Abstract
Background
Depressive symptoms and major depression are risk factors for clinical coronary heart disease (CHD) among CHD patients and among healthy individuals. It is less clear whether depression is related to the progression of atherosclerosis prior to the onset of CHD events.
Design
Longitudinal cohort study
Methods
149 middle-aged healthy women (113 white and 36 African American) who reported no heart disease, stroke, or diabetes were enrolled simultaneously in two ancillary studies of the Study Women’s Health across the Nation (SWAN) at the Pittsburgh site: the Mental Health Study and the SWAN Heart Study. These women were administered psychiatric interviews annually and coronary calcification computed tomography measures (CAC) on two occasions approximately 2¼ years apart.
Results
Women who had recurrent major depression (N = 33) had greater progression of CAC (logged difference scores) than did women with a single or no episodes, b = 0.09 (0.04), p = .01. The other significant covariates were BMI, SBP, initial CAC, and time between scans. Stratified analyses showed that the effect was obtained in those women who had any CAC at the first examination.
Conclusions
Recurrent major depression may be a risk factor for progression of atherosclerosis, especially in those who have at least some initial calcification. Women with a history of depression may be candidates for aggressive cardiovascular risk factor prevention therapy.
Keywords: Depression, coronary calcification, women, longitudinal
Depressive symptoms and diagnoses of major depression are risk factors for clinical coronary heart disease (CHD) among CHD patients and among healthy individuals (1). It is less clear whether depression is related to progression of atherosclerosis prior to the onset of CHD events. In the Multi-Ethnic Study of Atherosclerosis (MESA), the Pittsburgh Healthy Women Study, the Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research (EISNER) Study, Study of Women’s Health Across the Nation (SWAN), and a study of healthy Army personnel, depressive symptoms were unrelated to concurrent measures of coronary artery calcification (CAC), a measure of plaque burden that prospectively predicts clinical CHD (2–6). In these studies, well validated standardized scales of symptoms in the last 2 to 4 weeks were used, without diagnostic assessment of major depression being performed.
Perhaps longer term exposure to depression or experiencing more severe depression enough to warrant diagnosis of major depression is needed to show a relationship with CAC. In that regard, in the Whitehall II Study, reports of cognitive symptoms of depression at multiple examinations compared to one or no examinations was associated with CAC; these effects were observed in men and not in women (7). In the Rotterdam Aging Study, elderly participants who had both elevated concurrent depressive symptoms and met criteria for major depression had higher levels of CAC, relative to those with elevated symptoms only (8). Authors suggested that vascular changes in the brain may have lead to depression, but that the cross-sectional nature of the design did not allow for causal inferences. In Pittsburgh SWAN site, healthy African American and white middle-aged women with at least two episodes of major depression had any CAC, compared to women with a single or no episodes (9). In that analysis, depressive symptoms were not related to any CAC.
A number of pathways have been suggested to account for associations between depression and atherosclerosis, including life style factors, such as smoking and exercise, elevated weight and waist circumference, adverse lipid profile, and inflammation (10). Inflammatory markers, such as C reactive protein, are associated with depressive symptoms in a number of studies (11), but the effect may be bi-directional (12). That is, depressive symptoms may lead to increased inflammation, and inflammation may lead to increased depressive symptoms.
The present study tested the hypothesis that women who had at least two episodes of major depression would experience greater progression of CAC than women with only one or no episodes. The sample was composed of African American and white middle-aged women who had two assessments of CAC separated by 2¼ years and who reported no heart attack, stroke, or diabetes at the baseline CAC assessment. This hypothesis was tested in the full sample and then separately in women with and without any CAC at the initial evaluation. A secondary analysis tested whether CRP predicted CAC progression and constituted a potential pathway by which recurrent depression was related to CAC progression.
