Abstract
Social relationships strongly affect alcohol drinking in humans. Traditional laboratory rodents do not exhibit social affiliations with specific peers, and cannot adequately model how such relationships impact drinking. The prairie vole is a socially monogamous rodent used to study social bonds. The present study tested the prairie vole as a potential model for the effects of social affiliations on alcohol drinking. Same-sex adult sibling prairie voles were paired for five days, and then either separated into individual cages, or housed in pairs. Starting at the time of separation, the voles received unlimited access to alcohol in a two-bottle choice test versus water. Pair-housed siblings exhibited higher preference for alcohol, but not saccharin, than singly-housed voles. There was a significant correlation between the amount of alcohol consumed by each member of a pair when they were housed together (r = 0.79), but not when housed apart (r = 0.20). Following automated analysis of circadian patterns of fluid consumption indicating peak fluid intake before and after the dark phase, a limited access two-hour two-bottle choice procedure was established. Drinking in this procedure resulted in physiologically relevant blood ethanol concentrations (BECs) and increased Fos immunoreactivity in perioculomotor urocortin containing neurons (but not in nucleus accumbens or central nucleus of the amygdala). The high ethanol preference and sensitivity to social manipulation indicate that prairie voles can serve to model social influences on excessive drinking.
Keywords: c-Fos, circadian activity, ethanol self-administration, Microtus ochrogaster
INTRODUCTION
Social relationships and alcohol (ethanol) drinking have complex effects on each other. In one direction of these effects, social situations can affect alcohol intake patterns and quantities. For example, social stress or separation from a loved one through death or divorce can lead to increased alcohol intake (Hajema and Knibbe, 1998; Jose et al., 2000; Temple et al., 1991), while a supportive social network is a major aide for abstinent alcoholics (Groh et al., 2008; Kelly et al., 2008). There are many examples of effects in the opposite direction, where alcohol intake impacts social relationships. Male alcohol abuse is considered a causal risk factor for intimate partner violence (Heise, 1998; Leonard et al., 1985), and alcohol use is a commonly accepted cause of marital dissatisfaction and dissolution (for discussion of these findings, see Leonard and Eiden, 2009).
While adverse effects of social relationships can lead to increased drinking, many enjoyable relationships and circumstances may also lead to increased drinking. Alcohol is often considered a ‘social lubricant,’ such that there is a reciprocal relationship between the social network and individual drinking patterns (Park et al., 2008), and a person's social network is a primary factor in modulation of their alcohol use (Homish and Leonard, 2008).
Previously, there has not been an adequate laboratory rodent model to investigate socially facilitated drinking, or the effect of social relationships on alcohol consumption. It is difficult or impossible at this time to study the neural mechanisms in humans that are involved in influencing one another's drinking or peer pressure. A rodent model that exhibited behaviors relevant to elevated drinking in social situations would be invaluable.
The primary reason for the absence of an adequate rodent model is the lack of strong specific bonds in traditional laboratory rodents. While mice and rats do prefer social environments, particularly in adolescence (Douglas et al., 2004; Panksepp and Lahvis, 2007), can show signs of anxiety- and depression-like symptoms when they are socially isolated (Yates et al., 1991), and can show differences in alcohol intake dependent on their social housing conditions (Deehan et al., 2007; Doremus et al., 2005; Ehlers et al., 2007; Schenk et al., 1990), there is no evidence that they show strong pair bonds with, or prefer to spend time with, a particular individual.
In contrast, prairie voles (Microtus ochrogaster) are socially monogamous rodents that have been extensively studied because they form specific pair bonds. In the wild, mated pairs nest together, both parents participate in caring for offspring, and they typically mate for life (Getz et al., 1981). The pair bond can be demonstrated in the laboratory by more time spent with a partner than with a stranger in the partner preference test (Williams et al., 1992; for a review, see Young et al., 2008). While traditional laboratory animals can model altered behavior in response to social isolation, the prairie vole can be used to model more fully the formation, maintenance, and effects of a specific pair bond relationship.
While male-female pair bonds have been widely studied in prairie voles, the sibling bond is another important relationship. Under certain circumstances in the wild, a high percentage of juvenile prairie voles remain in the natal nest with their parents and siblings instead of dispersing (Carter and Roberts, 1997). In the lab, prairie voles exhibit signs of depression- and anxiety-like behaviors when separated from a partner or a same-sex sibling, such as in the elevated plus maze, sucrose consumption, and resident-intruder tests (Bosch et al., 2009; Grippo et al., 2007a; Grippo et al., 2007b; Pan et al., 2009). These bonds and the effects of separation can be used to examine the influences of specific social relationships on alcohol drinking. Here the sibling bond is explored, in order to avoid issues of pregnancy (or gonadectomy) that could independently affect alcohol consumption.
In our pilot experiments we observed a preference for alcohol intake in prairie voles (unpublished observations: Loftis et al., 2006), suggesting that voles can be useful for alcohol self-administration studies. Most other laboratory rodents that exhibit a high preference for alcohol are inbred or selectively bred lines of mice and rats. Therefore, the prairie vole, which remains outbred and exhibits a high degree of genetic diversity relative to these strains of mice and rats, might be valuable for examining genetic underpinnings of alcohol preference and intake, and for observing individual variation.
Interestingly, there is considerable overlap in a number of neural systems that have been implicated in pair bond formation in prairie voles and in alcohol intake. For example, the role of the dopamine system in pair bonding has been established in prairie voles (Aragona et al., 2003; Aragona et al., 2006; Wang et al., 1999), and it has been well-established that dopamine plays a role in response to alcohol and other addictive substances (Everitt et al., 2008; Robinson and Berridge, 1993; Robinson and Berridge, 2008; Wise and Bozarth, 1981). The commonalities and potential interplay of the dopamine system involved in these behaviors have been reviewed and discussed (Curtis et al., 2006). Additionally, vasopressin, a neuropeptide necessary for pair bond formation in male prairie voles (Winslow et al., 1993), has long been known to influence alcohol intake (Finkelberg et al., 1978). Similarly, the corticotropin-releasing factor (CRF) system plays a role in partner preference formation in male prairie voles (DeVries et al., 2002; Lim et al., 2007), as well as in coping with isolation or loss of a partner (Bosch et al., 2009; Grippo et al., 2007b), and has been shown in a large body of literature to regulate alcohol intake (for reviews, see Heilig and Koob, 2007; Ryabinin et al., 2002; Valdez and Koob, 2004). The overlap of the neural mechanisms for social bonding and alcohol intake implies that there could be some common regulation of these behaviors, and that they may affect one another.
The goal of these studies was to establish the prairie vole as a novel model to investigate the interrelation of social affiliations and alcohol drinking. The preliminary hypothesis was that the stress of separation from a partner would lead to increased drinking relative to voles that remained with their partner. The results described here show an effect in the opposite direction, implying that prairie voles prefer to drink alcohol more when they are with a partner, and that they may be a model for socially-facilitated excessive drinking. Subsequent experiments were performed to establish a procedure for limited access to alcohol useful for studies of pharmacological manipulations that could influence drinking behavior.
MATERIALS AND METHODS
Animals
The prairie vole colony was originally established from eight pairs generously provided by Dr. Joseph Lonstein at Michigan State University in March 2005. These voles originated from a colony at Emory University, which was derived from field-caught prairie voles in Illinois. Diversity in our colony was maintained by the generous donations of prairie voles from Dr. Phillip Smith at Texas Tech University in November 2007, and from Dr. Karen Bales at the University of California at Davis in February 2008.
Prior to any experimentation, prairie voles were housed in same-sex sibling groups after weaning at around 21 days, and maintained on a diet of mixed rabbit chow (LabDiet Hi-Fiber Rabbit), corn (Nutrena Cleaned Grains), and oats (Grainland Select Grains). All experiments took place in the vole colony room at the Portland Veterans Affairs Medical Center (VAMC). All animals were kept on a 12:12 hour light-dark cycle. All animals had ad libitum access to food and water throughout each experiment. Animals were alcohol- and experimentally-naïve, except where noted in Experiments 1 and 4.
In Experiment 1, we also used alcohol-naïve mice of C57BL/6J (C57) background bred in our animal colony at OHSU that were housed at four to five per cage with water and food constantly available, and C57 mice purchased from Jackson Laboratories and housed five per cage for one week prior to testing.
All animal care, breeding, and testing procedures were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health and approved by the local Institutional Animal Care and Use Committees at the VAMC and OHSU, Portland, OR, USA.
Drinking solutions and drugs
Fluids were available from 25 ml glass tubes with metal sipper tubes attached with a rubber stopper. For 24-hour access experiments, the bottles were filled to 25 ml, and for the limited access experiment the bottles were filled to 10 ml. After the period of consumption, the bottles were carefully removed to avoid spillage, and remaining volumes were read to the nearest 0.2 ml.
