Abstract
Background
Polymerase chain reaction (PCR) studies have demonstrated evidence of M. pneumoniae and C. pneumoniae in the lower airways of patients with asthma.
Objective
To test the hypothesis that clarithromycin would improve asthma control in individuals with mild-to-moderate persistent asthma that was not well-controlled despite treatment with low-dose inhaled corticosteroids (ICS).
Methods
Adults with an Asthma Control Questionnaire (ACQ) score ≥1.5 after a 4 week period of treatment with fluticasone propionate were entered into a PCR-stratified randomized trial to evaluate the effect of 16 weeks of either clarithromycin or placebo, added to fluticasone, on asthma control in individuals with or without lower airway PCR evidence of M. pneumoniae or C. pneumoniae.
Results
92 participants were randomized. Twelve (13%) subjects demonstrated PCR evidence of M. pneumoniae or C. pneumoniae in endobronchial biopsies; 80 were PCR negative for both organisms. In PCR positive participants, clarithromycin yielded a 0.4±0.4 unit improvement in the ACQ score, with a 0.1±0.3 unit improvement in those allocated to placebo. This between-group difference of 0.3±0.5 (p=0.6) was neither clinically nor statistically significant. In PCR negative participants, a non-significant between-group difference of 0.2±0.2 units (p=0.3) was observed. Clarithromycin did not improve lung function or airway inflammation but did improve airway hyperresponsiveness, increasing the methacholine PC20 by 1.2±0.5 doubling doses (p=0.02) in the study population.
Conclusion
Adding clarithromycin to fluticasone in adults with mild-to-moderate persistent asthma that was suboptimally-controlled by low-dose ICS alone did not further improve asthma control. Although there was an improvement in airway hyperresponsiveness with clarithromycin, this benefit was not accompanied by improvements in other secondary outcomes.
Keywords: asthma, infection, antibiotic
Introduction
Colonization of the upper and lower airways with typical and atypical bacterial pathogens has been postulated to be an important factor in the development and persistence of asthma (1, 2). Serologic studies have suggested that the atypical bacterium Chlamydophila pneumoniae (formerly Chlamydia pneumoniae) may be associated with an increased risk of asthma (3). Additionally, studies utilizing polymerase chain reaction (PCR) to identify atypical bacteria in patients with stable persistent asthma have indicated that approximately 56% manifest evidence of Mycoplasma pneumoniae or Chlamydophila pneumoniae in the upper or lower airway, a prevalence significantly higher than in the airway of healthy individuals (2, 4). While PCR evidence of lower airway M. pneumoniae or C. pneumoniae has been associated with increased airway inflammation, including increased numbers of mast cells in the airway and increased airway epithelial mucin production (2, 5), studies have failed to identify a specific clinical asthma phenotype in those asthmatics who demonstrate PCR positivity for these organisms.
The suggestion that chronic bacterial colonization or infection with atypical bacteria might play a significant role in asthma has raised the possibility that macrolide antibiotics could be of benefit in patients with persistent asthma and evidence of infection or colonization with these organisms. However, previous studies have not demonstrated uniform benefit to patients with asthma in this regard, with one study reporting that small, nonsustained improvements in peak flow and asthma control could be seen with 6 weeks of roxithromycin treatment in individuals with asthma and IgG seropositivity to C. pneumoniae (6), and another suggesting that only those with lower airway PCR evidence of M. pneumoniae or C. pneumoniae experienced improvements in lung function in response to 6 weeks of treatment with clarithromycin (7). However, clarithromycin has been shown to modulate sputum IL-8 concentrations and airway neutrophil activation in patients with refractory, noneosinophilic asthma (8), suggesting that certain phenotypic characteristics may predict clinical response to macrolide antibiotics in asthma. Notwithstanding, a systematic review of the use of macrolides in chronic asthma concluded that there is not currently enough evidence either to support or to reject the use of macrolides in chronic asthma, and that additional studies were needed to address this clinical question in patients with asthma and in subsets thereof, including patients with evidence of atypical bacterial colonization or infection (9). International asthma care guidelines also highlight this question as an area of ongoing uncertainty (10, 11).
To prospectively evaluate the interrelationship between lower airway PCR evidence of M. pneumoniae or C. pneumoniae, response to treatment with clarithromycin, and asthma phenotype, we conducted a randomized, controlled trial of clarithromycin versus placebo added to inhaled fluticasone propionate in patients with suboptimally-controlled asthma. We hypothesized that the addition of a macrolide antibiotic to an inhaled corticosteroid (ICS) would improve asthma control over that achieved with ICS alone, and we utilized a stratification-by-PCR design to test this hypothesis concurrently and independently in two separate groups of patients, those with and those without evidence of M. pneumoniae or C. pneumoniae in the lower airways.
Methods
The “Macrolides in Asthma” study (Clinicaltrials.gov identifier NCT00318708) was conducted by the National Heart, Lung and Blood Institute (NHLBI)-funded Asthma Clinical Research Network (ACRN) between July, 2006 and March, 2009 at ten sites throughout the United States. The study was approved by each site’s Institutional Review Board and all participants provided written informed consent. The study protocol was developed by the ACRN steering committee, reviewed and approved by an NHLBI-convened protocol review committee and monitored by an independent data and safety monitoring board (DSMB).
