Abstract
In recent years, the antifungal triazole posaconazole has become increasingly important for the prophylaxis and treatment of systemic mycoses. Although oral bioavailability of posaconazole can be enhanced by concomitant food intake and administration in divided daily doses, increased gastric pH or gut motility, as well as enzyme-inducing drugs, can result in lower plasma concentrations than expected. Whether therapeutic drug monitoring can reduce the risk of treatment failures by avoiding sub-therapeutic plasma concentrations needs further examination. Based on the ability of posaconazole to inhibit cytochrome P450 3A4, several drug interactions can be expected, especially with agents that undergo extensive first-pass effect through the gut and the liver. However, more information is needed regarding dose modifications during concomitant administration of posaconazole with drugs in certain categories, such as vinca alkaloids and retinoids, along with selected individual drugs such as everolimus.
Keywords: drug interactions, oral bioavailability, posaconazole, review, therapeutic drug monitoring
Introduction
Posaconazole (Figure 1) represents the latest development among the antifungal triazole derivatives, with its good tolerability and impressive activity against an extended spectrum of pathogens. In Europe, the drug has been approved for the treatment of invasive fungal infection (IFI), including second-line treatment of aspergillosis, fusariosis, chromoblastomycosis, mycetoma and coccidioidomycosis. Posaconazole is also approved for antifungal prophylaxis in neutropenic high-risk patients with acute myelogenous leukaemia (AML) or myelodysplastic syndrome (MDS) who are receiving cytotoxic chemotherapy and in immunosuppressed patients with graft-versus-host disease (GVHD) after peripheral blood stem cell transplant (PBSCT) [1]. The commercially available cherry-flavoured posaconazole suspension should be given with high-fat meals whenever possible because this greatly enhances bioavailability [2]. The pharmacokinetic behaviour of posaconazole is clearly different from that of other triazoles (Table 1) [3, 4]. Drug–drug interactions are similar to those of itraconazole, voriconazole or fluconazole at higher dosages because of the cytochrome P450 (CYP) 3A4-inhibiting properties these drugs have in common [5–8]. Whereas CYP2C9, CYP2C19 and other CYP isoenzymes remain unaffected, this is in contrast to the structurally related triazole voriconazole. Study results in healthy volunteers revealed low variability of plasma drug concentrations among individuals, but this variability may be more pronounced in the critical target population [9, 10]. Whether or not to recommend therapeutic drug monitoring (TDM) remains controversial [11].
Figure 1.
Posaconazole
Table 1.
Triazole antifungal agents: Comparison of pharmacokinetic data (modified from [3])
| Parameter | Fluconazole | Itraconazole | Voriconazole | Posaconazole |
|---|---|---|---|---|
| Formulations | I.v. infusion, p.o. capsules, p.o. solution | I.v. infusion (CDx), p.o. capsules, p.o. solution (CDx) | I.v. infusion (CDx), p.o. capsules, PO suspension | p.o. suspension |
| Maintenance dose for antifungal treatment | 400 mg i.v. and p.o.od | 200 mg i.v. and p.o. bid | 4 mg/kg i.v. bid; 200 mg p.o. bid | 400 mg bid |
| Absolute bioavailability (comment) | ≥90% (independent of food and gastric pH) | <55% (capsules dependent on food and gastric pH, in contrast to solution) | <90% (availability decreased by fat-rich foods) | 8%–47% (dose-dependent; availability increased by fat-rich foods) |
| Protein binding | 12% | 99.8% | 58% | 98%–99% |
| Half-life | 27 h | 21–64 h | 6 h | 25 h |
| Elimination | Renal≫faecal; primarily in unchanged form | Faecal≫renal; primarily as metabolites; (ω-1)-hydroxyitraconazole with antifungal activity | Renal≫faecal; primarily as inactive metabolites | Faecal≫renal; extensively in unchanged form |
| Metabolism | No | CYP3A4 | CYP2C19>2C9, 3A4 | UGT1A4 |
| CYP inhibition | CYP2C9>3A4;>2C19 | CYP3A4≫2C9 | CYP3A4, 2C19, >2C9 | CYP3A4 |
CDx: hydroxypropyl-β-cyclodextrin in itraconazole for injection (should not be used in patients with creatinine clearance [CLcr] < 30 ml min−1); β-cyclodextrin-sulfobutylether sodium in voriconazole for injection (should not be used in patients with CLcr < 50 ml min−1). Bid, twice daily; i.v., intravenous; od, once daily; p.o., oral. Table adapted from Lipp HP. Mycoses 2008; 51: 7–18, with permission [3].