The animals in each alcohol drinking experiment were given a two-bottle choice test, always with one bottle of tap water, and the other bottle containing one of the following solutions. Alcohol solutions (3%, 6%, or 10%) were made as volume/volume (v/v) concentrations from 95% ethanol and tap water. Saccharin and quinine concentrations were 0.05% and 0.0025%, respectively, weight/volume (w/v) in tap water. Each solution was made fresh every other day and stored in an airtight container, and the solutions were replaced in the drinking tube at the start of each consumption period.
Experiment 1 – Assessment of alcohol elimination rates in voles and mice
In order to test whether high alcohol intake in voles is not due to unusually high ethanol elimination rates, we compared blood ethanol concentration (BEC) in voles and C57 mice after an intraperitoneal (i.p.) injection of 2.5 g/kg of ethanol (20%, v/v). Twenty ethanol-naïve prairie voles (10 females and 10 males; weights 36.2 ± 1.4 g) with ages ranging from 83 to 109 days and 20 ethanol-naïve mice (9 females and 11 males; weights 22.8 ± 0.7 g; 72-103 days old) were euthanized either 30, 60, 120, or 180 minutes after the injection by an overdose of CO2, and trunk blood was collected.
In addition, a separate study of alcohol elimination rate was conducted in 35 voles (17 females and 18 males; weights 42.1 ± 1.0 g; 106-134 days old) and 40 mice (20 females and 20 males; weights 23.5 ± 0.6 g; 86 days old) that were not naïve to alcohol. Briefly, these animals were given continuous access to increasing concentrations of alcohol (3%, 6%, 10%) over 12 days in a two-bottle choice test with water as described in subsequent experiments, while half the animals were housed in pairs and the others were housed in isolation. Ethanol injections (2.5 g/kg) were given eleven days following the final access to drinking alcohol, and the animals were euthanized at the same relative times that the naïve animals were.
Blood samples were centrifuged at 5223 relative centrifugal force (RCF) for 10 minutes, after which serum was removed and stored at -20°C before processing. BEC was determined using an Analox Analyzer (Analox Instruments, Luneburg, MO, USA) and is reported in milligrams per deciliter (mg/dl).
Experiment 2 – Investigation of the effects of social separation on alcohol intake
The effect of social housing on alcohol intake was tested in 30 female (44.2 ± 1.2 g) and 32 male (44.3 ± 1.3 g) adult prairie voles, ranging from 68 to 85 days old on the first day of the experiment. First they were moved from their home cage into a new cage with one of the same-sex littermates, where they remained for five days, with water available from the drinking tubes. On the sixth day, the pairs were moved to new cages, where half of the pairs were kept together, and the other half of the pairs were separated into individual cages. In order to monitor drinking behavior of each subject, we created a cage that would house a pair of prairie voles in a manner that would allow each exclusive access to drinking solutions. A wire mesh divider down the center of the cage kept each of the paired voles in one half of the cage, where it had access to its own drinking tubes, but could still see, hear, smell and interact with the other vole through the mesh. The cage was approximately 26.7 × 26.7 × 13.3 cm, the mesh wire was less than 2 mm thick, and the distance between wires was 1.3 cm in the length dimension of the cage and 2.6 cm in the height dimension. The individual cages were approximately equal in size to one side of the mesh-divided cage (26.7 × 16.5 × 13.3 cm).
On the day the animals were moved to new cages, they began a continuous access two-bottle choice test with water and 3% ethanol. The position of the alcohol bottle relative to the water bottle was switched daily to avoid the potential effects of side preference. The choice test consisted of four days at each concentration (3%, 6%, 10%), given in increasing order to all animals. For all choice tests, consumption from both tubes was monitored every 24 hours, and preference for the solution relative to total fluid intake was calculated, in addition to dose of ethanol consumed per body weight (g/kg). Voles were weighed on the first day of the experiment, and every third day throughout. Following the last day of the choice test with ethanol, the bottles were all switched to water for 24 hours, and then a two-bottle choice tastant test began with 0.05% saccharin and water for two days, followed by 0.0025% quinine and water for two days.
Experiment 3 – Investigation of a circadian pattern of fluid consumption using a lickometer system
In order to designate the best time of day for a procedure for limited access to alcohol, fluid consumption was monitored throughout the circadian cycle to determine whether there existed a peak period of consumption in prairie voles, as has been observed in mice and rats (Aalto, 1986; Freund, 1970), and utilized to achieve high alcohol intake (Rhodes et al., 2005; Ryabinin et al., 2003; Sharpe et al., 2005). To examine drinking at regular short intervals without having to disturb the animals, we utilized a “lickometer” apparatus that would record the precise time of each lick on a drinking spout. The apparatus has been described previously (Ford et al., 2005). Briefly, it consisted of a raised, stainless steel rod floor beneath a four-sided Plexiglas box with a perforated lid for ventilation, nested inside a shoebox cage with bedding beneath the rod floor. Each animal had access to two drinking tubes, one containing water, and the other containing either saccharin or 10% ethanol, available through two holes in one side of the box. Modifications to the apparatus used by Ford et al. included the use of 50 ml conical polypropylene tubes fitted with a rubber stopper with a metal sipper tube, and the addition of a small Petri dish secured to the rod floor opposite the drinking tubes to contain food. The metal floor and sipper tubes created a circuit that was completed when the animal made contact with the sipper, which was recorded by a lickometer device (MED Associates, Inc., St. Albans, VT) interfaced to a computer with MED-PC IV software (MED Associates, Inc.) for collection of cumulative lick records. The tubes were filled at the start of each day, weighed, and secured to the cage. At the end of each session, the tubes were weighed again before refilling, to determine the amount of fluid that was consumed. Food was replenished at the start of each session. To avoid a potential entrainment of activity to the time of new food and fluid delivery, and to record a full 24 hours without interruption, each session started at a slightly different time each day (noted in Fig. 4).
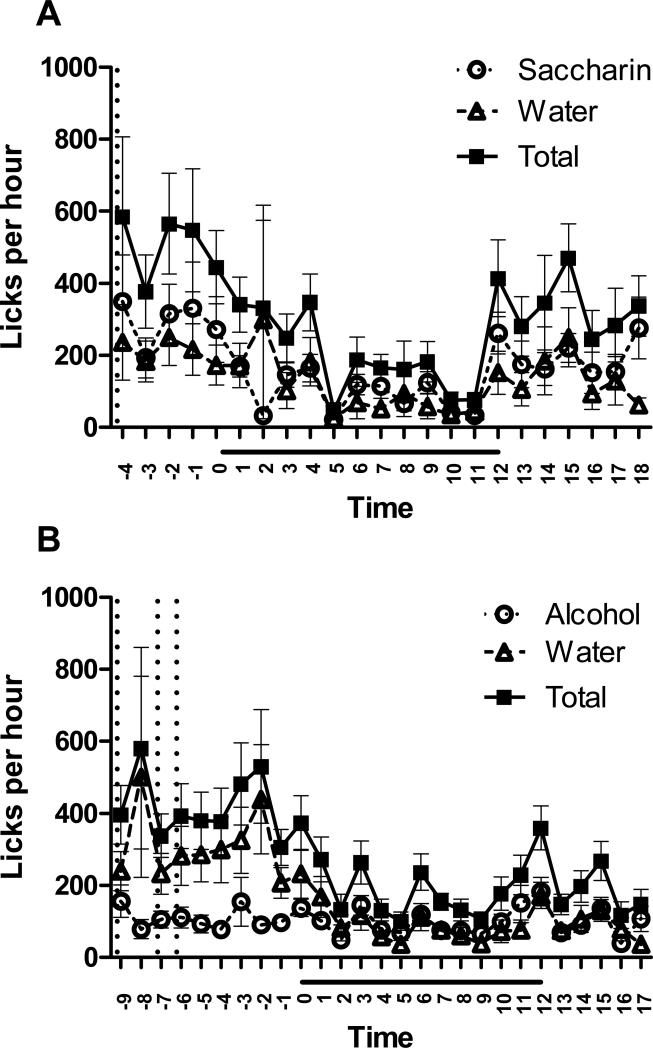
Figure 4. Fluid intake and the circadian cycle.
A) Average intake of saccharin, water, and total fluid, collapsed across days 3-4 of testing with saccharin. B) Average intake of ethanol, water, and total fluid, collapsed across days 1-4 of testing with ethanol. Values represent number of licks per hour for all animals, indicating mean ± SEM; n = 24. X-axis values represent number of hours from ‘lights off’ time. Horizontal black bars indicate the dark phase of the circadian cycle. Vertical dotted lines indicate the time at which fluids were replaced between successive test days.
