Abstract
Galanin-like peptide (GALP) was discovered in 1999 in the porcine hypothalamus and was found to be a 60 amino-acid neuropeptide. GALP shares sequence homology to galanin (1–13) in position 9–21 and can bind to, as well as activate, the three galanin receptor subtypes (GalR1-3). GALP-expressing cells are limited, and are mainly found in the arcuate nucleus of the hypothalamus (ARC) and the posterior pituitary. GALP-positive neurons in the ARC project to several brain regions where they appear to make contact with multiple neuromodulators. These neuromodulators are involved in the regulation of energy homeostasis and reproduction, anatomical evidence that suggests a role for GALP in these physiological functions. In support of this idea, GALP gene expression is regulated by several factors that reflect metabolic state including the metabolic hormones leptin and insulin, thyroid hormones, and blood glucose. Considerable evidence now exists to support the hypothesis that GALP has a role in the regulation of energy homeostasis and reproduction; and, that GALP’s role may be independent of the known galanin receptors. In this review we (1) provide an overview of the distribution of GALP, and discuss the potential relationship between GALP and other neuromodulators of energy homeostasis and reproduction, (2) discuss the metabolic factors that regulate GALP expression, (3) review the evidence for the role of GALP in energy homeostasis and reproduction, (4) discuss the potential downstream mediators and mechanisms underlying GALP’s effects, and (5) discuss the possibility that GALP may mediate it’s effects via an as yet unidentified GALP-specific receptor.
Discovery of Galanin-like Peptide: Relationship to Galanin
Galanin was originally isolated and characterized by Tatemoto et al [84]. Galanin has many functions both peripherally and centrally; chief among them galanin regulates hypothalamic secretagogues that alter the release of anterior pituitary hormones. Galanin also has regulatory actions on nociception, energy homeostasis (feeding, metabolism and body weight regulation), and learning and memory (for reviews see [1, 2]). Galanin exerts its effects on these physiological systems by activation of its three known receptors, designated GalR1, GalR2 and GalR3. Sixteen years after the discovery of galanin, Ohtaki and colleagues [65] isolated another peptide from the porcine hypothalamus that bound to the galanin receptors, GalR1 and GalR2. They dubbed this new peptide galanin-like peptide, or GALP. GALP not only bound to these two galanin receptors with relatively high affinity in vitro but by using a GTPγS binding assay, Ohtaki et al [65] demonstrated that GALP activated these receptors similarly to galanin, and it is now know that GALP also activates GalR3 [45]. In fact, GALP binds to and activates the GalR2 receptor with greater affinity than does galanin. Thus a peptide was discovered that bound to and activated galanin receptors in vitro. These exciting findings led researchers to investigate the genetic and structural relationships between galanin and GALP.
GALP and galanin share a 13 amino acid identity in their peptide sequence. The amino acids at position 9–21 of GALP are 100% similar to amino acids 1–13 of galanin. The first 13 amino acids in galanin’s structure comprise the minimum sequence required to bind to galanin receptors [7]. Despite the partially shared sequence identity, galanin and GALP are encoded by separate genes that are typically located on separate chromosomes. In humans, the GALP gene is located on chromosome 19 (19q12.13) while the galanin gene is on chromosome 11 (11q13.3). Although in rats both peptides’ genes are located on chromosome 1, GALP and galanin are still encoded by two separate genes (1q12 and 1q42, respectively). The mature GALP peptide is cleaved from a precursor of 115–120 amino acids, the exact number depending on the species. Furthermore, the GALP peptide shows a high degree of sequence identity in mice, rats, macaques, pigs and humans [10, 23, 28, 65].
Evolutionary Relationship Between GALP and Galanin
Alignment of the GALP peptide sequence in different species reveals that there are two highly conserved regions. The first is the previously mentioned region (amino acids 9–21) where the peptide has the potential to bind to galanin receptors. The second conserved region is unique to the GALP peptide and is between residues 38–54. Since the 38–54 sequence is distinctive to GALP alone, it is hypothesized to be a binding region for a putative GALP-specific receptor [65, 70], although the identity of a GALP-specific receptor has remained elusive. However, clues to the presence of a GALP-specific receptor can be gleaned from the distribution of GALP in the central nervous system (CNS).
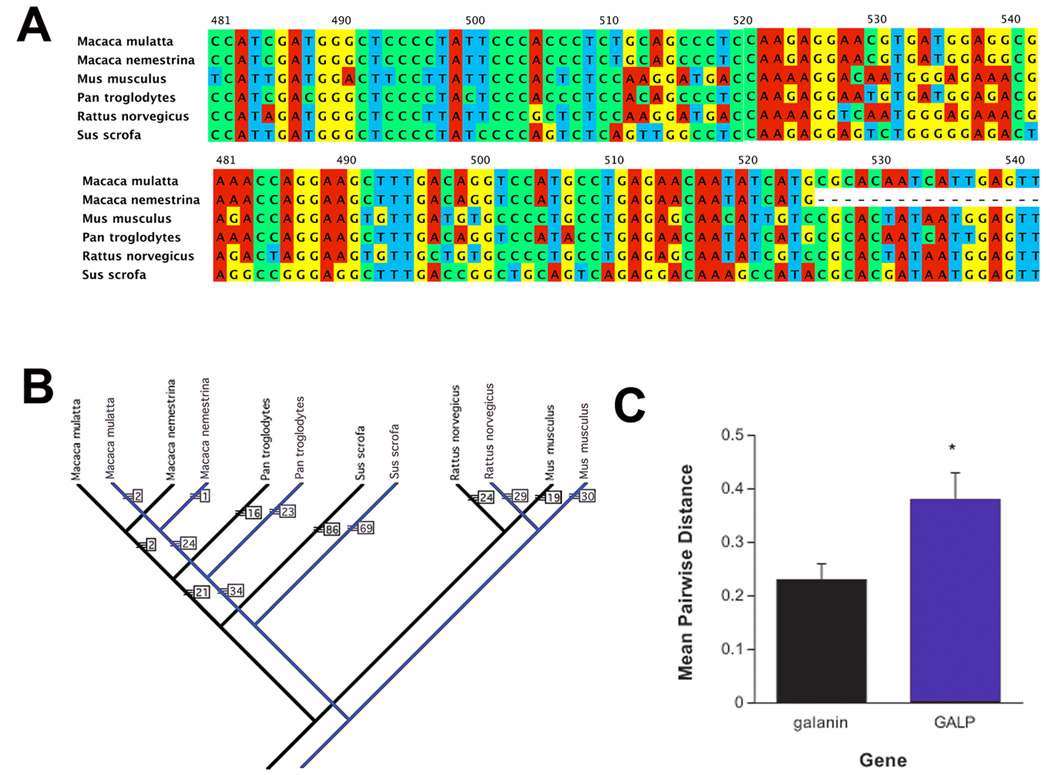
In order to more fully understand the evolutionary relationship between GALP and galanin, a molecular systematist (Dr. Timothy Evans, Grand Valley State University, Allendale, MI USA) was consulted to analyze the relationship between GALP and galanin genes. Nucleotide sequences were obtained from Genbank and aligned using ClustalW v. 2 [46]. Molecular phylogenies were constructed from each data set using PAUP* v. 4.10 [89]. Only species with complete nucleotide sequences for both GALP and galanin were utilized (Figure 1A). Sequences from Homo sapiens are excluded from the analyses. The exclusion of this species was decided upon due to the inability to obtain a quality sequence alignment. Poor sequence alignments with Homo sapiens were the result of considerable divergence in the gene sequences compared to the other species analyzed for both GALP and galanin. Because of the small sample size (six species), an exhaustive phylogenetic search was conducted, guaranteeing that the shortest evolutionary tree was found. A single most parsimonious tree was found for each data set (GALP and galanin; Figure 1B), and the trees were identical in their topology. For the GALP data set, the tree was 600 evolutionary steps, with a consistency index (CI) of 0.97 and for the galanin data set it was 530 steps (CI=0.99). The high CI values for these analyses indicate a high confidence in the phylogenies based on the available data (i.e. there is low conflict among different nucleotides for support of these phylogenetic trees).
Fig. 1.
A comparison of evolutionary relatedness between GALP and galanin. A) an example of the sequence alignment among galanin (top) and GALP (bottom) that demonstrates the higher degree of nucleotide substitution in GALP compared to galanin. Different colors in a column indicate nucleotide changes. B) Phylogenetic trees for galanin (black) and GALP (blue) from PAUP analyses of mean pairwise distances among aligned nucleotides. The numbers next to each arm indicate total number of changes to produce that clade. C). Non-parametric analyses revealed that GALP had a significantly (p < 0.05) higher PAUP value, indicating a greater rate of nucleotide substitutions compared to galanin.
