Abstract
The study had two aims--to determine the efficacy of a family-based cognitive-behavioral pain management intervention for adolescents with sickle cell disease (SCD) in (1) reducing pain and improving health-related variables and (2) improving psychosocial outcomes. Each adolescent and a family support person were randomly assigned to receive a brief pain intervention (PAIN) (n = 27) or a disease education attention control intervention (DISEASE ED) (n = 26) delivered at home. Assessment of primary pain and health-related variables (health service use, pain coping, pain-related hindrance of goals) and secondary psychosocial outcomes (disease knowledge, disease self-efficacy, and family communication) occurred at baseline (prior to randomization), post-intervention, and one-year follow-up. Change on outcomes did not differ significantly by group at either time point. When groups were combined in exploratory analyses, there was evidence of small to medium effects of intervention on health-related and psychosocial variables. Efforts to address barriers to participation and improve feasibility of psychosocial interventions for pediatric SCD are critical to advancing development of effective treatments for pain. Sample size was insufficient to adequately test efficacy, and analyses did not support this focused cognitive-behavioral pain management intervention in this sample of adolescents with SCD. Exploratory analyses suggest that comprehensive interventions, that address a broad range of skills related to disease management and adolescent health concerns, may be more effective in supporting teens during healthcare transition.
Keywords: adolescents, sickle cell disease, pain
Introduction
Sickle cell disease (SCD), a genetic blood disorder affecting 1 in 400 African American newborns, in which abnormal hemoglobin interferes with oxygen transportation and sickle-shaped red blood cells result in vaso-occlusions that may be painful, damaging to tissue, or both.1 Chronic, unpredictable pain is the most common complication of SCD, contributing to hospitalizations and interference with daily activities2 and often increasing during adolescence.3 In addition to pain, adolescents with SCD may experience chronic anemia, susceptibility to infection, pulmonary complications and acute chest syndrome, stroke risk, short stature and delayed puberty. 1,2 Consequently, SCD complications may compromise adolescents’ health status, quality of life, and emerging independence4, which may interfere with the important developmental process of adolescent transition to adulthood and adult SCD medical care.
While culturally and developmentally appropriate interventions to enhance disease management for adolescents with SCD are important for efforts to reduce pain and improve transition outcomes 5, few such interventions exist. Disease management has been targeted in a number of intervention studies for children, adolescents, and young adults.6–8 For example, Gil and colleagues 7 demonstrated that pain coping skills may be taught effectively, with increased practice of pain coping skills linked to fewer health care contacts, fewer school absences, and less interference with household activities. In addition, a family-based psychoeducation intervention to promote coping, and adjustment resulted in improved disease knowledge and family communication.6, 8 Thus, pain management techniques and family-based disease education may improve disease management for adolescents.
Further, research supports targeting the specific constructs of pain coping, disease knowledge and self-efficacy, and family communication to increase teen autonomy and promote effective disease management. Use of active SCD pain coping strategies are associated with adaptation9 ; however, adolescents with SCD show increased, but inconsistent, pain coping efforts over time, reflecting greater variability in the number of strategies used to manage SCD pain.10 Developing a sound understanding of SCD and disease-specific self-efficacy have been identified as a core factors in disease management1, transition readiness5, and health-promoting outcomes.11,12 Related to self-efficacy, the development and pursuit of personal goals have been shown to be affected by chronic conditions involving pain and may affect disease management.13, 14 Finally, family functioning has been associated with use of adaptive coping15 and improved adherence16 for children with SCD.
Despite the promise of this research, challenges to the development and implementation of adolescent-specific interventions include inconsistent adherence to medical treatment for SCD, poor attendance at SCD-specific disease education sessions, use of maladaptive pain coping strategies, and high drop out rates for older adolescents.8, 10 Broader economic and societal issues may result in financial, logistical or other barriers to participating in medical setting-based disease management interventions.17 Lastly, because SCD pain is most often managed at home18, teaching pain management strategies in a clinical setting may not promote the generalization of skills to their use in home and community settings.
In order to extend previous disease management intervention research, and to address the specific challenges of adolescents with SCD, a pain management program (PAIN) was developed and tested relative to an attention control disease education control group (DISEASE ED). PAIN consisted of established cognitive-behavioral techniques including relaxation, guided imagery, and positive coping self-statements. DISEASE ED addressed SCD complications, nutrition, and physical activity as well as communication skills and emergency planning. While the entire program, referred to as Teens Taking Control, incorporated components of previous interventions7, unique components were added to enhance cultural sensitivity and developmental focus on adolescents and their families, such as being home- and community-based, including support persons in SCD care, and using culturally relevant materials. 19 Previous disease management intervention research6–8 was also extended by testing a comprehensive set of health-related and psychosocial outcomes beyond the immediate post-intervention time period.
