Abstract
Background and Purpose
This work was undertaken to review the current cost-effectiveness analysis data on thrombolysis by intravenous (IV) therapy with recombinant tissue plasminogen activator (rtPA) for acute ischemic stroke.
Methods
PubMed was searched for articles published between 1995 and 2008. The cost-effectiveness analysis data from eight eligible studies were reviewed, paying particular attention to their modeling assumptions and the quality of the source data.
Results
The reviewed studies were from six countries: USA (n=2), UK (n=2), Canada (n=1), Australia (n=1), Spain (n=1), and Denmark (n=1); most were performed from the healthcare-system and/or societal perspectives. IV rtPA was associated with an acceptable increase in short-term cost [range: US$ 36-236/patient; US$ 29,148-55,591/quality-adjusted life-years (QALYs)], and a net long-term cost saving that was higher from a societal perspective (range: -US$ 12,043 to -US$ 630/patient; -US$ 207,253 to -US$ 21,938/QALYs) than from a healthcare-system perspective (range: -US$ 5,811 to -US$ 5,415/patient; -US$ 41,137 to -US$ 4,662/QALYs).
Conclusions
IV rtPA seems to be a cost-effective strategy for the management of acute ischemic stroke, and might reduce the associated healthcare costs as well as patients' disabilities. Further cost-effectiveness research and the development of a public health strategy are warranted to optimize the use of rtPA in Korea.
Keywords: thrombolysis, stroke, recombinant tissue plasminogen activator, cost-effectiveness analysis
Introduction
Stroke is the second leading cause of death in Korea, after cancer, accounting for around 15% of all deaths. A study with a nationally representative inpatient sample found that ischemic stroke was the most common subtype, accounting for 62.9% of all strokes, and that more than 62.8% of Korean patients with first-ever ischemic stroke survived to 6 years.1 However, patients who survive a stroke are often left with permanent disabilities and an impaired quality of life,2 and have significant needs for rehabilitation and long-term care that inevitability lead to increased healthcare costs.
There has been no systematic study of the cost or burden of illness incurred by strokes in Korea. However, it is highly likely that ischemic stroke places a considerable economic burden on society in terms of direct medical cost. Studies from Western countries estimate that stroke alone accounts for 2-5% of the total healthcare expenditure.3,4 Although the data are not directly comparable, considering the higher incidence of stroke relative to cardiovascular disease in Korea, the proportion of burden attributed to stroke care in Korea might be more than that in Western countries. This highlights a need for further economic evaluations to ensure that resources are allocated efficiently to the treatment of ischemic stroke in Korea.
Faced with soaring healthcare costs, which threaten the financial stability of National Health Insurance, there is an increasing demand in Korea for financial evaluations of healthcare interventions to enable the formulation and implementation of guidelines for clinical practice, and ischemic stroke is no exception. However, thus far there have been few cost-effectiveness studies conducted in Korea, primarily due to a lack of sufficient clinical and cost data. In contrast, numerous cost-effectiveness studies on ischemic stroke have been conducted in other countries, including primary prevention,5-8 diagnostic testing (echocardiography and carotid ultrasound,9,10 and screening for deep vein thrombosis by ultrasound11), mechanical thrombectomy,12 thrombolysis,13-20 secondary prevention,21-24 rehabilitation,25,26 and management systems.27,28
In this systematic review, we summarize the current data on the cost-effectiveness of intravenous (IV) thrombolysis by recombinant tissue plasminogen activator (rtPA; e.g., alteplase) for acute ischemic stroke. We have chosen this topic because it is not only a treatment with well-established efficacy,29,30 but improving the IV rtPA treatment rate and performance requires greater healthcare resource allocation. Two previous systematic reviews, published in 199931 and 2004,32 covered the cost-effectiveness of IV rtPA. However, given the considerable time that has elapsed since these studies were published, we believe that there is a considerable need to revisit the cost-effectiveness of rtPA, taking into account more results from various data sources and modeling approaches. The aim of this study was to summarize the results of the economic impact of IV rtPA, and to interpret the results in the context of the qualities and methods of the various studies.
Methods
An electronic, PubMed-based search of articles published between 1995 and 2008 was conducted using a combination of the following keywords: stroke, thrombolysis, plasminogen activator, cost-effectiveness, and cost. Additional studies were sought among the citations of the papers retrieved as a result of that initial search. The search included published, model-based, full-economic-evaluation studies regarding the use of IV thrombolysis by rtPA in a first-ever acute ischemic stroke setting. Exclusion criteria were non-English articles and review articles. After screening the abstracts and whole articles, eight were found to comply with the study criteria. The qualities of the selected studies were evaluated using a checklist based on other studies. Disagreements between the reviewers were resolved in a consensus meeting.
