Summary
Studies of singletons suggest that right-handed individuals may have higher levels of testosterone than do left-handed individuals. Prenatal testosterone levels are hypothesised to be especially related to handedness formation. In humans, female members from opposite-sex twin pairs may experience elevated level of prenatal exposure to testosterone in their intra-uterine environment shared with a male. We tested for differences in rates of left-handedness/right-handedness in female twins from same-sex and opposite-sex twin pairs. Our sample consisted of 4736 subjects, about 70% of all Finnish twins born in 1983–1987, with information on measured pregnancy and birth related factors. Circulating testosterone and estradiol levels at age 14 were available on 771 and 744 of these twins, respectively. We found significantly (p<.006) lower prevalence of left-handedness in females from opposite-sex pairs (5.3%) compared to females from same-sex pairs (8.6%). The circulating levels of neither testosterone nor estradiol related to handedness in either females or males. Nor were there differences in circulating testosterone or estradiol levels between females from opposite-sex and same-sex twin pairs. Birth and pregnancy related factors for which we had information were unrelated to handedness. Our results are difficult to fully explain by postnatal factors, but they offer support to theory that relates testosterone to formation of handedness, and in a population-based sample, are suggestive of effects of prenatal testosterone transfer.
Keywords: laterality, masculinisation, prenatal testosterone transfer, sex difference, testosterone
1. Introduction
Most humans are right-handed, while around 10% are left-handed (Gilbert and Wysocki, 1992). The origins of human handedness are unclear, although there is evidence that handedness is at least partly determined before birth. Ultrasound studies indicate that human fetuses prefer to move their right hand as early as 10 weeks of gestational age (Hepper et al., 1998), and this preference is reportedly maintained from 12 to 27 weeks of gestational age (McCartney and Hepper, 1999). Prenatal thumb sucking is related to newborn head position (Hepper et al., 1991) and even to handedness at 10–12 years age (Hepper et al., 2005). Although the formation of human handedness originates prenatally, genetic theories are inadequate to explain handedness. While specific chromosomal regions (Warren et al., 2006) and genes (Medland et al., 2005; Francks et al., 2007, but see Crow et al. 2009 and reply by Francks, 2009) are linked to handedness, twin studies indicate that only about a quarter of the variance in handedness is attributable to genetic effects (for a meta-analysis see Medland et al., 2006; Medland et al., 2009; Vuoksimaa et al, 2009). Some left-handedness may be of pathological origin (Dellatolas et al., 1993; Ramadhani et al., 2006), but in general, left-handedness is a natural phenomenon that correlates with lateralization of language functions (Knecht et al., 2000). Hormonal theories have been invoked to account for the development of handedness in addition to the genetic and pathological theories (for different theories of handedness see e.g. Beaton, 2003; for a meta-analysis of testosterone effects on laterality in animals see Pfannkuche et al., 2009).
The Geschwind-Behan-Galaburda (GBG) hypothesis (Geschwind and Behan, 1982; Geschwind and Galaburda, 1985) postulates that prenatal exposure to testosterone influences brain function and structures. According to the GBG hypothesis, high levels of testosterone may inhibit development of the left hemisphere and enhance development of the right hemisphere. This can shift handedness and language functions from the left hemisphere to the right, resulting in weaker dextrality or to left-handedness. In contrast to the GBG hypothesis, an alternative theory suggests that left-handedness is caused by decreased levels of testosterone (Witelson, 1991; Witelson and Nowakowski, 1991). This callosal theory proposes that low prenatal testosterone levels result in less regressive development of temporo-parietal regions of the brain, resulting in a larger isthmus of the corpus callosum and less functional asymmetry, thus increasing left-handedness.
Greater left-hand bias, assessed with the Edinburgh Handedness Inventory has been reported (Nass et al., 1987; Kelso et al., 2000) in girls with congenital adrenal hyperplasia (CAH), who are exposed to elevated levels of testosterone in utero. But two studies found that handedness of CAH girls did not differ from controls (Helleday et al., 1994; Mathews et al., 2004). Even if found, altered lateralization in special populations, such as CAH girls, might be secondary to complications of the disorder. Other studies have investigated the relationship between index/ring (2d:4d) finger ratio and handedness. Males exhibit lower 2d:4d ratios than females (Manning, 2002). Some consider this sexually dimorphic characteristic a putative marker of prenatal testosterone exposure. A low 2d:4d ratio has been associated with enhanced left hand performance as measured with hand skill tasks (Manning et al., 2000; Fink et al., 2004), offering some evidence linking higher prenatal testosterone levels (in a form of low 2d:4d ratio) to left hand preference, but the results have been mixed. One study found that non-right-handers had lower (more masculine) 2d:4d ratio than right-handers (Nicholls et al., 2008). Another study found that non-right-handed subjects had higher 2d:4d ratio than right-handed subject (Ypsilanti et al., 2008). In a large scale internet study with over 170,000 subjects, low 2d:4d in the right hand was associated with the left hand preference, whereas high 2d:4d in left hand was associated with left hand preference (Manning and Peters, 2009). Another study found that 2d:4d ratio was associated with hand preference, but due only to interaction with finger length (Jackson, 2008), and the difference between 2d:4d ratios in right and left hands has been associated with hand preference (Beaton et al., 2010). Other than CAH studies (usually of limited size samples) and the inconsistent studies concerning 2d:4d ratio, a relationship between high prenatal testosterone levels and left-handedness enjoys little support (see Bryden et al., 1994, but see also Previc, 1994).
The most striking evidence against the GBG hypothesis comes from a study associating prenatal testosterone in 2nd trimester amniotic fluid with cerebral lateralization in 10-year-old children: higher levels of prenatal testosterone were related to right-handedness (Grimshaw et al., 1995), not left-handedness as assumed by the GBG hypothesis. This study of healthy girls was consistent with callosal theory (Witelson and Nowakowski, 1991), which states that males with clinical conditions related to low testosterone levels in early development (e.g. Klinefelter syndrome), as well as male homosexuals (Lalumière et al., 2000), show increased odds of left-handedness compared to the general population. In support of callosal theory, Medland et al. (2005) found that left-handedness was more common in subjects whose variants of the androgen receptor gene were associated with lower levels of testosterone, but this study could not distinguish whether this result was a consequence of a direct effect of the androgen receptor gene or mediated by testosterone exposure.
Several studies have examined circulating testosterone levels of right- and left-handed adults. Right-handed (for writing and drawing) young adult women had higher salivary concentrations of testosterone than left-handed (for writing and drawing) women in a study of healthy subjects (Gadea et al., 2003). Similarly, another study that used the Crovitz and Zener handedness questionnaire, found higher salivary testosterone levels in right-handers versus left-handers in both men and women (Moffat and Hampson, 1996). In this study, ambidextrous subjects were categorized as left-handed. Because testosterone levels are highly genetic (Harris et al., 1998; Eriksson et al., 2005; Hoekstra et al, 2006), prenatal testosterone levels might also correlate with the circulating levels of testosterone later in life. Thus, it cannot be concluded whether the effect of testosterone in these studies is of prenatal or postnatal origin. Interestingly, a study that measured handedness as a right-left difference in grasp reflex indicated that testosterone levels, taken from the umbilical artery after birth, were higher in right-handers than in left-handers in newborns three to fives day after birth (Tan and Tan, 2001). Other studies have found no differences in salivary testosterone levels between right- and left-handers (Moffat and Hampson, 2000; Beaton et al., 2010).