Analyses of the average sequence distance among the species for each gene set show that GALP has a significantly (p < 0.05) greater number of nucleotide substitutions among species than does galanin (Figure 1C). The greater number of nucleotide substitutions in GALP provides evidence for two possible evolutionary scenarios for GALP and galanin. The first is that the GALP gene may pre-date the galanin gene in the evolution of these two systems, thereby having more time to accumulate nucleotide substitutions among the different animal lineages. The second is that the two genes are of the same “age,” but GALP is undergoing a greater rate of evolutionary change than is galanin. However, comparison of a larger number of species (particularly for GALP) would be necessary to strengthen either hypothesis. Regardless, the possibility that GALP is an older gene may lend some understanding to the considerable distribution of galanin in the CNS and periphery compared to GALP’s quite limited distribution.
Distribution of GALP
Expression of GALP mRNA and protein in the CNS
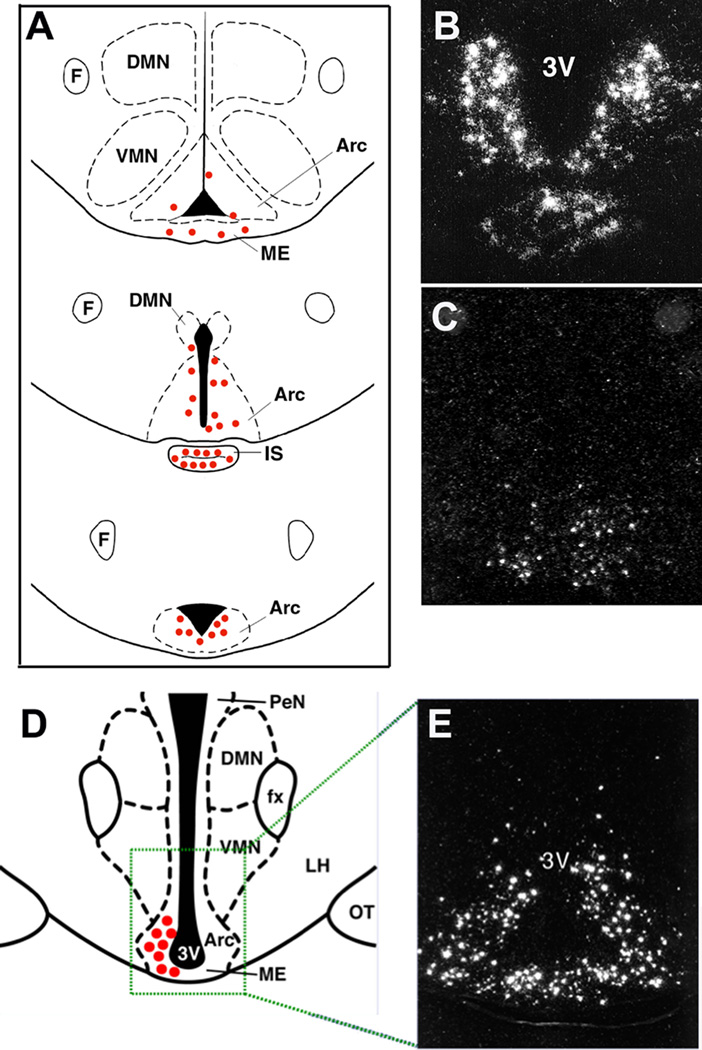
GALP was originally isolated from the porcine hypothalamus [65]. Subsequent studies have shown that the distribution of GALP in the CNS is restricted. Comparatively, galanin is more widespread within the brain and is found in several hypothalamic nuclei including the hypothalamic arcuate nucleus (ARC), the paraventricular hypothalamic nucleus (PVN), the peri-ventricular hypothalamic nucleus, the dorsomedial hypothalamic nucleus (DMH), the supraoptic nucleus (SON) of the hypothalamus, and the lateral hypothalamus. In contrast to galanin, cells producing GALP mRNA and protein are found only in the ARC, the median eminence and infundibular stalk, and the posterior pituitary of the rat [20, 31, 47, 78], mouse [30] and primate [10]. GALP is also expressed at very low levels in the ovine hypothalamus [29, 88] and although GALP mRNA is detected in the human brain, its distribution remains to be determined [65]. GALP mRNA in the rat ARC is first detected at postnatal days 8–10 [37, 67]. The levels of GALP mRNA increase during weaning and the peripubertal period, reaching a maximum level between days 25–40 with no obvious sex differences observed at any age. During development, there is also no apparent change in GALP’s distribution within the ARC.
Within the ARC of the rat, GALP is expressed in neurons that are located mostly in the posterior and periventricular zones (i.e. ventromedial ARC), extending from retrochiasmatic to premammillary regions (see Figure 2). The anatomical distribution of GALP-positive neurons in the ARC of mice varies slightly, with cells being found more laterally, ventrally and rostrally as compared to rats. Detailed electron microscopy studies show that GALP-expressing neurons in the ARC form complex synaptic relationships with both GALP immunopositive and immunonegative neurons [22].
Fig. 2.
Distribution of GALP in the brain. A) schematic diagram of the distribution of GALP mRNA-containing cells in the rat and mouse, B) in situ hybridization for GALP mRNA in the rat, C) in situ hybridization for GALP mRNA in the mouse, D) schematic diagram of the distribution of GALP mRNA-containing cells in the monkey, E) in situ hybridization for GALP mRNA in the monkey.
GALP-expressing neurons in the rat ARC may be a target for the orexigenic neuropeptides orexin and neuropeptide Y (NPY). Morphological studies show that orexin-immunoreactive fibers appear to make contact with GALP-positive neurons in the rat ARC, and 9% of these neurons express orexin-1 receptor protein [79]. NPY-positive fibers also project onto GALP-expressing neurons [80], that in the macaque express mRNA for NPY Y1 receptor [11]. These findings suggest that GALP may be a downstream target of orexin and NPY, although this is yet to be established.
In contrast to the neuronal expression in the ARC, cells expressing GALP in the posterior pituitary are pituicytes [20, 76]. Pituicytes are specialized astrocytes that are thought to modulate posterior pituitary hormone release by changing the amount of contact between axon terminals and fenestrated capillaries [25]. Thus pituicyte GALP may be involved in oxytocin and vasopressin release.
Expression of GALP mRNA and protein in the periphery
GALP-immunoreactivity (-ir) is also detected in blood, and this GALP-ir is thought to be able to enter the brain [35]. The pituicytes of the posterior pituitary may be a source of plasma GALP. GALP mRNA has also been detected in murine skin and thymus [74]. However, it is not known whether peripheral tissues can release GALP into the circulation, nor is the physiological relevance of circulating GALP understood. Thus, more detailed expression and function studies of GALP in the periphery are needed.
Phenotype and targets of GALP-expressing neurons
Several neuropeptides are expressed in the ARC, but GALP-positive cells may be an individual population of neurons, as these cells do not co-localize with NPY or somatostatin [78, 80]. However, Takenoya and colleagues [80] report that 3–12% of GALP neurons do contain α-melanocyte stimulating hormone (α-MSH), although this finding has not been supported by others [78], and thus a more detailed analysis of the phenotype of GALP containing neurons is required.
GALP-containing neurons in the rat ARC send projections to various areas of the forebrain, such as the bed nucleus of the stria terminalis and the lateral septal nucleus. In addition, a large proportion of GALP-ir fibers project to several nuclei within the hypothalamus including the ARC, the PVN, the medial preoptic area (mPOA), the lateral hypothalamus, and the peri-ventricular hypothalamic nucleus [78, 80]. GALP-ir nerve fibers appear to innervate numerous neuronal phenotypes known to regulate feeding and reproduction. GALP-innervated neurons include—but are not limited to—dopaminergic neurons in the ARC [33] orexin- and melanin-concentrating hormone-positive neurons in the lateral hypothalamus [82], and luteinizing hormone-releasing hormone (LHRH, also known as gonadotropin-releasing hormone, GnRH) expressing neurons in the mPOA [78, 81]. These observations provide anatomical evidence that these neurons may mediate some of the central actions of GALP.
Regulation of the GALP Gene in the CNS
The ARC is located in the ventral hypothalamus at a site that is unique, in that the ARC has a weak blood-brain-barrier. The ARC is therefore able to “monitor” blood constituents and alter the release of neuromodulators. Many of these neuromodulators are known to be involved in the regulation of reproduction and energy homeostasis. Thus GALP is located in a unique position in the ARC to be regulated by and respond to altered nutrients and/or metabolic hormones.
Metabolic hormones and fuels regulate the expression of GALP in the brain. Fasting is a negative metabolic state in which there is a sharp decline in blood levels of metabolic fuels (e.g. free fatty acids and glucose) and metabolic hormones (e.g. leptin and insulin). Accumulated evidence has demonstrated that GALP neurons are regulated by metabolic states [14, 16, 30, 31]. Fasting is known to reduce the expression of GALP mRNA in the ARC, and leptin treatment during a fast can reverse this effect [31]. Leptin-deficient ob/ob mice have profoundly reduced levels of GALP mRNA in the hypothalamus, but treating these animals centrally (intracerebroventricular; i.c.v.) with leptin completely restores the expression of GALP mRNA to the levels found in wild-type mice [30]. Rats and mice with dysfunctional leptin receptors (fa/fa and db/db, respectively) also have reduced expression of GALP mRNA [43, 71]. Moreover, in both the rat and macaque, virtually all GALP-containing neurons in the ARC express the leptin receptor [10, 78]. These observations indicate that the expression of GALP mRNA is regulated by direct actions of leptin on GALP-containing neurons in the brain. Other studies have indicated that additional metabolic hormones, such as insulin, also regulate GALP gene expression.