To enable comparison and summary of the study results, the incremental cost-effectiveness ratio (ICER) values and net cost of each study were recalculated to reflect the current values in US dollars (US$). If the study results were reported in a currency other than US$, the mean exchange rate during the year of publication was applied to convert them to US$.33 The data were then multiplied by the inflation rate in the USA between the year of publication and the year 2009 to reflect the current value.34 The results are presented according to the time horizon considered (1-year vs. 30-years or lifetime) and the perspective from which the study was taken (i.e., health system vs. societal).
Results
Study design and perspective
All of the studies included in this review employed a cost utility analysis (CUA). Utility analysis is viewed as a particularly useful technique because it allows for given treatment outcomes to be adjusted for the health-related quality of life, while simultaneously providing a generic outcome measure for comparison of costs and outcomes in different programs. The generic outcome, which is usually expressed in quality-adjusted life-years (QALYs), is determined by adjusting the length of time affected by the health outcome according to the utility value of the resulting level of health status. Other generic outcome measures, such as disability-adjusted life-years (DALYs) and healthy years equivalent, have been proposed as alternatives to the QALY.
Considering the importance of the perspective of an evaluation study, it was remarkable that seven of the eight studies explicitly mentioned the perspective used. Two studies were performed from the societal perspective (i.e., all costs and effects were included regardless of who incurred the costs and who obtained the effects),16,18 and four studies (and one other study that did not explicitly mention the perspective17) were performed from the healthcare-system perspective.12,13,15,19 The studies assessed only the direct medical costs of rtPA treatment for stroke. One study was performed from both the healthcare-system and societal perspectives.14
General characteristics
The studies provided data regarding the cost-effectiveness of thrombolysis treatment for stroke for six countries: USA (n=2), UK (n=2), Canada (n=1), Australia (n=1), Spain (n=1), and Denmark (n=1). The national healthcare systems in different countries appear diverse, and so the components of cost evaluated by these studies differ markedly.
Modeling techniques
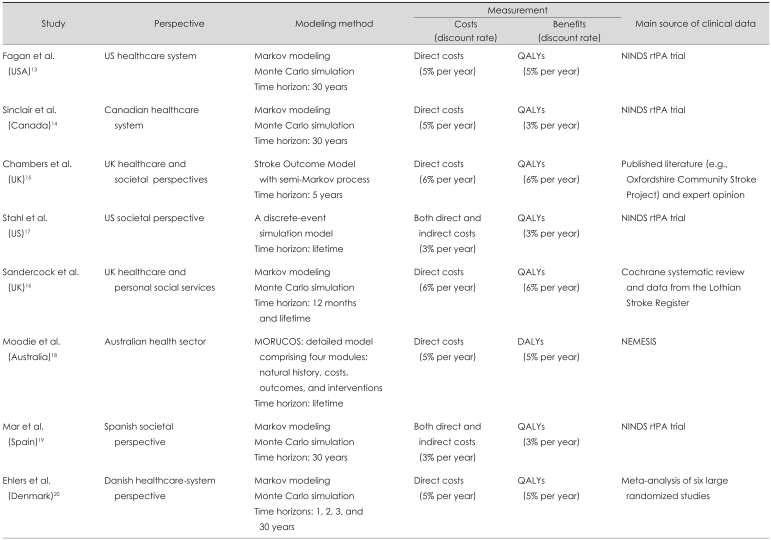
This systematic review concentrates on model-based economic evaluation studies. A model-based approach is necessary to estimate the long-term cost impact and consequences of interventions as well as the impact on the economic results of varying assumptions about risks of events, effectiveness of therapy, the cost of the intervention itself, and patient care.
Five studies used a Markov modeling approach to evaluate the cost-effectiveness of rtPA treatment for stroke. Another three studies used a discrete-event simulation model, the Model of Resource Utilization, Costs, and Outcomes for Stroke (MORUCOS), and the Stroke Outcome Model (SOM), but both of these simulation models use a semi-Markov modeling approach. Markov models are based on a series of "states" that a patient can occupy at a given point in time. Time elapses explicitly in a Markov model, with the probability of a patient occupying a given state assessed over a series of discrete time periods, called cycles. The length of these cycles vary with the disease and intervention being evaluated, but can be a certain number of months or even years. Each state in the model has a cost associated with it and, for CUA, a utility value. The duration of time during which the average patient occupies the various states in the model will-when weighted according to the relevant cost or utility-be used to calculate expected costs and outcomes. The rate with which patients move between the states in the model is determined by a set of transition probabilities.
