SUMMARY
Establishing synaptic connections often involves the activity-dependent withdrawal of off-target contacts. We describe an in vivo role for temporally patterned electrical activity, voltage-gated calcium channels, and CaMKII in modulating the response of Drosophila motoneurons to the chemorepellent Sema-2a during synaptic refinement. Mutations affecting the Sema-2a ligand, the plexin B receptor (plexB), the voltage-gated Ca(v)2.1 calcium channel (cac), or the voltage-gated Na(v)1 sodium channel (mlenap-ts;tipE) each result in ectopic neuromuscular contacts. Sema-2a interacts genetically with both of the channel mutations. The cac phenotype is enhanced by the Sema-2a mutation, and is suppressed by either plexB overexpression or patterned, low frequency (0.01Hz) bouts of electrical activity in the embryo. The calcium-dependent suppression of ectopic contacts also depends on the downstream activation of CaMKII. These results indicate a role for patterned electrical activity and presynaptic calcium signaling, acting through CaMKII, in modulating a retrograde signal during the refinement of synaptic connections.
INTRODUCTION
Neural connectivity depends on chemoattractive and chemorepulsive signals to guide axons to their synaptic targets. Early neural circuits are often imprecise, requiring the removal of off-target contacts through activity-dependent mechanisms (reviewed by Kano and Hashimoto, 2009). Activity-dependent refinement occurs in many contexts in vertebrate nervous systems, with compelling evidence found for the developing mammalian visual system (Eglen et al., 2003; Meister et al., 1991; Nicol et al., 2007; Penn et al., 1994; Schmidt and Tieman, 1985; Stellwagen and Shatz, 2002; Wiesel and Hubel, 1963a; Wiesel and Hubel, 1963b; Wong, 1999; Wong et al., 1993). Other examples include the Xenopus tectum, where visual activity regulates the addition and removal of synapses (Aizenman and Cline, 2007; Akerman and Cline, 2006). At the vertebrate neuromuscular junction (NMJ), the “Hebbian” matching of pre- and postsynaptic activity regulates the elimination of supernumerary junctions (Balice-Gordon and Lichtman, 1994; Redfern, 1970; Sanes and Lichtman, 1999; Sanes and Lichtman, 2001).
Although a role for neuronal activity in shaping synaptic connections was discerned over four decades ago (Wiesel and Hubel, 1963a; Wiesel and Hubel, 1963b), the mechanisms remain incompletely resolved. A potential mechanism emerges from in vitro studies, where the response of neurons to various chemotropic molecules was found to be modulated by electrical activity (Ming et al., 2001; Nicol et al., 2007). Here we examine this idea in vivo using a genetically-tractable model synapse.
The activity involved in synaptic refinement is not limited to sensory stimuli, but may also involve oscillatory activity in the embryo. Low frequency (<0.01 Hz) voltage oscillations occur in the prenatal visual system (Catsicas et al., 1998; Meister et al., 1991; Mooney et al., 1996; Sernagor and Grzywacz, 1996; Wong, 1999; Wong et al., 1993), the spinal cord (Gomez et al., 2001; Gomez and Spitzer, 1999; Gomez and Spitzer, 2000) and in motoneurons (Hanson and Landmesser, 2004; Hanson et al., 2008). Low frequency voltage oscillations also occur in Drosophila. Embryonic motoneurons fire bursts of action potentials at 100-200 second intervals during the last third of embryogenesis, when NMJs are established and refined (Crisp et al., 2008; Pereanu et al., 2007). Silencing electrical activity during this period results in aberrant motoneuron contacts throughout the bodywall musculature, that develop into ectopic NMJs (Jarecki and Keshishian, 1995; White et sal., 2001a; White et al., 2001b; this study).
Each Drosophila motoneuron selects its target with high fidelity through cell-specific responses to multiple muscle-expressed guidance cues (reviewed by Ruiz-Canada and Budnik, 2006). Superimposed on these expression patterns is the pan-muscular expression of globally acting chemoattractive and chemorepellent molecules, that control the stability of the correct neuromuscular innervation, and repel off-target contacts (Winberg et al., 1998). For example, all bodywall muscles secrete the chemorepulsive molecule Semaphorin-2a (Sema-2a), that is essential for the withdrawal of motoneuron contacts from off-target muscles (Matthes et al., 1995; Winberg et al., 1998). The corresponding receptor, expressed by all motoneurons and responsible for this response, is plexin B (plexB; Ayoob et al., 2006; Hu et al., 2001). Similarly, the homophilic IgCAM fasciclin 2 is expressed at all embryonic NMJs, but by contrast stabilizes synaptic contacts (Lin et al., 1994; Lin and Goodman, 1994). Synaptic connectivity thus involves a balance between multiple muscle-specific molecules that guide motoneuron growth cones to their correct targets, against a background of chemorepulsion mediated by pan-muscularly expressed Sema-2a (Winberg et al., 1998). Here we show that this repulsion is modulated by low frequency bouts of presynaptic electrical activity, acting through calcium channels and CaMKII.
Electrical activity plays a key role in the withdrawal of off-target motoneuron contacts. These contacts occur throughout the musculature of animals paralyzed with either the Na channel toxin tetrodotoxin, or by mutations that affect the Na(v)1 voltage-gated Na channel, such as para or mlenap-ts;tipE (Jarecki and Keshishian, 1995). The same phenotype occurs when depolarization is suppressed using potassium current shunts (White et al., 2001a; White et al., 2001b).
Ectopic contacts could arise through increased motoneuron sprouting, or alternatively through reduced motoneuron pruning. There is good evidence that the latter mechanism is involved. Miswired contacts associated with the loss of electrical excitability are readily withdrawn when activity is restored, provided it occurs within an early critical period (Jarecki and Keshishian, 1995). Moreover, the increased presence of ectopic NMJs in mutants of Sema-2a or its receptor plexB, described in this paper, suggests that withdrawal involves chemorepulsion. The ectopic contacts that remain post-embryonically mature into physiologically functional synapses, that express multiple presynaptic markers.
Here we show that bursts of action potentials spaced every 2-3 minutes are required presynaptically to prevent ectopic contacts. By contrast, suppressing postsynaptic depolarization has no effect on connectivity or refinement. The underlying mechanism involves an activity-dependent modulation of the neuron’s response to Sema-2a, acting through its presynaptic receptor plexin B. The modulation depends on presynaptic voltage-gated calcium channels, with CaMKII acting as a key downstream effector in the neuron. The ectopic contact phenotype is suppressed by episodically activating calcium permeable channels at a frequency and duration similar to the native pattern of embryonic activity. The results provide an in vivo demonstration of the role of patterned electrical activity in modulating the neuron’s response to a chemotropic factor, and provide insight into the molecular mechanisms involved in synaptic refinement.
RESULTS
Synaptic refinement depends on presynaptic membrane excitability
Loss of function (lof) mutations of para, the gene that encodes the alpha subunit of the Drosophila voltage-gated Na(v)1 channel, result in electrically silenced and paralyzed embryos (Ganetzky, 1986; Hong and Ganetzky, 1994; Jarecki and Keshishian, 1995). Electrical silencing of the embryo leads to the appearance of ectopically placed motoneuron contacts throughout the musculature (Jarecki and Keshishian, 1995; White et al., 2001a; White et al., 2001b; shown schematically in Fig. 1A. See also Figs. 2, 3, 5 and 6 for examples from both embryos and larvae of ectopic contacts). Depolarization on either side of the synapse can also be suppressed by expressing a reengineered potassium channel (EKO; Electrical Knock Out; White et al., 2001b; see also Fig. 1B). This also results in ectopic motoneuron contacts, indistinguishable from those arising from Na channel mutations (Fig. 1B, fourth bar, EKO presyn = elavC155-Gal4;UAS-EKO. Data shown for comparison are from White et al., 2001b). By contrast, suppressing muscle depolarization has no effect on connectivity (Fig 1B, fifth bar, EKO postsyn = MHC-Gal4;UAS-EKO). Thus, presynaptic depolarization is required during embryogenesis to prevent off-target contacts by motoneurons.
