Abstract
Background
ΔFosB is the best characterized transcription factor induced by chronic stimulation. Although previous studies have demonstrated that chronic passive ethanol exposure alters ΔFosB immunoreactivity (IR), the effect of chronic voluntary ethanol consumption on ΔFosB remains unknown. Furthermore, although previous studies have demonstrated that the opioid antagonist naltrexone reduces alcohol consumption in clinical and pre-clinical settings, the effect of naltrexone on FosB/ΔFosB has not been explored. Here we examined the effects of chronic voluntary ethanol intake and naltrexone on FosB/ΔFosB IR in striatal region and prefrontal cortex, and the effect of naltrexone on voluntary ethanol intake.
Methods
We utilized immunohistochemistry to define the changes in FosB/ΔFosB IR induced by chronic voluntary ethanol intake under a two-bottle intermittent access of 20% ethanol model and by systematic administration (intraperitoneal injection) of naltrexone in Sprague-Dawley rats.
Results
Chronic (15 drinking sessions in 35 days) voluntary ethanol intake robustly induces FosB/ΔFosB IR in nucleus accumbens core, dorsolateral striatum and orbitofrontal cortex, but not in nucleus accumbens shell, dorsomedial striatum and medial prefrontal cortex. Systemic administration of naltrexone for six days significantly reduced voluntary ethanol consumption and FosB/ΔFosB IR induced by chronic voluntary ethanol intake.
Conclusion
Our results suggest that chronic voluntary ethanol intake induces FosB/ΔFosB IR in a sub-region-specific manner and which involves activation of endogenous opioid system.
Keywords: FosB/Δ FosB, Alcohol, Opioid receptors
Introduction
Repeated exposures to abused drugs can cause several neuroadaptations in the mesocorticolimbic dopaminergic system. One such adaptation is the altered expression of transcription factors, which give rise to changes in gene expression and may lead to alterations in sensitivity to drugs of abuse (Kelz et al., 1999; Nestler et al., 2001; McClung et al., 2004). Regulation of gene expression is one of the mechanisms by which drugs of abuse can induce relatively long-lasting changes in the brain to cause a state of addiction. In particular, two transcription factors - ΔFosB and CREB (cAMP responsive element binding protein) have been implicated in addiction-related neural plasticity. Repeated exposures to drugs of abuse can lead to an increase in ΔFosB levels - an effect that persists for a relatively long time after the cessation of drug treatment (Hope et al., 1994; Nestler et al., 2001). ΔFosB is a protein formed by truncation of the C-terminal region of FosB protein (Nakabeppu and Nathans, 1991). Upon induction, ΔFosB heterodimerizes with Jun family proteins (Gentz et al., 1989; Nakabeppu and Nathans, 1991). ΔFosB subsequently forms an activator protein-1 binding complex, which can alter expression of other genes such as those encoding the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subunit of glutamate receptor, dynorphin and cyclin-dependent kinase-5 (Cdk5) (Chen et al., 1997; Kelz et al., 1999; Bibb et al., 2001). Therefore, drugs of abuse can produce long-term changes in brain neurochemistry through activation of these target genes by ΔFosB.
Despite this evidence, important questions remain unanswered. Although it is well documented that chronic exposure to abused drugs including alcohol increases ΔFosB expression in several areas of the mesocorticolimbic system of rat brains (Atkins et al., 1999; Ehrlich et al., 2002; McDaid et al., 2006b; Muller and Unterwald, 2005; Nye and Nestler, 1996; Pich et al., 1997; Perrotti et al., 2008; Zachariou et al., 2006, Perrotti et al., 2008), it still remains unknown whether chronic voluntary alcohol consumption can alter ΔFosB. Even though it has been shown previously that the opioid receptor antagonist naltrexone significantly reduces alcohol consumption in many studies in human and experimental animals (Bienkowski et al., 1999; Cichelli and Lewis, 2002; O'Malley et al., 2002; Coonfield et al., 2004; Stromberg et al., 2004; Zalewska-Kaszubska et al., 2008), study on the possible participation of opioid receptors in ΔFosB accumulation associated with drugs of abuse is lacking (Marttila et al., 2007, Kaste et al., 2009). Therefore, we conducted the current study to determine: 1) whether chronic voluntary alcohol consumption can alter FosB/ΔFosB, and 2) whether naltrexone induced reduction of alcohol consumption is associated with changes in FosB/ΔFosB.
Materials and Methods
All experiments were performed in accordance with the guidelines of the National Institutes of Health for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey, Newark, New Jersey.
Animals and Housing
Adult Sprague-Dawley (S-D) rats (n = 24, 250–350 g, at the start of the experiments, Taconic Farm, NY) were individually housed in ventilated Plexiglas cages. All rats were housed in a climate-controlled room (20–22°C). Rats were kept on a 12-h light/dark cycle (lights off at 5 p.m.). Food and water were available ad libitum.
Intermittent access to 20% ethanol using a two-bottle-choice drinking procedure
After acclimating to the homecage environment for 1 week, animals in ethanol-drinking groups were trained for self-administration of ethanol under a 2-bottle intermittent paradigm as described previously (Simms et al., 2008; Stuber et al., 2008). Briefly, animals had 24-hour concurrent access to two bottles, one with 20% ethanol (v/v) and another with water only starting on Monday afternoon. After 24 hours, the ethanol bottle was replaced with a second water bottle that was available for the next 24 hours. This pattern was repeated on Wednesdays and Fridays. On all other days the rats had unlimited access to two bottles of water. In each ethanol drinking session, the placement of the ethanol bottle was alternated to control for side preferences. The amount of ethanol or water consumed was determined by weighing the bottles before access and after 24 hours of access. Ethanol consumption was determined by calculating grams of alcohol consumed per kilogram of body weight. A bottle containing water in a cage without rats was used to evaluate the spillage due to the experimental manipulations during the test sessions. The spillage was always < 1.0 ml (< 2.5% of the total fluid intake). Animals in the control group were allowed to access to water and food without limitation. There were no significant differences in body weight between the control and the ethanol-drinking rats at the end of the experiments.
Naltrexone treatment
In this series of experiments, rats (n = 12) were first trained to drink ethanol voluntarily as described above. After 15 ethanol-drinking sessions, rats were randomly divided into two groups (n = 6 for each): one received naltrexone (2.0 mg/kg, i.p.) and the other received vehicle (saline, i.p.) respectively, for 6 consecutive days (Monday through Saturday). During the treatment days, rats had three ethanol-drinking sessions (day 1, Monday; day 3, Wednesday; and day 5, Friday). The amount of ethanol or water consumed was determined by weighing the bottles before access, after 30 min of access, and after 24 hours of access. We used 2.0 mg/kg naltrexone based on previous report showing that at this dose naltrexone effectively decreased ethanol consumption in rodents (Zalewska-Kazubska, et al., 2008). Naltrexone was dissolved in saline (1 ml/kg) immediately before each injection, and administered (i.p.) 30 min before ethanol and water bottles were presented.
