Summary
Soil respiration is the largest flux of carbon (C) from terrestrial ecosystems to the atmosphere. Here, we tested the hypothesis that photosynthesis affects the diurnal pattern of grassland soil-respired CO2 and its C isotope composition (δ13CSR).
A combined shading and pulse-labelling experiment was carried out in a mountain grassland. δ13CSR was monitored at a high time resolution with a tunable diode laser absorption spectrometer.
In unlabelled plots a diurnal pattern of δ13CSR was observed, which was not explained by soil temperature, moisture or flux rates and contained a component that was also independent of assimilate supply. In labelled plots δ13CSR reflected a rapid transfer and respiratory use of freshly plant-assimilated C and a diurnal shift in the predominant respiratory C source from recent (i.e. at least 1 d old) to fresh (i.e. photoassimilates produced on the same day).
We conclude that in grasslands the plant-derived substrates used for soil respiratory processes vary during the day, and that photosynthesis provides an important and immediate C source. These findings indicate a tight coupling in the plant–soil system and the importance of plant metabolism for soil CO2 fluxes.
Keywords: assimilate supply, δ13C, mountain grassland, pulse labelling, plant–soil carbon (C) transfer, soil respiration, tunable diode laser
Introduction
The soil is the largest source of CO2 in terrestrial ecosystems. Annual soil CO2 emissions are estimated to exceed the amount of CO2 produced by fossil fuel burning by an order of magnitude (Raich et al., 2002; Canadell et al., 2007). Thus, even minor changes in soil respiration (SR) are likely to impact the global carbon (C) cycle. For this reason there have been increasing efforts to improve our understanding of the environmental controls on soil respiratory fluxes in a changing environment. There is growing evidence that SR is not merely a function of soil temperature and moisture, but is also determined by photosynthetic assimilate supply (cf. recent reviews by Davidson et al., 2006; Högberg & Read, 2006; Trumbore, 2006; Bahn et al., 2009). While it is well documented that photosynthesis may influence SR from annual timescales to those of a few days, it is still unclear whether this also holds true for a diurnal timescale. Earlier attempts to derive such an effect from a hysteresis in the temperature–SR relationship (‘temperature-independent’ effect; cf. Tang et al., 2005; Liu et al., 2006, Vargas & Allen, 2008) have the major drawback that they do not permit a clear separation of potential influences of photosynthesis from those related to shifts in phase and amplitude in soil temperature with soil depth, and may thus be confounded by an arbitrary selection of the soil depth at which temperature is measured and to which SR is related (Reichstein et al., 2005; Pavelka et al., 2007; Graf et al., 2008; for detailed discussion cf. Bahn et al., 2008).
Isotopes are a useful tool for tracing the transfer of C in ecosystems (e.g. Ostle et al., 2000; Johnson et al., 2002; Carbone & Trumbore, 2007; Högberg et al., 2008) and thus have the potential to provide insights into the coupling of photosynthetic assimilation and soil respiratory fluxes. Earlier studies suggest that in forests the isotopic signal from tree photosynthesis, as modulated by changes in vapour pressure deficits, appears in SR within 1–10 d (Ekblad & Högberg, 2001; Bowling et al., 2002; Steinmann et al., 2004; Ekblad et al., 2005; Knohl et al., 2005; Mortazavi et al., 2005). More recent studies have attempted to detect such a relationship on a diurnal timescale. In forests, the carbon isotope composition (δ13C) of soil-respired CO2 (δ13CSR) may (Gessler et al., 2007; Kodama et al., 2008) or may not (Betson et al., 2007) follow a diel pattern. However, its immediate link to photosynthesis is still uncertain. The commonly observed 24-h periodicity in the δ13C of organic matter in leaf and twig phloem sap (Gessler et al., 2007; Wingate et al., 2007) is often dampened as carbohydrates are transported down the trunk, where apparent respiratory fractionation becomes more important for diurnal patterns of the δ13C of respired CO2 than changes in the δ13C of the organic substrate (Kodama et al., 2008).
Our study aimed to explore, for a grassland, whether recent photoassimilates are utilized in soil respiratory processes within a sufficiently short time to exert a diurnal influence on soil-respired CO2; whether there is a diurnal pattern of δ13CSR; and, if so, whether it is affected by freshly assimilated C. Using a tunable diode laser we monitored at a high time resolution (i.e. every 20 min) the diurnal course of the δ13C of soil-respired CO2 in a mountain grassland. We studied effects of assimilate supply and its potential short-term coupling to soil respiration by pulse-labelling canopy sections with highly 13C-enriched CO2 and tracing the subsequent response of δ13CSR; and by experimental shading of labelled and unlabelled plots over several days. We tested the hypotheses that freshly leaf-assimilated C is respired belowground within less than a day; that δ13CSR follows a diurnal pattern which is partly explained by soil temperature and moisture (cf. Kodama et al., 2008); and that shading reduces this diurnal variation and results in a closer coupling of δ13CSR to soil temperature. Our study demonstrates a direct diurnal link between photosynthesis and soil-respired CO2 in an intact ecosystem and indicates a diurnal variation in the C source of soil-respired CO2.
Materials and Methods
Site
The study was carried out on a mountain meadow at Kaserstattalm, Neustift, in the Austrian Central Alps. The site is located at 1820 m asl in the same meadow as described by Bahn et al. (2006) and thus exhibits the same land management, plant species composition and soil characteristics. In brief, the meadow is fertilized with manure in spring, cut once in late July or early August and lightly grazed in September. The dominating plant species include the grasses Anthoxanthum odoratum L. and Festuca rubra L., and the forbs Alchemilla vulgaris L., Leontodon hispidus L. and Trifolium repens L. All species are perennial and reached their peak of vegetative and generative development towards the end of July. The soil is a dystric cambisol on siliceous bedrock with a topsoil pH of 5.5. The meadow is characterized by a comparatively high productivity and high soil respiration rates, which are typical for nonwater-limited Central European mountain meadows (Bahn et al., 2008). In 2007 the meadow reached its peak biomass towards the end of July and was mowed at the beginning of August, immediately after the completion of the experiments.
