SUMMARY
Ca2+ is an essential and ubiquitous second messenger. Changes in cytosolic Ca2+ trigger events critical for tumorigenesis, such as cellular motility, proliferation and apoptosis. We show that an isoform of Secretory Pathway Ca2+-ATPase, SPCA2, is upregulated in breast cancer-derived cells and human breast tumors, and suppression of SPCA2 attenuated basal Ca2+ levels and tumorigenicity. Contrary to its conventional role in Golgi Ca2+ sequestration, expression of SPCA2 increased Ca2+ influx by a mechanism dependent on the store-operated Ca2+ channel Orai1. Unexpectedly, SPCA2-Orai1 signaling was independent of ER Ca2+ stores or STIM1 and STIM2 sensors, and uncoupled from Ca2+-ATPase activity of SPCA2. Binding of SPCA2 amino terminus to Orai1 enabled access of its carboxyl terminus to Orai1 and activation of Ca2+ influx. Our findings reveal a signaling pathway in which Orai1-SPCA2 complex elicits constitutive store-independent Ca2+ signaling that promotes tumorigenesis.
Keywords: Ca2+ signaling, tumorigenesis, basal calcium level, Orai1, STIM1 and STIM2, Ca2+-ATPase activity, store-independent calcium influx
INTRODUCTION
Basal Ca2+ concentrations are tightly controlled within a narrow submicromolar range by an array of Ca2+ channels and pumps that are susceptible to dysregulation in cancer. Transient changes in cytosolic Ca2+ induce downstream signaling events, which regulate a wide range of cellular functions (Berridge et al., 2003; Clapham, 2007; Roderick and Cook, 2008). Ca2+ signaling is required for every stage of the eukaryotic cell cycle, including activation and expression of transcriptional factors and cyclin-dependent kinases which are necessary for cell cycle progression (Hogan et al., 2003; Roderick and Cook, 2008), as well as centrosome duplication and separation (Fukasawa, 2007; Matsumoto and Maller, 2002). Crosstalk with other signaling mechanisms, such as the Ras pathway, regulates cell cycle transition and cell proliferation (Cook and Lockyer, 2006; Cullen and Lockyer, 2002). Dynamic regulation of Ca2+ signaling is achieved by cooperation of various cellular components including receptors, channels, transporters, buffering proteins, and downstream effectors (Berridge et al., 2003). Thus, inappropriate activation of Ca2+ influx channels or downregulation of Ca2+ efflux and sequestration mechanisms could increase basal Ca2+ to augment Ca2+ signaling and tumor cell proliferation. Alternatively, changes that deplete the ER Ca2+ store can confer cellular resistance to apoptosis (Monteith et al., 2007).
In most non-excitable cells, depletion of ER stores elicits sustained Ca2+ influx by store operated Ca2+ (SOC) entry, defining the major Ca2+ influx pathway. Upon the stimulation of cell surface receptors, depletion of ER Ca2+ results in release of Ca2+ from luminal EF hand domains of ER-localized STIM proteins, triggering their translocation to ER-plasma membrane junctions where they bind and activate Orai1, the pore subunit of Ca2+ release-activated Ca2+ (CRAC) channel, and resulting in refilling of ER stores (Cahalan et al., 2007; Gwack et al., 2007; Lewis, 2007; Vig and Kinet, 2007). Store-operated Ca2+ influx is essential for maintaining ER Ca2+ content at a precise level, and functions in various physiological processes such as gene transcription, cell cycle progression and apoptosis (Parekh and Putney, 2005). Dysfunction of store-operated Ca2+ signaling mediated by STIM and Orai1 leads to inhibition of physiological and pathophysiological activities including breast tumor cell migration and tumor metastasis (Yang et al., 2009), vascular smooth muscle cell proliferation and migration (Potier et al., 2009), and T cell activation and tolerance (Oh-Hora et al., 2008).
The Secretory Pathway Ca2+-ATPases (SPCA) are ATP-powered pumps that deliver Ca2+ and Mn2+ ions into the Golgi lumen for protein sorting, processing and glycosylation (Durr et al., 1998). In higher vertebrates, including human, this essential function is carried out by the ubiquitously expressed SPCA1 isoform, with orthologs in lower eukaryotes including yeast, nematode and fruitfly (Missiaen et al., 2007). A closely related second isoform, SPCA2, shares similar transport characteristics and appears at first glance to have a redundant role, given its absence in lower eukaryotes (Vanoevelen et al., 2005; Xiang et al., 2005). The limited tissue distribution of SPCA2 includes mammary epithelium, where it is sharply upregulated during lactation. Whereas SPCA1 showed a modest 2-fold induction upon lactation, SPCA2 increased by 35-fold and was localized to the luminal secretory cells of the mammary gland (Faddy et al., 2008).
We hypothesized that transformation of mammary epithelial cells to a cancerous phenotype would be accompanied by dysregulation of Ca2+ transporters and their downstream signaling pathways, augmenting proliferation and tumor formation. Furthermore, localized, inappropriate secretion of Ca2+ in the absence of calcium buffers, could result in microcalcifications which appear as radiographic “signatures” on mammograms used in diagnosis of breast cancer (Morgan et al., 2005). Although microcalcifications have been extensively used to characterize abnormalities in the breast tissue, a mechanistic understanding of the source of calcium and the specific pathways that lead to their deposition has remained elusive.
In this study we show that SPCA2 elicits constitutive Ca2+ signaling, mediated by Orai1, which correlates with oncogenic activities of mammary tumor cells. Unexpectedly, SPCA2-induced Ca2+ signaling was independent of its Ca2+ pump activity, and not regulated by store-depletion or STIM proteins. SPCA2 interacted with Orai1 by its N-terminus, and activated Ca2+ influx by the C-terminus. These findings reveal a previously unknown Ca2+ signaling mechanism in which Orai1 mediates store-independent Ca2+ influx, and dysregulation of SPCA2 constitutively activates this pathway leading to oncogenic activity of tumor cells.
RESULTS
Upregulation of SPCA2 Induces Oncogenic Signaling in Mammary Tumor Cells
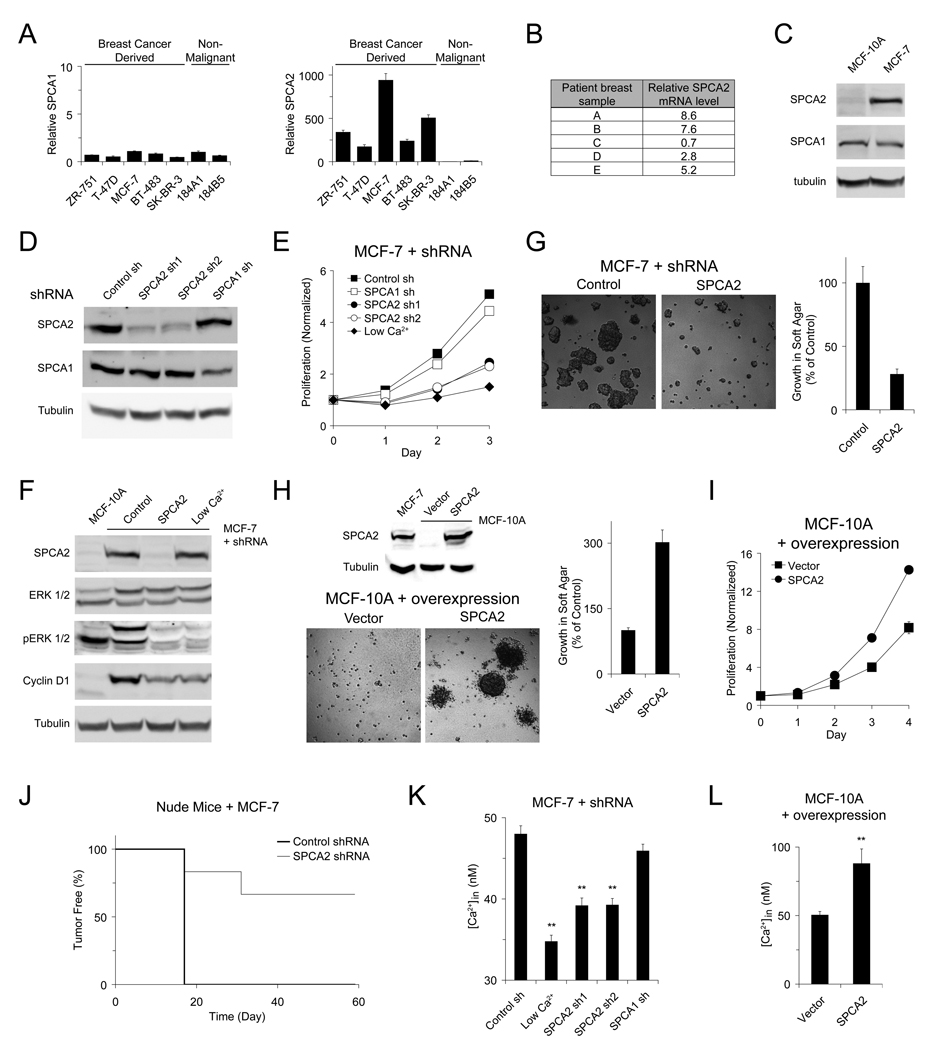
We investigated the expression of SPCA isoforms in a range of breast cancer-derived and non-malignant mammary epithelial cells by quantitative RT-PCR. In contrast to comparable mRNA levels of SPCA1, SPCA2 was highly upregulated in luminal-like breast cancer-derived cell lines (Figure 1A). Examination of mRNA levels in breast tissue from a small pool of breast cancer patients confirmed this upregulation (Figure 1B) and prompted us to mine data from microarray profiles of 295 primary human breast tumors: highest levels of SPCA2 were found in ERBB2+ tumors, among five transcriptional subtypes (Figure S1). Consistent with mRNA levels, protein expression of SPCA2 was higher in MCF-7 cells, a human breast adenocarcinoma cell line, relative to MCF-10A, a non-malignant human mammary epithelial cell line; in contrast, there was no increase in SPCA1 expression in MCF-7 (Figure 1C). We used lentiviral delivery of shRNA constructs to knock down expression of endogenous SPCA proteins in MCF-7 cells (Figure 1D). Proliferation was inhibited in SPCA2KD cells, with growth rates slower than mock transduced cells, and similar to cells growing in low extracellular Ca2+ (~0.1 mM). In contrast, SPCA1KD did not cause this growth phenotype (Figure 1E). The RAS-RAF-MEK-ERK1/2 pathway is known to play an essential role in cell proliferation and survival. Phosphorylation of ERK1/2 drives activation of transcriptional factors and expression of downstream proteins such as cyclin D1, which is essential for completion of G1/S transition (Coleman et al., 2004; Roderick and Cook, 2008). Levels of phospho-ERK and cyclin D1 reduced dramatically in SPCA2KD cells, as well as in cells incubated in low extracellular Ca2+, relative to control cells (Figure 1F).