Participants were eligible to enroll if they had a clinical diagnosis of asthma and either bronchodilator responsiveness, defined as an increase of 12% or greater in the forced expiratory volume in one second (FEV1) 15 minutes after the administration of two puffs of albuterol, or airway hyperresponsiveness, measured by the PC20 FEV1 to methacholine (the concentration of methacholine inducing a 20% fall in FEV1) of ≤ 16 mg/mL. Participants also were required to demonstrate suboptimally-controlled asthma at the time of enrollment, as defined by threshold scores on the Juniper Asthma Control Questionnaire (ACQ) of ≥ 1.5 in those not receiving inhaled corticosteroid (ICS)-containing treatments. Participants receiving ICS-containing treatments could be enrolled with an ACQ score ≥ 1.25 at enrollment or if in the opinion of the investigator the ACQ score was likely to be ≥ 1.25 at the end of the run-in period (12, 13). These values were derived from and validated in data from the “Gaining Optimal Asthma Control” trial of Bateman and colleagues (12, 14). All data were obtained using techniques and procedural standards employed in previous ACRN studies (15, 16).
After qualifying, participants were enrolled in a four-week run-in period in which they were treated with CFC-fluticasone propionate MDI (GlaxoSmithKline, Research Triangle Park, NC), 88mcg inhaled regularly twice daily, and inhaled CFC-albuterol sulfate, 180mcg as needed every four to six hours for relief of acute symptoms. If, at the end of the four week run-in period, participants demonstrated an ACQ score of ≥ 1.25, they were eligible to proceed to fiberoptic bronchoscopy for the purposes of endobronchial biopsy for characterization of lower airway PCR status for M. pneumoniae or C. pneumoniae. Fiberoptic bronchoscopy was performed according to standard investigative procedures (17, 18), and a standardized approach to biopsy was utilized, with between four and eight biopsies obtained from the lobar, segmental or subsegmental airways in the right lower and middle lobes. DNA was extracted from these biopsies according to standard methodology, and a nested quantitative PCR protocol was utilized, employing primers and probes specific for genomic DNA of the 16s ribosomal subunits of M. pneumoniae and C. pneumoniae, the M. pneumoniae P1 adhesin and the C. pneumoniae RNA polymerase 1 (4, 19-21). Based on the results of PCR testing, participants were stratified into one of two groups, either PCR positive (for any of the above genes) or PCR negative for both M. pneumoniae and C. pneumoniae. Within these two strata, participants were randomly allocated in a 1:1 distribution to the addition of either clarithromycin (DAVA Pharmaceuticals Inc., Fort Lee, NJ), 500mg capsule by mouth twice daily, or matched placebo (FMC Corporation, Philadelphia, PA) capsule by mouth twice daily, to continued regularly-scheduled fluticasone propionate and as-needed albuterol sulfate for 16 weeks (112 days). Both participants and study personnel were blinded to treatment allocation.
The primary outcome variable was the change in the 7-item ACQ score between the time of randomization and 16 weeks of study treatment, evaluated independently in each PCR stratum. The previously-established minimal clinically-important difference of a change in 0.5 units in ACQ score was used to identify treatment response (12). Secondary outcomes included change in lung function (forced expiratory volume in one second, morning and evening peak flow), change in rescue albuterol use, change in exacerbation number and frequency, change in PC20, and change in exhaled nitric oxide concentration. Main study conclusions were based the primary outcome variable, and corrections for multiple significance testing with regard to secondary outcomes were not prespecified (22).
It was estimated that 72 participants per PCR stratum would be needed to achieve 90% power to detect a difference of 0.5 in the change in the ACQ score between clarithromycin and placebo treatment arms in each of the two PCR strata, and an approximately equal distribution between PCR-positive and PCR-negative individuals was anticipated (2). Stratified repeated measures analysis of covariance (RM-ANCOVA) was utilized to analyze change in primary and secondary outcomes within each PCR stratum, with models adjusted for study site, sex, race, age, FEV1 % predicted and asthma duration. As a prespecified secondary analysis, RM-ANCOVA was also employed to analyze the difference between clarithromycin and placebo in the combined study population, irrespective of PCR status. Categorized threshold changes in ACQ were compared between treatment groups using Mantel-Haenszel chi-square tests, with additional analyses using Kaplan-Meier survival estimates to evaluate difference in time to achieving threshold changes in ACQ score. Logistic regression was used to identify predictors of PCR status, and predictors of response to clarithromycin were evaluated using linear regression. All analyses invoked the intent-to-treat paradigm, with truncation at the time of exacerbation or treatment failure in relevant analyses. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses.
Results
Baseline Characteristics of Participants
Two hundred and fifty-three participants met criteria for enrollment into the study, with 92 participants proceeding to randomization due to continued suboptimal asthma control at the end of the 4-week run-in period (Figure 1). Twelve (13%) of the 92 randomized participants demonstrated PCR evidence of M. pneumoniae on endobronchial biopsy, with one also demonstrating concurrent PCR evidence for C. pneumoniae. Eighty participants did not demonstrate PCR evidence of M. pneumoniae or C. pneumoniae. This proportion of PCR positive to negative participants was less than anticipated during trial planning, resulted in the PCR negative stratum being fully enrolled first, and suggested that eight bronchoscopies would be required to identify each additional PCR positive subject. On the basis of this information obtained during trial execution it was concluded that full enrollment of the PCR positive arm of the study was not feasible, and further enrollment and bronchoscopic characterization was discontinued. Across the two PCR strata, participants were well-matched with regard to physiologic and inflammatory biomarkers (Table 1).
