Abstract
Dietary and policy recommendations frequently focus on reducing saturated fatty acid consumption for improving cardiometabolic health, based largely on ecologic and animal studies. Recent advances in nutritional science now allow assessment of critical questions about health effects of saturated fatty acids (SFA). We reviewed the evidence from randomized controlled trials (RCTs) of lipid and non-lipid risk factors, prospective cohort studies of disease endpoints, and RCTs of disease endpoints for cardiometabolic effects of SFA consumption in humans, including whether effects vary depending on specific SFA chain-length; on the replacement nutrient; or on disease outcomes evaluated. Compared with carbohydrate, the TC:HDL-C ratio is nonsignificantly affected by consumption of myristic or palmitic acid, is nonsignificantly decreased by stearic acid, and is significantly decreased by lauric acid. However, insufficient evidence exists for different chain-length-specific effects on other risk pathways or, more importantly, disease endpoints. Based on consistent evidence from human studies, replacing SFA with polyunsaturated fat modestly lowers coronary heart disease risk, with ~10% risk reduction for a 5% energy substitution; whereas replacing SFA with carbohydrate has no benefit and replacing SFA with monounsaturated fat has uncertain effects. Evidence for the effects of SFA consumption on vascular function, insulin resistance, diabetes, and stroke is mixed, with many studies showing no clear effects, highlighting a need for further investigation of these endpoints. Public health emphasis on reducing SFA consumption without considering the replacement nutrient or, more importantly, the many other food-based risk factors for cardiometabolic disease is unlikely to produce substantial intended benefits.
Keywords: Cardiovascular disease, Diabetes mellitus, Diet, Nutrition, Saturated fatty acids, Fatty acids
Introduction
Reducing the consumption of saturated fatty acids (SFA) is a pillar of international dietary recommendations to reduce the risk of cardiovascular disease (CVD) [1–3]. The World Health Organization and the US Dietary Guidelines recommend consuming less than 10%E (percentage of total energy intake) from SFA [4], and the American Heart Association less than 7%E [3]. The strong focus on SFA as a risk factor for CVD originated in the 1960s and 1970s from lines of evidence including ecologic studies across nations, short-term metabolic trials in generally healthy adults assessing total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), and animal experiments that together appeared to provide consistent support that SFA intake increased the risk of coronary heart disease (CHD).
However, several critical questions have remained about the relationship between SFA consumption and CVD risk. First, do health effects of reducing SFA consumption vary depending on whether the replacement nutrient is carbohydrate (CHO), monounsaturated fat (MUFA), or polyunsaturated fat (PUFA)? A historical emphasis on low fat diets has produced drops in SFA consumption in the US and many other nations, but with concomitant increases in CHO, rather than MUFA or PUFA, as the replacement nutrient [1]. Is there strong evidence to support this dietary strategy? Second, do health effects of SFA vary depending on the chain-length, i.e. comparing 12-, 14-, 16-, and 18-carbon SFA? Current dietary recommendations generally focus on overall SFA consumption, without strong attention on specific SFA. Third, what is the relationship between SFA consumption and risk of stroke and type 2 diabetes mellitus? Historically, research on SFA has focused largely on CHD.
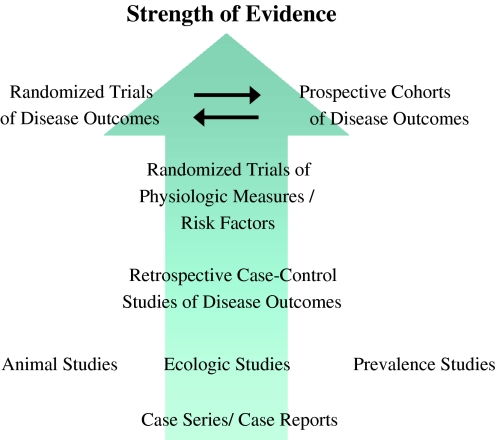
Advances in nutritional science in the last two decades now provide a substantial body of evidence to answer these questions (Fig. 1). These include well-conducted randomized controlled trials (RCTs) of SFA nutrient substitutions and multiple risk pathways as endpoints, including multiple lipid and also non-lipid risk factors (rather than only TC and LDL-C); and large prospective cohort studies and meta-analyses of RCTs of SFA consumption and clinical disease endpoints, that provide more direct evidence for effects on disease compared with changes in risk factors alone. Given the complementary strengths and limitations of these newer research paradigms, conclusions can be considered most robust when studies from each paradigm provide concordant evidence for health effects of SFA consumption. Together these research advances provide much stronger evidence for causal inference than data from prior available ecologic studies, limited metabolic studies, and animal experiments.
Fig. 1.
Advances in nutritional science research paradigms. For causal inference about how dietary habits affect chronic disease, the best evidence is derived from randomized controlled trials (RCTs) of multiple risk pathways, observed differences in disease endpoints in prospective cohort studies, and effects on disease endpoints in RCTs. Conclusions can be considered most robust when these complementary lines of evidence provide concordant results. Adapted with permission from Harris, Mozaffarian, et al. 2009 [90]
To elucidate the effects of SFA consumption on CVD risk based on the most current evidence, we reviewed the data from RCTs of multiple risk factors, large prospective cohort studies of disease endpoints, and RCTs of disease endpoints. When sufficient evidence was available, we particularly focused on the potentially different health effects of varying the replacement nutrient; of different chain-length SFA; and of specific effects on CHD, stroke, and diabetes.
Methods
Two investigators independently reviewed the literature for English-language articles published through Sep 2009 by performing searches of Medline, hand-searching of citation lists, and direct contact with experts. Inclusion criteria were any RCT or observational study in adults evaluating SFA consumption and the risk of CHD, stroke, or type 2 diabetes and related risk pathways, including lipids and lipoproteins, systemic inflammation, vascular function, and insulin resistance (1,254 identified articles). Search terms included “saturated fat(s)”, “lipoproteins”, “inflammation”, “blood pressure”, “vascular function”, “insulin resistance”, “cardiovascular diseases”, and “diabetes mellitus”. We focused on identifying RCTs of major risk factors, large prospective cohort studies of disease endpoints, and RCTs of disease endpoints, given strengths of these designs and their complementary limitations. We excluded a priori animal experiments, ecological studies, commentaries, general reviews, and case reports. Studies were independently considered by the two investigators for inclusion; rare differences were resolved by consensus.