Experimental set-up and instrumentation
In each of three consecutive campaigns during the period of peak biomass (14–25 July, 25–31 July and 31 July–4 August 2007) we studied one experimental block containing a control and a shaded plot as well as two plots that were pulse-labelled and subsequently shaded or left unshaded. Each plot contained a Plexiglas frame of 1 × 1 m inserted c. 3–5 cm into the soil, in whose centre a round soil chamber (diameter × height: 10 × 13 cm) made of stainless steel was fixed on a steel collar that had been inserted c. 1 (up to 2) cm into the soil at least 24 h before the start of measurements. The insertion depth was kept as low as possible to minimize severing of roots and the mycorrhizal system, while ensuring that no leaks occurred in the chamber–soil system. The vegetation inside the collars was removed by clipping when collars were installed to obtain the belowground respiration signal only. Both clipping and collar insertion may potentially lead to a reduction of assimilate supply to the soil section covered by the chamber system. Because, in the studied grassland, roots and mycorrhizal systems integrate over much larger areas than covered by the collar and CO2 may diffuse into the chamber from a larger soil volume than that located immediately underneath the collar, effects of clipping and shallow collar insertion on soil CO2 efflux have been shown to be minor (unpublished own data). While such small potential artefacts cannot be completely ruled out, it should be emphasized that in both cases a decrease in assimilate transport and thus a reduction in the assimilate-related signal of soil-respired CO2 would have occurred. This would have resulted in an underestimation of the amplitude of the diurnal patterns observed in the unshaded plots, which would not have altered the conclusions drawn from the study.
The soil chambers were configured as an open dynamic system, with a reference air inlet mounted close to the chamber air inlet, immediately outside the Plexiglas frame, slightly above the canopy. To minimize pressure fluctuations a vent following the design of Xu et al. (2006) was placed on top of each chamber. Air was pumped continuously at a rate of 0.45 l min−1 from chambers to the tunable diode laser instrument (TGA100A; Campbell Scientific, Logan, UT, USA), where 12C16O2 and 13C16O2 mixing ratios were continuously analysed. The laser of the TGA100A was cooled with a cooling aggregate (Cryotiger; Polycold Systems, Petaluma, CA, USA) and operated at a stabilized temperature of 91.3 K. The reference and sample detectors of the TGA100A were cooled thermoelectrically and kept at −2 and −5°C, respectively. Pressure within the optical cell of the instrument was maintained at 2 kPa. Fluctuations of ambient air temperature, leading inevitably to small fluctuations of instrument temperature and pressure and hence of stable isotope ratios, were corrected for by sampling three reference gas cylinders (325, 552 and 1010 ppmv CO2 in synthetic air; δ13C = − 45.5 ; Basi, Rastatt, Germany) every 10 min. The precision of each reference gas measurement was 0.06
; Basi, Rastatt, Germany) every 10 min. The precision of each reference gas measurement was 0.06 , and the accuracy over 24 h was within a range of approx. ±5
, and the accuracy over 24 h was within a range of approx. ±5 . The average instrument drift within each 10-min interval was 0.29
. The average instrument drift within each 10-min interval was 0.29 . Between two reference gas samplings, the inlet and outlet air of two chambers was sampled for 90 s each, of which the last 45 s was used for averaging. The instrument readings for the sample air were corrected for the instrument drift assuming a linear trend between two reference gas samplings and applying the true δ13C values of the reference gases. Within 20 min, all four soil respiration chambers were measured once. The respective δ13C values of soil respiration were obtained with an isotopic mass balance approach according to Eqn 1:
. Between two reference gas samplings, the inlet and outlet air of two chambers was sampled for 90 s each, of which the last 45 s was used for averaging. The instrument readings for the sample air were corrected for the instrument drift assuming a linear trend between two reference gas samplings and applying the true δ13C values of the reference gases. Within 20 min, all four soil respiration chambers were measured once. The respective δ13C values of soil respiration were obtained with an isotopic mass balance approach according to Eqn 1:
| Eqn 1 |
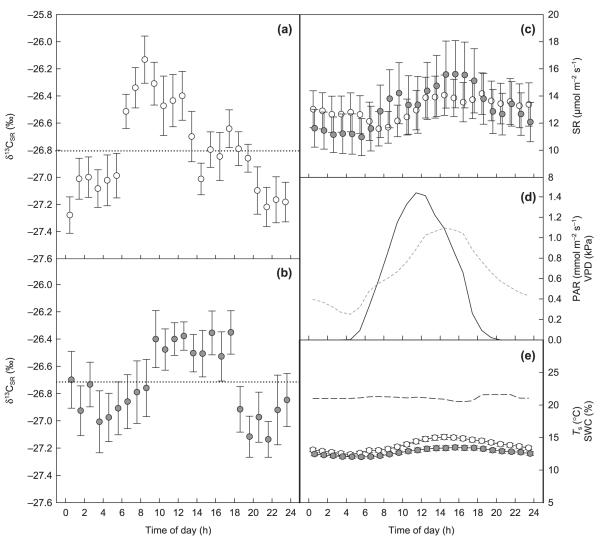
Thus, possible diurnal fluctuations of atmospheric δ13C were accounted for. To enable detection of possible significant trends in the diurnal variation of δ13CSR in unlabelled plots, we averaged the hourly means, each based on three measurements per treatment. Thereby we used the data for all campaigns and all days, for which complete or close to complete (i.e. not more than three missing values) diurnal data sets were available. For the aggregated data shown for the three unshaded and shaded plots (Fig. 1a,b), hourly means of 13–16 and 10–12 d were averaged, respectively. Thus each value shown in Fig. 1(a,b) is based on 30– 48 individual referenced measurements.
Fig. 1.
Averaged diurnal courses of (a, b) δ13C of soil-respired CO2 (δ13CSR) in (a) unshaded and (b) shaded plots in a mountain grassland, (c) soil respiration in unshaded (open circles) and shaded (closed circles) plots, (d) photosynthetically active radiation (PAR; black line) incident on unshaded plots and vapour pressure deficit (VPD; dashed grey line) and (e) soil temperatures (Ts) (Ts unshaded, open circles; Ts shaded, closed circles) and soil water content (SWC; dashed line) at 5 cm depth. Averages were calculated by aggregating hourly means (based on 20-min measurement intervals) obtained in three plots for each treatment during three separate field campaigns between 15 July and 4 August 2007 (for details see the Materials and Methods). Error bars indicate standard errors; n = 13–16 and 10–12 for three unshaded and three shaded plots, respectively. Horizontal dotted lines in (a) and (b) represent the daily mean of δ13CSR.