Insulin regulates the expression of GALP mRNA. Rats with streptozotocin-induced Type I diabetes have greatly diminished expression of GALP mRNA in the ARC, which can be corrected with either insulin or leptin treatment [16]. In fact, the two hormones have an additive effect to increase GALP mRNA levels in diabetic male rats. In addition, the administration of insulin directly into the brain during a fast stimulates the expression of GALP mRNA, which indicates that insulin also acts directly in the CNS to induce the expression of GALP mRNA. Leptin and insulin share a common signaling pathway in neurons within the ARC (e.g. phosphatidylinositol 3-kinase [64]), and it is conceivable that this common pathway is responsible for the similar actions of leptin and insulin on the expression of GALP mRNA [17]. The additive effects of insulin and leptin on GALP mRNA expression suggest that insulin may utilize other intracellular mechanisms, such as the Fox01 system, than does leptin to regulate GALP gene expression however this has not been clarified. Taken in concert, all of these studies demonstrate that metabolic hormones regulate GALP gene expression. Other data suggest that metabolic fuels also have the ability to regulate GALP mRNA, either directly or indirectly.
Circulating levels of metabolic fuels, such as glucose, are monitored by specialized neurons within the CNS. When rats are treated with a non-metabolically active form of glucose (2-D-deoxyglucose; 2DG) they experience a perceived negative metabolic (hypoglycemic) state [51]. Neurons within the ARC respond by stimulating orexigenic neuropeptides and inhibiting anorexigenic neuropeptides, thus increasing food intake. The ARC compensation for hypoglycemic states is mediated by specialized noradrenergic (NA) neurons in the hindbrain that are specifically glucoresponsive [15, 39, 68, 69]. In hypoglycemic states, hindbrain NA neurons increase signaling to the ARC (among other areas) and induce the aforementioned changes in neuropeptides. GALP mRNA within the ARC is significantly reduced when animals are treated with 2DG [14]. Furthermore, evidence shows that this 2DG effect on GALP neurons is dependant upon the hindbrain glucoresponsive NA neurons [14]. Thus GALP gene expression is regulated not only by metabolic hormones, but also by circulating levels of glucose. The question then becomes whether GALP is also regulated by other traditional modulators of ARC neuropeptides, such as steroid hormones.
Thyroid steroid hormones also influence the expression of GALP mRNA. Reduced circulating concentrations of thyroid hormones (caused by thyroidectomy) decrease hypothalamic levels of GALP mRNA, and replacement of thyroxine in thyroidectomized rats partially reverses this effect [9]. In addition, GALP delivered directly into the brain reduces the secretion of thyroid stimulating hormone [9], again indicating that GALP plays a role in the neuroendocrine regulation of the hypothalamo–pituitary–thyroid axis, further evidence that GALP regulates energy homeostasis. Some observations have also been made that GALP gene expression can be altered in response to other stimuli.
Evidence does suggest that GALP mRNA is increased in the ARC and pituitary in response to inflammatory stimuli [72, 73]. Furthermore, GALP mRNA levels in the pituitary, but not the ARC, are altered in response to osmotic stimuli, suggesting regulation of antidiuretic hormone (ADH) release by GALP within the pituitary [20, 38, 73, 76]. If GALP expressed in the pituitary is subsequently released into the circulation then alterations of pituitary GALP gene expression may alter circulating levels of GALP-ir. Kastin et al [35] demonstrated that fasting reduces peripheral GALP entry into the brain. However, the behavioral or physiological significance of this phenomenon has yet to be determined. It is important to note, however, that the expression of GALP in either the pituitary or the ARC is not regulated by circulating concentrations of glucocorticoids, sex steroids or growth hormone [9]; rather, ARC GALP’s gene expression appears to be influenced solely by factors that reflect metabolic state. Figure 3 shows a schematic of factors that influence GALP gene expression in the ARC.
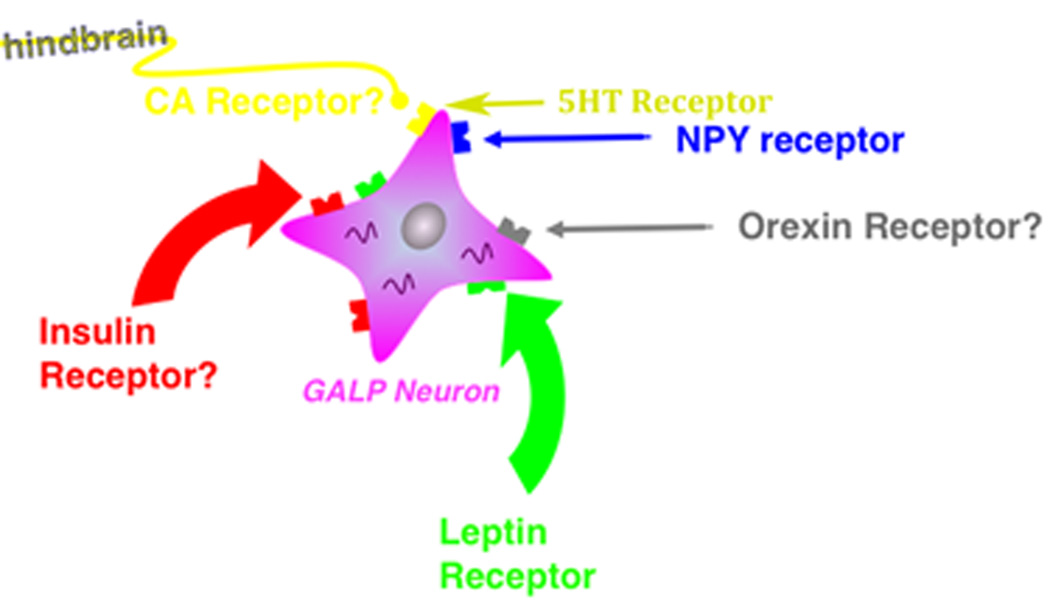
Fig. 3.
Factors that may influence hypothalamic GALP neurons. A schematic diagram that illustrates known and putative (question marks) regulators of GALP neuronal gene expression and/or neuronal activity. CA = catecholamine, 5HT = serotonin.
Central Actions of GALP
The expression of GALP in the ARC and pituitary is regulated by a number of factors including hormones such as leptin and insulin, and metabolic and osmotic challenges. These observations suggest a role for GALP in the central regulation of metabolism, reproduction and fluid intake. Although expression of GALP in the pituitary changes in response to an osmotic stimulus [20, 38, 73, 76], a direct action of GALP on fluid intake in vivo is yet to be determined, and to date the strongest evidence for central actions of GALP is in the regulation of metabolism and reproduction.
a) The effect of GALP on feeding, body weight and metabolism
GALP is regulated by changes in metabolic status (e.g. during fasting or leptin administration), thus a role for GALP in feeding and metabolism was proposed. There is now considerable evidence demonstrating that central administration of GALP has potent effects on food intake and body weight in rodents, although these actions are complex and are both species- and time-dependent. GALP was initially described as an orexigenic neuropepide since i.c.v. injection in rats acutely stimulates feeding (over 1 hour; [60]). Administration of GALP directly into the mPOA, PVN and DMH also increases food intake acutely at 1–2 hours, although for the PVN and DMH these effects are not seen consistently [44, 66, 75]. However, when rats are studied 24 hours after central administration of GALP, a decrease in food intake and body weight is observed [41, 48, 49]. These anorexigenic actions of GALP are also seen 24 hours after i.c.v. injection of GALP in mice [36, 41, 42, 55]. In contrast, the orexigenic effect of GALP observed in rats is species dependent as GALP does not acutely stimulate feeding in mice [41, 55]. The receptor responsible for the orexigenic actions of GALP in rats may be GalR1, as agonists for this receptor stimulate feeding in rats ([87]. In contrast, GalR2 and GalR3 are unlikely to be involved in the appetite stimulatory effect of GALP, as GalR2 and GalR2/3 preferring agonists (galanin 2–29 and AR-M1896 respectively), have no effect on food intake (at 1 h) in rats [56, 75, 87]
In C57BL/6 mice, central administration of GALP induces only transient reductions in food intake and body weight, as significant differences are observed only at 24 hours after the onset of chronic treatment with GALP (twice-daily injection for 4 days) [41]. In contrast, in leptin-deficient obese (ob/ob) mice, chronic administration of GALP for 14 days produces prolonged reductions in food intake and body weight [24]. Furthermore, the orexigenic effects of GALP are exacerbated in rats made obese after 12-weeks maintenance on a high-fat diet [83]. The increase in sensitivity to GALP in ob/ob mice may be due to an up-regulation in the number of galanin receptors (that may mediate GALP’s effects) in response to the reduction in GALP mRNA in the ARC of ob/ob mice [30]. Changes in GALP mRNA in the ARC have also been detected in rats fed a high-fat diet for 6 weeks. Whether these changes in GALP expression explain the enhanced responsiveness of diet-induced obese rats to GALP remains to be clarified, as GALP expression increases after a diet high in polyunsaturated fats while a diet high in saturated fats has no effect [13].