For the primary aim, we expected that, relative to DISEASE ED, participants in PAIN would demonstrate significant improvement in pain and health-related outcomes, specifically decreased pain and pain interference with daily activities, increased routine health service use, less pain-related hindrance of personal goals, and improved school attendance and pain coping following intervention and one year post-baseline. The second aim was to test the efficacy of PAIN in improving teen psychosocial variables important for adolescent/family disease management, including SCD knowledge, disease self-efficacy, and family cohesion following intervention and one year post-baseline.
Materials and Methods
Participants
The Human Subjects Committees of the appropriate Institutional Review Boards approved study procedures. Potential participants were identified through the patient registry of a comprehensive sickle cell center. Inclusion criteria were adolescents between 12 and 18 years old with SCD-SS or Sβ-thalassemia (variants of SCD with higher pain levels)1; and teens’ being able to recruit a support person (who could be a relative or a responsible friend) to attend all intervention sessions with them. Potential participants were excluded if they were receiving a medical treatment for SCD to reduce pain (e.g., hydroxyurea or transfusion therapy); did not speak English-; or did not have a caregiver that spoke English. Within a month of their clinic visits, eligible patients were sent a letter signed by the physician program director that invited them to consider participation. During the clinic visit, the families were approached and the study was explained. Those who missed clinic visits were contacted by phone. Researchers obtained informed consent/assent for families interested in the study in a location of their choice (typically their home), where families completed baseline study measures.
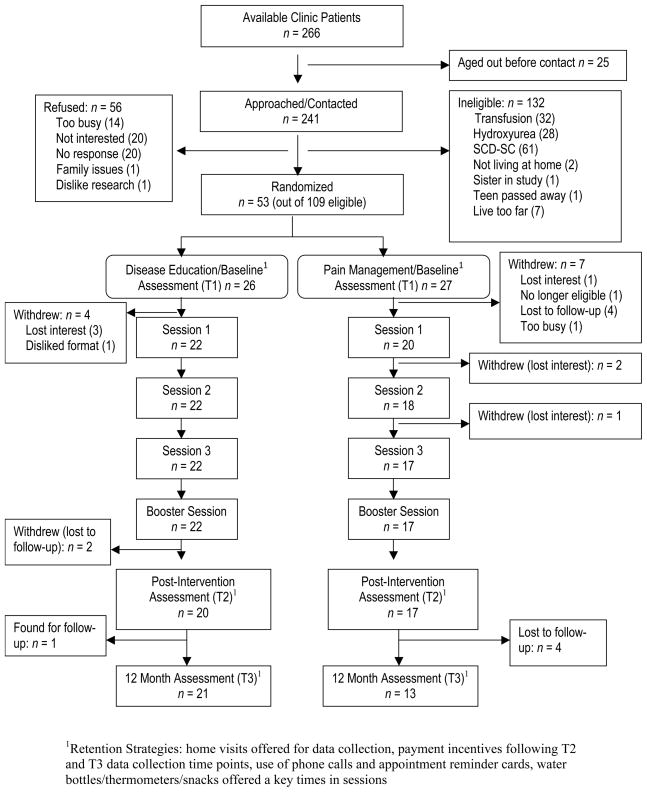
Figure 1 provides a summary of the participants’ progress through the stages of the intervention in addition to strategies used to retain participants. A sample of 53 eligible adolescents and caregivers entered the study, for a recruitment rate of 49%. When eligible participants were compared with those who enrolled in the intervention through independent samples t-tests, no significant gender or age differences were found. The participants were randomly assigned to either PAIN (n = 27) or DISEASE ED (n = 26).
Figure 1.
Progression of Participants through Teens Taking Control RCT
Demographic information on the study participants is presented in Table 1. Study groups did not differ statistically on any demographic variables, including initial treatment engagement and expectations. Participants withdrew from both study arms prior to engaging in the interventions at equivalent rates. Comparison via t-tests and chi-square tests for categorical variables on baseline variables including treatment expectations showed that those who did not complete the intervention were more likely to have family incomes that were < $50,000/year than those who did complete the intervention, t(48) = −2.25, p < .05, and that teens who did not complete the intervention had less knowledge of SCD, t(51) = −2.23, p < .05, than those who did complete the intervention.
Table 1.