In this experiment, 24 adult prairie voles (12 male, 12 female; 30.7 ± 0.9 g; 95-137 days old on the first day) were housed individually in the apparatus described. During the first four days, they had access to water and saccharin, followed by three days with only water available, and then four days with water and 10% ethanol available. The voles were weighed immediately before commencing the saccharin and ethanol experiments.
Preference for alcohol over water was calculated based on fluid consumption and recorded licks from each fluid, and alcohol dose consumed was calculated based on the weight of ethanol solution consumed.
Experiment 4 – Establishing a limited access two-bottle choice procedure
To determine whether prairie voles could voluntarily self-administer alcohol in quantities sufficient to produce substantial BECs and changes in neural activity as indicated by increased Fos immunoreactivity (IR) we established a two-hour limited-access procedure. The animals used here were the 26 of the same animals used in Experiment 2, and so were not naïve to alcohol, and continued to be pair- or singly-housed. In this study, begun 26 days after the last alcohol consumption, the voles were given a two-bottle choice test with 10% ethanol and water for two hours, starting at the onset of the light cycle (based on the results of Experiment 3), and repeated over four consecutive days. Preference for alcohol and dose consumed were calculated as described above, also subtracting the average volume missing from four control tubes in empty cages from the volume of fluid consumption for each solution.
Immediately after the end of the two-hour drinking session on the last day, animals were euthanized by CO2 inhalation, followed by decapitation. An additional 12 age-matched voles that were alcohol- and experimentally-naïve were euthanized at the same time. Trunk blood samples were collected for analysis of BEC, as described in Experiment 1. Brains were removed and fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) for 24 hours. Subsequently, brains were transferred to 20% sucrose in PBS with 0.1% sodium azide (NaN3) overnight, followed by 30% sucrose (in PBS with 0.1% NaN3) until slicing.
Brains were sliced into 30 μm floating sections in 0.1% NaN3 in PBS. Slices containing the nucleus accumbens (NAc), the lateral septum (LS), the central nucleus of the amygdala (CeA), and the perioculomotor urocortin-containing neurons (pIIIu) were chosen for immunohistochemistry (IHC). These brain regions were selected for analysis because they most frequently showed changes in Fos IR following alcohol administration in previous rodent studies (Bachtell et al., 1999; Sharpe et al., 2005; Vilpoux et al., 2009).
The Fos IHC protocol used here was based on previously published procedures in mice and rats (Ryabinin et al., 1999). Endogenous peroxidase activity was quenched with 0.3% peroxide in PBS, followed by blocking with goat serum in PBS/Triton-X 100. The slices were incubated overnight with a primary rabbit polyclonal antibody to c-Fos (Santa Cruz Biotechnology, Santa Cruz, CA, 1:2000). Slices were subsequently incubated in biotinylated anti-rabbit antibody, made in goat (Vector Laboratory Inc., Burlingame, CA), ABC solution (Vector Laboratory Inc.), and diaminobenzidine (Thermo Scientific, Rockford, IL) to visualize the stain.
In the NAc, CeA, and pIIIu, Fos IR was quantified by counting the number of cells stained above background. Counting was performed manually by a trained experimenter blind to the identification of the samples. In the LS, little or no staining was observed in tissues, and so this area was not quantified.
Statistical analyses
For each day of drinking, preference for alcohol over water was calculated by dividing the volume of alcohol consumed by the total volume of fluid consumed. Additionally, g/kg consumed was calculated for each session by dividing the grams of alcohol consumed (the density of alcohol multiplied by the v/v concentration multiplied by the volume consumed) by the weight of the animal in kilograms.
Several of the voles occasionally chewed through the rubber stopper of the drinking tubes, leading to spillage of the fluids. This behavior has not been observed in mice using the same equipment in our lab. On these occasions, animals that chewed through the stopper were removed from analysis for that day, and will be referred to as outliers. In addition, statistical outliers defined as animals having intake of at least one fluid more than two standard deviations from the mean intake were removed from analysis for that day (this information is included in the Results section). However, these individuals were included in analysis of Fos and BECs, where consumption was not used as a dependent variable, and outliers for measures of Fos IR and BEC were not removed, based on the intra-experiment reliability of these measures. The statistical results obtained with exclusion of outliers never contradicted the results obtained with them in the analyses.
In Experiment 1, alcohol elimination rates were determined by a regression line, and the slopes and intercepts of prairie voles and mice were compared with an F test. Effects of sex, housing, and age were assessed as appropriate using a two-factor ANOVA with sex, housing, or age (old or young) as one factor, time of BEC assessment as the other between-subjects factor, and BEC as the dependent variable.
For Experiment 2, group differences in preference and g/kg were determined by two-factor repeated measures ANOVA, with sex and housing condition as between-subject factors, and alcohol concentration as the repeated measure. Preference and g/kg were each averaged across the four days of drinking at each concentration of alcohol and used for the repeated measures analyses. A two-factor repeated measures ANOVA, with sex and housing condition as between-subject factors, and alcohol concentration as the repeated measure, was also used to analyze water intake (g/kg). Body weights were monitored throughout the experiments, but were not affected by the experimental manipulations, as was expected, and are therefore not described here. The tastant tests for saccharin and quinine were analyzed by two-way ANOVA with sex and housing condition as independent variables, and saccharin and quinine preference as dependent variables. Where appropriate, Fisher's PLSD was used for post-hoc comparisons, and tests of simple effects were used to discover the basis of interaction effects.
The correlation of alcohol consumption between sibling partners was analyzed in Experiment 2 to determine whether individual members of a pair drank similar amounts. Separate correlations were performed for pair-housed animals and separated partners, using the average g/kg consumed from the 10% ethanol solution by one member of a pair as the X variable and g/kg consumed by the partner as the Y variable. Pearson's r was computed, and a threshold α = 0.05 level of significance was applied to the correlation. The same test was applied to consumption of saccharin and quinine.
In Experiment 3, the number of licks was determined for each solution during each hour using SoftCR for Windows (MED Associates, Inc.). A software error caused nearly all of the data from the second day of the saccharin consumption study to not be recorded. As a result of this and the irregular drinking pattern observed on the first day of the experiment, which was likely due to the novelty of the cage and the saccharin, only the third and fourth days of saccharin consumption were examined. All four days of ethanol consumption were analyzed, excepting only the first hour of the first day, where unusually high numbers of licks for both fluids were recorded.
A repeated measures ANOVA was used to analyze consumption separately for each fluid (saccharin, ethanol, or water), and for the total amount of fluid (saccharin or ethanol plus water) consumed during each period. First the statistical test was applied to each day separately, with each hour as the repeated measure. Then the data were collapsed across all days of consumption, and again analyzed with each hour as the repeated measure. Fisher's PLSD was used for post hoc comparisons. The preference ratios calculated from lick data and fluid data were compared by a Pearson correlation.
In Experiment 4, induction of Fos by alcohol consumption was compared to that of naïve animals for each brain area investigated, using the Mann-Whitney test, since the measures were not normally distributed. For pIIIu, which showed significant Fos induction, correlational analyses were performed to examine the relationship between Fos IR and preference or alcohol consumption (g/kg), using the Spearman rank r test. This nonparametric analysis was also used to examine the relationship between BEC and preference or alcohol consumption since neither Fos nor BEC data were normally distributed. Naïve animals were not included in the correlational analyses. An α level of 0.05 was used for all tests.
RESULTS
Experiment 1 – Assessment of alcohol elimination rates in voles and mice
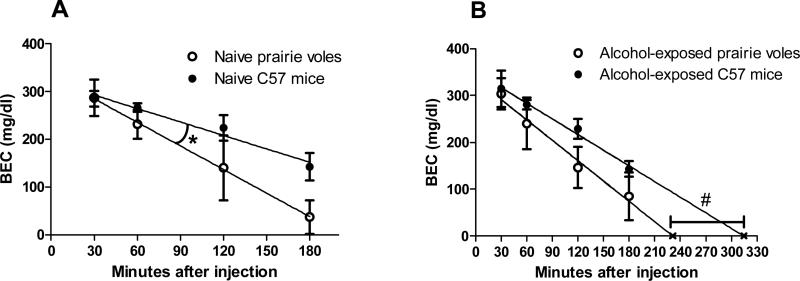
Our pilot experiments indicated that prairie voles exhibited high intakes of ethanol (unpublished observations: Loftis et al., 2006). To see whether these intakes could be due unusually high ethanol elimination rates, we compared BECs in prairie voles and C57 mice following an i.p. injection of 2.5 g/kg ethanol. We observed similar levels of behavioral responses (i.e. abnormal gait, loss of righting reflex, increased activity followed by sedation) in voles and mice after ethanol injections. Naïve C57 mice exhibited BECs and a rate of ethanol elimination in the expected range, based on previous reports (Grisel et al., 2002) (Fig. 1A). While the blood ethanol levels of prairie voles were near those of the mice, the rate of alcohol elimination of naïve C57s (slope of regression line: -0.94 ± 0.12) was slower than that of naïve prairie voles (slope of regression line: -1.65 ± 0.036) [F(1,4) = 29.90; p < 0.01] (Fig. 1A). However, in the alcohol-experienced animals, the elimination rates (slopes: -1.11 ± 0.14 for C57s, -1.40 ± 0.089 for prairie voles) were not significantly different [F(1,4) = 3.88; p = 0.12], while the intercepts were different [F(1,5) = 15.43; p < 0.05] (Fig. 1B). There was no difference in elimination rate between sexes or age groups in mice or voles in either study, or between housing conditions in the alcohol-experienced study, so the groups were combined.
