Abstract
The purpose of this article was to determine mast cell and neuropeptide nerve fiber numbers in joint capsules in posttraumatic contractures, as elevated numbers have been implicated in other fibrotic and contracture conditions. Twelve skeletally mature rabbits had intraarticular cortical windows removed from the medial and lateral femoral condyles and the knee joint immobilized. The contralateral unoperated limb served as a control. Equal numbers of rabbits were sacrificed 4 weeks after surgery or 40 weeks after the first surgery that included 32 weeks of remobilization. Six patients with chronic posttraumatic elbow joint contractures and six age-matched organ donor controls free of elbow contractures were also studied. Joint capsule myofibroblast, mast cell, and neuropeptide containing nerve fiber numbers were assessed with immunohistochemistry. The numbers of myofibroblasts, mast cells, and neuropeptide containing nerve fibers expressed as a percentage of total cells were significantly greater in the contracture capsules when compared to the control capsules at all time points (p < 0.0001). The range of percentages for the three components in the contracture capsules versus the controls were 41–48% versus 9–10%, 44–50% versus 11–13%, and 45–50% versus 10–12% for the acute and chronic stages of the rabbit model and the chronic stages in the human elbows, respectively. These data support the hypothesis that a myofibroblast–mast cell–neuropeptide fibrosis axis may underlie some of the pathologic changes in the joint capsule in posttraumatic contractures. Approaches designed to manipulate this axis, such as preventing degranulation of mast cells, warrant further investigation.
Keywords: contracture, joint capsule, myofibroblasts, mast cells, neuropeptides
Loss of joint motion, or joint contracture, is a common complication following injuries.1 The joint capsule is a key contributor to the formation of contractures and surgical releases to improve joint motion invariably include its removal.2 Although the anatomic importance of the joint capsule in the pathogenesis of joint contractures has been known for many years, only recently has there been description of the cellular changes associated with posttraumatic contractures.3–5 It was determined that the myofibroblast, a specialized fibro-blast expressing the contractile smooth muscle protein α-smooth muscle actin (α-SMA) and associated with contracture and fibrosis in many organ systems, is increased in numbers in joint capsules from posttraumatic contractures when compared to similar tissues from normal joints.4–6 This is true for the chronic stages of posttraumatic contractures in human elbow joints and a rabbit knee model of posttraumatic contractures.3–5 Myofibroblast numbers are increased very early in the contracture process, as early as 4 weeks after injury, in this rabbit knee model.7 The question remains as to what is contributing to this early and sustained increase in myofibroblast numbers.
One possibility contributing to the early and sustained increase in myofibroblast numbers is a mast cell–neuropeptide fibrosis axis as described in skin wound healing.8 Mast cells are widely distributed throughout connective tissues, including skin and joint capsules.8–14 Mast cell granules contain preformed mediators associated with fibrosis including platelet-derived growth factor-A, basic fibroblast growth factor and endothelin-1, while the profibrotic TGF-β1 is formed upon mast cell stimulation.9,12 It is generally believed that mast cells induce a fibrotic response via involvement of connective tissue fibroblasts and myofibroblasts.9,12,15,16 It has been well described that the neuropeptides Substance P and CGRP can cause mast cell degranulation.8,11,13,14,17,18 Peptidergic nerve terminalshavebeen detectedin close association with mast cells in skin, ligament, joint capsule, and the gut.10,11,13,14,19 It was recently reported that both the density of Substance P positive fibers and the number of mast cells were increased in the pathologic palmar fascia of patients with Dupuytren’s contracture when compared to nonpathologic palmar fascia of control individuals.19 Thus, the myofibroblast–mast cell–neuropeptide pathway may represent what is abnormal (myofibroblasts), how this abnormality comes about (mast cell degranulation liberating growth factors), and why this may occur (neuropeptide induced degranulation of mast cells).
As a first step to evaluate the potential involvement of this pathway in joint contracture development, the hypothesis that joint capsule mast cell and neuropeptide containing nerve fiber numbers are increased in posttraumatic contractures is tested. The objective is to evaluate this hypothesis in the acute and chronic stages of the rabbit knee model of posttraumatic contractures, as well as the chronic stages of human posttraumatic elbow contractures.