Figure 1. Upregulation of SPCA2 induces oncogenic signaling in mammary tumor cells.
mRNA levels were measured by quantitative real-time RT-PCRs and normalized to 18S rRNA in (A) a panel of breast epithelial cell lines relative to 184A1 and (B) in human breast tumor samples compared to matched normal surrounding breast tissue. n = 3 in (A). (C) Immunoblot of SPCA expression in MCF-10A and MCF-7 cells. Immunoblot (D) and normalized proliferation (E) of MCF-7 cells lentivirally transduced with shRNA against SPCA isoforms. n = 3 in (E). (F) Immunoblot of ERK 1/2 phosphorylation and Cyclin D1 expression in MCF-7 cells transduced with shSPCA2. Micrographs and normalized growth of (G) MCF-7 cells with SPCA2 knockdown or (H) MCF-10A cells with SPCA2 overexpression in soft agar. n = 3. Immunoblot showing relative SPCA2 expression levels in (H). (I) Normalized proliferation of MCF-10 cells with SPCA2 overexpression. n = 3. (J) Tumor incidence in nude mice injected with MCF-7 cells; n = 6, P = 0.005 (log-rank test). (K) Basal Ca2+ levels in MCF-7 cells with SPCA2 knockdown. From left to right: n = 80, 80, 77, 81, 69. ** P < 0.01 (Student’s t-test). (L) Basal Ca2+ levels in MCF-10A cells with SPCA2 overexpression. Vector, n = 23; SPCA2, n = 23. ** P < 0.01 (Student’s t-test). Error bars represent standard error (K and L) or standard deviation (A, E, G, H and I).
We examined the effects of depleting endogenous SPCA2 on the transformed phenotype of MCF-7 cells by monitoring growth of cells in soft agar. Fewer and smaller colonies were observed in soft agar seeded with SPCA2KD cells compared to control cells, and normalized results showed a clear reduction of growth in SPCA2KD cells (Figure 1G). Conversely, overexpression of SPCA2 in non-tumorigenic MCF-10A cells conferred the ability to form colonies in soft agar (Fig. 1H) and increased proliferation rate (Figure 1I). We also monitored tumor generation in nude mice injected subcutaneously in the flank with control or SPCA2KD MCF-7 cells. We show that SPCA2KD conferred only sporadic and delayed tumor formation relative to control (Figure 1J).
To determine whether oncogenic activity of endogenous SPCA2 was mediated by Ca2+ signaling, we measured basal cytoplasmic Ca2+ levels in control MCF-7 and SPCA2KD cells. We showed significant reduction of intracellular Ca2+ levels in SPCA2KD cells and in cells growing in low extracellular Ca2+, but not in SPCA1KD cells (Figure 1K). On the other hand, overexpression of SPCA2 in MCF-10A cells significantly increased basal Ca2+ concentration (Figure 1L). Although basal Ca2+ levels varied between cell lines, these levels could be modulated by SPCA2 expression levels. Thus, SPCA2 appears to play a role in regulating basal Ca2+, and upregulation of SPCA2 results in constitutive increase of basal Ca2+ and cell proliferation associated with oncogenesis.
SPCA2 Elicits Constitutive Ca2+ signaling Independent of Transport Function
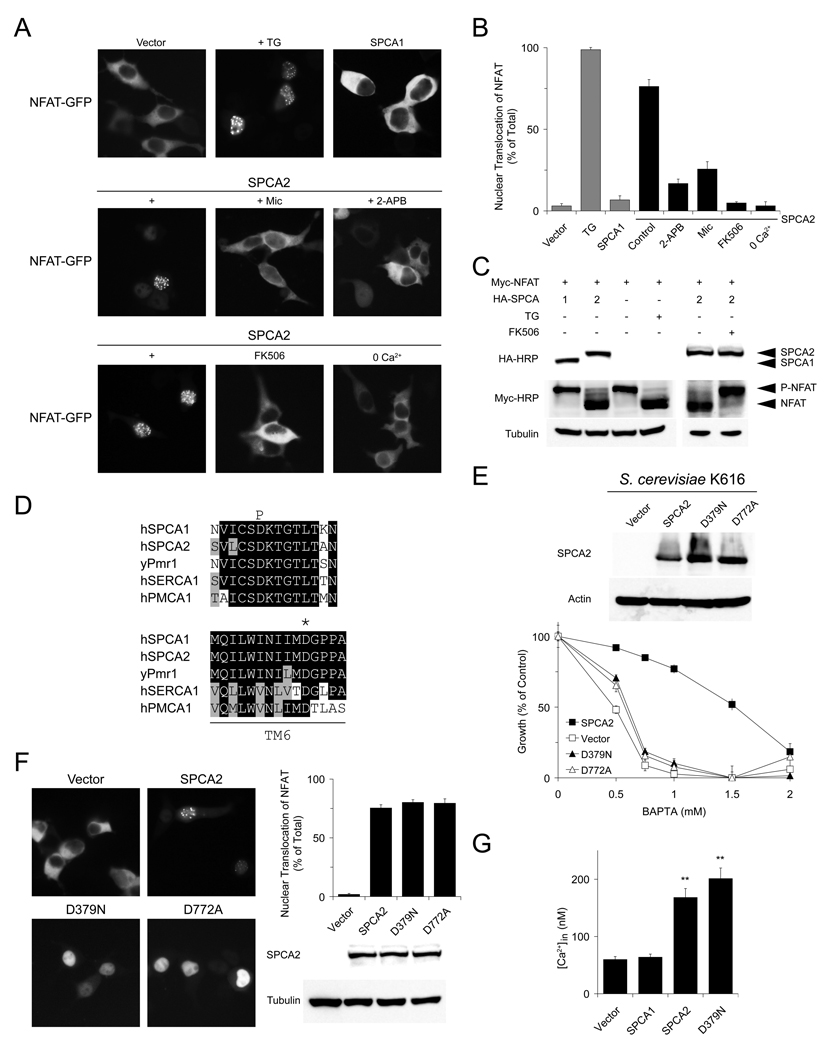
To investigate the molecular basis of SPCA2-induced Ca2+ signaling, we began by monitoring Ca2+-dependent localization of the nuclear factor of activated T cells, NFAT (Crabtree and Olson, 2002; Huang et al., 2006) in HEK293 cells where expression of SPCA2 is relatively low (Figure S2A). In resting cells GFP-tagged NFAT localized exclusively in the cytoplasm. Following treatment with thapsigargin, a blocker of sarco/endoplasmic reticulum Ca2+-ATPases (SERCA), depletion of ER Ca2+ resulted in store-operated Ca2+ entry, elevation of basal Ca2+ level and nuclear translocation of NFAT-GFP in nearly 100% of cells, as expected (Figures 2A–2B). Whereas transient expression of SPCA1 in HEK293 cells did not alter cytoplasmic localization of NFAT-GFP under resting conditions, transient expression of SPCA2 elicited nuclear translocation of NFAT-GFP in ~75% of cells. This was inhibited by store-operated Ca2+ channel blockers, miconazole (Clementi and Meldolesi, 1996) and 2-APB (Parekh and Putney, 2005) at the reported concentrations, and in low extracellular Ca2+, indicating Ca2+ entry through plasma membrane Ca2+ channels. Inhibition of the Ca2+-activated Ser/Thr phosphatase calcineurin by FK506 also prevented nuclear relocalization of NFAT-GFP in SPCA2-transfected cells (Figures 2A–2B). Accordingly, NFAT was predominantly dephosphorylated in TG treated- or SPCA2-expressing cells, but remained phosphorylated in cells transfected with SPCA1 or empty vector, and in FK506 treated-cells (Figure 2C).
Figure 2. SPCA2 elicits constitutive Ca2+ signaling independent of transport function.
