
Urine creatinine was measured by the modified kinetic Jaffé method. Serum creatinine was measured by using a kinetic rate Jaffé method with a Hitachi Model 704 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, Indiana) and was calibrated to account for laboratory differences across time and to standardize to creatinine measures at the Cleveland Clinic Research Laboratory (Cleveland, Ohio) (18). Estimated glomerular filtration rate was calculated from calibrated creatinine, age, sex, and race/ethnicity by using the Modification of Diet in Renal Disease Study formula (18).
Statistical analysis
Blood cadmium concentrations less than the limit of detection and urine cadmium concentrations missing completely at random were imputed as the median of each subject-specific posterior distribution of predicted levels obtained from a Marcov chain Monte Carlo by Gibbs sampling nested linear model, implemented with WinBUGS software (19, 20). This model consisted of a single model with 2 embedded, separate predictive equations for urine and blood, each based on strong cadmium determinants, including smoking, gender, and age, identified separately for blood and urine cadmium in smoking-specific strata by using a backward stepwise process (Web Appendix 1; this information is described in the first of 3 supplementary Appendixes referred to as “Web Appendix” in the text and posted on the Journal’s Web site (http://aje.oupjournals.org/)). The urine cadmium nested predictive equation was a standard linear model, whereas the blood cadmium nested predictive equation was a tobit linear model for truncated data. The WinBUGS code is provided in Web Appendix 2.
The median of the participants’ imputed missing completely at random urine cadmium was 0.40 μg/L (minimum–maximum: 0.02–4.96) (0.40 μg/g creatinine) (minimum–maximum: 0.11–3.29). The corresponding value in the measured one-third subsample was 0.39 μg/L (minimum–maximum: 0.01–36.78) (0.40 μg/g creatinine) (minimum–maximum: 0.02–282.9). The median of the participants’ imputed blood cadmium levels was 0.23 μg/L (minimum–maximum: 0.15–0.27) for NHANES 1999–2002 and 0.16 μg/L (minimum–maximum: 0.11–0.18) for NHANES 2003–2004.
Blood and urine cadmium concentrations were log-transformed for the analyses. Cutoffs for blood cadmium quintiles were based on weighted distributions in the whole study sample. Cutoffs for creatinine-corrected urine cadmium quintiles were based on weighted distributions in the originally measured one-third random subsample. Medians for trend tests and knots for spline terms were also defined based on measured urine cadmium concentrations.
Statistical analyses were conducted in the data set by including the imputed cadmium values and were repeated in the one-third random subsample with measured urine cadmium levels, with consistent findings (Web Appendix 3). Odds ratios and 95% confidence intervals for PAD compared quintiles 2–5 of blood- and creatinine-corrected urine cadmium with the lowest quintile. To assess nonlinear relations, we estimated the odds ratio for PAD by blood- and creatinine-corrected urine cadmium levels modeled using restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles of each cadmium distribution. We also evaluated the increase in the odds of PAD by comparing the 80th with the 20th percentiles of cadmium levels.
All analyses were stratified by gender. Statistical models were initially adjusted for age (restricted cubic splines), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), and survey year. Models were further adjusted for postmenopausal status for women (yes, no), body mass index (continuous), systolic blood pressure (continuous) and blood-pressure-lowering medication use (yes, no), diabetes (yes, no), smoking (never, former, current, and log-transformed serum cotinine), education (<high school, ≥high school), blood lead (continuous log-transformed), total cholesterol (continuous), high density lipoprotein cholesterol (continuous), cholesterol-lowering-medication use (yes, no), C-reactive protein (continuous log-transformed), and estimated glomerular filtration rate (continuous). P values for linear trend were obtained by including the medians for each cadmium quintile as continuous variables in the regression models. For urine cadmium models, we repeated the analyses using urine cadmium concentrations (μg/L) in models further adjusted for urine creatinine instead of using creatinine-corrected urine cadmium concentrations (μg/g), with similar findings (data not shown).
Exploratory interaction analyses were conducted by including the product of log-transformed blood- or creatinine-corrected urine cadmium regression terms with covariates, separately for men and women. We conducted sensitivity analysis by using a different cutoff for defining PAD by gender (ABPI of 0.90 for men and 0.85 for women), with similar results (not shown). The weighted prevalence of PAD among women when the lower cutoff was used was 3.2%.
Statistical analyses were performed by using the survey package in R software (21–23) to account for the complex sampling design and weights in NHANES 1999–2004 and to obtain appropriate standard errors for all estimates. All statistical tests were 2-sided.
RESULTS
The weighted prevalence of PAD was 4.5% for men and 5.1% for women. The overall geometric means of blood and urine cadmium were 0.44 μg/L and 0.31 μg/g creatinine (0.37 μg/L), respectively, for men and 0.50 μg/L and 0.44 μg/g (0.31 μg/L), respectively, for women. The Spearman correlation coefficients between blood cadmium (μg/L) and urine cadmium (μg/g creatinine) were 0.70 for men and 0.60 for women. For both men and women, participants with PAD, compared with participants without PAD, were older and were more likely to be current or former smokers; to have lower educational levels, diabetes, and chronic kidney disease; and to have higher levels of C-reactive protein, systolic blood pressure, blood lead, blood cadmium, and urine cadmium (Table 1).