MATERIALS AND METHODS
Institutional Animal Care Committee approval was obtained from the University of Calgary Animal Care Committee (Registration number M01042). Twelve skeletally mature New Zealand White female rabbits (5.6 ± 0.7 kg) were obtained (Reimans Furrier, St. Agatha, ON). All rabbits had the following surgical procedure under general anesthesia as previously described.5 Cortical windows (5 mm2) were removed from the nonarticular cartilage portion of the medial and lateral femoral condyles preserving the collateral ligament insertions. Bleeding from the condyles resulted in a stable intraarticular fracture. The knee joint was then immobilized with a 1.6 mm-diameter Kirschner wire (K-wire; Zimmer, Mississauga, ON) drilled through the tibia, passed posterior (extraarticular) to the knee joint, and bent around the femur with the knee at 150° of flexion. Six rabbits were sacrificed with an overdose of Euthanyl (MTC, Cambridge, ON) at 4 weeks after the procedure. Six rabbits had a second surgical procedure to remove the K-wire 8 weeks after the original procedure allowing remobilization of the knee for 32 weeks before being sacrificed. The rabbits were allowed free cage activity (0.1 m3) following all operations. The left knees were never surgically manipulated and served as controls.
The posterior joint capsule was isolated immediately after sacrifice by dissecting the gastrocnemius muscle from distal to proximal to the fibellae in the heads of the gastrocnemius. The medial and lateral collateral ligaments and the tibial insertion of the joint capsule were identified. The posterior loose connective tissue and popliteal neurovascular structures were dissected away from the capsule. The capsule was then removed going from the fibellae proximally, the tibia distally, and collateral ligaments medially and laterally, and placed in Optimal Cutting Temperature (OCT) embedding material (Sakura Finetek, Torrance, CA) for subsequent immunohistochemical examination. Tissues were stored at −80°C.
Institutional Conjoint Health Research Ethics Board approval from the University of Calgary was obtained (Registration number 15104). Human anterior capsules of elbows were obtained from six patients (five males, one female). The average age was 29 ± 12 years [mean ± standard deviation (SD); range 14–45 years] at the time of contracture release, and the release was performed at an average of 16 ± 6 months (range, 9–23 months) after injury. The average preoperative range of motion (ROM) in the flexion-extension arc was 48 ± 16° (range, 30–75°). The original injuries were intraarticular fractures; three patients with distal humerus fractures (one of which was an open fracture), one patient with a radial head fracture, one patient with a comminuted proximal ulna fracture, and one patient with a radial head fracture associated with an elbow dislocation. Control anterior capsules were obtained from six elbows (four males, two females) of postmortem organ donors (average age, 26 ± 15 years; range 15–52 years) free of contractures. All capsules were placed in OCT for subsequent immunohistochemical examination. The tissues were stored at −80°C.
A triple labeling immunohistochemical methodology was used for the myofibroblast, mast cell, and neuropeptides numbers in the joint capsules. Briefly, sections (8 μm thickness) of the frozen samples were cut, mounted on precoated glass slides, and pretreated with hyaluronidase. Ten percent normal goat serum in PBS was applied as a blocking agent. The monoclonal α-SMA antibody (clone asm-1, Röche Molecular Biochemicals, Laval, QC) was applied, incubated for 60 min at 37°C, and washed. The secondary antibody, a sheep antimouse IgG horseradish peroxidase (HRPO) conjugate (Röche), was applied to the sections (dilution 1:50) for 60 min at room temperature. The sections were washed, followed by application of the DAB/peroxide substrate (Röche). Then, CGRP or Substance P polyclonal guinea pig antibodies (Peninsula Laboratories, Houghton, MI) were applied overnight at 4°C followed by a Cy3 conjugated goat anti guinea pig secondary antibody (Peninsula Lab). Subsequently polyclonal tryptase or chymase goat antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were applied overnight followed by a donkey antigoat secondary antibody conjugated with Alexa Fluor 488 (Molecular Probes, Eugene, OR). Finally, 4′-6-diamidine-2-phenyl indole, or DAPI (Vector Laboratories, Burlington, ON) was applied to label nuclei.
