Abstract
The type II secretion system (T2SS) is a macromolecular complex spanning the inner and outer membranes of Gram-negative bacteria. Remarkably, the T2SS secretes folded proteins including multimeric assemblies like cholera toxin and heat-labile enterotoxin from Vibrio cholerae and enterotoxigenic Escherichia coli, respectively. The major outer membrane T2SS protein is the “secretin” GspD. Electron cryomicroscopy reconstruction of the V. cholerae secretin at 19 Å resolution reveals a dodecameric structure reminiscent of a barrel with a large channel at its center that appears to contain a closed periplasmic gate. The GspD periplasmic domain forms a vestibule with a conserved constriction, and binds to a pentameric exoprotein and to the trimeric tip of the T2SS pseudopilus. By combining our results with structures of the cholera toxin and T2SS pseudopilus, we provide a structural basis for a possible secretion mechanism of the T2SS.
Keywords: secretin, GspD, electron cryomicroscopy (cryoEM), type II secretion system (T2SS), XcpQ
One or more variants of the type II secretion system (T2SS) occur in many proteobacteria including several important pathogens of humans and plants1. One such pathogenic bacterium is Vibrio cholerae which uses its T2SS to secrete, amongst other proteins, the major virulence factor cholera toxin (CT) in a folded state from the periplasm across the outer membrane2,3. Heat-labile enterotoxin (LT), produced by enterotoxigenic Escherichia coli (ETEC) is related to CT in sequence and structure, and is also secreted by a T2SS4,5. Both CT and LT are heterohexamers composed of one A and 5 B subunits (AB5). Although the secretion signals of proteins secreted by the T2SS are still unknown, in the case of both the CT and LT toxins the secretion signal resides in the B-pentamer and not in the A-subunit6,7. Due to the apparent lack of a linear secretion signal sequence it has been suggested that the secretion signal is instead a structural motif present in the native or near-native protein structure8-11.
The T2SS is a multi-protein macromolecular machine that spans both the inner and outer membrane of the bacteria12,13. On its cytosolic side, the T2SS contains a secretion ATPase called GspE that is associated with an inner membrane platform comprising multiple copies of the proteins GspL, GspM, GspF and GspC. A second T2SS sub-assembly is the so-called pseudopilus consisting of the pseudopilins GspG, GspH, GspI, GspJ and GspK. The pseudopilus is thought to act as a plug and/or piston during the secretion of proteins and of toxins14-16.
The major outer membrane protein of the T2SS is the “secretin” GspD, which forms one of the largest multimeric assemblies in the outer membrane of bacteria. In Pseudomonas species, GspD is also called XcpQ; in Vibrio species EpsD. The secretins form a diverse family of proteins, occurring in a wide variety of bacteria, with a complex and variable multi-domain structure. Homologues of the T2SS-related secretins are critically important components of other macromolecular assemblies such as the type III secretion system (T3SS), the type IV pilus biogenesis system (T4PBS) and the filamentous phage assembly system17-26. These systems occur in a wide variety of bacteria and perform a large number of sophisticated functions, including bacteriophage extrusion, pilus extension and retraction, twitching motility, DNA uptake, injection of proteins directly into target cells, and secretion of proteins into the extracellular milieu.
Here we used electron cryomicroscopy (cryoEM) and single particle reconstruction to determine the 19 Å resolution structure of the T2SS secretin GspD from V. cholerae (VcGspD). Previous secretin reconstructions determined by cryoelectron microscopy have been reported with comparable resolutions23,21, however the VcGspD reconstruction reported here is structurally the most complete representation of a T2SS secretin to date. The V. cholerae secretin appears as a 200 Å tall dodecameric channel with a largely unobstructed periplasmic vestibule that contains a constriction site and is separated by a gate from an extracellular chamber that is connected to the extracellular milieu by a cap. Crystal structures of periplasmic GspD subdomains fit well within the contoured features of the cryoEM map. This conserved periplasmic domain is shown to bind to a T2SS exoprotein and also to the pseudopilus tip complex. By combining our results with structural data on a large exoprotein, the ∼85 kDa cholera toxin, and our recent crystal structure of the tip of the pseudopilus of the T2SS, we provide a structural basis for a possible secretion mechanism of exoproteins by the T2SS in which the secretin constriction site plays a critical role.
Results
Electron cryomicroscopy of recombinant V. cholerae GspD
Full-length VcGspD was over-expressed in E. coli. Intact channels were isolated from the membrane fraction and purified to homogeneity (Fig. 1a and b). The suitability of the preparation for analysis under cryogenic conditions was initially assayed by negative stain electron microscopy (data not shown). Purified VcGspD was prepared for cryomicroscopy using a vitrification robot (Vitrobot, FEI) and continuous carbon copper grids rather then holey carbon grids to circumvent particle orientation difficulties in ice (Fig. 1c). Approximately 20,000 vitrified VcGspD particles were initially selected in Ximdisp27 from electron micrographs and classified using reference-free computational alignment routines in SPIDER (Fig. 1c, inset)28.
Figure 1. Purification and electron cryomicroscopy of cholera toxin secretion channel VcGspD.
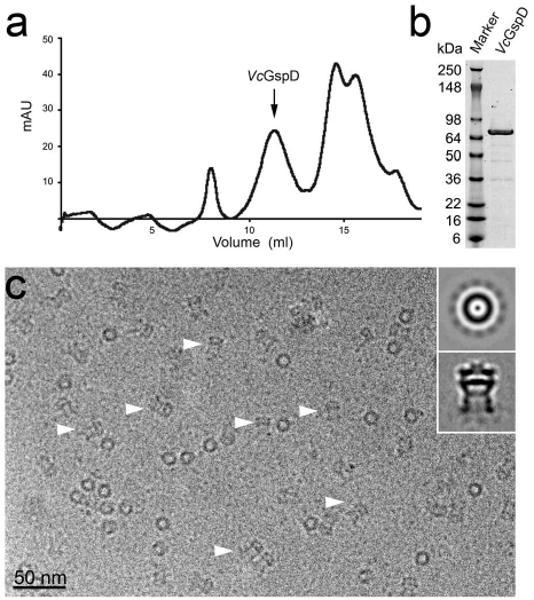
(a) VcGspD channels were purified to homogeneity by size-exclusion chromatography. Intact channels eluted as ∼0.9 MDa species (arrow). (b) Coomassie blue stained SDS-PAGE of purified VcGspD (∼74 kDa). (c) Electron micrograph of vitrified VcGspD channels. (inset, top panel) Representative class averages of VcGspD particles. In top views, looking down the channel axis, the particles appear as a ring with a punctate density located at the center of the channel and weaker densities “spokes” surround the channel. Side views (indicated with arrow heads in (c)) appear as long striated channels with a strong central density or “plug” that bisects the center of the channel (inset, bottom panel).