Table 1.
Characteristics of Participants by the Presence or Absence of PAD, National Health and Nutrition Examination Survey, 1999–2004a,b
| Men |
Women |
|||
| PAD (n = 245) | No PAD (n = 3,088) | PAD (n = 223) | No PAD (n = 2,900) | |
| Age, years | 67.6 (0.9) | 54.7 (0.2) | 67.9 (1.0) | 56.1 (0.3 |
| Menopause, % | 86.9 (2.8) | 66.1 (1.6) | ||
| Race/ethnicity, % | ||||
| White | 80.2 (2.8) | 80.5 (1.5) | 77.7 (2.9) | 78.2 (1.8) |
| Black | 13.2 (2.3) | 7.8 (0.7) | 15.0 (2.8) | 8.7 (1.1) |
| Mexican American | 4.1 (1.7) | 4.9 (0.7) | 3.0 (0.8) | 4.3 (0.8) |
| Education <high school, % | 35.2 (3.9) | 17.7 (1.0) | 28.2 (3.5) | 19.3 (0.9) |
| Smoking, % | ||||
| Former | 44.0 (3.8) | 33.6 (1.1) | 34.7 (4.4) | 25.1 (1.0) |
| Current | 42.5 (3.5) | 31.2 (1.5) | 21.4 (3.1) | 19.9 (1.0) |
| Serum cotinine, ng/mL | 1.63 (0.88, 3.05) | 0.70 (0.53, 0.91) | 0.28 (0.16, 0.48) | 0.22 (0.18, 0.27) |
| Body mass index, kg/m2 | 27.9 (0.4) | 28.4 (0.1) | 29.0 (0.5) | 28.1 (0.2) |
| C-reactive protein, mg/L | 3.30 (2.81, 3.86) | 1.76 (1.67, 1.86) | 3.94 (3.40, 4.56) | 2.45 (2.31, 2.60) |
| Total cholesterol, mg/dL | 200.7 (4.5) | 208.8 (1.3) | 216.8 (3.1) | 213.4 (1.1) |
| HDL cholesterol, mg/dL | 46.8 (1.0) | 47.0 (0.4) | 54.8 (1.2) | 59.5 (0.5) |
| Cholesterol medication, % | 35.3 (3.3) | 17.8 (0.9) | 28.2 (4.1) | 14.1 (0.7) |
| Systolic blood pressure, mm Hg | 136.0 (1.7) | 126.1 (0.5) | 141.7 (2.1) | 128.0 (0.5) |
| Blood pressure medication, % | 48.7 (3.6) | 24.3 (1.1) | 53.4 (3.9) | 27.9 (1.3) |
| Diabetes, % | 31.1 (4.2) | 11.4 (0.7) | 18.1 (3.2) | 9.0 (0.6) |
| GFR <60 mL/minute per 1.73 m2, % | 27.2 (3.3) | 6.6 (0.6) | 27.9 (3.3) | 9.9 (0.8) |
| Blood lead, μg/dL | 2.81 (2.60, 3.03) | 2.26 (2.19, 2.33) | 1.99 (1.82, 2.17) | 1.54 (1.47, 1.60) |
| Blood cadmium, μg/L | 0.65 (0.59, 0.71) | 0.43 (0.41, 0.45) | 0.62 (0.55, 0.69) | 0.50 (0.48, 0.52) |
| Urine cadmium, μg/Lc | 0.73 (0.56, 0.96) | 0.36 (0.33, 0.39) | 0.45 (0.35, 0.58) | 0.31 (0.28, 0.33) |
| Urine cadmium, μg/g creatininec | 0.67 (0.54, 0.82) | 0.30 (0.29, 0.32) | 0.51 (0.42, 0.61) | 0.44 (0.41, 0.47) |
Abbreviations: GFR, glomerular filtration rate; HDL, high density lipoprotein; PAD, peripheral arterial disease.
Percentages (standard errors) are given for categorical variables or weighted means (standard errors) for continuous variables, except for serum cotinine, C-reactive protein, blood lead and blood cadmium, and urine cadmium and creatinine-adjusted cadmium, for which geometric means (95% confidence intervals) are reported.
International system conversion factors: To convert urine and blood cadmium to nmol/L, multiply by 8.896; to convert creatinine-corrected urine cadmium to nmol/mmol creatinine, multiply by 1.006; to convert lead to μmol/L, multiply by 0.0483; to convert serum cotinine to nmol/L, multiply by 5.675.
Measured in a subsample only (n = 2,098).