Immunohistochemistry
Rats were sacrificed at 18 hours after the last drinking session. We choose this assay time point based on previous studies showing that 18–24 h after stimulus, the full-length FosB was degraded leaving the stable ΔFosB as the only FosB gene product (Chen et al., 1997; Perrotti et al., 2008; Ulery et al., 2006; Chen et al., 1995; Hope et al., 1994). Rats were overdosed with pentobarbital (i.p.) and transcardially perfused with cold-buffered saline followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4. The brains were removed and then transferred to a solution containing 20% sucrose in 4% paraformaldehyde overnight at 4°C for cryoprotection. Coronal sections (30 µm) were cut on a freezing microtome (Microm HM550, Walldorf, German) and then processed for immunohistochemistry. Brain sections were first treated with 0.3% hydrogen peroxide solution to inhibit endogenous peroxidase activity and then incubated for 30 min in a solution containing 0.3% Triton X-100 and 3% normal goat serum to minimize non-specific labeling. Then, the tissue sections were incubated in a solution containing 1% normal goat serum, 0.3% Triton-100 and pan-FosB antibody (1:3000, #sc-48; Santa Cruz Biotechnology, Santa Cruz, California) overnight, at 4 °C.
On the following day, sections were washed and placed for 40 min in a solution of biotinylated anti-rabbit antibody (1:200, Vector Laboratories, Burlingame, CA), again washed and placed for 40 min in 1:200 dilution of avidin–biotin complex from the ABC kit (Vector Laboratories, Burlingame, CA). After the final wash, antibody labeling was visualized with diaminobenzidine solution (Vector Laboratories, Burlingame, CA).Coded slides were used to count the number of FosB-immunoreactive cells. The code was not broken until analysis of an individual experiment was complete.
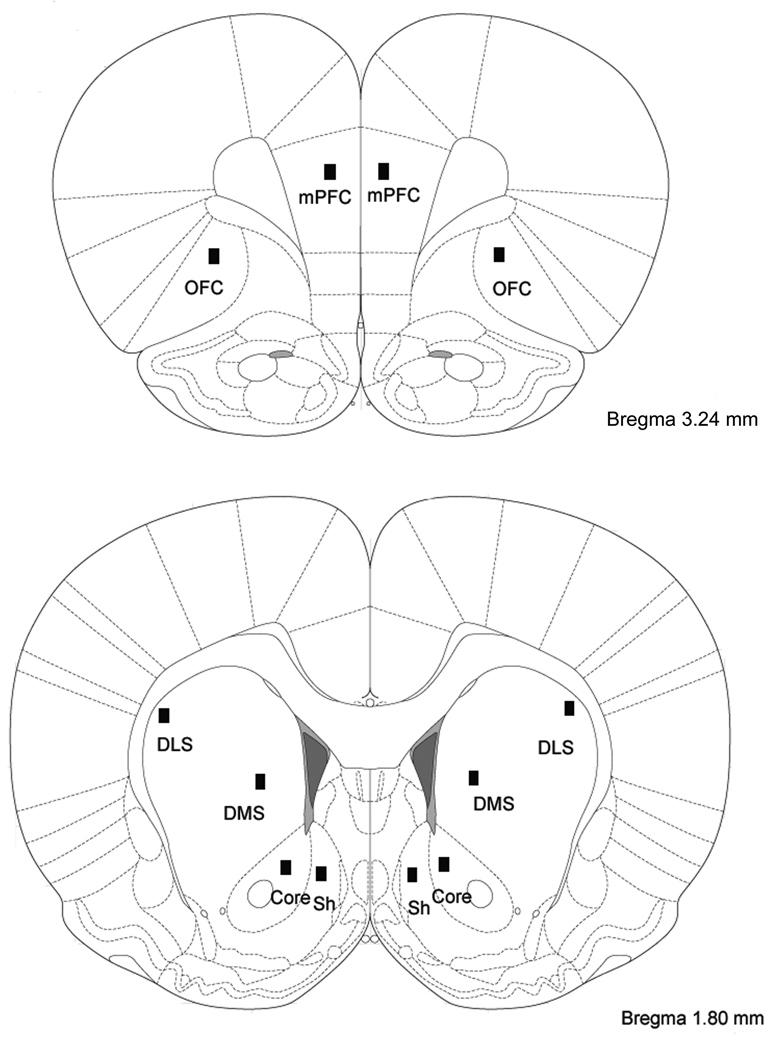
The total number of FosB/ΔFosB immunostaining was counted in several regions of interest, namely the medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), NAc (core and shell), dorsolateral striatum (DLS) and dorsomedial striatum (DMS, Figure 1). These brain regions were identified based on the Atlas of Paxinos and Watson (2005). The analysis was quantified using a computerized image-analysis system including Nikon DS-Ri1 digital camera, Nikon Eclipse 80i microscope, and a computer with a NIS-Elements BR 3.0 software. Images were captured using Nikon (DS-Ri1) camera under identical and calibrated exposure conditions. Two dimensional counts of labeled nuclei from each image [200× images (0.1 mm2 area) of FosB/ΔFosB-like immunoreactive nuclei within brain regions of interest] were determined without knowledge of treatment condition from three separate sections per animal using NIS-Elements BR 3.0 software.
Figure 1.
Schematic drawings of coronal sections of the rat brain based upon the atlas of Paxinos and Watson (2005), showing the different regions where quantification of ΔFosB positive nuclei was carried out. Counts were obtained from both hemispheres and from four sections for nucleus accumbens, DLS and DMS (AP 2.16, 1.92, 1.8 and 1.56) and from three sections within prefrontal cortex (AP 3.72, 3.24 and 3.00). mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; DLS, dorsolateral striatum; DMS, dorsomedial striatum; Core, Nucleus accumbens core; Sh, Nucleus accumbens shell.
Statistical Analyses
All data are expressed as mean ± SEM (standard error of the mean). Behavioral data were analyzed with the factors of treatment (saline or naltrexone) and days [baseline (0 day), day 1, 3, 5). Student-Newman-Keuls post hoc analysis was conducted using contrast analysis when day × treatment interaction was P < 0.05. Immunohistochemical results were analyzed using the Student’s t test.