Methods
Participants
The 149 women (113 white and 36 African Americans) in the analytic sample were enrolled simultaneously in two ancillary studies of SWAN at the Pittsburgh site: the Mental Health Study, which started with SWAN, (13) and the SWAN Heart Study, which began in follow-up year 4 (14). These women were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV) interviews at study entry (1996–1997) and annually thereafter. CAC was measured on two occasions several years apart (M= 2.23 (0.42) years). First CAC measure occurred in 2001–2003, whereas the second occurred in 2002 to 2005. At this site, SWAN women were recruited using random digit dialing and a voter’s registration list; initial eligibility criteria included being from white or African American ethnicity (self-identified) with the target of 1/3 minorities; ages 42 to 52 years, having had an intact uterus, menstruating within the prior 3 months, and not using reproductive hormones (15). Of the 463 women who enrolled in the Pittsburgh SWAN site, 443 also participated in the Mental Health Study and did not differ in sociodemographic factors and Center for Epidemiological Studies Depression (CES-D) scale scores (16) from those who did not participate.
Exclusionary criteria for the subclinical CVD study were self-reported heart disease (myocardial infarction, congestive heart failure, angina, intermittent claudication or revascularization), stroke, treated diabetes, current pregnancy, or use of hormone replacement therapy. Of the 280 women eligible to have electron beam tomography (EBT) evaluation of the heart, 216 agreed. Of these, 70% or 152 completed two EBT evaluations. One woman of the 152 reported a stroke prior to EBT procedures, and 2 did not participate in the Mental Health Study. Participants in the first EBT protocol were healthier at baseline than nonparticipants at the Pittsburgh site; they had lower blood pressure and waist-hip ratios, and were better educated, White, and nonsmokers, Ps < .05. No differences occurred in age, body mass index, and lipids. The better health of the participants is consistent with the selection criteria for the subclinical disease study. There were no differences on risk factors, age, ethnicity, or prevalence of major depression for the 149 women in the analysis who completed the second EBT vs those who had only one EBT.
Procedure
The Institutional Review Board of the University of Pittsburgh approved all procedures described herein. Written informed consent was obtained at the baseline visit as part of the SWAN protocol. The following assessments were performed at baseline and each annual follow-up visit: self-report and interviewer-administered questionnaires pertaining to medical, reproductive, and health history, lifestyle behaviors, medication use, and psychosocial factors; a fasting venipuncture; anthropometric measurements; and psychiatric diagnoses. Medical history was not verified against physician records. All blood samples were maintained at 4°C until spun and separated, and then frozen at −20° C and shipped on dry ice to the central laboratory (Medical Research Laboratories, Highland Heights, KY) for analysis (certified by the National Heart Lung and Blood Institute, Centers for Disease Control Lipid Standardization Part III program). To ensure accuracy and consistency of data collection, all phlebotomists and technicians were trained and annually certified according to a common protocol.
Cardiovascular Risk Factors
Body mass index was derived from in-clinic measures of weight and height. Waist circumference was measured at the level of the natural waist, defined as the narrowest part of the torso as seen from the anterior aspect; waist circumference was regressed onto BMI because of the high correlation between waist circumference and BMI. All lipid and lipoproteins were analyzed on EDTA-treated plasma. Total cholesterol and triglycerides were analyzed by enzymatic methods on a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN), and HDL-C was isolated using heparin-2M manganese chloride (17–19). CRP was measured using an ultra-sensitive rate immunonephelometry (Dade-Behring, Marburg, Germany). Blood pressure was measured twice with a minimum two-minute rest period between measures, with readings taken on the right arm, with the respondent seated and feet flat on the floor for at least 5 minutes prior to the measurement. Respondents had not smoked or consumed any caffeinated beverage within 30 minutes of blood pressure measurement. Appropriate cuff size was determined based on arm circumference. The two sequential blood pressure values were averaged. Smoking history was coded into current vs past or never smoker. Lipids and/or CRP were missing for 24 women at the baseline CAC measurement; for 20 of these women, data were carried forward at the visit immediately prior, whereas for 4, these values were carried forward from 2 to 4 visits prior to the baseline CAC measurement.