Figure 1. Alcohol elimination rate in prairie voles and C57BL/6J mice.
A) Blood ethanol concentration (BEC) following 2.5 g/kg i.p. injection of ethanol, in naïve prairie voles and mice (n = 4-5 per group per time point). B) BEC following 2.5 g/kg i.p. injection of ethanol in prairie voles and mice that had previously self-administered alcohol orally (n = 7-10 per group per time point). Values represent mean ± SEM; lines indicate linear regression, and rate of ethanol elimination; ‘X’ indicates X-intercept. * Slopes are significantly different (p < 0.01). # Intercepts are significantly different (p < 0.05).
Experiment 2 – Investigation of the effects of social separation on alcohol intake
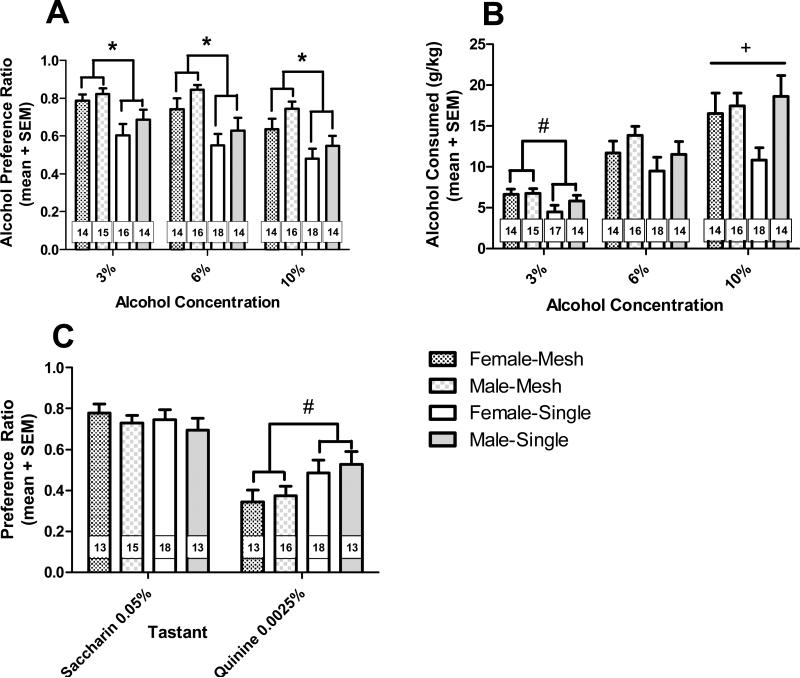
Prairie voles housed together with a mesh divider showed a higher preference for alcohol than voles that had been separated from a sibling partner (Fig. 2A). Accordingly, there was a significant main effect of housing on preference [F(1,55) = 15.2; p = 0.0003], and no significant effect of sex on preference. The housing effect was significant at each alcohol concentration tested [F(1,57) = 12.66, 13.16, 12.43 at 3%, 6%, 10%, respectively; p < 0.001]. There was a significant effect of concentration on preference [F(2,110) = 23.52; p < 0.0001], where the preference for 10% ethanol was significantly lower than the preference for 3% or 6% ethanol solutions. In contrast, there was no significant effect of housing condition on the dose of alcohol consumed, but there was a significant effect of concentration [F(2,112) = 92.84; p < 0.0001] such that a higher dose of alcohol was consumed at each increase in concentration (p < 0.0001 for each pairwise comparison). There was a significant interaction of sex and concentration [F(2,112) = 3.12; p = 0.048], and a test of simple effects revealed that the males consumed more alcohol than females, but only of 10% ethanol solution [F(1,58) = 4.12; p = 0.047]. There was a significant three-way interaction between housing condition, sex, and concentration [F(2,112) = 3.62; p = 0.030]; intake of 3% and 6% solutions reflects the higher alcohol preference demonstrated in pair-housed voles of both sexes, compared to isolated voles, but the pattern persists only in females for the 10% solution, while males show no effect of housing at this concentration.
Figure 2. Alcohol drinking in same-sex sibling pairs, housed together with a mesh divider, or separated from sibling.
A) Alcohol preference for females and males housed with a sibling (checkered bars) or separated from a sibling (solid bars), at increasing concentrations of ethanol (3%, 6%, 10%). B) Alcohol consumption (g/kg) for females and males housed with a sibling (checkered bars) or separated from a sibling (solid bars), at increasing concentrations of ethanol (3%, 6%, 10%). C) Saccharin and quinine preference for females and males housed with a sibling (checkered bars) or separated from a sibling (solid bars). Values represent mean + SEM; number of animals per group per concentration is indicated. * Effect of housing (p < 0.01). # Effect of housing (p < 0.05). + Effect of sex (p < 0.05).
Analysis of water intake revealed results complementary to alcohol intake. There was a significant effect of housing on amount of water consumed [F(1,110) = 12.97; p = 0.0007], such that the isolated animals drank more water than the pair-housed voles.
In the tastant test, there was no significant effect of sex or housing on preference for saccharin, though there was a significant effect of housing on preference for quinine [F(1,54) = 6.28; p = 0.015] (Fig. 2C). In this case, pair-housed voles showed less preference for (actually an avoidance of) quinine when compared to isolated voles, which exhibited an indifference to quinine, as the mean preference was near 50%. Several outliers in each group were removed from analysis on various days of testing, and so the final number in each group is indicated at each concentration in Fig. 2.
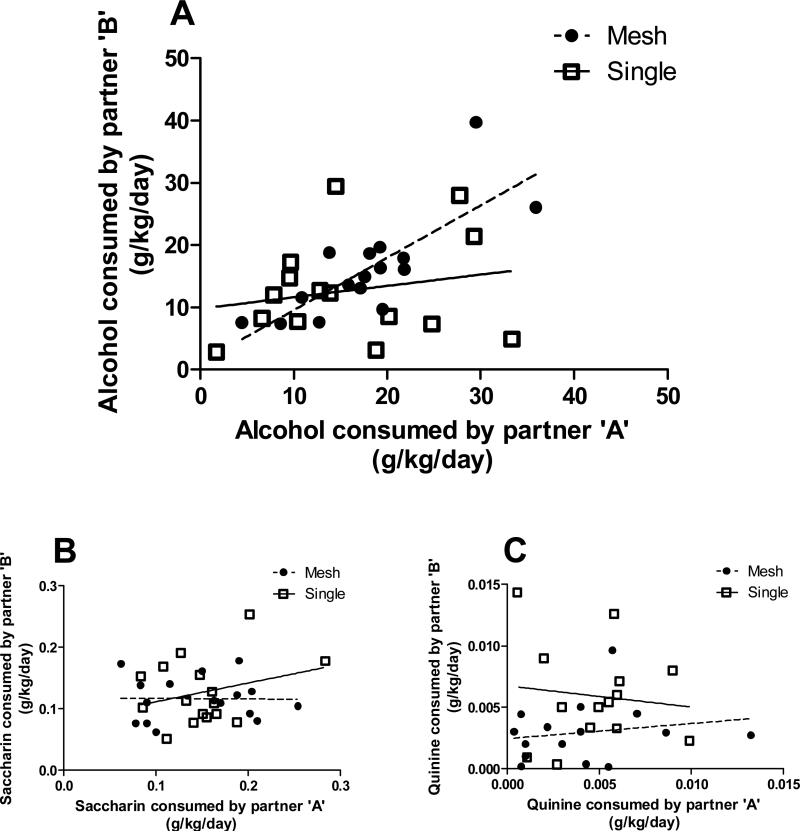
A strong correlation between alcohol consumption by one member of a pair and consumption by the other member of the pair was revealed between siblings housed together (r = 0.79; p = 0.0003; n = 16), but there was no correlation between consumption of siblings that were separated (r = 0.20; p = 0.46; n = 15) (Fig. 3A). The goodness of fit of the regression line (r2) of the relationship between alcohol consumption in the mesh-paired voles was 0.62, indicating that 62% of the variability in alcohol intake in partner ‘A’ could be could be accounted for by the variation in intake in partner ‘B’. However, there was no correlation of saccharin (Fig. 3B) or quinine (Fig. 3C) intake between partners.