RESULTS
Voluntary ethanol consumption increases FosB/ΔFosB-protein immunoreactivity in selective subregions of the rat brain
Given the dramatic induction of ΔFosB in rodent brain in response to chronic passive ethanol exposure (Perrotti et al., 2008), we were interested in determining the ability of voluntary ethanol consumption to induce ΔFosB in the brain. In this series of experiments, after acclimating to the homecage environment for 1 week, rats (n = 12) were divided into a control and an experimental group with matching body weights (control group: 276 ± 21 g, ethanol group: 279 ± 15 g, P = 0.93). In the experimental group, rats were trained for self-administration of 20% ethanol using the intermittent-access two-bottle choice drinking paradigm, as described in the Method section. One-way ANOVA analysis revealed that S-D rats under this drinking paradigm significantly escalated their ethanol intake over 2–3 drinking sessions (P < 0.001). This is in general agreement with previous reports in Long-Evans and Wistar rats (Simms et al., 2008; Stuber et al., 2008). Post hoc analysis revealed that the mean ethanol intake was significantly higher after the fourth drinking sessions than that in the first drinking session (2.82 ± 0.40 g ethanol/kg body weight/24 h). Following the fourth drinking session, there was no difference in the ethanol intake between any of the subsequent drinking sessions. That is, the rats had reached a stable consumption level of 5.63 ± 0.30 g ethanol/kg body weight/24 h. Ethanol consumption was terminated after 35 days (15 sessions) of ethanol-drinking. At 18 h after the last self-administration bout, rats were sacrificed and levels of FosB/ΔFosB protein were quantified by immunohistochemistry with a focus on striatal region and prefrontal cortex (Fig. 1), brain regions implicated in drug reward and addiction.
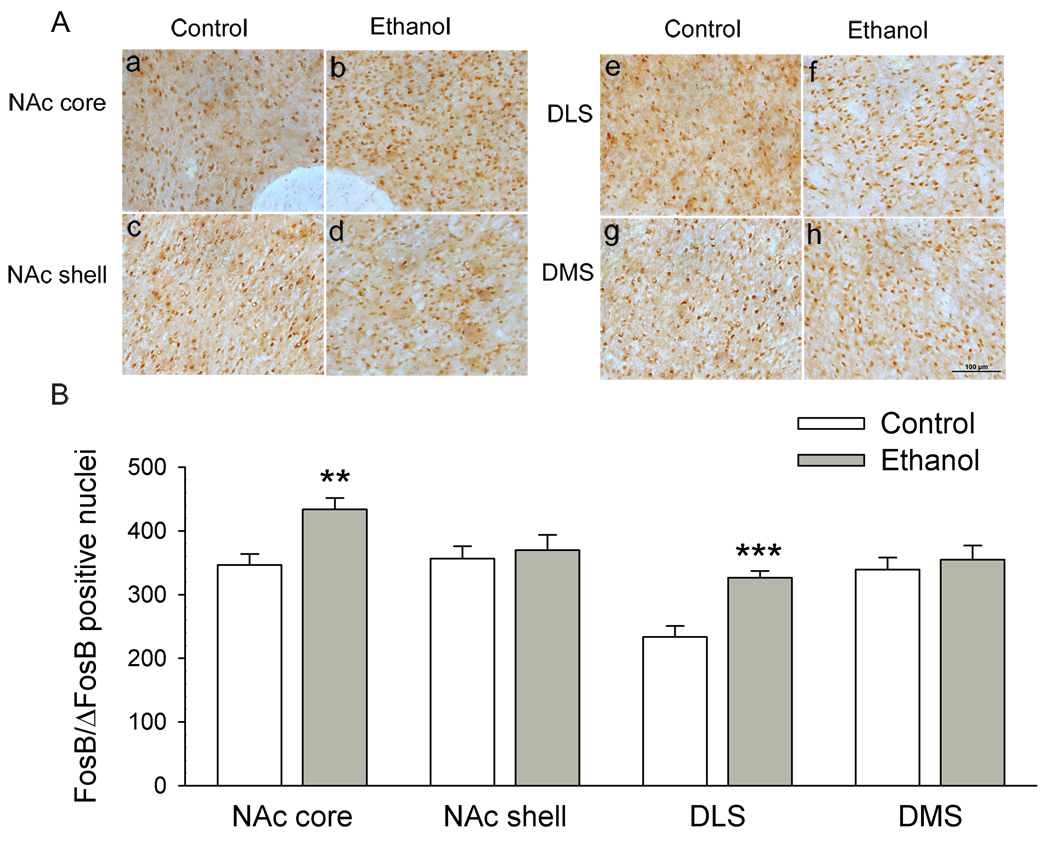
Chronic voluntary ethanol intakes significantly increased FosB/ΔFosB IR in the NAc core (t = −3.48, P = 0.002, Student’s t test, compared to ethanol-naïve rats; Fig. 2Aa, 2Ab), and in the dorsolateral striatum (DLS) (t = −4.79, P < 0.001, Fig. 2Ac, 2Ad). In the NAc core, the number of ΔFosB-like reactive nuclei was 346 ± 17 in ethanol naïve rats and 434 ± 18 in ethanol-drinking rats, respectively. In the DLS, it was 233 ± 17 in ethanol naïve rats and 326 ± 11 in ethanol-drinking rats, respectively (Fig. 2B). However, no such change was found in the NAc shell (t = −0.41, P = 0.68, Fig. 2Ae, 2Af), and in the dorsomedial striatum (DMS) (t = −0.52, P = 0.61, Fig. 2Ag, 2Ah). In the NAc shell, the number of FosB-like reactive nuclei was 356 ± 20 in ethanol naïve rats and 369 ± 25 in ethanol-drinking rats. In the DMS, it was 339 ± 18 in ethanol naïve rats and 355 ± 22 in ethanol-drinking rats, respectively (Fig. 2B).
Figure 2.
Chronic voluntary ethanol intake significantly increases ΔFosB IR within the NAc core (a–b) and DLS (e–f), but not in the NAc shell (c–d) and DMS (g–h). Data are expressed as mean ± SEM (n = 6 animals in each group). ** P < 0.01, *** P < 0.001 in comparison to control. Scale bar =100 µm.