Pharmacodynamic properties
As a potent inhibitor of the fungal CYP-dependent enzyme lanosterol 14α-demethylase, posaconazole blocks the synthesis of ergosterol and impairs the stability of the cell membrane. Because of possibly tighter binding to this key enzyme, posaconazole displays lower minimum inhibitory concentrations (MICs) for several fungal species compared with structurally related triazole derivatives (Table 2, adapted from [5]).
Table 2.
In vitro activity of posaconazole and other antifungal agents against clinical strains of moulds and yeasts [5]
| MIC90 values (µg ml−1) | |||
|---|---|---|---|
| Organism | POS | ITC | VRC |
| Aspergillus spp. | |||
| A. fumigatus | 0.5 | 1.0 | 0.5 |
| A. niger | 0.5 | 2.0 | 2.0 |
| A. flavus | 0.5 | 1.0 | 1.0 |
| A. terreus | 0.25 | 0.5 | 0.5 |
| Zygomycetes | |||
| Rhizopus spp. | 8.0 | 32.0 | 128.0 |
| Mucor spp. | 16.0 | 32.0 | 128.0 |
| Absidia spp. | 0.25 | 0.5 | 128.0 |
| Candida spp. | |||
| C. albicans | 0.063 | 0.25 | 0.063 |
| C. glabrata | 2.0 | 4.0 | 2.0 |
| C. parapsilosis | 0.25 | 0.5 | 0.125 |
| C. tropicalis | 0.25 | 0.5 | 0.5 |
| C. krusei | 1.0 | 1.0 | 0.5 |
| C. dubliniensis | 0.125 | 0.5 | 0.125 |
| Other organisms | |||
| Cryptococcus spp. | 0.25 | 0.5 | 0.125 |
| Fusarium oxysporum | 4.0 | ND | 16.0 |
| F. solani | 32.0 | ND | 32.0 |
ITC, itraconazole; MIC90, minimal inhibitory concentration at which the growth of 90% of isolates are inhibited; ND, not determined; POS, posaconazole; VRC, voriconazole. Table adapted from Keating GM. Posaconazole. Drugs 2005; 65: 1553–67, with permission [5].
Posaconazole exerts an extended spectrum of antifungal activity against various strains of clinically relevant moulds and yeasts. Susceptible strains include Cryptococcus neoformans, Candida spp. (including fluconazole-resistant strains) and several Aspergillus spp. (including A. terreus, which has been shown to be poorly managed by amphotericin B and its lipid formulations) [12–14]. Of the Candida spp., C. albicans and C. dubliniensis are reported to be the most susceptible to posaconazole [4] and C. glabrata the least susceptible [12]. However, relatively low posaconazole MIC values have been detected even in fluconazole-resistant C. glabrata isolates [15]. Unlike other, structurally related triazoles, posaconazole is active against various Zygomycetes species (Table 2) [5, 16]. In vitro studies have revealed that the combination of posaconazole and caspofungin provides at least additive efficacy against Aspergillus spp [17, 18]. Further research is needed to determine whether caspofungin-induced partial cell wall destruction may improve the penetration of posaconazole into the fungus. In contrast, the benefit of combining posaconazole and amphotericin B to improve efficacy against Aspergillus spp. remains questionable. Several study results have shown no additive or even antagonistic effects between triazole antifungal agents and polyene antimycotics [19].