Representative live images (A) and quantification of nuclear localization (B) of NFAT-GFP in HEK293 cells transfected with SPCA1 or SPCA2, or treated with drugs. n = 3 in (B). (C) Immunoblot showing phosphorylation status of NFAT following expression of SPCA1, SPCA2 or treatment with thapsigargin (TG) or FK506. (D) Alignments showing conserved aspartates in phosphorylation (P) domain (D379 in SPCA2) or transmembrane helix 6 (D772 in SPCA2) of P-type Ca2+-ATPases. (E) Immunoblot and normalized growth of yeast K616 expressing SPCA2 or mutants D379N and D772A in BAPTA medium. n = 4. (F) Representative live images, quantification of NFAT translocation in HEK293 cells and immunoblot showing expression of SPCA2 WT or mutants. n = 3. (G) Basal Ca2+ levels in HEK293 cells expressing SPCA1, SPCA2 or D379A mutant. From left to right, n = 57, 47, 46, 44. ** P < 0.01 (Student’s t-test). Error bars represent standard error (G) or standard deviation (B, E and F).
These findings were unexpected, given the known function of SPCA2 in pumping Ca2+ away from the cytoplasm into Golgi/vesicular stores. To determine whether constitutive Ca2+ signaling elicited by SPCA2 was dependent on its Ca2+ pumping ability, we generated two variants: mutant D379N lacks the conserved and essential aspartate that is transiently phosphorylated by ATP in the catalytic cycle, and mutant D772A disrupts a conserved and essential Ca2+ binding site (Figure 2D) (Wei et al., 2000). Both mutants failed to rescue growth of a yeast strain lacking endogenous Ca2+ pumps in BAPTA supplemented medium, consistent with loss of Ca2+-ATPase activity (Figure 2E), but retained ability to elicit constitutive NFAT translocation in HEK293 cells (Figure 2F). Also, mutant D379N induced growth of MCF-10A in soft agar similar to wild type SPCA2 (Figure S2B). Furthermore, introduction of SPCA2, either wild type or D379N mutant, resulted in elevated basal cytoplasmic Ca2+ levels in HEK293 cells, relative to cells transfected with empty vector or SPCA1 (Figure 2G). This was reminiscent of the effect of upregulation of endogenous SPCA2 on basal Ca2+ levels in MCF-7 cells (Figure 1K). We conclude that SPCA2, but not SPCA1, induces constitutive Ca2+ influx and signaling by a mechanism that is independent of its known function as a Ca2+-ATPase.
SPCA2-Mediated Ca2+ Signaling Is Store-Independent
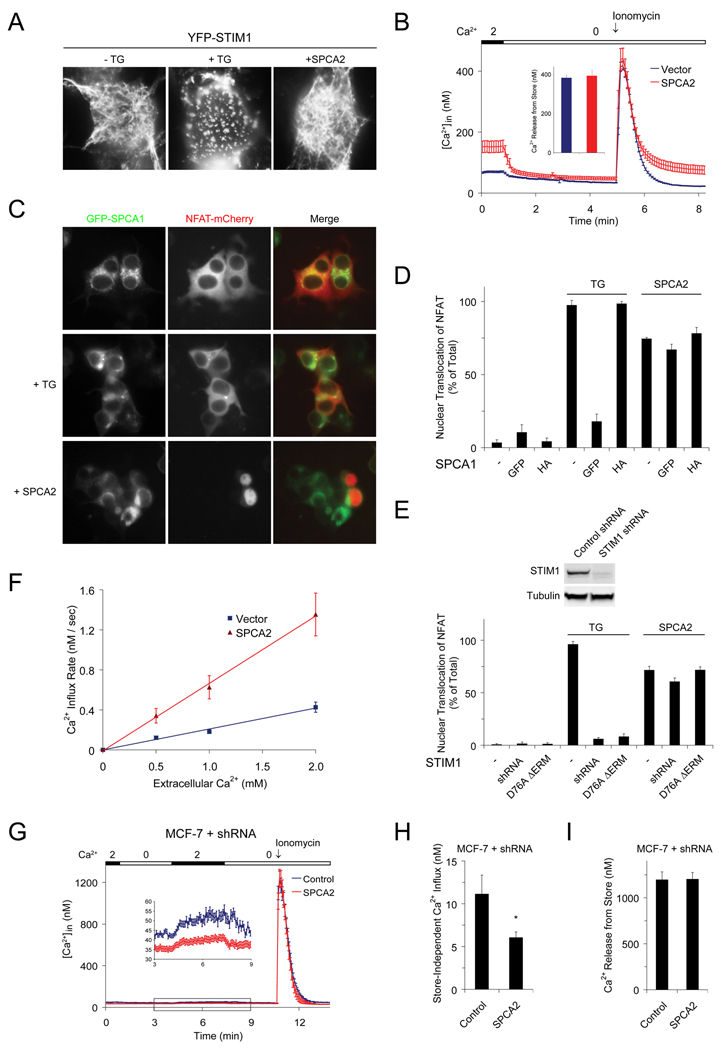
To investigate the possibility that SPCA2 could elicit ER Ca2+ store depletion leading to constitutive store operated Ca2+ entry (SOCE) we examined the status of the ER Ca2+ store. YFP-STIM1, the ER localized Ca2+ sensor protein, was present in a reticular, ER-like pattern in resting cells, and redistributed to punctae after store depletion with thapsigargin (Liou et al., 2005), as expected (Figure 3A). Transient transfection with SPCA2 did not elicit puncta formation of YFP-STIM1, suggesting that the ER store was not Ca2+ depleted (Figure 3A). Next, we directly measured the ER Ca2+ content by ionomycin treatment to completely release Ca2+ from intracellular stores. In Ca2+-free medium, peak levels of Ca2+ released by ionomycin were identical in cells transfected with SPCA2 or empty vector (Figure 3B and Figures S3A–S3B). In a different, independent approach, we used a thapsigargin-insensitive, ER localized Ca2+-ATPase to ensure that intracellular ER stores were replete with Ca2+. N-terminal GFP-tagged SPCA1 partially mislocalizes to the ER (Figure S3C) where it is functional in filling the stores and preventing nuclear translocation of NFAT-mCherry after thapsigargin treatment (Figure S3D and Figures 3C–3D). Despite co-expression with ER-localized GFP-SPCA1, SPCA2 was capable of eliciting nuclear translocation of NFAT-mCherry (Figures 3C–3D). HA-tagged SPCA1 localizes to the Golgi (Figure S3C) and does not interfere with thapsigargin-induced SOCE, nor with SPCA2-induced nuclear translocation of NFAT-mCherry (Figure 3D), indicating that Ca2+ signaling by SPCA2 is also independent of Golgi stores. It was previously reported that knock down of STIM1 or expression of the dominant negative mutant D76A ΔERM blocked SOC signaling (Huang et al., 2006; Roos et al., 2005), as seen by the failure of TG to elicit nuclear translocation of NFAT (Figure 3E and Figures S3E–S3F). Also, knockdown of STIM2, the feedback regulator of cytosolic and ER Ca2+ levels, was shown to lower basal Ca2+ concentration (Brandman et al., 2007) (Figures S3E and S3G). However, despite expression of dominant negative D76A ΔERM or knockdown of STIM1 and STIM2 expression, either singly or in combination, SPCA2 retained the ability to increase basal Ca2+ concentration and cause NFAT-GFP translocation to the nucleus, evidently by a STIM-independent Ca2+ signaling mechanism (Figure 3E and Figures S3F–S3G). We showed that SPCA2 expression in HEK293 cells resulted in Ca2+ influx from the extracellular medium, as shown by Mn2+ quench of intracellular pre-loaded Fura-2 as well as 45Ca2+ uptake (Figures S4A–S4B). Initial rates of uptake, monitored within the first 60 s, were significantly increased by expression of SPCA2 (Figure 3F and Figures S4D–S4I). Additionally, measurement of 45Ca2+ efflux and Fura2 fluorescence confirmed that SPCA2-induced elevation of intracellular Ca2+ did not result from decreased rates of Ca2+ efflux (Figures S4C–S4F). Consistent with these findings, knockdown of endogenous SPCA2 in MCF-7 cells also diminished store-independent Ca2+ entry without changing internal Ca2+ stores (Figures 3G–3I). Taken together, our results reveal a mechanism for SPCA2-mediated Ca2+ signaling that is independent of both ER and Golgi Ca2+ stores.
Figure 3. SPCA2 mediated Ca2+ signaling is store-independent.
(A) Localization of YFP-STIM1 in HEK293 cells following TG treatment or SPCA2 expression. (B) Representative Ca2+ traces following emptying of stores with 2 µM Ionomycin in HEK293 cells with or without SPCA2 expression. Vector, n = 40; SPCA2, n = 25. Cells were cultured in low Ca2+ medium (~0.1 mM) after transfection, followed by a 30 min incubation in normal Ca2+ (2 mM) immediately before calcium imaging experiments to allow restoration of stores (as described in “Supplemental Experimental Procedures - Calcium imaging”). (C) Representative images of GFP-SPCA1 and NFAT-mCherry following TG treatment or SPCA2 expression in HEK293 cells and (D) quantification of nuclear NFAT-mCherry translocation. n = 3 in (D). (E) Quantification of NFAT nuclear translocation following STIM1 knockdown or expression of dominant negative STIM1 mutant in cells treated with TG or expressing SPCA2. n = 3. (F) Initial rates of Ca2+ influx in HEK293 cells with or without SPCA2 expression, calculated from the experiments shown in Figure S4, with 0.5 mM, 1.0 mM or 2.0 mM extracellular Ca2+. Vector: n = 30 (0.5 mM), 25 (1.0 mM), 28 (2.0 mM); SPCA2: n = 28 (0.5 mM), 23 (1.0 mM), 25 (2.0 mM). Representative Ca2+ traces (G) and average intracellular Ca2+ concentration representing store-independent Ca2+ influx (H) and internal Ca2+ store content (I) in ControlKD and SPCA2KD MCF-7 cells. ControlKD, n = 36; SPCA2KD, n = 30. * P < 0.05 (Student’s t-test). Error bars represent standard error (B, F, G, H and I) or standard deviation (D and E).