Estrogen, as well as testosterone, may influence lateralization and formation of handedness. Studies concerning diethylstilbestrol (DES), a synthetic estrogen, have indicated that estrogen might have a masculinising effect on laterality to increase the probability for left-handedness. A study of 175 women exposed to DES prenatally found a higher incidence of left-handed writers (17.1%) compared to 219 controls (9.6%) (Scheirs and Vingerhoets, 1995). Another study of 65 women found significantly more left-handed writers among DES exposed subjects (17.5%) compared to unexposed subjects (4%) and further indicated that the exposure to DES before week nine of gestation was related to left-handedness (Smith and Hines, 2000). Also, one study found that 77 DES exposed women exhibited weaker right-handedness, measured with the Edinburgh Handedness Inventory, than 514 women who were not exposed to DES but there was no difference between these two groups of women when the comparison was made by using the laterality score of zero as a cutpoint (Schachter, 1994).
In animals, prenatal transfer of testosterone occurs between foetuses, and it has been shown that females adjacent to males in their uterine environment can be prenatally masculinised by testosterone exposure (Ryan and Vandenbergh, 2002). A parallel effect in humans may occur in females of brother-sister twin pairs, although there is no direct evidence of prenatal transfer of testosterone in humans. Some earlier studies have suggested that females with male co-twins can be masculinised in physiological and biological traits including spontaneous otoacoustic emissions and maternal fitness (McFadden, 1993; Lummaa et al., 2007; but see Rose et al, 2002).
To date, two studies (Elkadi et al., 1999; Ooki, 2006) have directly compared the rate of left-handedness between females from opposite-sex and same-sex twin pairs. Both studies tested the GBG hypothesis under the assumption that the prevalence of left-handedness would be increased in opposite-sex female twins if prenatal testosterone exposure occurs. These studies were not framed to test the competing callosal hypothesis, which predicts that the prevalence of left-handedness should be lower in opposite-sex female twins than in same-sex female twins.
No significant differences were found between 59 opposite-sex female and 40 same-sex female twins in strength of hand preference or in frequency of left-handedness measured with the Edinburgh Handedness Inventory (Elkadi et al., 1999). But subjects for that study were selected in an unusual, and seemingly inappropriate, way to enhance the proportion of left-handers in the study. Tested subjects from half of the twin pairs were selected from a larger sample using the criteria that one twin within each pair indicated a left hand preference for at least one of the following: hand used when dealing a deck of cards, writing hand, hand used for throwing a ball. Such selection makes generalization to comparisons of handedness within a population-based sample of same- and opposite-sex female twins uncertain.
A second twin study reported no significant difference in left-handedness between females from opposite-sex and same-sex pairs in two separate samples (Ooki, 2006). In the first sample (N=1131, age 11–12) the prevalence of the left-handedness in opposite-sex females was 5.6% and 11.6% for same-sex female twins. Similarly the rate of left footedness, another measure of laterality that correlates with language lateralization (Elias and Bryden, 1998), was lower in opposite-sex female twins (6.2%) compared to same-sex female twins (14.6%) and that difference was statistically significant (p= .034). In the other sample, results were opposite in direction, albeit not significantly so. The representativeness of this sample is also uncertain, because the prevalence of left-handedness was 22.1% and the prevalence of left-footedness was 18.7%, very different rates compared to large-scale studies reporting the prevalence of left-handedness is typically around 10% (Gilbert and Wysocki, 1992). In this sample, the age of the subjects (N=951) ranged from 1 to 15 years, despite the fact that handedness is not necessarily established in children below 6 years of age (Bryden et al., 2000). In the Ooki (2006) study, handedness was measured by mother’s report of which hand a twin used to write a letter and footedness by mother’s report of which foot a twin used to kick a ball. Twins who were reported to use both hands/feet were treated as left-handers/left-footed.
In addition, a recent study found no difference in the prevalence of left-handedness (as measured by the hand used for throwing or writing hand or self-reported handedness) between opposite-sex and same-sex twins in Australian (p=.052) and Dutch samples (p=.403), but this study did not report the prevalence of left-handedness or number of subjects in females from opposite-sex and same-sex pairs (Medland et al., 2009).
To sum up: earlier studies have suggested either decreases or increases in the prevalence of left-handedness due to elevated levels of prenatal testosterone, but only one study has used direct measures of testosterone in healthy subjects. Although the callosal hypothesis (Witelson, 1991; Witelson and Nowakowski, 1991) originally postulated that decreased levels of testosterone are related to left-handedness in males, this hypothesis has been extended to females with supporting evidence from the study of Grimshaw et al. (1995) along with studies that have indicated higher levels of activational testosterone in right-handers than in left-handers also in females (Moffat and Hampson, 1996; Gadea et al., 2003). Based on these earlier studies of healthy subjects with direct measures of testosterone, we hypothesise that both higher prenatal and activational levels of testosterone are related to right-handedness as measured by preference. Thus, our aim was to study whether the prevalence of left-handedness is lower/the prevalence of right-handedness is higher in females from opposite-sex twin pairs compared to females from same-sex twin pairs. We also investigated the relationship between handedness and circulating levels of testosterone and estradiol. Earlier studies have investigated the relationship between handedness and either organizational or activational effects of gonadal hormones, but our study included both an indirect measure of prenatal testosterone and information on activational levels of testosterone and estradiol. Some pregnancy and birth related variables have been reported to be associated with left-handedness. For example very low birth weight, greater maternal age and infant resuscitation have found to be associated with left-handedness (Powls et al., 1996; Williams et al., 1999; Bailey and McKeever, 2004). Moreover, monozygotic twins are known to have lower birth weight and more perinatal complications than dizygotic twins (Loos et al., 1998). Accordingly, we used birth data (Apgar score, birth weight, gestational age and maternal age at twins’ birth) as covariates to control for possible birth and prenatal development related differences between monozygotic and dizygotic twins and to restrict the possible effects of testosterone to those subjects whose left-handedness likely is not of pathological origin. Inclusion of information on gestational age is important, since it correlates linearly with prenatal testosterone levels in females (Finegan et al., 1992).
2. Methods
2.1. Subjects
The sample consisted of 4736 Finnish twins from the FinnTwin12 study, which includes all Finnish twins born in 1983–1987 (Kaprio et al., 2002). There were 749 monozygotic (MZ) females, 697 MZ males, 706 dizygotic (DZ) females, 784 DZ males, 755 females from opposite-sex pairs (OSF), and 730 males from opposite-sex pairs (OSM) and 315 same-sex twins whose zygosity could not be definitively determined due to missing or inconsistent replies to zygosity questions. Handedness was reported by the twins when they were 14 years old in a postal questionnaire. Zygosity was determined from a validated questionnaire (Sarna et al., 1978) supplemented by questions explicitly designed for younger twins (Goldsmith, 1991). In addition to handedness information, we obtained saliva samples for 771 twins from the 1986 and 1987 cohorts, a subset of twins who were selected for intensive lab studies from the general FinnTwin12 population. Two-thirds of all twins in the intensive sample were selected at random, while the remainder, assumed at elevated risk for alcohol problems, were selected based on their parents’ reports of alcohol use. Detailed information about FinnTwin12 study and selection of the intensively studied twins can be found elsewhere (Kaprio et al., 2002). We had handedness data for about 70% of Finnish twins born in 1983–1987. FinnTwin12 study protocols were approved by the IRB of Indiana University, Bloomington, IN, and by the ethical committee of the Helsinki and Uusimaa Hospital District. All twins and their parents gave written informed consent for their participation.
2.2. Handedness measures
Assessment of handedness was based on a postal questionnaire that was sent to twins at age 14. Twins were asked to self-report their handedness by answering two questions: 1) Are you: right-handed, left-handed or use both hands equally well ? (n=4736); 2) Do you write with your right hand? No or Yes (n=4734).