Microclimate data were recorded continuously at the site and logged at half-hourly intervals with an automated station (CR10X; Campbell Scientific), and included soil temperature (averaging soil thermocouple probe TCAV; Campbell Scientific) and moisture (ML2x; Delta-T Devices, Cambridge, UK) at 5 and 10 cm soil depth, as well as incident photosynthetically active radiation (PAR) (BF2H; Delta-T Devices), air temperature and air humidity at 2 m above ground level (C2.4; GWU-Umwelttechnik, Erftstadt, Germany). Furthermore, automatically recording soil temperature probes (S-TMB-M002 12-Bit Temp Smart Sensor; H21-002 Hobo Micro Station, Onset, MA, USA) were installed at 5 cm soil depth in the unshaded, shaded and labelled plots, and additional manual measurements of soil water content (ML2x; Delta-T Devices) were carried out at regular time intervals in all plots. Air pressure was continuously monitored with an automated soil respiration system (Li-8100; Li-Cor, Lincoln, NE, USA), which was also used for cross-referencing the CO2 efflux (SR) rates obtained by the tunable diode laser.
To assess the background spatial variability of SR rates, three additional collars were placed on each plot at a distance of 0.5–1 m from the Plexiglas frames, yielding a total of 18 collars for each of the shaded and unshaded treatments. Collar diameter and insertion were as described above. During each experiment, on 11 occasions SR was measured on each collar using a portable closed dynamic soil respiration system (SRC-1, EGM-4; PPSystems, Hitchin, Herts, UK). Next to each collar, soil temperature and soil moisture were recorded using a temperature probe attached to the SRC-1 chamber and a mobile soil moisture sensor (Theta Probe; Delta-T Devices), respectively. Pretreatment values of SR, soil temperature and moisture did not differ significantly amongst any of the treatments.
Experimental treatments
Pulse labelling was accomplished by covering canopy sections with a transparent (95% light transmission) Plexiglas chamber of 1 × 1 × 0.7 m and continuously adding a small amount of 99.9 atom % 13C-CO2 to the chamber air. The chamber was placed on Plexiglas frames that had been inserted 3–5 cm into the soil several days before the experiments (cf. the previous section). A rubber seal at the bottom of the chamber ensured gas tightness of the system. Pressurization of the system was avoided by using a 12-mm-diameter opening at the top of the chamber, which was closed after the chamber had been placed onto the frame, and a 4-mm-diameter venting tube inserted at the bottom of the chamber and through the sealing gasket. Four small fans mounted at the back of the chamber provided a circulation of air. The chamber air temperature was stabilized using ice packs mounted on the rear of the chamber in the air flow. The frozen ice packs also prevented condensation of water vapour on the chamber walls during measurements. During labelling, the temperature was maintained within 2°C of ambient, except for the last 20 min during the third labelling experiment, when the chamber air temperature reached values of up to 29°C, while ambient air temperature was near 22°C. The amount of 13CO2 added was manually regulated with a mass flow controller at average rates of c. 40 ml min−1 to keep the chamber CO2 mixing ratio at 600–800 ppmv throughout the labelling. This range was chosen to maximize photosynthetic uptake of the label, assuming that the short exposure of plants to elevated CO2 would not affect the allocation and respiratory use of C in the plant–soil system. This assumption is backed up by the fact that total soil CO2 efflux (SR) and its diurnal pattern did not differ between labelled and unlabelled plots. Also, the fact that the hourly means of the δ13C of soil-respired CO2 (δ13CSR) of unlabelled and labelled unshaded plots are well correlated (Fig. 4) appears to indicate that the labelling per se had no effect on the diurnal pattern of δ13CSR. CO2 concentrations of chamber air were continuously monitored using a 13CO2-sensitive and a 13CO2-insensitive infrared gas analyser (LCA4; ADC, Hoddesdon, UK). Pulse labelling was carried out on clear days during the morning hours, starting between 09:45 and 10:30 h CET. Altogether, three plots were labelled for a period of 100 min each.
Fig. 4.
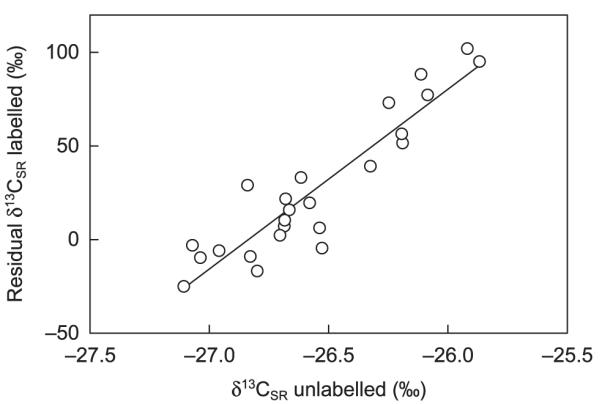
Relationship between hourly means of δ13C of soil-respired CO2 of unlabelled plots (δ13CSR unlabelled; x) and the residuals of the decay curve of labelled plots (residual δ13CSR labelled; y) for the unshaded treatments of the first experiment (y = 96.075x + 2578.2; R2 = 0.85, P < 0.001). Each hourly mean is based on an average of three hourly measurements for days with complete data sets (n = 7).
In labelling experiments where the soil system is not physically separated from the labelling chamber, diffusion of 13CO2 from the chamber into the soil is likely to occur during the labelling. Conversely, after the labelling is completed, back-diffusion of 13CO2 from the soil to the atmosphere takes place, producing a nonbiological δ13C signal. Thus, the measured isotopic signal after labelling results from a combination of back-diffusion of 13C from the label that diffuses into the soil during the labelling treatment and the isotopic signature of soil-respired CO2 (Högberg et al., 2008). To separate these effects we fitted a first-order decay function to the initial drawdown of δ13C after the labelling treatment was completed. To constrain this estimate of back-diffusion we also included two additional plots that were labelled for only 30 min. This model suggested back-diffusion time constants in the range of 4 to 16 min. By contrast, the first unambiguous physiological signal of increasing δ13C values occurred 1.5–4.5 h after the end of labelling (see also Results and Discussion). Thus, even in the plot with the highest back-diffusion time constant (16 min), virtually none of the initial diffused label was left in the soil when the first respiratory signal was detected.