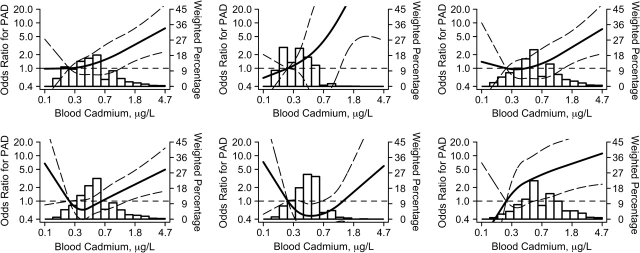
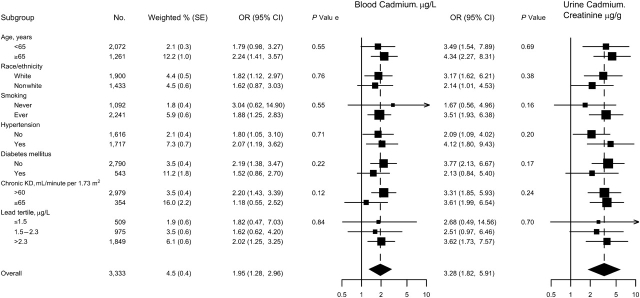
For men, we found a positive and statistically significant association of cadmium levels with the prevalence of PAD (Table 2, Figure 1), although the association was stronger for urine than for blood cadmium. The multivariable adjusted odds ratios for PAD comparing men in the highest versus the lowest quintiles of blood and urine cadmium were 1.82 (95% confidence interval (CI): 0.82, 4.05) and 4.90 (95% CI: 1.55, 15.54), respectively (Table 2). In spline regression models, the dose-response relation was progressive over the range of cadmium concentrations for blood (Figure 1) and urine cadmium (not shown). The P values for the nonlinear components in the restricted quadratic spline terms were 0.64 for blood and 0.32 for urine cadmium. When we assumed a log-linear dose-response relation, the odds ratios for PAD comparing the 80th with the 20th percentiles of blood and urine cadmium concentrations were 1.95 (95% CI: 1.28, 2.96) and 3.28 (95% CI: 1.82, 5.91), respectively. The increase in the prevalence of PAD with increasing blood and urine cadmium levels was consistent in participant subgroups, including age groups and never and ever smokers (Figures 1 and 2).
Table 2.
Odds Ratios and 95% Confidence Intervals for Peripheral Arterial Disease by Blood and Urine Cadmium Quintiles in Men (N = 3,333), National Health and Nutrition Examination Survey, 1999–2004a
| Participants, No. |
Model 1b |
Model 2c |
Model 3d |
|||||
| Cases | Noncases | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Blood Cadmium (μg/L) | ||||||||
| Quintile 1 (≤0.26) | 23 | 694 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Quintile 2 (0.26–0.3) | 17 | 434 | 0.84 | 0.34, 2.09 | 0.94 | 0.37, 2.36 | 0.85 | 0.33, 2.20 |
| Quintile 3 (0.4–0.5) | 30 | 535 | 0.97 | 0.50, 1.90 | 1.00 | 0.51, 1.93 | 0.83 | 0.41, 1.67 |
| Quintile 4 (0.6–0.7) | 83 | 787 | 1.84 | 0.93, 3.63 | 1.66 | 0.78, 3.53 | 1.20 | 0.52, 2.80 |
| Quintile 5 (≥0.8) | 92 | 638 | 3.79 | 2.04, 7.05 | 3.14 | 1.54, 6.40 | 1.82 | 0.82, 4.05 |
| P trende | <0.001 | <0.001 | 0.02 | |||||
| Urine Cadmium (μg/g Creatinine) | ||||||||
| Quintile 1 (<0.20) | 8 | 623 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Quintile 2 (0.20–0.30) | 34 | 778 | 1.81 | 0.68, 4.84 | 1.93 | 0.72, 5.19 | 1.66 | 0.63, 4.41 |
| Quintile 3 (0.31–0.43) | 59 | 743 | 2.35 | 0.89, 6.19 | 2.26 | 0.86, 5.91 | 1.66 | 0.62, 4.45 |
| Quintile 4 (0.44–0.68) | 74 | 636 | 3.90 | 1.60, 9.51 | 3.42 | 1.36, 8.60 | 2.31 | 0.87, 6.14 |
| Quintile 5 (≥0.69) | 70 | 308 | 9.18 | 3.29, 25.64 | 8.04 | 2.69, 24.02 | 4.90 | 1.55, 15.54 |
| P trende | <0.001 | <0.001 | <0.001 | |||||
Abbreviations: CI, confidence interval; OR, odds ratio.
International system conversion factors: To convert blood cadmium to nmol/L, multiply by 8.896; to convert creatinine-corrected urine cadmium to nmol/mmol creatinine, multiply by 1.006.
Adjusted for age (years), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), and survey year.
Further adjusted for education (<high school, ≥high school), postmenopausal status for women (yes, no), body mass index (kg/m2), blood lead (log μg/dL), C-reactive protein (log mg/L), total cholesterol (mg/dL), high density lipoprotein cholesterol (mg/dL), cholesterol-lowering medication (yes, no), systolic blood pressure (mm Hg), blood-pressure-lowering medication (yes, no), diabetes (yes, no), and estimated glomerular filtration rate (mL/minute per 1.73 m2).
Further adjusted for smoking (never, former, current) and serum cotinine (log ng/mL).
Two-sided P values for linear trend across quintiles of cadmium were obtained by including blood- or creatinine-corrected medians corresponding to each quintile as continuous variables in the linear regression models.