Effects on Cardiovascular Risk Factors
Lipids and Lipoproteins
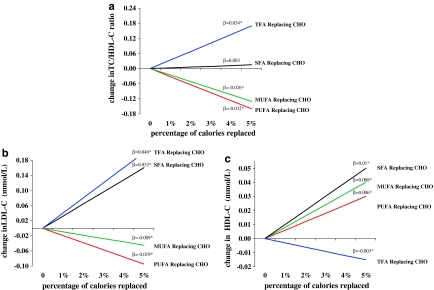
RCTs have established clear multiple effects of SFA consumption on circulating lipids and lipoproteins [5, 6]. Each of these effects varies depending on the comparison nutrient, i.e., the nutrient isocalorically replaced for SFA (Fig. 2). Compared with CHO, SFA intake raises TC and LDL-C, but also lowers triglycerides and raises high-density lipoprotein cholesterol (HDL-C). Given these conflicting directions of effects, effects on apolipoproteins or, even better, a more global risk marker such as the TC:HDL-C ratio may provide the best overall indication of potential effects on CHD risk. Compared with CHO, SFA intake has no significant effects on the TC:HDL-C ratio or ApoB levels, and raises ApoA1 levels. In contrast, consumption of PUFA or MUFA in place of SFA leads to lowering of TC, LDL-C, and ApoB; slight lowering (for PUFA) of HDL-C and ApoA1; little effect on triglycerides; and lowering of the TC:HDL-C ratio. Compared with trans fatty acids (TFA), SFA intake has minimal effects on LDL-C but raises HDL-C and lowers triglycerides and lipoprotein(a), with improvement in the TC:HDL-C ratio [7]. Thus, consideration of which nutrient is being replaced is essential when considering lipid effects or designing dietary guidelines or policy measures related to SFA consumption. Overall, the changes in lipid and apolipoprotein levels predict minimal effects on CHD risk when CHO replaces SFA, benefits when PUFA or MUFA replace SFA, and harms when TFA replace SFA.
Fig. 2.
Changes in blood lipid levels for consumption of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), or trans fatty acids (TFA) as an isocaloric replacement for carbohydrate (CHO) as a reference, based on two meta-analyses of randomized controlled feeding trials [5, 6]. β reflects the change for each 1% energy isocaloric replacement; *P < 0.05
Effects of SFA consumption on serum lipids and lipoproteins further vary according to which specific SFA is consumed (Fig. 3) [5]. With CHO consumption as the reference, lauric (12:0), myristic (14:0), and palmitic (16:0) acid raise TC and LDL-C, whereas stearic acid (18:0) does not. All SFA raise HDL-C, but HDL-raising effects are greater as chain-length decreases. Overall, the TC:HDL-C ratio is not significantly affected by myristic or palmitic acid consumption, is nonsignificantly decreased by stearic acid consumption, and is significantly decreased by lauric acid consumption (Fig. 3). These effects suggest little CHD benefit of replacing myristic, palmitic, or stearic acid with CHO, and potential harm of replacing lauric acid with CHO.
Fig. 3.
Changes in blood lipid levels for consumption of different chain-length saturated fatty acids (SFA) as an isocaloric replacement for carbohydrate (CHO), based on meta-analysis of randomized controlled feeding trials [5]. β reflects the change for each 1% energy isocaloric replacement; *P < 0.05
Systemic Inflammation
Inflammation independently increases risk of CVD and diabetes [8–11]. Compared with lipid effects, the influence of SFA consumption on inflammation is less well investigated, with mixed results. In a randomized cross-over trial, 20 healthy men consumed a high SFA (22%E SFA), a high MUFA (24%E MUFA), and a high CHO high PUFA (55%E CHO, 8%E PUFA) diet for 4 weeks [12]. At the end of each intervention period, participants were given a fat-rich breakfast (60%E fat) with similar fat composition to that of each diet. Consumption of a butter-rich breakfast (35%E SFA) had no effect on postprandial plasma levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6 or monocyte chemoattractant protein (MCP)-1, compared with an olive oil-rich breakfast (36%E MUFA) or a walnut-rich breakfast (16%E PUFA) [12]. In another cross-over trial of 50 healthy men, consumption of low-chain SFA (12:0–16:0) for 5 weeks (8%E) had no effect on fibrinogen, C-reactive protein (CRP), or IL-6 levels; similar consumption of stearic acid (18:0) increased plasma levels of fibrinogen, but not of CRP or IL-6, compared with CHO [13]. Among hypercholesterolemic subjects (n = 18), a one-month diet with 16.7%E from SFA (butter), compared with 12.5%E from PUFA (soybean oil), resulted in a trend toward higher macrophage production of TNF-α, without effects on IL-6 [14].
Observational studies investigating associations between SFA intake and markers of inflammation are limited [15, 16]. Among 4,900 US adults, dietary SFA intake was not cross-sectionally associated with CRP levels, after adjusting for other risk factors and lifestyle behaviors [15]. Other cross-sectional studies have been very small and/or not multivariable-adjusted [16]. Observational studies of circulating (e.g., plasma) or tissue (e.g., adipose) SFA levels [17, 18] are helpful for investigating effects of metabolism but not of SFA consumption, as circulating and tissue SFA are poorly reflective of dietary SFA due to endogenous synthesis and regulation by lipolysis, lipogenesis, and beta-oxidation [19–22]. Overall, the limited and mixed evidence precludes strong conclusions about potential pro-inflammatory effects of SFA consumption.
Blood Pressure, Endothelial Function, and Arterial Stiffness
Effects of dietary SFA on markers of vascular function including blood pressure, endothelial function, and arterial stiffness are similarly not well characterized [23]. A few observational studies have evaluated SFA intake and incidence of hypertension, with mixed results [24, 25]. Among 30,681 US men followed for 4 years, no significant associations were seen between SFA intake and incident hypertension, after adjusting for age, body mass index, and alcohol consumption [24]. In contrast, among 11,342 US men in the MRFIT study, SFA intake was cross-sectionally positively associated with systolic and diastolic blood pressure, after adjusting for risk factors and lifestyle behaviors, although no adjustments were made for other dietary fats, CHO, or protein [25].
Randomized controlled feeding trials ranging in duration from 3 weeks to 6 months have demonstrated mixed results of SFA intake compared with MUFA, PUFA, TFA, or CHO on measures of blood pressure, endothelial dysfunction, and/or arterial stiffness [23] (Table 1). Among nine trials assessing blood pressure, seven observed no differences between the different diets [26–34]. These trials evaluated a range of SFA consumption levels and replacement nutrients (Table 1). Improvements in BP were seen in two of five RCTs including a comparison to MUFA, one of five RCTs including a comparison to PUFA, and zero of four RCTs including a comparison to CHO. Among four trials assessing indices of endothelial function, three observed differences in brachial artery flow-mediated dilatation (FMD) between the different diets [31, 35–37]. Improvements in endothelial function were seen in two of three RCTs including a comparison to MUFA, one RCT including a comparison to PUFA, and one of three RCTs including a comparison to CHO; endothelial function was worsened in one RCT replacing SFA with TFA. In two trials evaluating arterial stiffness as assessed by pulse wave velocity (PWV) [31, 37], no effects of reducing SFA consumption were seen, including two RCTs including a comparison to MUFA, one RCT including a comparison to PUFA, and two RCTs including a comparison to CHO. Thus, evidence for effects of SFA consumption on vascular function is mixed, with no clear pattern based on underlying population characteristics, SFA consumption levels, or the comparison nutrient, and with most studies suggesting no effects.
Table 1.