Shading of unlabelled and labelled plots was achieved with tents of 3 × 3 m ground area and 2 m height. The tents were covered with nontransparent plastic sheets. Small slots at the bottom of the tent and the four corners facilitated an exchange of air. In the centre of the tent, where fluxes were measured, 5–8% of the incoming photosynthetically active radiation was incident on the vegetation, as measured with a PAR sensor (SunScan SS1; Delta-T Devices) during the course of a sunny day. Shading treatments were started 1 h after the pulse labelling was completed and lasted for 6–8 d.
Data analysis
The decay trend of δ13C on labelled plots was analysed using the regression function yielding the highest R2. To minimize the influence of back-diffusion immediately after labelling, regression analyses were based on δ13C values of less than 2000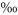 . The decay functions were used to calculate the mean residence time (MRT) of the label in soil-respired CO2 as the time required to reduce δ13C to 1/e times its initial value (2000
. The decay functions were used to calculate the mean residence time (MRT) of the label in soil-respired CO2 as the time required to reduce δ13C to 1/e times its initial value (2000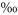 ). As the decay trend resulted both from a respiratory use of labelled C and a dilution of the labelled C pool by C assimilated after labelling, it was not possible to calculate how much label was respired how quickly. The decay functions were also used to separate the overall decay trend from possible diurnal patterns. This was done by calculating residuals of the respective decay curves shown in Table 1 in relation to observed values (i.e. observed minus predicted values).
). As the decay trend resulted both from a respiratory use of labelled C and a dilution of the labelled C pool by C assimilated after labelling, it was not possible to calculate how much label was respired how quickly. The decay functions were also used to separate the overall decay trend from possible diurnal patterns. This was done by calculating residuals of the respective decay curves shown in Table 1 in relation to observed values (i.e. observed minus predicted values).
Table 1.
Quantitative analysis of decay curves of δ13C of soil-respired CO2 in unshaded and shaded plots
| Peak 1 (h) | Peak 1 ( ) ) |
MRT (h) | Regression equations and statistics | |
|---|---|---|---|---|
| Unshaded 1 | 2.4 | 1518 | 48.7 | y = 45412x−1.0612, R2 = 0.973, P < 0.001 |
| Unshaded 2 | 6.1 | 1448 | 62.4 | y = 1996.8e−0.016x, R2 = 0.965, P < 0.001 |
| Unshaded 3 | 4.5 | 1405 | 60.0 | y = 1797.6e−0.0149x, R2 = 0.953, P < 0.001 |
| Shaded 1 | 13.0 | 1436 | 52.4 | y = 63073x−1.1242, R2 = 0.994, P < 0.001 |
| Shaded 2 | 16.5 | 1821 | 84.5 | y = 46882x−0.9365, R2 = 0.974, P < 0.001 |
| Shaded 3 | 18.5 | 1728 | 88.7 | y = 2547.4e−0.014x, R2 = 0.995, P < 0.001 |
| Unshaded mean ± SD | 4.3 ± 1.9 | 1437 ± 57 | 57.0 ± 2.7 | |
| Shaded mean ± SD | 16.0 ± 2.8 | 1684 ± 200 | 75.2 ± 6.9 |
Numbers 1–3 refer to the three independent experiments.
Peak 1 denotes the time (h since labelling ended) and δ13C value of the first peak of the decay curve (cf. Fig. 2a,b). Mean residence times of label in soil-respired CO2 (MRT, h since labelling ended) are based on an initial δ13C of 2000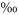 . Regression equations (x = h since start of experiment, y = δ13C) and statistics are shown for the best fitting curves.
. Regression equations (x = h since start of experiment, y = δ13C) and statistics are shown for the best fitting curves.
Results
The δ13C of soil-respired CO2 (δ13CSR) of control (i.e. unshaded and unlabelled) plots followed a distinct diurnal pattern, exhibiting significantly enriched δ13C values during the morning hours relative to those observed during the night, with intermediate values of δ13C in the afternoon and early evening (Fig. 1a). The sharp increase of δ13CSR occurred c. 1 h after sunrise and peaked at 08:30 h. The overall variability of δ13CSR was not explained by the soil CO2 efflux rate (R2 < 0.2), soil temperature at 5 or 10 cm depth (R2 < 0.1), soil water content (R2 < 0.2), air temperature (R2 < 0.1), air pressure (R2 < 0.1), vapour pressure deficit (R2 < 0.1) or PAR (R2 < 0.1), individually or in combination (R2 of a multiple linear regression < 0.2). Also, when δ13CSR was related to these parameters, no hysteresis was observed, which would have indicated an influence of lagged effects on the diurnal pattern, which could potentially also reduce the R2 of a possible relationship.
The shaded plots also showed a diurnal course in δ13CSR, but it was less pronounced than on the unshaded plots. δ13C values increased comparatively later in the morning (i.e. after 09:00 h) and remained well above the daily average for 9 h until the early evening (Fig. 1b). The average diurnal amplitude of δ13CSR decreased from 1.18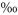 in the unshaded to 0.79
in the unshaded to 0.79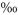 in the shaded plots, the daily average being very similar in the two treatments (δ13C = −26.82 and −26.73
in the shaded plots, the daily average being very similar in the two treatments (δ13C = −26.82 and −26.73 , respectively). The diurnal course of soil temperature in shaded plots was less pronounced than in the unshaded control plots (Fig. 1c) and did not explain the variability of δ13CSR (R2 < 0.1); nor did the soil CO2 efflux rate (R2 < 0.1). During the course of the shading experiments (lasting 6–8 d) no distinct changes in the diurnal pattern of δ13CSR were observed, which is also reflected in the comparatively small error bars in Fig. 1(b).
, respectively). The diurnal course of soil temperature in shaded plots was less pronounced than in the unshaded control plots (Fig. 1c) and did not explain the variability of δ13CSR (R2 < 0.1); nor did the soil CO2 efflux rate (R2 < 0.1). During the course of the shading experiments (lasting 6–8 d) no distinct changes in the diurnal pattern of δ13CSR were observed, which is also reflected in the comparatively small error bars in Fig. 1(b).