The sections were viewed under a Zeiss light microscope (Zeiss, Axioskop 2 plus, Toronto, ON). Images were captured (200×) from five randomly selected areas from each of four sections for each specimen with a digital camera (Zeiss, Axiocam). Image-Pro Plus (Media Cybernetics, Silver Spring, MD) was used to analyze the sections. Mast cell numbers were recorded for each area and then all numbers were averaged. The Substance P and CGRP nerve fibers were counted for each area and all numbers were averaged. To be counted, a nerve fiber must have a minimum length of 50 μm.19 Appropriate controls were assessed in all experimental series. Sections of skin are sources of blood vessels (α-SMA), mast cells, and neuropeptide containing nerve fibers, and these positive controls tested appropriately in both species.12,14,20 Negative controls included substituting primary antibodies with 3% bovine serum albumin.
Data are presented as mean ± SD. Statistical analysis used a paired t-test to evaluate differences between experimental and control rabbit knees at each time period and a t-test to evaluate contracture and control human data. Statistical significance was set at p = 0.05.
RESULTS
The rabbits lost 5–10% of their starting weight at the time of sacrifice at 4 weeks but had returned to pre-operative weights in the chronic group. All K-wires were intact. In the acute stages of the formation of joint contractures, significant differences in markers for myofibroblasts, mast cells, and neuropeptides were already evident in the rabbits sacrificed at 4 weeks (Table 1, Fig. 1). All four combinations of the mast cell and neuropeptide markers were evaluated. Although total cell numbers varied among the four groups, the total cell numbers were statistically different between contracture and control capsules within each marker combination only in the tryptase–CGRP combination (Table 1). The numbers of α-SMA-positive myofibroblasts, chymase, or tryptase-positive mast cells and neuropeptide containing nerve fibers expressed as a percentage of total cells were significantly greater in the contracture capsules when compared to the control capsules ( < 0.0001). The percentages ranged from 41–48% for the contracture capsules, whereas the controls had ranges from 9–10% for each of the markers.
Table 1.
Total Cell Counts and Percentages of Total Cell Numbers for α-SMA (Myofibroblast Marker), Mast Cell Markers (Chymase or Tryptase), and Neuropeptides (CRGP or Substance P) in the 4-Week Rabbit Group
| Total Cells | % SMA | % Chymase | % CGRP | |
|---|---|---|---|---|
| Contracture | 446 ± 73 | 46 ± 5 | 41 ± 3 | 43 ± 4 |
| Control | 398 ± 70 | 10 ± 1 | 9 ± 1 | 9 ± 1 |
| p = 0.33 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Chymase | % SP | |
| Contracture | 429 ± 66 | 48 ± 2 | 47 ± 3 | 47 ± 2 |
| Control | 378 ± 47 | 10 ± 1 | 10 ± 1 | 10 ± 1 |
| p = 0.24 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Tryptase | % SP | |
| Contracture | 372 ± 48 | 48 ± 2 | 46 ± 2 | 47 ± 2 |
| Control | 360 ± 30 | 10 ± 1 | 10 ± 1 | 10 ± 1 |
| p = 0.64 | p < .0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Tryptase | % CGRP | |
| Contracture | 441 ± 46 | 47 ± 1 | 44 ± 4 | 45 ± 2 |
| Control | 365 ± 33 | 10 ± 1 | 10 ± 1 | 10 ± 1 |
| p = 0.04 | p < 0.0001 | p < .0001 | p < 0.0001 |
Data are presented as mean ± SD.
Figure 1.
