SUMMARY
Peroxisome-proliferator activated receptor (PPAR) γ coactivator-1β (PGC-1β) is a transcriptional coactivator that induces hypertriglyceridemia in response to dietary fats through activating hepatic lipogenesis and lipoprotein secretion. The expression of PGC-1β is regulated by free fatty acids. Here we show that PGC-1β regulates plasma triglyceride metabolism through stimulating apolipoprotein C3 (APOC3) expression and elevating APOC3 levels in circulation. Remarkably, liver-specific knockdown of APOC3 significantly ameliorates PGC-1β-induced hypertriglyceridemia in mice. Hepatic expression of PGC-1β and APOC3 is reduced in response to acute and chronic treatments with nicotinic acid, a widely prescribed drug for lowering plasma triglycerides. Adenoviral-mediated knockdown of PGC-1β or APOC3 in the liver recapitulates the hypolipidemic effect of nicotinic acid. Proteomic analysis of hepatic PGC-1β transcriptional complex indicates that it stimulates APOC3 expression through coactivating orphan nuclear receptor ERRα and recruiting chromatin-remodeling cofactors. Together, these studies identify PGC-1β as an important regulator of the APOC3 gene cluster and reveal a mechanism through which nicotinic acid achieves its therapeutic effects.
INTRODUCTION
Elevated plasma triglyceride levels are a central component of dyslipidemia in metabolic syndrome and serve as an independent risk factor for cardiovascular disease (Brunzell, 2007; Cohen et al., 1998; Goldberg, 2001). Hypertriglyceridemia results from an imbalance of the production and catabolism of triglyceride-rich lipoprotein particles, particularly very low-density lipoprotein (VLDL). Genetic analyses of familial lipoprotein disorders as well as recent genome-wide association studies have revealed a number of candidate factors that contribute to the pathogenesis of hypertriglyceridemia (Breslow, 2000; Kathiresan et al., 2008; Saxena et al., 2007; Willer et al., 2008). Notably, single-nucleotide polymorphisms within the apolipoprotein (APO) gene cluster, which includes APOA1/C3/A4/A5 genes, have been repeatedly identified as risk alleles associated with hypertriglyceridemia in humans (Lai et al., 2005; van Dijk et al., 2004). Among these apolipoproteins, APOC3 and APOA5 have opposing effects on plasma triglyceride metabolism (van Dijk et al., 2004). Transgenic expression of APOC3 in mouse livers leads to pronounced hypertriglyceridemia, likely through inhibiting lipases that hydrolyze VLDL triglycerides, whereas deletion of APOC3 lowers plasma triglyceride levels in mice (Ito et al., 1990; Maeda et al., 1994). In contrast, APOA5 appears to have opposite effects on plasma triglyceride metabolism (Pennacchio et al., 2001). As such, balanced expression of apolipoprotein genes within this locus, particularly APOC3 and APOA5, is predicted to significantly influence plasma triglyceride homeostasis.
PGC-1β is a member of the PGC-1 family of transcriptional coactivators that regulates mitochondrial oxidative metabolism and diverse biological processes (Finck and Kelly, 2006; Handschin, 2009; Lin et al., 2005a). In the liver, PGC-1α regulates hepatic fasting response and coordinates key aspects of circadian metabolic rhythms (Lin et al., 2004; Liu et al., 2007; Yoon et al., 2001). We have previously shown that PGC-1β induces hyperlipidemia in response to dietary fats through enhancing hepatic lipogenesis and VLDL secretion (Lin et al., 2005b). The expression of PGC-1β is increased in the liver in response to short-term high-fat diet feeding in mice. Several factors have been demonstrated to interact with PGC-1β and stimulate hepatic lipogenesis and lipoprotein secretion, including sterol-response element binding protein (SREBP), liver-X receptor (LXR), and Foxa2 (Lin et al., 2005b; Wolfrum and Stoffel, 2006). Interestingly, systemic delivery of antisense oligonucleotide targeting PGC-1β leads to improved metabolic profile in the context of fructose-induced insulin resistance (Nagai et al., 2009). Given the prominent role of APOC3 and APOA5 in plasma triglyceride metabolism, we hypothesized that PGC-1β may regulate lipoprotein homeostasis through impinging on the APO gene cluster.
In the current study, we investigated the role of PGC-1β in apolipoprotein gene expression and determined the significance of APOC3 in mediating the hypertriglyceridemic effects of PGC-1β using in vivo RNAi knockdown. We also identified the PGC-1β/APOC3 pathway as a key hepatic target of nicotinic acid, a commonly prescribed triglyceride-lowering drug. Finally, we performed proteomic studies on PGC-1β transcriptional complexes isolated from mouse liver and identified transcriptional components that mediate the induction of APOC3 by PGC-1β.
