Abstract
Background
Studies of lung cancer disparities between American Indians and Alaska Natives (AIANs) and whites have yielded mixed results. No studies have investigated whether race-based differences in histology could explain survival disparities.
Methods
We obtained data on AIANs and whites with lung cancer from the 17 population-based cancer registries participating in the Surveillance, Epidemiology, and End Results (SEER) program from 1973–2006. We used logistic regression to determine whether race and other covariates were associated with histology, stage at diagnosis, and receipt of surgery. We used Cox regression to determine the risk of death associated with race, after adjusting for histology, stage, and other covariates.
Results
Histology, but not race, was associated with stage at diagnosis, and both race and stage were associated with histology. AIANs were less likely to receive surgery than whites, after adjusting for patient and tumor characteristics. Survival improved for both AIANs and whites after 2000, compared to the 1973–1999 period, but survival was consistently shorter for AIANs. The association between AIAN race and decreased survival was strongest in the later time period.
Conclusion
Lung cancer histology is associated with tumor characteristics, treatment, and survival. AIAN race is associated with tumor histology, receipt of surgery, and survival. Future studies with access to smoking data, patient comorbidity information, and health systems-level data will be able to identify factors responsible for the disparities observed in these analyses.
Keywords: lung cancer, survival, histology, health status disparities, operative therapy
American Indians and Alaska Natives (AIANs) experience disparities in incidence and survival compared to whites for several cancers,(1, 2) However, studies aimed at identifying lung cancer disparities between AIANs and whites have had mixed results. A nationwide study of lung cancer incidence found that incidence and stage at diagnosis did not differ between AIANs and whites, although 42% of AIANs were diagnosed before age 65, compared to 30% of whites.(3) Another study of AIANs in the Seattle-Puget Sound Surveillance, Epidemiology, and End Results (SEER) Registry from 1974 through 1989 observed that lung cancer stage distribution at diagnosis and survival were similar for AIANs and whites.(4) A later study using SEER data from 1988 through 1995 noted that AIANs with lung cancer had worse 5-year survival rates than any other racial/ethnic group.(5) In terms of differences in tumor characteristics, several studies have observed that the incidence of squamous cell lung cancer is higher in AIANs than whites.(3, 5) Because survival differs by histology type,(6) race-based differences in histology distribution may be mirrored in survival differences. However, the relationship of histology to survival disparities among AIANs is unknown.
To our knowledge, the existence and magnitude of disparities in lung cancer survival and cancer-directed surgery between AIANs and whites has not been established in a population-based sample. No recent nationwide studies have examined whether lung cancer stage at diagnosis and survival differs between AIANs and whites, and whether any differences are related to histology types. Further, the most recent study using SEER data only included patients diagnosed through 1995. These prior studies warrant updating because the overall survival of lung cancer patients has increased as detection and treatment methods improved over time.(7)
To address these knowledge gaps, we analyzed SEER data from 1973–2006 to determine if race, histology, and survival in lung cancer were associated among patients in two time periods and to document changes in lung cancer disparities between AIANs and whites over time.
Methods
Case Selection
Lung cancer cases were drawn from the 17 population-based cancer registries participating in the SEER program anytime from 1973–2006. The populations under SEER registry surveillance encompass 26% of the U.S. population, including 42% of the AIAN population, and are estimated to include greater than 95% of all incident cancers in their catchment areas.(8) All AIAN and white cases diagnosed with lung cancer (ICD-O 33.9 – 34.9) at age 21 or older were potentially eligible for inclusion in these analyses. We limited our analyses to non-small cell carcinoma cases diagnosed with one of five histology subtypes, comprised of the following ICD-O histology codes: adenocarcinoma (8140, 8251, 8255, 8260, 8310, 8323, 8480, 8481, 8570), bronchioloalveolar carcinoma (8250, 8252, 8253), large cell carcinoma (8012, 8031), squamous cell carcinoma (8052, 8070, 8071, 8072, 8073, 8074), and other non-small cell carcinoma (ONSCLC) (8010, 8020, 8022, 8032, 8033, 8046, 8050, 8490, 8550, 8560). Cases with in situ stage, unknown stage and/or unknown surgery status were also excluded from all analyses.