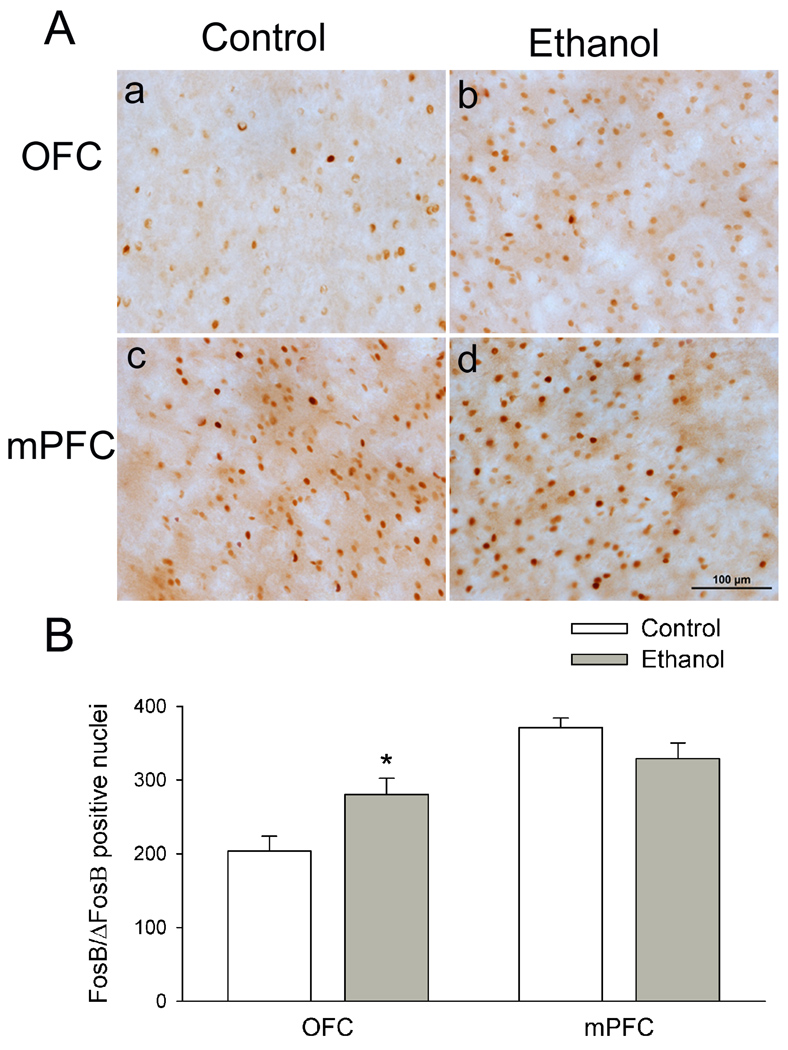
The prefrontal cortex is responsible for the organization of behavior. Disruptions in the prefrontal cortex network activity can lead to the loss of control over behavior, contributing to the impulsive and habitual behaviors associated with drug addiction including ethanol abuse. Therefore, beyond the striatal complex, we also compared FosB/ΔFosB expression in the ventral orbitofrontal cortex (OFC) and the medial prefrontal cortex (mPFC). Chronic voluntary ethanol intakes significantly increased the number of FosB/ΔFosB positive nuclei in the OFC (Fig. 3Aa, 3Ab) from 204 ± 20 in ethanol-naïve rats to 280 ± 22 (Student’s t test, t = −2.6, P = 0.019, Fig. 3B). Conversely, the numbers of FosB/ΔFosB positive nuclei in the mPFC in ethanol-naïve rats (371 ± 13) is similar to that (329 ± 21) in ethanol-drinking rats (t = 1.7, P = 0.11).
Figure 3.
Voluntary ethanol intake significantly increases ΔFosB levels in the OFC (a–b), but not the mPFC (c–d). Data are expressed as mean ± SEM (n = 6 animals in each group). * P < 0.05 in comparison to control. Scale bar = 100 µm.
Naltrexone treatment reduces voluntary ethanol intake and blocks ethanol-induced up-regulation of FosB/ΔFosB in the striatal region and OFC
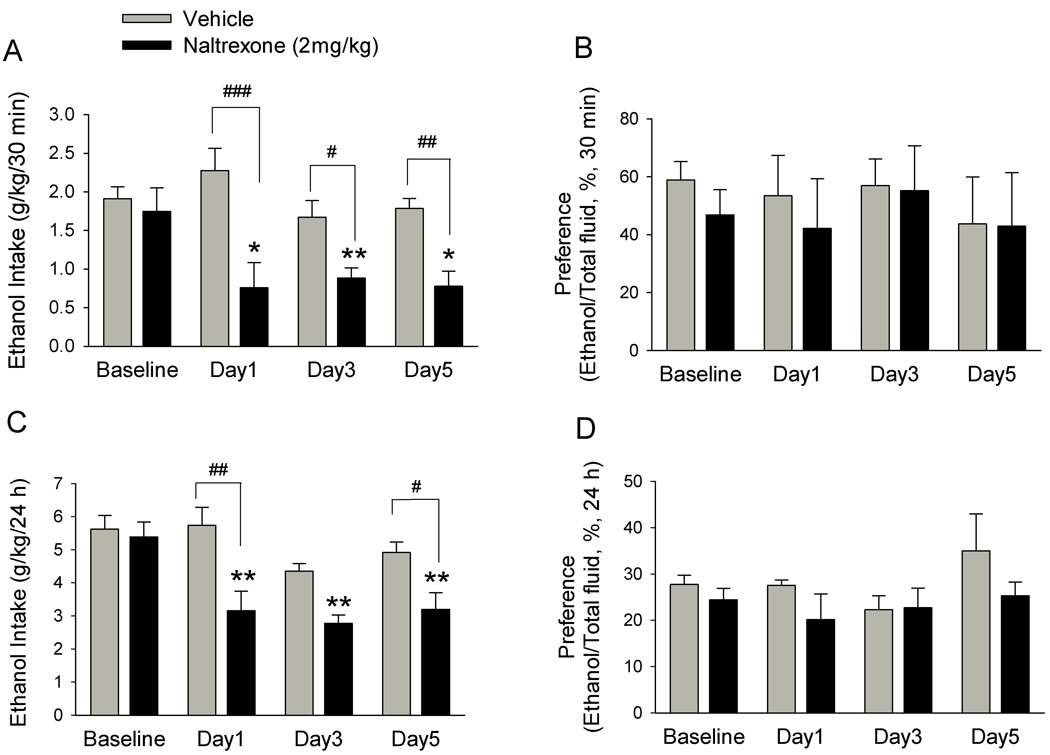
We next examined the effect of naltrexone. In this series of experiments, rats (n = 12) were first trained for self-administration of ethanol using the intermittent two-bottle drinking paradigm, as described in the Method section. After 35 days (15 sessions) of self-administration of ethanol, rats were divided into two groups: One group (n = 6) received naltrexone (2 mg/kg, i.p.), and the other (n = 6) received vehicle (saline, 1 ml/kg, i.p.), 30 min before the start of the ethanol self-administration session, once per day for 6 consecutive days. The ethanol consumed was measured on days 1, 3, and 5. There was not significant difference regarding their baseline ethanol drinking levels (saline group: 5.62 ± 0.42 g ethanol/kg body weight/24 h, naltrexone group: 5.39 ± 0.45 g ethanol/kg body weight/24 h, P = 0.67; Fig. 4 B).
Figure 4.