Effects of saturated fatty acids on blood pressure, endothelial function, and arterial stiffness in human feeding trials
| Study | Outcome | N | Duration | Design | Comparison | SFA replaced by | |||
|---|---|---|---|---|---|---|---|---|---|
| PUFA | MUFA | TFA | CHO | ||||||
| Margetts et al. [26] | Blood pressure | 54 | 6 weeks | Cross-over | 18%E SFA versus 15%E PUFA | ↔ | |||
| Puska et al. [27] | Blood pressure | 84 | 12 weeks | Parallel | 11%E SFA versus 8%E PUFA | ↔ | |||
| Sacks et al. [28] | Blood pressure | 21 | 6 weeks | Cross-over | 16%E SFA versus 14%E PUFA or 52%E CHO | ↔ | ↔ | ||
| Storm et al. [29] | Blood pressure | 15 | 3 weeks | Cross-over | 13%E 18:0 SFA versus 16%E 16:0 SFA or 51%E CHO | ↔ | |||
| Piers et al. [30] | Blood pressure | 8 | 4 weeks | Cross-over | 24%E SFA versus 23%E MUFA | ↔ | |||
| Sanders et al. [31] | Blood pressure | 110 | 6 months | Parallel | 17%E SFA versus 17%E MUFA or CHOa | ↔ | ↔ | ||
| Uusitupa et al. [33] | Blood pressure | 159 | 6 months | Parallel | 14%E SFA vs. 8%E PUFA, 11%E MUFA, or 53%E CHO | ↔ | ↔ | ↔ | |
| Lahoz et al. [32] | Blood pressure | 42 | 5 weeks | Consecutive diets-non randomized | 17%E SFA versus 21%E MUFA or 13%E PUFA | ↓ | ↓ | ||
| Rasmussen et al. [34] | Blood pressure | 162 | 3 months | Parallel | 18%E SFA versus 21%E MUFA | ↓ | |||
| de Roos et al. [35] | Endothelial function – FMD | 29 | 4 weeks | Cross-over | 23%E SFA versus 9%E TFA | ↓ | |||
| Fuentes et al. [36] | Endothelial function – FMD | 22 | 4 weeks | Cross-over | 20%E SFA versus 22%E MUFA or 57%E CHO | ↑ | ↔ | ||
| Keogh et al. [37] | Endothelial function – FMD | 40 | 3 weeks | Cross-over | 19%E SFA versus 19%E MUFA, 10%E PUFA, or 65%E CHO | ↑ | ↑ | ↑ | |
| Sanders et al. [31] | Endothelial function – FMD | 110 | 6 months | Parallel | 17%E SFA versus 17%E MUFA or CHOa | ↔ | ↔ | ||
| Keogh et al. [37] | Arterial stiffness – PWV | 40 | 3 weeks | Cross-over | 19%E SFA versus 19%E MUFA, 10%E PUFA, or 65%E CHO | ↔ | ↔ | ↔ | |
| Sanders et al. [31] | Arterial stiffness – PWV | 110 | 6 months | Parallel | 17%E SFA versus 17%E MUFA or CHOa | ↔ | ↔ | ||
Direction of effect on reported outcome (↑ increased; ↓ decreased; ↔ no effect)
CHO carbohydrate, MUFA monounsaturated fatty acids, FMD brachial artery flow-mediated dilatation, PUFA polyunsaturated fatty acids, PWV pulse wave velocity, SFA saturated fatty acids, TFA trans fatty acids, %E percentage of total energy intake
a%E not reported
Insulin Resistance and Diabetes
SFA has been considered a risk factor for insulin resistance and diabetes mellitus [38], but review of the current evidence indicates surprisingly equivocal findings. SFA consumption inconsistently affects insulin resistance in controlled trials (Table 2) and has not been associated with incident diabetes in prospective cohort studies (Fig. 4) [39–52]. Among generally healthy individuals, most RCTs show no differences in markers of glucose-insulin homeostasis comparing different intakes of SFA versus MUFA, PUFA, or CHO. Findings are more mixed among individuals having or predisposed to insulin resistance. In these individuals, improvements in markers of glucose-insulin homeostasis were seen in three of five RCTs including a comparison to MUFA, one of three RCTs including a comparison to PUFA, and one RCT including a comparison to CHO. Among all these trials, the great majority were short-term (up to several weeks) and surprisingly small (<20 subjects). The two largest trials (n = 162, n = 59) found SFA to worsen several indices of glucose-insulin homeostasis in comparison to MUFA (two trials) or CHO (one trial).
Table 2.
Effects of saturated fatty acids on insulin resistance in human feeding trials
| Study | Subjects | N | Duration | Design | Comparison | Outcomes and results | SFA replaced by | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PUFA | MUFA | TFA | CHO | |||||||
| Individuals Predisposed to Insulin Resistance | ||||||||||
| Christiansen et al. [42] | Obese (BMI 33.5 ± 1.2 kg/m2), type 2 diabetic, age 55 ± 3 years | Nine men; seven women | 6 weeks | Cross-over | Three isocaloric diets: all 30%E fat, with 20%E from SFA, MUFA, or TFA |
SFA versus MUFA: ↑ postprandial insulin by 78.9% and ↑ postprandial C-peptide by 41.8% (P < 0.05 for each) SFA versus TFA: no significant effects on postprandial insulin and C-peptide No significant effects on fasting insulin, fasting C-peptide, or fasting and postprandial glucose with any diet |
↓ | ↔ | ||
| Vessby et al. [43] | Moderately overweight (BMI 26.5 ± 3 kg/m2), age 48.5 ± 7.8 years | 86 men, 76 women | 3 months | Parallel | Two isocaloric diets: both ~37%E fat, with 17.6%E SFA, or 21.2%E MUFA; each group was further randomized to 3.6 g of either omega-3 fatty acids or olive oil |
SFA versus MUFA: ↓ insulin sensitivity by 23.8% (P = 0.05), and ↑ insulin levels by 30.3% (P = 0.06) during a FSIGTT No significant effects on acute insulin response, or glucose levels during a FSIGTT with either diet |
↓ | |||
| Summers et al. [44] | Obese (BMI 37 ± 6 kg/m2), type 2 diabetic, age 53.7 ± 11 years | Eight men; nine women | 5 weeks | Cross-over | Two diets: 42%E fat in SFA diet with 21%E from SFA, and 34%E fat in PUFA diet with 9%E from PUFA |
SFA versus PUFA: ↓ insulin sensitivity by 20.3% (P = 0.02) during an euglycemic clamp No significant effects on fasting glucose insulin, or triglycerides with either diet |
↓ | |||
| Vega-Lopez et al. [45] | Hyperlipidemic (LDL-cholesterol ≥ 130 mg/dl), moderately overweight (BMI 26 ± 2.4 kg/m2), age 63.9 ± 5.7 years | Five men; ten women | 5 weeks | Cross-over | Four isocaloric diets: all ~30%E fat, with 20%E from partially hydrogenated soybean (4.2%E TFA), soybean (12.5%E PUFA), palm (14.8%E SFA), or canola (15.4%E MUFA) | SFA versus PUFA, MUFA, or TFA: no significant effects on fasting insulin, fasting glucose, or HOMA | ↔ | ↔ | ↔ | |