Figure 3. Fluid consumption of prairie vole partners.
Correlation of consumption of A) alcohol (10% ethanol), B) saccharin, and C) quinine between siblings in each housing condition. Values represent the average dose of each substance consumed by an individual vole per day, during four days for alcohol and two days for saccharin and quinine.
Experiment 3 – Investigation of a circadian rhythm of fluid consumption
Prairie voles consumed fluids in the lickometer apparatus, and the number of licks were successfully recorded in this procedure. During alcohol consumption, a number of voles showed behavioral evidence of intoxication (i.e. abnormal gait, hind footslips, difficulty balancing while drinking). The general pattern of drinking for an individual vole consisted of discrete bouts of licks separated by long periods of time (often over an hour) without licking.
The graphical representation of the lickometer data combined for all animals shows that prairie voles generally drank more fluid during the light period than in the dark (Fig. 4). Time had a significant effect on saccharin consumption on day 3 [F(21,483) = 2.84; p < 0.0001], as well as water [F(21,483) = 1.78; p = 0.018] and total volume [F(21,483) = 2.84; p < 0.0001]. On day 4, time had a significant effect on saccharin licks [F(21,483) = 2.40; p = 0.005]. In the four-day test with ethanol, only the fourth day alone had significant differences over time, in water [F(23,506) = 1.58; p = 0.043] and total fluid [F(23,506) = 1.84; p = 0.010] licks.
When the data were collapsed across days, time had a significant effect on saccharin [F(21, 987) = 4.50; p < 0.0001] and total fluid [F(21, 987) = 2.52; p = 0.0002] licks (but not water) on the third and fourth days of saccharin consumption (Fig. 4A). There was a significant effect of time on ethanol [F(19,1805) = 1.93; p = 0.0094], water [F(19,1691) = 2.87; p < 0.0001], and total fluid [F(19,1691) = 3.28; p < 0.0001] licks during the four days of alcohol consumption when the data were collapsed across days (Fig. 4B). Post hoc tests revealed a number of hours with significantly elevated intakes. Peak drinking of saccharin occurred at -2 and -1 hours relative to ‘lights off,’ with slightly smaller peaks at 0, +12, and +18 hours from ‘lights off.’ Total drinking (saccharin and water combined) reflected the same peaks in the hours preceding ‘lights off’ (-2 – -1 and a lower peak at 0), but had a different peak following the dark phase at +15.
Peak drinking of ethanol occurred at +12 (‘lights on’), with slightly lower peaks at +11 and -3 hours from ‘lights off.’ In contrast, water intake during ethanol exposure was highest at -3, with a slightly lower peak at -2. As a result, the highest peaks for total fluid intake during these four days were at -3 and +12, with lower peaks at -2 and 0 hours from ‘lights off.’
The preference ratio for saccharin or alcohol compared to water was determined each day for each animal by the number of licks on each drinking tube, and by the mass of each fluid consumed. The average saccharin preference by fluid mass was 0.71 ± 0.028 (mean ± SEM), and the average ethanol preference was 0.45 ± 0.023. The correlation between preference calculated by fluid and by number of licks was significant for both saccharin (r = 0.85; p < 0.0001; Fig. 5A) and ethanol (r = 0.56; p < 0.0001; Fig 5B).
Figure 5. Solution preference ratios by fluid consumption and recorded licks.
Correlation of preference ratios for A) saccharin and B) 10% ethanol over water, calculated from fluid consumption by number of licks recorded (X-axis) and by weight (Y-axis) for each solution. Values represent individual animal preference for each day.
Experiment 4 – BEC and Fos immunoreactivity in the brain after limited access to alcohol
A limited access procedure was designed to determine whether voles consume pharmacologically relevant doses of alcohol, and whether BEC and Fos immunoreactivity indicating activation of particular brain regions would reflect alcohol intake. The 2-hour limited access was started immediately at the onset of the light cycle corresponding to the peak of ethanol consumption according to Experiment 3. On the final day of two-hour access to alcohol, voles showed a moderate preference averaging 0.60 ± 0.06 (range 0.048 – 0.996). They drank on average 1.99 ± 0.24 g/kg ethanol (range 0.015 – 3.933 g/kg), and exhibited an average BEC of 34.65 ± 7.01 mg/dl (range 13.1 – 133.3 mg/dl). There was no significant effect of sex or housing (mesh-housed or isolated) on alcohol preference, intake, or BEC, and so these animals were collapsed into one group for analysis.
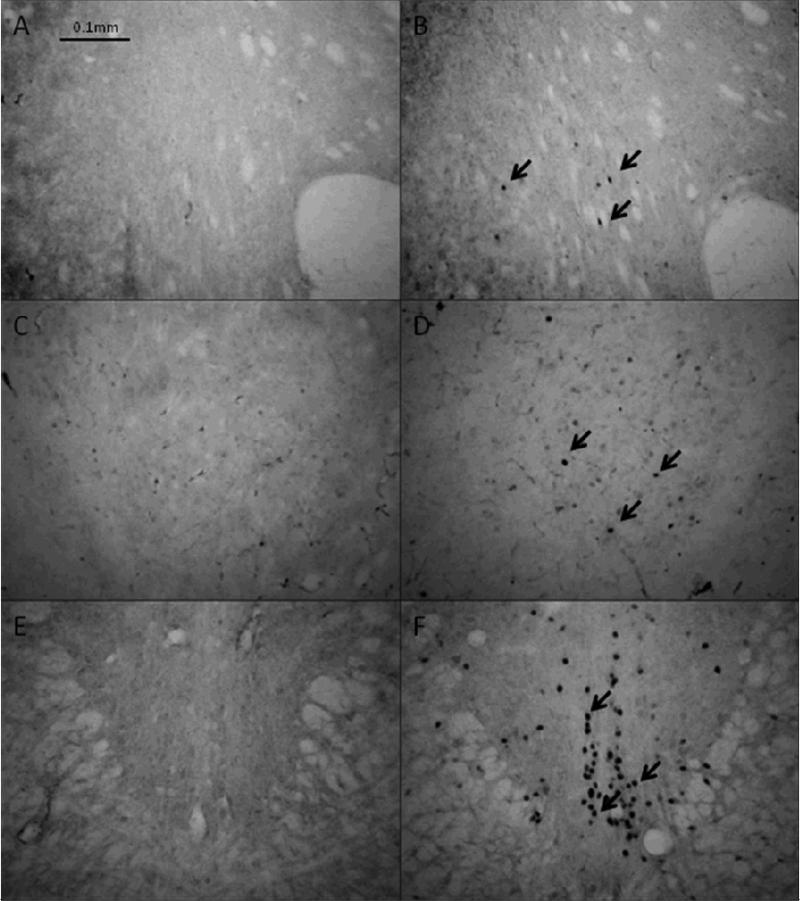
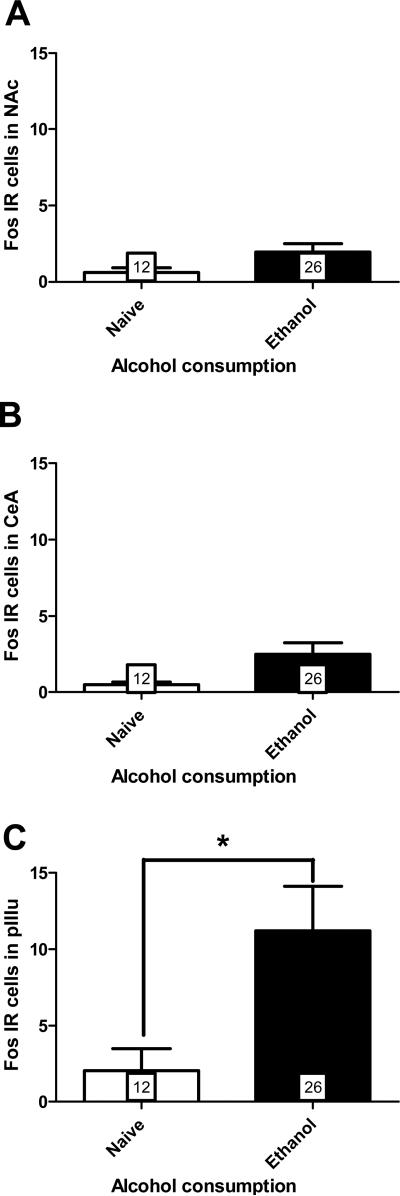
Representative micrographs of Fos staining are shown in Fig. 6. The presence of Fos staining was not observed in the lateral septum of any animals, and thus was not counted and is not shown here. Alcohol did not induce Fos above the level of naïve controls in NAc (Fig. 7A) or CeA (Fig. 7B). Alcohol did significantly induce Fos in the pIIIu, compared to naïve controls (p = 0.0065; Fig. 7C). There was no effect of sex or housing condition on Fos IR in any of the brain regions analyzed.