Depression
The SCID-IV for nonpatients was conducted at baseline to determine lifetime history and current diagnoses of major depression and annually thereafter to diagnose major depression in the past year. The SCID is a semistructured diagnostic interview designed to enable trained interviewers to determine lifetime and current diagnoses of psychiatric disorders, including mood, anxiety, and substance use disorders according to the DSM-IV (20). The current study classified women on the basis of the number of episodes of major depression (including current if applicable) by the time of the first EBT; presence of minor depression or dysthmia was not considered. Women were categorized into those who had at least 2 episodes vs 1 or none parallel to our prior cross-sectional analyses (9). The occurrence of at least two episodes makes it likely that the women were vulnerable to depression for a period of time.
The SCID-IV interviewers were trained clinicians with a Masters degree, PhD, or degree in psychiatric nursing who participated in a training session with the Biometrics Institute at the College of Physicians and Surgeons of Columbia University (New York, NY), conducted at least 10 practice interviews evaluated by the mental health study’s principle investigator (J.T.B.), and had taped interviews reviewed and “certified” by the Biometrics Institute. The k-score for lifetime diagnoses of major depression was 0.81, indicating adequate reliability.
CES-D scores were also available. Only 12 women had scores ≥ 16, indicative of at least mild depressive symptoms, and of these women, 8 had recurrent depression. A separate report is being prepared on the CES-D in relation to progression in the present sample combined with a second SWAN site, which did not administer the SCID. Thus, we are not reporting herein the results of the CES-D.
Coronary Calcification
Beginning in 2000 ( computed tomography scanner (C-150 Ultrafast CT Scanner; GE Imatron, San Francisco, CA) was used to quantify calcification in the coronary arteries (21). Several passes were performed: the first allowed an evaluation of the participant’s anatomy so that the landmarks for the coronary scans are identified. The second pass was for the coronary arteries, in which 30 to 40 contiguous 3 mm thick transverse images were obtained from the level of the aortic root to the apex of the heart. Images were obtained during a maximal breath hold using electrocardiographic triggering so each 100-millisecond exposure was obtained during the same phase of the cardiac cycle (60%) of the R-R interval). All scan data were saved to an optical disk for central scoring using a DICOM workstation and software by AcuImage, Inc (South San Francisco CA). This software program implements the Agatson scoring method. Coronary artery lesions were considered to be present when 3 or more pixels greater than 130 Hounsfield units (HU) were detected overlying the vessels of interest (22). Scoring resulted in total calcium, which was the sum of the individual scores for the 4 major epicardial coronary arteries. Under the supervision of a cardiologist, a technologist unaware of the depression evaluation scored the scans. Inter-rater correlation of coronary calcification scores was high, intraclass correlation coefficient= 0.99 (23).
Statistical Analyses
CRP was natural log transformed for analyses. As in MESA (24), change in CAC was measured by the difference between the natural log (second CAC + 25) and the natural log (first CAC + 25). Spearman rank order correlations examined the association between risk factors and initial CAC. Pearson correlations examined the association of risk factors with change in CAC. Stepwise forward linear regression models were used, with recurrent depression forced into the model, and forward selection on the significant covariates of first CAC score, age, ethnicity, BMI, waist circumference residualized for BMI, HDL-C, LDL-C, SBP, smoking status, and CRP. Because the interaction between baseline CAC and recurrent depression was significant, we stratified by groups and repeated the primary analyses. P-values < .05 were considered statistically significant.
Results
As expected given the inclusion criteria, the analytic sample was composed of healthy middle-aged women, with few current smokers and no one taking lipid lowering agents (Table 1). Sixty-four of the 149 women had any calcification at baseline, with a median Agaston score of 8.0 and M= 38.2 (69.3) among the 64. Thirty-three women had at least two episodes of major depression (11 current), 26 had a single episode (3 current), and 90 had no episodes by the time of the first CAC measures. Comparisons of women with and without recurrent depression showed that women with recurrent depression had at baseline more CAC, larger BMI and waist circumference, and reported more often taking anti-depressant medications for a “nervous” condition (33% vs 7.8%). Similar results had been obtained in the larger sample from which this subsample was drawn (9). Spearman rank order correlations were significant between baseline CAC scores and CRP, r = 0.30, p < .0001; BMI, r = 0.53, < .0001; HDL-C, r = −0.38, p < .0001, and SBP, r = 0.38, p < .0002. Age, waist circumference residualized for BMI, LDL-C, smoking status, and ethnicity were not related to initial CAC in this healthy sample, p > .10.