| Paniagua et al. [46] | Obese (BMI 32.6 ± 7.8 kg/m2), insulin resistant (as assessed by OGTT), age 62.3 ± 9.4 years | Four men; seven women | 28 days | Cross-over | Three isocaloric diets: 38%E fat and 47%E CHO in the two high-fat diets, with 23%E from SFA or MUFA, and 20%E fat and 65%E CHO in the low-fat diet (the latter as a replacement of SFA) |
SFA versus MUFA: ↑ HBA1c (P < 0.01), ↑ fasting glucose by 9.6% (P < 0.05), ↑ HOMA by 17.2% (P < 0.01), ↑ fasting proinsulin by 26.1% (P < 0.05), no significant effects on postprandial glucose, postprandial insulin, or postprandial GLP-1 SFA vs. CHO: ↑ HBA1c by 6.3% (P < 0.01), ↑ fasting glucose by 9.3% (P < 0.05), ↓ postprandial glucose by 51% (P < 0.05), ↓ postprandial insulin by 53% (P < 0.05), ↑ postprandial GLP-1 by 134.6% (P < 0.05), no significant effects on HOMA or fasting proinsulin No significant effects on fasting insulin or GLP-1, or the 60 min proinsulin:insulin ratio with any diet |
↓ | ↓ | ||
| Lithander et al. [47] | Hyperlipidemic (LDL 3.0–5.0 mmol/L), moderately overweight (BMI 25.9 ± 4.2 kg/m2), age 39.7 ± 13.9 years | 18 men | 3 weeks | Cross-over | Two isocaloric diets, both 38%E fat: 18%E SFA, 10%E MUFA and 7%E PUFA in the high SFA:USFA diet, and 13%E SFA, 12%E MUFA and 8%E PUFA in the low SFA:USFA diet | SFA versus PUFA + MUFA: No significant effects on fasting adiponectin | ↔ | ↔ | ||
| Healthy individuals | ||||||||||
| Schwab et al. [48] | Healthy, normal weight (BMI 21.4 ± 0.5 kg/m2), age 23.9 ± 1.2 years | 11 women | 4 weeks | Cross-over | Two isocaloric diets: all ~38%E fat, with 5%E from lauric acid (12:0 SFA), or 11.4%E from palmitic acid (16:0 SFA) | 12:0 SFA versus 16:0 SFA: no significant effects on insulin, glucose, acute insulin response, or insulin sensitivity index during a FSIGTT with either diet | ||||
| Fasching et al. [49] | Healthy, normal weight (BMI 22.4 ± 1.8 kg/m2), age 26 ± 3.5 years | 8 men | 1 week | Cross-over | Four isocaloric diets: 54%E fat and 35%E CHO in the three high-fat diets with 31.5%E from SFA, 28%E from PUFA and 22%E from MUFA, and 25%E fat and 64%E CHO in the high CHO diet | SFA versus PUFA, MUFA, or CHO: no significant effects on insulin, glucose, acute insulin response, or insulin sensitivity index during a FSIGTT with any diet | ↔ | ↔ | ↔ | |
| Louheranta et al. [50] | Healthy, normal weight (BMI 22.6 ± 0.6 kg/m2), age 22 ± 0.6 years | 14 women | 4 weeks | Cross-over | Two isocaloric diets: both ~38%E fat, with 18.5%E from SFA or MUFA | SFA versus MUFA: no significant effects on insulin, glucose, acute insulin response, or insulin sensitivity index during a FSIGTT with either diet | ↔ | |||
| Perez-Jimenez et al. [52] | Healthy, normal weight (BMI 22.87 ± 2.45 kg/m2), age 23.1 ± 1.8 years | 30 men, 29 women | 28 days | Cross-over | Baseline 28-day high SFA diet followed by Two randomized cross-over periods; all isocaloric diets: 38%E fat and 47%E CHO in the two high-fat diets, with 20%E from SFA or 22%E from MUFA, and 28%E fat and 57%E CHO in the low-fat diet (the latter as a replacement of SFA) |
SFA versus MUFA: ↑ fasting insulin by 134%, ↑ fasting free fatty acids by 40.5%, ↑ mean steady-state plasma glucose by 21.9%, ↓ in vitro basal glucose uptake by 61.3%, and ↓ in vitro insulin-stimulated glucose uptake by 55.3% (P < 0.001 for each) SFA versus CHO: ↑ fasting insulin by 119.7%, ↑ fasting free fatty acids by 40.5%, ↑ mean steady-state plasma glucose by 29%, ↓ in vitro basal glucose uptake by 57.1% %, and ↓ in vitro insulin-stimulated glucose uptake by 55.9% (P < 0.001 for each) No significant effects on fasting glucose with any diet |
↓ | ↓ | ||
| Lovejoy et al. [51] | Healthy, normal weight (BMI 23.5 ± 0.5 kg/m2), age 28 ± 2 years | 12 men; 13 women | 4 weeks | Cross-over | Three isocaloric diets: all 30%E fat, with 9%E from elaidic acid (TFA), oleic acid (MUFA), or palmitic acid (SFA) | SFA versus MUFA or TFA: no significant effects on insulin, glucose, acute insulin response, or insulin sensitivity index during a FSIGTT with any diet | ↔ | ↔ | ||
Direction of effect on biomarkers of insulin resistance (↑increased; ↓ decreased; ↔ no effect). If even one biomarker was affected, this was considered an effect; this might overestimate the effects of these dietary changes as often many other biomarkers were unaffected (detailed results are also provided)
BMI body mass index, CHO carbohydrate, FSIGTT frequently sampled intravenous glucose tolerance test, GLP-1 glucagon-like peptide-1, HBA1c glycosylated hemoglobin, HOMA homeostasis model assessment, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids, SFA saturated fatty acids, TFA trans fatty acids, USFA unsaturated fatty acids, %E percentage of total energy intake
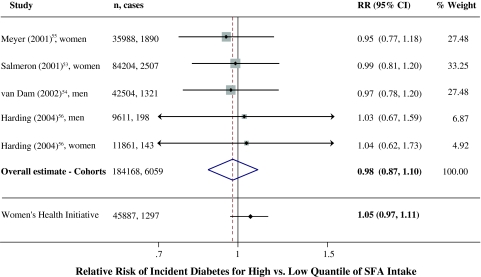
Fig. 4.
Relative risk of incident diabetes associated with consumption of saturated fat (SFA). Multivariable-adjusted results from prospective cohort studies and the overall pooled result using fixed-effects meta-analysis are shown. Results from the Women’s Health Initiative randomized controlled trial are also shown comparing controls (higher SFA intake) to the intervention group in which SFA was reduced by ~3.2%E over 8 years [79]. CI’s for Harding et al.[56] were estimated based on the numbers of cases
Significant additional insight into effects of dietary fats on glucose-insulin homeostasis can be gained from long-term studies evaluating actual onset of diabetes. Among four large prospective cohort studies, none found independent associations between consumption of either SFA (Fig. 4) or MUFA and onset of diabetes [53–56]. In contrast, three of four cohorts [54] observed lower incidence of diabetes with greater consumption of PUFA and/or vegetable fat [53, 55, 56]. In the large Women’s Health Initiative trial (n = 45,887), SFA intake was reduced in the intervention group from 12.7 to 9.5%E over 8 years as part of overall total fat reduction, largely replaced with CHO [57]. In this large RCT, this significant reduction in SFA consumption had no effect on fasting glucose, fasting insulin, homeostasis model assessment (HOMA) insulin resistance, or incident diabetes (RR = 0.95, 95% CI = 0.90–1.03).