Fagan et al.13 and Sinclair et al.14 developed a model to describe the short- and long-term outcomes of hospitalization. During hospitalization, the risk of symptomatic intracranial hemorrhage or death varied according to whether rtPA therapy was chosen. The 30-year time horizon was chosen in both of those studies. Sandercock et al.16 used a Markov modeling approach to predict the health and economic outcome of rtPA and standard care groups. The Markov model used age-specific mortality, risk of recurrent stroke, and stroke-specific case-fatality to estimate the probabilities of being dead, dependent, and independent at the beginning of each year. The Markov process was run repeatedly in 1-year cycles until the end of the cohort lifetime, and totals were computed for the accumulated health outcomes and costs. Mar et al.19 employed a cycle length of 1-year and the whole life as the time horizon. The probabilities of transition to other states changed depending on the type of stroke. The states of stroke were transitory, since at the end of the 1-year cycle all patients had moved to another state (i.e., death, autonomy, or disability). Ehlers et al.20 designed a decision tree with Markov modeling of the long-term consequences, which calculated the marginal cost-effectiveness ratio for time spans of 1, 2, 3, and 30-years. The model assumes that the patient can receive either thrombolysis or conservative treatment. Depending on the treatment instituted, the patient is exposed to a different risk of intracranial hemorrhage.
Other studies adopted modeling approaches other than the simple Markov modeling approach. Chambers et al.15 designed the SOM. The SOM comprises two modules: acute care and long-term care/prevention of recurrence among stroke survivors. A prototype primary prevention module has also been developed. The model was constructed so that results from the long-term care/prevention of recurrence module could be used as a payoff in the acute care module. This design allowed both the long-term consequences of acute events and interventions during acute care to be considered. Stahl et al.17 conducted a discrete-event simulation model of the process of stroke care from symptom onset through administration of rtPA. A literature review was performed to determine the process ties, performance of CT, health outcomes, and cost estimates. The model assumed that the patient received either a National Institute of Neurological Disorders and Stroke (NINDS)-compliant strategy (i.e., evaluation by an emergency physician within 10-minutes, interpretation of CT scans within 45-minutes, and administration of rtPA within 1-hour of presentation) or current practice. Moodie et al.18 designed the MORUCOS system, which is a detailed model consisting of four modules (natural history, costs, outcomes, and interventions) that was developed through a series of linked spreadsheets. In this model, interventions were assumed to be operating in a steady state (i.e., fully implemented and operating in accordance with efficacy potential), and they were applied to all eligible patients who presented during a 1-year period. Interventions were applied for a duration that realistically reflected their real-world use. The time horizon for tracking-associated costs and consequences extended over the lifetime of the target population (Table 1).
Table 1.
Summary of methodological approached used in cost-effectiveness studies of recombinant tissue plasminogen activator (rtPA) for the treatment of acute ischemic stroke
MORUCOS: Model of Resource Utilization, Costs, and Outcomes for Stroke, QALYs: quality-adjusted life-years, DALYs: disability-adjusted life-years, NINDS: National Institute of Neurological Disorders and Stroke, NEMESIS: North East Melbourne Stroke Incidence Study.
Data sources
Fagan et al.,13 Sinclair et al.,14 Stahl et al.,17 Mar et al.19 estimated the outcomes (with or without rtPA treatment) based on the NINDS trial, which was a randomized, double blind, placebo-controlled trial evaluating the short- and long-term outcomes of tissue plasminogen activator (tPA) in 624 patients with acute ischemic stroke. Moodie et al.18 used stroke incidence, mortality, and service utilization data from the North East Melbourne Stroke Incidence Study (NEMESIS), a community-based stroke incidence study that provides the most realistic picture of current-practice stroke care, including post acute care in Australia.
More efficacy data are available nowadays, such as that from the study by Chambers et al.,15 who used clinical data including trials, meta-analysis, and prospective cohort studies such as the Oxfordshire Community Stroke Project and the Northern Manhattan Stroke Study. Sandercock et al.16 estimated efficacy based on a systematic review of all relevant randomized trials supplemented by data from a local stroke registry, the Lothian Stroke Register (LSR). The LSR data items analyzed included the length of hospital stay, the score on the modified Rankin Scale (mRS), the occurrence of recurrent stroke, death from recurrent stroke, and death from all causes up to 12-months after the index stroke. Ehlers et al.20 extracted efficacy data from a meta-analysis of six large-scale, randomized, and placebo-controlled studies of thrombolytic therapy (Table 1).20
Outcomes considered
As described above, all of the studies reviewed were undertaken from different perspectives (i.e., societal, healthcare system, or mixed). Many experts consider a societal perspective to be highly appropriate for carrying out economic evaluations of healthcare interventions. However, only two of the studies were performed from the societal perspective and included direct and indirect costs.17,19 To measure direct costs, the studies used published literature and hospital records. Indirect costs were measured based on the average daily wages reported by the United States Bureau of Labor Statistics, the Sakontzen questionnaire, and the matrix of societal needs defined by a committee of social service experts from the province of Gipuzkoa, Spain.19 Other studies included only direct costs based on published literature, local surveys, expert opinions, and hospital records. Since the healthcare system in each country is different, the components of direct cost evaluated by these studies differ markedly. In our review, two studies used a microcosting approach and listed key unit costs and quantities of resources base on hospital records and literature.18,20 The precision in costing has also varied. The least-precise estimates are likely to be based on average per diems (or daily costs), while the most-precise estimates are likely to be based on microcosting, in which each component of the resource used (e.g., laboratory tests or drugs) is estimated and a unit cost is derived for each.