Figure 1.
Odds ratios for peripheral arterial disease (PAD) by blood cadmium levels, National Health and Nutrition Examination Survey, 1999–2004. Shown are adjusted odds ratios (solid lines) and 95% confidence intervals (curved dashed lines) based on restricted quadratic splines for log-transformed cadmium concentrations with knots at the 10th, 50th, and 90th percentiles. The reference value was set at the 10th percentile of cadmium distribution. Vertical bars represent the weighted histogram of cadmium distribution. Odds ratios for PAD were adjusted for survey year, age (years modeled as restricted cubic spline with 5 knots), sex, race/ethnicity, body mass index (kg/m2), low educational level (<high school, ≥high school), smoking status (never, former, current), serum cotinine (log ng/mL), diabetes mellitus (yes, no), menopause status (yes, no), systolic blood pressure (mm Hg), antihypertensive medication (yes, no), C-reactive protein (log mg/L), total cholesterol (mg/dL), high density lipoprotein cholesterol (mg/dL), cholesterol-lowering medication (yes, no), estimated glomerular filtration rate (mL/minute per 1.73 m2), and blood lead (log μg/dL). Men- and women-stratified analyses are shown in the top and bottom rows, respectively. Overall (both never and ever smokers together; left column) and analyses stratified by never smoker and ever smoker status (middle and right columns, respectively) are shown.
Figure 2.
Odds ratios (ORs) for peripheral arterial disease comparing the 80th with the 20th percentile of blood or urine cadmium distribution in men (N = 3,333), by participant characteristics, National Health and Nutrition Examination Survey, 1999–2004. The 80th and 20th percentiles of cadmium distributions were 0.80 and 0.26 μg/L, respectively, for blood cadmium and were 0.69 and 0.20 μg/g creatinine, respectively, for creatinine-corrected urine cadmium. Odds ratios and 95% confidence intervals (CIs) comparing the 80th with the 20th percentiles of the blood or urine cadmium distribution were calculated by assuming a log-linear relation. Odds ratios were adjusted for survey year, age (years modeled as restricted cubic splines with 5 knots), sex, race/ethnicity, body mass index (kg/m2), low educational level (<high school, ≥high school), smoking status (never, former, current), serum cotinine (log ng/mL), diabetes mellitus (yes, no), systolic blood pressure (mm Hg), antihypertensive medication (yes, no), C-reactive protein (log mg/L), total cholesterol (mg/dL), high density lipoprotein cholesterol (mg/dL), cholesterol-lowering medication (yes, no), estimated glomerular filtration rate (mL/minute per 1.73 m2), and blood lead (log μg/dL). Estimated 2-sided P values for interaction between log-linear cadmium with participant characteristics were computed by using the Wald test adjusting for complex design. KD, kidney disease; SE, standard error.
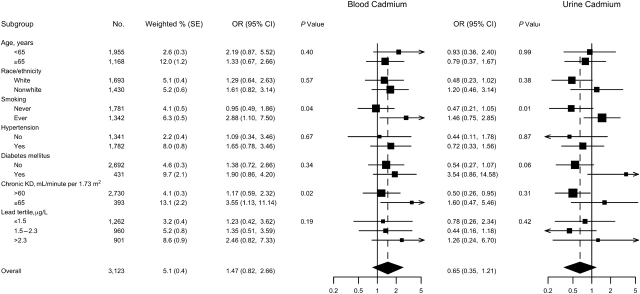
For women, the multivariable adjusted odds ratios for PAD comparing quintiles 2–5 versus the lowest quintile were 0.58 (95% CI: 0.22, 1.54), 0.61 (95% CI: 0.22, 1.69), 0.67 (95% CI: 0.36, 1.24), and 1.19 (95% CI: 0.66, 2.16), respectively, for blood cadmium; and 0.42 (95% CI: 0.13, 1.33), 0.32 (95% CI: 0.12, 0.85), 0.24 (95% CI: 0.09, 0.68), and 0.56 (95% CI: 0.18, 1.71) for urine cadmium (Table 3). This nonlinear pattern was also evident in spline regression models (Figure 1). The P values for the nonlinear components in the restricted quadratic splines were 0.05 for blood and 0.003 for urine cadmium. When we assumed a nonlinear dose-response relation modeled using restricted quadratic splines, the odds ratios for PAD comparing the 80th with the 20th percentiles of blood and urine cadmium concentrations were 1.47 (95% CI: 0.82, 2.66) and 0.65 (95% CI: 0.35, 1.21), respectively (Figure 3). In more detailed analyses, never smokers seemed to account for the nonlinear pattern of PAD with cadmium among women (Figure 1). For women ever smokers, the odds ratios for PAD comparing the 80th with the 20th percentiles of blood and urine cadmium concentrations were 2.88 (95% CI: 1.10, 7.50) and 1.46 (95% CI: 0.75, 2.85), respectively.
Table 3.