Thus, some evidence from short-term RCTs suggests that SFA consumption in place of MUFA may worsen glucose-insulin homeostasis, especially among individuals predisposed to insulin resistance. However, several long-term observational studies and one large RCT suggest no effect of SFA consumption on onset of diabetes. Further confirmatory results of either harm or no effect in additional appropriately powered studies are needed given the present inconsistency of effects across all studies.
Weight Gain and Adiposity
The role of total dietary fat in obesity has been widely studied due to its high energy content (9 kcal/g) and subsequent potential for weight gain [58–60]. Based on RCTs of weight loss with balanced-intensity interventions (i.e., all individuals receiving similar guidance and follow-up, with only the specific dietary advice varying) and prospective observational studies of weight gain, the %E from total fat does not have strong effects on adiposity compared with overall quality and quantity of foods consumed. Evidence for independent effects of specific dietary fats such as SFA on weight gain or adiposity are much more limited. In two large prospective cohort studies, increases in SFA consumption were associated with very small increases in abdominal circumference [61] or body weight during 8–9 years follow-up [62] compared with CHO, after adjusting for other risk factors and lifestyle and dietary behaviors.
Relationships with Cardiovascular Events
Coronary Heart Disease—Prospective Cohort Studies
Most individual prospective cohort studies have not observed an independent relationship between SFA consumption and incident CHD [63–67]. The relatively small number of published studies, among the many available international cohorts, also raises concern for potential publication bias (i.e., additional unreported null studies). Two recent systematic reviews and meta-analyses, the first including 9 cohorts (11 estimates) evaluating 160,673 individuals [64], and the second including 16 cohorts among 214,182 individuals [68], found no significant association between SFA intake and CHD risk. Comparing the highest to the lowest category of consumption, the pooled RRs in these two meta-analyses were 1.06 (95% CI = 0.96–1.15) and 1.07 (95% CI = 0.96–1.19), respectively. These meta-analyses suggest no overall effect of SFA consumption on CHD events. However, these studies were unable to separately evaluate whether consuming SFA might have different effects on CHD events depending on the nutrient replaced, as would be suggested by differing effects of SFA, depending on the comparison nutrient, on blood lipids and apolipoproteins (Fig. 2).
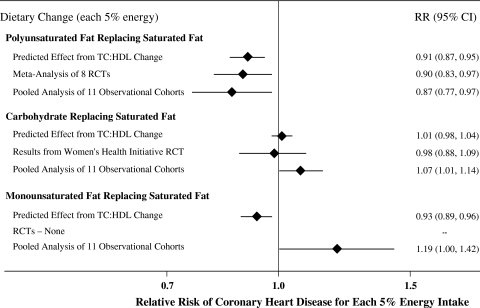
The best observational evidence to-date of this question is a recent pooled analysis of individual-level data from 11 prospective cohort studies across three continents, including 344,696 individuals with 5,249 CHD events over 4–10 years of follow-up [69]. In fully multivariable-adjusted analyses, SFA consumption was associated with higher CHD risk only in comparison to PUFA. In other words, only consumption of PUFA in place of SFA was associated with lower CHD risk, whereas in fact consumption of CHO or MUFA in place of SFA was associated with higher CHD risk or trends toward higher CHD risk (Fig. 5). These associations were similar when analyses were restricted to CHD deaths only, and were not different in subgroups stratified by either sex or age.
Fig. 5.
Effects on coronary heart disease (CHD) risk of consuming polyunsaturated fat (PUFA), carbohydrate (CHO), or monounsaturated fat (MUFA) in place of saturated fat (SFA). Predicted effects are based on changes in the TC:HDL-C ratio in short-term trials [5], coupled with observed associations between the TC:HDL-C ratio and CHD disease events in middle-aged adults [91]. Evidence for effects of dietary macronutrients on actual CHD events comes from a meta-analysis of eight randomized controlled trials (RCTs) for PUFA replacing SFA, including 13,614 participants with 1,042 CHD events [78]; and from the Women’s Health Initiative (WHI) RCT for CHO replacing SFA, including 46,558 individuals with 1,185 CHD events and ~3.2%E reduction in SFA over 8 years [79]. Evidence for observed relationships of usual dietary habits with CHD events comes from a pooled analysis of 11 prospective cohort studies, including 344,696 individuals with 5,249 CHD events [69]. Reproduced with permission from Mozaffarian et al., in press [78]
Coronary Heart Disease—Randomized Controlled Trials
Eight RCTs have investigated the effects of consuming PUFA (either total or linoleic acid, LA) in place of SFA on CHD events [70–77]. Most of these trials individually found no significant effects. A recent meta-analysis of these RCTs, including a total of 13,614 participants with 1,042 CHD events, found that CHD risk was lowered by 10% for each 5%E greater PUFA intake replacing SFA [78] (Fig. 5). Many of these trials have important limitations, including for example not being double-blind; incompletely assessing compliance; randomizing sites rather than individuals and having open enrollment and drop-out; and/or including vegetable oils that contained omega-3 PUFA of plant origin that may provide cardiovascular benefits unrelated to decreased SFA intake. Nonetheless, the overall findings from these RCTs of CHD endpoints are consistent with the results from prospective cohorts (Fig. 5).
One large RCT has tested the effect of reducing SFA consumption, replaced largely with CHO, on CHD events. As described, the Women’s Health Initiative trial randomized 46,558 women to lower total fat consumption, that included lowering of SFA consumption by ~3%E over 8 years, and largely replaced with CHO. Even though this was an unbalanced intervention (i.e., the intervention group received extensive dietary counseling, whereas the control group received usual care) that would generally bias toward risk-reduction in the intervention group, there were no significant effects on either incident CHD (RR = 0.93, 95%CI = 0.83–1.05) or total CVD (RR = 0.96, 95%CI = 0.89–1.03) [79]. This absence of benefit for substituting SFA with CHO is consistent with expected effects based on lipid changes (TC:HDL ratio) or observed relationships in prospective cohort studies (Fig. 5).
Stroke: Prospective Cohorts and Randomized Controlled Trials
Among five prospective cohort studies evaluating SFA consumption and incidence of stroke, one of three found SFA to be associated with lower risk of ischemic stroke [80–82], and one of three found SFA to be associated with lower risk of hemorrhagic stroke [80, 83, 84]. Four prospective cohorts have also observed protective associations between animal protein intake, that is often consumed together with SFA, and risk of hemorrhagic stroke [85]. A recent systematic review and meta-analysis of eight prospective cohorts also found that SFA consumption was associated with trends toward lower risk of stroke: comparing the highest to the lowest category of SFA intake, the RR was 0.81 (95% CI = 0.62–1.05) [68]. In the Women’s Health Initiative trial, reduction in SFA consumption did not have a significant effect on incident stroke over 8 years (RR = 1.02, 95% CI = 0.90–1.17) [79]. Thus, overall, SFA consumption does not appear to increase the risk of stroke, and in fact some studies suggest a protective effect. Further investigation of these effects, including independence from potential benefits of animal protein intake, is warranted.