Figure 1.
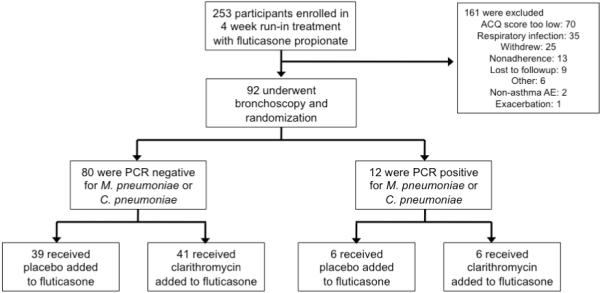
Enrollment, randomization and follow-up of participants
Table 1.
Baseline characteristics of the participants
| Characteristic | PCR− (n=80) |
PCR+ (n=12) |
p |
|---|---|---|---|
| Age | 39.3 ± 11.5 | 40.5 ± 12.3 | 0.8 |
| Female Sex, n (%) | 42 (53%) | 10 (83%) | 0.05 |
| African-American, n (%) | 19 (24%) | 6 (50%) | 0.08† |
| White, n (%) | 45 (56%) | 5(42%) | 0.3 |
| Duration of asthma, yrs | 24.0 ± 12.9 | 27.3 ± 12.7 | 0.4 |
| ACQ at randomization | 1.7 ± 0.7 | 1.7 ± 0.7 | 0.8 |
| Pre-albuterol FEV1 (L) | 2.67 ± 0.7 | 2.32 ± 0.7 | 0.1 |
| Pre-albuterol FEV1 % predicted | 76.2 ± 14.6 | 74.4 ± 11.6 | 0.7 |
| FEV1 reversibility (%) to 180mcg albuterol |
11.2 ± 10.5 | 14.8 ± 14.8 | 0.3 |
| AM Peak Flow 2-week average prior to visit 5 (liters/min) |
414.2 ± 114.9 | 384.5 ± 120.2 | 0.4 |
| PM Peak Flow 2-week average prior to visit 5 (liters/min) |
419.1 ± 117.2 | 389.2 ± 131.9 | 0.4 |
| PC20 (mg/ml)^ | 1.3 (1.5) | 1.4 (1.0) | 0.9 |
| IgE (IU/mL)^ | 142.0 (1.4) | 118.5 (1.4) | 0.7 |
| FeNO (ppb)+ | 14.7 (9.4, 24.0) | 15.4 (12.7, 24.6) | 0.5 |
Mean ± SD, except ^ Geometric mean (CV) reported, +Median (Q1,Q3) reported.
Fisher’s exact p-value
Change in Asthma Control in Response to Clarithromycin Treatment
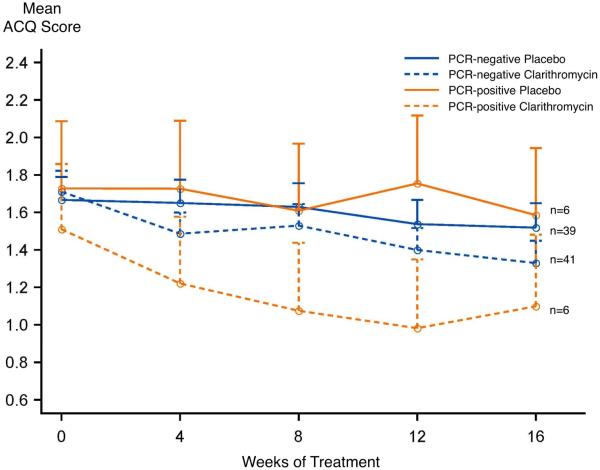
In those participants who were PCR negative for M. pneumoniae or C. pneumoniae (n=80), 16 weeks of clarithromycin added to fluticasone resulted in a 0.4±0.1 unit reduction in the ACQ (Figure 2), compared with a 0.2±0.1 unit reduction in those allocated to placebo plus fluticasone, indicative of a nonsignificant between-group difference of 0.2±0.2 units (p=0.3). In those participants who were PCR positive for mycoplasma or chlamydophila (n=12), 16 weeks of clarithromycin added to fluticasone resulted in a 0.4±0.4 unit reduction in the ACQ versus a 0.1±0.3 unit reduction in those allocated to placebo plus fluticasone, a between-group difference of 0.3±0.5 units (p=0.6) that was also not significant. When response to clarithromycin versus placebo was evaluated in all participants, irrespective of PCR status, 16 weeks of clarithromycin added to fluticasone resulted in a 0.4±0.1 unit reduction in the ACQ, with a 0.2±0.1 unit reduction in those allocated to placebo plus fluticasone, a between-group difference of 0.2±0.2 units (p=0.2).
Figure 2.
Change in ACQ score over 16 weeks, within PCR strata by treatment allocation. There was a between-group difference of 0.2±0.2 units (p=0.3) in those who were PCR-negative and 0.3±0.5 in those who were PCR-positive (p=0.6).