RESULTS
PGC-1β Induces Hepatic APOC3 Expression and Elevates Its Plasma Levels
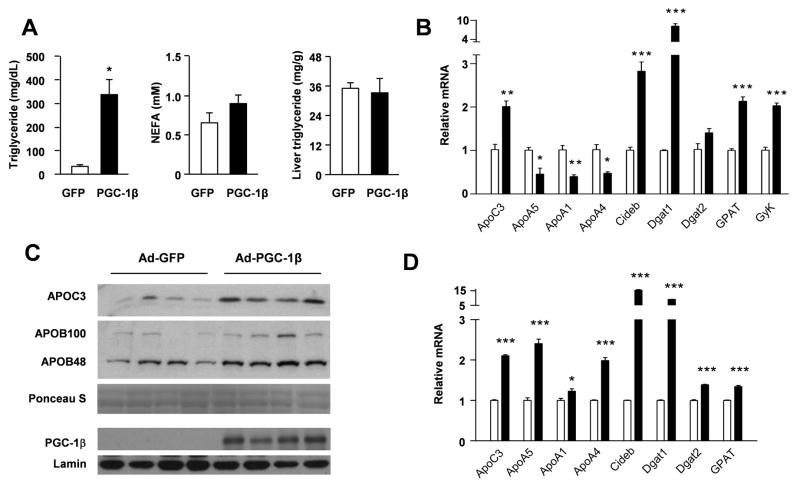
To determine whether PGC-1β regulates APO gene expression, we transduced C57/BL6 mice with recombinant adenoviral vectors expressing control (Ad-GFP) or PGC-1β (Ad-PGC-1β) via tail vein injection. Hepatic expression of PGC-1β significantly increases plasma triglyceride concentrations without affecting non-esterified fatty acid (NEFA) levels (Figure 1A). Unlike high-fat fed mice, liver triglyceride content is not affected by PGC-1β in chow-fed mice. Gene expression analysis indicates that PGC-1β increases APOC3 mRNA levels by approximately two-fold in the liver, while reducing APOA5 gene expression (Figure 1B). The expression of APOA1 and APOA4 is also decreased by PGC-1β. PGC-1β induces the expression of several genes involved in triglyceride synthesis, including diacylglycerol o-acyltransferase 1 (Dgat1), glycerol-3-phosphate acyltransferase (Gpat), glycerol kinase (GyK), as well as CideB, a factor recently found to regulates VLDL secretion (Ye et al., 2009). Consistently, immunoblotting analysis revealed that circulating APOC3 levels are significantly elevated in the plasma from mice transduced with Ad-PGC-1β (Figure 1C). In contrast, APOB protein levels (APOB48 and APOB100) remain largely unaltered.
Figure 1. Induction of APOC3 expression by PGC-1β in the liver and cultured primary hepatocytes.
(A) Plasma triglyceride and non-sterified fatty acids (NEFA) levels and liver triglyceride content in mice transduced with Ad-GFP (open) or Ad-PGC-1β (filled). * p<0.01.
(B) Realtime PCR analysis of total liver RNA from mice transduced with Ad-GFP or Ad-PGC-1β. Shown is fold-induction versus Ad-GFP group. Data represent mean ± s.e.m. (n=4). * p<0.05; **p<0.01; ***p<0.001.
(C) Immunoblot analysis of APOC3 and APOB proteins in serum samples and PGC-1β in liver nuclear extracts from mice transduced with Ad-GFP or Ad-PGC-1β (n=4). Ponceau S staining and Lamin immunoblot were shown as loading control for serum and nuclear extracts, respectively.
(D) Realtime PCR analysis of total RNA from primary hepatocytes transduced with Ad-GFP (open) or Ad-PGC-1β (filled). Shown is fold-induction versus Ad-GFP group. Data represent mean ± s.d. from a representative experiment. * p<0.05; *** p<0.001.
Similar to in vivo studies, PGC-1β stimulates APOC3 expression in transduced primary hepatocytes (Figure 1D). Compared to GFP control, mRNA levels of CideB, Dgat1, Dgat2, and GPAT are also elevated by PGC-1β. Unexpectedly, mRNA expression of APOA1, A4, and A5 is increased by PGC-1β in transduced hepatocytes, suggesting that certain physiological signals that impact on APO gene expression might be lacking in culture conditions. APOC3 is an inhibitor of lipoprotein lipases and suppresses triglyceride hydrolysis, whereas APOA5 appears to play an opposite role (Merkel et al., 2005; Wang et al., 1985). The induction of APOC3 by PGC-1β is consistent with its ability to elevate plasma triglyceride levels. Together, these results suggest that altered balance of apolipoproteins may contribute to PGC-1β-induced hypertriglyceridemia. In addition, this factor appears to differentially regulate the transcription of individual APO genes within the APOA1/C3/A4/A5 cluster.
Induction of APOC3 is Required for PGC-1β-induced Hypertriglyceridemia
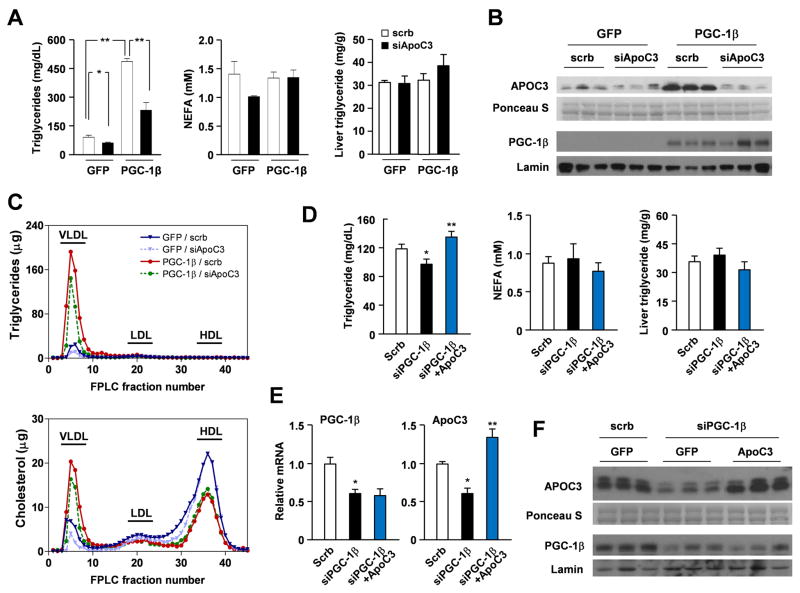
To determine the significance of APOC3 induction by PGC-1β in mediating hypertriglyceridemia, we used an RNAi knockdown approach to modulate APOC3 expression in the liver. We constructed a recombinant adenovirus that expresses a short hairpin RNA (shRNA) directed toward mouse APOC3 (siAPOC3). Compared to control, siAPOC3 adenovirus significantly reduces APOC3 mRNA levels in transduced primary hepatocytes (data not shown). We next transduced mice with Ad-GFP or Ad-PGC-1β adenoviruses in the presence of control or siAPOC3 adenoviruses. As expected, hepatic expression of PGC-1β increases total plasma triglycerides by approximately 4.7-fold. RNAi knockdown of APOC3 in the liver severely blunts PGC-1β-mediated hypertriglyceridemia by approximately 50% (Figure 2A). Gene expression analysis indicates that siAPOC3 reduces APOC3 mRNA expression and circulating APOC3 levels in transduced mice (Figure 2B and Supplemental Figure S1A). In contrast, the expression of APOA5 and other PGC-1β target genes, including CideB and Dgat1, is largely unaffected by siAPOC3 treatment. Lipoprotein profile analyses revealed that PGC-1β increases triglyceride concentrations almost exclusively in the VLDL fractions (Figure 2C). Normalization of plasma APOC3 levels with siAPOC3 significantly reduces VLDL triglyceride content. Interestingly, hepatic expression of PGC-1β also increases VLDL cholesterol, while reducing HDL cholesterol content. RNAi knockdown of APOC3 has modest effects on HDL cholesterol, whereas it lowers VLDL cholesterol content in both control and PGC-1β groups (Supplemental Figure S1B).