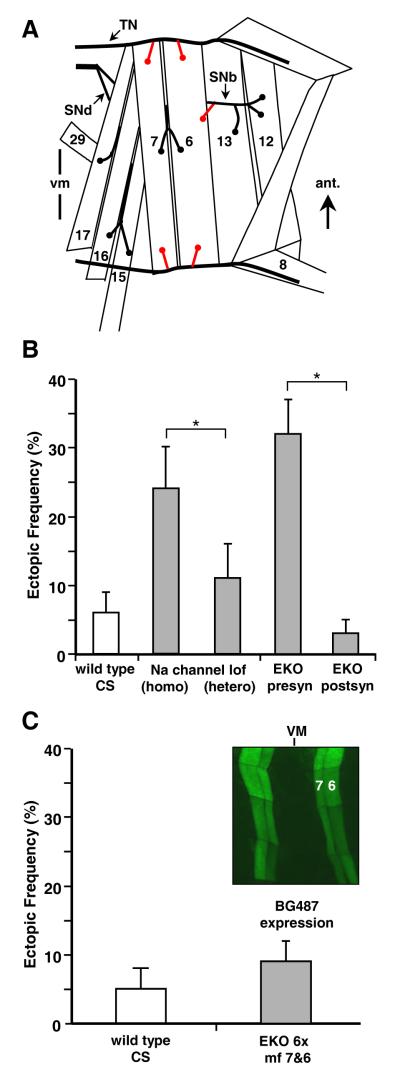
Figure 1. Ectopic neuromuscular contacts arise following presynaptic but not postsynaptic suppression of depolarization.
(A) The ventral bodywall muscle fibers and their innervation by the SNb (Segmental Nerve b, also referred to as ISNb), SNd (Segmental Nerve d) and TN (transverse nerve). The TN bypasses the ventral musculature, except for a branch to muscle 25. Shown in red are typical locations of ectopic contacts made onto muscle fibers 6 and 7 following electrical silencing (Jarecki and Keshishian, 1995). Anterior (ant) and ventral midline (vm) are indicated.
(B) Suppression of depolarization elevates the frequency of ectopic contacts in stage 17 embryos. The frequency on muscle fibers 6 and 7 is less than 10% in CS wild type embryos (CS; for this and subsequent figures, mean ± s.e.m. are shown). Embryos homozygous for a Na channel loss of function genotype that suppresses excitability (mlenap-ts;tipE), have a higher frequency of ectopic contacts compared to the CS wild type. The frequency of ectopic contacts in the lof heterozygotes (mlenap-ts;tipE/+) is not significantly different from wild type, a feature important for the genetic interaction screen. Suppression of depolarization using a potassium shunt channel (EKO; data for comparison are adapted from White et al., 2001b) results in a higher frequency of ectopic contacts when expressed presynaptically (EKO presyn= elavC155-Gal4;UAS-EKO). By contrast, no effect was observed when EKO was expressed postsynaptically (EKO postsyn=24B-Gal4;UAS-EKO).
(C) The phenotype in third instar larvae was examined following expression of up to 6 copies of EKO to suppress membrane excitability. BG487-Gal4 was used to drive expression in muscle fibers 6 and 7 (shown in the inset, 4 segments, using an EGFP reporter; VM = ventral midline). No increase in ectopic contacts was found despite the postsynaptic suppression of excitability.
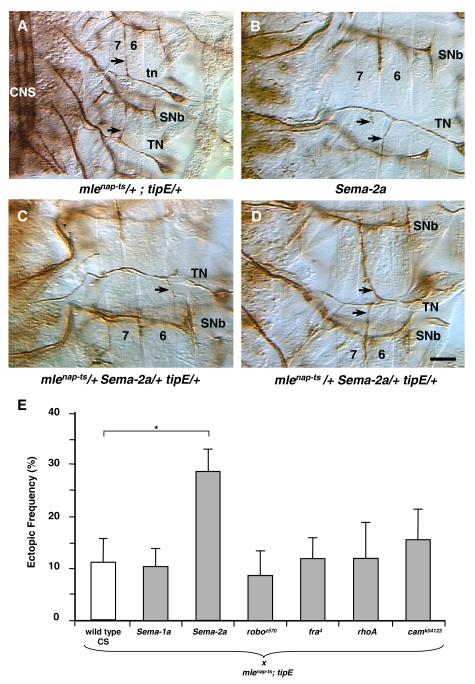
Figure 2. Ectopic contacts in stage 17 embryos from a genetic interaction test.
(A) In homozygous mlenap-ts;tipE mutants, the transverse nerve (TN) inappropriately forms contacts on ventral longitudinal muscle fibers 6 and 7 (arrows).
(B) Sema-2a mutant embryos have a similar ectopic contact phenotype (arrows).
(C, D) Sema-2a/+ mlenap-ts/+ tipE/+ triple heterozygotes reveal a genetic interaction between the Na channel activity mutations and Sema-2a. (A-D) Motoneuron projections immunolabeled with anti-fasciclin 2 in stage 17 embryos. Scale bar (A) = 16 micrometers; (B-D) = 10 micrometers.
(E) None of the candidate genes tested in the screen showed a genetic interaction with mlenap-ts /+;tipE/+ with the exception of Sema-2a/+. Single asterisk indicates p < 0.05.
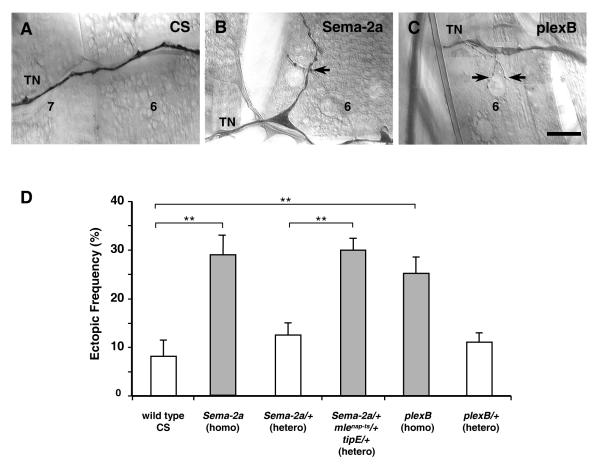
Fig. 3. Sema-2a and plexin B loss of function result in ectopic contacts in third instar larvae.
(A) Ectopic contacts on ventral longitudinal muscle fibers 6 and 7 are readily detected emerging from the transverse nerve (TN). They are present at <10% frequency in CS wild type larvae.
(B, C) Ectopic contacts (arrows) on muscle 6 in B) Sema-2aex59 and C) plexBKG00878 third instar larvae. Anti-HRP immunolabeling. Scale bar = 25 micrometers.
(D) Ectopic contact frequency in CS wild type, Sema-2aex59, Sema-2aex59/+, and Sema-2aex59/+ mlenap-ts/+ tipE/+ larvae. Also shown are results from plexBKG00878 and plexBKG00878/+ larvae. Double asterisk indicates p < 0.005.
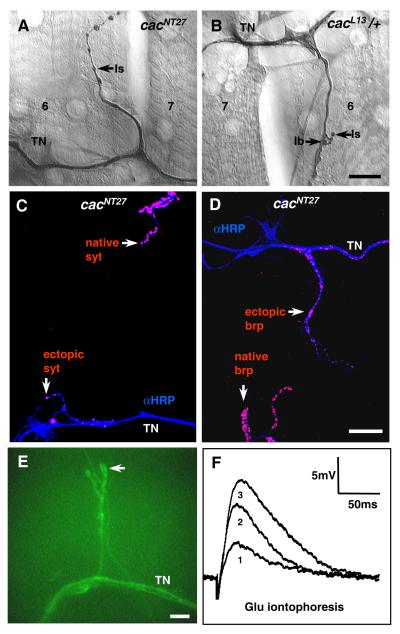
Figure 5. Morphology and functional characterization of ectopic contacts.
(A-B) Ectopic contact morphology in (A) cacNT27, and (B) cacL13/+ mutants. Scale bar = 25 micrometers. Arrows indicate type Ib and Is boutons on the ectopic contacts.
(C-D) In cacNT27, the ectopic contacts contain (C) the vesicular vSNARE protein Synaptotagmin (syt in red), as well as (D) the active zone-associated protein bruchpilot (brp in red). The motoneuron endings, labeled with anti-HRP, are shown in blue. Scale bar = 20 micrometers.
(E) An ectopic contact in a cacNT27 background revealed vitally with a fluorescently conjugated anti-HRP antibody for focal glutamate iontophoresis. The arrow indicates the position of the glutamate containing electrode. (F) Postsynaptic responses upon focal glutamate release from the electrode at increasing levels of iontophoretic current (traces 1-3). Scale bar = 10 micrometers.