2.3. Hormonal measures
Circulating testosterone and estradiol levels were determined from two saliva samples. Testosterone and estradiol concentrations were measured from the saliva supernatants, using commercially available ^125 I radioimmunoassay kits (Spectria) from Orion Diagnostica, Espoo, Finland. Testosterone within-assay variability (CV%) was 6.2% and between-assay variability was 9.7% at the level of 0.13 nmol/L (N=25). For estradiol, the average within-assay variability (CV%) was 13.2% (at the level of 5–20 pmol/L, n= 20) and between-assay variability was 13.5 % (at 5 pmol/L, n = 10). Testosterone and estradiol measures used in the analysis were adjusted for age, diurnal and seasonal effects. To reduce skewness, the testosterone and etradiol data used in the analyses were log transformed, but we report the non-transformed values in figures. Two saliva samples were collected from the twins in conjunction with a face to face psychiatric interview when the twins were 14 years old: the first sample was taken before interview and the second sample was taken after the interview. The procedure for collecting the saliva samples and determination of testosterone levels are described in detail elsewhere (Eriksson et al., 2005).
2.4. Pregnancy and birth related measures
The pregnancy and birth related factors (Apgar score at 5 minutes, birth weight, gestational age and mothers age at twins’ birth) were assessed in questionnaire items asked of the twins’ parents, when the twins were 11–12 years old. The validity of these measures is likely satisfactory, because in Finland, these measures are routinely collected by hospital staff at the time of birth and recorded on child health forms retained by the mother for subsequent visits to well-child clinics, which are universally used by the population at no cost. Apgar scores ranged from 0–10, birth weight from 700 to 4530 grams, gestational age from 26 to 42 weeks and mothers’ age at twins birth from 17.3 to 43.4 years. Due to missing values, samples sizes varied across measures (4105 for 5 minute Apgar score, 4574 for birth weight and 4405 for gestational age). Maternal age at twins’ birth was known for all subjects.
2.5. Statistical analyses
We used chi-square statistics to analyse the difference in the rate of left-handedness by sex and co-twins sex. Because the data was based on sampling families, we adjusted for the clustered observations (twins within families) in all analyses and report design-based F-values instead of uncorrected chi-square values (Rao and Scott, 1984). A logistic regression model was used to calculate the odds ratios (OR) of handedness between twins from same-sex and opposite-sex pairs. For testing the differences in circulating hormone levels and in pregnancy and birth related factors, we used an adjusted Wald test and clustered data were taken into account (Williams, 2000). For this reason, the reported numbers for design based degrees of freedom (df) refers to number of clusters (families) in the comparison. All analyses were performed using Stata software, version 9.1. (Stata, 2006).
3. Results
Based on their self-reported handedness, most of the subjects were right-handed (89%), while 9% were left-handed, and 2% were ambidextrous. Measured with self-reported writing hand, 10% were left-handed. There was significantly more left-handedness in males (11%) than in females (8%) (F(2, 4842) = 7.34, p< .001), whereas ambidextrousness was equally common in males (2%) and in females (1.4%). There were significantly more left-handed writers among males (12%) than females (9%) (F(1, 2420) = 11.66, p< .001). Most subjects were consistent right or left-handers (i.e. they reported same hand for both of the handedness questions), but 35 subjects who reported themselves right-handed were left-handed writers, and 13 of those who reported themselves left-handed were right-handed writers. We excluded those 48 subjects who reported inconsistent handedness, because they are likely to be those who have switched their writing-hand due to injury or subsequent to being encouraged or forced to write with their right hand. We excluded also all subjects, regardless of their writing hand, who reported themselves as ambidextrous (most of the ambidextrous subjects reported themselves as right-handed writers). In further analyses, we included only those subjects who reported themselves as consistent left- or right-handers (n=4605). The consistency of handedness and those groups included in further analyses can be seen in Table 1. The prevalence of left-handedness according to these criteria was 9.1%, and there were significantly more left-handers among males (10.7%) than in females (7.5%) (F(1,2412) = 13.58, p<.001). Of these 4605 twins, testosterone data were available for 771 and estradiol data for 744. The prevalence of left-handedness among twins for whom we had estradiol and testosterone measures was 8.7%, not different from the prevalence of left-handedness (9.2%) in subjects for whom we did not have hormonal measures (F(1,2412) = 0.14, p=.71).
Table 1.
Consistency of handedness.
| Writing hand |
||
|---|---|---|
| L | R | |
| Self-reported Handedness | ||
| R | 0.8% (35) | 99.2% (4186) |
| L | 97.0% (419) | 3.0% (13) |
| A | 27.2% (22) | 72.8% (59) |
R = right-handed, L = left-handed, A = ambidextrous.
The groups in bold font are included in the further analyses (consistent left- and right-handers). Percentages indicate the proportion of left- and right-handed writers among subjects who reported themselves as left-handed, right-handed or ambidextrous.
There were no differences in handedness between MZ and DZ same-sex females (F(1,733) = 0.06, p=.81) nor between MZ and DZ same-sex males (F(1,749) = 0.74, p=.39). Given that, we combined MZ and DZ same-sex females into one same-sex female (SSF) group and MZ and DZ males into one same-sex male (SSM) group. There were no significant differences in handedness between SSF twins and females from same-sex pairs with unknown zygosity (F(1,819) = 0.06, p=.81) nor between SSM twins and males from same-sex pairs with unknown zygosity (F(1,826) = 0.65, p=.42); and given that, we included females and males from same-sex pairs with unknown zygosity into SSF and SSM groups. Further, there were no significant differences between first (9.0%) and second born twins (9.2%) in left-handedness (F(1,2412) = 0.02, p=.89).
3.1. Estradiol and testosterone assays
As expected, males had significantly higher salivary testosterone levels than did females (F(1,412) = 197, p<.0001), and that difference was evident as well within opposite-sex twin pairs (F(1, 412) = 98, p<.0001). There were no significant differences in salivary testosterone levels between females from same-sex (SS) pairs (n=254) and females from opposite-sex (OS) pairs (n=117) (F(1,412) = 0.26, p=.61) or between males from same-sex pairs (n=291) and males from opposite-sex pairs (n=109) (F(1,412) = 0.10, p=.91) (Figure 1). Salivary testosterone did not differ significantly between right-handed and left-handed individuals in males (F(1,262) = 0.13, p=.72) or females (F(1,249) = 0.99, p=.32). Females had significantly higher salivary estradiol levels than males (F(1,407) = 52.49, p<.0001), and that sex difference was evident also in opposite-sex pairs (F(1,407) = 27,47, p< .0001). There were no significant differences in salivary estradiol levels between females from SS (n=244) and OS (n=114) pairs (F(1,407) = 0.03, p=.86) or between males from SS (n=278) and OS (n=108) pairs (F(1,407) = 0.64, p=.42) (Figure 2). Salivary estradiol did not differ significantly between right-handed and left-handed individuals in males (F(1,258) =0.35, p=.56.) nor in females (F(1,245) =1.51, p=.22).
Fig 1.
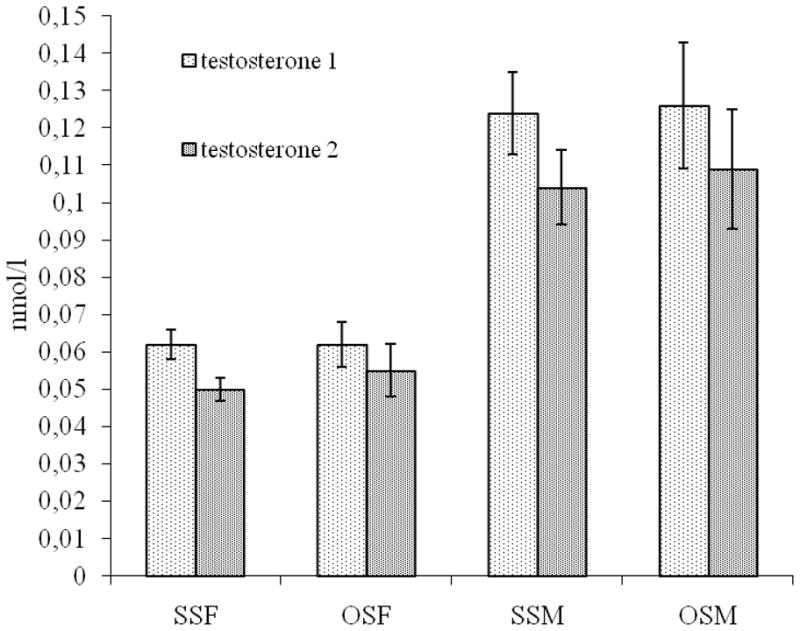
Mean testosterone levels (with 95% confidence intervals) of two saliva samples for same-sex female (SSF), opposite-sex female (OSF), same-sex male (SSM), and opposite-sex male (OSM) twins.