Table 1.
Sample Characteristics at Baseline According to Recurrent Depression History
| Recurrent Depression | |||
|---|---|---|---|
| Yes (n=33) | No (n=116) | Total Sample | |
| Mean (SD) Age | 50.2 (2.7) | 50.5 (2.5) | 50.5 (2.5) |
| N (%) African Americans | 8 (24.2) | 28 (24.1) | 36 (24.2) |
| N (%) Current Smokers | 3 (9.1) | 14 (12.1) | 17 (11.4) |
| Mean (SD) Body Mass Index (kg/m2) | 31.6 (7.7) | 28.6 (5.5)* | 29.2 (6.2) |
| Mean (SD) Waist Circumference (cm) | 96.6 (20.1) | 87.5 (13.3)* | 89.5 (15.5) |
| Mean (SD) HDL-C (mg/dl) | 55.2 (14.9) | 60.5 (14.1) | 59.3 (14.4) |
| Mean (SD) LDL-C (mg/dl) | 127.1 (42.7) | 122.2 (31.5) | 123.3 (34.2) |
| Mean (SD) C-Reactive Protein (mg/dl) | 4.93 (6.22) | 3.67 (4.29) | 3.95 (4.79) |
| Systolic Blood Pressure (mmHg) | 118.5 (20.4) | 113.7 (14.1) | 114.7 (15.7) |
| Calcification Score: | |||
| N (%) 0 | 14 (42.4) | 71 (61.2)* | 85 (57.0) |
| N (%) 1–9 | 6 (18.2) | 27 (23.3) | 33 (22.1) |
| N (%) ≥ 10 | 13 (39.4) | 18 (15.5) | 31 (20.8) |
p<.05 groups differ
Change in CAC scores was associated with the following measured at the first EBT exam: log CRP, r = 0.20, p = .01; BMI, r = 0.40, p < .0001; HDL-C, r = −0.25, p = .002; SBP, r = 0.34, p < .0001, time between exams, r = 0.20, p = .01, and first transformed CAC (natural log (score + 25)) score, r = 0.35, p < .0001.
Women who had recurrent major depression had greater increases in CAC than did women with a single or no episodes (M= 20.2 (33), median = 1.4, vs M = 5.9 (11), median = 0). In linear regression analyses adjusting for age, race, and time between scans, recurrent depression was a significant predictor, b = 0.15 (0.04), p = .001. Forward step-wise linear regression models (forcing recurrent depression) allowing significant covariates to come into the model showed that recurrent depression was associated with greater progression (Table 2). CRP did not enter the model; it was highly correlated with BMI in this sample, r = 0.40, p < .0001. When BMI was removed from the model, CRP still did not enter into the final model. When the no recurrent depression group was partitioned into groups with a single vs no episodes, recurrent depression remained significant in the forward stepwise model, b = .10 (0.04), p = .02, whereas single episode group did not differ from the no episode group, p = .11.
Table 2.
Linear Regression Model Predicting Change in Coronary Artery Calcification across 2.2 years.
| Variable | b (Standard Error) | p-value |
|---|---|---|
| Recurrent major depression (yes=1; no=0) | .09 (.04) | .03 |
| Body mass index (kg/m2) | .01 (.003) | .006 |
| Systolic blood pressure (mmHg) | .003 (.001) | .006 |
| Baseline calcification (log [CAC + 25]) | .10 (.03) | .001 |
| Time between scans (yrs) | .08 (.04) | .04 |
Major depression forced into model using forward step-wise linear regression to include potential covariates.
Both interactions between recurrent depression and any initial CAC yes/no, p < .05, or transformed initial CAC, p < .002, were significant. For women who had any initial CAC, mean change was 19.8 (30.0), with median of 4.1; among this group, those with a history recurrent depression increased 142 (204.3) percent on average, with a median percent increase of 74.7 percent compared to 69 (228.7) percent and −4.4 percent for women with no history of recurrent depression. For women with no initial CAC, mean change was 0.97 (2.67), with median of 0, with 2 of 14 women with a history of recurrent depression converting to having any CAC at follow-up, compared to 15 of the 56 with a history of recurrent depression.