Figure 2. Requirement of APOC3 in PGC-1β-induced hypertriglyceridemia.
(A) Plasma triglyceride and NEFA concentrations and liver triglyceride content in mice transduced with Ad-GFP or Ad-PGC-1β in combination with control (open) or siAPOC3 (filled) adenoviruses, as indicated. Four mice were included for each treatment. * p<0.01; ** p<0.01. See also Figure S1.
(B) Immunoblots of serum samples and liver nuclear extracts from mice transduced with indicated adenoviruses.
(C) Lipoprotein profile analysis. Plasma was fractionated by FLPC and triglycerides and cholesterol content in each fraction was measured.
(D) Plasma triglyceride and NEFA concentrations and liver triglyceride content in mice transduced with scrb+GFP (open), siPGC-1β+GFP (filled), or siPGC-1β+APOC3 (blue) adenoviral mixtures, as indicated.
(E) qPCR analysis of liver gene expression in transduced mice. Data in D-E represent mean ± s.e.m. (n=5). * p<0.05 scrb vs. siPGC-1β; ** p<0.01 siPGC-1β vs. siPGC-1β+APOC3.
(F) Immunoblots of APOC3 in serum samples and PGC-1β liver nuclear extracts from transduced mice.
We next examined whether PGC-1β is required for APOC3 expression and triglyceride regulation. Consistent with previous report (Lin et al., 2005b), RNAi knockdown of PGC-1β reduces plasma triglyceride levels following two days of high-fat feeding in transduced mice (Figure 2D). Hepatic APOC3 mRNA expression and circulating APOC3 protein levels were also decreased by expression of PGC-1β shRNA in the liver (Figure 2E–F). To determine if decreased APOC3 is responsible for lowering plasma triglyceride levels when PGC-1β is inhibited, we performed “rescue” studies using an adenoviral vector expressing mouse APOC3. As shown in Figure 2D–F, re-expression of APOC3 to levels slightly above its endogenous levels completely blocks triglyceride-lowering effects of PGC-1β knockdown. We conclude from these studies that the regulation of APOC3 by PGC-1β plays an important role in mediating its effects on plasma triglyceride metabolism.
Acute and Chronic Treatments with Nicotinic Acid Suppress Hepatic PGC-1β and APOC3 Expression
Fibrates and nicotinic acid (niacin) are two most commonly prescribed drugs for the treatment of hypertriglyceridemia (Brunzell, 2007; Gille et al., 2008). Fibrates act through nuclear receptor PPARα and stimulate fatty acid β-oxidation in peripheral tissues (Lefebvre et al., 2006). The therapeutic target of nicotinic acid remains elusive until recently with the discovery of G-protein coupled receptor GPR109A (also known as PUMAG and HM74) as its target (Soga et al., 2003; Tunaru et al., 2003; Wise et al., 2003). Binding of nicotinic acid to GPR109A leads to inhibition of lipolysis in adipocytes and lowers free fatty acids released by the adipose tissue. While it has been proposed that decreased circulating fatty acids contribute to reduced hepatic VLDL secretion and plasma triglycerides (Gille et al., 2008; Karpe and Frayn, 2004; Wang et al., 2001), the regulatory pathways that nicotinic acid engages to alter plasma lipid homeostasis are poorly understood. We have previously demonstrated that the expression of PGC-1β is responsive to fatty acids (Lin et al., 2005b). As such, we hypothesized that this coactivator may serve as a target for nicotinic acid action and mediate its therapeutic effects through modulating lipoprotein metabolism.