Statistical Analysis
All analyses were performed using SAS. We compared the distribution of age at diagnosis, year of diagnosis, sex, SEER historic stage, surgery status, and histology between AIANs and whites using a Chi-squared test. We used multinomial regression to test our hypothesis that race is associated with lung cancer histology type, using adenocarcinoma as the comparison category of the dependent variable. We used multinomial regression to test our hypothesis that race and histology type are predictors of stage at diagnosis, using localized/regional stage as the comparison category of the dependent variable. We used logistic regression models test our hypothesis that race and histology type are associated with receipt of cancer-directed surgery. Finally, we used Cox regression to test our hypothesis that race and histology type are associated with risk of death. All regression analyses were adjusted for sex, SEER site, and age at diagnosis. The multinomial regression on histology was additionally adjusted for stage, and the Cox regression analyses were additionally adjusted for stage and receipt of surgery. To account for advances in treatment for lung cancer, all regression analyses were adjusted for categorical year of diagnosis (1973–1999 and 2000–2006). We also constructed models including a race*time period interaction term to determine whether any association between race and histology differed by time period. This study involved de-identified, non-human subjects data only, and was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Results
Demographic characteristics for AIANs and whites with lung cancer are detailed in Table 1. AIANs were more likely than whites to be diagnosed before age 60 (30% vs. 23%, respectively, p < 0.0001), and more likely to be diagnosed with localized or regional stage disease (54% vs. 51%, respectively, p = 0.02). Among patients with regional stage disease, 42% of AIANs and 52% of whites received surgery (p < 0.0001). This disparity extended to patients diagnosed with distant stage disease, with 6% of AIANs and 10% of whites receiving surgery (p = 0.004). Finally, the distribution of histology types differed by race (p < 0.0001): the most common histology type among AIANs was squamous cell carcinoma, comprising 32% of cases, while adenocarcinoma was the most frequent histology in whites, comprising 39% of cases.
Table 1.
Demographic characteristics of lung cancer cases by race
| AIAN | white | total | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total | 1050 | 0.53 | 197896 | 99.47 | 198946 | 100.00 | p-value* |
| Age at diagnosis | |||||||
| <50 | 89 | 0.08 | 12805 | 0.06 | 12894 | 0.06 | <0.0001 |
| 50–59 | 229 | 0.22 | 33242 | 0.17 | 33471 | 0.17 | |
| 60–69 | 355 | 0.34 | 60877 | 0.31 | 61232 | 0.31 | |
| 70–79 | 278 | 0.26 | 65238 | 0.33 | 65516 | 0.33 | |
| 80+ | 99 | 0.09 | 25734 | 0.13 | 25833 | 0.13 | |
| Mean age (SD) | 65.11 | (10.94) | 67.43 | (10.94) | |||
| Year of diagnosis | |||||||
| 1973–1999 | 449 | 0.43 | 82359 | 0.42 | 82808 | 0.42 | 0.45 |
| 2000–2006 | 601 | 0.57 | 115537 | 0.58 | 116138 | 0.58 | |
| Sex | |||||||
| Male | 607 | 0.58 | 110293 | 0.56 | 110900 | 0.56 | 0.18 |
| Female | 443 | 0.42 | 87603 | 0.44 | 88046 | 0.44 | |
| SEER Historic Stage | |||||||
| Localized/Regional | 571 | 0.54 | 101109 | 0.51 | 101680 | 0.51 | 0.02 |
| Distant | 479 | 0.46 | 96787 | 0.49 | 97266 | 0.49 | |
| Surgery, localized/regional cases | |||||||