GALP administration in ob/ob mice leads to a greater reduction in body weight compared to vehicle-injected pair-fed mice [24]. This finding suggests that the loss of body weight after central administration of GALP in rodents is not due to a reduction in energy (food) intake alone, but that in addition, GALP may also affect energy expenditure. In support, central administration of GALP increases metabolic rate in rats, as measured by oxygen consumption [67]. Furthermore, i.c.v. injection of GALP in rats induces a rapid and prolonged (over 8 hours) increase in core body temperature that is mediated by prostaglandins [48]. An increase in body temperature is also observed in ob/ob mice after 14 days chronic treatment with GALP [24]. However, after acute GALP treatment in mice, a transient drop in body temperature is observed before a period of hyperthermia that lasts for approximately 8 hours after injection [55]. These effects of GALP on metabolism and body temperature may be due to activation of sympathetic nervous system as GALP administration to ob/ob mice increases the expression of uncoupling protein 1 (UCP-1) in brown adipose tissue [24].
The hypothesis that GALP has a physiological role in the regulation of metabolism has recently been tested. Mice deficient in GALP show no differences in body weight, feeding and temperature compared to control mice when maintained on a normal diet. However, GALP-deficient mice eat a reduced amount of food after a fast and gain less weight on a high-fat diet compared to control mice [12]. These data suggest that GALP signaling is not essential for the regulation of metabolism and energy homeostasis under normal dietary conditions, but GALP may be involved in the response to a metabolic challenge, such as during a fast or high-fat feeding.
Mechanisms of GALPs actions on feeding--orexigenic effects
Central administration of GALP induces a distinct pattern of cell activation in the rat brain. GALP administered i.c.v. increases fos protein expression in the SON, the parenchyma surrounding the peri-ventricular regions, the ependymal cells of the ventricles, and in the mPOA, the lateral hypothalamus, the DMH, and the nucleus tractus solitarius (NTS) of the brainstem [18, 49, 59]. However, in contrast to the rat, i.c.v. injection of GALP in mice fails to stimulate fos expression in the lateral hypothalamus and DMH and fos is observed only in the peri-ventricular regions, the meninges and the ependymal cells of the ventricles [56]. The lateral hypothalamus and DMH play important roles in the regulation of energy balance, and evidence suggests that GALP can acutely promote feeding in the rat via activation of orexigenic neurons (NPY and orexin) in these brain regions.
When administered i.c.v. in rats, GALP increases fos expression in NPY-containing neurons of the DMH [44] and it stimulates the release of NPY from hypothalamic explants in vitro [75]. As GALP injection directly into the DMH stimulates food intake (over 2 h) in rats [44], these findings suggest that GALP increases food intake via NPY release in the DMH. In support, blocking the actions of NPY inhibits the acute orexigenic effect of GALP in rats [44].
Morphological evidence for a relationship between GALP-positive fibers and orexin-containing neurons in the lateral hypothalamus has also been established [82]. A functional link for orexin in GALP’s actions is supported by the evidence that central administration of GALP activates orexin neurons in the lateral hypothalamus, and immunosuppression of endogenous orexin attenuates GALP-induced feeding at 1 hour in rats [32]. However, in contrast to the DMH, GALP injections into the lateral hypothalamus do not stimulate feeding in rats [44, 66] and therefore it is unlikely that GALP has direct actions on orexin neurons in this area. Thus, in summary, the orexigenic actions of GALP in rats may be partially due to the action of NPY and orexin in the DMH and lateral hypothalamus, respectively. Furthermore, as GALP fails to activate the DMH and lateral hypothalamus in mice, this may account for the lack of an orexigenic response to GALP in this species [56]. Direct injections of GALP into the mPOA of the rat also stimulate food intake (over 1 h), leading to the possibility that the mPOA may too play a crucial role in mediating the effects of GALP [66].
Mechanisms of GALPs actions on feeding--anorexigenic effects
As discussed above, evidence suggests that the acute orexigenic actions of GALP in rats may be mediated by NPY, orexin and dopamine (see below) acting within the hypothalamus. However, GALP has dichotomous actions on feeding, in that 24h after central administration of GALP a decrease in food intake and body weight, accompanied by a rise in body temperature is observed in rats and mice. These actions of GALP are reminiscent of those observed during infection or in response to an inflammatory stimulus. Interestingly, GALP expression is increased in response to acute and chronic inflammatory stimuli. Increases in GALP mRNA in the ARC and pituitary are observed after peripheral injection of the endotoxin lipopolysacchardie (LPS; [72, 73]). LPS is a component of gram-negative bacteria cell walls, and is a potent inducer of the acute inflammatory response. Like GALP, peripheral or central administration of LPS to rodents causes anorexia, body weight loss and fever (a prostaglandin-dependent rise in body temperature; [61]). LPS stimulates the expression of pro-inflammatory mediators in the brain such as interleukin-1 (IL-1; [86], [40]), and the actions of LPS on feeding and body temperature are partially mediated by this cytokine [50, 54, 62]. Central administration of GALP also increases the expression of IL-1α and IL-1β protein in the brains of rats and mice within macrophages of the meninges and choroid plexus, as well as within microglia located in the periventricular (e.g. hypothalamic) regions [57, 58]. These IL-1-expressing microglia cells are in close proximity to hypothalamic periventricular astrocytes that are activated in response to GALP [49] and these pituicytes also express cycloxygenase, an enzyme responsible for the synthesis of prostaglandins.
Similar to LPS, the anorectic and febrile actions of GALP in rats and mice are mediated by IL-1 [58]. The alterations in food intake, body weight, and core body temperature in response to GALP in rats are attenuated by central administration of the IL-1 receptor antagonist (IL-1RA). Furthermore, mice deficient in IL-1β, IL-1α/β or the IL-1 type I receptor (IL-1RI) are partially or fully resistant to the anorectic and febrile actions of GALP. However, the acute orexigenic action of GALP observed at 1 hour in rats is not mediated by IL-1, as rats treated i.c.v. with both IL-1RA and GALP consume the same amount of food as rats treated with GALP alone [58]. These data demonstrate that IL-1α and IL-1β mediate the anorectic and febrile actions of GALP via IL-1RI in rats and mice.
b) The effect of GALP on reproduction
Takatsu et al [78] first mapped the distribution of GALP cell bodies and fibers throughout the hypothalamus. Their report showed a dense population of GALP-ir fibers throughout the mPOA, an area known to regulate feeding and reproduction. Follow-up studies demonstrated that GALP-ir fibers are in close contact with GnRH cell bodies in the diagonal band of Broca (DBB) and the mPOA [59]. Matsumoto et al [59] further went on to demonstrate that i.c.v. GALP stimulates an increase in LH secretion in intact male rats. Further studies by Cunningham et al in the macaque [11] showed that not only does i.c.v. GALP stimulate LH secretion, but GALP specifically stimulates GnRH-mediated LH secretion. Thus GALP appears to stimulate GnRH release into the pituitary portal blood that subsequently increases gonadotropin secretion. The hypothesis that GALP acts on GnRH release is further supported by the observation that GALP-ir fibers are found in the internal, but not external, zone of the median eminence [78]. Thus, GALP does not appear to be a hyperphysiotropic agent, but rather GALP regulates GnRH release. Three independent studies [18, 49, 59] demonstrated that i.c.v. GALP stimulates fos in numerous brain areas (eg. The DBB and mPOA) that regulate GnRH release as well as behavioral aspects of reproduction in male rats.
While performing the fos induction study [18], it was observed that i.c.v. GALP-injected rats exhibited an excessive amount of genital grooming compared to control animals. This observation combined with the aforementioned GALP-elicited fos induction in the mPOA, led to the hypothesis that i.c.v. GALP may also stimulate male sex behavior. To test this hypothesis, male sexual behaviors were analyzed following i.c.v. injections of either GALP or vehicle. It was observed that i.c.v. GALP significantly—and profoundly—stimulated male sex behaviors [19, 36]. Interestingly, GALP was able to significantly increase both male sex behaviors and LH secretion in both intact and castrated male rats [19]. Furthermore, reports demonstrated that i.c.v. GALP infusion could restore LH secretion and male sex behaviors in rats with uncontrolled Type I diabetes [77]. In fact, i.c.v. GALP was able to restore these reproductive variables similarly to male rats with Type I diabetes given insulin and leptin replacement therapy. Thus, it appears that—in at least male rats—GALP stimulates both hormonal and behavioral reproduction independent of the male’s testosterone milieu. GALP gene expression is regulated by insulin, leptin, and blood glucose levels and central GALP actions stimulate hormonal and behavioral reproduction in male rats—even in the absence of metabolic signals. Thus, it appears that GALP is an intermediary between metabolic signals and reproductive status in males. Since the onset of puberty is so tightly regulated by metabolic status, it was then hypothesized that GALP played a role in the timing of the onset of puberty.