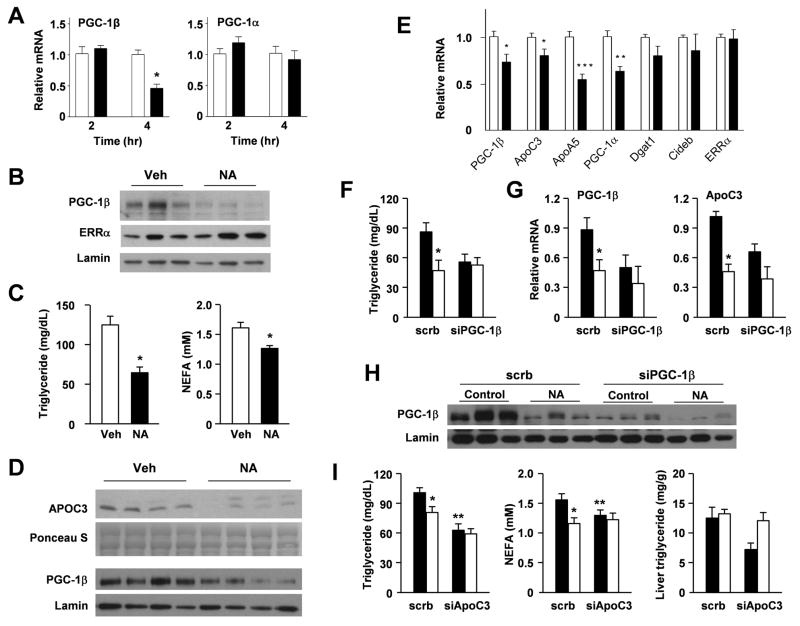
We next examined whether the expression of PGC-1β is regulated by acute and chronic nicotinic acid treatments. As expected, intraperitoneal injection of nicotinic acid caused a rapid decrease of circulating NEFA levels (Supplemental Figure S2A). While we observed a trend for plasma triglyceride to be lower immediately following a single dose of nicotinic acid administration, the data did not reach statistical significance. Gene expression analysis indicates that PGC-1α mRNA levels remain largely unchanged throughout this study. In contrast, PGC-1β mRNA as well as protein levels are significantly reduced 4 hrs following this treatment (Figure 3A–B). We did not observe a decrease of PGC-1β mRNA expression at earlier time points, likely reflecting the lag for fatty acids to exert their effects on PGC-1β gene expression in the liver. Consistent with a non-cell autonomous mechanism, nicotinic acid does not alter PGC-1β mRNA expression in cultured primary hepatocytes (Supplemental Figure S2B). To further evaluate the chronic effects of nicotinic acid, we fed C57/Bl6 mice with a high-fat diet with or without 1% nicotinic acid, as previously described (Hernandez et al., 2007). Chronic nicotinic acid treatment lowers plasma triglyceride and free fatty acid concentrations by 46% and 17%, respectively (Figure 3C). Compared to control diet, the expression of PGC-1β and APOC3 is significantly lower in the nicotinic acid-treated group (Figure 3D–E). Accordingly, circulating APOC3 protein levels are reduced in response to nicotinic acid. APOA5 and PGC-1α mRNA levels are also reduced in response to chronic niacin treatments.
Figure 3. Role of PGC-1β and APOC3 in mediating triglyceride-lowering effects of nicotinic acid.
(A) Realtime PCR analysis of total liver RNA from mice treated with vehicle (open, saline) or nicotinic acid (filled, 100 mg/kg, i.p.) for 2 or 4 hrs. * p<0.01.
(B) Immunoblotting analysis of liver nuclear extracts from mice treated with vehicle (Veh) or nicotinic acid (NA) for 4 hrs.
(C) Plasma triglyceride and NEFA concentrations in mice fed a high-fat diet containing no (open) or 1% (filled) nicotinic acid for three months (n=5). ** p<0.01.
(D) Immunoblotting analysis of APOC3 and PGC-1β in serum samples and liver nuclear extracts, respectively.
(E) Realtime PCR analysis of liver gene expression in mice in (C). * p<0.05; **p<0.01; ***p<0.001.
(F) Plasma triglyceride concentrations. C57/Bl6J male mice were fed control (filled) or chow containing 1% nicotinic acid (open) for two weeks and transduced with scrb or siPGC-1β adenoviruses for five days, as indicated.
(G) Realtime PCR analysis of liver gene expression. Data in F–G represent mean ± s.e.m. (n=4–5). * p<0.05 Veh vs. NA.
(H) Immunoblots of PGC-1β and Lamin in liver nuclear extracts from mice in (F). Note that PGC-1β is reduced in response to NA treatment and PGC-1β shRNA.
(I) Plasma triglyceride and NEFA concentrations and liver triglyceride content. C57/Bl6J male mice were fed control (filled) or chow containing 1% nicotinic acid (open) for two weeks and transduced with scrb or siAPOC3 adenoviruses for five days. Data represent mean ± s.e.m. (n=7–8). * p<0.05 Veh vs. NA; **p<0.01 scrb vs. siAPOC3. See also Figure S2.
Inhibition of Hepatic PGC-1β and APOC3 Mediates Triglyceride-lowering by Nicotinic Acid
To determine the significance of the PGC-1β/APOC3 pathway in mediating triglyceride-lowering effects of nicotinic acid, we transduced mice fed control or 1% nicotinic acid-containing chow with control or PGC-1β RNAi adenoviruses. While both nicotinic acid feeding and PGC-1β knockdown reduce plasma triglyceride levels, the combination of these two treatments does not lead to a further decrease in plasma triglycerides (Figure 3F). Liver triglyceride content is not affected by these treatments (data not shown). Realtime PCR analysis indicates that hepatic expression of PGC-1β and APOC3 is reduced by nicotinic acid and PGC-1β shRNA (Figure 3G–H). Similarly, adenoviral-mediated knockdown of APOC3 reduces plasma triglyceride levels by approximately 38% (Figure 3I). This hypotriglyceridemic effect is not augmented in mice fed nicotinic acid-containing chow, suggesting that downregulation of APOC3 significantly contributes to the triglyceride-lowering activity of nicotinic acid. Gene expression analyses revealed that APOC3 mRNA level is reduced following nicotinic acid and APOC3 shRNA treatments (Supplemental Figure S2C). Interestingly, PGC-1β expression appears to be slightly elevated when APOC3 is knocked down in the liver. We next examined whether adenoviral-mediated expression of PGC-1β can block the effects of nicotinic acid, we transduced control and nicotinic acid fed mice with GFP or PGC-1β adenoviruses. Nicotinic acid feeding results in 32% decrease in plasma triglyceride levels in mice transduced with Ad-GFP. In contrast, adenoviral-mediated expression of PGC-1β in the liver blocks the ability of nicotinic acid to lower plasma triglycerides (Supplemental Figure S2D). Together, these results suggest that hepatic PGC-1β/APOC3 pathway is a major contributor to the therapeutic action of nicotinic acid.