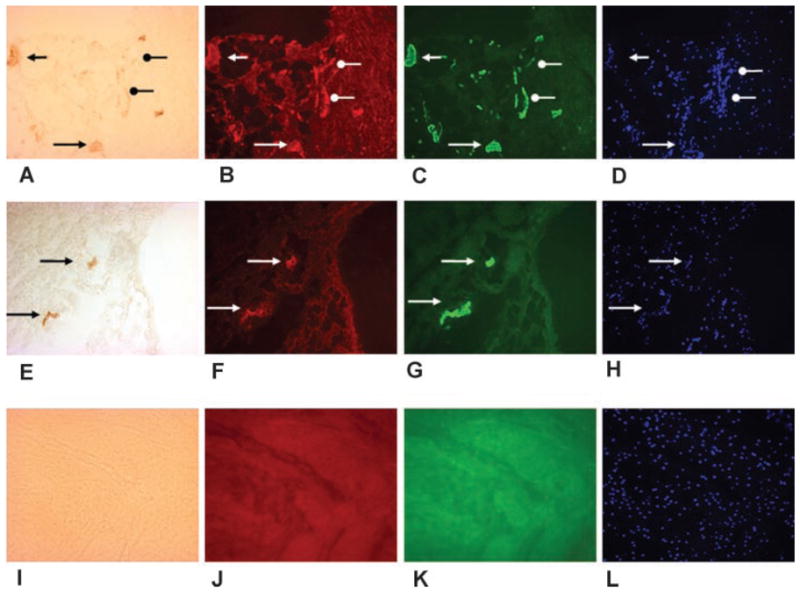
The rabbit posterior knee joint contracture capsule on the top row (A–D) and the contralateral control capsule on the middle row (E–H) are shown. The first column is α-SMA (A, E), the second column is the neuropeptide Substance P (B, F), the third column is the mast cell marker tryptase (C, G), and the fourth column is the nuclear marker DAPI (D, H). The images in each row are the same area with the only difference being the light source and filters. Obvious blood vessels are indicated with the black triangular arrow heads in the contracture (upper) and contralateral control (middle row) capsules (A, E). The corresponding areas are shown with the white triangular arrow heads, indicating colocalization of neuropeptides and mast cells with the blood vessels (B, C, D and F, G, H). Linear patterns consistent with myofibroblasts are indicated with the black circular arrow heads in the contracture capsule (A). The corresponding areas are shown with the white circular arrow heads, indicating colocalization of neuropeptides and mast cells with the myofibroblasts (B, C, D). There are no similar structures in the control capsule (middle row). The bottom row (I–L) is a negative control (omission of the primary antibodies) from a contracture capsule. The contracture knee had the femoral condyle fractures and 4 weeks immobilization (original magnification 200×).
In the chronic stages of joint contractures, significant differences in markers for myofibroblasts, mast cells, and neuropeptides were evident in the rabbits sacrificed after 32 weeks of remobilization, or 40 weeks after the index procedure (Table 2). All four combinations of the mast cell and neuropeptide markers were evaluated. Total cell numbers were not significantly different between the contracture and control knees for any combination of markers (Table 2). The numbers of α-SMA-positive myofibroblasts, chymase, or tryptase-positive mast cells and neuropeptide containing nerve fibers expressed as a percentage of total cells were significantly greater in the contracture capsules when compared to the control capsules (p < 0.0001). The percentages ranged from 44–50% for the contracture capsules, whereas the controls had ranges from 11–13% for each of the markers.
Table 2.
Total Cell Counts and Percentages of Total Cell Numbers for α-SMA, Mast Cell Markers, and Neuropeptides in the 32-Week Rabbit Group
| Total Cells | % SMA | % Chymase | % CGRP | |
|---|---|---|---|---|
| Contracture | 519 ± 133 | 48 ± 2 | 46 ± 3 | 45 ± 6 |
| Control | 512 ± 126 | 11 ± 1 | 11 ± 1 | 11 ± 1 |
| p = 0.62 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Chymase | % SP | |
| Contracture | 528 ± 48 | 49 ± 3 | 49 ± 4 | 48 ± 5 |
| Control | 535 ± 40 | 12 ± 1 | 12 ± 1 | 12 ± 1 |
| p = 0.28 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Tryptase | % SP | |
| Contracture | 549 ± 63 | 49 ± 1 | 47 ± 2 | 49 ± 3 |
| Control | 552 ± 57 | 12 ± 1 | 12 ± 1 | 13 ± 1 |
| p = 0.57 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Tryptase | % CGRP | |
| Contracture | 527 ± 54 | 50 ± 1 | 44 ± 4 | 47 ± 2 |
| Control | 541 ± 55 | 12 ± 1 | 13 ± 1 | 12 ± 1 |
| p = 0.11 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
Data are presented as mean ± SD.