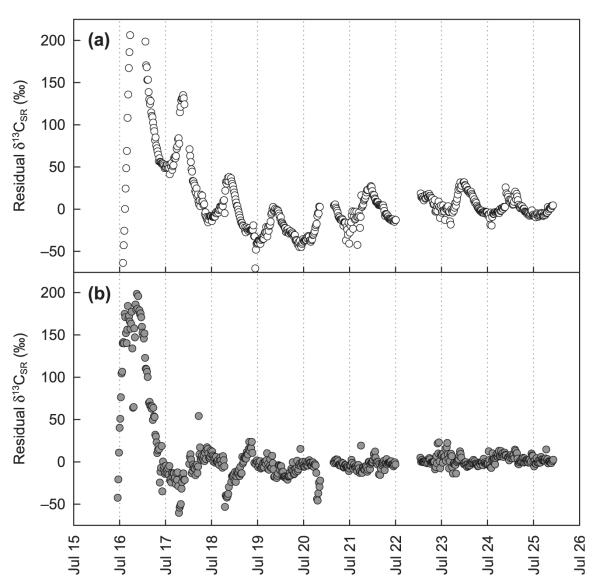
After pulse labelling, the δ13C of the sample air was highly enriched and declined rapidly (Fig. 2). δ13C increased again 1.5 h (unshaded plots) and 9.5 h (shaded plots) after pulse labelling was completed, and peaked 1 h (unshaded) and 3.5 h (shaded) later (inset in Fig. 2a,b; Table 1). Averaged over all three campaigns, the first peak occurred distinctly earlier in the unshaded as compared to the shaded plots (Table 1). Similarly, the subsequent decay trend of δ13CSR was more pronounced in the unshaded (MRT = 57.0 h) as compared with the shaded plots (MRT = 75.2 h; Table 1). In the unshaded plots, the overall decline of δ13CSR followed a diurnal pattern, with pronounced increases during the late night or early morning hours (mostly starting between midnight and sunrise) and peaks that normally occurred between 08:30 and 09:30 h (Fig. 2a). This pattern became more distinct when the overall decay trend was removed (Fig. 3a), which was also confirmed in two further experiments in separate plots, except during days with precipitation (not shown). In shaded plots, after the initial peak no consistent diurnal variation of δ13CSR was observed (Figs 2b, 3b). The diurnal pattern of the δ13CSR of unlabelled and labelled plots was well correlated for the unshaded treatment (Fig. 4), but not for the shaded one (R2 < 0.1).
Fig. 2.
Time course of (a, b) δ13C of soil-respired CO2 (δ13CSR) after pulse labelling in (a) an unshaded and (b) a shaded plot in a mountain grassland, (c) soil respiration in shaded (closed circles) and unshaded (open circles) plots (mean values of the respective control and the labelled plot, normalized for each plot to average pretreatment values), (d) photosynthetically active radiation (PAR; black line) incident on unshaded plots and vapour pressure deficit (VPD; dashed grey line) and (e) soil temperatures (Ts) (Ts unshaded, dashed grey line; Ts shaded, black line) and soil water content (SWC; dashed line) at 5 cm depth. Insets in (a) and (b) show the period immediately after labelling on an enlarged scale.
Fig. 3.
Diurnal variation of δ13C of soil-respired CO2 (δ13CSR) in labelled (a) unshaded and (b) shaded plots in the course of the first experiment, expressed as residuals of the respective decay curves shown in Table 1 in relation to observed values (Fig. 2a,b).
SR followed diurnal patterns in all plots (Figs 1c, 2c). No significant differences between unshaded and shaded plots could be detected, although the difference of c. 10% was close to significance after 6–8 d of shading, when all manually measured collars were pooled. This trend was probably a result of a decrease in temperature after shading (Figs 1e, 2e). The diurnal variation of hourly means of SR (Fig. 1c) was well explained by soil temperature in the unshaded (R2 = 0.64, P < 0.001) and particularly the shaded control plots (R2 = 0.85, P < 0.001). A progressive decrease in soil water content during the first campaign resulted in a decrease in the amount and amplitude of SR in both unshaded and shaded plots (Fig. 2c). Accordingly, the variability of SR was co-determined by soil temperature and moisture, and the percentage of variation explained by these two factors was 89% (P < 0.001; unshaded) and 79% (P < 0.001; shaded).
Discussion
Our study has shown that the δ13C of soil-respired CO2 (δ13CSR) in a grassland follows a diurnal pattern, confirming earlier similar observations for forests (Gessler et al., 2007; Kodama et al., 2008). The application of a tunable diode laser instrument (TDL) permitted a considerably higher time resolution of 13C:12C ratio measurements in CO2 (i.e. three referenced measurements per hour) than obtained for earlier studies, demonstrating the suitability of the TDL for investigating temporal dynamics of the carbon substrates utilized for respiratory processes in ecosystems (Bowling et al., 2003). We have shown that the background diurnal pattern of δ13CSR was not well explained by environmental conditions such as soil temperature, soil water content, PAR, air temperature, air pressure or soil CO2 efflux rate, individually or in combination (R2 < 0.2 in all cases). Thus we can also exclude measurement artefacts that might potentially induce an apparent diurnal shift in δ13CSR, including: potential effects of diurnal fluctuations in air temperature or pressure on instrument performance, such as effects on gas density and/or on the performance of the pump or detector; effects of soil CO2 efflux rates on concentration gradients in chambers, higher soil CO2 efflux rates decreasing the gradient and thus reducing gas diffusion (e.g. Davidson et al., 2002) and potentially altering isotopic diffusion fractionation; effects of pressure differences in the chamber–soil system (Lund et al., 1999). Pressure effects cannot be fully ruled out, as the arrangement of the pump in the system set-up may have created a slight continuous underpressure in the chambers. However, such an effect would be consistent throughout the experiment and should have resulted in a continuous offset of the values rather than effects on the diurnal courses. Diurnal effects of soil water content, which influences soil diffusivity (Moldrup et al., 2000) and the sorption of gases in the soil pore space and may thus also influence diffusive fractionation, were negligible throughout the experiments. This is important, as diffusive fractionation may be a major source of uncertainty in the interpretation of δ13CSR (Cerling et al., 1991; Bowling et al., 2008).