In side views, the VcGspD particles appear as striated elongated structures (indicated by arrow-heads in Fig. 1c and inset bottom panel). A strong band of density bisects the center of the VcGspD particle, and appears to separate the periplasmic and the extracellular domains of the channel. When viewed axially, along the channel pore axis, the VcGspD appears as a ring of density with weak radial densities, or “spokes” (Fig. 1c, inset top panel). These radial spokes were used to assign the rotational symmetry to VcGspD channels using real-space symmetry averaging and rotational power spectra analysis29 (Supplementary Fig. 1). This analysis strongly suggests that VcGspD is a dodecamer and is in agreement with mass estimates by size exclusion chromatography.
The three-dimensional (3D) reconstruction of VcGspD was performed in FREALIGN30 using the best ∼10,000 particles with an applied 12-fold rotational symmetry. The resulting density is ∼19 Å in resolution as determined by the 0.5 Fourier shell correlation (FSC) criteria (Supplementary Fig. 2). Two-dimensional back-projections of the VcGspD cryoEM map demonstrated a very good fit to the raw image data (data not shown). The density map was contoured at 3.4σ above noise, yielding a ∼0.9 MDa molecular mass consistent with 12 subunits of VcGspD per particle.
Architecture of the VcGspD secretin channel
The VcGspD cryoEM density reveals a cylindrical channel assembly, ∼155 Å in diameter, ∼200 Å in length and a girdled waist (Fig. 2). In side view, the periplasmic domain of the channel is at the bottom followed by an outer membrane domain, and an extracellular domain that is at the very top. The surface of the outer membrane domain appears smooth (possibly due to detergent and/or lipid molecules surrounding the transmembrane domain of the channel) while the surface of the periplasmic domain appears highly contoured and segmented with clear 12-fold symmetric features. The 12-fold radial spoke densities observed in projection averages are found radiating off the bottom of the periplasmic domain when the map is displayed at a slightly lower contour level (Fig. 1c, inset compared with Fig. 2c and Supplementary Fig. 1). These spoke features radiating off the periplasmic domain likely correspond to the disordered N-terminal signaling sequence that is retained by VcGspD in our E. coli expression system (see Methods section). Projection views of the VcGspD reconstruction reveal additional 12-fold symmetric features that can be observed throughout the architecture of the channel (Supplementary Fig. 1).
Figure 2. Three-dimensional electron cryomicroscopy reconstruction of VcGspD.

(a, c and d) Side, top and bottom views, respectively, of the dodecameric VcGspD reconstruction at 19 Å resolution. In side view, three domains are identified from bottom to top as the periplasmic domain, the outer membrane domain and extracellular cap. (b) A slice view through the channel reveals the periplasmic vestibule, a constriction, the periplasmic gate, an extracellular chamber and an extracellular gate. The extracellular chamber is ∼100 Å wide whereas the periplasmic vestibule is ∼75 Å in diameter. The periplasmic constriction narrows the vestibule to ∼55 Å.
Additional features become apparent in a cross section through the VcGspD channel (Fig. 2d). The periplasmic domain of the channel accounts for ∼125 Å out of the total 200 Å length of the channel. The opening to the periplasmic vestibule is ∼75 Å in diameter while its walls are ∼40 Å thick. Approximately two-thirds of the way into the periplasmic vestibule a constriction is identified that narrows the channel's internal diameter to ∼55 Å. A continuous density, which we refer to as the periplasmic gate, seals off the periplasmic vestibule from the extracellular chamber of the channel.
The outer membrane and the extracellular domains of VcGspD account for 75 Å out of the total 200 Å length of the channel. Immediately following the periplasmic gate, the extracellular domain of VcGspD appears as a chamber ∼100 Å in diameter (Fig. 2d). A puckered density that forms a second gate structure caps the top of this chamber. We refer to this second gate as the extracellular gate. In sharp contrast with the periplasmic gate that appears as a solid density, the extracellular gate seems to leave a small opening of ∼10 Å in diameter suggesting that this gate does not form a tight seal across the channel pore. The walls surrounding the extracellular chamber are ∼20 Å thick and appear smooth, possibly due to the presence of detergent and/or lipid molecules as discussed above, but also consistent with the predicted β-barrel topology for this domain of VcGspD and related secretins18.
Global aspects of our VcGspD architecture agree with the cryomicroscopy reconstruction23 of the T2SS secretin from Klebsiella oxytoca (KoGspD), also called PulD. The non-trypsinized KoGspD multimer was also a 12-fold symmetric channel with outer and inner diameters as observed in the VcGspD structure. However, the membrane-spanning segment of KoGspD was relatively poorly defined and the periplasmic vestibule of VcGspD is ∼80 Å longer than in the KoGspD study (Supplementary Fig. 3). This additional density has allowed us to place the recently determined crystal structures of the N-terminal subdomains of EcGspD31 into the VcGspD electron microscopy reconstruction as discussed below. The entrance to the periplasmic vestibule in the KoGspD reconstruction was ∼55 Å wide which we can now attribute to the constriction site in our more complete reconstruction. The extracellular gate in our VcGspD reconstruction is a novel feature that has not been previously observed in reported secretin reconstructions. We propose a critical role for this domain in the secretion mechanism of the T2SS as the final channel gate that allows exoprotein release into the extracellular environment (see Discussion).
The secretin architecture in diverse secretion systems
Members of the bacterial outer membrane secretin family are found in the T2SS, the T3SS, the T4PBS, and the filamentous phage secretion systems17,18. Amino acid sequence analysis was used to compare the domain organization of secretins from these four secretion systems (Fig. 3a and Supplementary Fig. 4). All members of the secretin family contain a defining large C-terminal core domain, called the “secretin domain” (Pfam family PF0026332, cyan in Fig. 3a and Supplementary Fig. 4). Variable regions, that are secretion system and species-specific, flank this defining core domain. The N-terminal region of members of the secretin family is modular and all of these secretins minimally contain (i) the N-terminal N0-like and (ii) the N3-like subdomains directly preceding the C-terminal defining secretin domain (Fig. 3a). These sequence-based analyses indicate a both considerable similarity and substantial differences in domain organization of secretins in the outer membrane of many bacterial secretion/biogenesis systems including the secretins from the T2SS, T3SS, T4BPS and the filamentous phage assembly system.