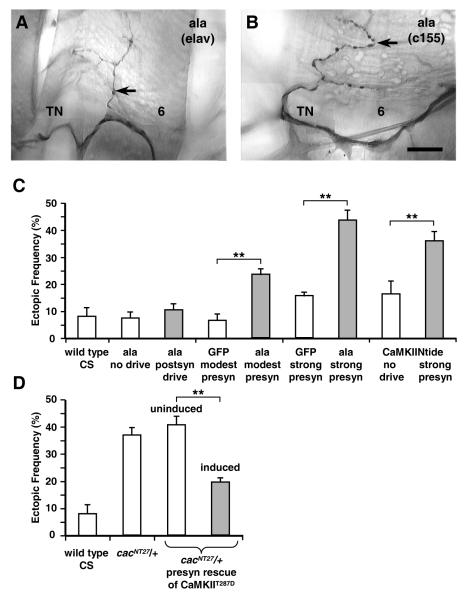
Figure 6. CaMKII function is required for normal connectivity.
(A-B) Inhibition of CamKII by presynaptic expression of the ala peptide results in ectopic contacts on muscle fibers 6 and 7. A) and B) compare the moderately-expressed pan-neural elav-Gal4 driver to the more strongly expressed elavc155-Gal4 driver. Scale bar = 25 micrometers.
(C) Ectopic contact frequency following expression of the ala inhibitor. Only presynaptic inhibition of CaMKII results in an increase in ectopic contacts. The stronger driver, elavc155-Gal4, further elevated the ectopic contact frequency. A similar effect was observed with presynaptic expression of the CaMKIINtide inhibitor.
(D) Expression of a constitutively active CaMKII (UAS-CaMKIIT287D) in a cacNT27 mutant background suppresses the ectopic contact phenotype (cacNT27/+;UAS-CaMKIIT287D/+;elav-GS-Gal4, UAS-CD8-GFP/+). Double asterisk indicates p < 0.005.
Earlier studies did not examine the effect of muscle silencing post-embryonically, due to the lethality of embryonic paralysis. However, by expressing the EKO channel in muscle fiber subsets we obtained partially paralyzed animals that survived to the third larval instar. Up to 6 copies of the EKO transgene were expressed in the ventral longitudinal muscle fibers 6 and 7 (Fig. 1A, and Fig. 1C inset). Expression of the GFP-tagged EKO channel was confirmed by its fluorescence and quantified by the measuring the resulting EKO-mediated current, using two electrode voltage clamp. In abdominal segments A2 and A3 the magnitude of the outward EKO current in muscle fiber 6 at +20 mV was 285 nA ± 5nA (mean ± s.e.m., as determined by current subtraction; White et al., 2001b). There was no effect on the number of ectopic contacts for either of the EKO expressing muscle fibers, nor were ectopic contacts observed at a higher than control frequency on neighboring muscle fibers (Fig. 1C; 9% ± 3% for EKO expressing fibers versus 5% ± 3% for controls). This confirms the results obtained with embryos (White et al., 2001b).
A genetic test of candidate genes involved in the refinement of connections
In vitro studies show that electrical activity can alter the response of a growth cone to several chemotropic molecules (Ming et al., 2001; Nishiyama et al., 2003). We therefore tested candidate chemotropic molecules that might be involved in the ectopic contact phenotype associated with Na channel mutants. The heterozygous Na channel mutation mlenap-ts/+;tipE/+ by itself does not have an ectopic contact phenotype (Fig 1B). This sensitized genetic background was used to test for genetic interactions, where the partial loss of function of an interacting gene (tested as the heterozygote) would lead to a dominant enhancement of the mlenap-ts/+;tipE/+ phenotype. While such genetic interaction tests demonstrate a functional relationship between two genes, they do not necessarily imply that the corresponding proteins interact directly. The genes tested included the chemorepulsive molecules Sema-1a and Sema-2a, the repulsive guidance receptor robo, and a component of the netrin receptor, frazzled. The screen was performed in stage 17 embryos (Fig. 2). Only Sema-2a/+ showed a genetic interaction with the heterozygous Na channel activity mutations, enhancing the ectopic contact phenotype in the triple heterozygote (mlenap-ts/+ Sema-2a/+ tipE/+ 29% ± 4%, Fig. 2E; compared to Sema-2a/+ 11% ± 6%, and mlenap-ts/+;tipE/+ 11% ± 4%, Fig. 1B; see also Figs. 2C-D for examples of the ectopic contacts).
We next examined third instar larvae to determine whether the ectopic contacts observed in the embryos persist through larval development (Fig. 3A-C). Consistent with the embryonic results, both Sema-2a homozygote and Sema-2a/+ mlenap-ts/+ tipE/+ triple heterozygote larvae have increased ectopic contacts (29% ± 5% and 30% ± 3%, respectively, compared to the CS wild type control of 8% ± 3%; Fig. 3D). In addition, hypomorphic mutations affecting the Sema-2a receptor, plexB had increased ectopic contacts (25% ± 4% compared to 11% ± 2% for the plexB/+ heterozygote; Fig. 3D). Triple heterozygotes of plexB/+ with mlenapts /+; tipE/+ were embryonic lethal and not tested.
The role of presynaptic calcium in motoneuron refinement
Having established a genetic interaction between mutations that affect neuronal excitability and the muscle-derived chemorepulsive molecule Sema-2a, we next sought to identify the effectors downstream of electrical activity that are involved in the Sema-2a phenotype. A compelling candidate is the calcium channel encoded by cacophony (cac; Brooks et al., 2003; Kawasaki et al., 2002; Kawasaki et al., 2000; Kawasaki et al., 2004; Rieckhof et al., 2003). The cac gene encodes the pore-forming alpha subunit of the Ca(v)2.1 voltage-dependent calcium channel, that is expressed at the active zones of motoneuron terminals (Kawasaki et al., 2002; Kawasaki et al., 2004; Rieckhof et al., 2003). In addition to reducing evoked transmission at the NMJ, cac mutants have defects in synaptic growth (Rieckhof et al., 2003). These phenotypes are rescued by presynaptic expression of a wild type cac transgene, demonstrating that cac functions in the motoneuron (Kawasaki et al., 2002; Rieckhof et al., 2003). However, no evidence was shown linking cac function to synaptic refinement.
Multiple alleles of cac were used to test whether third instar larvae have ectopic contacts. The cacNT27 allele is a hypomorphic allele with altered voltage gating, where a charged arginine residue is mutated to a neutral cysteine within the S4 voltage sensor (Rieckhof et al., 2003). The cacNT27 homozygotes have an elevated ectopic contact phenotype (42% ± 4% in cacNT27 vs. 8% ± 3% in CS wild type; see Fig. 4A, second bar: cacNT27; see also Fig. 5A-E). Surprisingly, larvae heterozygous for cacNT27 as well as the lethal allele cacL13 also had extensive ectopic contacts (37% ± 3% for cacNT27/+, Fig. 4A, third bar; 31% ± 3% for cacL13/+, Fig. 4B, fifth bar). Thus, even with one wild-type copy of cac, a marked defect in synaptic refinement was observed, suggesting that regulation of calcium levels is an important determinant of whether off-target contacts are stabilized or withdrawn.
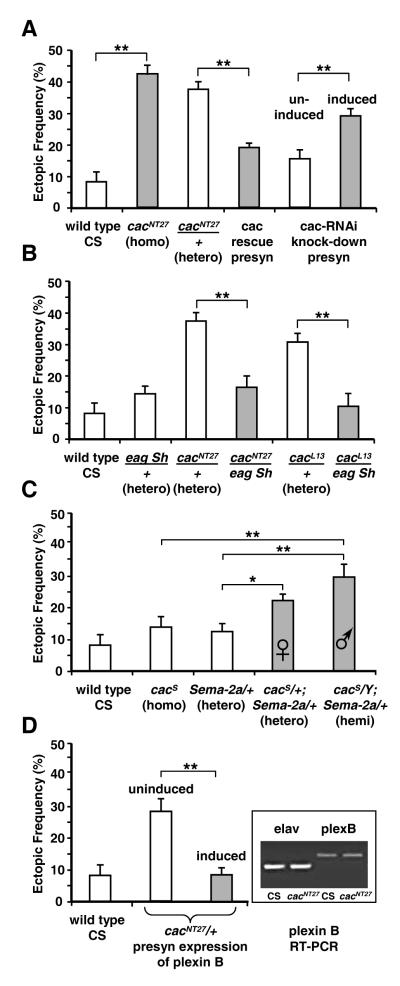
Fig. 4. Loss of function of the voltage gated calcium cacophony channel leads to ectopic contacts.