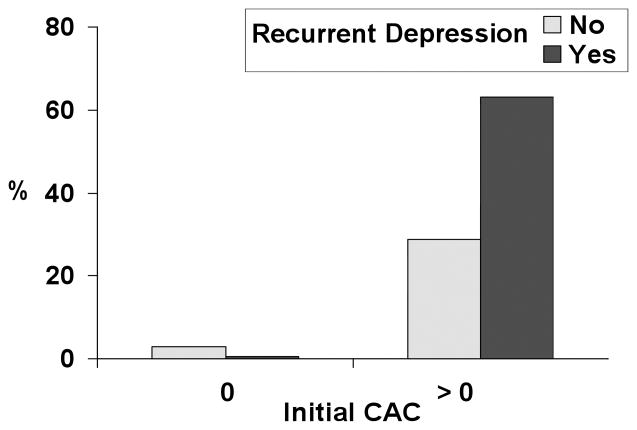
Stratified analyses showed that recurrent depression was associated with progression among the 64 women who had any initial CAC, b = 0.17 (0.07), p = .02. BMI, SBP, initial log CAC, and time between exams were also significant covariates in this model. Recurrent depression was not associated with the magnitude of increase in CAC among the 85 women with no CAC at the initial assessment, p = .73. In this analysis, high BMI, high SBP, and low CRP were significant covariates. To illustrate the pattern of results based on the above linear models, Figure 1 compares the proportion of women who increased ≥ 10 total CAC or less in relation to recurrent depression history and initial CAC score groups.
Figure 1.
Proportion of Women with Progression of ≥ 10 Total Calcium Score by Group.
Discussion
This study tested the hypothesis that African American and White women with recurrent major depression during their lives would show greater progression of coronary calcification than would women with a single or no episode of major depression. Results confirmed the hypothesis in analyses that adjusted for other risk factors for atherosclerosis. Furthermore, when analyses were stratified by whether women had any CAC at initial assessment, results showed that the associations were only apparent among the women who had initial CAC at baseline. Conversely, women who did not have any initial CAC were very unlikely to show progression. Thus, the results suggest that a history of recurrent major depression is associated with progression of CAC prior to the onset of clinical heart disease, in addition to being associated with prevalent CAC as reported in previous cross-sectional analyses (8–9). This is the first study to show that major depression is related to progression of coronary calcification. These results are unlikely to be due to vascular changes leading to depression, given the age of the sample. The findings also suggest that women who are most vulnerable to the effects of depression are those who already have some subclinical disease, perhaps indicating an acceleration of the atherosclerotic process in women with at least some disease. On the other hand, women with no detectable disease may be unlikely to convert to having any CAC, even in the presence of severe depression.
A secondary objective was to examine whether inflammation was a pathway by which depression would be related to progression of calcification. Our results showed that while CRP was related to progression of calcification in unadjusted models, it was not related to progression in models that included history of recurrent major depression history and other cardiovascular risk factors. This null result was not due to the high correlation between BMI and CRP because excluding BMI from the final model did not change the results.
Our study has several limitations. Results are specific to women in mid-life, who were free from self-reported heart disease, stroke, and diabetes at the initial exam and cannot be generalized to men. No physician verification of self-reported history was obtained. Recall that the majority of women did not have any CAC at baseline, in part because of the eligibility criteria to be in the protocol. Whether recurrent depression would be related to progression in samples with a higher prevalence of CAC or a higher prevalence of risk factors or in men is not clear from our analysis. However, among a large sample of patients who underwent bypass surgery, depressive symptom scores were related to greater saphenous graft disease progression over time (25). The pathways by which major depression may be linked to coronary calcification are also not clear from our analysis, given that the associations were not accounted for by standard risk factors. Other factors such as a wider array of inflammatory and coagulation factors and lipid particle size and number may be worthy of exploration as potential pathways. It is also important to examine whether there are shared genetic and/or environmental factors that account for both the risk of major depression and plaque burden early in women’s lives. Finally, major depression may serve to exacerbate the effects of other standard and novel risk factors to accelerate progression of atherosclerosis. However, because of the small sample size, we were not able to test this notion beyond stratification on presence/absence of initial calcification. Additional studies with a larger sample size are needed.