| None | 334 | 0.58 | 48425 | 0.48 | 48759 | 0.48 | <0.0001 |
| Cancer-directed | 237 | 0.42 | 52684 | 0.52 | 52921 | 0.52 | |
| Surgery, distant cases | |||||||
| None | 450 | 0.94 | 87028 | 0.90 | 87478 | 0.90 | 0.004 |
| Cancer-directed | 29 | 0.06 | 9759 | 0.10 | 9788 | 0.10 | |
| Histologic type | |||||||
| Adenocarcinoma | 324 | 0.31 | 76223 | 0.39 | 76547 | 0.38 | <0.0001 |
| Bronchioloalveolar carcinoma | 32 | 0.03 | 7482 | 0.04 | 7514 | 0.04 | |
| Large cell carcinoma | 59 | 0.06 | 13205 | 0.07 | 13264 | 0.07 | |
| Other non-small cell | 298 | 0.28 | 50030 | 0.25 | 50328 | 0.25 | |
| Squamous cell carcinoma | 337 | 0.32 | 50956 | 0.26 | 51293 | 0.26 | |
p-value for Chi-square test
AIAN: American Indians and Alaska Natives; SD: standard deviation
Table 2 displays the association between race and histology group. AIANs were significantly more likely than whites to be diagnosed with ONSCLC (OR = 1.22, 95% CI: 1.01 – 1.48) or squamous cell carcinoma (OR = 1.25, 95% CI: 1.02 – 1.52) versus adenocarcinoma, and the overall association between race and histology was significant (p = 0.02). Sex and stage were also significantly associated with histology type among all patients (both p<0.0001). Women were more likely to be diagnosed with bronchioloalveolar carcinoma, and less likely to be diagnosed with squamous cell carcinoma than men. Patients diagnosed with distant stage disease were less likely to have bronchioloalveolar, large cell, or squamous cell histologies than patients with localized/regional stage disease.
Table 2.
Association between race and histology
| Odds Ratios and 95% confidence intervals for Histology type | ||||||
|---|---|---|---|---|---|---|
| Adenocarcinoma | Bronchioloalveolar carcinoma | Large cell carcinoma | Other non-small cell | Squamous cell carcinoma | p-value | |
| AIAN vs. white race | 1 (reference) | 0.64 (0.37 – 1.09) | 1.29 (0.94 – 1.79) | 1.22 (1.01 – 1.48) | 1.25 (1.02 – 1.52) | 0.02 |
| Age at diagnosis | ||||||
| 1-year increments | 1 (reference) | 1.01 (1.01 – 1.01) | 0.78 (0.75 – 0.81) | 0.83 (0.81 – 0.85) | 0.52 (0.51 – 0.54) | <0.0001 |
| Sex | ||||||
| Female vs. male | 1 (reference) | 1.46 (1.39 – 1.53) | 0.78 (0.75 – 0.81) | 0.83 (0.81 – 0.85) | 0.52 (0.51 – 0.54) | <0.0001 |
| SEER Historic Stage | ||||||
| Distant vs. Localized/Regional | 1 (reference) | 0.25 (0.23 – 0.26) | 0.91 (0.87 – 0.94) | 1.28 (1.25 – 1.31) | 0.49 (0.47 – 0.50) | <0.0001 |
adjusted for SEER site, and year of diagnosis
AIAN: American Indians and Alaska Natives
As shown in Table 3, race was not significantly associated with stage at diagnosis, after adjusting for histology, SEER site, time period, and age at diagnosis. However, histology was significantly associated with stage at diagnosis. Patients with bronchioloalveolar carcinoma had a greatly reduced risk of distant-stage diagnosis compared to patients with adenocarcinoma (OR = 0.25, 95% CI: 0.23 – 0.26), and less substantial reductions in risk were observed for patients with large cell carcinoma (OR = 0.91, 95% CI: 0.87 – 0.94) and squamous cell carcinoma (OR = 0.49, 95% CI: 0.47 – 0.50). Patients with ONSCLC were more likely to be diagnosed at distant stage (OR = 1.28, 95% CI: 1.25 – 1.31) than patients with adenocarcinoma. We repeated these analyses including a race*histology interaction term, which was not statistically significant (data not shown) and was therefore removed from the model.