Pharmacokinetic behaviour
After oral administration of posaconazole, absolute bioavailability has been estimated to range from 8% to 47%. The low bioavailability may be explained by the high lipophilicity of posaconazole, possibly some first-pass effect and special conditions of the patients (e.g. malabsorption based on cytotoxic chemotherapy and bone marrow transplantation). Important factors that contribute to enhanced bioavailability include administration with high-fat food, absence of severe diarrhoea or mucositis, constitutive low gastric pH values, divided daily dosing, gamma-glutamyl transferase concentrations no greater than two times the upper level of normal and absence of highly potent enzyme-inducing drugs [20]. According to clinical pharmacokinetic study results with posaconazole 400 mg twice daily in healthy volunteers, on day 14 after the first and second daily doses, mean peak drug plasma concentrations (Cmax) (with coefficients of variation [%CV]) were 4.15 (20) µg ml−1 and 3.24 (19) µg ml−1, and mean times to Cmax were 5 and 9 h, respectively [9]. It has been recommended to consider twice daily rather than once daily dosing because the former increases the absolute bioavailability by about 98% [21]. A further increase in bioavailability can be achieved by administration of four rather than two divided doses. Administering more frequent divided doses is particularly important if the suspension cannot be administered with a high-fat or standard meal (Figure 2) [2, 21, 22].
Figure 2.
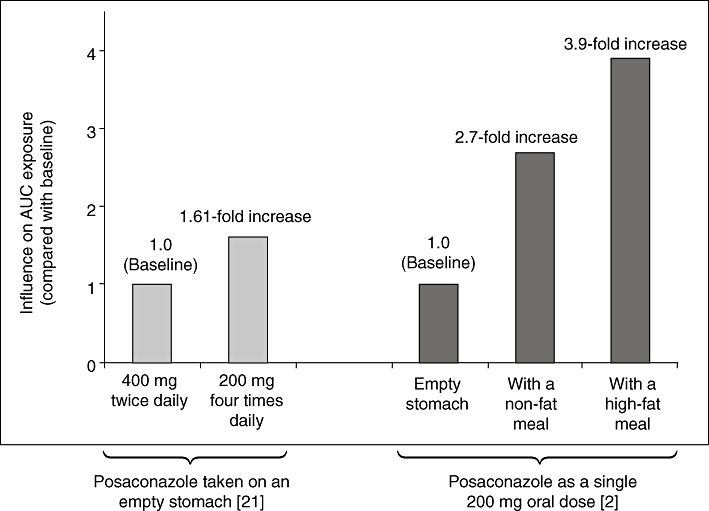
Posaconazole AUC exposure under different conditions [2, 21]. Posaconazole taken on an empty stomach [21]. Posaconazole as a single 200 mg oral dose [2]. AUC, area under the concentration–time curve
Based on its large volume of distribution (up to 2447 l) at steady-state conditions with 400 mg twice daily dosing [4], penetration of posaconazole into several tissues, including deeper compartments, appears to be extensive. There are no data available on the tissue distribution of posaconazole, although penetration into some compartments has been investigated. For example, posaconazole concentrations in pulmonary alveolar cells have been shown to be 31- to 42-fold higher than the corresponding plasma concentrations [23]. It has not yet been clearly shown to what extent posaconazole can pass the blood-brain barrier. However, based on the activity of posaconazole against fungal infections that target the central nervous system (CNS) significant penetration can occur [24, 25]. The role of P-glycoprotein (e.g. OATP1B1) has not yet been elucidated in detail. However, according to the study results published by Sansone-Parsons et al. a MDR1 single-nucleotide polymorphism was not associated with a significant change in posaconazole AUC [26]. Posaconazole is more than 98% bound to serum albumin in plasma, which indicates that haemodialysis has a limited effect on drug elimination [4]. Hepatic metabolism of posaconazole is minimal compared with that of the triazole antifungal agents, itraconazole and voriconazole (Table 1) [3]. Although CYP isoenzymes play a negligible role during posaconazole biodegradation, 20%–30% of the applied dose may be metabolized via UDP-glucuronosyltransferase UGT1A4 [27]. The corresponding glucuronide conjugates possess little antifungal activity [27].