To determine if GALP is involved in the timing of the onset of puberty, prepubertal male and female rats were given a constant i.c.v. infusion of GALP or vehicle [67]. Although GALP did not significantly alter the timing of the onset of puberty, GALP did increase LH secretion, feeding, metabolic rate and growth hormone secretion in male—but not female—rats. Thus, in rats it appears that GALP does not influence the timing of the onset of puberty but GALP does have sex-dependant regulatory effects on physiological changes associated with puberty [67]. Sex differences in the actions of GALP in prepubertal rats have also been demonstrated by other investigators [5]. Since GALP is known to “normalize” feeding and reproduction in rats and mice placed in negative metabolic states, in follow-up studies, researchers restricted food intake in prepubertal male and female rats to a level that is known to prevent the onset of puberty. GALP infusion in these studies showed that i.c.v. GALP could restore the onset of puberty, LH secretion, feeding and metabolic rate in male—but not female—rats [63]. Furthermore, these studies showed that i.c.v. GALP stimulates kiss1 gene expression in the ARC [63]. Kisspeptin expression in the ARC is known to be causal to, and obligatory for, the onset of puberty [34]. These data suggest a functional relationship between GALP and kisspeptin systems; however, putative interactions between GALP and kisspeptin still need to be fully explored. Regardless of potential kisspeptin involvement, GALP like leptin [6], has a gating effect on the onset of puberty but does not in and of itself stimulate the onset of puberty. These data have an apparent contradiction to recent observations that GALP knockout mice are fertile [12]. However, the lack of phenotype in knockout systems does not negate the importance of said neuropeptide. Furthermore, other neuropeptide knockouts have shown to exhibit minimal or lack of altered phenotypes due to developmental compensatory mechanisms—for example, those observed in NPY systems [21, 52]. The fact that GALP is a downstream target of leptin may diminish initial enthusiasm for its role in mediating the metabolic control of reproduction. Recently, melanin-concentrating hormone was shown to also play a role in this system [90]; however, other leptin targets such as NPY, pro-opiomelanocortin (POMC), and galanin among others have not. These observations further support the concept that GALP is an important intermediary between metabolic status and the maintenance of the reproductive system. It is also interesting to note that once again there appears to be a sex difference in GALP’s actions on regulating the onset of puberty that is dependant upon metabolic status. Explanations of these observations could include that perhaps, unlike the male, GALP effects in females may be gonadal steroid dependant. One possible explanation is that despite limited evidence [8] to the contrary, GALP neurons are regulated by gonadal steroids, such as estrogen. To date, no one has demonstrated either the presence or lack of presence of gonadal steroid receptors in GALP neurons. Alternatively, GALP’s primary target sites for effects on reproduction are themselves, sexually dimorphic. Possible neural mechanisms for GALP’s actions on reproduction and feeding will be discussed below.
Mechanisms of GALP’s actions on reproduction
In male rats, central injections of GALP have been shown to increase LH release and to activate fos expression throughout the diencephalon, including the mPOA [18, 49]). Central administration of GALP is known to stimulate GnRH-mediated LH secretion, food intake, and male-typical sexual behaviors, and many of these actions appear to be mediated via the mPOA [18, 19, 59, 66]. Furthermore in castrated males rats, i.c.v. administration of GALP stimulates sexual behaviors; however, GALP was not able to stimulate the ejaculatory response in these animals [19]. This characteristic of restoring mounts and intromissions, but not ejaculatory behaviors in castrated male rats is reminiscent of dopaminergic effects in the mPOA reported by others [3, 4, 26, 27].
Dopaminergic (DA) neurons within the anteroventral-periventricular nucleus (AVPV, incertohypothalamic system) project to the areas of the hypothalamus known to influence male-typical sexual behaviors (for review see [53]). In a recent study, Taylor et al [85] specifically eliminated dopaminergic afferents to the mPOA—presumably arising from the AVPV—and tested whether i.c.v. GALP could elicit its stimulatory effects on feeding and reproductive behaviors in male rats. It was reported that following the loss of DA input to the mPOA, i.c.v. GALP could still elicit a significant increase in LH secretion. However, i.c.v. GALP could no longer alter food intake or increase sex behavior in DA-lesioned rats compared to controls. The authors concluded that either the AVPV DA pathways are parallel to and obligatory for GALP’s effects on sex behaviors, or that the DA neurons are downstream targets of GALP neurons [85]. However, i.c.v. GALP was still able to significantly reduce body weight after 24 hrs in DA-lesioned rats similar to that reported previously by several labs [36, 41, 42, 48, 49, 55]. These observations further support the hypothesis that GALP’s effects to stimulate feeding and sex behavior are dependant upon hypothalamic dopamine neurons. However, this putative neuroendocrine pathway does not explain the known anorexigenic effects of i.c.v. GALP and the increase body temperature and metabolic rate. Figure 4 shows a schematic of the potential neuroendocrine interactions of GALP neurons with other regions and neuronal phenotypes within the diencephalon.
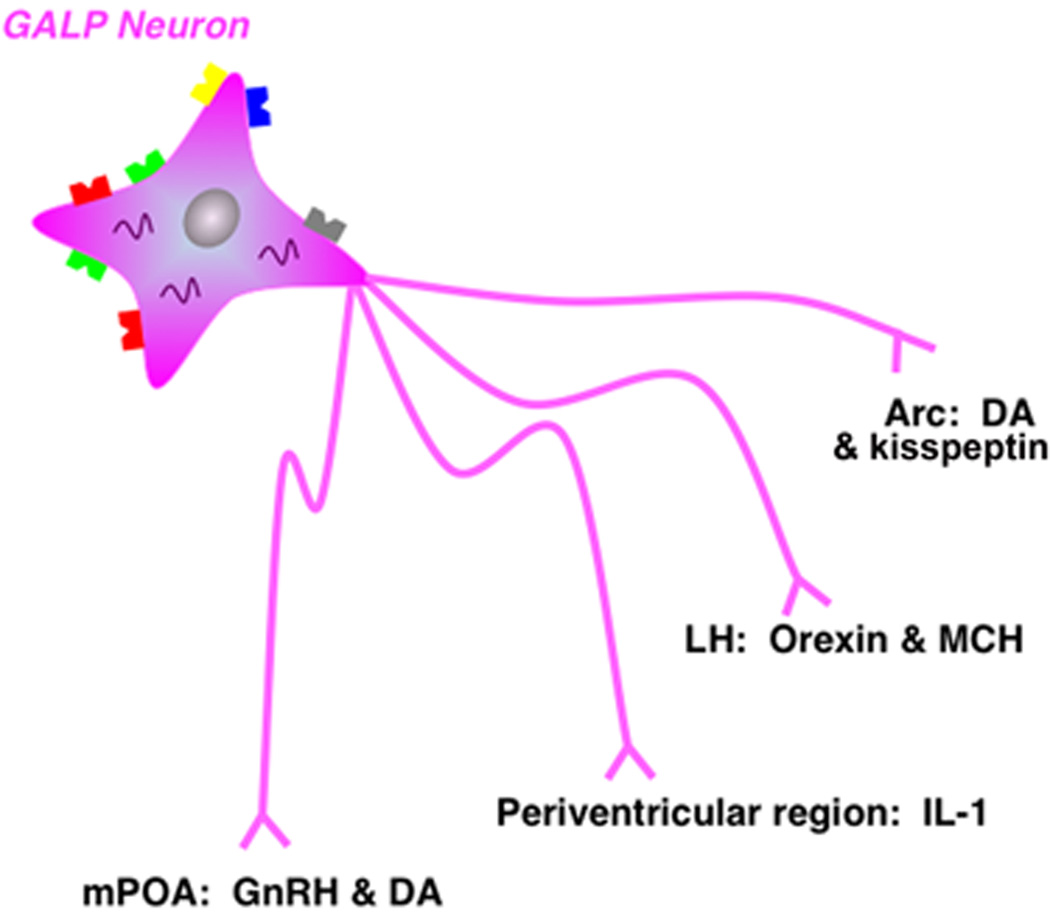
Fig. 4.
GALP innervates other diencephalic systems that regulate reproduction and energy homeostasis. A schematic diagram that illustrates the targets of hypothalamic GALP neurons and the targets’ phenotypes.