A prespecified secondary time-to-event analysis was conducted evaluating the effect of clarithromycin versus placebo, within PCR strata, evaluating time at which the minimal clinically-important difference of a 0.5 unit reduction in the ACQ score was achieved (Figure 3). In those participants who were PCR negative for M. pneumoniae or C. pneumoniae, there was no difference between treatment groups (log-rank chi-square = 0.79, p = 0.4). However, in participants who were PCR positive for M. pneumoniae or C. pneumoniae, there was weak evidence of a more rapid achievement of a reduction in ACQ score ≥ 0.5, with a log-rank chi-square = 3.55 (p = 0.06). When the analysis was conducted independent of PCR status, there was not evidence of a statistically-significant difference between the groups (log-rank chi-square = 2.39, p = 0.1).
Figure 3.
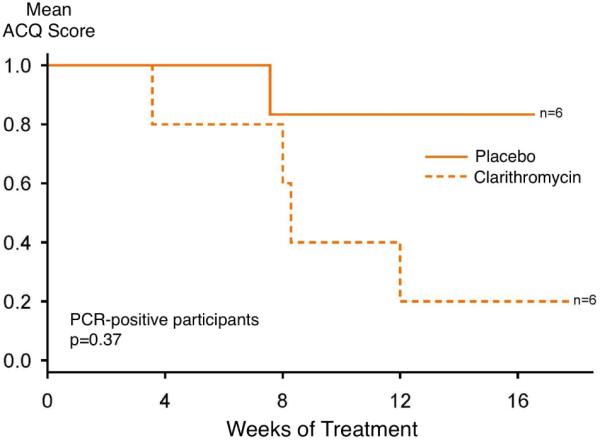
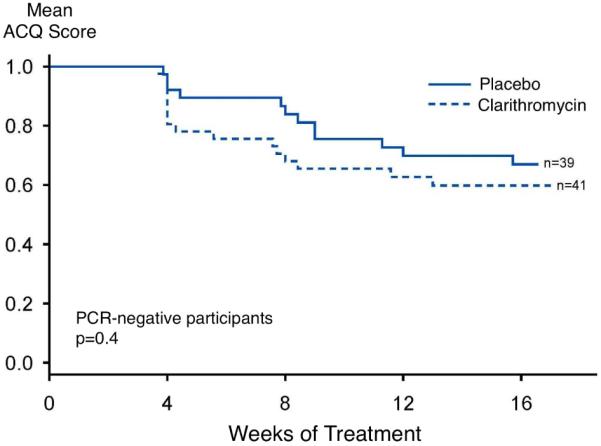
Time to achievement of a reduction in ACQ score of ≥ 0.5, within PCR-positive (left) and PCR-negative (right) groups.
Additional prespecified secondary analyses were performed to determine the proportion of participants who achieved reductions in the ACQ score of equal to or greater than the predefined minimal clinically-important difference of 0.5 units (12). When adjusted for PCR status, Mantel-Haenszel p values for the effect of clarithromycin versus placebo were p=0.1 for a change in ACQ of ≥ 0.5 (which occurred in 12 subjects treated with clarithromycin and 6 with placebo), p=0.06 for a change in ACQ of ≥ 0.75 (which occurred in 9 subjects treated with clarithromycin and 3 with placebo), and p=0.08 change in ACQ of ≥ 1.0 (which occurred in 7 subjects treated with clarithromycin and 2 with placebo), respectively.
Physiologic, Inflammatory and Clinical Parameters and Response to Clarithromycin Treatment
There was no significant effect of clarithromycin on markers of lung function including morning and evening peak flow, pre-albuterol FEV1 (L or % predicted) and maximum bronchodilator response (Table 2), all secondary outcomes. However, in those participants who were PCR negative for M. pneumoniae or C. pneumoniae, 16 weeks of clarithromycin added to fluticasone resulted in a 1.6±0.3 doubling dose increase in the PC20, with a 0.4±0.3 doubling dose increase in those allocated to placebo plus fluticasone, a between-group difference of 1.2±0.5 doubling doses (p=0.02) favoring clarithromycin. A differential effect of clarithromycin versus placebo on PC20 methacholine was not observed in those participants who were PCR positive for M. pneumoniae or C. pneumoniae, although a significant effect of clarithromycin was observed when data were analyzed independent of PCR status, with a between-group difference of 1.2±0.5 doubling doses (p=0.02).
Table 2.