Figure 3. The secretin architecture is conserved in different secretion systems.
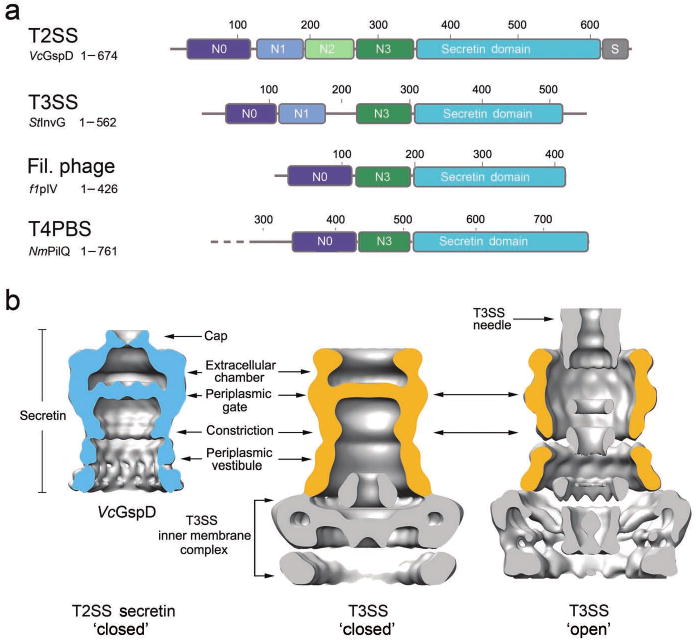
(a) Domain architecture of secretins from the T2SS, the T3SS, the filamentous phage assembly system and the T4 pilus biogenesis system (T4PBS). Members of the secretin super-family contain a C-terminal secretin core homology domain (cyan)17,18. The T2SS secretins generally contain four periplasmic subdomains, termed N0-N3. The N0 subdomain (dark blue) is located at the N-terminus and is followed by the three structurally homologous subdomains, N1-N3 (blue, green and dark green, respectively). A T2SS specific domain, termed the S-domain (grey), is located at the very C-terminus. Secretins from other systems share a similar architecture, composed of the secretin domain and at least two periplasmic subdomains that are structurally equivalent to N0 and N3 of VcGspD. (b) Structural comparison of the VcGspD density (blue, left) to single particle reconstructions of the T3SS in its close state (center) (EMDB 122426) and to the fully assembled T3SS needle complex in its open state (right) (EMDB 161724). The outer membrane T3SS secretin sits on top of a large inner membrane complex (gold, center and right). VcGspD appears to be in its closed state (left compared with center).
The domain architecture of secretins from the T2SS and T3SS appear most similar among these four systems, both containing N-terminal modules named the N0, N1 and N3 subdomains (Fig. 3a and Supplementary Fig. 4). The N0 subdomains of the T2SS and T3SS assume the same topology despite low amino acid sequence identity, with a fold related to the signaling domain of TonB-dependent receptors and bacteriophage tail proteins31,33. The structures of the T2SS N1 and N2 subdomains have the same KH domain fold and it has been predicted that the T2SS N3 subdomain has the same structure, which is also shared with the T3SS N1 and N3 subdomains (Supplementary Figs. 4 & 5). Interestingly, T3SS secretins lack the N2 subdomain that is found in the T2SS secretins.
Secretins from the T3SS have been studied by single particle electron microscopy as part of the assembled T3SS complex, in both its closed and open states (Fig. 3b)24-26. Like the T2SS, the T3SS is a large multi-protein complex that spans both the inner and outer membrane of bacteria. The T3SS base complex mainly consists of the outer membrane secretin, sitting on top of a T3SS inner membrane complex (Fig. 3b, center). The cryoEM reconstruction of the T2SS VcGspD secretin resembles that of the T3SS secretin in the closed state without needle (Fig. 3b, left compared with center). In both structures the large periplasmic gate appears closed and the ∼55 Å wide periplasmic constriction is apparent, approximately equally distant from the periplasmic gate. The reconstructions of the fully assembled T3SS 24,26 noticeably lack the extracellular gate observed in the VcGspD secretin. This feature may therefore be specific to the T2SS secretins, however, it is also possible that this domain was not resolved in the available T3SS reconstructions. Further structural studies will be required to dissect these structural differences.
The outer membrane pore-forming channel from the type IVa secretion system (T4aSS) has been recently characterized by X-ray crystallography34 and as part of the assembled T4aSS by cryoEM35. The T4aSS outer membrane channels do not appear genetically related to the secretin family of outermembrane channels34 (and indeed appear overall structurally unique, and also different from the type IVb secretion system36-38) nevertheless a side-by-side structural comparison does reveals some common global structural outside shape features), yet, the internal shape is distinctly different (compare e.g. Fig. 3b with Fig. 4c from ref. 34; Supplementary Fig. 6).
Figure 4. The periplasmic GspD domain contains a conserved N3 constriction and binds to the T2SS exoprotein and pseudopilus tip complex.