Evidence for GALP-specific Receptors
As stated above, GALP was originally identified as a potential endogenous ligand for the galanin receptors. In fact, GALP was originally shown to bind to and activate two of the galanin receptors, GalR1 and GalR2, in vitro and later, the GalR3 receptor in vivo [45]. However, there is controversy as to whether or not GALP acts through the galanin receptors in vivo. Both galanin and GALP act centrally to regulate energy homeostatic and reproductive systems. Although there is some similarity in function (e.g. stimulation of gonadotropin secretion and orexigenic effects in rats) there are also drastically opposing actions between the two peptides (e.g. GALP stimulates metabolic rate while galanin inhibits, GALP stimulates male sex behavior while galanin inhibits, GALP is anorexigenic in all species examined while galanin is only orexigenic). All of these actions of GALP are discussed in detail above. To investigate whether GALP requires the presence of the GalR1 and GalR2 receptors, Krasnow et al [42] tested the effects of exogenous GALP in galanin receptor knock-out mice. This report demonstrated that GALP had all of its functions in mice even in the absence of the GalR1 or GalR2 receptors as well as in the absence of both receptors. Furthermore, this study demonstrated that short fragments of the GALP peptide—including the galanin-related fragment—could not elicit GALP-specific effects in vivo. Thus, these authors concluded that endogenous GALP effects are not dependant upon either GalR1 or GalR2 receptors [42]. Another study tested GALP’s effects on GalR2/R3 receptors in mice and rats [55] and concluded that GALP’s effects are unlikely due to endogenous activation of either the GalR2 or GalR3 receptors. Though it is quite possible that GALP may have the ability to act through the three known galanin receptors, there is ample evidence to suggest that GALP may have its own, unique receptor. However, the identity of this receptor has yet to be determined.
Summary and Conclusion
The discovery of GALP has renewed interest and excitement in the galanin family of peptides. Galanin has long been known to influence many behavioral and physiological systems including energy homeostasis and reproduction. GALP is also involved in many of these systems, but appears to be a focal regulatory component of the interaction between metabolism and reproduction. GALP is a unique peptide compared to galanin in that GALP is only produced in one area of the CNS—namely the ARC—and the GALP gene is predominately regulated by metabolic signals. Furthermore, GALP itself appears to have a primary function in the regulation of energy homeostasis and reproduction. Thus, GALP appears to be a very important mediator between nutritional status and reproductive viability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartfai T, Fisone G, Langel U. Galanin and galanin antagonists: molecular and biochemical perspectives. Trends in pharmacological sciences. 1992;13:312. doi: 10.1016/0165-6147(92)90098-q. [DOI] [PubMed] [Google Scholar]
- 2.Bartfai T, Hokfelt T, Langel U. Galanin—a neuroendocrine peptide. Crit Rev Neurobiol. 1993;7:229–274. [PubMed] [Google Scholar]
- 3.Bitran D, Hull EM. Pharmacological analysis of male rat sexual behavior. Neurosci Biobehav Rev. 1987;11:365–389. doi: 10.1016/s0149-7634(87)80008-8. [DOI] [PubMed] [Google Scholar]
- 4.Bitran D, Hull EM, Holmes GM, Lookingland KJ. Regulation of male rat copulatory behavior by preoptic incertohypothalamic dopamine neurons. Brain Res Bull. 1988;20:323–331. doi: 10.1016/0361-9230(88)90062-7. [DOI] [PubMed] [Google Scholar]
- 5.Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Steiner RA, Aguilar E, Pinilla L, Tena-Sempere M. Effects of galanin-like peptide on luteinizing hormone secretion in the rat: sexually dimorphic responses and enhanced sensitivity at male puberty. Am J Physiol Endocrinol Metab. 2006;291:E1281–E1289. doi: 10.1152/ajpendo.00130.2006. [DOI] [PubMed] [Google Scholar]
- 6.Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 7.Crawley JN, Austin MC, Fiske SM, Martin B, Consolo S, Berthold M, Langel U, Fisone G, Bartfai T. Activity of centrally administered galanin fragments on stimulation of feeding behavior and on galanin receptor binding in the rat hypothalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:3695. doi: 10.1523/JNEUROSCI.10-11-03695.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham MJ, Krasnow SM, Gevers EF, Chen P, Thompson CK, Robinson IC, Smith MS, Clifton DK, Steiner RA. Regulation of galanin-like peptide gene expression by pituitary hormones and their downstream targets. J Neuroendocrinol. 2004;16:10–18. doi: 10.1111/j.1365-2826.2004.01118.x. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham MJ, Krasnow SM, Gevers EF, Chen P, Thompson CK, Robinson ICAF, Smith MS, Clifton DK, Steiner RA. Regulation of Galanin-Like Peptide Gene Expression By Pituitary Hormones and Their Downstream Targets. J Neuroendocrinol. 2003;15:1–9. doi: 10.1111/j.1365-2826.2004.01118.x. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham MJ, Scarlett JM, Steiner RA. Cloning and distribution of galanin-like peptide mRNA in the hypothalamus and pituitary of the macaque. Endocrinology. 2002;143:755–763. doi: 10.1210/endo.143.3.8661. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MJ, Shahab M, Grove KL, Scarlett JM, Plant TM, Cameron JL, Smith MS, Clifton DK, Steiner RA. Galanin-like peptide as a possible link between metabolism and reproduction in the macaque. J Clin Endocrinol Metab. 2004;89:1760–1766. doi: 10.1210/jc.2003-031628. [DOI] [PubMed] [Google Scholar]
- 12.Dungan Lemko HM, Clifton DK, Steiner RA, Fraley GS. Altered response to metabolic challenges in mice with genetically targeted deletions of galanin-like peptide. Am J Physiol Endocrinol Metab. 2008;295:E605–E612. doi: 10.1152/ajpendo.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dziedzic B, Szemraj J, Bartkowiak J, Walczewska A. Various dietary fats differentially change the gene expression of neuropeptides involved in body weight regulation in rats. J Neuroendocrinol. 2007;19:364–373. doi: 10.1111/j.1365-2826.2007.01541.x. [DOI] [PubMed] [Google Scholar]
- 14.Fraley GS. Immunolesions of glucoresponsive projections to the arcuate nucleus alter glucoprivic-induced alterations in food intake luteinizing hormone secretion, and GALP mRNA, but not sex behavior in adult male rats. Neuroendocrinology. 2006;83:97–105. doi: 10.1159/000094375. [DOI] [PubMed] [Google Scholar]
- 15.Fraley GS, Ritter S. Immunolesion of norepinephrine and epinephrine afferents to medial hypothalamus alters basal and 2-deoxy-D-glucose-induced neuropeptide Y and agouti gene-related protein messenger ribonucleic acid expression in the arcuate nucleus. Endocrinology. 2003;144:75–83. doi: 10.1210/en.2002-220659. [DOI] [PubMed] [Google Scholar]
- 16.Fraley GS, Scarlett JM, Shimada I, Teklemichael DN, Acohido BV, Clifton DK, Steiner RA. Effects of diabetes and insulin on the expression of galanin-like peptide in the hypothalamus of the rat. Diabetes. 2004;53:1237–1242. doi: 10.2337/diabetes.53.5.1237. [DOI] [PubMed] [Google Scholar]
- 17.Fraley GS, Scarlett JM, Teklemichael DN, Shimada I, Baumgartner JW, Acohido B, Clifton DK, Steiner RA. Regulation of Galanin-like Peptide Gene Expression by Insulin and Metabolic Fuels in the Hypothalamus of the Rat. Endocrine Society Annual Meeting; Philadelphia, PA. 2003. [Google Scholar]
- 18.Fraley GS, Shimada I, Baumgartner JW, Clifton DK, Steiner RA. Differential patterns of Fos induction in the hypothalamus of the rat following central injections of galanin-like peptide and galanin. Endocrinology. 2003;144:1143–1146. doi: 10.1210/en.2002-0114. [DOI] [PubMed] [Google Scholar]
- 19.Fraley GS, Thomas-Smith SE, Acohido BV, Steiner RA, Clifton DK. Stimulation of sexual behavior in the male rat by galanin-like peptide. Hormones and Behavior. 2004;46:551–557. doi: 10.1016/j.yhbeh.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara K, Adachi S, Usui K, Maruyama M, Matsumoto H, Ohtaki T, Kitada C, Onda H, Fujino M, Inoue K. Immunocytochemical localization of a galanin-like peptide (GALP) in pituicytes of the rat posterior pituitary gland. Neurosci Lett. 2002;317:65–68. doi: 10.1016/s0304-3940(01)02445-4. [DOI] [PubMed] [Google Scholar]