(A) Ectopic contacts occur with increased frequency in both homozygous and heterozygous cacNT27 third instar larvae. The phenotype is partially rescued by presynaptic expression of the wild type cac transgene (cac rescue = elavc155-Gal5;UAS-cac). The cac phenotype is phenocopied by presynaptic knockdown of cac using the RNAi transgene, driven with an inducible elav-GS-Gal4 driver. The uninduced and RU486-induced RNAi expression effects are compared (fifth and sixth bars).
(B) The hyperactivity mutations eag1 Sh133 partially suppress the elevated ectopic contact frequency observed in both cacNT27 and cacL13 mutants.
(C) Sema-2a genetically interacts with cacS, a weak hypomorphic allele of cac. The cacS homozygote and the Sema-2a heterozygote have ectopic contacts at frequencies similar to CS wild type larvae. However, the cacS/+;Sema-2a/+ double heterozygote female larvae have an elevated ectopic contact frequency. Male larvae, being hemizygous for cacS, have a more severe phenotype than the heterozygous females, with elevated ectopic contact frequency. Single asterisk indicates p < 0.05, double asterisk p < 0.005.
(D) The ectopic contact phenotype due to cac is suppressed by elevated plexin B expression in the neuron. GeneSwitch expression was used to drive UAS-plexB presynaptically in a cacNT27/+ background (cacNT27/+;; UAS-plexB / ElavGS-GAL4, UAS-CD8:GFP). RU486 induction (third bar) fully suppressed the cac phenotype (second bar) to wild type levels (first bar), double asterisk = p < 0.0001. The inset shows no change in the expression of plexB transcript between the CS wild type and cacNT27 mutant embryos, as determined by RT-PCR. Levels of the pan-neurally expressed elav transcript were monitored as a control for mRNA recovery.
To determine whether cac function is required presynaptically for proper refinement, we expressed a UAS-cac-RNAi construct in neurons using the inducible elav-GeneSwitch (GS) Gal4 pan-neuronal driver (Osterwalder et al, 2001). The UAS-transgene expression depends on the presence of RU486 (mifepristone), allowing tests in animals with identical genotypes. Compared to uninduced control larvae, inducing expression of the UAS-cac-RNAi in neurons led to an elevation in the frequency of ectopic contacts (29% ± 3%; Fig. 4A, fifth and sixth bars). Thus knocking down cac function in neurons leads to errors of refinement.
As a further test for a presynaptic role for cac, we reintroduced a wild-type copy of cac pan-neuronally in cacNT27 mutants to rescue the ectopic contact phenotype. Expression in neurons of UAS-cac in a cacNT27 /+ background reduced the frequency of ectopic contacts to 19% ± 2% (compared to 37% ± 3% in cacNT27/+ heterozygotes; Fig. 4A: third bar). The partial rescue is likely due to the modest cac expression levels reported for the UAS-cac transgenic line (Rieckhof et al., 2003).
We also examined whether elevated neuronal activity can suppress the ectopic contact phenotype seen in cac mutants. The eag Sh double mutation affects multiple potassium channels in both neurons and muscle, and results in neuromuscular hyperactivity (Budnik et al., 1990; Mosca et al., 2005; Zhong et al., 1992). These mutants were crossed with cacNT27 or cacL13 to generate transheterozygotes. Compared to either the cacNT27/+ or cacL13/+ heterozygotes, the corresponding cacNT27/eag Sh or cacL13/eag Sh transheterozygotes had a decrease in ectopic contacts (Fig. 4B; bars 3-6). The interaction between neuronal hyperactivity mutations and cac suggests that the ectopic contact phenotype observed with reduced neuronal activity is likely due to reduced calcium channel function.
We next asked whether calcium entry via cac was modulating Sema-2a signaling with respect to the elimination of ectopic contacts. This was examined by testing for a genetic interaction between Sema-2a and cac. The mild hypomorphic cacS allele has an ectopic contact frequency similar to that of control larvae (14% ± 3% in cacS vs. 8% ± 3% in WT; Fig. 4C, second bar: cacS). This allowed for genetic interaction tests with Sema-2a. As cac is on the X-chromosome, by sexing the larvae we collected Sema-2a/+ heterozygotes with two levels of cac loss of function. Heterozygous cacS/+;Sema-2a/+ females retain a wild type copy of the cac gene, and accordingly had a modest increase in ectopic contacts to 22% ± 2%. By contrast, the hemizygous males (cacS/Y;Sema-2a/+) lack the wild type copy and thus have a more severe phenotype (30% ± 4%; Fig. 4C; fourth and fifth bars). These results support the hypothesis that calcium signaling modulates the response of the motoneuron to a muscle-derived Sema-2a repulsive signal.
A plausible hypothesis to explain these results is that calcium regulates the motoneuron’s response to Sema-2a. Thus, the reduced calcium current of the cac mutation would down-regulate signaling, either mediated by the plexin B receptor, or in a parallel pathway. As a result of the reduced response to Sema-2a, the neurons would be less likely to withdraw off-target contacts. We asked whether elevated presynaptic expression of the plexin B receptor might suppress the ectopic contact phenotype of cac mutants. To control for genetic background effects, we employed the GeneSwitch inducible system to drive expression of UAS-plexB in the cacNT27/+ heterozygous mutant background (Fig. 4D). In the uninduced state, we observed a 28% ± 4% frequency of ectopic contacts on muscle fiber 6 and 7 in third instar larvae (Fig. 4D second bar). In larvae with plexin B expression induced by RU486, the frequency fell to 8% ± 2% (Fig. 4D third bar), showing a suppression of the ectopic contact phenotype to wild type levels (Fig. 4D first bar). This supports the view that the plexin B signal transduction pathway is regulated by presynaptic calcium entry through voltage-gated cac channels. The calcium-dependent modulation might be due to changes in the levels or function of the receptor itself, or one or more of the effectors downstream of plexin B.
To test whether plexB expression itself is regulated by neural activity we used RT-PCR to detect changes of plexB transcript levels in stage 17 homozygous cacNT27 embryos, as compared to WT (Fig. 4D, inset). This stage corresponds to the critical period when neural activity is required for synaptic refinement (Jarecki and Keshishian, 1995; White et al., 2001b). Using the level of pan-neurally expressed elav transcript as a control for mRNA recovery, we observed no difference in plexB transcript levels between the embryos of the two genotypes.
We also examined whether calcium entry through the cac channel might affect cell adhesion at the developing synapses. There is a genetic interaction between Sema-2a and the motoneuron-expressed IgCAM fasciclin 2 (fas2; Winberg et al., 1998). Furthermore, as fasciclin 2 is involved in the stabilization of neuromuscular contacts, we examined whether there are genetic interactions between cac and fas2. Since fasciclin 2 functions as a cell adhesion molecule (Lin et al., 1994), and elevated expression of fasciclin 2 in muscle can result in an ectopic contact phenotype (Davis et al., 1997; Winberg et al., 1998), we hypothesized that reduced fasciclin 2 levels would suppress the cac phenotype by destabilizing ectopic contacts. The fas2EB112/+ mutation reduces fasciclin 2 levels by 50%, without otherwise disrupting NMJ formation (more severe fas2 mutations destabilize all NMJs). The ectopic contact frequency in the double mutant was not significantly different from the cacNT27 heterozygote (fas2EB112/cacNT27 transheterozygotes, 44% +/− 4%, as compared to cacNT27/+, 37% ± 3%). This result supports the view that electrical activity functions specifically in the chemorepellent pathway mediated by Sema-2a.
Functional properties of the ectopic contacts
We next examined whether the ectopic contacts associated with reduced cac function are indeed functional synapses, as was previously found for the ectopic contacts that arise following muscle denervation (Chang and Keshishian, 1996). The ectopic contacts share many of the features of the native NMJs, including synaptic boutons of type Ib and Is morphology (Fig. 5A-B, arrows). The ectopic contacts also express fasciclin 2, an IgCAM that is normally expressed by embryonic motoneurons at the NMJ (Fig. 2A-D).