Future Research Directions
The multiple well-designed studies reviewed herein provide substantial evidence for health effects of SFA consumption. However, important questions remain. Although replacement of SFA with CHO appears to provide no overall CVD benefit, indirect lines of evidence suggest that effects could vary depending on overall CHO quality [86–88]. For example, replacing SFA with less processed, higher fiber, lower glycemic index CHO could provide benefit, whereas replacing SFA with more processed, lower fiber, higher glycemic index CHO might have no effects or even be harmful. Effects of replacing SFA with CHO could also vary with an individual's susceptibility to insulin resistance/metabolic syndrome, in whom adverse metabolic effects of highly refined CHO may be more pronounced. Evidence for effects of replacing SFA with MUFA is mixed. Such effects could vary depending on other constituents in MUFA-containing foods (e.g., animal fats vs. vegetable oils), for example due to potentially beneficial phytochemicals and flavanols contained in the latter but not the former. Each of these issues requires direct investigation. Additionally, whereas the substantial differences in blood lipid effects of different chain-length SFA are clear, blood lipids represent only one set of intermediate risk markers. Investigation of the effects of different chain-length SFA on other risk pathways and, more importantly, on disease endpoints is urgently needed to determine the extent to which dietary and policy recommendations should focus on specific SFA rather than overall SFA consumption. Additional investigation of effects of SFA consumption on blood pressure, endothelial function, insulin resistance, diabetes, and stroke (plus stroke subtypes) is also needed, including consideration of potential variation depending on both the replacement nutrients and specific chain-length SFA under consideration. Future research should also evaluate the health effects of specific foods consumed, i.e., SFA intake from different meats versus dairy versus tropical fats, as well as how individual factors, such as age, sex, lifestyle factors, predisposition to insulin resistance, or genetic variation, may alter such responses.
Conclusions
Current public health dietary recommendations often prioritize the reduction of SFA consumption to prevent CVD. A review of the current evidence, particularly findings from well-performed RCTs of risk pathways, large prospective cohorts of disease endpoints, and RCTs of disease endpoints, suggests that this focus may not produce the intended benefits. First of all, substantial evidence indicates that health effects of reducing SFA vary depending on the replacement nutrient. Based on the best evidence from human studies, replacing SFA with PUFA (e.g., vegetables, vegetable oils) lowers CHD risk, whereas replacing SFA with CHO has no benefits. Replacing SFA with MUFA has uncertain effects, based on mixed evidence within and across different research paradigms. Of note, the effects of replacing SFA with PUFA or CHO, but not MUFA, on clinical CHD endpoints could be relatively predicted from the effects of these nutrient substitutions on the TC:HDL-C ratio. Thus, policies that prioritize the reduction of SFA consumption without specifically considering the replacement nutrient may have little or no effects on disease risk, especially as the most common replacement in populations is often CHO.
Second, even under optimal replacement scenarios of SFA for PUFA, the magnitude of likely benefit warrants attention. RCTs of the blood TC:HDL-C ratio, prospective cohorts of disease endpoints, and RCTs of disease endpoints each converge on ~10% reduction in CHD events for 5%E substitution of SFA with PUFA. This approaches the maximal plausible risk reduction in most populations; in the US, for example, such benefit would require overall population decrease from the current 11.5 to 6.5%E SFA consumption. Thus, although recommendations to replace SFA with PUFA appear appropriate, the much larger CVD burdens caused by other dietary factors (e.g., low omega-3, low fruits and vegetables, high trans fat, and high salt) [89] appear to warrant much more attention. Finally, although investigation of individual nutrients provides important information on potential underlying mechanisms of health effects, people make decisions about eating whole foods that contain multiple macro- and micronutrients in various amounts. Thus, food-based scientific research and policy recommendations may be most relevant in the modern era to understand and reduce the pandemics of chronic disease occurring in nearly all nations.
Acknowledgments
Supported by the Searle Funds at The Chicago Community Trust and the Bill & Melinda Gates Foundation/World Health Organization Global Burden of Diseases, Injuries, and Risk Factors Study. The founders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Conflict of interest statement
R. Micha has no conflicts of interest to declare. D. Mozaffarian received ad hoc consulting honoraria (modest) from Nutrition Impact, Unilever, and SPRIM.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- BMI
Body mass index
- CHD
Coronary heart disease
- CHO
Carbohydrate
- CRP
C-reactive protein
- CVD
Cardiovascular disease
- FMD
Flow-mediated dilatation
- FSIGTT
Frequently sampled intravenous glucose tolerance test
- GLP-1
Glucagon-like peptide-1
- HBA1c
Glycosylated hemoglobin
- HDL
High-density lipoprotein
- HOMA
Homeostasis model assessment
- IL
Interleukin
- LA
Linoleic acid
- LDL
Low-density lipoprotein
- MCP
Monocyte chemoattractant protein
- MUFA
Monounsaturated fatty acids
- PUFA
Polyunsaturated fatty acids
- PWV
Pulse wave velocity
- RCT
Randomized controlled trial
- SFA
Saturated fatty acids
- TC
Total cholesterol
- TFA
Trans fatty acids
- TNF
Tumor necrosis factor
- USFA
Unsaturated fatty acids
- WHI
Women’s Health Initiative
- %E
Percentage of total energy intake
References
- 1.Centers for disease control, prevention Trends in intake of energy and macronutrients: United States, 1971–2000. Morb Mortal Wkly Rep. 2004;53(04):80–82. [PubMed] [Google Scholar]
- 2.World Health Organization Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation. World Health Org Tech Rep. 2003;916(i–viii):1–149. [PubMed] [Google Scholar]
- 3.Lichtenstein AH, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 4.Dietary Guidelines Advisory Committee (2005) Dietary Guidelines Advisory Committee report. http://www.health.gov/dietaryguidelines/dga2005/report/
- 5.Mensink RP, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr. 2009;63(Suppl 2):S22–S33. doi: 10.1038/sj.ejcn.1602976. [DOI] [PubMed] [Google Scholar]
- 7.Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol. 2009;5(6):335–344. doi: 10.1038/nrendo.2009.79. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 9.Albert CM, et al. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105(22):2595–2599. doi: 10.1161/01.CIR.0000017493.03108.1C. [DOI] [PubMed] [Google Scholar]
- 10.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–1491. doi: 10.1161/01.CIR.0000057810.48709.F6. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Gomez Y, et al. Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis. 2009;204(2):e70–e76. doi: 10.1016/j.atherosclerosis.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Baer DJ, et al. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79(6):969–973. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 14.Han SN, et al. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res. 2002;43(3):445–452. [PubMed] [Google Scholar]