About 77% of posaconazole is eliminated in the faeces (two-thirds as unchanged compound) and the remainder in the urine, mainly as glucuronidated metabolites [4, 28]. Comparing posaconazole plasma concentration in adult (18–64 years) and juvenile (<18 years) patients has shown no obvious differences, suggesting that age does not significantly influence the clinical pharmacokinetics of posaconazole [29].
Based on the route of drug elimination, no dose modification of posaconazole is needed in patients with renal impairment, even in those with creatinine clearance values of 20 ml min−1[30]. A post hoc subanalysis of a phase 3 trial in patients with IFI has recently confirmed that posaconazole is effective and well tolerated regardless of renal impairment [31]. According to a recent study, compared with matched subjects with normal hepatic function, Cmax (% CV) values were higher among subjects with moderate hepatic impairment (517 [80]vs. 724 [15] ng ml−1) but lower among subjects with severe hepatic impairment (608 [32]vs. 403 [31] ng ml−1) [33]. Pooled Cmax values for the hepatic function subjects were similar to the pooled normal group (607 [34]vs. 605 [35] ng ml−1), whereas there was an overall 36% increase in exposure (AUC) for the pooled hepatic impairment group compared with the pooled normal group [33]. While no dosage adjustment appears to be necessary, even in patients with severe hepatic impairment (Child-Pugh class C), the authors of the study suggest that physicians should continue to monitor posaconazole use in patients with hepatic impairment [33]. As yet, no recommendations have been made for dosage adjustment in patients with hepatic impairment.
As stated previously, patient-to-patient variability of posaconazole plasma concentrations was shown to be low in healthy volunteers [9]. However, mean peak plasma concentrations under steady-state conditions varied markedly among patients with IFIs. After administration of posaconazole 200 mg four times daily or 400 mg twice daily, patient Cmax concentrations ranged from 0 to 3710 ng ml−1 (mean, 817 ± 689 ng ml−1) [29], in accordance with study results published by Ullmann et al. [36]. Predisposing risk factors for lower-than-expected posaconazole plasma concentrations may include increased gastric pH values induced by concomitant cimetidine or a proton pump inhibitor (PPI), increased gastrointestinal motility, diarrhoea, mucositis, concomitant enzyme-inducing agents and non-compliance [3]. Similar results have been observed when posaconazole 200 mg three times daily was administered prophylactically [10]. However, the clinical impact of these parameters remains controversial.
Therapeutic drug monitoring
Determination of a relationship between plasma concentrations of posaconazole (or any antifungal agent) and efficacy comes from a combination of experimental models of fungal infections, preclinical experiments, pharmacodynamic and pharmacokinetic assessments, and in clinical settings and data. Preliminary data from an open-label multicentre trial in patients with invasive aspergillosis refractory to or intolerant of other antifungals indicate that lower Cmax and average plasma concentrations (Cavg) may be associated with a lower response rate [37]. More extensive clinical investigation is needed to determine whether target Cavg concentrations of 1.25 µg ml−1[37] have to be achieved to increase the probability of treatment success (Table 3) [11]. According to tentative recommendations for TDM, which have recently been published, trough concentrations of at least 0.5–1.5 µg ml−1 appear to be warranted in patients with IFI measured 4–7 days after the start of therapy [32]. Preliminary data from a retrospective analysis of cancer patients who received posaconazole as antifungal prophylaxis (200 mg three times daily) indicated a lower risk of proven or probable IFI in patients with plasma drug concentrations >700 ng ml−1 3–5 h after drug administration on day 7 (0%–1.88% IFI), vs. patients with plasma drug concentrations ≤700 ng ml−1 (3.87%–6.52% IFI) [10]. Meanwhile, the Food and Drug Administration has proposed a dosing algorithm that targets Cavg concentrations of posaconazole of 350 ng ml−1 and 700 ng ml−1 on days 2 and 7 during prophylaxis, respectively. The targets are based on two phase 3 trials concerning antifungal prophylaxis and patients with and without IFI despite posaconazole exposure (Table 4) [10, 20]. However, the algorithm is based on a very limited number of patients available for IFI, primarily from the trial focusing on GVHD. In a salvage study in patients with refractory invasive aspergillosis (IA) treated with posaconazole, a quartile analysis showed that mean Cmax (% CV) values as low as 411 (21) ng ml−1 (lower than the 700 ng ml−1 concentration identified in the FDA analysis) resulted in an efficacy rate of 53% [37], which is comparable or better than that seen with other agents [34, 38, 39]. Taking several factors into consideration, current TDM recommendations have suggested that a trough concentration >0.5 mg ml−1, 4–7 days after initiating prophylaxis is an appropriate target [32].