We find that in addition to fasciclin 2, the ectopic contacts express molecules associated with the presynaptic release machinery, including the vSNARE vesicle associated protein synaptotagmin (Fig. 5C), as well as the active zone associated ELKS/CAST protein bruchpilot (Fig. 5D; Kittel et al., 2006; Wagh et al., 2006). To test whether there are functional glutamate receptors at the ectopic sites, we vitally labeled cacNT27 motor endings of third instar larvae to visualize the ectopic contacts (Fig. 5E). Using focal glutamate iontophoresis we observed glutamatergic potentials that were dependent on the placement of the glutamate electrode to within 1 μm of the boutons, and whose amplitude scaled with the amount of iontophoretic current (Fig. 5F). This demonstrates that functional receptor sensitivity to glutamate is tightly localized opposite the ectopic contact, similar to the case for native terminals (Johansen et al., 1989). By contrast, using available antibody probes we could detect neither the postsynaptic adaptor protein Dlg nor dGluRIIA/IIC receptor subunits at the ectopic sites, despite robust labeling at the native synapses (not shown). Thus, although the ectopic contacts have functional properties and express presynaptic active-zone associated proteins, they may not have normally organized postsynaptic machinery.
CaMKII is a downstream effector of calcium signaling involved in synaptic refinement
Having demonstrated a requirement in the presynaptic terminals for cac in normal synaptic refinement, our focus shifted to identifying the downstream molecule(s) activated by calcium entry that might influence synaptic pruning. A likely candidate is the calcium/calmodulin-dependent protein kinase type II (CaMKII), that is expressed on either side of the synapse, and is involved in several forms of synaptic plasticity in this system (Griffith, 1997; Haghighi et al., 2003; Jin et al., 1998).
In order to suppress CaMKII function in a cell-specific fashion, we targeted both the inhibitory “ala” peptide, as well as the CaMKIINtide transgene to either side of the synapse (Griffith et al., 1993; Haghighi et al., 2003; Jin et al., 1998). We first examined the effect of expressing the ala inhibitory peptide with drivers that have two levels of expression. Using elav-Gal4, a pan-neuronal driver of relatively modest expression, we observed an elevation in the frequency of ectopic contacts (23% ± 3% vs. 8% ±3% in WT; Fig. 6C). This frequency increased substantially with a stronger driver, elavC155-Gal4, (44% ± 3%; Fig. 6C). The same driver was used to express CaMKIINtide presynaptically, and revealed a similar phenotype to that observed with ala expression (Fig. 6C). The ectopic contacts associated with reduced CaMKII function were similar to those of Sema-2a, plexB, cac, and mlenap-ts;tipE mutants (Fig. 6A-B). The parental lines were also tested as controls, and had no significant elevation of ectopic contacts (Fig. 6C, bars 2,4,6,8).
A presynaptic dependence for CaMKII was tested by expressing the inhibitory ala peptide postsynaptically in muscles using the pan-mesodermal 24B-Gal4 driver. In contrast to the presynaptic effects, the resulting ectopic contact frequency of 11% ± 2% was comparable to that of controls (8% ± 3%), indicating that inhibition of CaMKII in the muscle, as opposed to the motoneuron, has no significant effect (Fig. 6C, bar 3).
CaMKII could function downstream of cac to phosphorylate specific effectors. However, since CaMKII can also affect excitability (Haghighi et al., 2003; Wang et al., 1994), and can bind directly to mammalian Ca(v)2.1 channels (Jiang et al., 2008), the ectopic contact phenotype might be due to changes in neural activity, and thus upstream of cac. However, evoked transmission and spontaneous activity at the NMJ in animals where CaMKII was inhibited presynaptically by expression of the ala peptide were found to be unaffected (not shown), supporting the view that CaMKII acts downstream of neuronal activity and cac function (Fig. 6A-C).
In order to place CaMKII downstream of cac, we suppressed the elevated frequency of ectopic contacts seen in cacNT27 by expressing a constitutively active form of CaMKII in motoneurons. The transgenic line, UAS-CaMKIIT287D, contains a substitution of threonine at position 287 to aspartate, that mimics phosphorylation in the autoinhibitory domain, and renders CaMKII constitutively active (Jin et al., 1998; Wang et al., 1998). Expression of the transgene resulted in a 50% reduction in the ectopic contact frequency of cacNT27 (41% ± 3% versus 20% ± 2% for uninduced compared to induced larvae; Fig. 6D, third and fourth bars). Larvae with the same genotype, but without RU486 induction, had an ectopic contact frequency similar to the cacNT27 heterozygous mutants (37% ± 3% vs. 41% ± 3%, respectively; Fig. 6D, second and third bars). This positions CaMKII in the same signaling cascade as cac during neuromuscular refinement.
Role of periodic activity in governing synaptic refinement
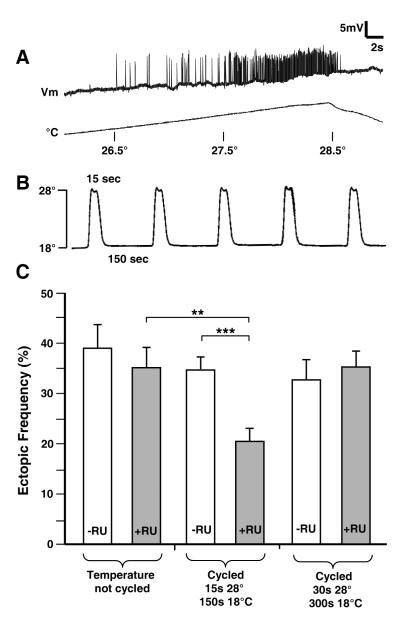
Embryos exhibit periodic bouts of motoneuron activity every 2-3 minutes (Crisp et al., 2008; Pereanu et al., 2007). We tested whether a similar pattern of motoneuron activity would suppress the ectopic contact phenotype of cac. The dTrpA1 gene encodes a temperature-regulated channel responsible for warmth sensing in Drosophila. The channel is permeable to mixed cations, including calcium (Hamada et al., 2008). The dTrpA1 channel was expressed in embryonic neurons and activated by temperature shifting the embryos in a PCR machine, from 18° C (where the channel is closed) to 28° C (where it is open; Pulver et al., 2009). This was done during the critical period for synaptic refinement (stages 16 and 17; Jarecki and Keshishian, 1995). The animals were then transferred to agar plates and reared at RT through the 3rd larval instar. Fig. 7A shows the induction of EJPs in a cacNT27 larva, temperature shifted from 26.5° to 28.5° C. Fig. 7B shows the observed temperatures within the PCR tube during a 1:10 duty cycle of 15s at 28° C, followed by 150s at 18° C, to emulate the WT frequency of embryonic neuromuscular activity (Pereanu et al., 2007). Fig. 7C shows the suppression of the ectopic contact phenotype due to cacNT27/+, as scored in 3rd instar larvae, when dTrpA1 is activated with the appropriate pattern. Embryos expressing the channel but without activation (i.e. held at a constant RT during development) showed no effect (first pair of bars). The effect of episodic (1:10 duty cycle) activation of dTrpA1 was next examined with two patterns of activity. A cycle of 15s at 28° C, followed by 150s at 18° C resulted in a reduction in ectopic contacts (second pair of bars). By contrast, a cycle of 30s at 28° C, followed by 300s at 18° C failed to suppress ectopic contacts (third pair of bars). Thus the episodic activation of a Ca-permeable channel in embryonic neurons, at a frequency that emulates the WT pattern of electrical activity, effectively promotes the withdrawal of off-target motoneuron contacts. These observations are consistent with a model where brief bouts of depolarization and calcium entry, with a 1-3 minute interval of quiescence, is sufficient to regulate the responsiveness of the motoneuron to retrograde chemorepulsion.
Figure 7. Low frequency activation of electrical activity is sufficient to promote synaptic refinement.
A) The dTrpA1 channel provides a conditional temperature sensitive permeability to mixed cations (including Ca), and was expressed in cac heterozygotes using the panneural elav GeneSwitch driver (cacNT27/+; UAS-dTrpA1/+; elav-GS-Gal4, UAS-CD8-GFP/+). The trace shows EJPs recorded from muscle fiber 6 in a 3rd instar larva, evoked as the temperature ramped from 26.5° to 28.5° C. The activation temperature closely matches the reported 27° C value (Pulver et al., 2009).