Figure 6. Fos immunoreactivity after limited access to alcohol.
Induction of Fos by alcohol self-administration was inspected by comparing immunohistochemical staining in brains of alcohol-naïve voles (A,C,E) to that of voles after two-hour access to alcohol (B,D,F) at 20X magnification. Very little induction was observed in the nucleus accumbens (A,B) or central nucleus of the amygdala (C,D), but there was apparent induction of Fos in the perioculomotor urocortin containing neurons (E,F). Arrows indicate examples of Fos positive nuclei.
Figure 7. Fos IR as a measure of alcohol consumption.
Fos immunoreactivity in limited-access alcohol drinking voles compared to naïve animals, in A) nucleus accumbens B) central nucleus of the amygdala and C) perioculomotor urocortin containing neurons. Values represent mean number of Fos-positive cells + SEM; number of animals per group is indicated. * Difference between groups (p < 0.01).
A Spearman correlation showed that variability in Fos IR in the pIIIu did not account for a significant portion of the variability observed in alcohol preference, but did account for 44% of the variation of alcohol consumption (rs = 0.66; p = 0.005). Similarly, the variation in BEC did not significantly account for the variation seen in preference, but there was a trend for a correlation with alcohol consumption (rs = 0.39; p = 0.058).
DISCUSSION
Alcohol intake in pair-housed or socially isolated siblings
The results of the experiments described here show that prairie voles exhibit a high intake of alcohol. During continuous access to the 10% ethanol solution, most groups consumed over 15 g/kg ethanol in 24 hours, and a number of individuals consumed over 20 g/kg (see Fig. 2B). In comparison, only C57 mice and C57 × FVB/NJ hybrids have been shown to consume comparable amounts of roughly 16-20 g/kg/day of 10% unsweetened ethanol, which is more than any other strains of mice (Blednov et al., 2005; Yoneyama et al., 2008). Our experiments show that the elimination rate of alcohol appears to be slightly higher in voles than in C57 mice; however, the voles do experience high levels of BECs in the same range as mice, and they also exhibit noticeable behavioral effects of intoxication. Moreover, although C57 mice show higher levels of alcohol consumption compared to other strains, a substantial number of recombinant inbred strains are known to have significantly lower or higher rates of alcohol elimination (Grisel et al., 2002; Phillips et al., 1994). Thus, while voles may metabolize ethanol faster than mice, this slightly faster metabolism alone cannot explain the high intake of alcohol observed in prairie voles, and should not serve as an obstacle in future studies. This idea is in agreement with high BECs observed in animals voluntarily consuming alcohol in our experiments. Therefore, prairie voles make a novel animal model for high alcohol preference and intake, which differs from traditional rodent models of high intake in that they are not inbred or selectively bred, and maintain a high degree of genetic variability.
Moreover, experiments examining the effects of social pairing or isolation on alcohol drinking behavior showed that prairie voles exhibit higher alcohol preference when they are housed together than when they have been separated from a sibling and are housed alone. The novelty of this surprising finding compared to other rodents that tend to increase drinking when in isolation (Advani et al., 2007; Daoust et al., 1985; Ehlers et al., 2007; Hall et al., 1998; Juarez and Vazquez-Cortes, 2003; McCool and Chappell, 2009; Nunez et al., 2002; Nunez et al., 1999; Rockman et al., 1989; Schenk et al., 1990; Wolffgramm and Heyne, 1991; Yanai and Ginsburg, 1976) indicates that the prairie vole is unique in the way its social circumstances affect alcohol intake. As such, this could be a valuable species to model socially-facilitated excessive or binge drinking. Future studies will attempt to elucidate whether the difference in alcohol intake is due to an increase in socially-housed voles, a decrease in isolated voles, or both, and to determine whether introduction of alcohol at a later time relative to separation from a partner will show the same effect of social circumstance.
The use of the mesh-divided cage that allowed observation of each vole's drinking behavior in Experiment 2 also allowed the animals to interact. One could theorize that the increased drinking is due to the stress of separating the voles by a mesh. However, this possibility is highly unlikely because in our pilot experiments we observed that voles do not show behavioral signs of ever having been separated when they are reunited after being mesh-separated, and, importantly, that vole siblings housed together without a mesh divider also have higher intakes than singly-housed voles (Anacker, Loftis and Ryabinin, unpublished results).
One of the most interesting findings of these experiments is the strong correlation of intake between siblings housed together, compared to the lack of correlation between siblings housed apart. This finding indicates that prairie voles housed together influence each other's alcohol intake. Future studies should investigate behavioral mechanisms of this phenomenon to see whether the paired voles drink alcohol at the same time, and whether one individual drives drinking of the pair, which can be assessed with an adaptation of the lickometer system utilized in Experiment 3 that would allow voles to be housed in pairs in the apparatus. These studies should not only address the mechanisms by which voles influence each other's drinking, but also whether this behavior has evolutionary origins. Prairie voles are a highly social species that choose to spend most of their time with another animal, rather than alone. This is exemplified by original field results that found pairs captured together in the same cages repeatedly (Getz et al., 1981). If the natural history of the species is based upon partners spending much of their time engaged in the same activities, then the traits that facilitate that behavior must be conserved in the species, and here extend to drinking alcohol together.
The facilitating effect of pair-housing on alcohol preference was specific, as it did not extend to saccharin, a rewarding, sweet-tasting solution. However, there was an effect of housing condition on quinine preference, where mesh-housed voles showed less preference for (in fact, an avoidance of) the bitter solution compared to isolated voles. While the reason for this remains unclear, this shows that pair-housed voles do not necessarily exhibit a higher preference for any substance over water, and that this was specific to alcohol in the current study. Importantly, only consumption of alcohol but not saccharin or quinine was correlated between pair-housed voles.
The variation in alcohol preference observed between animals is quite large, certainly due in large part to the high degree of genetic variation in the outbred prairie vole colony. In future studies, relatively large sample sizes may still be needed to observe significant effects in this population. The method of reading fluid volumes used here could also potentially have introduced error into the data. However, with care taken in handling, the amount of error due to the method of reading was minimized.
The effect of housing on alcohol intake reported in grams per kilogram body weight is similar in direction to the effect on alcohol preference, but of smaller magnitude. Meanwhile, the effect of housing on water intake was also significant, and in the opposite direction, with isolated animals drinking more water than pair-housed voles. The explanation of these combined results is that voles housed alone moderate their alcohol intake with more water, yielding a lower preference for alcohol in overall fluid intake, while not appreciably changing the dose they consume relative to pair-housed voles.
Taken together, the results of Experiment 2, and especially the correlation of drinking, show that prairie voles can serve as a unique animal model for the examination of the effects of social bonds on alcohol drinking behavior.
Circadian pattern of fluid consumption in voles
In natural environments, voles exhibit 2-6 hour ultradian or polyphasic activity cycles with bouts of activity followed by periods of rest, and with slightly higher diurnal activity during winter and slightly higher nocturnal activity during summer (Halle and Lehmann, 1987; Tamarin, 1985). Our study demonstrates that under a 12:12 hour light cycle, the circadian rhythms of fluid consumption in laboratory prairie voles are relatively flat with a tendency for higher diurnal activity. This pattern is quite different from the high intake of food, water, or alcohol observed in laboratory mice and rats during the dark period (Aalto, 1986; Agabio et al., 1996; Freund, 1970), but is consistent with other observations of increased diurnal activity and a shallower circadian rhythm in laboratory prairie voles relative to rats and other nocturnal rodents (Dewsbury, 1980; Taymans et al., 1997).
Analysis of number of licks and fluid intake indicated that voles exhibited no preference for ethanol. This was expected for single-housed animals based on our previous experiments. However, both analysis of licks and fluid consumption detected a clear preference for saccharin indicating the lickometer system reliably detects differences in fluid intake. Importantly, the lickometer system detected that peaks in fluid intake occurred at the same times each day, even though the tubes were removed at different times each day, and peaks occurred at least several hours after the fluids and food had been replenished (see Fig. 4B), indicating that this potential disruption did not disturb normal cycles of fluid intake. Moreover, the peak following the end of the dark phase is unlikely to have been influenced by any manipulations occurring during the light phase over 12 hours prior. The subsequent limited access procedure used in Experiment 4 was conducted during the two-hour period just after ‘lights on’ time, which would take advantage of relatively elevated levels of drinking, including the largest peak for alcohol intake.