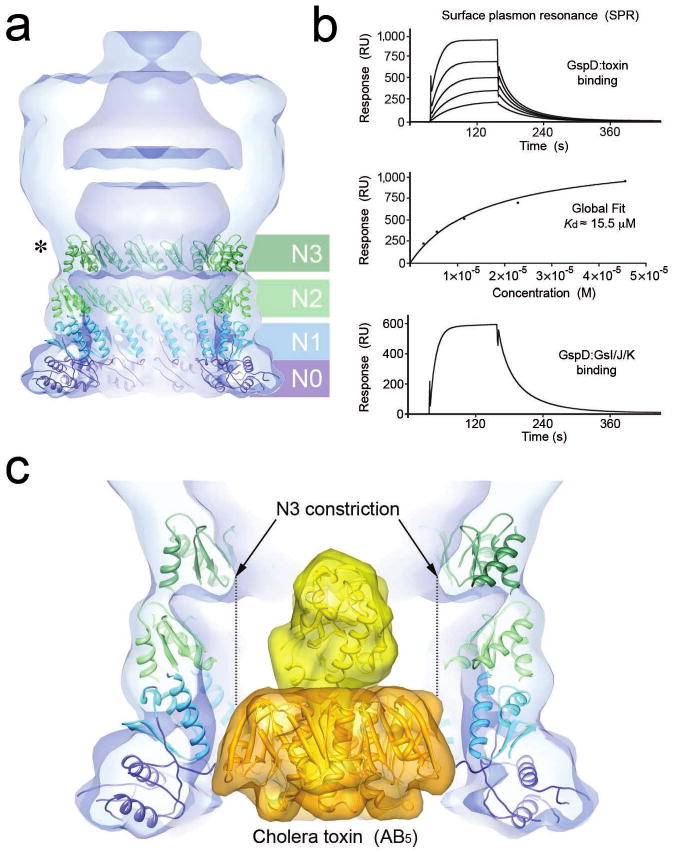
(a) Fitting of twelve member ring models of the VcGspD N-terminal periplasmic domains (N0-N3) into the VcGspD density map. The N0 domain (dark blue) and N1 domain (light blue) are anchored at the bottom of the VcGspD density map. This places the N2 domain (light green) into the central periplasmic domain density and the N3 domain (dark green) into the periplasmic constriction. This placement correlates well with protease cleavage experiments that cut at N3 (asterisk)23,33. (b) (top) Sensorgram showing binding of the B-pentamer of heat-labile enterotoxin (B5) to immobilized EcGspD. Relative units (RU, vertical) are plotted as a function of time (in seconds, horizontal). (middle) Global fit of equilibrium measurements (RE) depicted above versus B5 concentrations (M) gives a dissociation constant (Kd) of ∼15.5 μM. (bottom) Sensorgram showing the binding of the pseudopilus tip complex EcGspK-GspI-GspJ to immobilized EcGspD. (c) Fitting of the cholera toxin AB5 heterohexamer (42, PDB ID 1S5E) (A subunit yellow; B subunits gold) into the VcGspD periplasmic vestibule. The ∼65 Å wide cholera toxin molecule fits well within the ∼75 Å vestibule formed by the N0-N2 domains but would not fit though the ∼55 Å wide constriction composed of the N3 domain.
Molecular modeling of GspD N0-N3 and substrate interaction
The periplasmic domain of VcGspD density is modular. In cross-section, the walls of this domain that form the periplasmic vestibule of the channel appear as concentric rings of density (Fig. 2d). Indeed the periplasmic N-terminus of secretins from the T2SS contains four periplasmic subdomains N0-N3 (Fig. 3a and Fig. 4). We recently published the crystal structure of the N-terminal periplasmic subdomains N0-N1-N2 from the homologous ETEC GspD secretin31. The crystal structure revealed a modular architecture, composed of 3 consecutively linked domains with mixed α/β topology. The modular features resolved within the periplasmic domain of the VcGspD cryoEM map correspond well with the modular architecture observed in the crystal structures of periplasmic EcGspD. Although our crystal structure did not include N3, this domain is related in sequence to both N1 and N2 with strong predicted structural homology (31; Pfam family PF0395832).
Since the sequence similarity between EcGspD and VcGspD is very high, with 54% amino acid sequence identity for these subdomains (Supplementary Fig. 4), we used the crystal structure of periplasmic EcGspD to fit the N0-N1-N2-N3 subdomains. We first used the program SymmDock39 to generate 12-fold symmetrical rings (Fig. 4a). The N0 and N1 domains were treated as a single complex, as observed in the crystal structure of periplasmic EcGspD, whereas the N2 and N3 ring models were treated independently, since these are tethered by flexible linkers (Supplementary Fig. 4).
The fitted periplasmic ring models match well within the modular contours of the VcGspD cryoEM map (Fig. 4 and Supplementary Fig. 7). The N0-N1 ring fitted into the ∼40 Å lobe of density at the very bottom of the VcGspD periplasmic vestibule. The N2 and N3 rings fitted well directly above the N0-N1 ring (Fig. 4a). This arrangement matched the contours of the periplasmic domain, placing the N2 subdomain into the central segment of this region, and positioning the N3 subdomain snuggly into the volume encompassing the periplasmic constriction. The fit of the N3 subdomain at the periplasmic constriction is consistent with proteolysis studies of KoGspD23 (Supplementary Fig. 3).
To investigate the possible functional role of the GspD periplasmic domain, we characterized by surface plasmon resonance (SPR) the interactions between: (i) the periplasmic domain of the secretin from enterotoxigenic E. coli (specifically the fragment containing EcGspD subdomains N0-N1-N231, (ii) the B-pentamer of heat-labile enterotoxin (LT-B5) which is secreted by the T2SS of enterotoxigenic E. coli5 and (iii) the trimeric complex EcGspK-GspI-GspJ40 that forms the tip of the T2SS pseudopilus (Fig. 4b). Our analysis showed that the secretin EcGspD binds independently to exoprotein LT and to the pseudopilus tip complex. In contrast, we did not detect binding between LT and the pseudopilus tip complex (data not shown), which may reflect either a lack of interaction or a requirement for additional T2SS proteins. Together, these data suggest that the secretin periplasmic domain has an important functional role in recognition of both the exoprotein and the secretion machinery.
The N3 periplasmic domain fits into the constriction of the VcGspD density (Fig. 4a). The N3 periplasmic domain appears to be highly conserved at the amino acid level, even in distantly related secretins (Fig. 3a and Supplementary Fig. 4) suggesting that this domain plays a conserved mechanistic role in these secretion systems. Such a role is supported by a recent study that identified several amino acids within the N3 domain of the secretin pIV from the filamentous phage assembly system that resulted in a “leaky” channel phenotype when mutated41. To gain further insight into the mechanistic role of this conserved domain, we placed the structure of the cholera toxin (CT) heterohexamer AB542 into the VcGspD secretin density (Fig. 4c). Our analysis shows that the periplasmic vestibule of the secretin channel is large enough to accommodate the CT although it is a snug fit. The ∼65 Å wide CT structure fits well within the ∼75 Å wide vestibule created by the N0-N2 domains without any obvious steric hindrance. In sharp contrast, the CT will not be able to fit through the constriction formed by N3 (∼55 Å diameter) without inducing a major conformational change in the secretin. The N3 subdomain may therefore be important for T2SS exoprotein recognition and secretion at least for larger exoproteins like the ∼85 kDa cholera toxin and heat-labile enterotoxin (LT). For smaller exoproteins, like e.g. the ∼36 kDa pectate lyase from Dickeya dadantii (Erwinia chrysanthemi)43, there might be a different process in operation since such small proteins would not be blocked by the T2SS constriction. It is of interest that the diameter of the GspK-GspI-GspJ tip of the pseudopilus is ∼54 Å40, i.e. essentially the same as the cross-section of the VcGspD constriction. Therefore in such cases the tip of the pseudopilus might be interacting with the constriction and cause conformational changes leading to opening of the gate in the T2SS secretin channel.