B) Embryos were immersed in halocarbon 700 oil in PCR tubes, and temperature cycled in a PCR machine, as shown. This pattern emulates the episodic bouts of neuromuscular activity that occur in embryos.
C) Temperature cycling dTrpA1 expressing embryos suppresses the ectopic contact phenotype of cacNT27/+ mutants. As the dTrpA1 channel was expressed using the RU486 inducible elav-GS-Gal4 driver, animals of identical genotype and temperature cycling are compared, differing by their expression of dTrpA1. Embryos were cycled in a PCR machine from stage 16 until hatching, followed by larval development on agar plates at RT. The first and second bars show no difference in ectopic contacts in cacNT27/+ larvae without (white) or with (gray) RU486 dTrpA1induction when held at RT. The third and fourth bars compare the suppression of ectopic contacts in animals cycled with a 1:10 duty cycle, of 15s at 28°C followed by 150s at 18° C (as in B), either in the absence (white), or in the presence (gray) of RU486 induction of dTrpA1, where a suppression of the cacNT27/+ phenotype results. The third and fourth bars show the same experiment and duty cycle, but with a pattern of 30s at 28° C followed by 300s at 18° C, with no suppression observed. Double asterisk indicates p <0.007; triple asterisk indicates p <0.0003.
DISCUSSION
At the larval NMJ electrical activity affects synaptic connectivity (Jarecki and Keshishian, 1995; White et al., 2001b; this study), motoneuron terminal size (Ataman et al., 2008; Budnik et al., 1990; Zhong et al., 1992; Zhong and Wu, 2004), and synaptic homeostasis (Davis et al., 1998; Davis et al., 1997; Dickman and Davis, 2009; Frank et al., 2009; Paradis et al., 2001). A possible effector downstream of activity for each of these phenomena is calcium, entering the cell through voltage gated ion channels.
We show that the voltage-gated calcium channel encoded by the cac gene is critically involved in the activity-dependent refinement of connections at the Drosophila NMJ. Mutations of cac have an ectopic contact phenotype similar to those for mutations affecting Na channels, Sema-2a, plexin B, or due to inhibition of CaMKII. Our data provide genetic evidence for a mechanism where episodic activation of a voltage-dependent calcium influx regulates the motoneuron’s response to the retrograde chemorepellent Sema-2a. A likely player in this modulation is the Ca-dependent kinase CaMKII, where loss of function in the motoneuron results in an ectopic contact phenotype as severe as that seen with the cac mutations.
Semaphorin signaling and CaMKII
The receptor plexin B, or its downstream signaling cascade are the likely targets of the molecules that modulate the response to Sema-2a. The ectopic contact phenotype due to loss of cac function is suppressed by increased expression of plexB in the motoneuron. This suggests that in cac mutants there is reduced levels and/or activity of plexB (or possibly changes affecting its downstream effectors). However, our RT-PCR experiments show that loss of function of cac does not alter plexB transcript levels. Nevertheless, presynaptic electrical activity and calcium entry through the cac channel may regulate plexB post-transcriptionally. The regulation could include activating and/or recruiting kinases that phosphorylate the plexB receptor or its complex (Giordano et al., 2002). Also, receptor localization may also be dynamically regulated, as activation of the GTPase Rac targets PlexB1 to the membrane (Vikis et al., 2002). Moreover, proteolytic cleavage of PlexB1 enhances the receptor’s response to its ligand Sema-4D (Artigiani et al., 2003). The cleavage site is conserved in the Drosophila plexB protein (personal communication, A. Kolodkin). Activity could also act on proteins downstream of the plexB receptor.
We found that elevated expression of CaMKII in the motoneuron suppressed the ectopic contact phenotype of cac, indicating that CaMKII operates downstream of the channel. While a putative plexB signaling cascade has been proposed for Drosophila (Hu et al., 2001), it is not known whether any of the proteins is directly dependent on CaMKII for its function. To test these ideas in the future, key reagents (such as antibody probes) for biochemical tests examining the posttranslational modification of the signaling molecules in the plexB pathway would need to be developed.
Synaptic activity and refinement
Synaptic refinement often depends on an activity-dependent Hebbian matching mechanism. Competition favors neurons with strong synaptic drive, that evoke synchronous pre- and postsynaptic action potential activity (reviewed in Kano and Hashimoto, 2009; see also Katz and Shatz, 1996; Stent, 1973).
Although the ectopic contacts we observed are likely functional, based on the expression patterns of synaptic proteins and physiological tests, it is unlikely that a competitive mechanism is involved in their removal. Normal connectivity still occurs when muscle depolarization in blocked in the embryo, ruling out a synchronous, activity-matching mechanism (Jarecki and Keshishian, 1995; White et al., 2001b; this study). This is also consistent with a study that found no synaptic competition at the Drosophila NMJ (Cash et al., 1992). Instead, we propose that episodic neural activity is involved in a synaptic target selection mechanism.
We succeeded in suppressing the ectopic contact phenotype by periodically activating dTrpA1 channels (that are permeable to Ca). Significantly, the rescue depended on using a pattern similar to that observed in the embryo. Thus 15s of activation followed by 150s of rest was effective, while 30s of activation followed by 300s of rest was not, although the total amount of time at both temperatures was the same for both cases. Thus, normal synaptic refinement functions effectively when calcium levels oscillate on a 2-3 minute timescale. Given our observation that calcium signaling modulates the responsiveness of the motoneuron to retrograde Sema-2a signals, we favor a model where naturally arising calcium oscillations periodically alter the motoneuron’s response to the chemorepellent. We propose that episodic electrical activity is a strategy used by growth cones to manage a response to seemingly contradictory guidance cues. Thus, the neuron’s response to retrograde chemorepellents such as Sema-2a would rise and fall with the periodic bouts of electrical activity that drive calcium entry.
Motoneuron growth cone filopodia extend and withdraw on timescales ranging from 1-10 min (Keshishian et al., 1993; Murray et al., 1998). When the neuron is electrically quiet, a reduced response to Sema-2a would favor exploratory contacts. With electrical activity the transient responsiveness to Sema-2a would bias filopodial withdrawal from off-target muscles, where there is less chemoaffinity than for the target muscles. This would promote the progressive refinement of the motoneuron projections. There is precedence for a rapid modulation of chemotropic signal processing in vertebrate neurons. Episodic changes in electrical activity and cAMP levels are essential for proper EphrinA responses by retinal growth cones, as was found for explant cultures of the superior colliculus (Nicol et al., 2007).
The general significance of this mechanism will require further tests in vivo. It is also possible that chemoattractive signals are also regulated in an activity-dependent and episodic fashion. Temporal modulation of chemotropism would add an additional layer of mechanistic control over axon guidance and synaptic refinement.
EXPERIMENTAL PROCEDURES
Drosophila stocks
We thank the following for stocks and reagents: V. Budnik, U. Mass. Medical School, Worcester. MA: 1) BG487 Gal4. Drosophila Stock Center, Bloomington, IN: 1) cn1 P{ry+t7.2=PZ} Sema-2a03021/CyO; ry506. 2) y1 w67c23; P{w+mC=lacW}camk04213/CyO. 3) wa Nfa-g; rho1E3.10/CyO. P. Garrity, Brandeis U., Waltham, MA: UAS-dTrpA1. C. Goodman UC Berkeley, CA: 1) fra3/CyO, Wg-β-gal. 2) fra4/CyO, Wg-β-gal. 3) roboZ570/CyO. 4) roboZ1772/CyO. 5) roboGA285/CyO. L. Griffith, Brandeis U., Waltham, MA: 1) UAS-ala. 2) UAS-CaMKIIT287D. 3) UAS-CaMKIINtide. A. Kolodkin, Johns Hopkins Med. School, Baltimore, MD: 1) Sema-1aP1/CyO, elav-β-gal. 2) Sema-2aex59/CyO, Act[5C] - β-gal. 3) plexBKG00878/spa-pol. 4) UAS-plexB. T. Littleton, MIT, Cambridge, MA: cacNT27. R. Ordway, Penn State, State College, PA: 1) cacL13/FM7i-GFP. 2) cacS. 3) UAS-cac1-EGFP-786C. The Vienna Drosophila RNAi Center, Vienna, Austria: 1) w1118; UAS-cac-RNAi/CyO. The wild type control was Canton S. Embryos lacking a β-gal balancer were rebalanced with either CyO, elav β-gal or CyO, Wg β-gal. GeneSwitch inducible drivers were activated by either raising larvae on food containing 2.5, or for embryonic induction by feeding the parents 10 μg/ml mifepristone for 4 days prior to egg collection (RU486; Osterwalder et al., 2001).