Effect of clarithromycin on outcomes in PCR-stratified and aggregate analyses
| Variable | Clarithromycin Effect PCR − Participants Mean ± SE |
p | Clarithromycin Effect PCR + Participants Mean ± SE |
p | Clarithromycin Effect Independent of PCR Mean ± SE |
p |
|---|---|---|---|---|---|---|
| Physiologic | ||||||
| Pre-albuterol FEV1 (L) |
−0.02 ± 0.1 | 0.8 | +0.04 ± 0.2 | 0.9 | 0.0 ± 0.1 | 0.8 |
| Pre-albuterol FEV1 (% predicted) |
+0.2 ± 1.8 | 0.9 | +1.0 ± 3.9 | 0.8 | +0.1 ± 1.6 | 1.0 |
| Morning Peak Flow (L/min) |
+3.4 ± 6.4 | 0.6 | −9.3 ± 10.8 | 0.4 | +2.4 ± 8.6 | 0.8 |
| Evening Peak Flow (L/min) |
−0.3 ± 6.6 | 0.9 | −1.8 ± 13.0 | 0.9 | +0.8 ± 9.0 | 0.9 |
| Methacholine PC20 doubling dose |
+1.2 ± 0.5 | 0.02 | +0.9 ± 1.8 | 0.6 | +1.2 ± 0.5 | 0.01 |
| Inflammatory | ||||||
| FeNO (ppb) | −3.4 ± 4.5 | 0.5 | −11.4 ± 11.9 | 0.3 | −4.6 ± 4.2 | 0.3 |
| Clinical | ||||||
| Asthma Quality of Life Questionnaire Score |
+0.2 ± 0.2 | 0.4 | −0.1 ± 0.6 | 0.8 | +0.2 ± 0.2 | 0.5 |
| Rescue albuterol use (puffs/day) |
−0.6 ± 0.3 | 0.09 | −0.4 ± 0.5 | 0.4 | −0.6 ± 0.3 | 0.06 |
Treatment with clarithromycin did not alter bronchodilator responsiveness or improve the concentration of exhaled nitric oxide, nor did it improve quality of life as measured by the Asthma Quality of Life Questionnaire. However, a weak association between a reduction in the need for rescue bronchodilator and clarithromycin treatment was seen in the study population as a whole, with an overall reduction in need for rescue albuterol of 0.6 ± 0.3 puffs per day (−0.7 puffs/day in clarithromycin-treated participants versus −0.1 puffs/day with placebo), with a p=0.06. With regard to upper airway disease, there was no significant effect of clarithromycin on the development of self-reported sinusitis or rhinitis during the study (p=0.1).
Finally, to determine if pre-randomization sputum eosinophils or neutrophils were predictive of response to clarithromycin, we modeled the effect of clarithromycin on our primary outcome (ACQ) independent of PCR status with regard to 1) sputum eosinophils stratified as ≤3% or >3%, 2) percent sputum eosinophils treated as a continuous variable and 3) percent sputum neutrophils treated as a continuous variable. There were no statistically-significant differences in the effect of clarithromycin on asthma control with regard to the percent of either of these cell types in induced sputum, with p=0.8 for eosinophils dichotomized at 3%, p=1.0 for eosinophils (continuous) and p=0.5 for neutrophils (continuous).
Serology, PCR Status and Response to Clarithromycin
Of those randomized subjects with available serologic data (n=81), 36 (44%) were seropositive for C. pneumoniae IgA, 54 (67%) for C. pneumoniae IgG and 3 (4%) for C. pneumoniae IgM. Serology for M. pneumoniae demonstrated that 55 (68%) were seropositive for M. pneumoniae IgG and 1 for M. pneumoniae IgM. Serologic status (dichotomized) was not found to be predictive of endobronchial biopsy PCR positivity; a positive C. pneumoniae IgA status was only 62.5% sensitive and 57.5% specific for positive endobronchial biopsy PCR for C. pneumoniae, and a positive M. pneumoniae IgG status was 88% sensitive and 34% specific for endobronchial biopsy M. pneumoniae PCR positivity. Serologic status (positive or negative) was not predictive of improvement in ACQ score with clarithromycin treatment, with the C. pneumoniae IgA F=2.22 (p=0.2) and the M. pneumoniae IgG F=0.80 (p=0.4)). In analyses in which serologic results were treated as continuous variables, no significant association was observed between increasing concentrations of either C. pneumoniae IgA or M. pneumoniae IgG and the effect of clarithromycin on ACQ (data not shown).
Adverse Events
Participants allocated to clarithromycin were not more likely than those allocated to placebo to experience drug-related adverse events, including increased likelihood of gastrointestinal symptoms or respiratory tract infections.
Discussion
This PCR-stratified, double-blind, randomized, controlled trial of clarithromycin or placebo added to fluticasone in patients with suboptimally-controlled persistent asthma demonstrated that there is not a beneficial effect on asthma control of adding clarithromycin to inhaled fluticasone in patients similar to those entered into this trial. The PCR-stratified approach employed herein allowed us to test the effect of clarithromycin on asthma control independently in patients who did and did not have molecular evidence of atypical bacteria in the lower airways. Given full enrollment of the PCR negative stratum, we have robustly demonstrated a lack of effect in those who do not demonstrate PCR evidence of Mycoplasma pneumoniae or Chlamydophila pneumoniae on endobronchial biopsy, as well as in the study population as a whole when analyzed independent of PCR status. However, the underenrollment of the PCR positive stratum resulted in inadequate power to robustly test the effect of clarithromycin in the PCR positive subpopulation, leaving the question of efficacy in this group of asthmatics as of yet unanswered. Our findings address an area of uncertainty in asthma pharmacotherapy (9-11) and provide evidence that clarithromycin should not be considered as an addition to inhaled corticosteroids to improve disease control in patients with suboptimally-controlled mild-to-moderate persistent asthma who are PCR negative for C. pneumoniae and M. pneumoniae, a group that constituted 87% of the participants enrolled in this study and therefore possibly the majority of the adult asthma population as well. Additionally, our results suggest that C. pneumoniae and M. pneumoniae serologic evaluation is of minimal clinical utility in this setting, as serology predicted neither PCR status nor response to clarithromycin.