Table 3.
Association between race, histology, and stage at diagnosis; and association with surgery
| Odds Ratio* (95% CI) | Odds Ratio† (95% CI) | |
|---|---|---|
| distant vs. localized/regional | surgery vs. no surgery | |
| Race | ||
| AIAN vs. white | 0.92 (0.79 – 1.07) | 0.68 (0.55 – 0.83) |
| Sex | ||
| Female vs. male | 0.88 (0.86 – 0.90) | 1.05 (1.03 – 1.07) |
| Histology | ||
| Adenocarcinoma | 1 (reference) | 1 (reference) |
| Bronchioloalveolar carcinoma | 0.25 (0.23 – 0.26) | 3.15 (2.97 – 3.35) |
| Large cell carcinoma | 0.91 (0.87 – 0.94) | 0.57 (0.54 – 0.59) |
| Other non-small cell | 1.28 (1.25 – 1.31) | 0.30 (0.29 – 0.31) |
| Squamous cell carcinoma | 0.49 (0.47 – 0.50) | 0.63 (0.61 – 0.65) |
adjusted for year of diagnosis, SEER site, and age at diagnosis
adjusted for year of diagnosis, SEER site, age at diagnosis, and SEER historic stage
AIAN: American Indians and Alaska Natives, CI: confidence interval
Table 3 also displays the association between race and histology with receipt of surgery. AIANs were less likely to receive surgery than whites (OR = 0.68, 95% CI: 0.55 – 0.83). Histology type was also associated with surgery; patients with bronchioloalveolar carcinoma were more likely to receive surgery (OR = 3.15, 95% CI: 2.97 – 3.35) and patients with ONSCLC were less likely to receive surgery (OR = 0.30, 95% CI: 0.29 – 0.31) than those with adenocarcinoma. Women were less likely than men to be diagnosed with distant disease (OR = 0.88 (95% CI: 0.86 – 0.90), and more likely to receive surgery (OR = 1.05, 95% CI: 1.03 – 1.07).
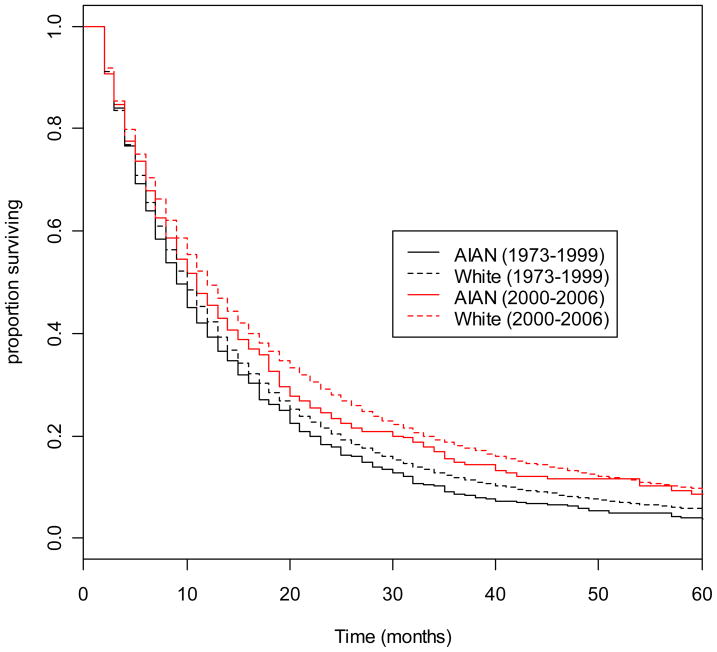
Table 4 summarizes results of the survival analysis. Compared to whites, AIANs had an increased risk of death (Hazard Ratio (HR) = 1.09, 95% CI: 1.01 – 1.19). Histology was significantly associated with risk of death, with the most substantial difference observed in cases with bronchioloalveolar carcinoma, who had a HR of 0.68 (95% CI: 0.66 – 0.70) compared to cases with adenocarcinoma. Statistically significant, but clinically minor survival differences were observed for large cell carcinoma, ONSCLC, and squamous cell carcinoma. We repeated these analyses including a race*histology interaction term, and then a race*time period interaction term. Neither interaction term was statistically significant (data not shown) and were therefore removed from the models. Figure 1 displays Kaplan-Meier survival curves for AIANs and whites, stratified by year of diagnosis category. Although both AIANs and whites diagnosed in later years had longer survival times than patients diagnosed in earlier years, whites consistently have longer survival than AIANs in both year of diagnosis categories, and across all timepoints.