SPCA2 Interacts with Orai1 to Mediate Ca2+ Entry
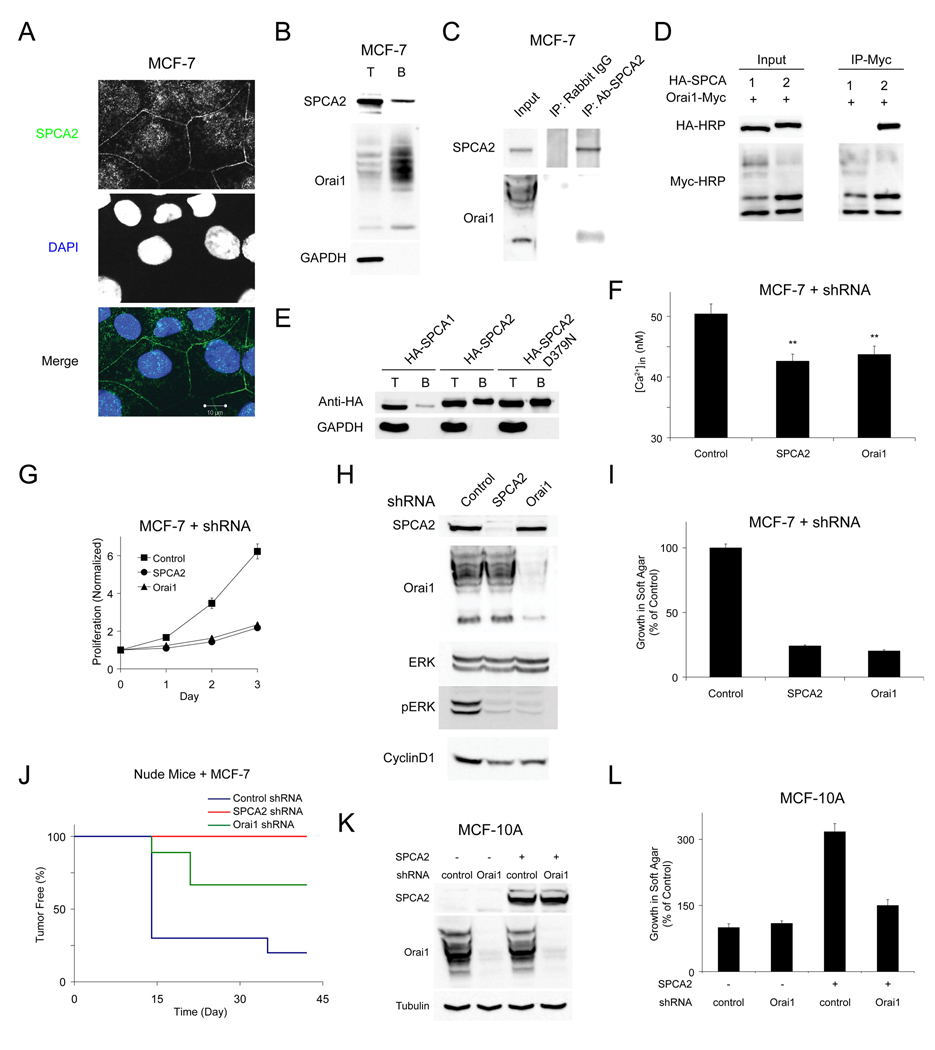
Our results suggested that SPCA2 elicited Ca2+ influx through plasma membrane Ca2+ channels. Immunofluorescence and cell surface biotinylation showed partial localization of endogenous SPCA2 to the plasma membrane in MCF-7 cells, where it has the potential to elicit Ca2+ influx (Figures 4A–4B). Next, we sought evidence for physical interaction between SPCA2 and candidate Ca2+ channels. Although SPCA2 mediated Ca2+ influx was independent of ER stores, we observed co-immunoprecipitation of the endogenous store-operated channel Orai1 and native SPCA2 in MCF-7 cells (Figure 4C). We verified and extended these findings using epitope-tagged proteins expressed in HEK293 cells: we could document robust co-immunoprecipitation of Orai1-Myc and HA-SPCA2 (Figure 4D). Consistent with the specificity of the Orai1-SPCA2 interaction, HA-SPCA1 did not co-immunoprecipitate with Orai1 (Figure 4D). Similar to endogenous protein, up to 10% of HA-SPCA2 could be labeled by cell surface biotinylation, including both wild type and the pump-inactive D379N mutant; in contrast, SPCA1 was barely detectable (Figure 4E). Surface residence of SPCA2 correlated with total expression levels, and with elevation of basal Ca2+ (Figure S5A). We also used cell surface biotinylation to confirm that a portion of SPCA2-complexed Orai1 was found at the plasma membrane (Figures S5B–S5C). Although SPCA2 preferentially interacted with lower molecular weight bands of post-translationally modified Orai1 as has been reported for STIM1 (Park et al., 2009; Vig et al., 2006), we confirmed that all forms of Orai1 reached the plasma membrane where they could be biotinylated (Figure 4B). Epitope-tagged SPCA2 and Orai1 also partially co-localized by confocal immunofluorescence microscopy (Figure S5D). Unlike STIM (Yeromin et al., 2006), interaction between SPCA2 and Orai1 was not affected by store depletion with thapsigargin, consistent with a store-independent regulation of Orai1 function (Figure S5E). Supporting this possibility, neither WT STIM1 nor the constitutively active STIM1 mutant (D76A) (Huang et al., 2006; Liou et al., 2005) was in the same protein complex as SPCA2 (Figure S5F). These findings point to Orai1 as a likely candidate for mediating SPCA2-regulated, store-independent Ca2+ influx in breast cancer cells.
Figure 4. SPCA2 interacts with Orai1 to mediate Ca2+ entry.
(A) Confocal micrographs of immunofluorescence staining of endogenous SPCA2 in MCF-7 cells showing partial plasma membrane localization. (B) Cell surface biotinylation of endogenous SPCA2 and Orai1 in MCF-7 cells. T and B represent total lysate and biotinylated fraction, respectively. (C) Co-immunoprecipitation of endogenous SPCA2 and Orai1 in MCF-7 cells. (D) Co-immunoprecipitation of HA-SPCA with Orai1-Myc following expression in HEK293. (E) Cell surface biotinylation of HA-tagged SPCA1, SPCA2 or D379N SPCA2 expressed in HEK293. (F) Basal Ca2+ in MCF-7 cells after knock down of endogenous SPCA2 or Orai1. ControlKD, n = 47; SPCA2KD, n = 54; Orai1KD, n = 52. ** P < 0.01 (Student’s t-test). (G) Normalized proliferation of MCF-7 cells with SPCA2 or Orai1 knockdown. n = 3. (H) Immunoblot of ERK 1/2 phosphorylation and Cyclin D1 expression in MCF-7 cells with SPCA2 or Orai1 knockdown. (I) Normalized growth of MCF-7 cells with SPCA2 or Orai1 knockdown in soft agar. n = 3. (J) Tumor incidence in nude mice injected with MCF-7 cells; ControlKD, n = 10; SPCA2KD, n = 8, P = 0.007 (log-rand test); Orai1KD, n = 9, P = 0.045 (log-rank test). Immunoblot (K) and normalized growth in soft agar (L) of MCF-10A cells with knockdown of Orai1 in control and cells overexpressing SPCA2. n = 3 in (L). Error bars represent standard error (F) or standard deviation (G, I and L).
To evaluate this possibility, we suppressed the expression of endogenous Orai1 in MCF-7 cells: Orai1KD lowered basal Ca2+ to levels comparable to effects of depleting endogenous SPCA2 (Figure 4F). In addition, Orai1KD in MCF-7 cells suppressed cell proliferation (Figure 4G) and inhibited the RAS pathway, as did SPCA2KD (Figures 4H and S5G). Furthermore, Orai1KD suppressed colony formation in soft agar, to a similar extent as SPCA2KD (Figure 4I), and tumor generation in nude mice (Figure 4J). Simultaneous knockdown of both Orai1 and SPCA2 did not confer additive phenotypes (Figures S5H, S5J and S5K). In MCF-10A cells overexpressing SPCA2, cell transformation and elevation of basal Ca2+ level were reversed by knockdown of Orai1, consistent with a role for Orai1 downstream of SPCA2 (Figures 4K–4L and Figure S5L). As expected for a store-independent mechanism of Orai1 activation, depletion of STIM1 (Figures S5M–S5O) or STIM2 (Figures S5I–S5K), the upstream activators of Orai1 in SOCE signaling, did not confer comparable phenotypes in MCF-7 cells. Taken together, our data point to promotion of tumorigenic pathways by SPCA2 in breast cancer cells, mediated by interaction with Orai1.