- 21.Gehlert DR, Thompson LK, Hemrick-Luecke SK, Shaw J. Monoaminergic compensation in the neuropeptide Y deficient mouse brain. Neuropeptides. 2008;42:367–375. doi: 10.1016/j.npep.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Guan JL, Kageyama H, Wang QP, Takenoya F, Kita T, Matsumoto H, Ohtaki T, Shioda S. Electron microscopy examination of galanin-like peptide (GALP)-containing neurons in the rat hypothalamus. Regul Pept. 2005;126:73–78. doi: 10.1016/j.regpep.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Gundlach AL, Burazin TC, Larm JA. Distribution, regulation and role of hypothalamic galanin systems: renewed interest in a pleiotropic peptide family. Clin Exp Pharmacol Physiol. 2001;28:100–105. doi: 10.1046/j.1440-1681.2001.03411.x. [DOI] [PubMed] [Google Scholar]
- 24.Hansen KR, Krasnow SM, Nolan MA, Fraley GS, Baumgartner JW, Clifton DK, Steiner RA. Activation of the sympathetic nervous system by galanin-like peptide--a possible link between leptin and metabolism. Endocrinology. 2003;144:4709–4717. doi: 10.1210/en.2003-0748. [DOI] [PubMed] [Google Scholar]
- 25.Hatton GI. Pituicytes, glia and control of terminal secretion. J Exp Biol. 1988;139:67–79. doi: 10.1242/jeb.139.1.67. [DOI] [PubMed] [Google Scholar]
- 26.Hull EM, Bitran D, Pehek EA, Warner RK, Band LC, Holmes GM. Dopaminergic control of male sex behavior in rats: effects of an intracerebrally-infused agonist. Brain Res. 1986;370:73–81. doi: 10.1016/0006-8993(86)91106-6. [DOI] [PubMed] [Google Scholar]
- 27.Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iismaa TP, Shine J. Galanin and galanin receptors. Results Probl Cell Differ. 1999;26:257–291. doi: 10.1007/978-3-540-49421-8_12. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal J, Manley TR, Ciofi P, Clarke IJ. Reduction in adiposity affects the extent of afferent projections to growth hormone-releasing hormone and somatostatin neurons and the degree of colocalization of neuropeptides in growth hormone-releasing hormone and somatostatin cells of the ovine hypothalamus. Endocrinology. 2005;146:4776–4785. doi: 10.1210/en.2005-0622. [DOI] [PubMed] [Google Scholar]
- 30.Juréus A, Cunningham MJ, Li D, Johnson LL, Krasnow SM, Teklemichael DN, Clifton DK, Steiner RA. Distribution and regulation of galanin-like peptide (GALP) in the hypothalamus of the mouse. Endocrinology. 2001;142:5140–5144. doi: 10.1210/endo.142.12.8542. [DOI] [PubMed] [Google Scholar]
- 31.Juréus A, Cunningham MJ, McClain ME, Clifton DK, Steiner RA. Galanin-like peptide (GALP) is a target for regulation by leptin in the hypothalamus of the rat. Endocrinology. 2000;141:2703–2706. doi: 10.1210/endo.141.7.7669. [DOI] [PubMed] [Google Scholar]
- 32.Kageyama H, Kita T, Toshinai K, Guan JL, Date Y, Takenoya F, Kato S, Matsumoto H, Ohtaki T, Nakazato M, Shioda S. Galanin-like peptide promotes feeding behaviour via activation of orexinergic neurones in the rat lateral hypothalamus. J Neuroendocrinol. 2006;18:33–41. doi: 10.1111/j.1365-2826.2005.01382.x. [DOI] [PubMed] [Google Scholar]
- 33.Kageyama H, Takenoya F, Hori Y, Yoshida T, Shioda S. Morphological interaction between galanin-like peptide- and dopamine-containing neurons in the rat arcuate nucleus. Regulatory peptides. 2008;145:165. doi: 10.1016/j.regpep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser UB, Kuohung W. KiSS-1 and GPR54 as new players in gonadotropin regulation and puberty. Endocrine. 2005;26:277–284. doi: 10.1385/ENDO:26:3:277. [DOI] [PubMed] [Google Scholar]
- 35.Kastin AJ, Akerstrom V, Hackler L. Food deprivation decreases blood galanin-like peptide and its rapid entry into the brain. Neuroendocrinology. 2001;74:423–432. doi: 10.1159/000054708. [DOI] [PubMed] [Google Scholar]
- 36.Kauffman AS, Buenzle J, Fraley GS, Rissman EF. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm Behav. 2005;48:141–151. doi: 10.1016/j.yhbeh.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Kawagoe R, Yamamoto Y, Kubo K, Dobashi K, Asayama K, Ueta Y, Shirahata A. Postnatal development of galanin-like peptide mRNA expression in rat hypothalamus. Regul Pept. 2008;145:133–140. doi: 10.1016/j.regpep.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Kawasaki M, Saito J, Hashimoto H, Suzuki H, Otsubo H, Fujihara H, Ohnishi H, Nakamura T, Ueta Y. Induction of the galanin-like peptide gene expression in the posterior pituitary gland after acute osmotic stimulus in rats. Neurosci Lett. 2007;419:125–130. doi: 10.1016/j.neulet.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Koegler FH, Ritter S. Feeding induced by pharmacological blockade of fatty acid metabolism is selectively attenuated by hindbrain injections of the galanin receptor antagonist, M40. Obes Res. 1996;4:329–336. doi: 10.1002/j.1550-8528.1996.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 40.Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–548. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- 41.Krasnow SM, Fraley GS, Schuh SM, Baumgartner JW, Clifton DK, Steiner RA. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology. 2003;144:813–822. doi: 10.1210/en.2002-220982. [DOI] [PubMed] [Google Scholar]
- 42.Krasnow SM, Hohmann JG, Gragerov A, Clifton DK, Steiner RA. Analysis of the contribution of galanin receptors 1 and 2 to the central actions of galanin-like peptide. Neuroendocrinology. 2004;79:268–277. doi: 10.1159/000079632. [DOI] [PubMed] [Google Scholar]
- 43.Kumano S, Matsumoto H, Takatsu Y, Noguchi J, Kitada C, Ohtaki T. Changes in hypothalamic expression levels of galanin-like peptide in rat and mouse models support that it is a leptin-target peptide. Endocrinology. 2003;144:2634–2643. doi: 10.1210/en.2002-221113. [DOI] [PubMed] [Google Scholar]
- 44.Kuramochi M, Onaka T, Kohno D, Kato S, Yada T. Galanin-like peptide stimulates food intake via activation of neuropeptide Y neurons in the hypothalamic dorsomedial nucleus of the rat. Endocrinology. 2006;147:1744–1752. doi: 10.1210/en.2005-0907. [DOI] [PubMed] [Google Scholar]
- 45.Lang R, Berger A, Santic R, Geisberger R, Hermann A, Herzog H, Kofler B. Pharmacological and functional characterization of galanin-like peptide fragments as potent galanin receptor agonists. Neuropeptides. 2005;39:179–184. doi: 10.1016/j.npep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 47.Larm JA, Gundlach AL. Galanin-like peptide (GALP) mRNA expression is restricted to arcuate nucleus of hypothalamus in adult male rat brain. Neuroendocrinology. 2000;72:67–71. doi: 10.1159/000054573. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence CB, Baudoin FM, Luckman SM. Centrally administered galanin-like peptide modifies food intake in the rat: a comparison with galanin. J Neuroendocrinol. 2002;14:853–860. doi: 10.1046/j.1365-2826.2002.00846.x. [DOI] [PubMed] [Google Scholar]
- 49.Lawrence CB, Williams T, Luckman SM. Intracerebroventricular galanin-like peptide induces different brain activation compared with galanin. Endocrinology. 2003;144:3977–3984. doi: 10.1210/en.2003-0391. [DOI] [PubMed] [Google Scholar]
- 50.Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–R98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- 51.Li AJ, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci. 2004;19:2147–2154. doi: 10.1111/j.1460-9568.2004.03287.x. [DOI] [PubMed] [Google Scholar]
- 52.Lin S, Boey D, Couzens M, Lee N, Sainsbury A, Herzog H. Compensatory changes in [125I]-PYY binding in Y receptor knockout mice suggest the potential existence of further Y receptor(s) Neuropeptides. 2005;39:21–28. doi: 10.1016/j.npep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luheshi G, Miller AJ, Brouwer S, Dascombe MJ, Rothwell NJ, Hopkins SJ. Interleukin-1 receptor antagonist inhibits endotoxin fever and systemic interleukin-6 induction in the rat. Am J Physiol. 1996;270:E91–E95. doi: 10.1152/ajpendo.1996.270.1.E91. [DOI] [PubMed] [Google Scholar]