Immunolabeling of embryos and larvae
Embryos from 2h egg lays were raised at 25° C for 16 hours to reach late stage 16/early stage 17 (Campos-Ortega and Hartenstein, 1997). They were dechorionated in 50% laundry bleach for 2.5 minutes, and fixed for 25 minutes in a 1:1 mix of heptane:4% paraformaldehyde. Both layers were replaced with a 1:1 mix of methanol:heptane, and vortexed to devitellinize. Following a methanol wash, the embryos were stored at −20° C prior to immunolabeling.
Embryos were rehydrated in steps with a mix of PBTween (PBS + 0.1% Tween 20) and methanol in ratios of: 1:3, 1:1, 3:1, and washed in PBTween. PBTween + 1% bovine serum albumin was used for blocking. Embryos were incubated overnight at 4°C with a both fasciclin 2 monoclonal antibody 1D4, and anti-beta galactosidase. Embryos were washed using PBTween, blocked with PBTween + 2% secondary antibody-specific serum, and incubated in secondary antibody for 2 hours at room temperature. Labeling was revealed with an ABC amplification kit (Vectastain) using diaminobenzidine. Following whole mount immunolabeling the embryos were made to adhere to a thin film of glycerol on a slide. Incisions were made using micropipets at the posterior and anterior ends. The embryo was then dorsally incised and the bodywall reflected. The viscera were removed and the specimen secured with a coverslip.
Larvae were fillet dissected as previously described (Johansen et al., 1989), fixed in 4% paraformaldahyde for 1 hr and washed in PBS. Primary antibodies were diluted in PBS with 0.3% Triton X100 (TBS). Larvae were labeled with anti-HRP (Jackson Lab), anti-discs large (V. Budnik, U. Massachusetts Medical Center, Worcester, MA), synaptotagmin (H. Bellen, Baylor College of Medicine, Houston, TX), anti-Fas2, anti-anti-bruchpilot, dGlurIIA monoclonal antibodies (Iowa Hybridoma Stock Center), and dGlurIIC (A. DiAntonio, Washington U., St. Louis, MO). Secondary antibodies used were either HRP- (Jackson Lab), Alexa488-, Alexa568, or Alexa647 conjugated (Molecular Probes). HRP labeling was revealed by diaminobenzidine reaction with an ABC kit (Vectastain). Confocal imaging of fluorescent preparations was with either a Zeiss 510 Meta or BioRad 1024 confocal microscope, with Imaris image processing software (Bitplane, Zurich, Switzerland). The scoring and statistical analysis of ectopic contacts made onto the ventral longitudinal muscle fibers 6 and 7 was as previously described (Jarecki and Keshishian, 1995). Data are presented as mean ± s.e.m, with significance by T test.
Electrophysiology
Single electrode recordings of excitatory junction potentials were performed using 20-30 MΩ sharp microelectrodes filled with 3M KCl. Motoneuron-specific excitation of the ventral longitudinal muscle fibers was with 2 μm suction electrodes placed at the nerve branch locations described by Lnenicka and Keshishian (2000). Physiological saline contained (in mM) NaCl 140, KCl 5, CaCl2 1, NaHCO3 4, MgCl2 6, TES 5, trehalose 5, sucrose 50 (pH 7.2). Two electrode voltage clamp recording of EKO currents was performed in the above saline, modified by omitting CaCl2 and adding 0.5 mM EGTA to block all inward Ca currents. Recordings were made with a Dagan 8500 voltage clamp, using a −80 mV holding potential, with command potentials stepped in 10 mV increments to +30 mV. For the current subtraction measurements 4 mM 4-aminopyridine was used to block the EKO current (Mosca et al., 2005; White et al., 2001b). All data were collected and analyzed using pClamp 9 software (Axon Instruments). Iontophoresis of glutamate at synaptic contacts was performed with motor endings vitally revealed by incubation for 1 h at room temperature with FITC-labeled anti-HRP, diluted 1:100 in saline, followed by a 30 minute wash in fresh saline (Chang and Keshishian, 1996; Johansen et al., 1989).
RT-PCR
Dechorionated stage 17 embryos of WT and cacNT27 genotypes were frozen on dry ice, homogenized in Trizol (Invitrogen) and total RNA was purified according to manufacturer’s instructions. Four independent RNA extractions were done for each genotype. cDNA was generated from 1 μg of total RNA using an oligo (dT)18 primer and Superscript II (Invitrogen). PCR primers include: elav primers (5′ end) 5′-TAATGCTGCCCA ATGGACTAGGAGC-3′ and (3′ end) 5′-GGTGCTGCAGGTCAGCTTCAAGACCAACAAA-3′; and from plexB primers (5′ end) 5′-TTTCTCTATGAGGCCATCTGTAAAC-3′ and (3′ end) 5′-ATTGAAATGCTGGTGATCCTCCG-3′.
Manipulation of oscillatory activity
Stage 16 embryos (Campos-Ortega and Hartenstein, 1997) of the genotype cacNT27/+; UAS-dTrpA1/+; elav-GS-Gal4, UAS-CD8GFP/+ were collected at 18° C. Control of dTrpA1 expression in embryos by RU486 induction was as described above. ~ 50 eggs were pooled in a PCR tube containing 20μl of Halocarbon 700 oil (Halocarbon Products Corp., River Edge, NJ USA), and cycled in a PCR machine between 18° and 28° C until hatching (~ 18.5 hrs), followed by rearing at RT until 3rd instar. Cycles of 15s at 28° C followed by 150s at 18° C, or of 30s at 28° C followed by 300s at 18° C were used (Fig. 7B). Control embryos without RU486 exposure underwent the same temperature cycling. EJPs were recorded from the ventral longitudinal muscle 6 in segments A2-A4 of filleted 3rd instar animals. A manifold perfusion system was used to deliver cooled or heated saline, monitored by a thermocouple mounted next to the animal.