Amino Terminus of SPCA2 Interacts with Orai1
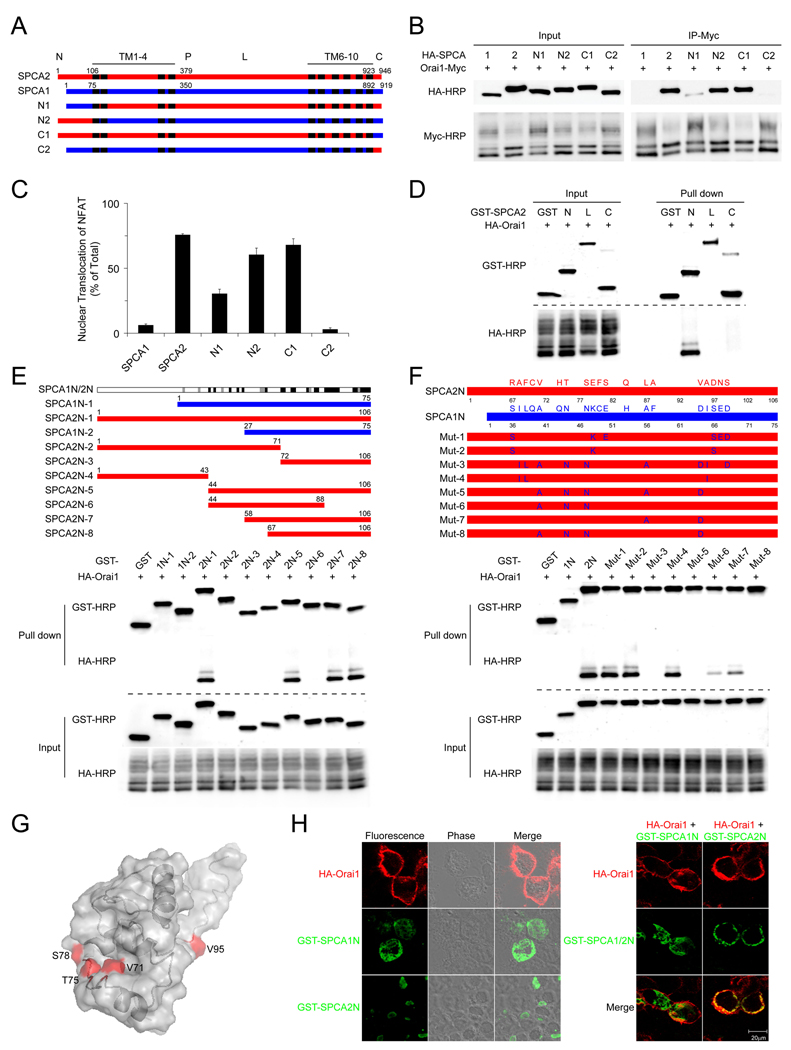
To dissect the molecular determinants of the SPCA2-Orai1 interaction, we evaluated the efficiency of co-immunoprecipitation between a series of SPCA chimeric proteins and Orai1. In each case, the ability of a chimeric protein to co-immunoprecipitate with Orai1 correlated with ability to elicit NFAT translocation, suggesting that physical interaction between the two proteins was required for Ca2+ signaling. Chimeras containing the SPCA2 N-terminus showed stronger binding with Orai1 and more effective NFAT translocation (Figure 5A–5C).
Figure 5. N-terminal domain of SPCA2 interacts with Orai1.
(A) Schematic of SPCA chimeras. N1 and C1 have the N- and C-termini of SPCA2 replaced by corresponding regions of SPCA1. N2 and C2 have N- and C-termini of SPCA1 replaced by corresponding regions of SPCA2. “P” indicates the conserved aspartate that is transiently phosphorylated by ATP in the catalytic cycle. “L” represents intracellular loop. (B) Interaction between Orai1 and SPCA chimeras was examined by co-immunoprecipitation in HEK293 cells. (C) Quantification of NFAT nuclear translocation in HEK293 cells expressing SPCA chimeras described in (B). n = 3, error bars represent standard deviation. (D) Interaction between SPCA2 N-terminus (N: aa1–106), intracellular loop (L: aa353–733), C-terminus (C: aa923–946) and Orai1 was examined by GST pull down in HEK293 cells. (E) Mapping of regions in SPCA1/2 that interact with Orai1. GST-SPCA1/2 N-terminal fragments were coexpressed with HA-Orai1 in HEK293 cells, and interaction with Orai1 was examined by GST pull down. Sequence conservation between SPCA1 and SPCA2 is showed on top, with black, gray and white bars representing identical, similar and different amino acids, respectively, as defined by ClustalW. (F) Screening of SPCA2 N-terminus for amino acids critical for the interaction with Orai1 in HEK293 cells. Point mutations in SPCA2 N-terminus convert amino acids to the equivalent residues in SPCA1 N-terminus. (G) Predicted 3D structure of SPCA2 N-terminus with residues essential for interaction with Orai1 shown in red. (H) Localization of SPCA1 / 2 N-termini, with or without coexpression of Orai1.
Next, we examined physical interaction between Orai1 and major intracellular soluble domains of SPCA2, including N- and C-termini and the large intracellular loop, which contains the iconic aspartate of P-type ATPases (D379). Of these, only the N-terminus was able to pull down Orai1 (Figure 5D). To further map regions within the SPCA2 N-terminus responsible for binding to Orai1, we performed GST pull downs between Orai1 and a series of SPCA1 and SPCA2 fragments. SPCA1 N-terminus did not bind to Orai1, consistent with an absence of functional interaction between SPCA1 and Orai1 (Figure 5E). A region of 40 amino acids within the N-terminus of SPCA2 was able to effectively interact with Orai1 (Figure 5E; construct SPCA2N-8, aa67–106). Surprisingly, this region was highly conserved between the two isoforms, with ~50% amino acid identity (Figure S6A). We therefore conducted mutational replacement of amino acids in SPCA2 N-terminus with the equivalent residues in SPCA1, and identified four amino acids that together were critical for interaction between SPCA2 N-terminus and Orai1 (Figure 5F). Three-dimensional structure of SPCA2 N-terminus, predicted by I-TASSER server (Wu et al., 2007), suggested that Val71, Thr75 and Ser78 were spatially clustered, whereas Val95 was on the remote side (Figure 5G and Figures S6B–S6C). Finally, we evaluated the effect of Orai1 expression on the intracellular localization of N-terminal fragments of SPCA1 and SPCA2 in HEK293 cells. When expressed alone, both fragments were localized intracellularly, with SPCA1 N-terminal fragment diffusely distributed in the cytosol, and the SPCA2 N-terminus concentrated in the perinuclear region. While the co-expression of Orai1 did not change localization of SPCA1 N-terminus, there was a redistribution of SPCA2 N-terminus to the cell surface, providing additional evidence that the N-terminal domain of SPCA2, but not SPCA1 interacts physically with Orai1 (Figure 5H).
Cooperation of SPCA2 N- and C-termini in Ca2+ Signaling
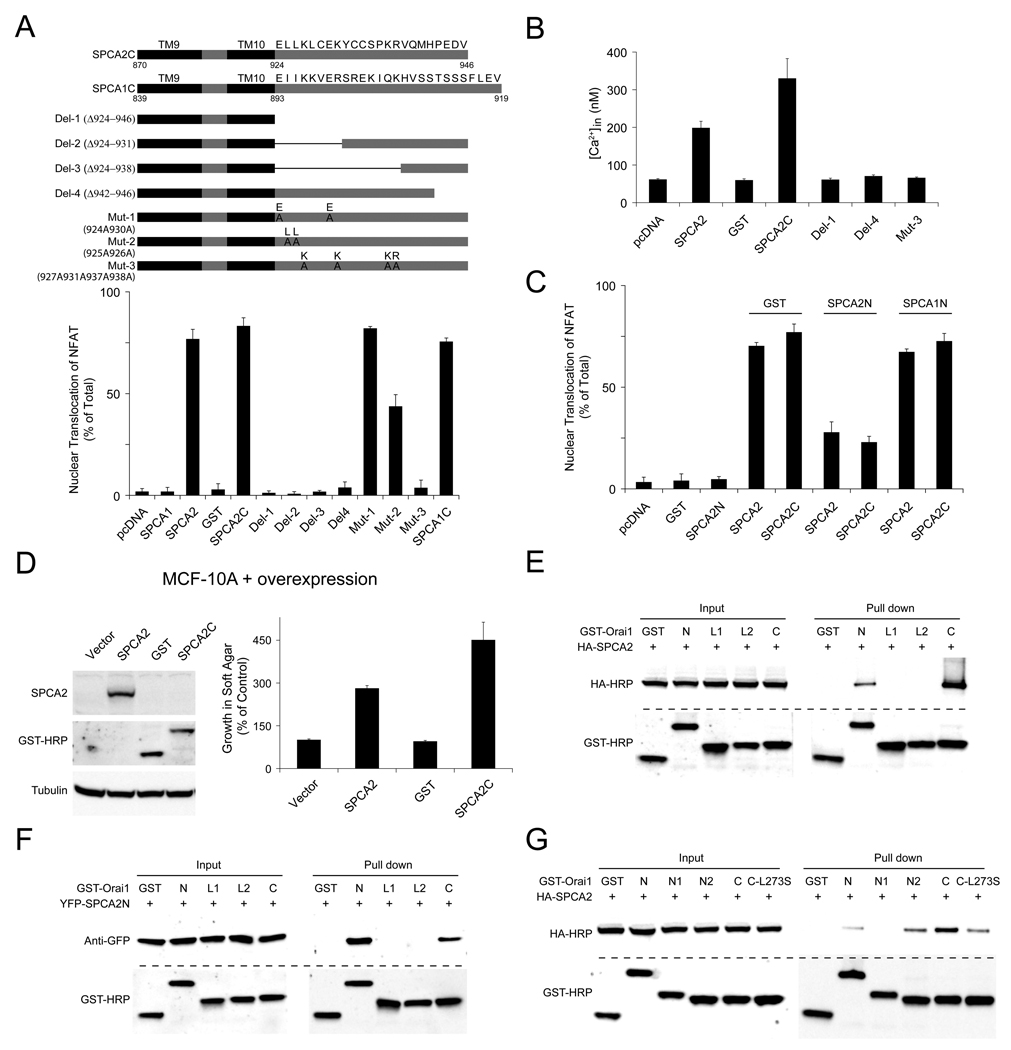
We further investigated the molecular mechanism of SPCA2-activated Ca2+ signaling. We noticed that the isolated N-terminus of SPCA2 did not elicit NFAT translocation despite being able to interact directly with Orai1, while C-terminus was sufficient to induce NFAT translocation when it was linked to 2 or more transmembrane domains and targeted to the membrane (Figure 6A and Figures S7A–S7B). Surprisingly, a similar membrane anchored construct containing SPCA1 C-terminus was also able to activate constitutive Ca2+ signaling even though full length SPCA1 could not (Figure 6A). In addition, both SPCA1 and SPCA2 membrane-anchored C-terminal domains physically interacted with Orai1 (Figure S7C). We hypothesized that access of SPCA C-terminus to Orai1 was blocked in the full-length proteins, and binding of SPCA2 N-terminus to Orai1 led to exposure of C-terminus and activation of downstream Ca2+ signaling. Analysis of deletion and point mutants of SPCA2 C-terminus identified essential functional residues including several positive charges (lysines and arginines) and a putative PDZ binding domain (Figure 6A and Figure S7D), conserved between human, rat and mouse SPCA proteins (Figure S7E). We then measured intracellular Ca2+ concentrations upon expression of SPCA2 C-terminal constructs in HEK293 cells. Basal Ca2+ level was elevated dramatically by expression of SPCA2 C-terminus, but remained the same as GST control when lysines (arginines) were mutated or putative PDZ domain was deleted (Figure 6B). N-terminal domain of SPCA2, but not SPCA1, had a dominant negative effect, dramatically inhibiting NFAT translocation induced by full length SPCA2 or the C-terminus (Figure 6C), whereas SOCE was not blocked by SPCA2 N-terminus or full length with C-terminus deleted (SPCA2 Δ924–946)(Figure S7F). Importantly, expression of membrane-anchored SPCA2 C-terminus in MCF-10A cells was able to induce cell transformation, consistent with the fact that it was identified to be the functional domain of SPCA2 and elicited constitutive Ca2+ signaling (Figure 6D). STIM1 CRAC activation domain (Park et al., 2009) showed a similar effect, supporting the role of Ca2+ signaling in cell transformation (Figures S7G–S7H).