Table 4.
Survival analysis of race, histology and risk of death; stratified by and adjusted for year of diagnosis
| Race | Hazard Ratio* (95% CI) |
|---|---|
| AIAN vs. white | 1.09 (1.01 – 1.19) |
| Sex | |
| Female vs. male | 0.85 (0.85 – 0.86) |
| Histology | |
| Adenocarcinoma | 1 (reference) |
| Bronchioloalveolar carcinoma | 0.68 (0.66 – 0.70) |
| Large cell carcinoma | 1.15 (1.12 – 1.17) |
| Other non-small cell | 1.10 (1.09 – 1.12) |
| Squamous cell carcinoma | 1.05 (1.03 – 1.06) |
adjusted for SEER site, year of diagnosis, SEER historic stage, andsurgery
AIAN: American Indians and Alaska Natives, CI: confidence interval
Figure 1.
Kaplan-Meier survival curves comparing all-cause survival between AIANs and whites among lung cancer cases diagnosed from 1973–1999, and from 2000–2006.
Discussion
The goal of these analyses was to determine whether race is associated with lung cancer histology, and whether race and histology are associated with death among lung cancer patients in a population-based sample. We found that AIAN race was associated with lung cancer histology type and survival, and that survival time differed according to histology type. Further, the association between AIAN race and lung cancer histology, as well as survival disparities between AIANs and whites, appeared to persist even after adjustment for time period, age, treatment, and stage at diagnosis. Our observation that histology was associated with stage at diagnosis is in line with current understanding of lung cancer development and progression. For example, bronchioloalveolar carcinoma was more likely than adenocarcinoma to be diagnosed at an early stage, consistent with the slow growth typical of bronchioloalveolar tumors.(9) Likewise, we noted that squamous cell tumors were most likely diagnosed at an early stage, congruent with the fact that these tumors often arise near the central airway,(10) and thus may be symptomatic earlier than tumors in peripheral airways.
Smoking behavior could explain the higher incidence of squamous cell carcinomas among AIANs compared to whites. The majority of lung cancers diagnosed among non-smokers or infrequent smokers are adenocarcinomas or bronchioloalveolar carcinomas.(11) The prevalence of smoking is in AIANs varies widely by age and tribe,(12) but the Nationwide Tobacco Use Supplement to the Current Population Survey (TUS-CPS) found that a higher proportion of AIANs smoke than whites. The 2003 TUS-CPS data show that among AIANs, 34% of men and 30% of were current smokers, compared to 21% of white men and 18% of white women.(13) Thus, it is not surprising that the incidence of smoking-related lung cancer histology is also higher in AIANs. Our analyses were unable to account for smoking behavior because SEER does not collect smoking data, but future studies should address the role of smoking in the race-histology association.
As illustrated in Figure 1, survival time increased from the earlier to later time periods in both AIAN and whites, although survival among AIANs was still shorter. A case-control study on AIANs diagnosed with cancer in Montana from 1984–1993 found that AIANs were less likely to receive surgery than whites, but that survival among lung cancer cases did not vary by race.(14) In partial contrast, we observed disparities between whites and AIANs from 1973 – 2006 in both receipt of surgery and risk of death. This survival disparity trend may partially be due to racial disparities in access that changed over time. In the 1970s and 1980s, modern treatments such as advanced radiation techniques, adjuvant chemotherapy, and chemoradiation for regional stage disease and palliative chemotherapy for distant stages did not exist. In recent years, lung cancer survival has increased due to advances in surgical techniques,(15–17) the adoption of adjuvant chemotherapy,(18–21) the increased frequency of chemotherapy treatment in advanced stages,(22) and stage migration due to novel staging methods.(23) As medical practice advanced, the existing racial gap in access to treatments may have widened and resulted in the survival disparities observed in recent time periods. More research is required to determine whether AIANs have reduced access to emerging lung cancer treatments compared to whites, and whether any access gap is reflected in survival disparities.