Fig 2.
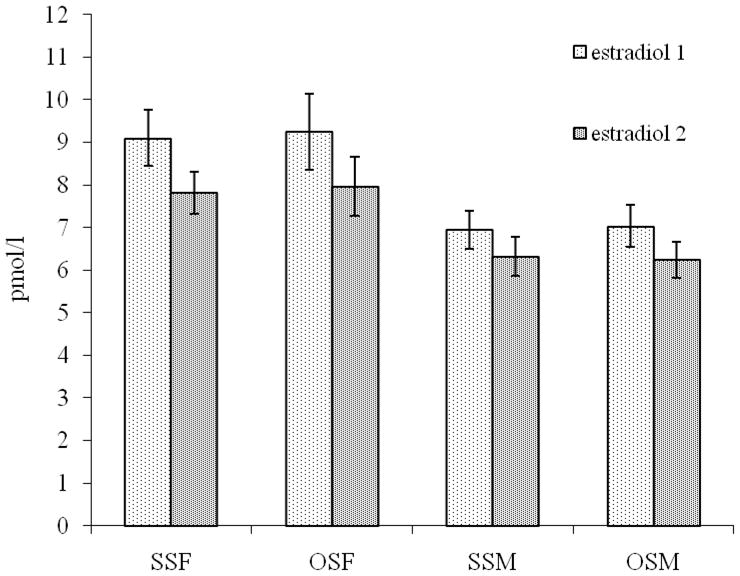
Mean estradiol levels (with 95% confidence intervals) of two saliva samples for same-sex female (SSF), opposite-sex female (OSF), same-sex male (SSM), and opposite-sex male (OSM) twins.
Because one third of the subjects with testosterone and estradiol measures were selected because of their parents’ elevated self-reported alcohol problems, we tested whether there were differences between these subjects and those randomly selected in prevalence of left/right-handedness and in hormone levels. Although there were more left-handers in the subjects whose selection criterion was their parents’ high alcohol related problems than in subjects who were selected at random, that difference was not statistically significant (10.2% vs 8.3%; F(1,407) =.69, p=.41). Neither testosterone levels (F(1,412) =2.21, p=.14), nor estradiol (F(1,407) =.34, p=.56) levels differed between randomly selected subjects and subjects who were selected for their parents’ elevated alcohol problems. The non-significant differences in testosterone and estradiol levels between left-and right-handed subjects were similar when we included only those subjects who were randomly selected (data not shown).
3.2. Handedness in twins from same-sex and opposite-sex pairs
There was a decreased prevalence of left-handedness in OSF twins (5.3%) compared to SSF twins (8.6%), whereas in males there was no difference between twins from OS and SS pairs (Table 2). Further, the 5.3% prevalence of left-handedness in OSF twins was significantly lower (F(1,1095) = 5.26, p=.02) when tested only against same-sex dizygotic females, of whom 8.4% were left-handed. The prevalences of left-handedness were similar in twins who had estradiol and testosterone measures (n=771; 8.6% in SSF, 4.4% in OSF, 9.7% in SSM and 11.1% in OSM twins). We then compared concordance/discordance of handedness across same-sex and opposite-sex twin pairs. There were 2192 pairs for these analyses. Discordant handedness of co-twin was equally common in SSF (14.0%) and OSF twins (14.6%), but in males, OSM twins (14.6%) were less likely to have a co-twin discordant for handedness than were SSM twins (18.9%) (Table 3). Left- and right-handed subjects did not differ by Apgar scores, birth weight, gestational age or mother’s age at twins’ birth (Table 4). Similarly, there were no differences between left-handed and right-handed subjects when females and males were considered separately. Since there was a trend for a difference in gestational age between left- and right-handed subjects, and also a trend for a difference in birth weight in females, we used logistic regression models to analyse the effects of co-twins’ sex and gestational age, and also birth weight in females, on handedness (n=2202). Having a male co-twin increased the probability of right-handedness in females (OR 1.22, 95% Confidence interval 1.01–1.48, p=.04), while neither gestational age (OR 1.03, 95%CI 0.95–1.12, p=.42) nor birth weight (OR 1.00, 95%CI 1.00–1.00, p=0.48) had an effect on handedness. In the model without gestational age and birth weight (n=2315), the OR for right-handedness in OSF twins was 1.29 (95%CI 1.07–1.56). In males, neither having a female co-twin (OR 1.00, 95%CI 0.87–1.16, p=.97) nor gestational age (OR 1.03, 95%CI 0.98–1.09, p=.27) had any relation with handedness (n=2199).
Table 2.
Prevalence of right- and left-handedness in same-sex and opposite-sex twins (number of subjects in parentheses).
| SSF | OSF | p-value SSF vs. OSF | SSM | OSM | p-value SSM vs. OSM | |
|---|---|---|---|---|---|---|
| Handedness | ||||||
| R | 91.4% (1443) | 94.7% (698) | 0.006 | 89.2% (1413) | 89.5% (632) | 0.823 |
| L | 8.6% (135) | 5.3% (39) | 10.8% (171) | 10.5% (74) | ||
SSF = same-sex female, OSF = opposite-sex female, SSM = same-sex male, OSM = opposite-sex male. R = right-handed, L = left-handed.
Table 3.
Pairwise handedness in 2192 twin pairs. Proportion of right hand concordant, left hand concordant, and discordant pairs (number of pairs in parentheses).
| SSF pairs | OS pairs | SSM pairs | p-value SSF vs. OS pairs | p-value SSM vs. OS pairs | |
|---|---|---|---|---|---|
| Right hand concordant | 84.7% (642) | 84.8% (574) | 79.8% (604) | 0.360 | 0.032 |
| Discordant | 14.0% (106) | 14.6% (99) | 18.9% (143) | ||
| Left hand concordant | 1.3% (10) | 0.6% (4) | 1.3% (10) |
SSF = same-sex female, OS = opposite-sex, SSM = same-sex male.
From 99 discordant OS pairs the left-handed twin was female in 31 pairs.
Table 4.
Birth and pregnancy related factors (means and 95% confidence intervals (in parentheses)) in left- and right-handed subjects.
| Left-handed | Right-handed | p-value | |
|---|---|---|---|
| Apgar score 5 min | 8.50 (8.30–8.70) | 8.51 (8.43–8.59) | 0.94 |
| Birth weight – males (grams) | 2740 (2671–2808) | 2754 (2728–2781) | 0.69 |
| Birth weight – females (grams) | 2585 (2494–2675) | 2664 (2639–2689) | 0.09 |
| Gestational age (weeks) | 36.71 (36.46–36.95) | 36.94 (36.85–37.04) | 0.06 |
| Maternal age at twins’ birth (years) | 29.60 (29.11–30.09) | 29.67 (29.47–29.87) | 0.78 |
Means for Apgar scores, gestational age and maternal age are for all subjects. The means for birth weight are separately for males and females due to significant sex difference in birth weight.
4. Discussion
First, the prevalence of left-handedness in our study is comparable to other studies that have indicated around 10% prevalence of left-handedness (Gilbert and Wysocki, 1992; Medland et al., 2003; Peters et al., 2006). Also the sex difference in left-handedness is consistent with earlier studies (for meta-analyses Papadatou-Pastou et al., 2008, see also Vuoksimaa and Kaprio, in press; Sommer et al., 2008).