Discussion
The mechanism of protein secretion by the T2SS is essentially unknown. The T2SS contains a pseudopilus, which has been suggested to act either as a plug to close the outer membrane pore or as a piston involved in pushing toxins and other secreted proteins through the secretin pore14-16. Our electron cryomicroscopy reconstruction of the secretin VcGspD clearly identifies a periplasmic gate and the channel appears to be in its closed state (Figs. 2, 3 and 4). Since the channel is already closed it seems likely that the function of the pseudopilus in secretion would be to act as a piston to push exoproteins out, yet, at the same time may serve as a plug to seal the pore once the periplasmic channel gate is opened.
The crystal structures of all pseudopilins have been recently determined including the ternary complex of the GspK-GspI-GspJ at the very tip of the pseudopilus40,44-47. In the following paragraphs, we combine this wealth of recent structural information with our new electron cryomicroscopy structure of the secretin to show how the piston mechanism for toxin secretion by the T2SS could work, with the secretin constriction playing a key role (Fig. 5).
Figure 5. Piston driven mechanism for protein secretion.

Illustration of key structural steps proposed to occur during protein secretion across the outer membrane by the type 2 secretion system. (left) The T2SS secretin (blue) is in its closed state. Large exoproteins, such as the cholera toxin AB5 heterohexamer (gold), are proposed to interact with the pseudopilus tip complex (grey) positioned at the inner membrane beneath the secretin channel, or bind directly to the secretin. (middle) The pseudopilus would then extend acting like a piston to push the toxin into the periplasmic vestibule of the secretin and onto the constriction. (right) The interaction between the N3 domain (constriction) and large exoproteins like the toxin could act as the trigger to open the periplasmic gate, allowing exoproteins to enter the extracellular chamber of the secretin. The final step would involve opening of the extracellular gate, allowing exoproteins to be secreted (See also Discussion).
The piston secretion model could start with the tip of the pseudopilus interacting with cholera toxin and aligning its helix axis with the pore axis of the secretin. It is also possible that the toxin, and other exoproteins in general, may be recruited initially into the secretin periplasmic vestibule followed by a second step in which the growing pseudopilus translocates the exoprotein through the secretion machinery14 (Fig. 5, left). Our binding data (Fig. 4b) indicate that exoproteins are recruited directly by the secretin by binding to the periplasmic domain of the channel (illustrated in Fig. 5, left). At this stage, the secretin itself is in its closed state. Next, the pseudopilus begins to polymerize and extend, pushing the toxin into the periplasmic vestibule of the secretin (Fig. 5, middle). The periplasmic vestibule of the secretin is wide enough to accommodate the toxin and the pseudopilus. Once the toxin or the tip of the pseudopilus reaches the N3 domain of the secretin (the constriction) the channel will need to undergo a conformational change to open the pore and allow the toxin to be secreted (Fig. 5, right). This conformational change may resemble the conformational change that was observed in the T3SS upon needle attachment (Fig. 3b, middle panel compared with right panel; see also26). Namely, the secretin N3 constriction would move outward, expanding the constriction from ∼55 Å to a larger diameter of ∼70-90 Å to let the toxin and pseudopilus pass. At the same time, the periplasmic gate would open and the toxin could then enter the extracellular chamber of the secretin (Fig. 5, right). No pseudopilus component of the T2SS has ever been observed outside the cell under physiological conditions48-50. It is therefore likely that the tip of the pseudopilus never passes the periplasmic gate of the secretin, and is used as a piston to push exoproteins into the periplasmic chamber of the secretin channel, possibly also to induce a conformational change in the secretin by interactions with the N3 constriction. Upon opening of the periplasmic gate, the pseudopilus may move the exoprotein through the opened gate and at the same time remain extended serving as a plug to prevent channel leakage. Once the exoprotein is loaded into the extracellular chamber, the extracellular gate opens and the exoprotein can be released. After the exoprotein is released, the extracellular and periplasmic gates are likely to close, and the pseudopilus would no longer be required as a plug.
Our structures support a piston driven secretion mechanism with a critical role for the constriction site in the periplasmic vestibule of GspD. This model is in line with current structural and biochemical studies. However, we must note, that whereas secretin interaction with the exoprotein has been reported before14 and is also shown here, it is still not known whether (i) the pilus directly interacts with exoproteins with the aid of additional T2SS proteins or (yet-to-be-identified) adapters; and, (ii) other T2SS proteins, such as GspC51,52, may play a key role in inducing conformational changes of the secretin. Future studies must be carried out to answer these questions regarding the intriguing T2SS.
Methods
Expression and purification of recombinant VcGspD channels
The full-length gene of V. cholerae GspD (VcGspD) was cloned into pET-22b(+) vector from pMMB710 plasmid (a generous gift from Michael Bagdasarian, Michigan State University) to encode a C-terminal hexahistidine tag. VcGspD was expressed in Tuner(DE3)pLacI strain by growing the cells to A600=0.8 and induced with 0.1 mM IPTG. Cells were harvested 3 h post-induction, resuspended in 20 mM Tris-HCl pH 7.8, 300 mM NaCl and disrupted using a French press. The membrane fraction was isolated by ultracentrifugation and membrane proteins were solubilized using N-dodecyl-N,N′-dimethylammonio-1-propanesulfonate (SB3-12). VcGspD was purified using Ni-affinity and size-exclusion chromatography (Fig. 1a and b). The N-terminal sequencing results (not shown) indicated that the signal peptide was intact. This also occurs upon replacement of the native signal VcGspD sequence with pelB signal sequence. Those results suggest that VcGspD is targeted to the inner membrane in our expression system, similar to KoGspD in the absence of a cognate pilotin protein GspS53. It should be noted that no pilotin homologs have been identified so far in the V. cholerae genome.