Analyses of Hepatic PGC-1β Transcriptional Complex in the Regulation of APOC3 Expression
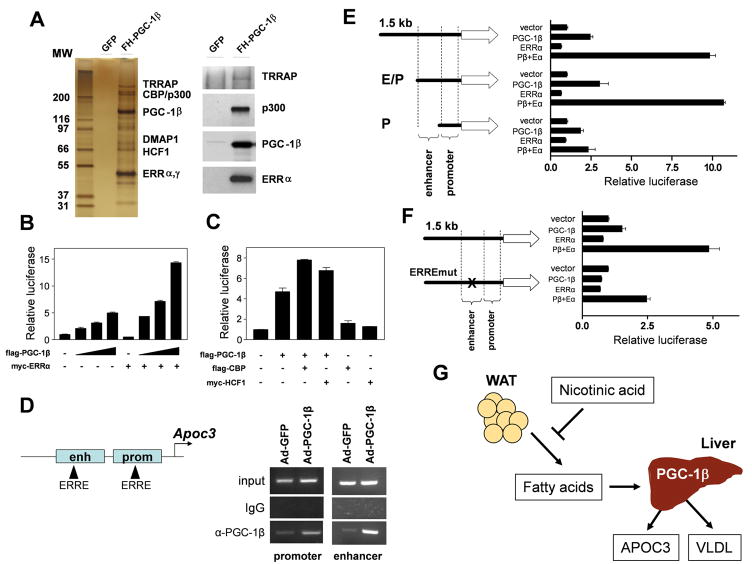
PGC-1β functions through interacting with transcription factors as well as chromatin-remodeling cofactors, including LXR, SREBP, PPARα, and Foxa2, in the regulation of hepatic metabolism (Lin et al., 2003; Lin et al., 2005b; Wolfrum and Stoffel, 2006). To date, the components of the PGC-1β transcriptional complex in the liver remain to be elucidated. To identify novel factors associated with PGC-1β, we used a recombinant adenoviral vector to direct the expression of Flag- and HA-tagged PGC-1β in mouse livers. We purified the PGC-1β transcriptional complex from liver nuclear extracts with anti-Flag and anti-HA affinity matrix and identified individual proteins using mass spectrometry. PGC-1β is present in a nearly stoichiometric manner with ERRα, an orphan nuclear receptor that has been shown to physically interact with PGC-1β (Kamei et al., 2003) (Figure 4A). In addition, we identified several chromatin-remodeling factors in this complex, including host cell factor 1 (HCF1), CBP/p300 histone acetyltransferase as well as TRRAP, a component of the mediator complex. The presence of TRRAP, p300, and ERRα in the PGC-1β transcriptional complex was further confirmed in immunoblotting analyses using specific antibodies against these factors (Figure 4A).
Figure 4. Regulation of APOC3 gene expression by the PGC-1β transcriptional complex.
(A) Identification of proteins in the PGC-1β transcriptional complex. Affinity-purified complexes from livers of mice transduced with Ad-GFP or Ad-PGC-1β were separated by SDS-PAGE. Individual bands were excised and proteins identified by mass spectrometry (left panel). Immunoblotting analysis of the PGC-1β complex using indicated antibodies (right panel).
(B) Coactivation assays of mouse APOC3 promoter with different amounts of PGC-1β plasmid in the presence or absence of ERRα in transiently transfected McArdle RH7777 hepatoma cells.
(C) Coactivation analysis of the mouse APOC3 gene promoter by PGC-1β, HCF1, and CBP. For B–C, shown are representative experiments from at least three independent experiments. Data represent mean ± s.d.
(D) ChIP analysis. Chromatin extracts from transduced livers were immunoprecipitated with anti-PGC-1β antibody or IgG. The precipitated genomic DNA were PCR-amplified using primers flanking ERRE located in the enhancer and proximal promoter regions.
(E) Coactivation assays of truncated APOC3 promoter reporters. RH7777 cells were transiently transfected with reporter constructs spanning 1.5 kb, 750 bp (enhancer and promoter regions, E/P) or 350 bp (proximal promoter only, P) in combination with ERRα in the presence or absence of PGC-1β.
(F) Coactivation assays of APOC3 reporter constructs with mutant ERRE. For E–F, shown are representative experiments from at least three independent experiments. Data represent mean ± s.d.
(G) Model illustrating the PGC-1β/APOC3 pathway in mediating the hypolipidemic effects of nicotinic acid.