We found, as hypothesised, lower prevalence of left-handedness/higher prevalence of right-handedness in females from OS pairs than in females from SS pairs. This result is consistent with theory that links higher levels of prenatal testosterone to right-handedness. Although not impossible, it is very improbable that postnatal effects fully explain our results. One previous twin study found a lower prevalence of left footedness in females from opposite-sex pairs compared to females from same-sex pairs (Ooki, 2006). Relating to laterality, another twin study found that OSF twins had a right ear advantage on a dichotic listening task more often than SSF twins (Cohen-Bendahan et al., 2004). Since right ear advantage is indicative of left hemisphere language lateralization and left hemisphere language lateralization is more common in right-handers than in left-handers, the Cohen-Bendahan et al. (2004) results are in line with our finding of increased prevalence of right-handedness in female twins with male co-twins. A recent study of singletons has found a positive relationship between prenatal testosterone, as measured from amniotic fluid at 2nd trimester, and left hemisphere language lateralization, in form of right ear advantage in dichotic listening task, at age six (Lust et al., 2010).
Some studies of 2d:4d ratio have been interpreted as inconsistent with our results and our inferences: low 2d:4d ratio is related to left hand preference (Nicholls et al., 2008) or enhanced left hand skill (Manning et al., 2000; Fink et al., 2004), and, if low 2d:4d ratio reflects low prenatal testosterone levels, these results contradict the interpretation we make of our data. But is 2d:4d ratio a reliable proxy for prenatal testosterone levels? In fact, there is no direct evidence that 2d:4d ratio reflects prenatal exposure to testosterone (see Berenbaum et al., 2009; Wallen, 2009). To date, two small-sample studies have reported that OSF twins have masculinised 2d:4d ratio (van Anders et al., 2006; Voracek and Dressler, 2007), whereas a study of 212 females from opposite-sex pairs and 237 females from same-sex pairs found no difference in 2d:4d between these two groups of females (Medland et al., 2008). Our results are in contrast with this negative finding considering finger ratios, but we note that prenatal testosterone effects, if they exist, are probably time dependent. Moreover, testosterone affecting handedness, which correlates with language lateralization (Knecht et al., 2000) presumably influences brain function in some way (e.g., by affecting expression of the androgen receptor gene in brain). The gene expression is tissue specific, so brain/cognitive function and physical characteristics do not necessarily have to correspond exactly.
Although OSF twins differed significantly from SSF twins in the prevalence of left-handedness/right-handedness in our study, in some sense this effect is not masculinisation, because the prevalence of left-handedness in OSF twins was not in the direction of males (i.e. left-handedness is more common in males). Nevertheless, our results, which are unlikely to be wholly a result of postnatal socialization, suggest that having a male co-twin increases the probability of right-handedness in female twins. Thus, the present results can be interpreted as evidence for prenatal testosterone transfer. It is possible that the effects of testosterone on handedness may be opposite for males and females. For example, in spatial abilities in males increased testosterone levels are related to poorer spatial abilities, whereas in females, increased levels of testosterone are related to better spatial abilities (Gouchie and Kimura, 1991; Aleman et al., 2004; for a meta-analysis of CAH individuals see Puts et al., 2008). Moreover, the effect of prenatal testosterone on language lateralization is suggested to be sex-specific (Lust et al., 2010). Similarly, it is possible that increased levels of testosterone have sex-specific effects on handedness: causing left-handedness in males and right-handedness in females. Why did we not find a difference in the prevalence of left-/right-handedness in males from opposite- and same-sex pairs? If there is prenatal transfer of testosterone from one fetus to another, it could be expected that right-handedness would be more common in males from same-sex pairs since males from those twin pairs are exposed to extra testosterone levels from their male co-twin. One possible explanation why this was not found in our study could be that the additional testosterone from a twin brother does not much increase male fetal testosterone levels, since males produce testosterone on their own substantially more than females. For example, there is evidence that sex of co-twin affects females, but not males in other sexually dimorphic traits; females with male co-twins are masculinised in mental rotation ability, but males with female co-twins do not differ from males with male co-twins (Vuoksimaa et al., in press).
Our results indicate that the different prevalence of left-handedness in SSF and OSF twins is not attributable to activational levels of sex hormones, since there were no significant differences in testosterone and estradiol levels in SSF and OSF twins at age 14 years. Our results show that in twins there exists a similar sex difference in fluctuating levels of testosterone and estradiol as in singletons, and that the sex difference is not affected by the sex of a co-twin. This is in line with an earlier study (Cohen-Bendahan et al., 2005a). The activational levels of testosterone and estradiol were not related to handedness in our study; thus, we could not confirm earlier reports of higher levels of activational testosterone in right-handers compared to left-handers (Moffat and Hampson, 1996, Gadea et al., 2003). Compared to our study with testosterone data on 771 subjects, the studies of singletons have comprised of somewhat smaller samples: 80 and 48 participants in the studies of Moffat and Hampson (1996) and Gadea et al. (2003), respectively. One notable difference in hormonal measures between these two studies and the present study was that our hormonal levels were measured at puberty, a period when testosterone levels are elevated in males. The studies of Moffat and Hampson (1996) and Gadea et al. (2003) measured testosterone levels from young adults. Accordingly it is possible that right-handers have higher testosterone levels than left-handers in adulthood, but not during puberty. But not all singleton studies have found differences in testosterone levels between left- and right-handers (Moffat and Hampson, 2000; Beaton et al, 2010).
Neither birth order, zygosity (MZ vs. DZ), pregnancy nor birth related complications had an effect on handedness. The similar prevalence of left-handedness in MZ and DZ twins and between first and second born twins are in line with previous studies (Medland et al., 2003; Medland et al., 2009; Vuoksimaa et al., 2009). MZ twins are known to have lower birth weights and more perinatal complications than DZ twins (Loos et al., 1998) and likewise, second-born twins are more liable to prolonged birth and perinatal morbidity (Armson et al., 2006). Our results suggest that the birth and pregnancy related differences (for which we had information) between MZ and DZ twins are not related to formation of handedness. Moreover, our results show that the difference in the prevalence of left-handedness between females from same-sex and opposite-sex pairs remained when we tested the difference including only dizygotic females. This indicates that our results are not explained by other differences (for which we did not have information) between monozygotic and dizygotic twinning, such as chorionicity. These findings also suggest that pathological left-handedness is rare in the general population. Importantly, the difference in the prevalence of right-handedness between females with male co-twins and females with female co-twins remained after controlling for gestational age, which correlates positively with prenatal testosterone levels in females (Nagamani et al., 1979; Finegan et al., 1992). In our study, right-handers had greater gestational age than left-handers, but this difference was very small and only marginally significant.
Intrauterine position has been suggested to be related to handedness in singletons (for a review see Previc, 1991); thus it could be speculated that if intrauterine position is associated with handedness in twins, then the higher frequency of left-handedness in males could cause more right-handedness in females in opposite-sex pairs. In our study most of the left-handed females, both in SSF and OSF group, were from handedness discordant pairs: that suggests that the left-handedness in males from opposite-sex pairs is not the cause for right-handedness in females from these pairs, although we did not have information about the intrauterine position of twins.
Our results, assuming that prenatal testosterone exposure in opposite-sex twin pairs does occur, are consistent with the callosal hypothesis of handedness, which relates increased levels of testosterone with right-handedness; conversely, our results contrast to the GBG model of handedness, which predicts increased levels of testosterone to be related with left-handedness. In line with our result, earlier singleton studies have reported higher levels of testosterone in right-handed females compared to left-handed females (Moffat and Hampson, 1996; Gadea et al, 2003) and one study found that prenatal testosterone, taken from amniotic fluid in 2nd trimester, correlated with right-handedness (Grimshaw et al., 1995).