Naltrexone significantly decreases voluntary ethanol intake. Naltrexone (2.0 mg/ kg, i.p.,) or vehicle was administered to two different groups of rats on each of 6 consecutive days, 30 min before the start of ethanol- or water-drinking session. The effect of naltrexone or vehicle on ethanol consumption was measured on day 1, 3, and 5 and compared with baseline drinking levels. Naltrexone (black bars) but not vehicle (grey bars) administration significantly decreased ethanol consumption (g/kg) compared with baseline drinking levels at 30 min (A) and 24 h (C) after the onset of drinking. There is no significant difference regarding the baseline drinking levels between the naltrexone and vehicle groups. Saline administration had no significant effect on ethanol consumption compared with baseline drinking levels at 30 min (A) or 24 h (C) after the onset of drinking. The values are expressed as mean ± SEM (two-way ANOVA followed by Newman-Keuls post hoc test). * P < 0.05, ** P < 0.01 compared with baseline drinking levels, # P < 0.05, ## P < 0.01, ### P < 0.001 compared with vehicle. n = 6 animals in each group.
Naltrexone but not vehicle treatment reduced ethanol intake in rats on all the ethanol-drinking days (1, 3, and 5). The two-way ANOVA analysis revealed a significant group effect on ethanol consumption at all points (30 min: F1, 46 = 25.34, P < 0.001, Fig. 4A; 24 h: F1, 46 = 18.95, P < 0.001, Fig. 4C), two-way interaction effect for group × day at all points (30 min: F3, 46 = 2.98, P = 0.046; 24 h: F3, 46 = 2.97, P = 0.049) and day effect (F3,46= 7.9, P < 0.001) at 24 h, but no day effect (F3,46= 2.79, P = 0.056) at 30 min. The main effect of group together with the effect of a group × day interaction revealed that naltrexone decreased ethanol consumption at 30 min (Fig. 4A) and 24 h (Fig. 4C) in all drinking sessions during the naltrexone treatment period. The persistent naltrexone-induced reduction in ethanol intake shown in days 1, 3 and 5, indicates the lack of tolerance to naltrexone. Post hoc analysis comparing treatment factors also revealed that the amount of ethanol consumed by naltrexone-treated rats was significant less than that by saline-treated rats at 30 min point on day 1, 3 and 5, and at 24 h point on day 1 and 5. Conversely, in the vehicle-treated group, ethanol consumption did not significantly change at all time points (30 min: Fig. 4A; 24 h: Fig. 4C). Naltrexone treatment did not decrease the total fluid intake at 24-h time point in any of the drinking days (F1, 46 =0.49, P = 0.49, Table 1), although it significantly decreased water and total fluid intake at 30-min time point (water: F1, 46 = 4.55, P = 0.046; total fluid: F1, 46 =24.14, P < 0.001, Table 1). Furthermore, naltrexone-induced reduction in the proportion of ethanol over the total fluid consumed was not significant (30 min: F1, 46 = 0.47, P = 0.49; 24 h: F1, 46 = 3.17, P = 0.08, Fig. 4B, 4D).
Table 1.
Water and total fluid measured during the first 30 mins and 24 h after onset of drinking
| Fluid intake in 30 mins after onset of drinking |
||||||
|---|---|---|---|---|---|---|
| Day 1 |
Day 2 |
Day 3 |
||||
| Water, ml | Total fluid, ml | Water, ml | Total fluid, ml | Water, ml | Total fluid, m | |
| Saline | 4.1 ± 1.3 | 8.2 ± 0.6 | 2.2 ± 0.7 | 5.3 ± 0.4 | 1.9 ± 0.9 | 5.2 ± 0.8 |
| Naltrexone | 3.0 ± 0.9 | 4.8 ± 1.2** | 0.7 ± 0.3 | 2.8 ± 0.3* | 0.4 ± 0.2 | 2.1 ± 0.3** |
| Fluid intake in 24 h after onset of drinking |
||||||
| Day 1 |
Day 2 |
Day 3 |
||||
| Water, ml | Total fluid, ml | Water, ml | Total fluid, ml | Water, ml | Total fluid, ml | |
| Saline | 29.5 ± 2.8 | 40.8 ± 4.0 | 28.3 ± 3.2 | 36.2 ± 2.7 | 26.3 ± 3.8 | 37.4 ± 4.0 |
| Naltrexone | 33.8 ± 4.6 | 42.0 ± 3.6 | 30.1 ± 5.1 | 38.8 ± 5.4 | 29.7 ± 4.4 | 39.4 ± 3.08 |
Naltrexone but not vehicle administration significantly decreased 30 min water intake and total fluid on day 3 and day 5 compared with saline treatment. However, the effect on water intake and total fluid was abolished 24 h after naltrexone treatment. The values are expressed as mean fluid intake ± SEM (two-way ANOVA followed by Newman-Keuls post hoc test.
P < 0.05,
P < 0.01 compared with saline, n = 6.
Remarkably, S-D rats consumed large quantities of ethanol in the first 30 min of ethanol access, reached a baseline level of 1.83 ± 0.016 g/kg/30 min, which is a significant portion of ethanol intake in 24 h. That is in just 2.1% of 24 h (30 min is 2.1% of 24 h), ~32% of 24 h ethanol intake was drunk. Our finding is consistent with a recent report showing that Long-Evens rats consumed large quantities of ethanol very rapidly (1.39 ± 0.11 g/kg) in the first 30 min of the session, which corresponded to ~25% of the total ethanol consumed within 24 h (Carnicella et al., 2009). These data suggest that rats become more inclined to ethanol drinking as soon as it’s available, and then slowly steers away from it.