BECs and Fos induction after limited access to alcohol
A portion of tested prairie voles (6/26) showed pharmacologically-relevant high BECs in the limited access procedure, ranging from 74 – 133 mg/dl, while the rest had negligible BECs (total range 13.1 – 133.3 mg/dl). The BECs showed a trend toward a significant correlation with alcohol dose consumed, although this correlation was weakened by the number of voles that exhibited low BECs even after consuming large quantities of alcohol in the two-hour session. However, the timing of each animal's drinking can influence the BEC that is determined at the end of the drinking period. For example, BECs may not show a strong correlation with consumption when some individuals drink at the beginning of the access period. If that is the case, then moderate amounts of alcohol consumed within the first part of the session would not be expected to induce significant BECs at the time the blood samples were taken (Livy et al., 2003).
Since BEC was not strongly correlated with alcohol consumption in the present experiment, the possibility that Fos activation could add another measure of alcohol consumption was investigated. Previous findings showed that various alcohol self-administration procedures in mice and rats lead to consistent induction of Fos in the perioculomotor urocortin containing neurons (pIIIu), and less consistent Fos induction in the nucleus accumbens (NAc), central nucleus of the amygdala (CeA), or reduction of Fos in the lateral septum (LS) (Bachtell et al., 1999; Sharpe et al., 2005; Topple et al., 1998; Vilpoux et al., 2009). In agreement with results of previous studies, pIIIu exhibited a significant Fos response in alcohol-drinking voles. Neither the NAc nor CeA showed significant induction in alcohol-exposed animals following a two-hour two-bottle choice test, compared to naïve controls. It should be noted that there were procedural differences between this and previous studies, since the voles were killed following two-hour access to ethanol, as opposed to 90 minutes after 30-minute access to ethanol, where animals consumed larger doses of ethanol in a shorter period. It could be also theorized that since Fos immunoreactivity in alcohol-drinking voles was compared to naïve control voles and not similarly-treated matched voles, this could have obscured our ability to detect differences in some of the brain regions. However, our naïve controls had virtually no Fos immunoreactivity in NAc and CeA. Therefore, this lack of immunoreactivity should actually improve our ability to see ethanol-induced Fos activation and does not explain lack of activation in NAc and CeA. With larger samples sizes or the ability to use a parametric test, we may have been able to reveal a significant difference between naïve and alcohol-exposed animals in these brain regions, but the magnitude of this response would remain minimal (Fig. 7), and the physiological relevance of such a small level of activation is questionable.
In contrast, the pIIIu exhibited strong induction of Fos, which was correlated with alcohol intake in individual voles. As with BEC, the fact that the predictive ability of Fos for alcohol intake levels was not stronger can be explained in part by differential timing of onset and peak drinking between individuals. Fos IR is increased starting at 60 minutes after alcohol exposure (Chang et al., 1995). Thus, since the animals were killed at 120 minutes after the alcohol was introduced to the cages, Fos IR may only have been evident in individuals that drank physiologically significant quantities of alcohol within the first hour of the session, but not in animals that tended to drink majority of alcohol at the end of the drinking session. Future studies on the microstructure of drinking behavior within a session may help elucidate details of the relationships of Fos and BEC with alcohol consumption over time. Since ethanol effects on c-Fos expression in pIIIu neurons are mediated by several mostly unknown signal transduction mechanisms (Bachtell et al., 2002), BEC remains a much more direct measure of alcohol consumption than Fos IR. However, the significant increase in Fos IR in this brain region after voluntary alcohol intake indicates that prairie voles consume alcohol in quantities sufficient to produce central effects.
The fact that there was no effect of housing condition on alcohol preference, or any of the other measures observed here, indicates that this limited access procedure may not be sufficient for showing differences due to social circumstances. One possible reason for this may be that the voles housed together influence each other to increase alcohol preference and intake in an ongoing process throughout the day, and that two hours is not enough time for them to coordinate or influence each other's drinking.
Importantly, the limited access procedure described here can be used to test effects of pharmacological manipulations (which often have transient effects that are difficult to demonstrate in a continuous access procedure), in order to elucidate the roles of neurotransmitter systems involved in alcohol drinking in voles, and to test potential drug treatments for alcoholism. In fact, our lab has demonstrated that naltrexone, an opioid receptor antagonist and approved treatment for human alcoholics, decreases alcohol intake in this limited access procedure (Anacker and Ryabinin, 2010). Future studies will utilize this procedure in elucidating the neurobiological factors involved in socially-facilitated excessive drinking, as well as determining potential therapeutic targets.
General conclusions
The experiments described here delineate two different procedures for studying alcohol consumption in prairie voles. The first is a 24-hour access procedure (Experiment 2), and the second is a two-hour limited access procedure that can be useful for testing effects of drugs, or involvement of certain neural substrates on alcohol drinking (Experiment 4). Notably, prairie voles prefer alcohol more when they are housed together in the 24-hour procedure, so they may be useful to model socially-facilitated excessive drinking behavior.
It is tempting to speculate on the neural substrates that underlie the behaviors demonstrated here, although they were not investigated in these experiments. For example, it was recently demonstrated that female (but not male) voles separated from a sibling showed elevated HPA axis reactivity, in increased levels of CRF IR in the paraventricular nucleus of the hypothalamus, and increased ACTH and corticosterone following a stressor (Grippo et al., 2007b). Also relevant to HPA axis activity, alcohol consumption elevates mRNA expression levels of CRF receptors (CRFR1 and CRFR2) and AVP in the hypothalamus, and these are speculated to contribute to a ‘stop signal’ (Pickering et al., 2007) for drinking via negative feedback. Altogether, a hyper-reactive HPA axis in isolated voles could more easily activate the stop signal pathway, leading to decreased drinking in separated compared to paired animals.
Additionally, the anhedonia described in both sexes of the isolated voles (Grippo et al., 2007b) may be extended to include lack of perceived reward from alcohol, leading to decreased alcohol consumption as seen in the current experiments. In contrast, paired voles may have greater NAc dopamine D1-like receptor levels (Aragona et al., 2006) than isolated voles, and may experience a greater reward due to dopamine release in the NAc when drinking alcohol under these social circumstances, ultimately leading to greater drinking after learning the reward value of alcohol. Interestingly, the strong correlation in alcohol intake of voles housed together relates to a feature of human relationships. A study by Homish and Leonard (2005) found that married couples who drank not only at similar levels, but also drank together at the same time, reported higher levels of marital satisfaction than those that did not. This “congruent drinking” is also a striking finding from the current study in prairie voles, and suggests further studies.
In conclusion, the experiments described here suggest that the prairie vole could be a valuable animal for investigating the interplay between social relationships and alcohol intake. In particular, this species could be used to model socially-facilitated excessive drinking. Several procedures and measures developed in the described studies will allow us to comprehensively examine the behavioral and neural mechanisms underlying the phenomenon of socially-facilitated excessive drinking in the future.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health grants AA016886 and AA016647 to AER, and training grant AA007468-22. We would like to thank Erika Spangler and Zuzzie Kapasova for technical assistance, Drs. Deborah Finn, Matthew Ford, Sue Carter and Larry Young for valuable consultations, Drs. Joseph Lonstein, Phillip Smith and Karen Bales for donating prairie voles for our colony, and the Veterinary Medical Unit at the Portland VA Medical Center for the care and maintenance of our vole colony.