Descriptive Summary of Participants and Treatment Expectations/Engagement
| PAIN (n = 17) | DISEASE ED (n = 20) | |
|---|---|---|
| Participant | ||
| Male gender | 9 (52.9%) | 6 (30%) |
| Ethnicity: African-American | 17 (100%) | 19 (95%) |
| Age, M (SD) | 14.24 (1.79) | 14.10 (1.71) |
| Grade, M (SD) | 8.18 (1.94) | 7.8 (1.74) |
| TONI standardized score | 90.53 (7.17) | 90.40 (14.64) |
| Caregiver | ||
| Education: < high school | 11 (64.7%) | 12 (60%) |
| Relationship status: Married | 11 (64.7%) | 10 (50%) |
| Employment | ||
| Employed full-time | 6 (35.3%) | 10 (50%) |
| Employed part-time | 8 (47.1%) | 9 (45%) |
| Not employed outside home | 3 (17.6%) | 1 (5%) |
| Income | ||
| <$50,000 | 9 (52.9%) | 11 (55%) |
| >$50,000 | 8 (47.1%) | 9 (45%) |
| Treatment expectations, M (SD) | ||
| Adolescent baseline (T1) | 4.41 (.80) | 4.21 (.98) |
| Caregiver baseline (T1) | 4.29 (.99) | 4.47 (.70) |
| Interventionist rating of engagement, M (SD) | ||
| Adolescents | 4.24 (.65) | 4.21 (.68) |
| Support person | 4.01 (.76) | 4.33 (.60) |
Measures
A General Information Form and the Test of Nonverbal Intelligence-3 (TONI-3)20 were administered at baseline to assess sociodemographic variables by caregiver report and cognitive functioning of the teen with SCD with a valid and reliable performance-based measure. The remaining measures were administered at baseline (T1), post-intervention (T2) and one-year follow-up (T3).
Primary Pain and Health-Related Variables
Pain diary
Teens completed a daily paper-and-pencil pain diary based on an earlier format21 in which they indicated whether or not pain was present and answered yes/no questions regarding healthcare utilization and medication use over the previous 24-hours. Percentage of days with pain was calculated.
Medical chart review
Medical records were reviewed for the 12-month period prior to and following baseline to calculate a routine health service use summary (number of SCD clinic visits, and other outpatient clinic visits).
School attendance
School attendance records were obtained for the 12-months prior to study entry and the 12-months following. Absenteeism was defined as the percent of school days missed out of the total possible school days during each 12-month period.
Health-Related Hindrance Inventory.22
A subset (n = 14) completed this measure, as it was under development at the start of the study. Teens listed six personally important goals and rated the extent their pain, other symptoms, and how taking care of their health affected their ability to achieve goals. Responses were on a Likert-type scale from 0–6, with 6 indicating greatest impact. The pain hindrance of goals score was used (α = .86).
Coping Strategies Questionnaire.9
This measure, used in prior pain intervention studies, assessed the strategies employed by teens during episodes of SCD-related pain. The measure consists of 80 items to which teens responded on a 7-point Likert-type scale from never (0) to always (6). Total coping attempts (α = .93) was used as an outcome variable.
Psychosocial Variables
SCD knowledge was measured using two scales
The SCD Knowledge Questionnaire23 is a 20-item, true/false measure used in prior studies of youth with SCD (α = .38). The SCD Transition Knowledge Questionnaire24 is a 25-item multiple-choice measure that assessed teen knowledge of SCD relevant to preparation for transition to adult SCD services; it was available for use only after the commencement of data collection (n = 30; α = .70).
Disease Self-efficacy Scale was adapted for this study from a cancer-specific scale26
This 20-item scale assessed teen and caregiver confidence in their ability to manage interactions with health care providers while receiving health care in various medical settings. Responses were given on a Likert-type scale from 1–5, with higher scores indicating increased confidence (α = .93).
Child Health Questionnaire27, completed by caregivers, consists of 28 items that assess 14 different concepts regarding teen and family physical and psychosocial well-being. There is evidence of adequate reliability and validity of this measure in pediatric populations27. The Family Cohesion scale, which rates a family’s ability to get along with one another using 7 items rated on a 5-point Likert-type scale, was used for this study (α = .64).
Treatment Measures
Expectations and Engagement were evaluated for the purpose of evaluating the role of treatment variables in retention and outcomes for treatment groups. For expectations, teens and caregivers rated how effective they believed the intervention would be in helping them manage SCD on a 5-point scale, ranging from 1 ‘won’t work’ to 5 ‘definitely will work’. This measure was administered in a self-report format prior to the intervention. After every session, the primary interventionist rated both the teen and the support person’s levels of engagement during the session on a 5-point scale, ranging from 1 ‘not at all engaged’ to 5 ‘extremely engaged’. Responses were averaged across sessions.