Electron cryomicroscopy and image processing
VcGspD was prepared for electron cryomicroscopy by applying a 2 μl drop of sample (∼0.05 mg ml-1) to a negatively charged Quantifoil holey carbon specimen grid (Quantifoil, Germany) overlayed with a continuous layer of carbon. The sample was blotted with filter paper and plunged into liquid ethane using a Vitrobot (FEI, Hilsboro, Oregon). The frozen specimen was loaded to a Gatan cryo holder and inserted into a FEI Tecnai F20 microscope, equipped with a field emission gun operated at 200 kV under low dose conditions. Images were recorded on film (Kodak SO-163) at a magnification of 50,000× and defocus values ranging from 1.5 to 5.0 μm. The film was digitized with a 6.9 μm step size, and binned twice, yielding a final pixel size of 2.54 Å per pixel. Thon rings in the power spectra were used to select only those micrographs free of drift or significant astigmatism. The contrast transfer function (CTF) parameters were determined for each micrograph using the program CTFTILT54. Approximately 20,000 particles were selected using Ximdisp27 and processed in SPIDER28 for generating multivariate reference free class averages. Rotational symmetry of VcGspD was determined from axially oriented particles, using real space symmetry averaging in EMAN55 and rotational power spectra analysis using RotaStat29 (Supplementary Fig. 1).
Three-dimensional reconstruction and refinement of VcGspD
A low-resolution density map of VcGspD was initially obtained from negatively stained particles in SPIDER28 and used as an initial search model for our best 10,000 particle images in FREALIGN ver. 730. Search and refine protocols were used in FREALIGN to determine initial Euler angles and x, y shifts relative to the initial search model for each particle and then refined with an applied C12 rotational symmetry through consecutive rounds of parameter refinement and three-dimensional reconstruction until no improvement in alignment parameters were observed. The nominal resolution of the final reconstruction was estimated from where the Fourier shell correlation (FSC) curve fell to a value of 0.556, corresponding to 19 Å (Supplementary Fig. 2). The final map was filtered to 19 Å and normalized using MAPMAN57. The map was visualized and prepared for figures using UCSF Chimera58 at a contour level of 3.4σ, corresponding to an approximate 0.9 MDa volume59. Figure 2c was prepared using a 2.4σ contour level to show the “spoke” features observed in projection averages. Our C12 symmetry assignment of VcGspD was supported by comparing the results obtained when alternative symmetries were applied to the reconstruction (Supplementary Fig. 1).
Creation of periplasmic VcGspD rings, cholera toxin and pseudopilus models
The modeling of C12 symmetry periplasmic VcGspD rings was performed using the program SymmDock39 using methods previously described47. A model of the pseudopilus was used based on the helical parameters from the GspK-GspI-GspJ complex, by adding additional pseudopilins to the tip40. A molecular model for the export of a secreted protein by the T2SS was then obtained by aligning the helical axis of the pseudopilus with the 12-fold symmetry axis of the GspD reconstruction, and placing the structure of cholera toxin42 on top of the GspK-GspI-GspJ tip with the 5-fold axis of symmetry coinciding with the 12-fold and helical axes(Fig. 5).
Fitting of models into VcGspD reconstruction
Periplasmic VcGspD ring models were fit into the density map of VcGspD as follows. The 10 lowest energy ring models for each the N0/1, N2 and N3 domains calculated by SymmDock were initially placed into the density map of VcGspD (Supplementary Fig. 7). The N0/N1 domain was treated as a single complex and the ring models for this domain was placed at the very bottom of the periplasmic domain of the VcGspD map, with the N-terminus pointed downward (toward the periplasm). The N2 and N3 ring models were treated separately as tethered by flexible linkers and placed in an N- to C-terminal (head-to-tail) fashion. These initial placements were optimized by automated fitting procedures in UCSF Chimera58. The ring models for each domain with the best correlation to the experimental map were selected. The final N0-N3 periplasmic domain model gave a cross-correlation score of 0.65 compared the experimental map (Supplementary Fig. 7).
Surface plasmon resonance studies
The B-pentamer of heat-labile enterotoxin (LTB5), the EcGspD fragment 1-237 (peri-GspD) corresponding to the N0-N1-N2 subdomains, and the trimeric pseudopilus tip complex EcGspK-GspI-GspJ were each expressed and purified as described31,40,60. peri-GspD was immobilized on a CM5 research-grade sensor chip (GE Healthcare) by amine coupling chemistry using the manufacturer's protocols and LTB5 and EcGspK-GspI-GspJ were used as the analyte. SPR measurements were carried out in HBS-EP buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% P-20 surfactant) at 25° C using Biacore T100 system (GE Healthcare). The interaction of LTB5 with peri-GspD was confirmed using an immobilized LTB5 and peri-GspD as analyte; however, in this case no steady state binding was observed due to homophilic GspD interactions (data not shown). Data were analyzed with Biacore T100 Evaluation software, ver. 2.0.1.
Supplementary Material
Acknowledgments
We thank the Murdock Charitable Trust and the Washington Research Foundation for generous support of our electron cryomicroscopy facility. We are grateful to Ji Sun, Melissa Gonen, Breanna Vollmar, and Stewart Turley for contributions to the earlier stages of this work; Michael Bagdasarian for a VcGspD-containing plasmid; and Jaclyn DelaRosa for assistance with protein preparation. We thank Alexey J. Merz (University of Washington) for helpful discussions. We thank Natalia Korotkova and Paul Wallace for discussion of SPR experiments. Part of this work was conducted at the University of Washington NanoTech User Facility, a member of the NSF National Nanotechnology Infrastructure Network (NNIN). This research is supported by the National Institutes of Health grant AI34501. TG is a Howard Hughes Medical Institute Early Career Scientist. The authors declare that none have a financial interest related to this work.
Footnotes
Accession codes: The density map of VcGspD has been deposited at the EM Database accession number EMD-1763.
Author contributions: TG and WGJH designed the research. KVK cloned, expressed, purified protein samples, constructed the molecular models of the periplasmic rings of VcGspD and of the pseudopilus, and performed SPR experiments. SLR collected and processed the cryomicroscopy data and prepared all figures. All authors wrote the manuscript.