Seven of the studies reviewed herein used QALYs as the health outcome summary measure to compare health outcomes of different types. A QALY is a year lived whilst weighted by the quality of life during that time. A patient's quality of life depends on his or her current health state.13-17,19,20 Fagan et al.,13 Sinclair et al.,14 Chamber et al.,15 Stahl et al.,17 Sandercock et al.,16 and Ehlers et al.20 categorized the health state based on the mRS as follows: no symptoms (R0), no significant disability (R1), minimal disability (R2), moderate disability (R3), moderate to severe disability (R4), severe disability (R5), and dead. Mar et al.19 used the European Quality of Life Questionnaire (EuroQol) with a random sampling of patients to determine values for the utilities of a health state. Moodie et al.18 used DALYs to estimate the health gain attributable to each intervention (Table 1).
Cost-effectiveness results of the studies
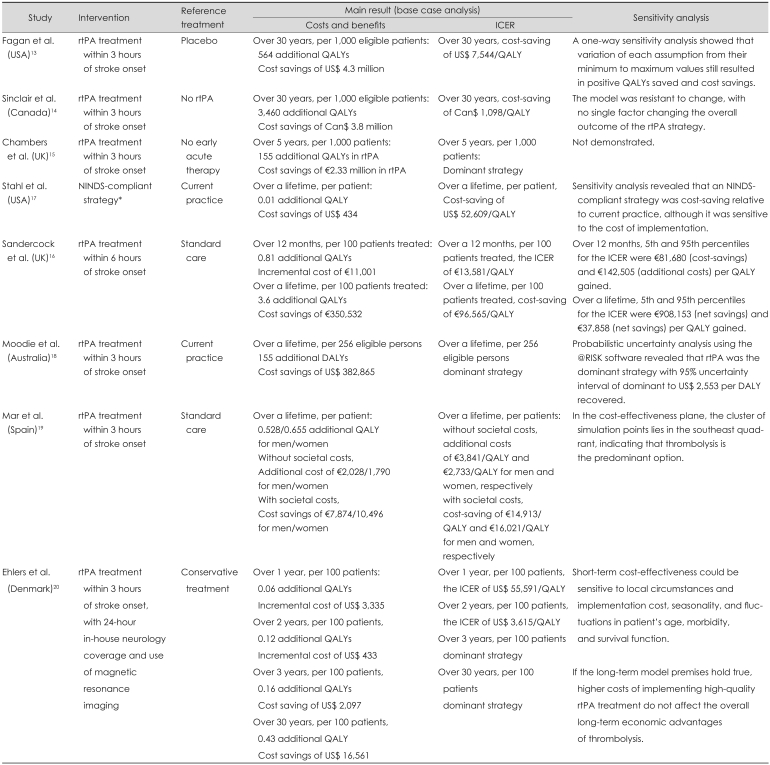
All of the studies reviewed herein concluded that thrombolysis treatment can be a cost-effective strategy, especially in the long term. Fagan et al.13 estimated an increase in hospitalization costs of US$ 1.7-million and decreases in rehabilitation and nursing home costs of US$ 1.4-million and US$ 4.8-million, respectively, per 1,000 eligible treatment patients, for a healthcare system that includes care facilities from acute through to long term. Sinclair et al.14 estimated a lifetime cost difference of 3.8-million Canadian dollars (Can$) in favor of tPA versus no tPA (Can$-3,800/patient). In the hypothetical cohort, tPA treatment resulted in 13,130-QALYs, versus 9,670-QALYs with no tPA treatment. This translated into a net benefit of 3,460 additional QALYs per 1,000 patients (i.e., 3.46-QALYs/patient). Chamber et al.15 demonstrated that rtPA in acute ischemic stroke administered within 3-hours of symptom onset could result in 155 additional QALYs per 1,000 patients, at a cost saving of €2.33-million, suggesting that in treated patients, the savings related to disability and long-term care considerably outweigh any potential extra costs of acute therapy, given a broad cost perspective and a time horizon of 5-years. Stahl et al.17 found that the NINDS-compliant strategy including t-PA treatment resulted in an average QALYs value of 3.64, versus 3.63 QALYs for the base case, at an approximate cost of US$ 434/patient.