Procedures
A two-group, randomized treatment design was used. Randomization occurred at the end of the baseline assessment. Through consultation with psychologists with expertise in pediatric SCD and with the treatment team at the center, manualized interventions were developed for each study arm. Interventions were 4 sessions in length (3 sessions, 2 weeks apart with a booster session one month later). Both interventions included discussion of SCD and disease management with the participant and support person, completion of daily paper-and-pencil pain diaries, homework, review of homework, and biweekly check-in telephone calls. Support persons encompassed a host of family members (predominantly mothers, but also siblings, uncles, grandparents and cousins). All sessions were led by two interventionists, either doctoral students in clinical psychology or psychologists. The 90-minute sessions were held primarily in the homes of the families. To ensure cultural sensitivity and “fit” of the intervention to the individual teen/family, interventionists were trained to be responsive to issues that arose during the sessions and to flexibly apply the manualized intervention to teens’ specific SCD issues. Participants (adolescents and caregivers) each received $20 gift cards after completing measures post-intervention (T2) and at the 12-month follow-up assessment (T3).
Pain Management Intervention
PAIN consisted of training in deep breathing/relaxation, positive coping statements, and guided imagery. Relaxation was taught through instruction on evaluating bodily tension, even in the presence of SCD pain, and how to systematically relax muscles. Positive coping statements were addressed by drawing attention to participants’ thoughts about their ability to deal with pain, which were then evaluated as positive or negative. Instruction was provided as to how coping statements may influence pain outcomes favorably or not, and teens and support persons were taught to develop positive coping statements for use during pain episodes. Guided imagery was taught in successive stages: 1) inducing in the teens and support persons a state of deep relaxation accompanied by guided imagery; 2) leading the teen through guided imagery based on teen’s image preferences; 3) training the support person to direct the guided imagery; and 4) creation of a guided imagery audiotape, directed by the support person for practice and future use.
Disease Education Intervention
DISEASE ED session content was developed from existing psychoeducational programs for youth with SCD28,29 as well as guidelines for health care maintenance for youth with SCD.30 Sessions utilized a variety of modalities and included a cathartic activity (e.g., writing or drawing about SCD), information about SCD and its management, adolescent health issues in terms of SCD impact, and effective communication with health care providers.
Assurance of Procedural Consistency and Integrity
Methods used to ensure consistent implementation of study procedures were: (1) a detailed treatment manual describing each study procedure; (2) each session was audiotaped; and (3) interventionists met weekly in supervision with licensed psychologists (authors: JR, LB, LS) using audiotapes as a basis for supervision. Adherence to treatment protocols was determined by having audiotapes reviewed by trained adherence raters using an adherence checklist of key components. Adherence raters (doctoral clinical psychology students) assessed a random sample of 25% of all sessions, evenly distributed across sequence of sessions and study arms, with re-checking for 25% of sessions, and any disagreements discussed to agreement. Total adherence to study procedures was 96.57%. Manual departures were minor and included only three specific omissions (homework discussion, session overview, and specific question on SCD diagnosis).
Data Analysis Plan
Description of the treatment groups on demographic and disease-related variables identified substantial skew for all variables with the exception of total coping attempts; therefore, non-parametric statistics were used as appropriate. Group comparisons, using t-tests or Χ2 analyses as appropriate, were conducted to identify potential control variables. Because there were no significant differences among variables at baseline (see Table 1), change scores were compared between the groups with no covariates. Wilcoxon Mann-Whitney tests, the equivalent independent sample t-tests, were used to examine group differences in identified outcomes for change scores from baseline assessment to T2 and from baseline assessment to T3. Health service utilization and school attendance change scores were based on one year prior to baseline to one year post-intervention. A t-test was used for total coping attempts. For exploratory analyses, paired sample Wilcoxon Mann-Whitney tests were used to compare scores at baseline to T2, and scores from baseline to T3, for the entire sample. A paired t-test was used for exploratory analyses on total coping attempts. Because the tests of significance were affected by the small sample size, effect sizes (Cohen’s d31) were reported for each outcome with small effect sizes = .20 to .49, medium effect sizes = .50 to .79, and large effect sizes > .80.
Results
Group Comparisons
Change in scores from baseline to T2 and to T3 did not differ significantly between the treatment groups for the pain and health-related outcomes (see Table 2). In fact, small effect sizes, in favor of PAIN, were identified for percent of days with pain from baseline to T2 and for routine health service use from baseline to T3. Pain-related hindrance of goals decreased for both groups, with a slightly greater decrease (small effect) for DISEASE ED from baseline to T3. Likewise, percent of school days missed showed favorable changes (small effect) in DISEASE ED from baseline to T3.
Table 2.