Figure 6. Cooperation of SPCA2 N- and C- terminal domains in Ca2+ signaling.
(A) SPCA2 C-terminus was sufficient to activate Ca2+ signaling. Function of deletion and point mutants of SPCA2 C-terminal domain were examined by NFAT translocation assay in HEK293 cells. Full length SPCA proteins were HA-tagged, all C-terminus fragments shown were GST-tagged. n = 3. (B) Basal intracellular Ca2+ concentrations in HEK293 cells, with the expression of GST-tagged deletion and point mutants of SPCA2 C-terminal domain described in (A). From left to right, n = 49, 45, 46, 48, 40, 54, 59. (C) Effects of N-terminal fragments of SPCA proteins on NFAT translocation induced by SPCA2 full length or C-terminal fragment shown in (A). n = 3. (D) Immunoblot and normalized cell growth in soft agar of MCF-10A cells transduced with vector, SPCA2, GST, and membrane-anchored SPCA2 C-terminus (GST-tagged). n = 3. (E and F) Interaction between Orai1 N- (N: aa1–91)), C- (C: aa255–301) termini, intra- (L1: aa141–177), extracellular (L2: aa198–234) loops and SPCA2 full length or N-terminus. (G) Interaction between Orai1 full-length and subregions of N-terminus (N: aa1–91; N1: aa1–47; N2: aa48–91), C-terminus (C: aa255–301), C-terminus mutation (C-L273S) and SPCA2. Error bars represent standard error (B) or standard deviation (A, C and D).
We next expressed various domains of Orai1 as GST fusions, including full-length, N- and C-termini, extra- and intracellular loops together with full length or N-terminus SPCA2. Both N- and C-termini, but not the loops of Orai1 were able to pull down both full length and N-terminus of SPCA2, revealing that SPCA2 and Orai1 interacted within the cytoplasm (Figures 6E–6F). We then mapped subregions of Orai1 N- and C-termini to further explore SPCA2 interaction domains (Figure 6G). GST pull down experiments identified a fragment (aa 48–91) of the Orai1 N-terminus that bound SPCA2 with a higher affinity than the full-length N-terminus. Mutation L273S, previously shown to disrupt coiled-coil domain of the C-terminus of Orai1 (Muik et al., 2008), severely reduced the interaction with SPCA2 (Figure 6G). Taken together, we propose a model for SPCA2 interaction with Orai1 and activation of Ca2+ signaling. We suggest that the N-terminus of SPCA2 binds Orai1, resulting in a conformational change and exposure of the C-terminus, which can interact with Orai1 either directly or together with other proteins to activate Ca2+ influx (Figure S7I).
DISCUSSION
Role of SPCA2 and Orai1 in Breast Tumorigenicity
Our findings reveal a mechanism for activation of the so-called SOCE channel Orai1 that is independent of ER and Golgi Ca2+ stores and sensors. This store-independent mode of endogenous Orai1 activation in breast cancer derived MCF-7 cells underlies constitutive Ca2+ signaling, proliferation and anchorage-independent growth and implicates a hitherto unrecognized role for Orai1 in breast tumorigenicity. We also identified a role for SPCA2 in tumorigenicity and revealed a functional link to RAS signaling. The RAS-ERK pathway regulates cell cycle progression and cell proliferation, and it is well known that hyperactivation of the RAS gene family correlates with various human cancers (Bos, 1989). GTP-exchange factors (GEFs) and GTPase-activating proteins (GAPs) control activity of RAS by regulating the balance of GTP binding and hydrolysis (Donovan et al., 2002; Downward, 1996). Recent studies have suggested that GEFs and GAPs can be regulated by different Ca2+ signals, such as amplitude of the Ca2+ signals and frequency of Ca2+ oscillation (Cook and Lockyer, 2006). By monitoring activation of ERK and expression of the downstream protein Cyclin D1, we revealed a correlation between SPCA2 and Orai1-mediated increase of basal Ca2+ levels and constitutive activation of RAS signaling in MCF-7 cells, placing SPCA2-Orai1 pathway in the RAS signaling network.
Mechanism of Orai1-Mediated Ca2+ Signaling Induced by SPCA2
It has been reported that STIM1 and Orai1 mediate CRAC currents in endothelial cells, and knockdown of either elicits cell cycle arrest (Abdullaev et al., 2008). Another recent study implicated a store-dependent role for Orai1 in cell migration of the metastatic breast cancer line MDA-MB-231, based on a requirement for STIM1 (Yang et al., 2009). We note that SPCA2 expression is very low in MDA-MB-231 (data not shown), consistent with a Ca2+ signaling mechanism distinct from the store-independent pathway reported here. While the importance of SOC signaling is well established, the store independent Ca2+ signaling described in our study suggests that multiple mechanisms may invoke Orai1 activation. Interaction between SPCA2 and Orai1 was not affected by ER store-depletion and activation of SOC signaling, and SOCE was not inhibited by expression of SPCA2, supporting that SPCA-induced signaling may function independently of SOC pathway and different pools or fine subdomains of Orai1 are involved in the two pathways.
ER-localized Ca2+ sensor STIM proteins, which regulate SOCE, did not physically interact with SPCA2 or participate in regulation of SPCA2-Orai1 signaling pathway. In addition, internal Ca2+ store content was not depleted by suppression or overexpression of SPCA2. Thus, it remains to be determined how the store-independent, Orai1-mediated mechanism of Ca2+ influx is regulated. One possibility is that signaling activity of SPCA2 is regulated by its trafficking between Golgi and plasma membrane. Interaction with Orai1 at cell surface may be dependent on a specific conformation of SPCA2 which could be regulated by kinase-mediated phosphorylation, Ca2+ binding or changes in pH between extracellular and Golgi lumen. Removal of a potential PDZ-binding motif in the last four residues of the C-terminal tail of SPCA2 abolished Ca2+ signaling, suggesting that interaction with scaffold proteins may be important for activation of this signaling pathway.