We observed significant associations between sex and lung cancer tumor characteristics and survival. Interestingly, female sex and AIAN race appeared to have opposite associations with lung cancer histology and survival. This is consistent with the hypothesis that smoking behavior, and/or smoking-related comorbidities could be associated with histology type and survival, because women tend to smoke less than men, and as noted above, AIANs smoke more than whites.(13)
This study has several important limitations. Although the SEER database provided a large number of population-based AIAN lung cancer patients, SEER does not collect information on smoking behavior, clinical performance status at diagnosis, or treating surgeon experience with regards to lung cancer, all of which are strongly linked to lung cancer survival.(24–27) Survival outcomes also depend on the volume of lung cancer surgeries performed at medical centers and the quality of supportive care for patients recovering from surgery and receiving chemotherapy,(27, 28) However, this systems-level information is also not available in SEER. Therefore, while we can document differences in lung cancer histology and survival between AIANs and whites, we cannot determine whether these disparities are caused by race-specific differences in access to quality care, or by race-based differences in the prevalence of comorbid conditions (such as diabetes, smoking behavior, and smoking-related disorders) that may complicate treatment, recovery, and survival.
Although SEER is a good source for cancer data on a large number of AIANs, future studies of the clinical attributes and prognosis of lung cancer in AIANs could supplement SEER data with detailed smoking information, including type of cigarette smoked and pack-years. This enrichment of SEER data could be achieved by administering a survey to rapidly-identified lung cancer cases reported to SEER. These studies could also collect data on comorbidities, in order to control for the effect of overall health on survival outcomes. In addition, SEER does not capture information on tribal membership, which may be associated with health-related behaviors and access to care. Our adjustment for SEER site partially accounts for geographic differences, but is unable to completely account for differences between AIAN tribes. Future studies should include factors related to access to quality care, such as hospital volume and provider experience. These studies could differentiate between biological, access, and behavior-based causes for the observed disparities in survival.
In conclusion, our analyses describe differences in lung cancer histology, treatment, and survival between AIANs and whites. Although both racial groups have experienced improvements in survival over time, survival disparities persist and may be increasing, even after adjusting for stage, histology, and treatment. Future studies should consider patient comorbidities and access to quality care as potential confounders of the association between AIAN race and shortened lung cancer survival.
Acknowledgments
Supported by grant 1UO1 CA114642, Native People for Cancer Control, a Community Networks Program funded by the National Cancer Institute, Bethesda, MD. None of the authors report any financial disclosures.
References
- 1.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004 Mar–Apr;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 2.Wiggins CL, Espey DK, Wingo PA, et al. Cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008 Sep 1;113(5 Suppl):1142–1152. doi: 10.1002/cncr.23734. [DOI] [PubMed] [Google Scholar]
- 3.Bliss A, Cobb N, Solomon T, et al. Lung cancer incidence among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008 Sep 1;113(5 Suppl):1168–1178. doi: 10.1002/cncr.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugarman JR, Dennis LK, White E. Cancer survival among American Indians in western Washington State (United States) Cancer Causes Control. 1994 Sep;5(5):440–448. doi: 10.1007/BF01694758. [DOI] [PubMed] [Google Scholar]
- 5.Wang SJ, Fuller CD, Thomas CR., Jr Ethnic disparities in conditional survival of patients with non-small cell lung cancer. J Thorac Oncol. 2007 Mar;2(3):180–190. doi: 10.1097/JTO.0b013e318031cd4e. [DOI] [PubMed] [Google Scholar]
- 6.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007 Dec 10;25(35):5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 7.Horner MR, LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; [accessed March 22, 2010]. [serial online]. Available from: http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 8.National Cancer Institute. [accessed March 22, 2010];Surveillance, Epidemiology, and End Results (SEER) Program Website [serial online] Available from: http://www.seer.cancer.gov.