References
- 1.Cianciotto NP. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 2005;13:581–588. doi: 10.1016/j.tim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Hirst TR, Sanchez J, Kaper JB, Hardy SJ, Holmgren J. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc Natl Acad Sci U S A. 1984;81:7752–6. doi: 10.1073/pnas.81.24.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streatfield SJ, et al. Intermolecular interactions between the A and B subunits of heat-labile enterotoxin from Escherichia coli promote holotoxin assembly and stability in vivo. Proc Natl Acad Sci U S A. 1992;89:12140–4. doi: 10.1073/pnas.89.24.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sixma TK, et al. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 5.Tauschek M, Gorrell RJ, Strugnell RA, Robins-Browne RM. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc Natl Acad Sci U S A. 2002;99:7066–71. doi: 10.1073/pnas.092152899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirst TR, Holmgren J. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci U S A. 1987;84:7418–22. doi: 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leece R, Hirst TR. Expression of the B subunit of Escherichia coli heat-labile enterotoxin in a marine Vibrio and in a mutant that is pleiotropically defective in the secretion of extracellular proteins. J Gen Microbiol. 1992;138:719–24. doi: 10.1099/00221287-138-4-719. [DOI] [PubMed] [Google Scholar]
- 8.Chapon V, Simpson HD, Morelli X, Brun E, Barras F. Alteration of a single tryptophan residue of the cellulose-binding domain blocks secretion of the Erwinia chrysanthemi Cel5 cellulase (ex-EGZ) via the type II system. J Mol Biol. 2000;303:117–23. doi: 10.1006/jmbi.2000.4103. [DOI] [PubMed] [Google Scholar]
- 9.Francetic O, Pugsley AP. Towards the identification of type II secretion signals in a nonacylated variant of pullulanase from Klebsiella oxytoca. J Bacteriol. 2005;187:7045–55. doi: 10.1128/JB.187.20.7045-7055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voulhoux R, Taupiac MP, Czjzek M, Beaumelle B, Filloux A. Influence of deletions within domain II of exotoxin A on its extracellular secretion from Pseudomonas aeruginosa. J Bacteriol. 2000;182:4051–8. doi: 10.1128/jb.182.14.4051-4058.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun P, Tommassen J, Filloux A. Role of the propeptide in folding and secretion of elastase of Pseudomonas aeruginosa. Mol Microbiol. 1996;19:297–306. doi: 10.1046/j.1365-2958.1996.381908.x. [DOI] [PubMed] [Google Scholar]
- 12.Johnson TL, Abendroth J, Hol WG, Sandkvist M. Type II secretion: from structure to function. FEMS Microbiol Lett. 2006;255:175–186. doi: 10.1111/j.1574-6968.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 13.Michel GPF, Voulhoux R. The Type II Secretory System (T2SS) in Gram-negative Bacteria: A Molecular Nanomachine for Secretion of Sec and Tat-Dependent Extracellular Proteins. In: Wooldridge K, editor. Bacterial Secreted Proteins: Secretory Mechanisms and Role in Pathogenesis. Caister Academic Press; 2009. pp. 67–92. [Google Scholar]
- 14.Shevchik VE, Robert-Baudouy J, Condemine G. Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J. 1997;16:3007–3016. doi: 10.1093/emboj/16.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filloux A, Michel G, Bally M. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol Rev. 1998;22:177–98. doi: 10.1111/j.1574-6976.1998.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 16.Sandkvist M. Biology of type II secretion. Molecular Microbiology. 2001;40:271–283. doi: 10.1046/j.1365-2958.2001.02403.x. [DOI] [PubMed] [Google Scholar]
- 17.Martin PR, Hobbs M, Free PD, Jeske Y, Mattick JS. Characterization of pilQ, a new gene required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol Microbiol. 1993;9:857–868. doi: 10.1111/j.1365-2958.1993.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 18.Genin S, Boucher CA. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 19.Brok R, et al. The C-terminal domain of the Pseudomonas secretin XcpQ forms oligomeric rings with pore activity. J Mol Biol. 1999;294:1169–1179. doi: 10.1006/jmbi.1999.3340. [DOI] [PubMed] [Google Scholar]
- 20.Collins RF, Davidsen L, Derrick JP, Ford RC, Tonjum T. Analysis of the PilQ secretin from Neisseria meningitidis by transmission electron microscopy reveals a dodecameric quaternary structure. J Bacteriol. 2001;183:3825–32. doi: 10.1128/JB.183.13.3825-3832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opalka N, et al. Structure of the filamentous phage pIV multimer by cryo-electron microscopy. J Mol Biol. 2003;325:461–70. doi: 10.1016/s0022-2836(02)01246-9. [DOI] [PubMed] [Google Scholar]
- 22.Burghout P, et al. Structure and electrophysiological properties of the YscC secretin from the type III secretion system of Yersinia enterocolitica. J Bacteriol. 2004;186:4645–54. doi: 10.1128/JB.186.14.4645-4654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chami M, et al. Structural insights into the secretin PulD and its trypsin-resistant core. J Biol Chem. 2005;280:37732–41. doi: 10.1074/jbc.M504463200. [DOI] [PubMed] [Google Scholar]
- 24.Hodgkinson JL, et al. Three-dimensional reconstruction of the Shigella T3SS transmembrane regions reveals 12-fold symmetry and novel features throughout. Nat Struct Mol Biol. 2009;16:477–85. doi: 10.1038/nsmb.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marlovits TC, et al. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature. 2006;441:637–40. doi: 10.1038/nature04822. [DOI] [PubMed] [Google Scholar]
- 26.Marlovits TC, et al. Structural insights into the assembly of the type III secretion needle complex. Science. 2004;306:1040–2. doi: 10.1126/science.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JM. Ximdisp--A visualization tool to aid structure determination from electron microscope images. J Struct Biol. 1999;125:223–8. doi: 10.1006/jsbi.1998.4073. [DOI] [PubMed] [Google Scholar]
- 28.Frank J, et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116:190–9. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 29.Kocsis E, Cerritelli ME, Trus BL, Cheng N, Steven AC. Improved methods for determination of rotational symmetries in macromolecules. Ultramicroscopy. 1995;60:219–28. doi: 10.1016/0304-3991(95)00070-2. [DOI] [PubMed] [Google Scholar]