Between-Group Comparisons
| PAIN (n = 17) M (SD) | DISEASE ED (n = 20) M (SD) | Group Differences in Change Scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T2 – T1 | T3 – T1 | |||
| p | d | p | d | |||||||
| SCD-related Variables | ||||||||||
| % Pain Days | 24.94 (29.64) | 16.60 (16.57) | 16.71 (23.03) | 15.03 (25.92) | 17.29 (23.21) | 7.84 (12.31) | .19 | .48 | .86 | −.04 |
| % Interference with activities | 11.84 (19.15) | 7.67 (11.26) | 11.40 (22.75) | 9.86 (12.49) | 7.38 (12.46) | 5.08 (4.55) | .63 | −.10 | .47 | −.21 |
| Pain-related goal hindrance | 2.38 (2.03) | 1.53 (1.39) | 1.87 (1.39) | 2.21 (1.55) | 1.58 (1.57) | 1.41 (1.21) | .90 | .18 | .39 | −.48 |
| Routine health service use | 5.35 (5.37) | -- | 7.0 (9.60) | 6.15 (7.11) | -- | 6.55 (7.04) | -- | -- | .67 | .25 |
| % School days missed | 10.01 (7.92) | -- | 13.83 (14.33) | 13.27 (12.94) | -- | 11.94 (9.25) | -- | -- | .32 | −.41 |
| Coping attempts | 65.82 (36.11) | 70.35 (36.41) | 75.67 (32.26) | 60.63 (34.44) | 67.32 (26.15) | 64.17 (32.23) | .62 | .11 | .95 | .04 |
| Teen Psychosocial Variables | ||||||||||
| Disease self-efficacy | 3.62 (.91) | 3.67 (.92) | 4.16 (.51) | 3.54 (.90) | 4.07 (.65) | 3.90 (.78) | .06 | −.64 | .92 | .09 |
| SCD transition knowledge | 72.0 (15.61) | 81.41 (9.37) | 86.15 (6.85) | 68.50 (19.37) | 81 (17.45) | 81.05 (16.10) | .65 | −.40 | .89 | −.11 |
| SCD knowledge | 81.18 (11.39) | 85.29 (11.25) | 89.23 (6.07) | 81.25 (10.99) | 82.25 (11.97) | 82.89 (11.10) | .47 | .33 | .17 | .51 |
| Family cohesion | 68.24 (26.63) | 73.24 (18.54) | 80.77 (21.97) | 67.50 (20.74) | 72.37 (17.03) | 69.72 (21.99) | .65 | .03 | .37 | .39 |
Note: All comparisons used non-parametric tests due to skewed variables with the exception of Coping Attempts.
Change in psychosocial variables did not differ significantly between the treatment groups (see Table 2). Small to medium effect sizes were noted, with PAIN showing relatively larger increases in disease self-efficacy from baseline to T2 and in SCD knowledge from baseline to T3. Although both groups improved, small effect sizes in favor of PAIN were also identified for SCD knowledge from baseline to T2 and family cohesion from baseline to T3. For DISEASE ED, a medium effect was found for change from baseline to T2 in teen SCD transition knowledge although both groups showed improved scores.
Exploratory Analyses
Because no significant group differences were found for the pain, health-related, and psychosocial variables, post-hoc analyses were conducted to examine changes over time for the treatment groups combined (see Table 3). For pain and health-related variables, no statistically significant findings in the expected direction were found. Small effect sizes were found for decrease in percent of days with pain from baseline to T3, decrease in percentage of days with interference with daily activities at both time points, and decrease in pain-related hindrance of goals at both time points.
Table 3.
Within-Group Comparisons on Intervention Outcomes for Teens Taking Control
| Total Sample (N = 37) M (SD) | Comparisons Across Time | ||||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 to T2 | T1 to T3 | |||
| p | d | p | d | ||||
| SCD-related Variables | |||||||
| % Days with pain | 19.58 (27.75) | 16.98 (20.16) | 11.44(17.69) | .51 | .11 | .91 | .35 |
| % Interference with activities | 10.77 (15.69) | 7.51(11.76) | 7.65 (14.91) | .22 | .24 | .47 | .20 |
| Pain-related goal hindrance | 2.30 (1.74) | 1.56 (1.46) | 1.58 (1.28) | .10 | .46 | .12 | .47 |
| Routine health service use | 5.78 (6.30) | -- | 6.76 (8.20) | -- | -- | .73 | .13 |
| % School days missed | 11.99 (11.21) | -- | 12.73 (11.47) | -- | -- | .59 | −.07 |
| Coping attempts | 63.08 (34.83) | 68.75 (30.99) | 68.77(32.19) | .09 | .17 | .47 | .17 |
| Teen Psychosocial Variables | |||||||
| Disease self-efficacy | 3.58 (.89) | 3.88 (.80) | 4.01 (.68) | .04 | .35 | .03 | .55 |
| SCD transition knowledge | 70.13 (17.51) | 81.19 (14.14) | 83.13 13.24) | .00 | .69 | .00 | .84 |
| SCD knowledge | 81.22 (11.02) | 83.65 (11.59) | 85.47(9.78) | .12 | .21 | .05 | .41 |
| Family cohesion | 67.84 (23.29) | 72.78 (17.50) | 74.35(22.31) | .09 | .24 | .13 | .29 |
Note: All comparisons are made using non-parametric tests due to skewed variables with the exception of Coping Attempts
For psychosocial variables, adolescents showed statistically significant improvements in disease self-efficacy (small effect sizes) and SCD transition knowledge (medium to large effect sizes) at both time points. Small effect sizes at both time points were also present, although statistical significance was not reached, for increase in SCD knowledge and increase in family cohesion. All psychosocial variables changed in the expected direction.