While there was no effect of adding clarithromycin to fluticasone on physiologic measures of airflow or bronchodilator response, there was a clinically- and statistically-significant effect of clarithromycin on airway hyperresponsiveness in PCR negative participants, as indicated by a doubling of the concentration of methacholine required to produce a 20% decline in FEV1 in those allocated to clarithromycin. This occurred despite the fact that all participants were being treated concurrently with fluticasone and suggests that non-antibiotic effects of clarithromycin on airway smooth muscle functional or inflammatory phenotype can be seen even in patients treated with inhaled corticosteroids. The improvement in airway hyperresponsiveness with clarithromycin in asthma patients already receiving inhaled corticosteroids is of interest, augments prior reports from small uncontrolled clinical studies (23, 24), and supports prior observations that macrolides might modulate this effect through non-antimicrobial pathways including alteration of cholinergic signaling pathways or attenuation of endothelin-1 expression by airway epithelial cells (25-27).
Additionally, while clarithromycin did not have a beneficial effect on exhaled nitric oxide or asthma-specific quality of life, it was weakly associated with a reduction in the need for rescue albuterol use, both in PCR negative subjects and in the population as a whole. Of additional interest (although not definitive) were our findings with regard to the time to achievement of a clinically-significant improvement in asthma control, where those participants who were PCR positive achieved a 0.5-unit improvement in the ACQ more rapidly than did those who were PCR negative. Similarly clarithromycin treatment was associated with a greater likelihood of achieving improvements in the ACQ that exceeded the minimal clinically-important difference. While these results are subject to the limitation of the small sample size in the PCR positive stratum and are secondary outcomes, they raise the possibility that there may be a relevant interaction between PCR status and response to clarithromycin.
The results of this study are likely generalizable to most adults with mild to moderate persistent asthma, although extrapolation to severe disease may not be appropriate given the lack of enrollment of participants with severe asthma into this trial. The PCR negative stratum (which comprised 87% of study participants) of the study was adequately powered to detect an effect of clarithromycin on asthma control and did not. A similar lack of effect was observed in analyses in which all participants (independent of PCR status) were included, indicating a lack of benefit of clarithromycin overall. Additionally, we enrolled adults with asthma that remained suboptimally controlled despite low-dose inhaled corticosteroid monotherapy. This subset of individuals likely comprises a substantial proportion of the asthmatic population (14), and for them guidelines recommend either increasing the dose of inhaled corticosteroids or adding a second asthma controller medication such as a long-acting beta2 agonist (10, 11). In this context, our findings indicate that clarithromycin should not be considered as the next therapeutic step in mild and moderate persistent asthmatics. While weak trends were observed toward a favorable effect of clarithromycin on asthma control in PCR positive patients, these findings were not statistically significant and can not be considered conclusive. Thus, while there may be a beneficial effect of clarithromycin in those with evidence of M. pneumoniae or C. pneumonia or in other patient populations not included in our study (e.g. those with more severe or neutrophil-predominant asthma (8)), further research will be required to definitively assess the role of macrolide antibiotics in these subpopulations.
Certain features of the study design should be considered when interpreting these results. Clarithromycin was chosen because, when compared with other macrolides, it is preferentially concentrated in the lung epithelial lining fluid (28, 29), it may be less likely to contribute to antimicrobial resistance than other members of the macrolide family (30, 31), its side effect profile with extended treatment period has been described (32, 33), it is unlikely to significantly alter fluticasone pharmacokinetics (34), and it has been previously used in asthma (7). However, it is possible that the antimicrobial or anti-inflammatory effects might have been greater had another member of the macrolide class been chosen. Of note, the absence of a significant improvement in asthma control in both the entire study population and the PCR negative subset suggests that an effect of clarithromycin on glucocorticoid metabolism, similar to that previously described with the macrolide antibiotic troleandomycin (35, 36), was not present.
Another novel feature of this study is the choice of asthma control, a composite variable which differs from quantitative physiologic or inflammatory parameters, as the primary outcome variable. This outcome measure was chosen for several reasons: it is patient-centered, taking clinical variables such as symptoms and beta-agonist use into account, it is a reliable technique for assessing asthma disease activity and control, and it incorporates a quantitative measure of airflow in the FEV1. It is possible that the use of a composite variable could have obscured the effect of clarithromycin in one or more clinical or physiologic domains, but we did not show a clear benefit of clarithromycin on lung function, and only a weak trend toward a reduction in rescue bronchodilator use was observed.
Complete enrollment of the PCR positive arm of this trial was hampered by a lower-than-anticipated prevalence of PCR positivity for M. pneumoniae and C. pneumoniae. A previous study indicated that PCR evidence of these organisms could be found in 56% of adults with asthma, with 30% demonstrating these organisms in the lower airway (2), whereas in this trial, overall lower airway positivity was 13%. While the reason for this reduced prevalence in our study is not clear, the prior report of Martin and colleagues suggested the possibility of a reduction in the likelihood of PCR positivity in those participants who were using ICS (2). Since the run-in and treatment periods of this study exposed all participants to a fixed continuous dose of fluticasone, it is possible that this treatment reduced the prevalence of PCR positivity for M. pneumoniae and C. pneumoniae in our study population. Given that the technical approaches to obtaining and processing endobronchial biopsies used in this study were similar to those previously employed by investigators in this area (2, 7), we believe it is unlikely that technical factors alone explain the difference. Whatever the cause, given the fact that the PCR positive group did not have adequate enrollment to robustly test the effect of supplemental clarithromycin in this group, it remains unknown if clarithromycin is of clinical benefit in patients who are PCR positive for either M. pneumoniae and C. pneumoniae.