Based on the function of a series of chimeras and mutant proteins, we propose a model in which cooperation of N- and C-termini of SPCA2 is required for Orai1-mediated Ca2+ signaling. Whereas the N-terminus of SPCA2 binds strongly to Orai1, the C-terminus elicits activation of Ca2+ influx. Although the Orai1 binding domain within the SPCA2 N-terminus is highly conserved with the corresponding region of SPCA1, no interaction was detected between SPCA1 N-terminus and Orai1. Replacement of four residues within the minimal Orai1 binding domain of SPCA2 N-terminus (Val71, Thr75, Ser78 and Val95) to the corresponding less hydrophobic or charged residues in SPCA1 abolished the interaction with Orai1. Interestingly, C-terminal constructs of both SPCA isoforms, anchored to the membrane by a minimum of two transmembrane helices, were able to elicit Ca2+ influx and signaling. Consistent with this, critical amino acids within the C-terminus were conserved in both isoforms from rat, mouse and human. Therefore, we propose a mechanism in which accessibility of SPCA C-termini is blocked in the full-length protein and binding of the N-terminus to Orai1 is required for functional availability of the C-terminus. Consistent with this hypothesis, we find that expression of the soluble N-terminal domain from SPCA2, but not SPCA1, has a dominant negative effect in blocking activation of Ca2+ signaling. Long-range conformational interactions between the N-terminus and other cytosolic domains have been noted in SPCA and other P-type pumps, as well as changes in accessibility of the C-terminal tail (Huster and Lutsenko, 2003; Lecchi et al., 2005; Wei et al., 1999).
Physiological and Pathophysiological Perspectives of SPCA2-Induced Ca2+ Signaling
The conventional role of ATP-powered Ca2+ pumps is to scavenge and extrude cytoplasmic Ca2+ in order to terminate a signal, and as a prerequisite for additional signaling events. Unexpectedly, high levels of expression of the Ca2+ efflux pump SPCA2 increased, rather than lowered basal cytoplasmic Ca2+ levels and conversely, attenuation of SPCA2 expression was accompanied by a decrease in basal Ca2+. We speculate that this unconventional mechanism may be physiologically important in eliciting high rates of transcellular Ca2+ flux during lactation (Lee et al., 2006) and in other Ca2+ secreting tissues, including salivary glands and intestinal epithelia, where SPCA2 is expressed at high levels. Total calcium concentration in milk, can reach up to 100 millimolar, five to six orders of magnitude greater than typical cytoplasmic concentrations (~0.1µM). Thus, there must be energy dependent transport processes for effective transcellular movement of Ca2+ from blood into milk. In mammary gland, a 30-fold transcriptional increase in the plasma membrane Ca2+ pump isoform, PMCA2, is accompanied by apical efflux of Ca2+ into milk (Reinhardt et al., 2004). Compared to modest changes in SPCA1 levels, SPCA2 was found to be upregulated during pregnancy (~8-fold), and dramatically upon lactation (~35-fold on day 1). Furthermore, SPCA2 expression was restricted to the luminal cells of lactating glands (Faddy et al., 2008). Our findings raise the possibility that SPCA2 traffics to the basolateral membrane where it can interact with Ca2+ channels to elicit Ca2+ influx and promote transcellular Ca2+ transport.
The unusual role of SPCA2 in activation of Ca2+ influx supersedes its ATP-dependent Ca2+ sequestering activity and may be a raison d’etre for its redundant expression along with SPCA1, in mammals and higher vertebrates. It is noteworthy that in lower eukaryotes (yeast, worm, fly) and vertebrates (fish), there is only a single ubiquitously expressed SPCA protein, which functions in transporting Ca2+ and Mn2+ into the secretory pathway. The advent of the SPCA2 gene in higher eukaryotes including frog, mouse, rat and human may correlate with a newly required role in Ca2+ signaling. At the molecular level, a longer and divergent N-terminus appears to have endowed SPCA2 with the ability to interact with unique partners, and discrete cell and tissue specific distribution would appear to regulate its function. Whereas in lactation, an exquisitely orchestrated developmental program ensures a coordinated regulation of Ca2+ pumps, channels, receptors and buffers, there is emerging appreciation for a pronounced dysregulation of these processes in breast-derived tumor cells. For example, an aberrant switch in heterotrimeric G protein preference by the Calcium Sensing Receptor, CaSR, in breast cancer cells leads to stimulation of cAMP signaling and increased secretion of PTHrP, which in turn is believed to contribute to a “vicious cycle” or feed forward loop of bone metastasis and osteolysis (Mamillapalli et al., 2008). Thus, the upregulation of SPCA2 in the altered signaling environment of tumor cells may result in constitutive Ca2+ signaling and cell growth. Inappropriate secretion of Ca2+ from these cells, in the absence of calcium buffers, could lead to microcalcifications that are diagnostic of breast cancer. Finally, the separation of signaling function from transport activity in SPCA2, evidenced by our findings, is highly unusual in pumps. An extreme case is SUR1, an ABC transporter that lacks known transport activity but is essential for conferring ATP sensitivity to KATP channels in insulin secreting pancreatic β cells (Aittoniemi et al., 2009).
In summary, we identified a store-independent SPCA2-Orai1 signaling pathway. Upregulation of SPCA2 led to constitutively active Ca2+ signaling and correlated with oncogenic activity in breast cancer. Both SPCA2 and Orai1 emerge as druggable targets of therapeutical potential in the treatment of some breast cancer subtypes.
EXPERIMENTAL PROCEDURES
Materials and additional experimental procedures are described in the Supplemental Data.
SPCA2 Analysis in Human Breast Tumors
A gene expression dataset consisting of the microarray profiles of 295 primary human breast tumors (van de Vijver et al., 2002) was obtained from Rosetta Inpharmatics (Seattle, WA). Tumors were assigned to transcriptional subtypes based on their gene expression profiles (Luminal A [n=88], Luminal B [n=81], Normal-like [n=31], Basal-like [n=46] and ERBB2+ [n=49]) as described (Chang et al., 2005). One probe on the array (annotated as KIAA0703) corresponded to SPCA2. Tumors were grouped by transcriptional subtype and analyzed for SPCA2 expression. Statistical significance between groups was assessed by comparing medians using the Kruskall-Wallis test followed by Dunn’s Multiple Comparison Test (Prism version 5, Graphpad Inc.).
NFAT Translocation Assay
Monitoring of nuclear translocation of NFAT was performed 24 hrs post-transfection in HEK293 cells. Fresh medium was added 2 hrs before the start of the experiment. Localization of NFAT-GFP and NFAT-mCherry in cells was examined by fluorescent microscopy. 100–300 cells were manually counted on each coverslip, 3 wells for each condition, and the fraction of cells with nuclear NFAT was calculated. Where indicated, 2 µM thapsigargin was added for 30 minutes, 10 µM miconazole was used for 1 hr, and 50 µM 2-APB was used for 1 hr.
Calcium Imaging and Mn2+ quench
Cells were loaded with Fura-2 AM at 1 µg/ml in calcium recording buffer (126 mM NaCl, 2 mM MgCl2, 4.5 mM KCl, 10 mM Glucose, 20 mM Hepes pH 7.4, 2 mM CaCl2; no CaCl2 was added for the 0 Ca2+ buffer) for 30 min at RT. After loading, cells were rinsed in same calcium recording buffer without Fura-2 for 20 min. Cells were excited at 340 nm and 380 nm, and Fura AM emission at 505 nm was monitored. Intracellular Ca2+ concentration was calculated based on the ratio of 340/380 nm. For Mn2+ quench of Fura-2 fluorescence, 0.5mM of Mn2+ was added to nominally Ca2+-free buffer. Cells were excited at 360 nm, the isosbestic point of Fura-2, and emission at 505nm was monitored. The average fluorescence of 10 time points (50s) before Mn2+ addition was set as 100%.
Construction, Production and Infection of Lentiviruses and Retroviruses
Replication-incompetent lentivirus was used to package shRNA for knockdown in MCF-7 cells and HEK293 cells. Cells were incubated with viruses for 48hrs and selected with puromycin (2–4 µg/ml).
Retroviral gene transfer and expression system (Clontech, Mountain View, CA) was used for stable expression of SPCA2 in MCF-10 cells. SPCA2 gene was cloned into pLXRN vector to package retroviruses. Viruses were collected 48 hrs after transfection, and added to MCF-10A cells. Cells were treated with G418 (400 µg/ml) after 48 hrs infection and selected cells were used to assay proliferation and colony formation in soft agar.
Cell Proliferation Assay
Proliferation was monitored using Celltiter 96® Aqueous One Solution cell proliferation assay kit (Promega, Madison, WI) according to manufacturer’s instructions. Briefly, 0.5–1 ×104 cells were plated into a 96-well plate. After every 24 hrs, 20 µl of Celltiter 96® Aqueous One Solution reagent was added to each well, and incubated for 2 hrs at 37°C, 5% CO2. The absorbance at 490 nM was recorded using a 96-well plate reader.
Colony Formation in Soft Agar
Colony formation in soft agar assay was performed using CytoSelect™ 96-well cell transformation assay kit (Cell Biolabs, San Diego, CA). In a 96-well plate, 0.5–1 ×104 cells were resuspended in DMEM containing 0.4% agar and 10% FBS, and layered on to a base agar consisting of DMEM with 0.6% agar and 10% FBS. Following solidification, growth medium was added on to the cell agar layer. 1–2 weeks later, colonies were imaged under the microscope, the agar layer was solubilized and cells were lysed and quantified with CyQuant®GR dye. The plate was read in a FLUOstar Optima plate reader (BMG Labtechnologies) using a 485/520 nm filter set.
Tumor Formation in Nude Mice
Female 4- to 6- week old athymic nude mice (NCI) were received by the animal facility personnel and acclimated at the facility for 2 weeks. Estrogen pellets (SE-121, 0.72 mg/pellet, 60 days release) were obtained from Innovative Research of America. For each animal, a pellet was implanted into the back of the neck through a 1 cm cut and the wound was closed by a wound clip. After 3 days of implantation, the animals were ready to be injected with cells. MCF-7 cells transduced with control or SPCA2 shRNA or Orai1 shRNA were trypsinized and diluted to 1.5–3 × 107 cells per ml in PBS. 3 × 106 cells per animal were injected subcutaneously into the flank of each of 6–10 mice. The incidence of tumor formation was recorded in each animal once per week, starting 14–18 days after injection. Animal care was in accordance with institutional guidelines. One animal with subsequent tumor necrosis was euthanized, others were sacrificed after 10 weeks of observation.