- 15.King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. 2003;92(11):1335–1339. doi: 10.1016/j.amjcard.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Lennie TA, et al. Dietary fat intake and proinflammatory cytokine levels in patients with heart failure. J Card Fail. 2005;11(8):613–618. doi: 10.1016/j.cardfail.2005.06.434. [DOI] [PubMed] [Google Scholar]
- 17.Petersson H, et al. Relationships between serum fatty acid composition and multiple markers of inflammation and endothelial function in an elderly population. Atherosclerosis. 2009;203(1):298–303. doi: 10.1016/j.atherosclerosis.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Real JM, et al. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care. 2003;26(5):1362–1368. doi: 10.2337/diacare.26.5.1362. [DOI] [PubMed] [Google Scholar]
- 19.Poppitt SD, et al. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis. 2005;4:30. doi: 10.1186/1476-511X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Q, et al. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86(1):74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 21.Baylin A, Campos H. The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol. 2006;17(1):22–27. doi: 10.1097/01.mol.0000199814.46720.83. [DOI] [PubMed] [Google Scholar]
- 22.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47(5):348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22(1):18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- 24.Ascherio A, et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86(5):1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 25.Stamler J, et al. Relationship to blood pressure of combinations of dietary macronutrients: findings of the multiple risk factor intervention trial (MRFIT) Circulation. 1996;94(10):2417–2423. doi: 10.1161/01.cir.94.10.2417. [DOI] [PubMed] [Google Scholar]
- 26.Margetts BM, et al. Blood pressure and dietary polyunsaturated and saturated fats: a controlled trial. Clin Sci (Lond) 1985;69(2):165–175. doi: 10.1042/cs0690165. [DOI] [PubMed] [Google Scholar]
- 27.Puska P, et al. Dietary fat and blood pressure: an intervention study on the effects of a low-fat diet with two levels of polyunsaturated fat. Prev Med. 1985;14(5):573–584. doi: 10.1016/0091-7435(85)90078-7. [DOI] [PubMed] [Google Scholar]
- 28.Sacks FM, et al. Effect of dietary fats and carbohydrate on blood pressure of mildly hypertensive patients. Hypertension. 1987;10(4):452–460. doi: 10.1161/01.hyp.10.4.452. [DOI] [PubMed] [Google Scholar]
- 29.Storm H, et al. Comparison of a carbohydrate-rich diet and diets rich in stearic or palmitic acid in NIDDM patients: effects on lipids, glycemic control, and diurnal blood pressure. Diabetes Care. 1997;20(12):1807–1813. doi: 10.2337/diacare.20.12.1807. [DOI] [PubMed] [Google Scholar]
- 30.Piers LS, et al. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr. 2003;90(3):717–727. doi: 10.1079/BJN2003948. [DOI] [PubMed] [Google Scholar]
- 31.Sanders T, Lewis F, Frost G (2009) Impact of the amount and type of fat and carbohydrate on vascular function in the RISCK study. Proc Nutr Soc, vol 68 (in press)
- 32.Lahoz C, et al. Effects of dietary fat saturation on eicosanoid production, platelet aggregation and blood pressure. Eur J Clin Invest. 1997;27(9):780–787. doi: 10.1046/j.1365-2362.1997.1860735.x. [DOI] [PubMed] [Google Scholar]
- 33.Uusitupa MI, et al. Long-term effects of four fat-modified diets on blood pressure. J Hum Hypertens. 1994;8(3):209–218. [PubMed] [Google Scholar]
- 34.Rasmussen BM, et al. Effects of dietary saturated, monounsaturated, and n-3 fatty acids on blood pressure in healthy subjects. Am J Clin Nutr. 2006;83(2):221–226. doi: 10.1093/ajcn/83.2.221. [DOI] [PubMed] [Google Scholar]
- 35.de Roos NM, Bots ML, Katan MB. Replacement of dietary saturated fatty acids by trans fatty acids lowers serum HDL cholesterol and impairs endothelial function in healthy men and women. Arterioscler Thromb Vasc Biol. 2001;21(7):1233–1237. doi: 10.1161/hq0701.092161. [DOI] [PubMed] [Google Scholar]
- 36.Fuentes F, et al. Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann Intern Med. 2001;134(12):1115–1119. doi: 10.7326/0003-4819-134-12-200106190-00011. [DOI] [PubMed] [Google Scholar]
- 37.Keogh JB, et al. Flow-mediated dilatation is impaired by a high-saturated fat diet but not by a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2005;25(6):1274–1279. doi: 10.1161/01.ATV.0000163185.28245.a1. [DOI] [PubMed] [Google Scholar]
- 38.Eyre H, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109(25):3244–3255. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 39.Galgani JE, et al. Effect of the dietary fat quality on insulin sensitivity. Br J Nutr. 2008;100(3):471–479. doi: 10.1017/S0007114508894408. [DOI] [PubMed] [Google Scholar]
- 40.Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feskens EJ, et al. Dietary factors determining diabetes and impaired glucose tolerance: a 20-year follow-up of the Finnish and Dutch cohorts of the seven countries study. Diabetes Care. 1995;18(8):1104–1112. doi: 10.2337/diacare.18.8.1104. [DOI] [PubMed] [Google Scholar]
- 42.Christiansen E, et al. Intake of a diet high in trans monounsaturated fatty acids or saturated fatty acids: effects on postprandial insulinemia and glycemia in obese patients with NIDDM. Diabetes Care. 1997;20(5):881–887. doi: 10.2337/diacare.20.5.881. [DOI] [PubMed] [Google Scholar]
- 43.Vessby B, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU study. Diabetologia. 2001;44(3):312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 44.Summers LK, et al. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia. 2002;45(3):369–377. doi: 10.1007/s00125-001-0768-3. [DOI] [PubMed] [Google Scholar]
- 45.Vega-Lopez S, et al. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am J Clin Nutr. 2006;84(1):54–62. doi: 10.1093/ajcn/84.1.54. [DOI] [PubMed] [Google Scholar]
- 46.Paniagua JA, et al. A MUFA-rich diet improves postprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J Am Coll Nutr. 2007;26(5):434–444. doi: 10.1080/07315724.2007.10719633. [DOI] [PubMed] [Google Scholar]
- 47.Lithander FE, et al. No evidence of an effect of alterations in dietary fatty acids on fasting adiponectin over 3 weeks. Obesity (Silver Spring) 2008;16(3):592–599. doi: 10.1038/oby.2007.97. [DOI] [PubMed] [Google Scholar]
- 48.Schwab US, et al. Lauric and palmitic acid-enriched diets have minimal impact on serum lipid and lipoprotein concentrations and glucose metabolism in healthy young women. J Nutr. 1995;125(3):466–473. doi: 10.1093/jn/125.3.466. [DOI] [PubMed] [Google Scholar]
- 49.Fasching P, et al. No effect of short-term dietary supplementation of saturated and poly- and monounsaturated fatty acids on insulin secretion and sensitivity in healthy men. Ann Nutr Metab. 1996;40(2):116–122. doi: 10.1159/000177904. [DOI] [PubMed] [Google Scholar]
- 50.Louheranta AM, et al. A high-stearic acid diet does not impair glucose tolerance and insulin sensitivity in healthy women. Metabolism. 1998;47(5):529–534. doi: 10.1016/S0026-0495(98)90235-9. [DOI] [PubMed] [Google Scholar]