Discussion
The primary goals of this study were to extend the literature on disease management for adolescents with SCD by testing a developmentally and culturally appropriate, family and community-based, cognitive-behavioral intervention. No significant differences between the PAIN group and the DISEASE ED attention control condition were identified at post-intervention and long-term follow-up assessments. Small to medium effect sizes in favor of PAIN were noted on a number of variables, particularly percentage of days with pain, routine health service use, SCD knowledge, and family cohesion, but DISEASE ED also showed favorable outcomes. In terms of exploratory combined group analyses, additional variables important for disease management and transition showed small to medium effect sizes, namely, disease self-efficacy, SCD transition knowledge, and family cohesion.
Findings were contrary to expectations. Low recruitment rates and attrition reduced power of analyses to detect group differences. While considerable effort was made to address barriers to participation and engagement by making the intervention developmentally and culturally relevant, a host of factors may influence family decisions about initiating and maintaining participation in disease management intervention studies. Severity of SCD, for example, may be one consideration in low retention. Specifically, significant numbers of potential participants were on transfusion therapy or hydroxyurea treatment to manage pain and other SCD complications, thus reducing the potential pool of eligible participants. These ineligible patients were more likely to have significant pain and other complications. As a result, the current sample was more likely to have mild to moderate pain in contrast to severe pain. Yet, adolescents with increased pain and complications may be more motivated to remain enrolled in a disease management study and to engage in daily practice. This limitation that was not anticipated originally as the study was designed prior to the wider use of hydroxyurea therapy for pain management in SCD. Future evaluations may target those with more severe disease.
Furthermore, of those approached, only 49% participated, and those with lower family incomes and less SCD knowledge were more likely to drop out of the trial. These difficulties with recruitment and retention highlight the significant challenges of enrolling African American adolescents and their families in intervention research. Sociodemographic barriers, mistrust and misunderstanding of the role of research in advancing interventions, and lack of perceived benefits to participation may explain low enrollment and differential drop-out. Strategies such as reducing demands on participants, offering incentives for participation, and remaining in contact via telephone call and reminder notes, as implemented in this study, were insufficient to overcome barriers to engagement. Studies of decision-making around enrollment in clinical trials for pediatric SCD, and the role of perceived barriers and benefits will allow future researchers to more successfully engage adolescents and their caregivers in potentially beneficial treatment programs.
In an effort to improve retention, demands on participants were reduced by designing a brief intervention and using telephone follow-up and home-based sessions. Brevity of intervention programs reduces burden, but it may limit potential effectiveness by reducing scope and depth of the intervention and limiting necessary, intensive, daily practice of skills. Further examination of results, however, suggests directions for future intervention research in pediatric SCD. In particular, significant changes for combined groups across time and high drop-out rates (especially for the PAIN group) highlight the importance of considering the “whole” teen in preparation for transition. Information from program evaluations completed at the time 2 post-intervention and the time 3 one-year follow-up assessments are also informative. Almost all teens and caregivers reported that they would recommend the intervention to a friend and the majority (over 50%) reported their interventions to be moderately to extremely helpful and interesting, regardless of arm of study. In contrast, post-intervention, significantly fewer teens in PAIN rated it as enjoyable (Χ2(4) = 9.52, p = .049). Moreover, 29% of caregivers in PAIN found it helpful in managing pain post-intervention compared to 65% in DISEASE ED (Χ2(2) = 5.44, p = .066). Evaluations at the one-year follow-up assessment were consistent with post-intervention feedback. Thus, a more comprehensive, family-based approach, which includes provision of health-specific information and psychoeducation to enhance teen disease knowledge as well as self-efficacy, is indicated. Consistent with standards proposed by the American Association of Pediatrics 30 for the care of children and adolescents with SCD, programs should consider addressing SCD management, adolescent health care, and health care provider communication as essential components for preparing adolescents for transition to adult care.