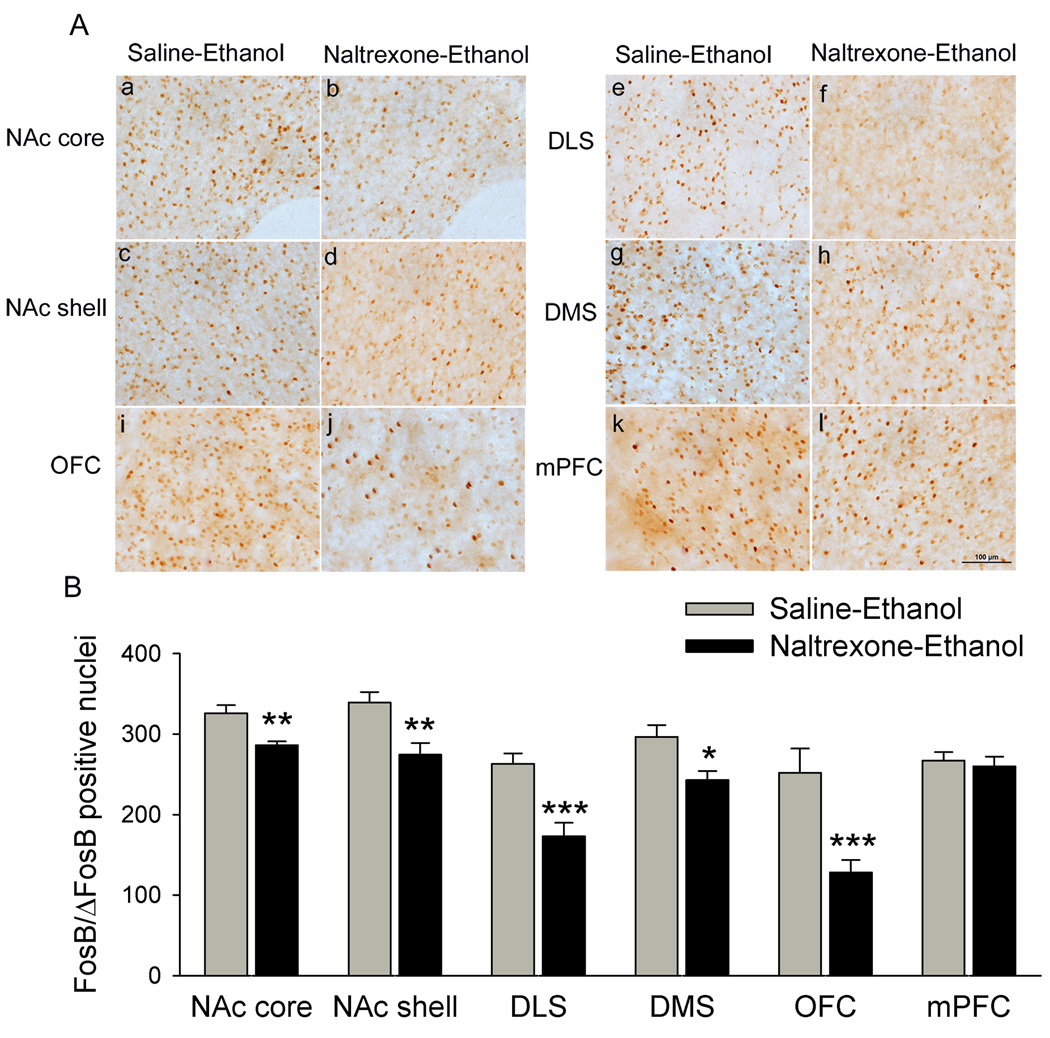
At the end of above consumption experiment, and at 18 h after the last self-administration bout, these rats were sacrificed and effects of naltrexone on FosB/ΔFosB induced by voluntary ethanol intake were examined. Naltrexone treatments dramatically decreased FosB/ΔFosB IR in the NAc core [t = 3.99, P = 0.002, Fig. 5 Aa, 5 Ab], NAc shell [t = 3.22, P = 0.007, Fig. 5 Ac, 5 Ad], DLS [t = 4.17, P < 0.001, Fig. 5 Ae, 5 Af], DMS [t = 2.92, P = 0.01, Fig. 5 Ag, 5 Ah] and OFC [t = 4.07, P < 0.001, Fig. 5 Ai, 5 Aj]. However, naltrexone did not significantly change the levels of FosB/ΔFosB IR in the mPFC [t = 0.42, P > 0.05, Fig. 5 Ak, 5 Al].
Figure 5.
Naltrexone treatment blocks ethanol-induced up-regulation of ΔFosB IR in the striatal region and OFC. Counts of ΔFosB immunoreactive nuclei in animals subjected to chronic ethanol treated with saline, and in animals subjected to chronic ethanol treated with naltrexone (2 mg/kg, i.p.) were statistically distinguishable. A decrease in ΔFosB immunoreactive nuclei was observed in NAc core (a–b), DLS (e–f), OFC (i–j) in animals subjected to chronic ethanol treated with naltrexone compared with animals subjected to chronic ethanol treated with saline. Furthermore, significant decrease in ΔFosB immunoreactive nuclei was also seen in the NAc shell (c–d) and DMS (g–h) after chronic naltrexone treatment compared with animals subjected to chronic ethanol treated with saline. However, there is no significant change in ΔFosB levels in the mPFC in animals subjected to chronic ethanol treated with naltrexone (k–l) compared with animals subjected to chronic ethanol treated with saline. Scale bar = 100 µm. Data are expressed as mean ± SEM (n = 5 animals in each group). * P < 0.05, ** P < 0.01, *** P < 0.001 versus saline-ethanol. Scale bar = 100 µm.
DISCUSSION
We reported here that chronic voluntary ethanol intake robustly induces FosB/ΔFosB IR in NAc core, dorsolateral striatum (DLS) and orbitofrontal cortex (OFC), but not in NAc shell, dorsomedial striatum (DMS) and medial prefrontal cortex (mPFC) of S-D rats. This is somewhat different from a previous study showing that passive ethanol exposure induced high levels of ΔFosB IR broadly within striatal complex and mPFC (Perrotti et al., 2008). This difference indicates that the pathways activated by voluntary drinking of ethanol are different from those activated by forced exposure. In support of this notion, a previous study has suggested that some structures activated by involuntary alcoho1 administration can be affected by the stress of this procedure and not by the pharmacological effect of the drug itself (Ryabinin et al., 1999).
NAc core may contribute to conditioned stimulus-supported drug-seeking behavior (Everitt and Robbins, 2005). Likewise, dorsal striatum may contribute to the compulsive or habit-like nature of drug consumption (Vanderschuren et al., 2005). The lateral and medial parts of dorsal striatum have distinct anatomical inputs and outputs and therefore different functions (Voorn et al., 2004). For instance, endogenous brain-derived neurotrophic factor in DLS but not in DMS controls voluntary ethanol intake (Jeanblanc et al., 2009). In line with these findings, we demonstrated that voluntary ethanol intake induced pronounced accumulation of FosB/ΔFosB IR in NAc core and DLS, but not in NAc shell and DMS. Our results provide further support to the notion that the induction of ΔFosB IR in related brain regions is a common chronic adaptation to virtually all drugs of abuse (McClung et al., 2004).
OFC is a key brain region implicated in regulating goal-directed behavior and impulsivity (Krawczyk, 2002). Abstinent cocaine users show hypofunction within OFC (Volkow and Fowler, 2000), and ΔFosB levels in OFC induced by self-administration of cocaine are several-fold higher than that by yoked cocaine administration (Winstanley et al., 2007). In keeping with their findings, current study showed that voluntary ethanol intake induced FosB/ΔFosB IR in OFC.