Sandercock et al.16 showed that over the cohort lifetime, applying rtPA becomes the dominant strategy. Treatment with rtPA was more effective (gain in QALYs of 3.63 per 100 patients treated), less expensive than standard treatment (cost savings of €350,532), and resulted in a reduced cost of €96,565/QALY. Moodie et al.18 estimated that aspirin intervention was effective for reducing stroke mortality, but required a higher overall cost because of increased stroke survival (US$ 1.7-million), whereas rtPA effectively reduced post-stroke disability and led to long-term cost savings (US$ 0.4-million). Mar et al.19 calculated that the ICER obtained with thrombolytic treatment had values of €3,841/QALY and €2,733/QALY for men and women, respectively, without consideration of societal cost, but produced a net saving of €14,913/QALY for men and €16,021/QALY for women when the societal costs were incorporated in the model. Ehlers et al.20 calculated the ICER for 1, 2, 3, and 30-years. In that study, short-term thrombolysis (first year) increased the net health costs, but the picture changed after 2-years when, given the assumptions about effect and costs, thrombolysis became cost-effective. In the long term (30-years), IV thrombolysis with rtPA was the dominant strategy compared with conservative treatment (Table 2).
Table 2.
Summary of the results of cost-effectiveness studies of rtPA for acute ischemic stroke
ICER: incremental cost-effectiveness ratio, NINDS-compliant strategy: evaluation by emergency physician within 10 minutes, interpretation of CT scans within 45 minutes, and administration of rtPA within 1 hour of presentation.
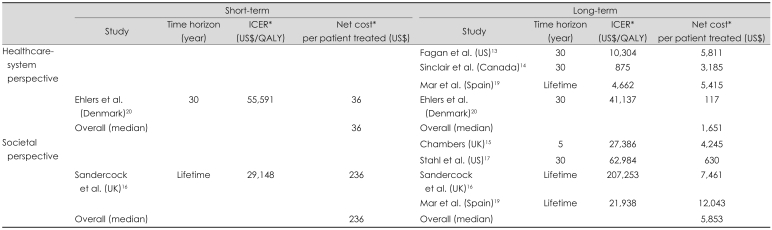
The overall results are summarized in Table 3. IV rtPA was associated with an acceptable increase in short-term cost (ranges: US$ 36-236/patient, US$ 29,148-55,591/QALY), and a net long-term cost saving that was higher from a societal perspective (ranges: -US$ 12,043 to -US$ 630/patient, -US$ 207,253 to -US$ 21,938/QALY) than from a healthcare-system perspective (ranges: -US$ 5,811 to US$ 5,415/patient, -US$ 41,137 to US$ 4,662/QALY).
Table 3.
Summary of the ICER value and net cost per patient treated according to the time horizon considered and the study perspective
*ICER value, additional cost, and cost-saving were recalculated whilst considering the exchange rate during the year of publication and the inflation rate between the year of publication and 2009. A negative value denotes a cost saving.
ICER: incremental cost-effectiveness ratio.
Discussion
Our study shows that IV rtPA is associated with an acceptable increase in short-term cost and a net cost saving in the long term; more favorable estimates were obtained when involving the societal perspective. At the same time, we also found considerable variations in the methodological approaches, data used, and results obtained in previous studies on the cost-effectiveness of IV rtPA. This indicates the need for careful interpretation of the individual studies in light of their methodological robustness, and further study to evaluate the cost-effectiveness of IV rtPA in the context of Korean data.
Health economic evaluation is considered increasingly important in health policy decision making, including insurance coverage and resource allocation, and in turn has huge influences on clinical practice. However, since the results of cost-effectiveness could be susceptible to modeling assumptions, critical appraisal and judicious interpretation of cost-effectiveness research is essential, and these analyses should also be systematically updated as new research emerges.
To our knowledge, the present study is the most extensive systematic review of the cost-effectiveness of the use of rtPA in acute ischemic stroke by modeling approach. Economic evaluation using the modeling approach has unique strengths and drawbacks when compared to economic evaluation alongside clinical trials. Although modeling approaches were unable to provide high internal validity, which can be optimized in randomized clinical trials, they rarely examine the long-term consequences of a given intervention. However, they could be utilized in situations where the relevant clinical trials and observational data are constrained by the range of outcome data collected or the length of follow-up. Thus in practice, modeling approaches may have better external validity, and are therefore highly desirable in health economic evaluations.35
The greatest strength of our study is the comprehensiveness of the data reviewed, which were from six countries that used various sources of clinical and cost data, and adopted various perspectives and modeling assumptions (i.e., short- and long-term time horizons). Despite these differences in model structure and input variables, the analyzed studies demonstrated that applying rtPA is cost-effective, in all cases producing cost savings in the long-term.