In addition to the brief intervention, another design limitation may have been the use of two contrasting study arms rather than a single experimental intervention contrasting with a control condition. A wait-list control design may have been more effective in testing effects of a single intervention. However, fewer families may have agreed to participate if randomization led to no treatment. Moreover, doing this would have limited the ability to discover the differential promising results from DISEASE ED. These promising results, suggesting that comprehensive intervention may be more clinically significant, are important. Although there is general agreement that SCD pain management programs are needed, even the most effective programs show only modest effects in targeted pain, pain coping, and disease knowledge outcomes that are not necessarily linked to broader functional outcomes. 6–8
Designed as a family-based intervention, this study ultimately included a wide range of family members in its implementation, reflecting the strength of the African American family and its ability to mobilize on behalf of youth with sickle cell disease.32 Although transition planning encourages greater independence on the part of adolescents in managing chronic illness11, this study points to the continued importance of the family in supporting both greater independence among the adolescents, as well as an important source of physical, emotional, financial, and logistical support in the day to day management of sickle cell disease. However, developing and implementing disease management interventions such as those described in this study is a resource and time intensive process, particularly when multiple family members are encouraged to participate. The challenges involved in engaging youth with chronic conditions and their family members have been identified for other pediatric populations, including children with cancer and adolescent survivors of childhood cancer.33,34 Suggested strategies for improving participation include modified research designs, overcoming practical barriers to participation, and improving incentives. As the numbers of youth with chronic illnesses increases, progress in developing effective transition programs is indispensable to better prepare them for productive adult lives and to support them and their families in the process.
Acknowledgments
This research was funded by National Heart, Lung, and Blood Institute (U54 30117 to J.R.). The authors wish to acknowledge (in alphabetical order): Katelyn Abrams, Kimberly Bennett, Jennifer Brereton, Stacey Carpenter, Renee Cecil, Lauren C. Daniel, Janet Fithian, Abbas F. Jawad, Anne E. Kazak, Kathleen Lemanek, Kristin Loiselle, Rebecca Martin, D. Colette Nicolaou, Robert Noll, Cindy Phillips, Elizabeth Pulgaron, Jillian Schneider, Beverley Slome-Weinberger, Naketa Thigpen, Kim Whitley-Smith, and Thananya Wooden for their contributions to this project. We are also grateful to the adolescents and families for their generous participation in this research.
Footnotes
Portions of this work were presented at the National Child Healthy Psychology Conference in April, 2008.
References
- 1.National Institutes of Health, National Heart, Lung, and Blood Institute, Division of Blood Diseases and Resources. The Management of Sickle Cell Disease. National Institutes of Health; 2002. [Google Scholar]
- 2.Lemanek KL, Ranalli MA, Green K, et al. In: Handbook of pediatric psychology. 3. Roberts MC, editor. New York: Guilford; 2003. pp. 321–341. [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease: Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 4.Palermo TM, Schwartz L, Drotar D, et al. Parental report of health-related quality of life in children with sickle cell disease. J Behav Med. 2002;25:269–283. doi: 10.1023/a:1015332828213. [DOI] [PubMed] [Google Scholar]
- 5.Telfair J, Ehiri JE, Loosier PS, et al. Transition to adult care for adolescents with sickle cell disease: Results of a national survey. Int J Adolesc Med Health. 2004;16:47–64. doi: 10.1515/ijamh.2004.16.1.47. [DOI] [PubMed] [Google Scholar]
- 6.Baskin ML, Collins MH, Brown F, et al. Psychosocial considerations in sickle cell disease (SCD): The transition from adolescence to young adulthood. J Clin Psychol Med Settings. 1998;5:315–341. [Google Scholar]
- 7.Gil KM, Anthony KK, Carson JW, et al. Daily coping practice predicts treatment effects in children with sickle cell disease. J Pediatr Psychol. 2001;26:163–173. doi: 10.1093/jpepsy/26.3.163. [DOI] [PubMed] [Google Scholar]
- 8.Kaslow NJ, Collins MH, Rashid FL, et al. The efficacy of a pilot family psychoeducational intervention for pediatric sickle cell disease (SCD) Fam Syst Health. 2000;8:381–404. [Google Scholar]
- 9.Gil KM, Williams DA, Thompson RJ, et al. Sickle cell disease in children and adolescents: The relation of child and parent pain coping strategies to adjustment. J Pediatr Psychol. 1991;16:643–663. doi: 10.1093/jpepsy/16.5.643. [DOI] [PubMed] [Google Scholar]