- 55.Man PS, Lawrence CB. The effects of galanin-like peptide on energy balance, body temperature and brain activity in the mouse and rat are independent of the GALR2/3 receptor. J Neuroendocrinol. 2008;20:128–137. doi: 10.1111/j.1365-2826.2007.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Man PS, Lawrence CB. The Effects of Galanin-Like Peptide on Energy Balance, Body Temperature and Brain Activity in the Mouse and Rat Are Independent of the GALR2/3 Receptor. Journal of neuroendocrinology. 2008;20:128. doi: 10.1111/j.1365-2826.2007.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Man PS, Lawrence CB. Galanin-like peptide: a role in the homeostatic regulation of energy balance? Neuropharmacology. 2008;55:1–7. doi: 10.1016/j.neuropharm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Man PS, Lawrence CB. Interleukin-1 mediates the anorexic and febrile actions of galanin-like Peptide. Endocrinology. 2008;149:5791–5802. doi: 10.1210/en.2008-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumoto H, Noguchi J, Takatsu Y, Horikoshi Y, Kumano S, Ohtaki T, Kitada C, Itoh T, Onda H, Nishimura O, Fujino M. Stimulation effect of galanin-like peptide (GALP) on luteinizing hormone-releasing hormone-mediated luteinizing hormone (LH) secretion in male rats. Endocrinology. 2001;142:3693–3696. doi: 10.1210/endo.142.8.8432. [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto Y, Watanabe T, Adachi Y, Itoh T, Ohtaki T, Onda H, Kurokawa T, Nishimura O, Fujino M. Galanin-like peptide stimulates food intake in the rat. Neurosci Lett. 2002;322:67–69. doi: 10.1016/s0304-3940(01)02515-0. [DOI] [PubMed] [Google Scholar]
- 61.McCarthy DO, Kluger MJ, Vander AJ. The role of fever in appetite suppression after endotoxin administration. Am J Clin Nutr. 1984;40:310–316. doi: 10.1093/ajcn/40.2.310. [DOI] [PubMed] [Google Scholar]
- 62.Miller AJ, Hopkins SJ, Luheshi GN. Sites of action of IL-1 in the development of fever and cytokine responses to tissue inflammation in the rat. Br J Pharmacol. 1997;120:1274–1279. doi: 10.1038/sj.bjp.0701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohr M, Madison FN, Fraley GS. Galanin-like peptide is sufficient to maintain the onset of puberty in food restricted rats. Society for Neuroscience Annual Meeting; Washington D.C.. 2008. [Google Scholar]
- 64.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 65.Ohtaki T, Kumano S, Ishibashi Y, Ogi K, Matsui H, Harada M, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J Biol Chem. 1999;274:37041–37045. doi: 10.1074/jbc.274.52.37041. [DOI] [PubMed] [Google Scholar]
- 66.Patterson M, Murphy KG, Thompson EL, Smith KL, Meeran K, Ghatei MA, Bloom SR. Microinjection of galanin-like peptide into the medial preoptic area stimulates food intake in adult male rats. J Neuroendocrinol. 2006;18:742–747. doi: 10.1111/j.1365-2826.2006.01473.x. [DOI] [PubMed] [Google Scholar]
- 67.Rich N, Reyes P, Reap L, Goswami R, Fraley GS. Sex differences in the effect of prepubertal GALP infusion on growth, metabolism and LH secretion. Physiol Behav. 2007;92:814–823. doi: 10.1016/j.physbeh.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 69.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- 70.Robinson JK, Bartfai T, Langel U. Galanin/GALP receptors and CNS homeostatic processes. CNS Neurol Disord Drug Targets. 2006;5:327–334. doi: 10.2174/187152706777452281. [DOI] [PubMed] [Google Scholar]
- 71.Saito J, Ozaki Y, Kawasaki M, Ohnishi H, Okimoto N, Nakamura T, Ueta Y. Galanin-like peptide gene expression in the hypothalamus and posterior pituitary of the obese fa/fa rat. Peptides. 2004;25:967–974. doi: 10.1016/j.peptides.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Saito J, Ozaki Y, Kawasaki M, Ohnishi H, Okimoto N, Nakamura T, Ueta Y. Induction of galanin-like peptide gene expression in the arcuate nucleus of the rat after acute but not chronic inflammatory stress. Brain Res Mol Brain Res. 2005;133:233–241. doi: 10.1016/j.molbrainres.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 73.Saito J, Ozaki Y, Ohnishi H, Nakamura T, Ueta Y. Induction of galanin-like peptide gene expression in the rat posterior pituitary gland during endotoxin shock and adjuvant arthritis. Brain Res Mol Brain Res. 2003;113:124–132. doi: 10.1016/s0169-328x(03)00129-3. [DOI] [PubMed] [Google Scholar]
- 74.Santic R, Schmidhuber SM, Lang R, Rauch I, Voglas E, Eberhard N, Bauer JW, Brain SD, Kofler B. Alarin is a vasoactive peptide. Proc Natl Acad Sci U S A. 2007;104:10217–10222. doi: 10.1073/pnas.0608585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seth A, Stanley S, Dhillo W, Murphy K, Ghatei M, Bloom S. Effects of galanin-like peptide on food intake and the hypothalamo-pituitary-thyroid axis. Neuroendocrinology. 2003;77:125–131. doi: 10.1159/000068648. [DOI] [PubMed] [Google Scholar]
- 76.Shen J, Larm JA, Gundlach AL. Galanin-like peptide mRNA in neural lobe of rat pituitary. Increased expression after osmotic stimulation suggests a role for galanin-like peptide in neuron-glial interactions and/or neurosecretion. Neuroendocrinology. 2001;73:2–11. doi: 10.1159/000054615. [DOI] [PubMed] [Google Scholar]
- 77.Stoyanovitch AG, Johnson MA, Clifton DK, Steiner RA, Fraley GS. Galanin-like peptide rescues reproductive function in the diabetic rat. Diabetes. 2005;54:2471–2476. doi: 10.2337/diabetes.54.8.2471. [DOI] [PubMed] [Google Scholar]
- 78.Takatsu Y, Matsumoto H, Ohtaki T, Kumano S, Kitada C, Onda H, Nishimura O, Fujino M. Distribution of galanin-like peptide in the rat brain. Endocrinology. 2001;142:1626–1634. doi: 10.1210/endo.142.4.8089. [DOI] [PubMed] [Google Scholar]
- 79.Takenoya F, Aihara K, Funahashi H, Matsumoto H, Ohtaki T, Tsurugano S, Yamada S, Katoh S, Kageyama H, Takeuchi M, Shioda S. Galanin-like peptide is target for regulation by orexin in the rat hypothalamus. Neurosci Lett. 2003;340:209–212. doi: 10.1016/s0304-3940(03)00120-4. [DOI] [PubMed] [Google Scholar]
- 80.Takenoya F, Funahashi H, Matsumoto H, Ohtaki T, Katoh S, Kageyama H, Suzuki R, Takeuchi M, Shioda S. Galanin-like peptide is co-localized with alpha-melanocyte stimulating hormone but not with neuropeptide Y in the rat brain. Neurosci Lett. 2002;331:119. doi: 10.1016/s0304-3940(02)00867-4. [DOI] [PubMed] [Google Scholar]
- 81.Takenoya F, Guan JL, Kato M, Sakuma Y, Kintaka Y, Kitamura Y, Kitamura S, Okuda H, Takeuchi M, Kageyama H, Shioda S. Neural interaction between galanin-like peptide (GALP)- and luteinizing hormone-releasing hormone (LHRH)-containing neurons. Peptides. 2006;27:2885–2893. doi: 10.1016/j.peptides.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 82.Takenoya F, Hirayama M, Kageyama H, Funahashi H, Kita T, Matsumoto H, Ohtaki T, Katoh S, Takeuchi M, Shioda S. Neuronal interactions between galanin-like-peptide- and orexin- or melanin-concentrating hormone-containing neurons. Regul Pept. 2005;126:79–83. doi: 10.1016/j.regpep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Tan HM, Gundlach AL, Morris MJ. Exaggerated feeding response to central galanin-like peptide administration in diet-induced obese rats. Neuropeptides. 2005;39:333–336. doi: 10.1016/j.npep.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 84.Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 85.Taylor A, Madison FN, Fraley GS. Galanin-like peptide stimulates feeding and sexual behaviors via dopaminergic fibers within the medial preoptic area of adult male rats. Journal of Chemical Neuroanatomy. 2009;37:105–111. doi: 10.1016/j.jchemneu.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 86.van Dam AM, Poole S, Schultzberg M, Zavala F, Tilders FJ. Effects of peripheral administration of LPS on the expression of immunoreactive interleukin-1 alpha, beta, and receptor antagonist in rat brain. Ann N Y Acad Sci. 1998;840:128–138. doi: 10.1111/j.1749-6632.1998.tb09557.x. [DOI] [PubMed] [Google Scholar]
- 87.Wang J, Akabayashi A, Yu HJ, Dourmashkin J, Alexander JT, Silva I, Lighter J, Leibowitz SF. Hypothalamic galanin: control by signals of fat metabolism. Brain research. 1998;804:7. doi: 10.1016/s0006-8993(98)00632-5. [DOI] [PubMed] [Google Scholar]
- 88.Whitelaw CM, Robinson JE, Chambers GB, Hastie P, Padmanabhan V, Thompson RC, Evans NP. Expression of mRNA for galanin, galanin-like peptide and galanin receptors 1–3 in the ovine hypothalamus and pituitary gland: effects of age and gender. Reproduction. 2009;137:141–150. doi: 10.1530/REP-08-0266. [DOI] [PubMed] [Google Scholar]
- 89.Wilgenbusch JC, Swofford D. Inferring evolutionary trees with PAUP*. Curr Protoc Bioinformatics. 2003;Chapter 6 doi: 10.1002/0471250953.bi0604s00. Unit 6 4. [DOI] [PubMed] [Google Scholar]
- 90.Wu M, Dumalska I, Morozova E, van den Pol A, Alreja M. Melanin-concentrating hormone directly inhibits GnRH neurons and blocks kisspeptin activation, linking energy balance to reproduction. Proc Natl Acad Sci U S A. 2009;106:17217–17222. doi: 10.1073/pnas.0908200106. [DOI] [PMC free article] [PubMed] [Google Scholar]