ACKNOWLEDGMENTS
We thank V. Budnik, C. Goodman, P. Garrity, L. Griffith, A. Kolodkin, T. Littleton, and R. Ordway for generously providing Drosophila stocks and reagents. We also thank Rebecca Delventhal for her contribution to the plexin B analysis. We thank Elke Stein, Robert Wyman, and Weimin Zhong, as well as members of the Keshishian lab, for their advice and comments on the manuscript. The research was supported by grants from the NIH (5R01NS031651 and 1R21NS053807), and from the NSF (IBN0641915) to HK, and by a predoctoral fellowship from the Ford Foundation to RAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aizenman CD, Cline HT. Enhanced visual activity in vivo forms nascent synapses in the developing retinotectal projection. J Neurophysiol. 2007;97:2949–2957. doi: 10.1152/jn.00452.2006. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci. 2006;26:5117–5130. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigiani S, Barberis D, Fazzari P, Longati P, Angelini P, van de Loo JW, Comoglio PM, Tamagnone L. Functional regulation of semaphorin receptors by proprotein convertases. J Biol Chem. 2003;278:10094–10101. doi: 10.1074/jbc.M210156200. [DOI] [PubMed] [Google Scholar]
- Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoob JC, Terman JR, Kolodkin AL. Drosophila Plexin B is a Sema-2a receptor required for axon guidance. Development. 2006;133:2125–2135. doi: 10.1242/dev.02380. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372:519–524. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- Brooks IM, Felling R, Kawasaki F, Ordway RW. Genetic analysis of a synaptic calcium channel in Drosophila: intragenic modifiers of a temperature-sensitive paralytic mutant of cacophony. Genetics. 2003;164:163–171. doi: 10.1093/genetics/164.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. 2nd edn Springer; Berlin ; New York: 1997. [Google Scholar]
- Cash S, Chiba A, Keshishian H. Alternate neuromuscular target selection following the loss of single muscle fibers in Drosophila. J Neurosci. 1992;12:2051–2064. doi: 10.1523/JNEUROSCI.12-06-02051.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol. 1998;8:283–286. doi: 10.1016/s0960-9822(98)70110-1. [DOI] [PubMed] [Google Scholar]
- Chang TN, Keshishian H. Laser ablation of Drosophila embryonic motoneurons causes ectopic innervation of target muscle fibers. J Neurosci. 1996;16:5715–5726. doi: 10.1523/JNEUROSCI.16-18-05715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp S, Evers JF, Fiala A, Bate M. The development of motor coordination in Drosophila embryos. Development. 2008;135:3707–3717. doi: 10.1242/dev.026773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic analysis of the mechanisms controlling target selection: target-derived Fasciclin II regulates the pattern of synapse formation. Neuron. 1997;19:561–573. doi: 10.1016/s0896-6273(00)80372-4. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen SJ, Demas J, Wong RO. Mapping by waves. Patterned spontaneous activity regulates retinotopic map refinement. Neuron. 2003;40:1053–1055. doi: 10.1016/s0896-6273(03)00808-0. [DOI] [PubMed] [Google Scholar]
- Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B. Neurogenetic analysis of Drosophila mutations affecting sodium channels: synergistic effects on viability and nerve conduction in double mutants involving tip-E. J Neurogenet. 1986;3:19–31. doi: 10.3109/01677068609106892. [DOI] [PubMed] [Google Scholar]
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Spitzer NC. Regulation of growth cone behavior by calcium: new dynamics to earlier perspectives. J Neurobiol. 2000;44:174–183. [PubMed] [Google Scholar]
- Griffith LC. Drosophila melanogaster as a model system for the study of the function of calcium/calmodulin-dependent protein kinase II in synaptic plasticity. Invert Neurosci. 1997;3:93–102. doi: 10.1007/BF02480364. [DOI] [PubMed] [Google Scholar]
- Griffith LC, Verselis LM, Aitken KM, Kyriacou CP, Danho W, Greenspan RJ. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Milner LD, Landmesser LT. Spontaneous rhythmic activity in early chick spinal cord influences distinct motor axon pathfinding decisions. Brain Res Rev. 2008;57:77–85. doi: 10.1016/j.brainresrev.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Ganetzky B. Spatial and temporal expression patterns of two sodium channel genes in Drosophila. J Neurosci. 1994;14:5160–5169. doi: 10.1523/JNEUROSCI.14-09-05160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron. 2001;32:39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Jarecki J, Keshishian H. Role of neural activity during synaptogenesis in Drosophila. J Neurosci. 1995;15:8177–8190. doi: 10.1523/JNEUROSCI.15-12-08177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc Natl Acad Sci U S A. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Griffith LC, Murphey RK. Presynaptic calcium/calmodulin-dependent protein kinase II regulates habituation of a simple reflex in adult Drosophila. J Neurosci. 1998;18:8955–8964. doi: 10.1523/JNEUROSCI.18-21-08955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Halpern ME, Johansen KM, Keshishian H. Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci. 1989;9:710–725. doi: 10.1523/JNEUROSCI.09-02-00710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Collins SC, Ordway RW. Synaptic calcium-channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony. J Neurosci. 2002;22:5856–5864. doi: 10.1523/JNEUROSCI.22-14-05856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000;20:4885–4889. doi: 10.1523/JNEUROSCI.20-13-04885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Zou B, Xu X, Ordway RW. Active zone localization of presynaptic calcium channels encoded by the cacophony locus of Drosophila. J Neurosci. 2004;24:282–285. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H, Chiba A, Chang TN, Halfon MS, Harkins EW, Jarecki J, Wang L, Anderson M, Cash S, Halpern ME, et al. Cellular mechanisms governing synaptic development in Drosophila melanogaster. J Neurobiol. 1993;24:757–787. doi: 10.1002/neu.480240606. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Lin DM, Fetter RD, Kopczynski C, Grenningloh G, Goodman CS. Genetic analysis of Fasciclin II in Drosophila: defasciculation, refasciculation, and altered fasciculation. Neuron. 1994;13:1055–1069. doi: 10.1016/0896-6273(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Lnenicka GA, Keshishian H. Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J Neurobiol. 2000;43:186–197. [PubMed] [Google Scholar]
- Matthes DJ, Sink H, Kolodkin AL, Goodman CS. Semaphorin II can function as a selective inhibitor of specific synaptic arborizations. Cell. 1995;81:631–639. doi: 10.1016/0092-8674(95)90084-5. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Mooney R, Penn AA, Gallego R, Shatz CJ. Thalamic relay of spontaneous retinal activity prior to vision. Neuron. 1996;17:863–874. doi: 10.1016/s0896-6273(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Mosca TJ, Carrillo RA, White BH, Keshishian H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc Natl Acad Sci U S A. 2005;102:3477–3482. doi: 10.1073/pnas.0406164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, Merritt DJ, Brand AH, Whitington PM. In vivo dynamics of axon pathfinding in the Drosophilia CNS: a time-lapse study of an identified motorneuron. J Neurobiol. 1998;37:607–621. [PubMed] [Google Scholar]
- Nicol X, Voyatzis S, Muzerelle A, Narboux-Neme N, Sudhof TC, Miles R, Gaspar P. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10:340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;424:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Penn AA, Wong RO, Shatz CJ. Neuronal coupling in the developing mammalian retina. J Neurosci. 1994;14:3805–3815. doi: 10.1523/JNEUROSCI.14-06-03805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereanu W, Spindler S, Im E, Buu N, Hartenstein V. The emergence of patterned movement during late embryogenesis of Drosophila. Dev Neurobiol. 2007;67:1669–1685. doi: 10.1002/dneu.20538. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern PA. Neuromuscular transmission in new-born rats. J Physiol. 1970;209:701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. Presynaptic N-type calcium channels regulate synaptic growth. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Canada C, Budnik V. Introduction on the use of the Drosophila embryonic/larval neuromuscular junction as a model system to study synapse development and function, and a brief summary of pathfinding and target recognition. Int Rev Neurobiol. 2006;75:1–31. doi: 10.1016/S0074-7742(06)75001-2. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Schmidt JT, Tieman SB. Eye-specific segregation of optic afferents in mammals, fish, and frogs: the role of activity. Cell Mol Neurobiol. 1985;5:5–34. doi: 10.1007/BF00711083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Grzywacz NM. Influence of spontaneous activity and visual experience on developing retinal receptive fields. Curr Biol. 1996;6:1503–1508. doi: 10.1016/s0960-9822(96)00755-5. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Stent GS. A physiological mechanism for Hebb’s postulate of learning. Proc Natl Acad Sci U S A. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikis HG, Li W, Guan KL. The plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 2002;16:836–845. doi: 10.1101/gad.966402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wang J, Renger JJ, Griffith LC, Greenspan RJ, Wu CF. Concomitant alterations of physiological and developmental plasticity in Drosophila CaM kinase II-inhibited synapses. Neuron. 1994;13:1373–1384. doi: 10.1016/0896-6273(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Palmer G, Griffith LC. Regulation of Drosophila Ca2+/calmodulin-dependent protein kinase II by autophosphorylation analyzed by site-directed mutagenesis. J Neurochem. 1998;71:378–387. doi: 10.1046/j.1471-4159.1998.71010378.x. [DOI] [PubMed] [Google Scholar]
- White B, Osterwalder T, Keshishian H. Molecular genetic approaches to the targeted suppression of neuronal activity. Curr Biol. 2001a;11:R1041–1053. doi: 10.1016/s0960-9822(01)00621-2. [DOI] [PubMed] [Google Scholar]
- White BH, Osterwalder TP, Yoon KS, Joiner WJ, Whim MD, Kaczmarek LK, Keshishian H. Targeted attenuation of electrical activity in Drosophila using a genetically modified K(+) channel. Neuron. 2001b;31:699–711. doi: 10.1016/s0896-6273(01)00415-9. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. J Neurophysiol. 1963a;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963b;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Mitchell KJ, Goodman CS. Genetic analysis of the mechanisms controlling target selection: complementary and combinatorial functions of netrins, semaphorins, and IgCAMs. Cell. 1998;93:581–591. doi: 10.1016/s0092-8674(00)81187-3. [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Budnik V, Wu CF. Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci. 1992;12:644–651. doi: 10.1523/JNEUROSCI.12-02-00644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Neuronal activity and adenylyl cyclase in environment-dependent plasticity of axonal outgrowth in Drosophila. J Neurosci. 2004;24:1439–1445. doi: 10.1523/JNEUROSCI.0740-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]