- 10.Gil KM, Wilson JJ, Edens JL. The stability of pain coping strategies in young children, adolescents, and adults with sickle cell disease over an 18-month period. Clin J Pain. 1997;13:110–115. doi: 10.1097/00002508-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Bandura A. Self-efficacy: The exercise of control. New York: W.H. Freeman; 1997. [Google Scholar]
- 12.Edwards R, Telfair J, Cecil H, et al. Self-efficacy as a predictor of adult adjustment to sickle cell disease: One year outcomes. Psychosom Med. 2001;63:850–858. doi: 10.1097/00006842-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Affleck G, Tennen H, Urrows S, et al. Fibromyalgia and women's pursuit of personal goals: A daily process analysis. Health Psychol. 1998;17:40–47. doi: 10.1037//0278-6133.17.1.40. [DOI] [PubMed] [Google Scholar]
- 14.Palermo TM, Witherspoon D, Valenzuela D, et al. Development and validation of the Child Activity Limitations Interview: A measure of pain-related functional impairment in school-age children and adolescents. Pain. 2004;109:461–470. doi: 10.1016/j.pain.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Kliewer W, Lewis J. Family influences on coping processes in children and adolescents with sickle cell disease. J Pediatr Psychol. 1995;20:511–525. doi: 10.1093/jpepsy/20.4.511. [DOI] [PubMed] [Google Scholar]
- 16.Barakat LP, Smith-Whitley K, Ohene-Frempong K. Treatment adherence in children with sickle cell disease: Disease-related risk and psychosocial resistance factors. J Clin Psychol Med Settings. 2002;9:201–210. [Google Scholar]
- 17.Barbarin OA, Christian M. The social and cultural context of coping with sickle cell disease: I. A review of biomedical and psychosocial issues. J Black Psychol. 1999;25:277–293. [Google Scholar]
- 18.Beyer JE, Simmons LE. Home treatment of pain for children and adolescents with sickle cell disease. Pain Manag Nurs. 2004;5:126–135. doi: 10.1016/j.pmn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz L, Radcliffe J, Barakat LP. Assessment of health-related hindrance in teens with sickle cell disease. Memphis, TN. Poster presented at the meeting of the NIH Conference of National Centers of Sickle Cell Disease; 2006. [Google Scholar]
- 20.Brown L, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence. 3. Austin, TX: Pro-Ed; 1997. [Google Scholar]
- 21.Gil KM. Behavioral assessment of sickle cell disease pain. J Health Soc Policy. 1994;5:19–38. doi: 10.1300/J045v05n03_03. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LA, Radcliffe J, Barakat LP. Development of a culturally sensitive pediatric pain management intervention for African-American adolescents with sickle cell disease. Child Health Care. 2007;36(3):267–283. doi: 10.1080/02739610701377954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong FD, Lemanek KL, Pegelow CH. Impact of lifestyle disruption on parent and child coping, knowledge, and parental discipline in children with sickle cell anemia. Child Health Care. 1993;22:89–203. [Google Scholar]
- 24.Newland JA. Factors influencing independence in adolescents with sickle cell disease. J Child Adolesc Psychiatr Nurs. 2008;21:77–185. doi: 10.1111/j.1744-6171.2008.00149.x. [DOI] [PubMed] [Google Scholar]
- 26.Best M, Streisand R, Catania L. Parental distress during pediatric leukemia and posttraumatic stress symptoms (PTSS) after treatment ends. J Pediatr Psychol. 2001;26(5):299–307. doi: 10.1093/jpepsy/26.5.299. [DOI] [PubMed] [Google Scholar]
- 27.Landgraf JM, Abetz L, Ware JE. The CHQ User’s Manual. Boston, MA: The Health Institute, New England Medical Center; 1996. [Google Scholar]
- 28.Project HEALTH Boston. STRIVE curriculum. Boston, MA: Project Health; 2002. [Google Scholar]
- 29.Hazzard A, Celano M, Collins M. Effects of STARBRIGHT World on knowledge, social support, and coping in hospitalized children with sickle cell disease and asthma. Child Health Care. 2002;31:69–86. [Google Scholar]
- 30.American Academy of Pediatrics. Health supervision for children with sickle cell disease. Pediatrics. 2002;109:526–535. doi: 10.1542/peds.109.3.526. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 32.Radcliffe J, Barakat LP, Boyd RC. Family systems issues in pediatric sickle cell disease. In: Brown Ronald T., editor. Handbook of Pediatric Psychosocial Oncology. New York: Guilford; 2006. pp. 496–513. [Google Scholar]
- 33.Lutz Stehl M, Kazak AE, Alderfer MA, Rodriguez A, Hwang W, Pai AL, Boeving A, Reilly A. Conducting a randomized clinical trial of an psychologyical intervention for parents/caregivers of children with cancer shortly after diagnosis. J Pediatr Psychol. 2009;34:803–816. doi: 10.1093/jpepsy/jsn130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tercyak KP, Donze JR, Prahlad S, Mosher RB, Shad AT. Identifying, recruiting, and enrolling adolescent survivors of childhood cancer into a randomized controlled trial of health promotion: Preliminary experiences in the survivor health and resilience education (SHARE) program. J Pediatr Psychol. 2006;31:252–261. doi: 10.1093/jpepsy/jsj013. [DOI] [PubMed] [Google Scholar]