In the chronic stages of joint contractures, significant differences in markers for myofibroblasts, mast cells and neuropeptides were evident in the human elbow joint capsules (Table 3, Fig. 2). All four combinations of the mast cell and neuropeptide markers were evaluated. Total cell numbers were statistically different between contracture and control capsules in all groups (Table 3). The numbers of α-SMA-positive myofibroblasts, chymase, or tryptase-positive mast cells and neuropeptide containing nerve fibers expressed as a percentage of total cells were significantly greater in the contracture capsules when compared to the control capsules ( < 0.0001). The percentages ranged from 45–50% for the contracture capsules, whereas the controls had ranges from 10–12% for each of the markers.
Table 3.
Total Cell Counts and Percentages of Total Cell Numbers for α-SMA, Mast Cell Markers, and Neuropeptides in the Human Anterior Elbow Capsules
| Total Cells | % SMA | % Chymase | % CGRP | |
|---|---|---|---|---|
| Contracture | 475 ± 58 | 50 ± 2 | 47 ± 1 | 49 ± 1 |
| Control | 338 ± 36 | 10 ± 1 | 10 ± 1 | 10 ± 1 |
| p = 0.001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Chymase | % SP | |
| Contracture | 484 ± 70 | 48 ± 1 | 46 ± 2 | 47 ± 1 |
| Control | 370 ± 13 | 11 ± 1 | 10 ± 1 | 11 ± 1 |
| p = 0.007 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Tryptase | % SP | |
| Contracture | 489 ± 34 | 46 ± 2 | 45 ± 2 | 45 ± 2 |
| Control | 369 ± 37 | 12 ± 1 | 11 ± 1 | 11 ± 1 |
| p = 0.0002 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| Total Cells | % SMA | % Tryptase | % CGRP | |
| Contracture | 549 ± 40 | 49 ± 1 | 47 ± 1 | 48 ± 2 |
| Control | 321 ± 40 | 11 ± 1 | 11 ± 1 | 11 ± 1 |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
Data are presented as mean ± SD.
Figure 2.
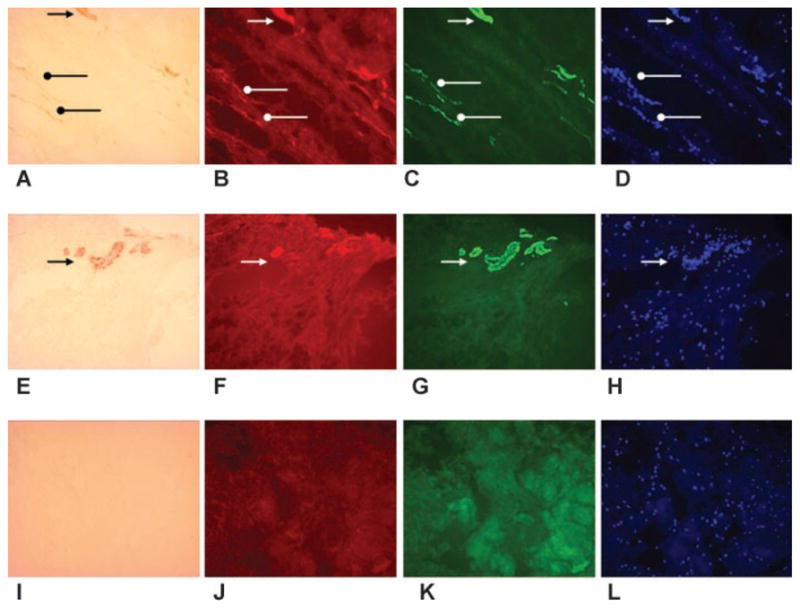
Immunohistochemistry of the human anterior elbow joint capsule showing the contracture capsule on the top row (A–D) and the control capsule on the middle row (E–H). The first column is α-SMA (A, E), the second column is the neuropeptide CGRP (B, F), the third column is the mast cell marker chymase (C, G), and the fourth column is the nuclear marker DAPI (D, H). The images in each row are the same area with the only difference being the light source and filters. Obvious blood vessels are indicated with the black triangular arrow heads in the contracture (upper) and control (middle row) capsules (A, E). The corresponding areas are shown with the white triangular arrow heads, indicating colocalization of neuropeptides and mast cells with the blood vessels (B, C, D and F, G, H). Linear patterns consistent with myofibroblasts are indicated with the black circular arrow heads in the contracture capsule (A). The corresponding areas are shown with the white circular arrow heads, indicating colocalization of neuropeptides and mast cells with the myofibroblasts (B, C, D). There are no similar structures in the control capsule (middle row). The bottom row (I–L) is a negative control (omission of the primary antibodies) from a contracture capsule (original magnification 200×).