There are a number of possible mechanisms underlying a diurnal pattern of δ13CSR. The first set of mechanisms relates to diurnal changes in carbon source, which could be caused by: diurnal shifts in the δ13C of photosynthates, as induced, for example, by diurnal patterns of transitory starch accumulation and remobilization (Tcherkez et al., 2004; Göttlicher et al., 2006; Gessler et al., 2007) or effects of variable vapour pressure deficit on photosynthetic discrimination against 13C (Brugnoli et al., 1988; Farquhar et al., 1989; Ekblad & Högberg, 2001; Bowling et al., 2002); mixing of fresh photoassimilates with older metabolites during the transport of assimilates belowground, as has been suggested for trees (e.g. Betson et al., 2007; Gessler et al., 2007; Kodama et al., 2008); diurnal shifts in the respiratory utilization of different substrates for respiration and related differences in δ13C (e.g. the δ13C of lipids is distinctly more depleted than that of sugars; cf. Bowling et al., 2008). A further set of potential mechanisms relates to diurnal changes in respiratory C isotope fractionation. This has been documented for leaves (Hymus et al., 2005; Werner et al., 2007), but so far not for root or microbial components of soil respiration. Finally, diurnal patterns of δ13CSR could be caused by diurnal shifts in the contribution of autotrophic and heterotrophic components to soil respiration, which may differ in the isotopic signature of respired CO2 (Bowling et al., 2008).
From the pulse-labelling experiment we can conclude that under sunny conditions photoassimilates were transported and respired belowground within less than 2 h after the pulse labelling started. This is backed up by measurements of δ13C of root-respired CO2 and of biomass, as well as sucrose of roots that were harvested immediately after the labelling was completed and already showed highly enriched δ13C values originating from the label (data not shown). Earlier pulse-labelling studies have suggested similarly fast transfer rates of assimilated C to roots and the soil in short-stature vegetation (Ostle et al., 2000; Johnson et al., 2002; Carbone & Trumbore, 2007). The high δ13C values of soil-emitted air immediately after labelling are probably attributable to back-diffusion of labelled CO2 from the soil (Högberg et al., 2008), a replacement of labelled by ambient air resulting in a pronounced initial drawdown of δ13C. In unshaded plots c. 1.5–4.5 h after labelling, δ13C increased distinctly, which suggests that a significant amount of the C acquired during the labelling was respired. After another 1–1.5 h, δ13CSR C declined again, indicating that less of the labelled assimilates was used in respiration. A similar though delayed pattern was found in plots that were shaded after labelling, suggesting that the transfer and/or respiratory use of recent photoassimilates was slowed down by reduced subsequent photosynthesis. Interestingly, on the following days a diurnal pattern of δ13CSR occurred in the unshaded, but not in the shaded labelled plots, with distinct peaks mostly in the morning at c. 09:00 h (Fig. 3a). This pattern again suggests that carbohydrates that were assimilated during the labelling were preferentially used during the late night and early morning, while afterwards those derived from photosynthesis on the same day were the more important substrate for respiration during the rest of the day. No such diurnal variation of δ13CSR was observed in the shaded labelled plots (Fig. 3b). From this we conclude that photoassimilates trigger the variable use of labelled versus unlabelled assimilates on the same day. It is possible that such a variable use of different carbohydrate pools for respiration is also related to diurnal patterns of transitory starch accumulation and remobilization (see previous paragraph).
Control plots (i.e. unshaded and unlabelled) showed a diurnal pattern of δ13CSR that was similar to that of the unshaded labelled plots (Fig. 4), with a slight increase after midnight and a more rapid enrichment of δ13C after sunrise, δ13CSR peaking at c. 09:00 h. As the δ13C of leaves is often more negative than that of the soil (Bowling et al., 2008), the decline of δ13CSR after 09:00 h can again be attributed to an increased respiratory utilization of recent photoassimilates. However, such a pattern could also be the result of diurnal shifts in the contribution of autotrophic and heterotrophic components of soil respiration (cf. discussion above). There is limited evidence that the δ13C of root respiration is distinctly more negative than that of any other flux component contributing to ecosystem respiration (Bowling et al., 2008). A diurnal shift between autotrophic and heterotrophic components of soil respiration may be partly caused by temperature changes (Boone et al., 1998), but it is probably also assimilate driven, as the microbial component of soil respiration, including mycorrhizas, has been shown to respond more rapidly and sensitively to variable assimilate supply than roots (Bahn et al., 2006; Heinemeyer et al., 2006; Moyano et al., 2007).
In this context it is interesting that in shaded unlabelled plots a diurnal variability of δ13CSR was also observed, with more enriched δ13C values between 9:30 and 17:30 h, and more depleted values during the evenings and night-time. This diurnal course was not related to soil temperature and photosynthesis (which was eliminated by shading) and thus reflects an independent periodicity in the belowground plant–soil system.
Surprisingly, shading had no significant effect on SR, which contrasts with results from studies in C4-dominated grasslands (Craine et al., 1999; Wan & Luo, 2003). It can be assumed that SR in shaded plots was sustained by larger amounts of carbohydrates typically stored in roots, tubers and rhizomorphs of perennial mountain plants. Delayed peaks of δ13CSR and higher MRT of label in soil-respired CO2 in labelled shaded versus unshaded plots may have partly been caused by a lack of dilution of the labelled C pool as a result of the absence of photosynthesis, but they may have also been partly related to an increased use of older carbohydrates stored in intermediate or storage pools (Carbone & Trumbore, 2007).
Conclusion
This study has explored the coupling of processes in the plant–soil assimilatory–respiratory continuum with high time resolution measurements of δ13C, testing the effects of assimilate supply on δ13CSR in a combined shading and pulse-labelling experiment. In the grassland studied, our results indicate that: there is a temperature- and flux-rate-independent diurnal pattern of δ13CSR, δ13C being more enriched during the daytime and more depleted during the night-time; in the absence of a supply of fresh photoassimilates (during shading) the diurnal variation in δ13CSR is delayed and dampened but persists, possibly indicating a diurnal temperature- and assimilate-independent shift in the proportional contribution of autotrophic and heterotrophic components to soil respiration; freshly plant-assimilated C is rapidly transferred belowground and respired there, and is preferentially used for soil respiratory processes from the late morning hours onwards, whereas photosynthates originating from previous days are a predominant respiratory substrate during the night-time and early morning hours. Further studies on the diurnal patterns of carbohydrate metabolism and C transfer in the plant–soil system are needed to better evaluate and interpret these observed patterns.