Notably, the numbers of FosB/ΔFosB positive cells in this study are significantly lower than the levels reported in a previous study (Perrotti et al. 2008). The difference might be resulted mainly from the different methods used by these investigators and us in calculating positive particles. Interestingly, despite the huge difference in number, the levels of FosB/ΔFosB positive cells in our study were similar to that presented by these investigators. Furthermore, a recent study using a method similar to ours reported that FosB/ΔFosB positive nuclei in NAc core of AA rats are ~150 (Kaste et al., 2009). It is worthy mentioned that the levels of FosB/ΔFosB positive nuclei in NAc core in ethanol experienced animals are 434 in the first series of experiment (as shown Fig. 2B), but are 325 in the second series of experiment (as shown in Fig. 5B). By comparing these two figures, one might question whether the level of FosB/ΔFosB in the latter experiment has been elevated. Nevertheless, caution should be taken when doing this kind of comparison, since these figures were collected from two different series of experiments. Importantly, a control group was included in each series of experiments. The data indicate that levels of FosB/ΔFosB IR in ethanol drinking animals were higher than ethanol naïve animals and naltrexone treatments significantly decreased the levels of FosB/ΔFosB IR induced by voluntary alcohol intake in NAc core.
The antiserum (#sc-48; Santa Cruz Biotechnology, Santa Cruz, California) used in our experiment was raised to the N terminus of FosB and recognizes both FosB and ΔFosB. However, ΔFosB but not FosB or other Fos-related antigens is known to be stably expressed following chronic drugs of abuse (Nestler, 2008). Importantly, in the current study, rats were sacrificed at 18 h after the last drinking bout. We choose this assay time point based on previous studies showing that 18–24 h after stimulus, the full-length FosB was degraded, leaving the stable ΔFosB as the only FosB gene product (Hope et al., 1994; Chen et al., 1995, 1997; Perrotti et al., 2008; Ulery et al., 2006). Therefore, the positive particles recognized by the antiserum under our experimental conditions are most likely ΔFosB. Nevertheless, since the rats were sacrificed 18 h after the last drinking session, they may be experiencing withdrawal from ethanol. Therefore, this would result in a mixed signal reflecting ethanol-induced ΔFosB and anxiety-induced full-length FosB. Similarly, since naltrexone decreased ethanol intake, these animals may experience less withdrawal and less withdrawal-induced anxiety. Therefore, the decrease in FosB-IR in these animals may be due to decrease anxiety.
We reported that systematic administration of naltrexone significantly reduced excessive voluntary ethanol intake in S-D rats under the intermittent-access of 20% ethanol paradigm. This is in line with that seen in Long-Evens and Warsaw High Preferring rats where naltrexone is most effective in decreasing ethanol intake in rats that are consuming high amounts of ethanol (Simms et al., 2008; Zalewska-Kaszubska et al., 2008). Our results of naltrexone at a dose of 2 mg/kg are in agreement with previous studies showing that naltrexone at similar doses (1.8 mg/kg or 2.0 mg/kg) suppressed ethanol intake, and possibly by affecting the μ opioid receptor activity (Mhatre et al, 2004; Zalewska-Kaszubska et al, 2008). However, this dose of naltrexone has been shown to have effects in general consummatory behavior. Therefore, it is necessary to test the effects of lower doses of naltrexone in future studies, because lower doses of naltrexone are more selective for μ opioid receptors and these studies may provide more clues for which opioid receptor subtypes are responsible for these effects.
Our previous studies suggested that naloxone, an antagonist of opioid receptors similar to naltrexone attenuates ethanol consumption via antagonizing the down-regulation of CaM kinase IV and the phosphorylation of CREB in the striatal region induced by forced ethanol exposure (Li et al., 2003; 2008) and that naloxone increases the firing of VTA dopamine neurons by inhibiting VTA GABAergic interneurons (Xiao et al., 2007; Xiao and Ye, 2008). In the present study, we observed that naltrexone treatment significantly attenuated FosB/ΔFosB IR in NAc core, NAc shell, DLS, DMS and OFC. Moreover, the attenuation is more significant in DLS and OFC than NAc core.
It has been shown that μ opioid receptor agonists indirectly increase dopamine release in NAc and caudate putamen (Di Chiara and Imperato, 1988; Job et al., 2007) and that the induction of ΔFosB by psychostimulants is mediated by D1 dopamine receptor pathway (Muller et al., 2005). Furthermore, naltrexone attenuates striatum dopamine levels, which are increased by systemic administration of ethanol (Acquas et al., 1993; Benjamin et al., 1993; Gonzales and Weiss 1998). Therefore, naltrexone-induced attenuation of dopamine levels may contribute to its attenuation of FosB/ΔFosB IR upregulation observed in current study. However, little is known regarding the role of opioid receptor-dopamine link in frontal cortex in modulating the behavioral effects of ethanol. It has been demonstrated that D1 dopamine receptors are expressed at much lower levels in prefrontal cortex than in dorsal striatum and NAc (Boyson et al., 1986; Diop et al., 1988). We found that voluntary ethanol intake induced high levels of FosB/ΔFosB IR in OFC but not in mPFC. The mechanism underlying the difference between these two areas warrants further investigation.
In summary, the present study demonstrates that chronic voluntary consumption of large quantities of ethanol induces FosB/ΔFosB expression selectively in the subregions of the striatum and the prefrontal cortex, which is reversed by naltrexone treatment. The pattern of the induction of FosB/ΔFosB found in current study is somewhat different from those observed by forced ethanol exposure. This suggests that voluntary drinking may activate pathways that are different from those activated by forced ethanol exposure. Therefore, study on voluntary drinking may be more relevant in the search to discover treatments for alcoholism, since human drinks alcohol voluntary, in most, if not all of the cases. Further investigation into the potential pathways mediating ΔFosB in the related brain regions as well as endogenous opioid system is important for a more comprehensive understanding of the role of this transcription factor in the regulation of gene expression produced by voluntary ethanol intake.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Alcohol and Alcoholism (NIAAA) AA 016964, AA015925 and AA016618 to JHY. There are no conflicts of interest, including specific financial interests and relationships and affiliations relevant to this manuscript.