Nonetheless, these results have several clinical implications. Current American Heart Association recommendations are to screen women for depression only if they are heart disease patients (26). Our findings suggest that it would be useful to screen all women who are entering a period of increasing cardiovascular risk, i.e. around the time of menopause. Screening tools such as the Patient Health Questionnaire-9 are available and could be incorporated into standard practice (27). At present, we do not have clinical trial evidence that intervening on depression among coronary patients alters later risk for recurrent events (28–29) and we do not have prevention trials available. Thus, a better alternative for healthy women who report multiple episodes of major depression may be aggressive cardiovascular risk factor therapy to prevent calcification. This is particularly the case because even high normal levels of lipids and blood pressure as well as smoking in healthy mid-life women are related to progression of calcification (30–31).
In sum, lifetime history of recurrent major depression was a predictor of progression of coronary calcification in African American and White women in mid-life, well before the usual onset of clinical heart disease in women. This observation was particularly true in women who already had at least some calcification in the coronary arteries. Women with history of major depression may benefit from both close monitoring and aggressive risk factor treatment.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Mental Health (NIMH), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants MH059689; NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NIMH, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 – 1999; Joel Finkelstein, PI 1999-present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI 1994–2009; Howard Kravitz, PI 2009-present; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee: Chris Gallagher, Chair Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN.
Supported by the National Institutes of Health
Abbreviations list
- SWAN
Study of Women’s Health Across the Nation
- CAC
Coronary calcification
- BMI
Body mass index
- SBP
Systolic blood pressure
- CHD
Coronary heart disease
- MESA
Multi-ethnic study of atherosclerosis
- EISNER
Early identification of subclinical atherosclerosis by noninvasive imaging research
- CRP
C-reactive protein
- SCID-IV
Structured clinical interview for DSM-IV axis I disorders
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- CES-D
Center for epidemiological studies depression
- CVD
Cardiovascular disease
- EBT
Electron beam tomography
- HU
Hounsfield units
- EDTA
Ethylenediaminetetraacetic acid
- HDL-C
High density lipoprotein cholesterol
- LDL-C
Low density lipoprotein cholesterol
Footnotes
All authors report no conflict of interest.
References
- 1.Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- 2.Diez Roux AV, Ranjit N, Powell L, Jackson S, Lewis TT, Shea S, Wu C. Psychosocial factors and coronary calcium in adults without clinical cardiovascular disease. Ann Intern Med. 2006;144(11):822–831. doi: 10.7326/0003-4819-144-11-200606060-00008. [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Owens JF, Edmundowicz D, Lee L, Kuller LH. Positivie and negative attributes and risk for coronary and aortic calcification in healthy women. Psychosom Med. 2006;68:355–361. doi: 10.1097/01.psy.0000221274.21709.d0. [DOI] [PubMed] [Google Scholar]
- 4.Kop WJ, Berman DS, Gransar H, Wong ND, Miranda-Peats R, White MD, Shin M, Bruce M, Krantz DS, Rozanski A. Social network and coronary artery calcification in asymptomatic individuals. Psychosom Med. 2005;67:343–352. doi: 10.1097/01.psy.0000161201.45643.8d. [DOI] [PubMed] [Google Scholar]
- 5.O’Malley PG, Jones DL, Feuerstein IM, Taylor AJ. Lack of correlation between psychosocial factors and subclinical coronary artery disease. N Engl J Med. 2000;343(18):1298–1304. doi: 10.1056/NEJM200011023431803. [DOI] [PubMed] [Google Scholar]