- 30.Grigorieff N. FREALIGN: high-resolution refinement of single particle structures. J Struct Biol. 2007;157:117–25. doi: 10.1016/j.jsb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Korotkov KV, Pardon E, Steyaert J, Hol WG. Crystal structure of the N-terminal domain of the secretin GspD from ETEC determined with the assistance of a nanobody. Structure. 2009;17:255–65. doi: 10.1016/j.str.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finn RD, et al. Pfam: clans, web tools and services. Nucleic Acids Res. 2006;34:D247–51. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spreter T, et al. A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nat Struct Mol Biol. 2009;16:468–76. doi: 10.1038/nsmb.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandran V, et al. Structure of the outer membrane complex of a type IV secretion system. Nature. 2009;462:1011–5. doi: 10.1038/nature08588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fronzes R, et al. Structure of a type IV secretion system core complex. Science. 2009;323:266–8. doi: 10.1126/science.1166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christie PJ, Vogel JP. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 2000;8:354–60. doi: 10.1016/s0966-842x(00)01792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent CD, et al. Identification of the core transmembrane complex of the Legionella Dot/Icm type IV secretion system. Mol Microbiol. 2006;62:1278–91. doi: 10.1111/j.1365-2958.2006.05446.x. [DOI] [PubMed] [Google Scholar]
- 38.Ensminger AW, Isberg RR. Legionella pneumophila Dot/Icm translocated substrates: a sum of parts. Curr Opin Microbiol. 2009;12:67–73. doi: 10.1016/j.mib.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:W363–7. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korotkov KV, Hol WG. Structure of the GspK-GspI-GspJ complex from the enterotoxigenic Escherichia coli type 2 secretion system. Nat Struct Mol Biol. 2008;15:462–8. doi: 10.1038/nsmb.1426. [DOI] [PubMed] [Google Scholar]
- 41.Spagnuolo J, et al. Identification of the gate regions in the primary structure of the secretin pIV. Mol Microbiol. 2010;76:133–150. doi: 10.1111/j.1365-2958.2010.07085.x. [DOI] [PubMed] [Google Scholar]
- 42.O'Neal CJ, Amaya EI, Jobling MG, Holmes RK, Hol WG. Crystal structures of an intrinsically active cholera toxin mutant yield insight into the toxin activation mechanism. Biochemistry. 2004;43:3772–82. doi: 10.1021/bi0360152. [DOI] [PubMed] [Google Scholar]
- 43.Creze C, et al. The crystal structure of pectate lyase peli from soft rot pathogen Erwinia chrysanthemi in complex with its substrate. J Biol Chem. 2008;283:18260–8. doi: 10.1074/jbc.M709931200. [DOI] [PubMed] [Google Scholar]
- 44.Köhler R, et al. Structure and assembly of the pseudopilin PulG. Mol Microbiol. 2004;54:647–64. doi: 10.1111/j.1365-2958.2004.04307.x. [DOI] [PubMed] [Google Scholar]
- 45.Yanez ME, Korotkov KV, Abendroth J, Hol WG. Structure of the minor pseudopilin EpsH from the Type 2 Secretion system of Vibrio cholerae. J Mol Biol. 2008;37:91–103. doi: 10.1016/j.jmb.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanez ME, Korotkov KV, Abendroth J, Hol WG. The crystal structure of a binary complex of two pseudopilins: EpsI and EpsJ from the type 2 secretion system of Vibrio vulnificus. J Mol Biol. 2008;375:471–86. doi: 10.1016/j.jmb.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korotkov KV, et al. Calcium is essential for the major pseudopilin in the type 2 secretion system. J Biol Chem. 2009;284:25466–70. doi: 10.1074/jbc.C109.037655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durand E, et al. Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure. J Bacteriol. 2003;185:2749–58. doi: 10.1128/JB.185.9.2749-2758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durand E, et al. XcpX controls biogenesis of the Pseudomonas aeruginosa XcpT-containing pseudopilus. J Biol Chem. 2005;280:31378–89. doi: 10.1074/jbc.M505812200. [DOI] [PubMed] [Google Scholar]
- 50.Vignon G, et al. Type IV-like pili formed by the type II secreton: specificity, composition, bundling, polar localization, and surface presentation of peptides. J Bacteriol. 2003;185:3416–28. doi: 10.1128/JB.185.11.3416-3428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouley J, Condemine G, Shevchik VE. The PDZ domain of OutC and the N-terminal region of OutD determine the secretion specificity of the type II out pathway of Erwinia chrysanthemi. J Mol Biol. 2001;308:205–219. doi: 10.1006/jmbi.2001.4594. [DOI] [PubMed] [Google Scholar]
- 52.Korotkov K, Krumm BE, Bagdasarian M, Hol WG. Structural and Functional Studies of EpsC, a Crucial Component of the Type 2 Secretion System from Vibrio cholerae. J Mol Biol. 2006;363:311–321. doi: 10.1016/j.jmb.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 53.Guilvout I, Chami M, Engel A, Pugsley AP, Bayan N. Bacterial outer membrane secretin PulD assembles and inserts into the inner membrane in the absence of its pilotin. Embo J. 2006;25:5241–9. doi: 10.1038/sj.emboj.7601402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mindell JA, Grigorieff N. Accurate determination of local defocus and specimen tilt in electron microscopy. J Struct Biol. 2003;142:334–47. doi: 10.1016/s1047-8477(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 55.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 56.Stewart PL, Chiu CY, Haley DA, Kong LB, Schlessman JL. Review: resolution issues in single-particle reconstruction. J Struct Biol. 1999;128:58–64. doi: 10.1006/jsbi.1999.4176. [DOI] [PubMed] [Google Scholar]
- 57.Kleywegt GJ, Jones TA. xdlMAPMAN and xdlDATAMAN - programs for reformatting, analysis and manipulation of biomacromolecular electron-density maps and reflection data sets. Acta Crystallogr D Biol Crystallogr. 1996;52:826–8. doi: 10.1107/S0907444995014983. [DOI] [PubMed] [Google Scholar]
- 58.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 59.Matthews BW. Solvent content of protein crystals. J Mol Biol. 1968;33:491–7. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell DD, Pickens JC, Korotkov K, Fan E, Hol WG. 3,5-Substituted phenyl galactosides as leads in designing effective cholera toxin antagonists; synthesis and crystallographic studies. Bioorg Med Chem. 2004;12:907–20. doi: 10.1016/j.bmc.2003.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