also found large variations in the estimates of ICER among the studies, some of which can be explained. First, economic modeling is highly susceptible to assumptions about clinical parameters implicit in a model, including the life expectancy, mortality hazard ratio, and utility. For example, the Fagan et al.13 study projected that long-term mortality rates beyond the first poststroke year are the same across all five levels of mRS poststroke disability. However, the subsequent UK Lothian cohort study36 and the Swedish Riks-Stroke cohort study37 have shown that long-term life expectancy decreases monotonically as the mRS level increases. In this sense, we can assume that the Fagan et al.13 study probably underestimated the effectiveness of IV rtPA. On the other hand, in other large trials conducted in Europe [i.e., the European Cooperative Acute Stroke Study (ECASS) and ECASS-II],38 IV rtPA was not as effective as was shown in the NINDS rtPA trial. So, in this regard, we can assume that earlier cost-effectiveness studies based on the NINDS trial,13,14,17 including that of Fagan et al.,13 could have overestimated the cost-effectiveness of IV rtPA relative to later studies that were based on systematic reviews or meta-analyses.16,20 Second, economic modeling is also highly susceptible to the other assumptions used in the model, including the model structure, cost of care, and discount rate. Fagan et al.13 and Sinclair et al.14 used the same clinical data, but produced hugely different QALYs and cost-saving results. Differences in the modeling methods and costs of care between the USA and Canada could explain this difference. We therefore consider that more valid and consistent methods should be used in future cost-effectiveness analysis or IV rtPA.
While the cost-effectiveness of rtPA treatment appears very convincing across studies, economic evaluations need to be checked in each country due to intercountry variations in clinical practice patterns and healthcare resources. Cost-effectiveness analysis requires information on an intervention's effectiveness and country-specific sources of epidemiological and resource utilization data, most of which are not readily available in Korea. Future research should include direct economic evaluation or rtPA use from a Korean perspective.
Apart from the theoretical cost-effectiveness of the drug, implementation of the strategy is another factor. The proportion of eligible patients who are receiving this costly but simultaneously cost-saving IV rtPA treatment in Korea is not well known, but it was reported for other countries that only about 20% of stroke patients arrive in time to be treated with thrombolysis, and only 1-8% of all stroke patients are actually treated with IV rtPA.39 Interorganizational, intraorganizational, medical, and psychological barriers hamper the broad implementation of thrombolysis for acute ischemic stroke.40 If the cost-effectiveness of rtPA is accepted, every single measure should be considered in order to increase the proportion of patients who may benefit from the treatment. Since a short door-to-door time is critical for the clinical effectiveness of rtPA treatment, investment in improving the system for delivering emergency healthcare and public education should be considered. Since the administration of rtPA requires pretreatment imaging41 and careful follow-up, the appropriate care setting and availability of a trained physician are essential. Designation of stroke centers (both primary and comprehensive) to improve the organization and delivery of care to stroke patients might be considered from the cost-effectiveness perspective.42,43
In summary, based on a comprehensive review of the available literature, we conclude that IV rtPA is a cost-effective strategy for the management of acute ischemic stroke that might reduce healthcare costs as well as patient disability. Further cost-effectiveness research and development of a public health strategy is warranted to optimize the use of rtPA in Korea.
Acknowledgements
This study was supported by a grant (A060171) of the Korea Health 21 R&D project, Ministry of Health, Welfare and Family Affairs, Republic of Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kim HC, Choi DP, Ahn SV, Nam CM, Suh I. Six-year survival and causes of death among stroke patients in Korea. Neuroepidemiology. 2009;32:94–100. doi: 10.1159/000177034. [DOI] [PubMed] [Google Scholar]
- 2.Kim JS, Choi-Kwon S, Kwon SU, Lee HJ, Park KA, Seo YS. Factors affecting the quality of life after ischemis stroke: young versus old patients. J Clin Neurol. 2005;1:59–68. doi: 10.3988/jcn.2005.1.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman L, van der Meulen JH, Limburg M, Habbema JD. Costs of medical care after first-ever stroke in The Netherlands. Stroke. 1995;26:1830–1836. doi: 10.1161/01.str.26.10.1830. [DOI] [PubMed] [Google Scholar]