In conclusion, this study demonstrated that there is not a beneficial effect on asthma control or lung function of adding clarithromycin to fluticasone in adults with persistent, suboptimally-controlled asthma. There was a significant reduction in airway hyperresponsiveness seen with clarithromycin treatment in this study, occurring in the absence of concordant improvements in multiple other clinical and physiologic parameters. While our findings do not support a role for clarithromycin in the treatment of suboptimally-controlled asthma, particularly in those without evidence of mycoplasma or chlamydophila in the lower airway, further studies are warranted to characterize the role of microbial communities in the asthmatic airway (37) and to determine if evidence of bacterial colonization or infection in the lower airway is predictive of asthma phenotype or clinical improvement with antibiotic treatment.
Clinical Implications
The “Macrolides in Asthma” trial evaluated if clarithromycin improved control of mild-to-moderate persistent asthma above that achieved with low-dose fluticasone alone. Clarithromycin did not improve asthma control, lung function or quality of life, but did improve airway hyperresponsiveness.
Capsule Summary
The addition of clarithromycin in adults with mild-to moderate persistent asthma that is suboptimally-controlled by low-dose ICS alone does not further improve asthma control.
Acknowledgement
The authors wish to acknowledge the study participants for their contributions, the ACRN coordinator staff for execution of the protocol, and Robert A. Smith, PhD of the NHLBI for his support of this trial.
Grant Funding: NIH/NHLBI U10 HL074227, 074231, 074204, 074212, 074073, 074206, 074208, 074225, 074218, NIH/NHLBI K23 HL04385, NIH UL1-RR025011
Abbreviations
- ACRN
Asthma Clinical Research Network
- ACQ
asthma control questionnaire
- CFC
chlorofluorocarbon
- DSMB
Data and Safety Monitoring Board
- FeNO
exhaled nitric oxide
- FEV1
forced expiratory volume in ones second
- ICS
inhaled corticosteroids
- IgE
immunoglobulin E
- IL
interleukin
- NHLBI
National Heart, Lung and Blood Institute
- PCR
polymerase chain reaction
- PC20 FEV1
the concentration of methacholine inducing a 20% fall in FEV1
- RM-ANCOVA
repeated measures analysis of covariance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007 Oct 11;357(15):1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 2.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001 Apr;107(4):595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 3.Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult-onset asthma. JAMA. 1991;266(2):225–30. [PubMed] [Google Scholar]
- 4.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 5.Kraft M, Adler KB, Ingram JL, Crews AL, Atkinson TP, Cairns CB, et al. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. Eur Respir J. 2008 Jan;31(1):43–6. doi: 10.1183/09031936.00103307. [DOI] [PubMed] [Google Scholar]
- 6.Black PN, Blasi F, Jenkins CR, Scicchitano R, Mills GD, Rubinfeld AR, et al. Trial of roxithromycin in subjects with asthma and serological evidence of infection with Chlamydia pneumoniae. Am J Respir Crit Care Med. 2001 Aug 15;164(4):536–41. doi: 10.1164/ajrccm.164.4.2011040. [DOI] [PubMed] [Google Scholar]
- 7.Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002 Jun;121(6):1782–8. doi: 10.1378/chest.121.6.1782. [DOI] [PubMed] [Google Scholar]
- 8.Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008 Jan 15;177(2):148–55. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 9.Richeldi L, Ferrara G, Fabbri LM, Lasserson TJ, Gibson PG. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2005;(3):CD002997. doi: 10.1002/14651858.CD002997.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. U.S. Department of Health and Human Services; National Institutes of Health; National Heart Lung and Blood Institute; National Asthma Education and Prevention Program; Bethesda, MD: 2007. Report No.: NIH Publication Number 07-4051. [Google Scholar]
- 11.Global Strategy for Asthma Management and Prevention 2008 Update. 2008 [cited December 7, 2009]; Available from: http://www.ginasthma.org/Guidelineitem.asp??l1=2&l2=1&intId=1561.