Acknowledgements
We thank Dominik Steigner for maintaining the tunable diode laser in the field, Vincent Saxl, Valentina Danese, Zofia Garajova, Thomas Ladreiter-Knauss and Sebastian Waldhuber for assistance with the experimental set-up, Karin Bianchi for helping with the preparation of graphs, and Gert Bachmann (University of Vienna) for lending the 12C and 13C sensitive IRGAs used for monitoring CO2 concentrations during labelling. We gratefully acknowledge comments on the manuscript by Richard Norby and three anonymous referees. This study was financially supported by the Austrian Science Fund (FWF) (P18756-B16) and the Tiroler Wissenschaftsfonds.
References
- Bahn M, Knapp M, Garajova Z, Pfahringer N, Cernusca A. Root respiration in temperate mountain grasslands differing in land use. Global Change Biology. 2006;12:995–1006. [Google Scholar]
- Bahn M, Kutsch W, Heinemeyer A. Synthesis: emerging issues and challenges for an integrated understanding of soil carbon dynamics. In: Kutsch W, Bahn M, Heinemeyer A, editors. Soil carbon dynamics. An integrated methodology. Cambridge University Press; Cambridge, UK: 2009. in press. [Google Scholar]
- Bahn M, Rodeghiero M, Anderson-Dunn M, Dore S, Gimeno S, Drösler M, Williams M, Ammann C, Berninger F, Flechard C, et al. Soil respiration in european grasslands in relation to climate and assimilate supply. Ecosystems. 2008;11:1352–1367. doi: 10.1007/s10021-008-9198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betson NR, Göttlicher SG, Hall M, Wallin G, Richter A, Högberg P. No diurnal variation in rate or carbon isotope composition of soil respiration in a boreal forest. Tree Physiology. 2007;27:749–756. doi: 10.1093/treephys/27.5.749. [DOI] [PubMed] [Google Scholar]
- Boone RD, Nadelhoffer KJ, Canary JD, Kaye JP. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature. 1998;396:570–572. [Google Scholar]
- Bowling DR, McDowell N, Bond B, Law B, Ehleringer J. 13C content of ecosystem respiration is linked to precipitation and vapor pressure deficit. Oecologia. 2002;131:113–124. doi: 10.1007/s00442-001-0851-y. [DOI] [PubMed] [Google Scholar]
- Bowling DR, Pataki DE, Randerson JT. Carbon isotopes in terrestrial ecosystem pools and CO2 fluxes. New Phytologist. 2008;178:24–40. doi: 10.1111/j.1469-8137.2007.02342.x. [DOI] [PubMed] [Google Scholar]
- Bowling DR, Sargent SD, Tanner BD, Ehleringer J. Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem–atmosphere CO2 exchange. Agricultural and Forest Meteorology. 2003;118:1–19. [Google Scholar]
- Brugnoli E, Hubick KT, von Caemmerer S, Wong SC, Farquhar GD. Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiology. 1988;88:1418–1424. doi: 10.1104/pp.88.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadell JG, Le Quéré C, Raupach MR, Field CB, Buitenhuis ET, Ciais P, Conway TJ, Gillett NP, Houghton RA, Marland G. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proceedings of the National Academy of Sciences, USA. 2007;104:18866–18870. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone MS, Trumbore SE. Contribution of new photosynthetic assimilates to respiration by perennial grasses and shrubs: residence times and allocation patterns. New Phytologist. 2007;176:124–135. doi: 10.1111/j.1469-8137.2007.02153.x. [DOI] [PubMed] [Google Scholar]
- Cerling TE, Solomon DK, Quade J, Bowman JR. On the isotopic composition of carbon in soil carbon dioxide. Geochimica et Cosmochimica Acta. 1991;55:3403–3405. [Google Scholar]
- Craine JM, Wedin DA, Chapin FS., III Predominance of ecophysiological controls on soil CO2 flux in a Minnesota grassland. Plant and Soil. 1999;207:77–86. [Google Scholar]
- Davidson EA, Janssens IA, Luo Y. On the variability of respiration in terrestrial ecosystems: moving beyond Q10. Global Change Biology. 2006;12:154–164. [Google Scholar]
- Davidson EA, Savage K, Verchot LV, Navarro R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agricultural and Forest Meteorology. 2002;113:21–37. [Google Scholar]
- Ekblad A, Boström B, Holm A. Forest soil respiration rate and δ13C is regulated by recent above ground weather conditions. Oecologia. 2005;143:136–142. doi: 10.1007/s00442-004-1776-z. [DOI] [PubMed] [Google Scholar]
- Ekblad A, Högberg P. Natural abundance of 13C in CO2 respired from forest soils reveals speed of link between tree photosynthesis and root respiration. Oecologia. 2001;127:305–308. doi: 10.1007/s004420100667. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology. 1989;40:503–537. [Google Scholar]
- Gessler A, Keitel C, Kodama N, Weston C, Winters AJ, Keith H, Grice K, Leuning R, Farquhar GD. Δ13C of organic matter transported from the leaves to the roots in Eucalyptus delegatensis: short-term variations and relation to respired CO2. Functional Plant Biology. 2007;34:692–706. doi: 10.1071/FP07064. [DOI] [PubMed] [Google Scholar]
- Göttlicher S, Knohl A, Wanek W, Buchmann N, Richter A. Short-term changes in carbon isotope composition of soluble carbohydrates and starch: from canopy leaves to the root system. Rapid Communications in Mass Spectrometry. 2006;20:653–660. doi: 10.1002/rcm.2352. [DOI] [PubMed] [Google Scholar]
- Graf A, Weihermüller L, Huisman JA, Herbst M, Bauer J, Vereecken H. Measurement depth effects on the apparent temperature sensitivity of soil respiration in field studies. Biogeosciences. 2008;5:1175–1188. [Google Scholar]
- Heinemeyer A, Ineson P, Ostle N, Fitter AH. Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature. New Phytologist. 2006;171:159–170. doi: 10.1111/j.1469-8137.2006.01730.x. [DOI] [PubMed] [Google Scholar]