To investigate the role of PGC-1β and its associated proteins in APOC3 gene transcription, we constructed a luciferase reporter plasmid that contains 1.5-kb upstream of the transcriptional start site of mouse APOC3 gene. As shown in Figure 4B, PGC-1β stimulates APOC3 promoter activity in transiently transfected hepatoma cells in a dose-dependent manner. While ERRα alone modestly affect reporter gene activity, it robustly potentiates the ability of PGC-1β to transactivate the APOC3 promoter. Similarly, CBP and HCF1 also augment the stimulatory effects of PGC-1β on this reporter (Figure 4C). Previous studies have demonstrated that APOC3 promoter is regulated by several transcription factors, including HNF4α, RORα, RXR, and FoxO1 (Altomonte et al., 2004; Mishiro et al., 2009; Raspe et al., 2001; Vu-Dac et al., 1998; Zannis et al., 2001). Our results suggest that PGC-1β may coactivate ERRα on the APOC3 promoter and recruit additional chromatin-remodeling factors to stimulate its transcription. Previous work has identified a transcriptional enhancer located upstream of the APOC3 gene (Bisaha et al., 1995). We found that two putative ERRα binding sites (ERRE) are located within the enhancer and the proximal promoter regions (Figure 4D). The ERRE in the enhancer was previously shown to regulate APOA4 gene expression (Carrier et al., 2004). Chromatin-immunoprecipitation (ChIP) assays indicates that PGC-1β is recruited to the proximity of these ERRα binding sites. Coactivation studies using truncated mutant promoters indicate that the enhancer sequence is required for the synergistic activation of APOC3 reporter activity by ERRα and PGC-1β (Figure 4E). Consistently, mutation of ERRE in the enhancer region severely diminished the ability of PGC-1β to coactivate ERRα on the APOC3 promoter (Figure 4F). These results identified ERRα and chromatin-remodeling cofactors as components of the PGC-1β transcriptional complex that regulates APOC3 gene transcription.
DISCUSSION
We identified the PGC-1β/APOC3 pathway as a hepatic target of nicotinic acid that mediates its triglyceride-lowering effects (Figure 4G). Nicotinic acid signals through its receptor on adipocytes, leading to inhibition of lipolysis and lower circulating NEFA levels. The latter decreases the expression of PGC-1β and its target gene APOC3 in the liver and reduces plasma APOC3 concentrations. Importantly, adenoviral-mediated knockdown of PGC-1β or APOC3 does not further reduce plasma triglyceride concentrations beyond nicotinic acid treatments. These findings suggest that the suppression of PGC-1β and APOC3 in the liver is responsible for a significant portion of the therapeutic activity of nicotinic acid.
Genetic polymorphisms within the APOA1/C3/A4/A5 gene cluster confers significant risk for hypertriglyceridemia in humans. Our studies indicate that PGC-1β is a key component of the regulatory network that governs apolipoprotein gene expression within this locus. First, PGC-1β differentially regulates the expression of APO genes in the liver. While PGC-1β reduces mRNA levels of APOA5, APOA1, and APOA4 in transduced mouse liver, it stimulates APOC3 expression and significantly elevates plasma APOC3 levels. The dichotomous effects of PGC-1β on APOC3 and APOA5 expression are consistent with its ability to significantly raise plasma triglyceride levels. At the functional level, RNAi knockdown of APOC3 expression in the liver blunts the hypertriglyceridemic effect of PGC-1β, whereas re-expression of APOC3 blocks lipid-lowering effects of PGC-1β shRNA. We previously demonstrated that PGC-1β mediates hyperlipidemic effects of dietary fats through induction of hepatic lipogenesis and VLDL secretion (Lin et al., 2005b). Our current study indicates that PGC-1β also modulates catabolism of VLDL triglycerides via its regulation of APOC3. In addition, this regulatory pathway appears to be functional under both chow and high-fat dietary conditions. The exact molecular mechanisms that mediate the suppression of APOA5 by PGC-1β remain unknown. It is possible PGC-1β alters local chromatin structure in a manner that favors transcriptional activation of the APOC3 gene. Alternatively, PGC-1β may function as a transcriptional repressor and directly inhibits APOA5 transcription. Given the strong association of genetic polymorphisms within the APOA1/C3/A4/A5 locus and plasma triglyceride concentrations in humans, it is possible that transcriptional regulation by PGC-1β may underlie the influence of certain genetic polymorphisms on hypertriglyceridemia. Future work should address whether PGC-1β differentially regulates APO gene expression on distinct polymorphic alleles.
Recent studies have identified GPR109A, a Gi-coupled receptor, as the molecular target that mediates the therapeutic function as well as certain side effects of nicotinic acids (Benyo et al., 2005; Tunaru et al., 2003). GPR109A is highly expressed in the adipose tissue and it negatively modulates lipolysis. Since fatty acids provide key substrates for triglyceride synthesis in the liver, a logical model for the hypotriglyceridemic activity of nicotinic acid is that reduced flux of fatty acids leads to decreased VLDL assembly and secretion (Gille et al., 2008; Karpe and Frayn, 2004; Wang et al., 2001). Other targets that mediate the therapeutic action of nicotinic acid have not been identified. We found that acute and chronic nicotinic acid treatments suppress hepatic PGC-1β and APOC3 gene expression. These observations strongly suggest that nicotinic acid may impinge on a broader program of lipid metabolism to achieve its therapeutic benefits. In this case, inhibition of hepatic PGC-1β and APOC3 is expected to lower plasma triglycerides through reducing the production while enhancing the catabolism of triglyceride-rich lipoproteins. Since nicotinic acid does not appear to directly regulate PGC-1β in cultured primary hepatocytes, the inhibition of its expression is likely secondary to the suppression of adipose lipolysis and reduced NEFA levels.