References
- Acquas E, Meloni M, Di Chiara G. Blockade of delta-opioid receptors in the nucleus accumbens prevents ethanol-induced stimulation of dopamine release. Eur J Pharmacol. 1993;230:239–241. doi: 10.1016/0014-2999(93)90809-v. [DOI] [PubMed] [Google Scholar]
- Atkins JB, Atkins J, Carlezon WA, Chlan J, Nye HE, Nestler EJ. Region-specific induction of ΔFosB by repeated administration of typical versus atypical antipsychotic drugs. Synapse. 1999;33:118–128. doi: 10.1002/(SICI)1098-2396(199908)33:2<118::AID-SYN2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847(2):157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Benjamin D, Grant ER, Pohorecky LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res. 1993;621:137–140. doi: 10.1016/0006-8993(93)90309-b. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nelster EJ, Greenguard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature (Lond) 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W, Koros E. Ethanol-reinforced behaviour the rat: effects of naltrexone. Eur J Pharmacol. 1999;374:321–327. doi: 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Nye HE, Kelz MB, Hiroi N, Nakabeppu Y, Hope BT, Nestler EJ. Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol Pharmacol. 1995;48:880–889. [PubMed] [Google Scholar]
- Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of ΔFosB induced in brain by chronic treatments. J Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichelli MJ, Lewis MJ. Naloxone nonselective suppression of drinking of ethanol, sucrose saccharin and water by rats. Pharmacol Biochem Behav. 2002;72:699–706. doi: 10.1016/s0091-3057(02)00736-0. [DOI] [PubMed] [Google Scholar]
- Coonfield DL, Kiefer SW, Ferraro FM, III, Sinclair JD. Ethanol palatability and consumption by high ethanol-drinking rats: manipulation of the opioid system with naltrexone. Behav Neurosci. 2004;118:1089–1096. doi: 10.1037/0735-7044.118.5.1089. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop L, Gottberg E, Briere R, Grondin L, Reader TA. Distribution of dopamine D1 receptors in rat cortical areas, neostriatum, olfactory bulb and hippocampus in relation to endogenous dopamine contents. Synapse. 1988;2:395–405. doi: 10.1002/syn.890020406. [DOI] [PubMed] [Google Scholar]
- Davidson D, Amit Z. Naltrexone block acquisition of voluntary ethanol intake in rats. Alcohol Clin Exp Res. 1997;21:677–683. [PubMed] [Google Scholar]
- Ehrlich ME, Sommer J, Canas E, Unterwald EM. Periadolescent mice show enhanced ΔFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Gentz R, Rauscher FJ, 3rd, Abate C, Curran T. Parallel association of Fos and un leucine zippers juxtaposes DNA binding domains. Science (Wash DC) 1989;243:1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, Tang A, Hall FS, Sora I, Uhl GR, Bergeson SE, Gonzales RA. Mu (mu) opioid receptor regulation of ethanol-induced dopamine response in the ventral striatum: evidence of genotype specific sexual dimorphic epistasis. Biol Psychiatry. 2007;62:627–634. doi: 10.1016/j.biopsych.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaste K, Kivinummi T, Piepponen TP, Kiianmaa K, Ahtee L. Differences in basal and morphine-induced FosB/DeltaFosB and pCREB immunoreactivities in dopaminergic brain regions of alcohol-preferring AA and alcohol-avoiding ANA rats. Pharmacol Biochem Behav. 2009;92:655–662. doi: 10.1016/j.pbb.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen JS, Carlezon WA, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self SW. Expression of the transcriptional factor ΔFosB in the brain controls sensitivity to cocaine. Nature (Lond) 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci Biobehav Rev. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Li J, Bian WL, Xie GQ, Cui SZ, Wu ML, Li YH, Que LL, Yuan XR. Chronic ethanol intake-induced changes in open-field behavior and calcium/calmodulin-dependent protein kinase IV expression in nucleus accumbens of rats: naloxone reversal. Acta Pharmacol Sin. 2008;29:646–652. doi: 10.1111/j.1745-7254.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- Li J, Li YH, Yuan XR. Changes of phosphorylation of cAMP response element binding protein in rat nucleus accumbens after chronic ethanol intake: naloxone reversal. Acta Pharmacol Sin. 2003;24:930–936. [PubMed] [Google Scholar]
- Marttila K, Petteri Piepponen T, Kiianmaa K, Ahtee L. Accumbal FosB/DeltaFosB immunoreactivity and conditioned place preference in alcohol-preferring AA rats and alcohol-avoiding ANA rats treated repeatedly with cocaine. Brain Res. 2007;1160:82–90. doi: 10.1016/j.brainres.2007.05.036. [DOI] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. ΔFosB: A molecular switch for long-term adaptation in the brain. Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McDaid J, Graham MP, Napier TC. Methamphetamine-induced sensitization differentially alters pCREB and ΔFosB throughout the limbic circuit of the mammalian brain. Mol Pharmacol. 2006;70:2064–2074. doi: 10.1124/mol.106.023051. [DOI] [PubMed] [Google Scholar]
- Mhatre M, Pruthi R, Hensley K, Holloway F. 5-HT3 antagonist ICS 205-930 enhances naltrexone's effects on ethanol intake. Eur J Pharmacol. 2004;491:149–156. doi: 10.1016/j.ejphar.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. D1 dopamine receptors modulate ΔFosB induction in rat striatum after intermittent morphine administration. J Pharmacol Exp Ther. 2005;314:148–154. doi: 10.1124/jpet.105.083410. [DOI] [PubMed] [Google Scholar]
- Mumberg D, Lucibello FC, Schuermann M, Muller R. Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally distinct antagonistic proteins. Genes Dev. 1991;5:1212–1223. doi: 10.1101/gad.5.7.1212. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. ΔFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol dependent subjects and activates the hypothalamic-pituitary adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Academic Press Inc.; 2005. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of ΔFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Williams-Hemby L, Whitlow C, Bowen C, Samson HH. Metabolic mapping of the effects of oral alcohol self-administration in rats. Alcohol Clin Exp Res. 1998;22:176–182. [PubMed] [Google Scholar]
- Ryabinin AE, Wang YM, Freeman P, Risinger FO. Selective effects of alcohol drinking on restraint-induced expression of immediate early genes in mouse brain. Alcohol Clin Exp Res. 1999;23:1272–1280. doi: 10.1111/j.1530-0277.1999.tb04288.x. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav. 2004;78:743–750. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, DiLeone FJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. ΔFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Hemby L, Grant KA, Gatto GJ, Porrino LJ. Metabolic mapping of the effects of chronic voluntary ethanol consumption in rats. Pharmacol Biochem Behav. 1996;54:415–423. doi: 10.1016/0091-3057(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Williams-Hemby L, Porrino LJ. Low and moderate doses of ethanol produce distinct patterns of cerebral metabolic changes in rats. Alcohol Clin Exp Res. 1994;18:982–988. doi: 10.1111/j.1530-0277.1994.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Xiao C, Ye JH. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of mu-opioid receptors. Neurosci. 2008;153:240–248. doi: 10.1016/j.neuroscience.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjević K, Ye JH. Effects of ethanol on midbrain neurons: role of opioid receptors. Alcohol Clin Exp Res. 2007;31:1106–1113. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fos immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, et al. ΔFosB: An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Gorska D, Dyr W, Czarnecka E. Voluntary alcohol consumption and plasma beta-endorphin levels in alcohol-preferring rats chronically treated with naltrexone. Physiol Behav. 2008;93:1005–1010. doi: 10.1016/j.physbeh.2008.01.007. [DOI] [PubMed] [Google Scholar]