Immunofluorescence
Cultured HEK293 and MCF-7 cells on coverslips were pre-extracted with PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, and 2 mM MgCl2, pH 6.8) containing 0.025% saponin for 2 min, and then washed twice for 2 min with PHEM buffer containing 0.025% saponin and 8% sucrose. The cells were fixed with a solution of 4% PFA and 8% sucrose in PBS for 30 min at room temperature, and blocked with a solution of 1% BSA and 0.025% saponin in PBS for 1 hr. Primary antibodies were diluted 1:500 in 1% BSA and incubated with the cells for 1 hr. Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) and Alexa Fluor 568 goat anti-mouse IgG were used at a 1:1,000 dilution for 30 min. Cells were mounted onto slides using Dako Fluorescent Mounting Medium. Slides were imaged on a Zeiss LSM510-Meta confocal microscope. In Figure 4A, anti-SPCA2 was used. In Figure 5H, anti-SPCA2, anti-SPCA1 and anti-HA were used to detect SPCA2N, SPCA1N and HA-Orai1. In Figure S3C, anti-Myc, anti-SPCA1 and anti-Golgi97 was used to detect Myc-STIM1, HA-SPCA1 and Golgi 97. In Figure S5D, anti-SPCA2 and anti-HA were used to detect Myc-SPCA2 and HA-Orai1. In Figure S7B, anti-GST and anti-HA were used to detect GST-SPCA2C and HA-Orai1.
Co-Immunoprecipitation and GST Pull Down
Co-immunoprecipitation (co-IP) and GST pull down assay in HEK293 cells were performed 24 hrs after transfection. Co-IP in MCF-7 cells for endogenous proteins was performed 24–48 hrs after seeding cells. Cells were lysed in lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM Na3EDTA, 1 mM EGTA, 5 mM Na4P2O7, 1 mM Na3VO4, 10 mM NaF, supplemented with 1% Triton X-100 and protease inhibitor cocktail (Roche)). 1 / 10 of the lysate was saved as “input”. For co-IP, cell lysate was incubated 1 hr with GammaBind Plus Sepharose (GE Healthcare, Waukesha, WI) for pre-clearance, and 1–4 hrs with antibodies (anti-Myc or anti-SPCA2) at 4°C. GammaBind beads were added and incubated for 1 hr at 4°C. For GST pull down assay, cell lysate was incubated 2hrs with Glutathione Sepharose 4B. Beads were washed using lysis buffer supplemented with 1% Triton X-100 before SDS-PAGE and immunoblotting. 1/2 of the “input” and 1/2 to 1/8 of the Co-IP / pull down fraction were loaded to SDS-PAGE gels. To co-IP cell surface Orai1 with SPCA2, cells were biotin labeled, lysed and immunoprecipitated using anti-SPCA2 antibody. The proteins on the GammaBind beads were eluted with lysis buffer containing 1% SDS, and incubated with neutravidin resin overnight at RT. Beads were washed in the same buffer. Only the portion of Orai1 that bound to SPCA2 at the cell surface was detected using after SDS-PAGE and immunoblotting. 1/2 of the “input” and 1/2 of the biotinylated fraction were loaded to SDS-PAGE gels.
Functional Complementation in Yeast
Yeast growth assays were performed as described before (Xiang et al., 2005). The yeast strain K616 (pmr1Δpmc1Δcnb1Δ) was used as host for plasmids expressing SPCA2. Freshly grown cells were inoculated into each well of 96-well plates at 0.05 OD600 nm. Plates were incubated overnight at 30°C, resuspended by agitation and OD600 nm was measured using a FLUOstar Optima plate reader.
Three-Dimensional Structure Prediction
I-TASSER method was used to predict 3D-protein structure from the primary amino acid sequence of SPCA2 N-terminus. I-TASSER was ranked as the No.1 server in recent CASP7 and CASP8 experiments (Critical Assessment of protein Structure Prediction).
Article Highlights.
SPCA2, but not SPCA1, is highly upregulated in breast cancer cells.
SPCA2 elicits elevation of basal Ca2+ levels mediated by Orai1.
SPCA2 functions independently of its ATPase activity or store-operated Ca2+ entry.
SPCA2-induced Ca2+ signaling is necessary for cell proliferation and tumorigenesis.
Supplementary Material
Acknowledgements
We thank Dr. Paul Worley and Dr. Guo Huang (Johns Hopkins University) for kind gifts of plasmids expressing Orai1, STIM1, and NFAT. This work was supported by grants from the National Institution of Health GM62142 to R. R., National Health and Medical Research and Cancer Council Queensland 569644 to G. M. and S. R. T., Department of Defense-Center of Excellence Grant W81XWH-04-1-0595 to S. S., laboratory startup funds from the Albert Einstein College of Medicine to P. K., American Heart Association Pre-doctoral fellowship 0815058E to M. F., and American Psychological Association scholarship to H. F. and D. G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res. 2008;103:1289–1299. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aittoniemi J, Fotinou C, Craig TJ, de Wet H, Proks P, Ashcroft FM. Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci. 2009;364:257–267. doi: 10.1098/rstb.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J, Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van't Veer LJ, Bartelink H, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci U S A. 2005;102:3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Clementi E, Meldolesi J. Pharmacological and functional properties of voltage-independent Ca2+ channels. Cell Calcium. 1996;19:269–279. doi: 10.1016/s0143-4160(96)90068-8. [DOI] [PubMed] [Google Scholar]
- Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol. 2004;5:355–366. doi: 10.1038/nrm1365. [DOI] [PubMed] [Google Scholar]
- Cook SJ, Lockyer PJ. Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39:101–112. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109 Suppl:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Lockyer PJ. Integration of calcium and Ras signalling. Nat Rev Mol Cell Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- Donovan S, Shannon KM, Bollag G. GTPase activating proteins: critical regulators of intracellular signaling. Biochim Biophys Acta. 2002;1602:23–45. doi: 10.1016/s0304-419x(01)00041-5. [DOI] [PubMed] [Google Scholar]
- Downward J. Control of ras activation. Cancer Surv. 1996;27:87–100. [PubMed] [Google Scholar]
- Durr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy HM, Smart CE, Xu R, Lee GY, Kenny PA, Feng M, Rao R, Brown MA, Bissell MJ, Roberts-Thomson SJ, et al. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem Biophys Res Commun. 2008;369:977–981. doi: 10.1016/j.bbrc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–924. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Huster D, Lutsenko S. The distinct roles of the N-terminal copper-binding sites in regulation of catalytic activity of the Wilson's disease protein. J Biol Chem. 2003;278:32212–32218. doi: 10.1074/jbc.M305408200. [DOI] [PubMed] [Google Scholar]
- Lecchi S, Allen KE, Pardo JP, Mason AB, Slayman CW. Conformational changes of yeast plasma membrane H(+)-ATPase during activation by glucose: role of threonine-912 in the carboxy-terminal tail. Biochemistry. 2005;44:16624–16632. doi: 10.1021/bi051555f. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: targets for breast cancer. Biochim Biophys Acta. 2006;1765:235–255. doi: 10.1016/j.bbcan.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem. 2008;283:24435–24447. doi: 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Maller JL. Calcium, calmodulin, and CaMKII requirement for initiation of centrosome duplication in Xenopus egg extracts. Science. 2002;295:499–502. doi: 10.1126/science.1065693. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Dode L, Vanoevelen J, Raeymaekers L, Wuytack F. Calcium in the Golgi apparatus. Cell Calcium. 2007;41:405–416. doi: 10.1016/j.ceca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- Morgan MP, Cooke MM, McCarthy GM. Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors? J Mammary Gland Biol Neoplasia. 2005;10:181–187. doi: 10.1007/s10911-005-5400-6. [DOI] [PubMed] [Google Scholar]
- Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425–2437. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem. 2004;279:42369–42373. doi: 10.1074/jbc.M407788200. [DOI] [PubMed] [Google Scholar]
- Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, Raeymaekers L, Wuytack F. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J Biol Chem. 2005;280:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, Kinet JP. The long and arduous road to CRAC. Cell Calcium. 2007;42:157–162. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Chen J, Rosas G, Tompkins DA, Holt PA, Rao R. Phenotypic screening of mutations in Pmr1, the yeast secretory pathway Ca2+/Mn2+-ATPase, reveals residues critical for ion selectivity and transport. J Biol Chem. 2000;275:23927–23932. doi: 10.1074/jbc.M002618200. [DOI] [PubMed] [Google Scholar]
- Wei Y, Marchi V, Wang R, Rao R. An N-terminal EF hand-like motif modulates ion transport by Pmr1, the yeast Golgi Ca(2+)/Mn(2+)-ATPase. Biochemistry. 1999;38:14534–14541. doi: 10.1021/bi9911233. [DOI] [PubMed] [Google Scholar]
- Wu S, Skolnick J, Zhang Y. Ab initio modeling of small proteins by iterative TASSER simulations. BMC Biol. 2007;5:17. doi: 10.1186/1741-7007-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+,Mn(2+)-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J Biol Chem. 2005;280:11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.