Proteomic analysis of the PGC-1β transcriptional complex in the liver reveals surprising diversity of chromatin-remodeling factors that stably associate with this coactivator. Similar to PGC-1α, PGC-1β physically interacts with the CBP/p300 family of histone acetyltransferases as well as TRRAP, a component of the mediator complex (Puigserver et al., 1999; Wallberg et al., 2003). In addition, PGC-1β associates with HCF1, a scaffold protein that was previously found to interact with PGC-1 coactivators and associate with histone methyltransferases (Lin et al., 2002; Wysocka et al., 2003). As such, it is likely that the recruitment of HCF1 to PGC-1β target genes leads to specific changes in histone methylation patterns on their promoters. Notably, we found that ERRα is the only DNA-binding transcription factor that is abundantly and stably present in the PGC-1β protein complex. This finding is consistent with the uniquely high affinity between the PGC-1 coactivators and the ERR family of nuclear receptors (Huss et al., 2002; Kamei et al., 2003; Schreiber et al., 2003). At the molecular level, PGC-1β coactivates ERRα to stimulate APOC3 promoter activity, primarily through an ERRα binding site located within the APOC3 enhancer. Because PGC-1β differentially regulates genes within the APOA1/C3/A4/A5 locus, it is possible that this coactivator participates in the formation of high-order regulatory complexes that exert both positive and negative regulation on individual apolipoprotein genes.
EXPERIMENTAL PROCEDURES
In vivo adenoviral transduction and metabolic analyses
C57/Bl6J male mice were transduced with purified adenoviruses through tail vein injection (0.15 OD per mouse), as previously described (Li et al., 2008). All adenoviruses were titered in mice and monitored for the expression of GFP and adenoviral gene AdE4 to ensure similar doses were administered in metabolic studies. For nicotinic acid feeding, rodent chow (Harlan Teklad, #7012) was mixed with the compound at 1% (w/w), as previously described (Hernandez et al., 2007). Plasma triglyceride and non-esterified fatty acid concentrations were measured 5–7 days following transduction using commercial assay kits (Sigma, Wako Diagnostics). Hepatic gene expression was analyzed by qPCR using specific primers (Table S1). For lipoprotein profile analysis, pooled plasma samples from two transduced mice were fractionated by fast protein liquid chromatography. The concentrations of triglyceride and cholesterol in each fraction were analyzed.
Affinity purification of PGC-1β transcriptional complex
Liver nuclear extract was prepared from ten mice transduced with Ad-GFP or Ad-Flag/HA-PGC-1β adenoviruses. Sequential steps of immunoprecipitation were performed using anti-Flag (Sigma) and anti-HA (Roche) affinity matrix followed by eluting with 50 μg/ml Flag and HA peptides, respectively. Eluted protein complex was analyzed by SDS-PAGE. Following colloidal blue staining, individual bands were excised for protein identification by mass spectrometry. Immunoblotting was performed using anti-p300 (sc-584, Santa Cruz Biotechnology), anti-ERRα (ab16363, Abcam), and anti-TRRAP (sc-11411), anti-APOC3 (sc-50378), anti-APOB48/APOB100 (K23300R, Biodesign), anti-Lamin (Cell Signaling, #2032), and anti-PGC-1β antibodies.
ChIP assay
Chromatin immunoprecipitation was performed essentially as described (Li et al., 2008). For chromatin lysates from mouse livers, liver nuclei were isolated and then cross-linked in 1% formaldehyde for 10 minutes followed by sonication. After pre-cleared with Protein-G agarose beads, chromatin lysates were immunoprecipitated using antibodies against PGC-1β or control rabbit IgG in the presence of BSA and salmon sperm DNA. Beads were extensively washed before reverse cross-linking. DNA was purified using a purification kit (Qiagen) and subsequently analyzed by PCR using primers flanking the enhancer or the proximal promoter regions (Table S1).
Reporter gene assays
Rat McArdle RH7777 hepatoma cells were transiently transfected with indicated plasmids using Lipofectamine. Equal amounts of DNA were used for all transfection combinations by adding appropriate vector DNA. Relative luciferase activities were determined 48 hours following transfection. All transfection experiments were repeated at least three times in duplicates. Data represent mean ± s.e.m.