The anatomical correlates of handedness in the human brain are located in motor cortex (Hammond, 2002), but asymmetry differences in cerebellum between left- and right-handers have been reported as well (Snyder et al., 1995). We propose that future studies should investigate whether there are anatomical or functional brain differences between SS and OS female twins in these brain areas. Also the anatomy of corpus callosum, which according to callosal hypothesis was originally hypothesised to differ between right-handed and non-consistent right-handed males (Witelson, 1985: Witelson, 1991) could be studied in SS and OS female twins. A meta-analysis of seven studies indicated that corpus callosum is larger in left-handers than in right-handers (Driesen and Raz, 1995); although the effect size of that difference was small and further, more recent brain imaging studies have not replicated a relationship between handedness and corpus callosum anatomy (Peters et al., 2002; Luders et al., 2003; see also Beaton, 1997). To date, only one study has investigated the anatomical brain differences in female twins from OS and SS pairs: females from opposite-sex pairs had significantly bigger global brain volumes in cerebellum, grey matter, white matter as well in total brain volume (Peper et al., 2009), but that result was at least partly explained by lower birth weight in twins from SS pairs compared to twins from OS pairs. While males have greater grey and white matter and greater global brain volumes, these differences were not related to handedness in a study of 465 healthy adults (Good et al., 2001).
One limitation of our study is the fact that we did not have a direct measure of prenatal testosterone. In fact, there are no studies where prenatal transfer of testosterone in human twins has been confirmed. The effects of prenatal testosterone might occur directly via the feto-fetal route or through the maternal-fetal route. One study found no difference in maternal testosterone levels between mothers who where expecting same-sex twins and mothers who were expecting opposite-sex twins (Cohen-Bendahan et al., 2005), which suggests that the prenatal transfer of testosterone is not occurring through the maternal route. But that result awaits replication, and we note that the testosterone levels in the Cohen-Bendahan et al. (2005) study were taken relatively late, at 24 and 32 weeks of gestation. It is known that testosterone levels in male fetuses are elevated from 8 to 24 weeks of gestation (for a review see Collaer and Hines, 1995). It is also suggested that the amniotic fluid would be a better source than maternal serum for studies concerning the effects of prenatal testosterone exposure (Van de Beek et al., 2004).
To conclude, our results showed decreased prevalence of left-handedness/increased prevalence of right-handedness in female twins who have male co-twins in a large population based sample of Finnish twins born in 1983–1987. This provides indirect evidence to support the idea of prenatal transfer of testosterone in humans. We did not find differences in activational levels of testosterone or estradiol between left- and right-handers. Our results do not support the pathological etiology for left-handedness in general.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Bronk E, Kessels RPC, Koppeschaar HPF, van Honk J. A single administration of testosterone improves visuospatial ability in young women. Psychoneuroendocrinology. 2004;29:612–617. doi: 10.1016/S0306-4530(03)00089-1. [DOI] [PubMed] [Google Scholar]
- Armson BA, O’Connell C, Persad V, Joseph KS, Young DC, Baskett TF. Determinants of perinatal mortality and serious neonatal morbidity in the second twin. Obstet Gynecol. 2006;108:556–564. doi: 10.1097/01.AOG.0000227747.37184.0a. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of planum temporal asymmetry and morphology of the corpus callosum to handedness, gender, and dyslexia: a review of the evidence. Brain Lang. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The nature and determinants of handedness. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. MIT Press; Cambridge, MA: 2003. pp. 105–158. [Google Scholar]
- Beaton AA, Rudling N, Kissling C, Taurines R, Thome J. Digit ratio (2D:4D), salivary testosterone, and handedness. Laterality. 2010;20:1–20. doi: 10.1080/13576500903410369. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. Fingers as a marker of prenatal androgen exposure. Endocrinology. 2009;150:5119–5124. doi: 10.1210/en.2009-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryden MP, McManus IC, Bulman-Fleming MB. Evaluating the empirical support for the Geschwind-Behan-Galaburda model of cerebral lateralization. Brain Cogn. 1994;26:103–167. doi: 10.1006/brcg.1994.1045. [DOI] [PubMed] [Google Scholar]
- Bryden PJ, Pryde KM, Roy EA. A developmental analysis of the relationship between hand preference and performance: II. A performance-based method of measuring hand preference in children. Brain Cogn. 2000;43:60–64. [PubMed] [Google Scholar]
- Cohen-Bendahan CC, Buitelaar JK, van Goozen SH, Cohen-Kettenis PT. Prenatal exposure to testosterone and functional cerebral lateralization: a study in same-sex and opposite-sex twin girls. Psychoneuroendocrinology. 2004;29:911–916. doi: 10.1016/j.psyneuen.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van Goozen SH, Buitelaar JK, Cohen-Kettenis PT. Maternal serum steroid levels are unrelated to fetal sex: a study in twin pregnancies. Twin Res Hum Genet. 2005;8:173–177. doi: 10.1375/1832427053738764. [DOI] [PubMed] [Google Scholar]
- Collaer ML, Hines M. Human behavioral sex differences: a role for gonadal hormones during early development? Psychol Bull. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Close JP, Dagnall AM, Priddle TH. Where and what is the right shift factor or cerebral dominance gene? A critique of Francks et al (2007) Laterality. 2009;14:3–10. doi: 10.1080/13576500802574984. [DOI] [PubMed] [Google Scholar]
- Dellatolas G, Luciani S, Castresana A, Remy C, Jallon P, Laplane D, Bancaud J. Pathological left-handedness. Left-handedness correlatives in adult epileptics. Brain. 1993;116:1565–1574. doi: 10.1093/brain/116.6.1565. [DOI] [PubMed] [Google Scholar]
- Driesen NR, Raz N. The influence of sex, age, and handedness on corpus callosum morphology: a meta analysis. Psychobiology. 1995;23:240–247. [Google Scholar]
- Elias LJ, Bryden MP. Footedness is a better predictor of language lateralisation than handedness. Laterality. 1998;3:41–51. doi: 10.1080/713754287. [DOI] [PubMed] [Google Scholar]
- Elkadi S, Nicholls ME, Clode D. Handedness in opposite and same-sex dizygotic twins: testing the testosterone hypothesis. Neuroreport. 1999;10:333–336. doi: 10.1097/00001756-199902050-00023. [DOI] [PubMed] [Google Scholar]
- Eriksson CJP, Kaprio J, Pulkkinen L, Rose RJ. Testosterone and alcohol use among adolescent male twins: testing between-family associations in within-family comparisons. Behav Genet. 2005;35:359–368. doi: 10.1007/s10519-005-3228-x. [DOI] [PubMed] [Google Scholar]
- Finegan JAK, Niccols GA, Sitarenios G. Relations between prenatal testosterone levels and cognitive abilities at 4 years. Dev Psychol. 1992;28:1075–1089. [Google Scholar]
- Fink B, Manning JT, Neave N, Tan U. Second to fourth digit ratio and hand skill in Australian children. Biol Psychol. 2004;67:375–384. doi: 10.1016/j.biopsycho.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Francks C. Understanding the genetics of behavioural and psychiatric traits will only be achieved through a realistic assessment of their complexity. Laterality. 2009;14:11–16. doi: 10.1080/13576500802536439. [DOI] [PubMed] [Google Scholar]