- 4.Saka O, Serra V, Samyshkin Y, McGuire A, Wolfe CC. Cost-effectiveness of stroke unit care followed by early supported discharge. Stroke. 2009;40:24–29. doi: 10.1161/STROKEAHA.108.518043. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahim S. Cost-effectiveness of stroke prevention. Br Med Bull. 2000;56:557–570. doi: 10.1258/0007142001903201. [DOI] [PubMed] [Google Scholar]
- 6.Lindgren P, Buxton M, Kahan T, Poulter NR, Dahlöf B, Sever PS, et al. Cost-effectiveness of atorvastatin for the prevention of coronary and stroke events: an economic analysis of the Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA) Eur J Cardiovasc Prev Rehabil. 2005;12:29–36. [PubMed] [Google Scholar]
- 7.Lundkvist J, Ekman M, Kartman B, Carlsson J, Jönsson L, Lithell H. The cost-effectiveness of candesartan-based antihypertensive treatment for the prevention of nonfatal stroke: results from the Study on COgnition and Prognosis in the Elderly. J Hum Hypertens. 2005;19:569–576. doi: 10.1038/sj.jhh.1001857. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan PW, Arant TW, Ellis SL, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics. 2006;24:1021–1033. doi: 10.2165/00019053-200624100-00009. [DOI] [PubMed] [Google Scholar]
- 9.Meenan RT, Saha S, Chou R, Swarztrauber K, Krages KP, O'Keefee-Rosetti M, et al. Effectiveness and cost-effectiveness of echocardiography and carotid imaging in the management of stroke. Evid Rep Technol Assess (Summ) 2002:1–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Meenan RT, Saha S, Chou R, Swarztrauber K, Pyle Krages K, O'Keeffe-Rosetti MC, et al. Cost-effectiveness of echocardiography to identify intracardiac thrombus among patients with first stroke or transient ischemic attack. Med Decis Making. 2007;27:161–177. doi: 10.1177/0272989X06297388. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RD, Murray PK. Cost-effectiveness of screening for deep vein thrombosis by ultrasound at admission to stroke rehabilitation. Arch Phys Med Rehabil. 2005;86:1941–1948. doi: 10.1016/j.apmr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Patil CG, Long EF, Lansberg MG. Cost-effectiveness analysis of mechanical thrombectomy in acute ischemic stroke. J Neurosurg. 2009;110:508–513. doi: 10.3171/2008.8.JNS08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagan SC, Morgenstern LB, Petitta A, Ward RE, Tilley BC, Marler JR, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. NINDS rt-PA Stroke Study Group. Neurology. 1998;50:883–890. doi: 10.1212/wnl.50.4.883. [DOI] [PubMed] [Google Scholar]
- 14.Sinclair SE, Frighetto L, Loewen PS, Sunderji R, Teal P, Fagan SC, et al. Cost-Utility analysis of tissue plasminogen activator therapy for acute ischaemic stroke: a Canadian healthcare perspective. Pharmacoeconomics. 2001;19:927–936. doi: 10.2165/00019053-200119090-00004. [DOI] [PubMed] [Google Scholar]
- 15.Chambers MG, Koch P, Hutton J. Development of a decision-analytic model of stroke care in the United States and Europe. Value Health. 2002;5:82–97. doi: 10.1046/j.1524-4733.2002.52011.x. [DOI] [PubMed] [Google Scholar]
- 16.Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UK NHS costs. Stroke. 2004;35:1490–1497. doi: 10.1161/01.STR.0000126871.98801.6E. [DOI] [PubMed] [Google Scholar]
- 17.Stahl JE, Furie KL, Gleason S, Gazelle GS. Stroke: effect of implementing an evaluation and treatment protocol compliant with NINDS recommendations. Radiology. 2003;228:659–668. doi: 10.1148/radiol.2283021557. [DOI] [PubMed] [Google Scholar]
- 18.Moodie ML, Carter R, Mihalopoulos C, Thrift AG, Chambers BR, Donnan GA, et al. Trial application of a Model of Resource Utilization, Costs, and Outcomes for Stroke (MORUCOS) to assist priority setting in stroke. Stroke. 2004;35:1041–1046. doi: 10.1161/01.STR.0000125012.36134.89. [DOI] [PubMed] [Google Scholar]
- 19.Mar J, Begiristain JM, Arrazola A. Cost-effectiveness analysis of thrombolytic treatment for stroke. Cerebrovasc Dis. 2005;20:193–200. doi: 10.1159/000087204. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers L, Andersen G, Clausen LB, Bech M, Kjolby M. Cost-effectiveness of intravenous thrombolysis with alteplase within a 3-hour window after acute ischemic stroke. Stroke. 2007;38:85–89. doi: 10.1161/01.STR.0000251790.19419.a8. [DOI] [PubMed] [Google Scholar]
- 21.Wade WE. Cost-effectiveness of venous thrombosis prophylaxis following ischemic stroke: an assessment of currently available literature. Thromb Res. 1998;89:199–202. doi: 10.1016/s0049-3848(97)00313-7. [DOI] [PubMed] [Google Scholar]