Odds Ratios and 95% Confidence Intervals for Peripheral Arterial Disease by Blood and Urine Cadmium Quintiles in Women (N = 3,123), National Health and Nutrition Examination Survey, 1999–2004a
| Participants, No. |
Model 1b |
Model 2c |
Model 3d |
|||||
| Cases | Noncases | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Blood Cadmium (μg/L) | ||||||||
| Quintile 1 (≤0.26) | 22 | 384 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Quintile 2 (0.26–0.3) | 17 | 392 | 0.61 | 0.23, 1.58 | 0.61 | 0.23, 1.61 | 0.58 | 0.22, 1.54 |
| Quintile 3 (0.4–0.5) | 33 | 586 | 0.63 | 0.23, 1.71 | 0.64 | 0.23, 1.75 | 0.61 | 0.22, 1.69 |
| Quintile 4 (0.6–0.7) | 79 | 927 | 0.81 | 0.41, 1.58 | 0.76 | 0.40, 1.42 | 0.67 | 0.36, 1.24 |
| Quintile 5 (≥0.8) | 72 | 611 | 1.66 | 0.92, 3.00 | 1.49 | 0.86, 2.58 | 1.19 | 0.66, 2.16 |
| P trende | <0.001 | <0.001 | 0.01 | |||||
| Urine Cadmium (μg/g Creatinine) | ||||||||
| Quintile 1 (<0.20) | 10 | 130 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Quintile 2 (0.20–0.30) | 23 | 465 | 0.44 | 0.14, 1.37 | 0.43 | 0.13, 1.36 | 0.42 | 0.13, 1.33 |
| Quintile 3 (0.31–0.43) | 37 | 773 | 0.35 | 0.14, 0.92 | 0.34 | 0.13, 0.94 | 0.32 | 0.12, 0.85 |
| Quintile 4 (0.44–0.68) | 61 | 887 | 0.29 | 0.11, 0.73 | 0.30 | 0.11, 0.80 | 0.24 | 0.09, 0.68 |
| Quintile 5 (≥0.69) | 92 | 645 | 0.78 | 0.30, 2.00 | 0.80 | 0.28, 2.31 | 0.56 | 0.18, 1.71 |
| P trende | 0.004 | 0.008 | 0.22 | |||||
Abbreviations: CI, confidence interval; OR, odds ratio.
International system conversion factors: To convert blood cadmium to nmol/L, multiply by 8.896; to convert creatinine-corrected urine cadmium to nmol/mmol creatinine, multiply by 1.006.
Adjusted for age (years), race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), and survey year.
Further adjusted for education (<high school, ≥high school), postmenopausal status for women (yes, no), body mass index (kg/m2), blood lead (log μg/dL), C-reactive protein (log mg/L), total cholesterol (mg/dL), high density lipoprotein cholesterol (mg/dL), cholesterol-lowering medication (yes, no), systolic blood pressure (mm Hg), blood-pressure-lowering medication (yes, no), diabetes (yes, no), and estimated glomerular filtration rate (mL/minute per 1.73 m2).
Further adjusted for smoking (never, former, current) and serum cotinine (log ng/mL).
Two-sided P value for linear trend across quintiles of cadmium were obtained by including blood- or creatinine-corrected medians corresponding to each quintile as continuous variables in the linear regression models.
Figure 3.
Odds ratios (ORs) for peripheral arterial disease comparing the 80th with the 20th percentile of blood or urine cadmium distribution in women (N = 3,123), by participant characteristics, National Health and Nutrition Examination Survey, 1999–2004. The 80th and 20th percentiles of cadmium distributions were 0.80 and 0.26 μg/L, respectively, for blood cadmium and were 0.69 and 0.20 μg/g creatinine, respectively, for creatinine-corrected urine cadmium. Odds ratios and 95% confidence intervals (CIs) comparing the 80th with the 20th percentiles of the blood or urine cadmium distribution were calculated based on restricted quadratic splines for log-transformed cadmium concentrations with knots at the 10th, 50th, and 90th percentiles. Odds ratios were adjusted for survey year, age (years modeled as restricted cubic splines with 5 knots), sex, race/ethnicity, body mass index (kg/m2), low educational level (<high school, ≥high school), smoking status (never, former, current), serum cotinine (log ng/mL), diabetes mellitus (yes, no), menopause status (yes, no), systolic blood pressure (mm Hg), antihypertensive medication (yes, no), C-reactive protein (log mg/L), total cholesterol (mg/dL), high density lipoprotein cholesterol (mg/dL), cholesterol-lowering medication (yes, no), estimated glomerular filtration rate (mL/minute per 1.73 m2), and blood lead (log μg/dL). Estimated 2-sided P values for the interaction between log-cadmium spline terms with participant characteristics were computed by using the Wald test adjusting for complex design. KD, kidney disease; SE, standard error.
DISCUSSION
In this representative sample of the US population, high cadmium levels were associated with an increased prevalence of PAD, but the association between cadmium and PAD at low cadmium exposures was markedly different in men and women. Among men, including never and ever smokers, the prevalence of PAD increased progressively throughout the whole range of cadmium exposure. The association between cadmium exposures and PAD among men never smokers is important because it rules out confounding by smoking. Among women, cadmium levels showed a U-shaped relation with PAD, although the nonlinear component mostly reflected the association in women never smokers.