- 6.Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, Jacobs E, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in African American women: The SWAN heart study. Psychosom Med. 2006;68:362–368. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- 7.Hamer M, Kivimaki M, Lahiri A, Marmot MG, Steptoe A. Persistent cognitive depressive symptoms are associated with coronary artery calcification. Atherosclerosis. 2010;210:209–213. doi: 10.1016/j.atherosclerosis.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiemeier H, van Dijck W, Hofman A, Witteman JCM, Stijnen T, Breteler MMB. Relationship between atheroscerlosis and late-life depression: the Rotterdam Study. Arch Gen Psychiatry. 2004;61:369–376. doi: 10.1001/archpsyc.61.4.369. [DOI] [PubMed] [Google Scholar]
- 9.Agatisa PK, Matthews KA, Bromberger JT, Edmundowicz D, Chang YF, Sutton-Tyrrell K. Coronary and aortic calcification in women with a history of major depression. Arch Intern Med. 2005;165:1229–1236. doi: 10.1001/archinte.165.11.1229. [DOI] [PubMed] [Google Scholar]
- 10.Miller GE, Freeland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 11.Howren MB, Lamkin DM, Suls J. Associations of depression with c-reactive protein, IL-1, and IL-6: a meta analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 12.Matthews KA, Schott LL, Bromberger JT, Cyranowski JM, Everson-Rose SA, Sowers M. Are there bi-directional associations between depressive symptoms and c-reactive protein in mid-life women? Brain Behav Immun. 2010;24(1):96–101. doi: 10.1016/j.bbi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromberger JT, Kravitz HM, Wei HL, Brown C, Youk A, Powell LH, Matthews KA. History of depression and women’s current health and functioning in midlife. General Hospital Psychiatry. 2005;27:200–208. doi: 10.1016/j.genhosppsych.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: Findings from the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118:1234–1240. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowers M, Crawford S, Sternfeld, Morganstein D, Gold E, Greendale GA. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, editors. Menopause: Biology and Pathobiology. New York, NY: Academia Press; 2000. pp. 175–188. [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 17.Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control- National Heart, Lung and Blood Institute Lipid Standardization Program: an approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–135. [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Stein EA, Steiner PM, Gartside PS, Glueck CJ. Development and evaluation of a method for quantitation of plasma high-density-lipoprotein cholesterol. Clin Chem. 1978;24:1112–1115. [PubMed] [Google Scholar]
- 20.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 21.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 22.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 23.Sutton-Tyrrell K, Kuller LH, Edmundowicz D, Feldman A, Holubkov R, Givens L, Matthews KA. Usefulness of electron beam tomography to detect progression of coronary and aortic calcium in middle-aged women. Am J Cardiol. 2001;87(5):560–564. doi: 10.1016/s0002-9149(00)01431-4. [DOI] [PubMed] [Google Scholar]
- 24.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects. Results from the Multi-Ethnic Study of Atherosclerosis. Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 25.Wellenius GA, Mukamal KJ, Kulshreshtha A, Asonganyi S, Mittleman MA. Depressive symptoms and the risk of atherosclerotic progression among patients with coronary artery bypass grafts. Circulation. 2008;117:2313–2319. doi: 10.1161/CIRCULATIONAHA.107.741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Heart Association’s Guidelines At-A-Glance for Preventing Heart Disease and Stroke in Women. [Accessed November 30, 2009];The American Heart Association Web Site. http://www.americanheart.org/
- 27.Kroenke OK, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3016. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 29.Frasure-Smith N, Lesperance F, Prince RH, Verrier P, Garber RA, Juneau M, Wolfson C, Bourassa MG. Randomized trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet. 1997;350(9076):473–479. doi: 10.1016/S0140-6736(97)02142-9. [DOI] [PubMed] [Google Scholar]
- 30.Matthews KA, Kuller LH, Chang Y, Edmundowicz D. Premenopausal risk factors for coronary and aortic calcification: a 20-year follow-up in the health women study. Prev Med. 2007;45(4):302–308. doi: 10.1016/j.ypmed.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuller LH, Matthews KA, Edmundowicz D, Chang Y. Incident coronary artery calcium among postmenopausal women. Atherosclerosis. 2008;200:278–285. doi: 10.1016/j.atherosclerosis.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]