DISCUSSION
It was determined that markers for mast cells and neuropeptide containing nerve fibers are significantly elevated in the joint capsule of patients with posttraumatic elbow contractures and in affected knees of a rabbit model of posttraumatic joint contractures when compared to similar tissues obtained from joints without contractures. In the contracture capsules, the number of α-SMA-positive cells, a marker of myofibroblasts, was significantly elevated. In most cases, the myofibroblast, mast cell, and neuropeptide markers localized to the same areas (Figs. 1 and 2). In the rabbit model of posttraumatic contractures, these changes are present in the acute stages and appear to be maximized by 4 weeks postinjury. Interestingly, the changes persist at similar levels in the chronic stages of the rabbit model, and reflect what is seen in the chronic stages of the human condition.
Conditions such as Dupuytren’s contracture of the hand and hypertrophic skin wound healing also show an association with elevated numbers of mast cells and neuropeptide containing nerve fibers. Schubert et al.19 reported that the pathologic palmar fascia has significantly increased numbers of mast cells and Substance P containing nerve fibers when compared to normal palmar fascia. Myofibroblast numbers have been reported to be increased in the pathologic palmar fascia of Dupuytren’s contracture by other authors.6,21 An evaluation of hypertrophic human skin wounds showed increased Substance P levels.22 In related work by these authors, they report that mast cell numbers are increased in human hypertrophic wounds and in the skin wounds of red Duroc pigs, a model of hypertrophic scarring, when compared to normal skin taken from the subjects.23 In the red Duroc model of hypertrophic skin wound healing, myofibroblast numbers were elevated early, but returned to control levels by 3 months.23
This project regarding mast cells and neuropeptides in joint capsule adds to the knowledge pertaining to other adaptations previously reported by our research group, and further validates the animal model of posttraumatic contractures. The joint capsule develops increased numbers of myofibroblasts, and mRNA and protein levels increased for collagens, MMPs, TGF-β1, and CTGF or decreased for TIMPs in chronic stages of human elbow posttraumatic contractures and in the animal model.4,5,24–26 The changes in these cellular, matrix molecule, and enzyme and growth factor profiles occur very early in the animal model, supporting the concept that the process leading to joint contractures starts very quickly after the inciting event.7
The total cellularity in the joint capsules was similar in some cases comparing contracture to control samples or was significantly elevated in the contracture capsules, even in the chronic posttraumatic contractures. These findings are in contrast to what has been published for a healing medial collateral ligament (MCL) where cellularity increases in the first several weeks following injury (acute), but then markedly decreases over the next several months (chronic).27 One obvious difference is the type of injury. In the studies from our laboratory on human elbow capsules, the injuries were all periarticular fractures with only one dislocation and it is unlikely that the joint capsule was physically disrupted in these scenarios, except for the single dislocation. In the posttraumatic contracture animal model, the joint capsule is not transected; the injury is the intraarticular fracture. Thus, the capsule changes may be considered in part, adaptive. However, in the rabbit knee MCL model, a segment of ligament tissue is removed and the resulting gap is filled by the healing process.27 In the process of filling the gap in the MCL, a large influx and proliferation of cells and blood vessels occurs contributing to the increased cellularity. It would appear a different process is taking place in the joint capsule in the posttraumatic contracture model; total cellularity does not change or tends to increase, even in chronic stages. This is currently being addressed to further understand the mechanisms involved.