Among men and women, high cadmium levels were associated with increased prevalence of PAD. Several mechanisms may explain a role for cadmium in promoting atherosclerosis. Cadmium may indirectly increase the formation of reactive oxygen species (3) and interfere with antioxidative stress responses by binding metallothionein (3, 24), a low-molecular-weight protein that regulates zinc homeostasis and acts as a free radical scavenger (3, 25, 26). Other mechanisms that could contribute to atherosclerosis include partial agonism for calcium channels, direct vasoconstrictor action, inhibition of vasodilator substances such as nitric oxide, activation of the sympathetic nervous system, and cadmium-related epigenetic mechanisms (27–31). In vitro, cadmium causes endothelial dysfunction; in vivo, it accelerates atherosclerotic plaque formation (4).
In addition to experimental evidence, a growing body of epidemiologic evidence supports cadmium as a cardiovascular risk factor (32). In prospective NHANES analysis, increasing urinary cadmium levels were associated with increased cardiovascular and coronary heart disease mortality in men over 12 years of follow-up (8). Among young women in Austria, participants with increasing serum cadmium levels had substantially increased intima-media thickness (4). In cross-sectional NHANES analyses, cadmium exposure was related to increased blood pressure (33), albuminuria and decreased estimated glomerular filtration rate (34), electrocardiogram-diagnosed myocardial infarction (7), and PAD (5, 6), although the associations were not reported by gender.
In our stratified analysis, an increase in the prevalence of PAD was evident throughout the range of cadmium exposure for men ever smokers and never smokers and for women ever smokers. Among women never smokers, however, cadmium at very low concentrations showed an inverse association with PAD. We do not have a good explanation for this inverse association. Smoking, diet, occupation, and ambient air in urban and industrial areas are the main sources of cadmium exposure in the population. Among smokers, tobacco is the major source of cadmium (35). Among nonsmokers, cadmium sources and exposure routes could be very different for men and women. Occupational exposures (the metal and mining industry, transportation, and repairing services) (36) could be important for men. Cadmium dietary sources, including leafy/root vegetables, grains, shellfish, and offal (1, 35), could be more important for women. These differences in exposure sources could translate into different effective dose because cadmium absorption through the respiratory tract is 10%–50% compared with 5%–20% through the gastrointestinal tract (35). In addition, respiratory and dietary exposure routes to cadmium may have different metabolic pathways and health effects.
Even under similar conditions of cadmium exposure, women tend to have higher urine, blood, and renal cortex cadmium levels compared with men (10). Women may absorb more cadmium through the gastrointestinal tract because of a higher prevalence of decreased iron stores, which increases expression of the divalent metal transporter DMT-1 (10), the nonheme iron intestinal transporter that also mediates cadmium transport (35). Women could also be genetically predisposed to have higher cadmium levels. For instance, blood cadmium heritability was 65% in Swedish same-sex female twins who were nonsmokers compared with only 13% among male twins who were nonsmokers (37). In 2,926 adult twins living in Australia, suggestive linkage peaks related to red blood cell cadmium levels were found in chromosomes 2, 18, 20, and X (38). Interestingly, a minor linkage peak in chromosome 2 was close to the gene SLC11A1, which encodes a divalent metal transporter. In our study, consistent with previous studies, women had higher blood and urine cadmium levels than men at similar levels of cadmium predictors, including smoking status. In addition to increased internal cadmium dose, women may have higher expression of the metallothionein IIA gene (39), which could explain differential atherosclerosis-related cadmium toxicity for men and women.
In cadmium-polluted areas, including the Itai-Itai disease area in Japan, women had increased total mortality and an increased incidence of kidney and bone disease (11–16). The Itai-Itai disease affected mostly women in the Jinzu river basin in Japan during 1910 to 1945. The disease was characterized by renal tubular dysfunction and bone mass loss related to extremely high cadmium levels in contaminated rice and water (15). In another cadmium-polluted area in Japan, the dose-response relation between urine cadmium and total mortality was also stronger for women (hazard ratios ranged from 2.03 to 3.11) compared with men (hazard ratios, 1.11 to 1.32) (16). For heart failure, however, cadmium-associated mortality was somewhat higher in men than in women (hazard ratio = 1.97 vs. 1.59) (16). In a cadmium-polluted area in Belgium, the hazard ratio for bone fracture for a doubling in 24-hour urine cadmium excretion was 1.73 for women compared with 1.20 for men (13).
On the basis of these data from cadmium-polluted areas, it has been argued that women are more susceptible to the toxic effects of cadmium. Our study, however, was performed in a population exposed to relatively low cadmium levels, with potentially different patterns of toxicity. Indeed, using data from NHANES III, Menke et al. (8) found the same pattern of progressively increasing coronary heart disease mortality with increasing urine cadmium in men but a U-shaped relation for women. These findings indicate that epidemiologic studies of cadmium should report detailed dose-response analyses separately for men and women and also by smoking status.