- Francks C, Maegawa S, Lauren J, Abrahams BS, Velayos-Baeza A, Medland SE, Colella S, Groszer M, McAuley EZ, Caffrey TM, Timmusk T, Pruunsild P, Koppel I, Lind PA, Matsumoto-Itaba N, Nicod J, Xiong L, Joober R, Enard W, Krinsky B, Nanba E, Richardson AJ, Riley BP, Martin NG, Strittmatter SM, Möller HJ, Rujescu D, St Clair D, Muglia P, Roos JL, Fisher SE, Wade-Martins R, Rouleau GA, Karayiorgou M, Geschwind DH, Ragoussis J, Kendler KS, Airaksinen MS, Oshimura M, DeLisi LE, Monaco AP. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol Psychiatry. 2007;12:1129–39. doi: 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea M, Gomez C, Gonzalez-Bono E, Salvador A, Espert R. Salivary testosterone is related to both handedness and degree of linguistic lateralization in normal women. Psychoneuroendocrinology. 2003;28:274–287. doi: 10.1016/s0306-4530(02)00020-3. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Behan P. Left-handedness: association with immune disease, migraine, and developmental learning disorder. Proc Natl Acad Sci U S A. 1982;79:5097–5100. doi: 10.1073/pnas.79.16.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I A hypothesis and a program for research. Arch Neurol. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- Gilbert AN, Wysocki CJ. Hand preference and age in the United States. Neuropsychologia. 1992;30:601–608. doi: 10.1016/0028-3932(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. A zygosity questionnaire for young twins: a research note. Behav Genet. 1991;21:257–269. doi: 10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Grimshaw GM, Bryden MP, Finegan JAK. Relations between prenatal testosterone and cerebral lateralization in children. Neuropsycholoy. 1995;9:68–79. [Google Scholar]
- Hammond G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neurosci Biobehav Rev. 2002;26:285–292. doi: 10.1016/s0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Harris JA, Vernon PA, Boomsma DI. The heritability of testosterone: a study of Dutch adolescent twins and their parents. Behav Genet. 1998;28:165–171. doi: 10.1023/a:1021466929053. [DOI] [PubMed] [Google Scholar]
- Helleday J, Siwers B, Ritzén M, Hughdahl K. Normal lateralization for handedness and ear advantage in a verbal dichotic listening task in women with congenital adrenal hyperplasia (CAH) Neuropsychologia. 1994;32:875–880. doi: 10.1016/0028-3932(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Hepper PG, McCartney GR, Shannon EA. Lateralised behaviour in first trimester human foetuses. Neuropsychologia. 1998;36:531–534. doi: 10.1016/s0028-3932(97)00156-5. [DOI] [PubMed] [Google Scholar]
- Hepper PG, Shahidullah S, White R. Handedness in the human fetus. Neuropsychologia. 1991;29:1107–1111. doi: 10.1016/0028-3932(91)90080-r. [DOI] [PubMed] [Google Scholar]
- Hepper PG, Wells DL, Lynch C. Prenatal thumb sucking is related to postnatal handedness. Neuropsychologia. 2005;43:313–315. doi: 10.1016/j.neuropsychologia.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Boomsma DI. Heritability of testosterone levels in 12-year-old twins and its relation to pubertal development. Twin Res Hum Genet. 2006;9:558–565. doi: 10.1375/183242706778025071. [DOI] [PubMed] [Google Scholar]
- Jackson C. Prediction of hemispheric asymmetry as measured by handedness from digit length and 2D:4D digit ratio. Laterality. 2008;13:34–50. doi: 10.1080/13576500701692507. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res. 2002;5:366–371. doi: 10.1375/136905202320906101. [DOI] [PubMed] [Google Scholar]
- Kelso WM, Nicholls ME, Warne GL, Zacharin M. Cerebral lateralization and cognitive functioning in patients with congenital adrenal hyperplasia. Neuropsychology. 2000;14:370–378. doi: 10.1037//0894-4105.14.3.370. [DOI] [PubMed] [Google Scholar]
- Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Lalumière ML, Blanchard R, Zucker KJ. Sexual orientation and handedness in men and women: a meta-analysis. Psychol Bull. 2000;126:575–592. doi: 10.1037/0033-2909.126.4.575. [DOI] [PubMed] [Google Scholar]
- Loos R, Derom C, Vlietinck R, Derom R. The East Flanders prospective twin survey (Belgium): a population-based register. Twin Res. 1998;1:167–175. doi: 10.1375/136905298320566131. [DOI] [PubMed] [Google Scholar]
- Luders E, Rex DE, Narr KL, Woods RP, Jancke L, Thompson PM, Mazziotta JC, Toga AW. Relationship between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- Lummaa V, Pettay JE, Russell AF. Male twins reduce fitness of female co-twins in humans. Proc Natl Acad Sci U S A. 2007;104:10915–10920. doi: 10.1073/pnas.0605875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lust JM, Geuze RH, Van de Beek C, Cohen-Kettenis PT, Groothuis AGG, Bouma A. Sex specific effect of prenatal testosterone on language lateralization in children. Neuropsychologia. 2010;48:536–540. doi: 10.1016/j.neuropsychologia.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Manning JT. Digit ratio: a pointer to fertility, behaviour, and health. New Brunswick: Rutgers University Press; 2002. [Google Scholar]
- Manning JT, Trivers RL, Thornhill R, Singh D. The 2nd:4th digit ratio and asymmetry of hand performance in Jamaican children. Laterality. 2000;5:121–132. [PubMed] [Google Scholar]
- Mathews GA, Fane BA, Pasterski VL, Conway GS, Brook C, Hines M. Androgenic influences on neural asymmetry: handedness and language lateralization in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2004;29:810–822. doi: 10.1016/S0306-4530(03)00145-8. [DOI] [PubMed] [Google Scholar]
- McCartney G, Hepper P. Development of lateralized behaviour in the human fetus from 12 to 27 weeks’ gestation. Dev Med Child Neurol. 1999;41:83–86. doi: 10.1017/s0012162299000183. [DOI] [PubMed] [Google Scholar]
- McFadden D. A masculinizing effect on the auditory systems of human females having male co-twins. Proc Natl Acad Sci U S A. 1993;90:11900–11904. doi: 10.1073/pnas.90.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medland SE, Duffy DL, Spurdle AB, Wright MJ, Geffen GM, Montgomery GW, Martin NG. Opposite effects of androgen receptor CAG repeat length on increased risk of left-handedness in males and females. Behav Genet. 2005;35:735–744. doi: 10.1007/s10519-005-6187-3. [DOI] [PubMed] [Google Scholar]
- Medland SE, Duffy DL, Wright MJ, Geffen GM, Hay DA, Levy F, van-Beijsterveldt CE, Willemsen G, Townsend GC, White V, Hewitt AW, Mackey DA, Bailey JM, Slutske WS, Nyholt DR, Treloar SA, Martin NG, Boomsma DI. Genetic influences on handedness: data from 25,732 Australian and Dutch twin families. Neuropsychologia. 2009;47:330–337. doi: 10.1016/j.neuropsychologia.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medland SE, Duffy DL, Wright MJ, Geffen GM, Martin NG. Handedness in twins: joint analysis of data from 35 samples. Twin Res Hum Genet. 2006;9:46–53. doi: 10.1375/183242706776402885. [DOI] [PubMed] [Google Scholar]
- Medland SE, Loehlin JC, Martin NG. No effects of prenatal hormone transfer on digit ratio in a large sample of same- and opposite-sex dizygotic twins. Pers Individ Dif. 2008;44:1225–1234. [Google Scholar]
- Medland SE, Wright MJ, Geffen GM, Hay DA, Levy F, Martin NG, Duffy DL. Special twin environments, genetic influences and their effects on the handedness of twins and their siblings. Twin Res. 2003;6:119–130. doi: 10.1375/136905203321536245. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. Salivary testosterone levels in left- and right-handed adults. Neuropsychologia. 1996;34:225–233. doi: 10.1016/0028-3932(95)00090-9. [DOI] [PubMed] [Google Scholar]
- Nagamani M, McDonough PG, Ellegood JO, Manesh VB. Maternal and amniotic fluid steroids throughout human pregnancy. Am J Obstet Gynecol. 1979;13:674–680. doi: 10.1016/0002-9378(79)90649-5. [DOI] [PubMed] [Google Scholar]