The results of this study have to be interpreted with some caution. The myofibroblast numbers are likely an overestimate as a double labeling technique to differentiate α-SMA due to myofibroblasts from smooth muscle containing blood vessels was not used. The percentages are slightly higher than what we reported previously.3–5 The nerve fiber counts are limited by the fact that the same fiber may have been counted more than once if it undulated in an out of the plane of the section. However, the number count can be viewed as a surrogate for neuropeptide nerve fiber containing density. The density of fibers in relation to the number of cells in a given area is probably more important than defining the exact number of uniquely individual fibers.
The observations of this study are consistent with the hypothesis that a myofibroblast–mast cell–neuropeptide axis may be an underlying mechanism contributing to posttraumatic joint contractures.8,13,18 Mast cells have been implicated in other conditions of fibrosis because they contain granules with profibrotic growth factors.9 The neuropeptides represent an interesting possibility that can connect the observations of increased pain, swelling, and joint contracture formation. It has been well described that Substance P and CGRP can cause mast cell degranulation, and this is in part their contribution to neurogenic inflammation represented by pain and swelling.8,11,13,14,17,18 In addition, the mast cell factors liberated can contribute to fibrosis as well. However, the extent of the changes was maximal after 4 weeks postinjury and one cannot make any interpretations regarding “cause and effect” relationships between the three components addressed in this study. Nonetheless, this work serves as the basis for future experiments to extend these observations from an association relationship to potential cause-and-effect relationships. One such strategy will be to use methods to limit mast cell function to determine the effect on joint contracture formation, severity, and reversibility. Validation of the involvement of this axis in posttraumatic contractures may lead to development of new interventions to prevent or inhibit their occurrence.
Acknowledgments
Funding for the study was received from the Canadian Institutes of Health Research (K.A.H.), the Health Research Foundation (K.A.H.), the Alberta Heritage Foundation for Medical Research (K.A.H.), and the Calgary Foundation–Grace Glaum Professorship (D.A.H.). A preliminary report of these results contained in the manuscript in abstract form received the Founder’s medal for the best paper at the Canadian Orthopaedic Research Society annual meeting June 1, 2007 at Halifax, NS.
References
- 1.Doornberg JB, Jupiter JB. The posttraumatic stiff elbow: a historical perspective of treatment. In: Jupiter JB, editor. The stiff elbow. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006. pp. 1–7. [Google Scholar]
- 2.O’Driscoll SW. Clinical assessment and open and arthroscopic surgical treatment of the stiff elbow. In: Jupiter JB, editor. The stiff elbow. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006. pp. 9–19. [Google Scholar]
- 3.Germscheid NM, Hildebrand KA. Regional variation is present in elbow joint capsules following injury. Clin Orthop. 2006;450:219–224. [PMC free article] [PubMed] [Google Scholar]
- 4.Hildebrand KA, Zhang M, van Snellenberg W, et al. Myofibroblastnumbers are elevated in human elbow joint capsules following trauma. Clin Orthop. 2004;419:189–197. doi: 10.1097/00003086-200402000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res. 2004;22:313–320. doi: 10.1016/j.orthres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 7.Hildebrand KA, Zhang M, Germscheid NM, et al. Cellular, matrix and growth factor components of the joint capsule are modified early in the process leading to post-traumatic contracture formation in a rabbit model. Acta Orthop. 2008;79:116–125. doi: 10.1080/17453670710014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottwald T, Coerper S, Schaffer M, et al. The mast cell-nerve axis in wound healing: a hypothesis. Wound Repair Regen. 1998;6:8–20. doi: 10.1046/j.1524-475x.1998.60104.x. [DOI] [PubMed] [Google Scholar]
- 9.Gruber B. Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2003;5:147–153. doi: 10.1007/s11926-003-0043-3. [DOI] [PubMed] [Google Scholar]