Our study has several limitations, including a cross-sectional design, the possibility of different residual confounding by gender, and the use of single determinations of cadmium biomarkers. Furthermore, 20% of study participants had blood cadmium levels lower than the limit of detection, and two-thirds of study participants had urine cadmium levels missing completely at random. We attempted to overcome these limitations by using a prediction model to simultaneously impute the missing cadmium data. Finally, for smokers, we cannot discard residual confounding by long-term, cumulative smoking dose. However, the associations remained similar after adjustment for serum cotinine, a biomarker of ongoing smoking, among both men and women. Strengths of the study include the large sample size that enabled us to explore effect modification within gender strata, the availability of information on multiple risk factors for PAD, the careful standardization of study protocols and extensive quality control for the examination and laboratory procedures in NHANES, and the representative nature of the study sample.
In conclusion, gender differences in the association between cadmium and PAD in a representative sample of US adults were dependent on dose and smoking status. Cadmium was consistently associated with increased prevalence of PAD at high levels of exposure and among ever smokers, supporting the role of cadmium as a cardiovascular risk factor for both men and women. Among never smokers, cadmium was progressively associated with PAD in men, whereas the dose-response relation was U-shaped for women. These gender differences among never smokers need to be interpreted cautiously given wide confidence intervals and conflicting literature. Further experimental and prospective epidemiologic research is needed to understand whether these differences are related to differential cadmium toxicity, differential measurement error, or differential residual confounding for men and women. Our results, however, add to current concerns about cadmium exposure as an independent and important risk factor for atherosclerotic cardiovascular disease.
Supplementary Material
Acknowledgments
Author affiliations: Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Maria Tellez-Plaza, Ana Navas-Acien, A. Richey Sharrett, Eliseo Guallar); Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Maria Tellez-Plaza, Ana Navas-Acien); Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, Maryland (Maria Tellez-Plaza, Ana Navas-Acien, A. Richey Sharrett, Eliseo Guallar); Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Ciprian M. Crainiceanu); Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland (A. Richey Sharrett, Eliseo Guallar); and Department of Cardiovascular Epidemiology and Population Genetics, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain (Maria Tellez-Plaza, Eliseo Guallar).
This work was supported by the National Heart, Lung, and Blood Institute, Bethesda, Maryland (grant R01 HL090863); the National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (grants 2 and 3 ES007198, ES012673, and ES015597); and the National Institute for Occupational Safety and Health, Atlanta, Georgia (grant T42 OH0008428 from the Education and Research Center for Occupational Safety and Health at the Johns Hopkins Bloomberg School of Public Health) and the National Institute of Neurological Disorders and Stroke (grant R01NS060910), Bethesda, Maryland. Maria Tellez-Plaza was also supported by the 2009 Louis I. and Thomas D. Dublin Award for the Advancement of Epidemiology and Biostatistics (Johns Hopkins Bloomberg School of Public Health).
Selected data from this manuscript were presented at the following conferences: the International Society for Environmental Epidemiology Conference, Dublin, Ireland, August 26–29, 2009; and the American Heart Association Cardiovascular Disease Epidemiology and Prevention Conference, San Francisco, California, March 2–6, 2010.
Conflict of interest: none declared.
Glossary
Abbreviations
- ABPI
ankle-brachial blood pressure index
- CI
confidence interval
- NHANES
National Health and Nutrition Examination Survey
- PAD
peripheral arterial disease
References
- 1.US Department of Health and Human Services. Toxicological profile for cadmium. Atlanta, GA: Public Health Services, US Department of Health and Human Services; 1999. ( http://www.atsdr.cdc.gov/toxprofiles/tp5.html). (Accessed July 13, 2010) [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. Lyon, France: IARC; 1994. pp. 119–238. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; vol 58. [Google Scholar]
- 3.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12(10):1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 4.Messner B, Knoflach M, Seubert A, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol. 2009;29(9):1392–1398. doi: 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- 5.Navas-Acien A, Selvin E, Sharrett AR, et al. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109(25):3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]
- 6.Navas-Acien A, Silbergeld EK, Sharrett R, et al. Metals in urine and peripheral arterial disease. Environ Health Perspect. 2005;113(2):164–169. doi: 10.1289/ehp.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environ Res. 2008;106(2):284–286. doi: 10.1016/j.envres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Menke A, Muntner P, Silbergeld EK, et al. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect. 2009;117(2):190–196. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishijo M, Satarug S, Honda R, et al. The gender differences in health effects of environmental cadmium exposure and potential mechanisms. Mol Cell Biochem. 2004;255(1-2):87–92. doi: 10.1023/b:mcbi.0000007264.37170.39. [DOI] [PubMed] [Google Scholar]