- 51.Lovejoy JC, et al. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care. 2002;25(8):1283–1288. doi: 10.2337/diacare.25.8.1283. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Jimenez F, et al. A Mediterranean and a high-carbohydrate diet improve glucose metabolism in healthy young persons. Diabetologia. 2001;44(11):2038–2043. doi: 10.1007/s001250100009. [DOI] [PubMed] [Google Scholar]
- 53.Salmeron J, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73(6):1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 54.van Dam RM, et al. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care. 2002;25(3):417–424. doi: 10.2337/diacare.25.3.417. [DOI] [PubMed] [Google Scholar]
- 55.Meyer KA, et al. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care. 2001;24(9):1528–1535. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- 56.Harding AH, et al. Dietary fat and the risk of clinical type 2 diabetes: the European prospective investigation of Cancer-Norfolk study. Am J Epidemiol. 2004;159(1):73–82. doi: 10.1093/aje/kwh004. [DOI] [PubMed] [Google Scholar]
- 57.Tinker LF, et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women’s Health Initiative randomized controlled dietary modification trial. Arch Intern Med. 2008;168(14):1500–1511. doi: 10.1001/archinte.168.14.1500. [DOI] [PubMed] [Google Scholar]
- 58.Willett WC, Leibel RL. Dietary fat is not a major determinant of body fat. Am J Med. 2002;113(Suppl 9B):47S–59S. doi: 10.1016/S0002-9343(01)00992-5. [DOI] [PubMed] [Google Scholar]
- 59.Pirozzo S, et al. Should we recommend low-fat diets for obesity? Obes Rev. 2003;4(2):83–90. doi: 10.1046/j.1467-789X.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 60.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. Eur J Clin Nutr. 1995;49(2):79–90. [PubMed] [Google Scholar]
- 61.Koh-Banerjee P, et al. Prospective study of the association of changes in dietary intake, physical activity, alcohol consumption, and smoking with 9-y gain in waist circumference among 16,587 US men. Am J Clin Nutr. 2003;78(4):719–727. doi: 10.1093/ajcn/78.4.719. [DOI] [PubMed] [Google Scholar]
- 62.Field AE, et al. Dietary fat and weight gain among women in the nurses’ health study. Obesity (Silver Spring) 2007;15(4):967–976. doi: 10.1038/oby.2007.616. [DOI] [PubMed] [Google Scholar]
- 63.Mozaffarian D. Effects of dietary fats versus carbohydrates on coronary heart disease: a review of the evidence. Curr Atheroscler Rep. 2005;7(6):435–445. doi: 10.1007/s11883-005-0060-y. [DOI] [PubMed] [Google Scholar]
- 64.Mente A, et al. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169(7):659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 65.Oh K, et al. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses’ health study. Am J Epidemiol. 2005;161(7):672–679. doi: 10.1093/aje/kwi085. [DOI] [PubMed] [Google Scholar]
- 66.Leosdottir M, et al. Dietary fat intake and early mortality patterns–data from The Malmo Diet and Cancer Study. J Intern Med. 2005;258(2):153–165. doi: 10.1111/j.1365-2796.2005.01520.x. [DOI] [PubMed] [Google Scholar]
- 67.Pietinen P, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men: the alpha-tocopherol, beta-carotene cancer prevention study. Am J Epidemiol. 1997;145(10):876–887. doi: 10.1093/oxfordjournals.aje.a009047. [DOI] [PubMed] [Google Scholar]
- 68.Siri-Tarino P, et al. A meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91(3):535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jakobsen MU, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89(5):1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dayton S, et al. Controlled trial of a diet high in unsaturated fat for prevention of atherosclerotic complications. Lancet. 1968;2(7577):1060–1062. doi: 10.1016/S0140-6736(68)91531-6. [DOI] [PubMed] [Google Scholar]
- 71.Leren P. The Oslo diet-heart study: eleven-year report. Circulation. 1970;42(5):935–942. doi: 10.1161/01.cir.42.5.935. [DOI] [PubMed] [Google Scholar]
- 72.Turpeinen O, et al. Dietary prevention of coronary heart disease: the Finnish mental hospital study. Int J Epidemiol. 1979;8(2):99–118. doi: 10.1093/ije/8.2.99. [DOI] [PubMed] [Google Scholar]
- 73.Burr ML, et al. Diet and reinfarction trial (DART): design, recruitment, and compliance. Eur Heart J. 1989;10(6):558–567. doi: 10.1093/oxfordjournals.eurheartj.a059528. [DOI] [PubMed] [Google Scholar]
- 74.Watts GF, et al. Effects on coronary artery disease of lipid-lowering diet, or diet plus cholestyramine, in the St Thomas’ Atherosclerosis Regression Study (STARS) Lancet. 1992;339(8793):563–569. doi: 10.1016/0140-6736(92)90863-X. [DOI] [PubMed] [Google Scholar]
- 75.Miettinen M, et al. Dietary prevention of coronary heart disease in women: the Finnish mental hospital study. Int J Epidemiol. 1983;12(1):17–25. doi: 10.1093/ije/12.1.17. [DOI] [PubMed] [Google Scholar]
- 76.Frantz ID, Jr, et al. Test of effect of lipid lowering by diet on cardiovascular risk. the Minnesota Coronary Survey. Arteriosclerosis. 1989;9(1):129–135. doi: 10.1161/01.atv.9.1.129. [DOI] [PubMed] [Google Scholar]
- 77.Council Medical Research Controlled trial of soya-bean oil in myocardial infarction. Lancet. 1968;2(7570):693–699. [PubMed] [Google Scholar]
- 78.Mozaffarian D, Micha R, Wallace SK (2010) Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med (in press) [DOI] [PMC free article] [PubMed]
- 79.Howard BV, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 80.He K, et al. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ. 2003;327(7418):777–782. doi: 10.1136/bmj.327.7418.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seino F, et al. Dietary lipids and incidence of cerebral infarction in a Japanese rural community. J Nutr Sci Vitaminol. 1997;43(1):83–99. doi: 10.3177/jnsv.43.83. [DOI] [PubMed] [Google Scholar]
- 82.Gillman MW, et al. Inverse association of dietary fat with development of ischemic stroke in men. JAMA. 1997;278(24):2145–2150. doi: 10.1001/jama.278.24.2145. [DOI] [PubMed] [Google Scholar]
- 83.Iso H, et al. Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation. 2001;103(6):856–863. doi: 10.1161/01.cir.103.6.856. [DOI] [PubMed] [Google Scholar]
- 84.Iso H, et al. Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am J Epidemiol. 2003;157(1):32–39. doi: 10.1093/aje/kwf166. [DOI] [PubMed] [Google Scholar]
- 85.Ding EL, Mozaffarian D. Optimal dietary habits for the prevention of stroke. Semin Neurol. 2006;26(1):11–23. doi: 10.1055/s-2006-933305. [DOI] [PubMed] [Google Scholar]
- 86.Jacobs DR, Gallaher DD., Jr Whole grain intake and cardiovascular disease: a review. Curr Atheroscler Rep. 2004;6(6):415–423. doi: 10.1007/s11883-004-0081-y. [DOI] [PubMed] [Google Scholar]
- 87.Thomas D, EJ Elliott (2009) Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst Rev (1):CD006296 [DOI] [PMC free article] [PubMed]
- 88.Livesey G, et al. Glycemic response and health–a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87(1):258S–268S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 89.Danaei G et al (2009) The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 6(4):e1000058. doi:10.1371/journal.pmed.1000058 [DOI] [PMC free article] [PubMed]
- 90.Harris WS, et al. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139(4):804S–819S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lewington S, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]