- Högberg P, Högberg MN, Göttlicher SG, Betson NR, Keel SG, Metcalfe DB, Campbell C, Schindlbacher A, Hurry V, Lundmark T, et al. High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytologist. 2008;177:220–228. doi: 10.1111/j.1469-8137.2007.02238.x. [DOI] [PubMed] [Google Scholar]
- Högberg P, Read DJ. Towards a more plant physiological perspective on soil ecology. Trends in Ecology & Evolution. 2006;21:548–554. doi: 10.1016/j.tree.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Hymus GJ, Maseyk K, Valentini R, Yakir D. Large daily variation in 13C-enrichment of leaf-respired co2 in two Quercus forest canopies. New Phytologist. 2005;167:377–384. doi: 10.1111/j.1469-8137.2005.01475.x. [DOI] [PubMed] [Google Scholar]
- Johnson D, Leake JR, Ostle N, Ineson P, Read DJ. In situ 13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. NewPhytologist. 2002;153:327–334. [Google Scholar]
- Knohl A, Werner RA, Brand WA, Buchmann N. Short-term variations in δ13C of ecosystem respiration reveals link between assimilation and respiration in a deciduous forest. Oecologia. 2005;142:70–82. doi: 10.1007/s00442-004-1702-4. [DOI] [PubMed] [Google Scholar]
- Kodama N, Barnard R, Salmon Y, Weston C, Ferrio J, Holst J, Werner R, Saurer M, Rennenberg H, Buchmann N, et al. Temporal dynamics of the carbon isotope composition in a Pinus sylvestris stand: from newly assimilated organic carbon to respired carbon dioxide. Oecologia. 2008;156:737–750. doi: 10.1007/s00442-008-1030-1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Edwards NT, Post WM, Gu L, Ledford J, Lenhart S. Temperature-independent diel variation in soil respiration observed from a temperate deciduous forest. Global Change Biology. 2006;12:2136–2145. [Google Scholar]
- Lund CP, Riley WJ, Pierce LL, Field CB. The effects of chamber pressurization on soil-surface CO2 flux and the implications for NEE measurements under elevated CO2. Global Change Biology. 1999;5:269–281. [Google Scholar]
- Moldrup P, Olesen T, Schjonning P, Yamaguchi T, Rolston DE. Predicting the gas diffusion coefficient in undisturbed soil from soil water characteristics. Soil Science Society of America Journal. 2000;64:94–100. [Google Scholar]
- Mortazavi B, Chanton JP, Prater JL, Oishi AC, Oren R, Katul G. Temporal variability in 13C of respired CO2 in a pine and a hardwood forest subject to similar climatic conditions. Oecologia. 2005;142:57–69. doi: 10.1007/s00442-004-1692-2. [DOI] [PubMed] [Google Scholar]
- Moyano FE, Kutsch WL, Schulze E-D. Response of mycorrhizal, rhizosphere and soil basal respiration to temperature and photosynthesis in a barley field. Soil Biology and Biochemistry. 2007;39:843–853. [Google Scholar]
- Ostle N, Ineson P, Benham D, Sleep D. Carbon assimilation and turnover in grassland vegetation using an in situ 13CO2 pulse labelling system. Rapid Communications in Mass Spectrometry. 2000;14:1345–1350. doi: 10.1002/1097-0231(20000815)14:15<1345::AID-RCM22>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Pavelka M, Acosta M, Marek M, Kutsch W, Janous D. Dependence of the Q10 values on the depth of the soil temperature measuring point. Plant and Soil. 2007;292:171–179. [Google Scholar]
- Raich JW, Potter CS, Bhagawati D. Interannual variability in global soil respiration, 1980–94. Global Change Biology. 2002;8:800–812. [Google Scholar]
- Reichstein M, Subke J-A, Angeli AC, Tenhunen JD. Does the temperature sensitivity of decomposition of soil organic matter depend upon water content, soil horizon, or incubation time? Global Change Biology. 2005;11:1–14. [Google Scholar]
- Steinmann KTW, Siegwolf R, Saurer M, Körner C. Carbon fluxes to the soil in a mature temperate forest assessed by 13C isotope tracing. Oecologia. 2004;141:489–501. doi: 10.1007/s00442-004-1674-4. [DOI] [PubMed] [Google Scholar]
- Tang J, Baldocchi DD, Xu L. Tree photosynthesis modulates soil respiration on a diurnal time scale. Global Change Biology. 2005;11:1298–1304. [Google Scholar]
- Tcherkez G, Farquhar G, Badeck F, Ghashghaie J. Theoretical considerations about carbon isotope distribution in glucose of C3 plants. Functional Plant Biology. 2004;31:857–877. doi: 10.1071/FP04053. [DOI] [PubMed] [Google Scholar]
- Trumbore S. Carbon respired by terrestrial ecosystems – recent progress and challenges. Global Change Biology. 2006;12:141–153. [Google Scholar]
- Vargas R, Allen ME. Environmental controls and the influence of vegetation type, fine roots and rhizomorphs on diel and seasonal variation in soil respiration. New Phytologist. 2008;179:460–471. doi: 10.1111/j.1469-8137.2008.02481.x. [DOI] [PubMed] [Google Scholar]
- Wan S, Luo Y. Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment. Global Biogeochemical Cycles. 2003;17:1–12. [Google Scholar]
- Werner C, Hasenbein N, Maia R, Beyschlag W, Máguas C. Evaluating high time-resolved changes in carbon isotope ratio of respired CO2 by a rapid in-tube incubation technique. Rapid Communications in Mass Spectrometry. 2007;21:1352–1360. doi: 10.1002/rcm.2970. [DOI] [PubMed] [Google Scholar]
- Wingate L, Seibt U, Moncrieff JB, Jarvis PG, Lloyd J. Variations in 13C discrimination during CO2 exchange by Picea sitchensis branches in the field. Plant, Cell & Environment. 2007;30:600–616. doi: 10.1111/j.1365-3040.2007.01647.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Furtaw MD, Madsen RA, Garcia RL, Anderson DJ, McDermitt DK. On maintaining pressure equilibrium between a soil CO2 flux chamber and the ambient air. Journal of Geophysical Research. 2006;111 (D08S10): doi: 10.1029/2005JD006435. [Google Scholar]