- 10.Buckley MG, Gallagher PJ, Walls AF. Mast cell subpopulations in the synovial tissue of patients with osteoarthritis: selective increase in the numbers of tryptase-positive, chymase-negative mast cells. J Pathol. 1998;186:67–74. doi: 10.1002/(SICI)1096-9896(199809)186:1<67::AID-PATH132>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Foreman JC. Substance P and calcitonin gene-related peptide: effects on mast cells and in human skin. Int Arch Allergy Appl Immunol. 1987;82:366–371. doi: 10.1159/000234229. [DOI] [PubMed] [Google Scholar]
- 12.Maurer M, Theoharides T, Granstein RD, et al. What is the physiological function of mast cells? Exp Dermatol. 2003;12:886. doi: 10.1111/j.0906-6705.2003.0109a.x. [DOI] [PubMed] [Google Scholar]
- 13.Hart DA, Frank CB, Bray RC. Inflammatory processes in repetitive motion and overuse syndromes: potential role of neurogenic mechanisms in tendons and ligaments. In: Gordon SL, Blair SJ, Fine LJ, editors. Repetitive motion disorders of the upper extremity. 1. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995. pp. 247–262. [Google Scholar]
- 14.Foreman JC. Peptides and neurogenic inflammation. Br Med Bull. 1987;43:386–400. doi: 10.1093/oxfordjournals.bmb.a072189. [DOI] [PubMed] [Google Scholar]
- 15.Lee YS, Vijayasingam S. Mast cells and myofibroblasts in keloid: a light microscopic, immunohistochemical and ultrastructural study. Ann Acad Med Singapore. 1995;24:902–905. [PubMed] [Google Scholar]
- 16.Gailit J, Marchese MJ, Kew RR, et al. The differentiation and function of myofibroblasts is regulated by mast cell mediators. J Invest Dermatol. 2001;117:1113–1119. doi: 10.1046/j.1523-1747.2001.15211.x. [DOI] [PubMed] [Google Scholar]
- 17.Schaffer M, Beiter T, Becker HD, et al. Neuropeptides: mediators of inflammation and tissue repair? Arch Surg. 1998;133:1107–1116. doi: 10.1001/archsurg.133.10.1107. [DOI] [PubMed] [Google Scholar]
- 18.Hart DA, Frank CB, Kydd A, et al. Neurogenic, mast cell, and gender variables in tendon biology: potential role in chronic tendinopathy. In: Maffulli N, Renstrom P, Leadbetter WB, editors. Tendon injuries. 1. London: Springer-Verlag; 2005. pp. 40–48. [Google Scholar]
- 19.Schubert TEO, Weidler C, Borisch N, et al. Dupuytren’s contracture is associated with sprouting of substance P positive nerve fibres and infiltration by mast cells. Ann Rheum Dis. 2006;65:414–415. doi: 10.1136/ard.2005.044016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foreman JC. Substance P and calcitonin gene-related peptide: effects on mast cells and in human skin. Int Arch Allergy Appl Immunol. 1987;82:366–371. doi: 10.1159/000234229. [DOI] [PubMed] [Google Scholar]
- 21.Berndt A, Kosmehl H, Katenkamp D, et al. Appearance of the myofibroblastic phenotype in Dupuytren’s disease is associated with a fibronectin, laminin, collagen type IV and tenascin extracellular matrix. Pathobiology. 1994;62:55–58. doi: 10.1159/000163879. [DOI] [PubMed] [Google Scholar]
- 22.Scott JR, Muangman PR, Tamura RN, et al. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar. Plastic Reconstruct Surg. 2005;115:1095–1102. doi: 10.1097/01.prs.0000156151.54042.da. [DOI] [PubMed] [Google Scholar]
- 23.Harunari N, Zhu KQ, Armendariz RT, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006;32:669–677. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebrand KA, Zhang M, Hart DA. High rate of joint capsule matrix turnover in chronic human elbow contractures. Clin Orthop. 2005;439:228–234. doi: 10.1097/01.blo.0000177718.78028.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hildebrand KA, Zhang M, Hart DA. Joint capsule matrix turnover in a rabbit model of chronic joint contractures: correlation with human contractures. J Orthop Res. 2006;24:1036–1043. doi: 10.1002/jor.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hildebrand KA, Zhang M, Hart DA. Myofibroblast upregulators are elevated in joint capsules in post traumatic contractures. Clin Orthop. 2007;456:85–91. doi: 10.1097/BLO.0b013e3180312c01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank CB, Amiel D, Akeson WH. Healing of the medial collateral ligament of the knee. Acta Orthop Scand. 1983;54:917–923. doi: 10.3109/17453678308992934. [DOI] [PubMed] [Google Scholar]