REFERENCES
- Aalto J. Circadian drinking rhythms and blood alcohol levels in two rat lines developed for their alcohol consumption. Alcohol. 1986;3(1):73–5. doi: 10.1016/0741-8329(86)90074-1. [DOI] [PubMed] [Google Scholar]
- Advani T, Hensler JG, Koek W. Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol. 2007;10(5):595–607. doi: 10.1017/S1461145706007401. [DOI] [PubMed] [Google Scholar]
- Agabio R, Cortis G, Fadda F, Gessa GL, Lobina C, Reali R, Colombo G. Circadian drinking pattern of Sardinian alcohol-preferring rats. Alcohol Alcohol. 1996;31(4):385–8. doi: 10.1093/oxfordjournals.alcalc.a008166. [DOI] [PubMed] [Google Scholar]
- Anacker AMJ, Ryabinin AE. Biological contribution to social influences on alcohol drinking: evidence from animal models. Int J Environ Res Public Health. 2010;7(2):473–493. doi: 10.3390/ijerph7020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23(8):3483–90. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9(1):133–9. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302(2):516–24. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847(2):157–65. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J x FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005;29(11):1949–58. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34(6):1406–15. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Roberts RL. The psychobiological basis of cooperative breeding in rodents. In: Solomon NG, French JA, editors. Cooperative breeding in mammals. Cambridge Press; New York: 1997. pp. 231–66. [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679(1):89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Res. 2006;1126(1):76–90. doi: 10.1016/j.brainres.2006.07.126. [DOI] [PubMed] [Google Scholar]
- Daoust M, Chretien P, Moore N, Saligaut C, Lhuintre JP, Boismare F. Isolation and striatal (3H) serotonin uptake: role in the voluntary intake of ethanol by rats. Pharmacol Biochem Behav. 1985;22(2):205–8. doi: 10.1016/0091-3057(85)90378-8. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr., Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcohol Clin Exp Res. 2007;31(10):1692–8. doi: 10.1111/j.1530-0277.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27(6):705–14. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Wheel running behavior in 12 species of muroid rodents. Behav Proc. 1980;5:271–280. doi: 10.1016/0376-6357(80)90007-8. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29(10):1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45(3):153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121(1):111–9. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–35. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelberg F, Kalant H, Blanc AE. Effect of vasopressin-like peptides on consumption of ethanol by the rat. Pharmacol Biochem Behav. 1978;9(4):453–8. doi: 10.1016/0091-3057(78)90040-0. [DOI] [PubMed] [Google Scholar]
- Freund G. Alcohol consumption and its circadian distribution in mice. J Nutr. 1970;100(1):30–6. doi: 10.1093/jn/100.1.30. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS, Gavish L. The mating system of the prairie vole Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007a;69(2):149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007b;32(8-10):966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Metten P, Wenger CD, Merrill CM, Crabbe JC. Mapping of quantitative trait loci underlying ethanol metabolism in BXD recombinant inbred mouse strains. Alcohol Clin Exp Res. 2002;26(5):610–6. [PubMed] [Google Scholar]
- Groh DR, Jason LA, Keys CB. Social network variables in alcoholics anonymous: a literature review. Clin Psychol Rev. 2008;28(3):430–50. doi: 10.1016/j.cpr.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajema KJ, Knibbe RA. Changes in social roles as predictors of changes in drinking behaviour. Addiction. 1998;93(11):1717–27. doi: 10.1046/j.1360-0443.1998.931117179.x. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998;139(3):210–6. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Halle S, Lehmann U. Circadian activity patterns, photoperiodic responses and population cycles in voles. Oecologia (Berlin) 1987;71:568–572. doi: 10.1007/BF00379299. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30(8):399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise LL. Violence against women: an integrated, ecological framework. Violence Against Women. 1998;4(3):262–90. doi: 10.1177/1077801298004003002. [DOI] [PubMed] [Google Scholar]
- Homish GG, Leonard KE. Marital quality and congruent drinking. J Stud Alcohol. 2005;66(4):488–96. doi: 10.15288/jsa.2005.66.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homish GG, Leonard KE. The social network and alcohol use. J Stud Alcohol Drugs. 2008;69(6):906–14. doi: 10.15288/jsad.2008.69.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose BS, van Oers HA, van de Mheen HD, Garretsen HF, Mackenbach JP. Stressors and alcohol consumption. Alcohol Alcohol. 2000;35(3):307–12. doi: 10.1093/alcalc/35.3.307. [DOI] [PubMed] [Google Scholar]
- Juarez J, Vazquez-Cortes C. Alcohol intake in social housing and in isolation before puberty and its effects on voluntary alcohol consumption in adulthood. Dev Psychobiol. 2003;43(3):200–7. doi: 10.1002/dev.10133. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Brown SA, Abrantes A, Kahler CW, Myers M. Social recovery model: an 8-year investigation of adolescent 12-step group involvement following inpatient treatment. Alcohol Clin Exp Res. 2008;32(8):1468–78. doi: 10.1111/j.1530-0277.2008.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard KE, Bromet EJ, Parkinson DK, Day NL, Ryan CM. Patterns of alcohol use and physically aggressive behavior in men. J Stud Alcohol. 1985;46(4):279–82. doi: 10.15288/jsa.1985.46.279. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Eiden RD. Marital and family processes in the context of alcohol use and alcohol disorders. Annual Review of Clinical Psychology. 2009;3:285–310. doi: 10.1146/annurev.clinpsy.3.022806.091424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, Young LJ. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Horm Behav. 2007;51(4):508–15. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Bussell C, Pagel RL, Ryabinin AE. Alcohol drinking in the prairie vole. Alcohol Clin Exp Res. 2006;30(6):66. [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33(2):273–82. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez MJ, Rivas M, Riveiro P, Suarez J, Balboa J, Nunez LA, Rey-Mendez M, Freire-Garabal M. Effects of nefazodone on voluntary ethanol consumption induced by isolation stress in young and aged rats. Pharmacol Biochem Behav. 2002;73(3):689–96. doi: 10.1016/s0091-3057(02)00875-4. [DOI] [PubMed] [Google Scholar]
- Nunez MJ, Riveiro P, Becerra MA, De Miguel S, Quintans MR, Nunez LA, Legazpi MP, Mayan JM, Rey-Mendez M, Varela M, Freire-Garabal M. Effects of alprazolam on the free-choice ethanol consumption induced by isolation stress in aged rats. Life Sci. 1999;64(20):PL213–7. doi: 10.1016/s0024-3205(99)00130-7. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454(1):67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6(7):661–71. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Sher KJ, Krull JL. Risky drinking in college changes as fraternity/sorority affiliation changes: a person-environment perspective. Psychol Addict Behav. 2008;22(2):219–29. doi: 10.1037/0893-164X.22.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18(4):931–41. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Pickering C, Avesson L, Liljequist S, Lindblom J, Schioth HB. The role of hypothalamic peptide gene expression in alcohol self-administration behavior. Peptides. 2007;28(12):2361–71. doi: 10.1016/j.peptides.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman GE, Gibson JE, Benarroch A. Effects of environmental enrichment on voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1989;34(3):487–90. doi: 10.1016/0091-3057(89)90545-5. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Bachtell RK, Heinrichs SC, Lee S, Rivier C, Olive MF, Mehmert KK, Camarini R, Kim JA, Koenig HN, Nannini MA, Hodge CW, Roberts AJ, Koob GF. The corticotropin-releasing factor/urocortin system and alcohol. Alcohol Clin Exp Res. 2002;26(5):714–22. [PubMed] [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology (Berl) 2003;165(3):296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Wang YM, Freeman P, Risinger FO. Selective effects of alcohol drinking on restraint-induced expression of immediate early genes in mouse brain. Alcohol Clin Exp Res. 1999;23(7):1272–80. doi: 10.1111/j.1530-0277.1999.tb04288.x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol. 1990;7(4):321–6. doi: 10.1016/0741-8329(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Tsivkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29(8):1419–26. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Tamarin RH, editor. Biology of New World Microtus. The American Society of Mammalogists; Boston: 1985. [Google Scholar]
- Taymans SE, DeVries AC, DeVries MB, Nelson RJ, Friedman TC, Castro M, Detera-Wadleigh S, Carter CS, Chrousos GP. The hypothalamic-pituitary-adrenal axis of prairie voles (Microtus ochrogaster): evidence for target tissue glucocorticoid resistance. Gen Comp Endocrinol. 1997;106(1):48–61. doi: 10.1006/gcen.1996.6849. [DOI] [PubMed] [Google Scholar]
- Temple MT, Fillmore KM, Hartka E, Johnstone B, Leino EV, Motoyoshi M. A meta-analysis of change in marital and employment status as predictors of alcohol consumption on a typical occasion. Br J Addict. 1991;86(10):1269–81. doi: 10.1111/j.1360-0443.1991.tb01703.x. [DOI] [PubMed] [Google Scholar]
- Topple AN, Hunt GE, McGregor IS. Possible neural substrates of beer-craving in rats. Neurosci Lett. 1998;252(2):99–102. doi: 10.1016/s0304-3940(98)00574-6. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79(4):671–89. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33(6):945–69. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR. Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci. 1999;113(3):602–11. doi: 10.1037//0735-7044.113.3.602. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26(3):339–49. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–8. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain substrates for reinforcement and drug self-administration. Prog Neuropsychopharmacol. 1981;5(5-6):467–74. doi: 10.1016/0364-7722(81)90028-x. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol Biochem Behav. 1991;38(2):389–99. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- Yanai J, Ginsburg BE. Increased sensitivity to chronic ethanol in isolated mice. Psychopharmacologia. 1976;46(2):185–9. doi: 10.1007/BF00421390. [DOI] [PubMed] [Google Scholar]
- Yates G, Panksepp J, Ikemoto S, Nelson E, Conner R. Social isolation effects on the “behavioral despair” forced swimming test: effect of age and duration of testing. Physiol Behav. 1991;49(2):347–53. doi: 10.1016/0031-9384(91)90055-s. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–60. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148(4):401–10. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]