- 12.Juniper EF. Asthma control questionnaire. Background, analysis and administration. QOL Technologies LTD; Bonham, West Sussex, UK: 2004. [Google Scholar]
- 13.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999 Oct;14(4):902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 14.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004 Oct 15;170(8):836–44. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 15.Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005 Apr 14;352(15):1519–28. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler ME, Kunselman SJ, Chinchilli VM, Bleecker E, Boushey HA, Calhoun WJ, et al. Effect of beta2-adrenergic receptor polymorphism on response to longacting beta2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial. Lancet. 2009 Nov 21;374(9703):1754–64. doi: 10.1016/S0140-6736(09)61492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busse WW, Wanner A, Adams K, Reynolds HY, Castro M, Chowdhury B, et al. Investigative bronchoprovocation and bronchoscopy in airway diseases. Am J Respir Crit Care Med. 2005 Oct 1;172(7):807–16. doi: 10.1164/rccm.200407-966WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarjour NN, Peters SP, Djukanovic R, Calhoun WJ. Investigative use of bronchoscopy in asthma. Am J Respir Crit Care Med. 1998 Mar;157(3 Pt 1):692–7. doi: 10.1164/ajrccm.157.3.9705020. [DOI] [PubMed] [Google Scholar]
- 19.Gaydos CA, Quinn TC, Eiden JJ. Identification of Chlamydia pneumoniae by DNA amplification of the 16S rRNA gene. J Clin Microbiol. 1992;30:796–800. doi: 10.1128/jcm.30.4.796-800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaydos CA, Roablin PM, Hammerschlag MR, Hyman C, Eiden JJ, Schachter J, et al. Diagnostic utility of PCR-enzyme immunoassay, culture and serology for detection of Chlamydia pneumoniae in symptomatic and asymptomatic patients. J Clin Microbiol. 1994;32:903–5. doi: 10.1128/jcm.32.4.903-905.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson J, Marmion BP, Kok T, Antic R, Harris RJ. Confirmation of fatal Mycoplasma pneumoniae infection by polymerase chain reaction detection of adhesin gene in fixed lung tissue (letter) J Inf Dis. 1994;170:1052–3. doi: 10.1093/infdis/170.4.1052. [DOI] [PubMed] [Google Scholar]
- 22.Pocock SJ. Clinical trials with multiple outcomes: a statistical perspective on their design, analysis, and interpretation. Control Clin Trials. 1997 Dec;18(6):530–45. doi: 10.1016/s0197-2456(97)00008-1. discussion 46-9. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu T, Kato M, Mochizuki H, Tokuyama K, Morikawa A, Kuroume T. Roxithromycin reduces the degree of bronchial hyperresponsiveness in children with asthma. Chest. 1994 Aug;106(2):458–61. doi: 10.1378/chest.106.2.458. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T, Kato M, Mochizuki H, Takei K, Maeda S, Tokuyama K, et al. Roxithromycin attenuates acid-induced cough and water-induced bronchoconstriction in children with asthma. J Asthma. 1997;34(3):211–7. doi: 10.3109/02770909709068191. [DOI] [PubMed] [Google Scholar]
- 25.Tamaoki J, Tagaya E, Sakai A, Konno K. Effects of macrolide antibiotics on neurally mediated contraction of human isolated bronchus. J Allergy Clin Immunol. 1995 Apr;95(4):853–9. doi: 10.1016/s0091-6749(95)70129-x. [DOI] [PubMed] [Google Scholar]
- 26.Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, et al. Erythromycin and clarithromycin attenuate cytokine-induced endothelin-1 expression in human bronchial epithelial cells. Eur Respir J. 1998 Jul;12(1):57–63. doi: 10.1183/09031936.98.12010057. [DOI] [PubMed] [Google Scholar]
- 27.Takizawa H, Desaki M, Ohtoshi T, Kawasaki S, Kohyama T, Sato M, et al. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med. 1997 Jul;156(1):266–71. doi: 10.1164/ajrccm.156.1.9612065. [DOI] [PubMed] [Google Scholar]
- 28.Patel KB, Xuan D, Tessier PR, Russomanno JH, Quintiliani R, Nightingale CH. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob Agents Chemother. 1996 Oct;40(10):2375–9. doi: 10.1128/aac.40.10.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhanel GG. Antibacterial drivers of resistance. Treat Respir Med. 2005;4(Suppl 1):13–8. doi: 10.2165/00151829-200504001-00005. [DOI] [PubMed] [Google Scholar]
- 30.Bergman M, Huikko S, Huovinen P, Paakkari P, Seppala H. Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2006 Nov;50(11):3646–50. doi: 10.1128/AAC.00234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyde TB, Gay K, Stephens DS, Vugia DJ, Pass M, Johnson S, et al. Macrolide resistance among invasive Streptococcus pneumoniae isolates. JAMA. 2001 Oct 17;286(15):1857–62. doi: 10.1001/jama.286.15.1857. [DOI] [PubMed] [Google Scholar]
- 32.Dautzenberg B, Piperno D, Diot P, Truffot-Pernot C, Chauvin JP. Clarithromycin in the treatment of Mycobacterium avium lung infections in patients without AIDS. Clarithromycin Study Group of France. Chest. 1995 Apr;107(4):1035–40. doi: 10.1378/chest.107.4.1035. [DOI] [PubMed] [Google Scholar]
- 33.Kadota J, Mukae H, Ishii H, Nagata T, Kaida H, Tomono K, et al. Long-term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitis. Respir Med. 2003 Jul;97(7):844–50. doi: 10.1016/s0954-6111(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 34.GlaxoSmithKline . Data on File, Fluticasone Propionate, NN1996/00005/00, Study FLTA1001 Report Summary. 1997. pp. 1–9. [Google Scholar]
- 35.Fost DA, Leung DY, Martin RJ, Brown EE, Szefler SJ, Spahn JD. Inhibition of methylprednisolone elimination in the presence of clarithromycin therapy. J Allergy Clin Immunol. 1999 Jun;103(6):1031–5. doi: 10.1016/s0091-6749(99)70175-2. [DOI] [PubMed] [Google Scholar]
- 36.Szefler SJ, Rose JQ, Ellis EF, Spector SL, Green AW, Jusko WJ. The effect of troleandomycin on methylprednisolone elimination. J Allergy Clin Immunol. 1980 Dec;66(6):447–51. doi: 10.1016/0091-6749(80)90004-4. [DOI] [PubMed] [Google Scholar]
- 37.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]