- 10.Vahter M, Akesson A, Lidén C, et al. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Hellström L, Elinder CG, Dahlberg B, et al. Cadmium exposure and end-stage renal disease. Am J Kidney Dis. 2001;38(5):1001–1008. doi: 10.1053/ajkd.2001.28589. [DOI] [PubMed] [Google Scholar]
- 12.Nishijo M, Nakagawa H, Morikawa Y, et al. Mortality in a cadmium polluted area in Japan. Biometals. 2004;17(5):535–538. doi: 10.1023/b:biom.0000045734.44764.ab. [DOI] [PubMed] [Google Scholar]
- 13.Staessen JA, Roels HA, Emelianov D, et al. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Lancet. 1999;353(9159):1140–1144. doi: 10.1016/s0140-6736(98)09356-8. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Zhu G, Jin T, et al. Changes in bone mineral density 10 years after marked reduction of cadmium exposure in a Chinese population. Environ Res. 2009;109(7):874–879. doi: 10.1016/j.envres.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Kasuya M. Recent epidemiological studies on itai-itai disease as a chronic cadmium poisoning in Japan. Water Sci Technol. 2000;42(7-8):147–155. [Google Scholar]
- 16.Nakagawa H, Nishijo M, Morikawa Y, et al. Urinary cadmium and mortality among inhabitants of a cadmium-polluted area in Japan. Environ Res. 2006;100(3):323–329. doi: 10.1016/j.envres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. National Health and Nutrition Examination Survey. Questionnaires, datasets, and related documentation. Atlanta, GA: Centers for Disease Control and Prevention; 2010. ( http://www.cdc.gov/nchs/nhanes.htm.) (Accessed July 13, 2010) [Google Scholar]
- 18.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50(6):918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Lunn DJ, Thomas A, Best N, et al. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 20.Spiegelhalter D, Thomas A, Best A, et al. WinBUGS version 1.4 user manual. Cambridge, United Kingdom: MRC Biostatistics Unit; 2003. ( http://www.mrc-bsu.cam.ac.uk/bugs/winbugs/manual14.pdf). (Accessed July 14, 2010) [Google Scholar]
- 21.R-development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. ( http://R-project.org). (Accessed July 14, 2010) [Google Scholar]
- 22.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9(1):1–19. [Google Scholar]
- 23.Lumley T. “Survey: analysis of complex survey samples”. R package version 3.16. Seattle, WA: University of Washington; 2009. ( http://cran.r-project.org/web/packages/survey/index.html). (Accessed July 14, 2010) [Google Scholar]
- 24.Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19(4-5):529–535. [PubMed] [Google Scholar]
- 25.Bell SG, Vallee BL. The metallothionein/thionein system: an oxidoreductive metabolic zinc link. Chembiochem. 2009;10(1):55–62. doi: 10.1002/cbic.200800511. [DOI] [PubMed] [Google Scholar]
- 26.Maret W. Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. Biometals. 2009;22(1):149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- 27.Varoni MV, Palomba D, Gianorso S, et al. Cadmium as an environmental factor of hypertension in animals: new perspectives on mechanisms. Vet Res Commun. 2003;27(suppl 1):807S–810S. doi: 10.1023/b:verc.0000014277.06785.6f. [DOI] [PubMed] [Google Scholar]
- 28.Bilgen I, Oner G, Edremitlioğlu M, et al. Involvement of cholinoceptors in cadmium-induced endothelial dysfunction. J Basic Clin Physiol Pharmacol. 2003;14(1):55–76. doi: 10.1515/jbcpp.2003.14.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Jiang G, Xu L, Song S, et al. Effects of long-term low-dose cadmium exposure on genomic DNA methylation in human embryo lung fibroblast cells. Toxicology. 2008;244(1):49–55. doi: 10.1016/j.tox.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Takiguchi M, Achanzar WE, Qu W, et al. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp Cell Res. 2003;286(2):355–365. doi: 10.1016/s0014-4827(03)00062-4. [DOI] [PubMed] [Google Scholar]
- 31.Poirier LA, Vlasova TI. The prospective role of abnormal methyl metabolism in cadmium toxicity. Environ Health Perspect. 2002;110(suppl 5):793S–795S. doi: 10.1289/ehp.02110s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satarug S, Garrett SH, Sens MA, et al. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118(2):182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, et al. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) Environ Health Perspect. 2008;116(1):51–56. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navas-Acien A, Tellez-Plaza M, Guallar E, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol. 2009;170(9):1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordberg GF, Nogawa K, Nordberg M, et al. Cadmium. In: Nordberg GF, Fowler BF, Nordberg M, et al., editors. Handbook on the Toxicology of Metals. Amsterdam, the Netherlands: Elsevier; 2007. pp. 445–486. [Google Scholar]
- 36.Yassin AS, Martonik JF. Urinary cadmium levels in the U S working population, 1988–1994. J Occup Environ Hyg. 2004;1(5):324–333. doi: 10.1080/15459620490445499. [DOI] [PubMed] [Google Scholar]
- 37.Björkman L, Vahter M, Pedersen NL. Both the environment and genes are important for concentrations of cadmium and lead in blood. Environ Health Perspect. 2000;108(8):719–722. doi: 10.1289/ehp.108-1638287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitfield JB, Dy V, McQuilty R, et al. Genetic effects on toxic and essential elements in humans: arsenic, cadmium, copper, lead, mercury, selenium and zinc in erythrocytes. Environ Health Perspect. 2010;118(6):776–782. doi: 10.1289/ehp.0901541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon CS, Kountouri AM, Mayer C, et al. Mononuclear cell metallothionein mRNA levels in human subjects with poor zinc nutrition. Br J Nutr. 2007;97(2):247–254. doi: 10.1017/S0007114507328614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.