- Nass R, Baker S, Speiser P, Virdis R, Balsamo A, Cacciari E, Loche A, Dumic M, New M. Hormones and handedness: left-hand bias in female congenital adrenal hyperplasia patients. Neurology. 1987;37:711–715. doi: 10.1212/wnl.37.4.711. [DOI] [PubMed] [Google Scholar]
- Nicholls MER, Orr CA, Yates MJ, Loftus AM. A new means of measuring index/ring finger (2D:4D) ratio and its association with gender and hand preference. Laterality. 2008;13:71–91. doi: 10.1080/13576500701751287. [DOI] [PubMed] [Google Scholar]
- Ooki S. Non genetic factors associated with human handedness and footedness in Japanese twin children. Environ Health Prev Med. 2006;11:304–312. doi: 10.1007/BF02898021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadatou-Pastou M, Martin M, Munafo MR, Jones GV. Sex differences in left-handedness: a meta-analysis of 144 studies. Psychol Bull. 2008;134:677–699. doi: 10.1037/a0012814. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, van Baal GCM, Schnack HG, van Leeuwen M, Boomsma DI, Kahn RS, Hulshof Pol HE. Does having a twin-brother make for a bigger brain? Eur J Endocrinol. 2009;160:739–746. doi: 10.1530/EJE-08-0915. [DOI] [PubMed] [Google Scholar]
- Peters M, Oeltze S, Seminowicz D, Steinmetz H, Koeneke S, Jäncke L. Division of the corpus callosum into subregions. Brain Cogn. 2002;50:62–72. doi: 10.1016/s0278-2626(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Peters M, Reimers S, Manning JT. Hand preference for writing and associations with selected demographic and behavioral variables in 255,100 subjects: the BBC internet study. Brain Cogn. 2006;62:177–189. doi: 10.1016/j.bandc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Pfannkuche KA, Bouma A, Groothuis TGG. Does testosterone affect lateralization of brain and behaviour? A meta-analysis in humans and other animal species. Phil Trans R Soc B. 2009;364:929–942. doi: 10.1098/rstb.2008.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powls A, Botting N, Cooke RW, Marlow N. Handedness in very-low-birthweight (VLBW) children at 12 years of age: relation to perinatal and outcome variables. Dev Med Child Neurol. 1996;38:594–602. doi: 10.1111/j.1469-8749.1996.tb12124.x. [DOI] [PubMed] [Google Scholar]
- Previc FH. A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychol Rev. 1991;98:299–334. doi: 10.1037/0033-295x.98.3.299. [DOI] [PubMed] [Google Scholar]
- Previc FH. Assessing the legacy of the GBG model. Brain Cog. 1994;26:174–180. doi: 10.1006/brcg.1994.1047. [DOI] [PubMed] [Google Scholar]
- Puts DA, McDaniel MA, Jordan CL, Breedlove SM. Spatial ability and prenatal androgens: meta-analyses of congenital adrenal hyperplasia and digit ratio (2D:4D) studies. Arch Sex Behav. 2008;37:100–111. doi: 10.1007/s10508-007-9271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadhani MK, Koomen I, Grobbee DE, van Donselaar CA, Marceline van Furth A, Uiterwaal CS. Increased occurrence of left-handedness after severe childhood bacterial meningitis: support for the pathological left-handedness hypothesis. Neuropsychologia. 2006;44:2526–2532. doi: 10.1016/j.neuropsychologia.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Rao JNK, Scott AJ. On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat. 1984;12:46–60. [Google Scholar]
- Rose RJ, Kaprio J, Winter T, Dick DM, Viken RJ, Pulkkinen L, Koskenvuo M. Femininity and fertility in sisters with twin brothers: prenatal androgenization? Cross-sex socialization? Psychol Sci. 2002;13:263–267. doi: 10.1111/1467-9280.00448. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- Sarna S, Kaprio J, Sistonen P, Koskenvuo M. Diagnosis of twin zygosity by mailed questionnaire. Hum Hered. 1978;28:241–254. doi: 10.1159/000152964. [DOI] [PubMed] [Google Scholar]
- Schachter SC. Handedness in women with intrauterine exposure to diethylstilbestrol. Neuropsychologia. 1994;32:619–623. doi: 10.1016/0028-3932(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Scheirs JG, Vingerhoets AJ. Handedness and other laterality indices in women prenatally exposed to DES. J Clin Exp Neuropsychol. 1995;17:725–730. doi: 10.1080/01688639508405162. [DOI] [PubMed] [Google Scholar]
- Smith LL, Hines M. Language lateralization and handedness in women prenatally exposed to diethylstilbestrol (DES) Psychoneuroendocrinology. 2000;25:497–512. doi: 10.1016/s0306-4530(00)00005-6. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Bilder RM, Wu H, Bogerts B, Lieberman JA. Cerebellar volume asymmetries are related to handedness: a quantitative MRI study. Neuropsychologia. 1995;33:407–419. doi: 10.1016/0028-3932(94)00125-9. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Somers M, Boks MP, Kahn RS. Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization. Brain Res. 2008;24:76–88. doi: 10.1016/j.brainres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Stata. Stata statistical software: Release 9.1. College Station, Texas: Stata Corporation; 2006. [Google Scholar]
- Tan U, Tan M. Testosterone and grasp-reflex differences in human neonates. Laterality. 2001;6:181–192. doi: 10.1080/713754405. [DOI] [PubMed] [Google Scholar]
- Van Anders SM, Vernon PA, Wilbur CJ. Finger-length ratios show evidence of prenatal hormone-transfer between opposite-sex twins. Horm Behav. 2006;49:315–319. doi: 10.1016/j.yhbeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Van de Beek C, Thijssen JHH, Cohen-Kettenis PT, Van Goozen SHM, Buitelaar JK. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure. Horm Behav. 2004;46:663–669. doi: 10.1016/j.yhbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Voracek M, Dressler SG. Digit ratio (2D:4D) in twins: heritability estimates and evidence for a masculinized trait expression in women from opposite-sex pairs. Psychol Rep. 2007;100:115–126. doi: 10.2466/pr0.100.1.115-126. [DOI] [PubMed] [Google Scholar]
- Vuoksimaa E, Koskenvuo M, Rose RJ, Kaprio J. Origins of handedness: a nationwide study of 30161 adults. Neuropsychologia. 2009;47:1294–1301. doi: 10.1016/j.neuropsychologia.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoksimaa E, Kaprio J. Sex differences in left-handedness exists also in Scandinavia and in twins: comment on Papadatou-Pastou, Martin, Munafo & Jones. Psychol Bull. 2008 doi: 10.1037/a0018972. in press. [DOI] [PubMed] [Google Scholar]
- Vuoksimaa E, Kaprio J, Kremen WS, Hokkanen L, Viken RJ, Tuulio-Henriksson A, Rose RJ. Having a male co-twin masculinises mental rotation performance in females. Psychol Sci. doi: 10.1177/0956797610376075. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Does finger fat produce sex difference in second to fourth digit ratios. Endocrinology. 2009;150:4819–4822. doi: 10.1210/en.2009-0986. [DOI] [PubMed] [Google Scholar]
- Warren DM, Stern M, Duggirala R, Dyer TD, Almasy L. Heritability and linkage analysis of hand, foot, and eye preference in Mexican Americans. Laterality. 2006;11:508–524. doi: 10.1080/13576500600761056. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Witelson SF. The brain connection: the corpus callosum is larger in left-handers. Science. 1985;229:665–668. doi: 10.1126/science.4023705. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Neural sexual mosaicism: sexual differentiation of the human temporo-parietal region for functional asymmetry. Psychoneuroendocrinology. 1991;16:131–153. doi: 10.1016/0306-4530(91)90075-5. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Nowakowski RS. Left out axons make men right: a hypothesis for the origin of handedness and functional asymmetry. Neuropsychologia. 1991;29:327–333. doi: 10.1016/0028-3932(91)90046-b. [DOI] [PubMed] [Google Scholar]
- Ypsilanti A, Ganou M, Koidou I, Grouios G. Digit ratio (2D:4D) in individuals with intellectual disability: investigating the role of testosterone in the establishment of cerebral lateralisation. Laterality. 2008;13:527–544. doi: 10.1080/13576500802117164. [DOI] [PubMed] [Google Scholar]