- 9.Zell JA, Ou SH, Ziogas A, et al. Epidemiology of bronchioloalveolar carcinoma: improvement in survival after release of the 1999 WHO classification of lung tumors. J Clin Oncol. 2005 Nov 20;23(33):8396–8405. doi: 10.1200/JCO.2005.03.0312. [DOI] [PubMed] [Google Scholar]
- 10.Franklin WA, Chanin T, Gonzalez A. Molecular and Cellular Pathology of Lung Cancer. In: Pass HI, Carbone DP, Minna, et al., editors. Lung Cancer: Principles and Practices. 3. 2005. [Google Scholar]
- 11.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007 Oct;7(10):778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 12.Eichner JE, Cravatt K, Beebe LA, et al. Tobacco use among American Indians in Oklahoma: an epidemiologic view. Public Health Rep. 2005 Mar–Apr;120(2):192–199. doi: 10.1177/003335490512000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis WW, Hartman AM, Gibson JT. National Cancer Institute; [accessed March 22, 2010]. Trends in Smoking Prevalence by Race based on the Tobacco Use Supplement to the Current Population Survey. [monograph online]. Available from: http://www.fcsm.gov/07papers/Davis.VII-C.pdf. [Google Scholar]
- 14.Dennis TD. Cancer stage at diagnosis, treatment, and survival among American Indians and non-American Indians in Montana. Cancer. 2000 Jul 1;89(1):181–186. doi: 10.1002/1097-0142(20000701)89:1<181::aid-cncr24>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Daniels LJ, Balderson SS, Onaitis MW, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients with stage I lung cancer. Ann Thorac Surg. 2002 Sep;74(3):860–864. doi: 10.1016/s0003-4975(02)03764-5. [DOI] [PubMed] [Google Scholar]
- 16.Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg. 1995 May;109(5):997–1001. doi: 10.1016/S0022-5223(95)70326-8. discussion 1001–1002. [DOI] [PubMed] [Google Scholar]
- 17.Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg. 1999 Jul;68(1):194–200. doi: 10.1016/s0003-4975(99)00467-1. [DOI] [PubMed] [Google Scholar]
- 18.Chhatwani L, Cabebe E, Wakelee HA. Adjuvant treatment of resected lung cancer. Proc Am Thorac Soc. 2009 Apr 15;6(2):194–200. doi: 10.1513/pats.200807-068LC. [DOI] [PubMed] [Google Scholar]
- 19.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004 Jan 22;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 20.Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol. 2007 Apr 20;25(12):1553–1561. doi: 10.1200/JCO.2006.09.5570. [DOI] [PubMed] [Google Scholar]
- 21.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006 Sep;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 22.Lang K, Marciniak MD, Faries D, et al. Trends and predictors of first-line chemotherapy use among elderly patients with advanced non-small cell lung cancer in the United States. Lung Cancer. 2009 Feb;63(2):264–270. doi: 10.1016/j.lungcan.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Morgensztern D, Goodgame B, Baggstrom MQ, et al. The effect of FDG-PET on the stage distribution of non-small cell lung cancer. J Thorac Oncol. 2008 Feb;3(2):135–139. doi: 10.1097/JTO.0b013e3181622c2c. [DOI] [PubMed] [Google Scholar]
- 24.Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janjigian YY, McDonnell K, Kris MG, et al. Pack-years of cigarette smoking as a prognostic factor in patients with stage IIIB/IV nonsmall cell lung cancer. Cancer. 2009 Feb 1;116(3):670–675. doi: 10.1002/cncr.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol. 2009 Dec 1;27(34):5816–5822. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003 Nov 27;349(22):2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 28.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001 Jul 19;345(3):181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]