- 22.Sarasin FP, Gaspoz JM, Bounameaux H. Cost-effectiveness of new antiplatelet regimens used as secondary prevention of stroke or transient ischemic attack. Arch Intern Med. 2000;160:2773–2778. doi: 10.1001/archinte.160.18.2773. [DOI] [PubMed] [Google Scholar]
- 23.Matchar DB, Samsa GP, Liu S. Cost-effectiveness of antiplatelet agents in secondary stroke prevention: the limits of certainty. Value Health. 2005;8:572–580. doi: 10.1111/j.1524-4733.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 24.Shah H, Gondek K. Aspirin plus extended-release dipyridamole or clopidogrel compared with aspirin monotherapy for the prevention of recurrent ischemic stroke: a cost-effectiveness analysis. Clin Ther. 2000;22:362–370. doi: 10.1016/S0149-2918(00)80041-7. [DOI] [PubMed] [Google Scholar]
- 25.Kane K, Andary MT, Turk M, Goldberg G. Cost-effectiveness in stroke rehab. Arch Phys Med Rehabil. 1996;77:521. doi: 10.1016/s0003-9993(96)90045-3. [DOI] [PubMed] [Google Scholar]
- 26.Keith RA. Rehabilitation after stroke: cost-effectiveness analyses. J R Soc Med. 1996;89:631–633. doi: 10.1177/014107689608901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Exel NJ, Koopmanschap MA, Scholte op, Niessen LW, Huijsman R. Cost-effectiveness of integrated stroke services. QJM. 2005;98:415–425. doi: 10.1093/qjmed/hci065. [DOI] [PubMed] [Google Scholar]
- 28.Launois R, Giroud M, Mégnigbêto AC, Le Lay K, Présenté G, Mahagne MH, et al. Estimating the cost-effectiveness of stroke units in France compared with conventional care. Stroke. 2004;35:770–775. doi: 10.1161/01.STR.0000117574.19517.80. [DOI] [PubMed] [Google Scholar]
- 29.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 30.National Institute for Health and Clinical Excellence. Stroke: Diagnosis and initial management of acute stroke and transient ischaemic attack (TIA); 2008. [PubMed] [Google Scholar]
- 31.Holloway RG, Benesch CG, Rahilly CR, Courtright CE. A systematic review of cost-effectiveness research of stroke evaluation and treatment. Stroke. 1999;30:1340–1349. doi: 10.1161/01.str.30.7.1340. [DOI] [PubMed] [Google Scholar]
- 32.Tseng MC, Chang KC. Cost-effectiveness analysis of tissue plasminogen activator for acute ischemic stroke: a comparative review. Acta Neurol Taiwan. 2004;13:149–155. [PubMed] [Google Scholar]
- 33.Korean Exchange Bank [cited] Available from: http://inflationdata.com/
- 34.Inflationdata.com. [cited] Available from: http://inflationdata.com/
- 35.Buxton MJ, Drummond MF, Van Hout BA, Prince RL, Sheldon TA, Szucs T, et al. Modelling in economic evaluation: an unavoidable fact of life. Health Econ. 1997;6:217–227. doi: 10.1002/(sici)1099-1050(199705)6:3<217::aid-hec267>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 36.Slot KB, Berge E, Dorman P, Lewis S, Dennis M, Sandercock P Oxfordshire Community Stroke Project, the International Stroke Trial (UK): Lothian Stroke Register. Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ. 2008;336:376–379. doi: 10.1136/bmj.39456.688333.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eriksson M, Norrving B, Terént A, Stegmayr B. Functional outcome 3 months after stroke predicts long-term survival. Cerebrovasc Dis. 2008;25:423–429. doi: 10.1159/000121343. [DOI] [PubMed] [Google Scholar]
- 38.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Second European-Australasian Acute Stroke Study Investigators. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (EC-ASS II) Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 39.Dirks M, Niessen LW, Huijsman R, van Wijngaarden J, Minkman MM, Franke CL, et al. Promoting Acute Thrombolysis for Ischaemic Stroke (PRACTISE) Int J Stroke. 2007;2:151–159. doi: 10.1111/j.1747-4949.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 40.Choi JC, Kang SY, Kang JH, Ko YJ, Bae JM. Are in-Hospital Delays Important Obstacles In Thrombolytic Therapy Following Acute Ischemic Stroke? J Clin Neurol. 2007;3:71–78. doi: 10.3988/jcn.2007.3.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bang OY. Multimodal MRI for ischemic stroke: from acute therapy to preventive strategies. J Clin Neurol. 2009;5:107–119. doi: 10.3988/jcn.2009.5.3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alberts MJ, Hademenos G, Latchaw RE, Jagoda A, Marler JR, Mayberg MR, et al. Recommendations for the establishment of primary stroke centers. Brain Attack Coalition. JAMA. 2000;283:3102–3109. doi: 10.1001/jama.283.23.3102. [DOI] [PubMed] [Google Scholar]
- 43.Alberts MJ, Latchaw RE, Selman WR, Shephard T, Hadley MN, Brass LM, et al. Recommendations for comprehensive stroke centers: a consensus statement from the Brain Attack Coalition. Stroke. 2005;36:1597–1616. doi: 10.1161/01.STR.0000170622.07210.b4. [DOI] [PubMed] [Google Scholar]