Supplementary Material
Acknowledgments
We are grateful to S. Gu, A.A. Baker, and L. Yu for technical assistance and members of the laboratory for discussions. We thank Drs. D.P. Kelly and T. Leone for ERRα adenovirus, the Michigan Diabetes Research and Training Center for core support, and the University of Cincinnati Mouse Metabolic Phenotyping Center for performing lipoprotein analysis. This work was supported by National Institutes of Health (DK077086 and HL097738, J.D.L.) and Career Development Award from the American Diabetes Association (J.D.L.). S.L. is supported by Scientist Development Grant from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest. 2004;114:1493–1503. doi: 10.1172/JCI19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, Moers A, Pfeffer K, Offermanns S. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115:3634–3640. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaha JG, Simon TC, Gordon JI, Breslow JL. Characterization of an enhancer element in the human apolipoprotein C-III gene that regulates human apolipoprotein A-I gene expression in the intestinal epithelium. J Biol Chem. 1995;270:19979–19988. doi: 10.1074/jbc.270.34.19979. [DOI] [PubMed] [Google Scholar]
- Breslow JL. Genetics of lipoprotein abnormalities associated with coronary artery disease susceptibility. Annu Rev Genet. 2000;34:233–254. doi: 10.1146/annurev.genet.34.1.233. [DOI] [PubMed] [Google Scholar]
- Brunzell JD. Clinical practice. Hypertriglyceridemia. N Engl J Med. 2007;357:1009–1017. doi: 10.1056/NEJMcp070061. [DOI] [PubMed] [Google Scholar]
- Carrier JC, Deblois G, Champigny C, Levy E, Giguere V. Estrogen-related receptor alpha (ERRalpha) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. J Biol Chem. 2004;279:52052–52058. doi: 10.1074/jbc.M410337200. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Raz I, Merin G, Mozes B. Comparison of factors associated with 30-day mortality after coronary artery bypass grafting in patients with versus without diabetes mellitus. Israeli Coronary Artery Bypass (ISCAB) Study Consortium. Am J Cardiol. 1998;81:7–11. doi: 10.1016/s0002-9149(97)00797-2. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- Goldberg IJ. Clinical review 124: Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab. 2001;86:965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- Handschin C. The biology of PGC-1alpha and its therapeutic potential. Trends Pharmacol Sci. 2009;30:322–329. doi: 10.1016/j.tips.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Wright SD, Cai TQ. Critical role of cholesterol ester transfer protein in nicotinic acid-mediated HDL elevation in mice. Biochem Biophys Res Commun. 2007;355:1075–1080. doi: 10.1016/j.bbrc.2007.02.079. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- Ito Y, Azrolan N, O'Connell A, Walsh A, Breslow JL. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990;249:790–793. doi: 10.1126/science.2167514. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci U S A. 2003;100:12378–12383. doi: 10.1073/pnas.2135217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Frayn KN. The nicotinic acid receptor--a new mechanism for an old drug. Lancet. 2004;363:1892–1894. doi: 10.1016/S0140-6736(04)16359-9. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CQ, Parnell LD, Ordovas JM. The APOA1/C3/A4/A5 gene cluster, lipid metabolism and cardiovascular disease risk. Curr Opin Lipidol. 2005;16:153–166. doi: 10.1097/01.mol.0000162320.54795.68. [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu C, Li N, Hao T, Han T, Hill DE, Vidal M, Lin JD. Genome-wide coactivation analysis of PGC-1alpha identifies BAF60a as a regulator of hepatic lipid metabolism. Cell Metab. 2008;8:105–117. doi: 10.1016/j.cmet.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005a;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem. 2002;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- Lin J, Tarr PT, Yang R, Rhee J, Puigserver P, Newgard CB, Spiegelman BM. PGC-1beta in the regulation of hepatic glucose and energy metabolism. J Biol Chem. 2003;278:30843–30848. doi: 10.1074/jbc.M303643200. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005b;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Maeda N, Li H, Lee D, Oliver P, Quarfordt SH, Osada J. Targeted disruption of the apolipoprotein C-III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J Biol Chem. 1994;269:23610–23616. [PubMed] [Google Scholar]
- Merkel M, Loeffler B, Kluger M, Fabig N, Geppert G, Pennacchio LA, Laatsch A, Heeren J. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem. 2005;280:21553–21560. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- Mishiro T, Ishihara K, Hino S, Tsutsumi S, Aburatani H, Shirahige K, Kinoshita Y, Nakao M. Architectural roles of multiple chromatin insulators at the human apolipoprotein gene cluster. Embo J. 2009;28:1234–1245. doi: 10.1038/emboj.2009.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Loffler MG, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O'Malley B, Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- Raspe E, Duez H, Gervois P, Fievet C, Fruchart JC, Besnard S, Mariani J, Tedgui A, Staels B. Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORalpha. J Biol Chem. 2001;276:2865–2871. doi: 10.1074/jbc.M004982200. [DOI] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha) J Biol Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun. 2003;303:364–369. doi: 10.1016/s0006-291x(03)00342-5. [DOI] [PubMed] [Google Scholar]
- Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- van Dijk KW, Rensen PC, Voshol PJ, Havekes LM. The role and mode of action of apolipoproteins CIII and AV: synergistic actors in triglyceride metabolism? Curr Opin Lipidol. 2004;15:239–246. doi: 10.1097/00041433-200406000-00002. [DOI] [PubMed] [Google Scholar]
- Vu-Dac N, Gervois P, Torra IP, Fruchart JC, Kosykh V, Kooistra T, Princen HM, Dallongeville J, Staels B. Retinoids increase human apo C-III expression at the transcriptional level via the retinoid X receptor. Contribution to the hypertriglyceridemic action of retinoids. J Clin Invest. 1998;102:625–632. doi: 10.1172/JCI1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg AE, Yamamura S, Malik S, Spiegelman BM, Roeder RG. Coordination of p300-mediated chromatin remodeling and TRAP/mediator function through coactivator PGC-1alpha. Mol Cell. 2003;12:1137–1149. doi: 10.1016/s1097-2765(03)00391-5. [DOI] [PubMed] [Google Scholar]
- Wang CS, McConathy WJ, Kloer HU, Alaupovic P. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J Clin Invest. 1985;75:384–390. doi: 10.1172/JCI111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Basinger A, Neese RA, Shane B, Myong SA, Christiansen M, Hellerstein MK. Effect of nicotinic acid administration on hepatic very low density lipoprotein-triglyceride production. Am J Physiol Endocrinol Metab. 2001;280:E540–547. doi: 10.1152/ajpendo.2001.280.3.E540. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–9874. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006;3:99–110. doi: 10.1016/j.cmet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;17:896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Li JZ, Liu Y, Li X, Yang T, Ma X, Li Q, Yao Z, Li P. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 2009;9:177–190. doi: 10.1016/j.cmet.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zannis VI, Kan HY, Kritis A, Zanni EE, Kardassis D. Transcriptional regulatory mechanisms of the human apolipoprotein genes in vitro and in vivo. Curr Opin Lipidol. 2001;12:181–207. doi: 10.1097/00041433-200104000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.