Table 3.
Posaconazole peak (Cmax) and average (Cavg) concentrations may be of predictive value in patients with invasive aspergillosis treated with 400 mg twice daily [37]
| Cmax (µg ml−1) | Cavg (µg ml−1) | Response |
|---|---|---|
| 0.142 | 0.134 | 24% (4 of 17) |
| 0.467–0.852 | 0.411–0.719 | >53% (18 of 34) |
| 1.48 | 1.25 | 75% (12 of 16) |
Table 4.
Clinical pharmacokinetics of oral posaconazole in allogeneic haematopoietic stem cell transplant recipients with GVHD and neutropenic patients receiving chemotherapy for AML or MDS with or without IFI [10, 20]
| Prophylaxis during neutropenia | Prophylaxis during GVHD | |||
|---|---|---|---|---|
| POS (ng ml−1) | Without IFI (n = 188) | With IFI (n = 6) | Without IFI (n = 241) | With IFI (n = 5) |
| Mean Cavg ± SD | 586 ± 379 | 457 ± 169 | 1131 ± 759 | 669 ± 543 |
| Median Cavg (range) | 486 (92–1945) | 454 (254–679) | 922 (0–3650) | 611 (158–1562) |
| Mean Cmax ± SD | 633 ± 413 | 498 ± 194 | 1514 ± 970 | 755 ± 644 |
| Median, Cmax (range) | 531 (92–2400) | 468 (254–781) | 1360 (0–4420) | 635 (158–1800) |
AML, acute myelogenous leukaemia; GVHD, graft-versus-host disease; IFI, invasive fungal infection; MDS, myelodysplastic syndrome; POS, posaconazole.
According to study results based on juvenile patients aged >18 years, there is some evidence that Cavg (with SD) values were similar to adults: 776 (769) ng ml−1vs. 817 (689) ng ml−1, respectively. In addition, the Cavg values for the youngest and oldest patients (aged 8 and 17 years, respectively) were similar [29]. As a consequence, TDM recommendations for adult patients may be translated to paediatric patients in the near future.
Clinical efficacy for prophylaxis and treatment of IFI
Based on the impressive results of a phase 3 randomized, multicentre trial involving 600 patients, comparing posaconazole (200 mg three times daily) with fluconazole for IFI prophylaxis in high-risk patients with GVHD after allogeneic haematopoietic stem cell transplantation, the extended-spectrum triazole was approved for this indication [40]. In this trial, posaconazole was superior to fluconazole in the prevention of proven or probable aspergillosis (2.3% vs. 7.0%, P = 0.006) [40]. In addition, the number of IFI-related deaths was significantly lower with posaconazole treatment (Table 5) [41], and there were no significant differences in drug-related adverse events (AEs) [40].
Table 5.
Numbers needed to treat with posaconazole to prevent IFI and death [41]
| Antifungal prophylaxis during neutropenia | Antifungal prophylaxis during GVHD | |||||
|---|---|---|---|---|---|---|
| Clinical outcome | Posaconazole 200 mg tid | Fluconazole 400 mg od; itraconazole 200 mg bid | NNT | Posaconazole 200 mg tid | Fluconazole 400 mg od | NNT |
| IFI | 2.3% | 8.4% | 16 | 5.3% | 9% | 27* |
| Invasive aspergillosis | 0.7% | 6.7% | 17 | 2.3% | 7% | 21 |
| Death due to IFI | 1.6% | 5.4% | 27 | 0.7% | 3.7% | 33 |
| Death due to any cause | 14.5% | 21.5% | 14 | 25.2% | 28.1% | 35* |
Not significant. Bid, twice daily; IFI, invasive fungal infection; GVHD, graft-versus-host disease; NNT, number needed to treat; od, once daily; tid, three times daily.
Another trial included patients undergoing chemotherapy for AML or MDS who developed febrile neutropenia. Antifungal prophylaxis with posaconazole (200 mg three times daily) was associated with a significantly lower incidence of breakthrough fungal infection (2% vs. 8%) and proven or probable infections caused by Aspergillus spp. than prophylaxis with either fluconazole (400 mg day−1) or itraconazole (200 mg twice daily) (Table 5) [41]. In addition, prophylaxis with posaconazole was associated with significantly fewer deaths due to fungal infection or any cause [42]. The incidence of AEs was similar between treatment groups [42].
Study results published by Walsh et al. [37] found posaconazole (800 mg daily) to be active against invasive aspergillosis or other systemic mycoses in patients with disease progression or intolerance to conventional amphotericin B or its lipid formulations. Similar results have been obtained in patients with refractory coccidioidomycosis, CNS cryptococcal infections and chromoblastomycosis [1, 5]. In addition, posaconazole was confirmed to be useful as salvage therapy in patients with zygomycosis, according to a retrospective study including 91 patients with approximately 75% proven and 25% probable IFIs [43]. At 12 weeks after initiation of treatment, 60% of patients reported overall success (46% and 14% of patients reached partial or complete response, respectively), which is impressive based on the limited spectrum of therapeutic alternatives for this pathogen [43]. Although these results support the use of posaconazole as an extended-spectrum triazole antifungal agent even in refractory aspergillosis, a head-to-head comparison between posaconazole (400 mg twice daily) and other agents (e.g. caspofungin) for the treatment of refractory mycoses is still lacking.
In patients with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS) who have oropharyngeal candidiasis, posaconazole (200 mg on day 1, followed by 100 mg daily) was associated with a significantly higher mycological success rate by day 42 than fluconazole at the same dosage. In addition, patients receiving posaconazole showed a trend towards lower clinical relapse rates. The incidence of AEs was comparable between the two groups [44]. Further data indicated that posaconazole (400 mg orally once or twice daily) appeared to be a very encouraging agent in fluconazole- or itraconazole-refractory oropharyngeal and oesophageal candidiasis, with 75% of 176 patients achieving a clinical response [45].
Clinical experience with posaconazole in paediatric patients is still very limited. According to a multicentre retrospective survey in 10 patients (median age 10.4 years, range 3.6–15.5 years), clinical adverse effects were mild to moderate with a median daily dosage of 20.5 mg kg−1 (95% CI 14.6, 25.8). The underlying infections included zygomycosis (5), mould infection (3), aspergillosis (1) and chronic disseminated candidiasis (1). The usefulness of posaconazole is reflected by an overall response rate of 60% and an overall survival at 3 months after the end of treatment of 70% (7/10) [46].
Tolerability
Posaconazole has been associated with a low incidence of AEs. No obvious differences in drug tolerability have been observed between young (18–45 years) and elderly patients (>65 years) [26].
The safety of posaconazole was assessed in an analysis of 428 patients who received posaconazole in two phase 2/3 open-label clinical trials [47]. Among the 109 patients who received long-term (≥6 months) posaconazole 400 mg twice daily, the most common treatment-related AEs reported were headache (9%), nausea (7%) and anorexia, raised serum glutamic pyruvic transaminase (SGPT) concentrations and abdominal pain (all 5%) [47].
Another analysis of 18 clinical trials in 448 healthy volunteers who received posaconazole 50–1200 mg day−1 reported that the incidence of treatment-related adverse events appeared to be unrelated to dose [35].
There are no specific data on distribution and frequency of adverse events in paediatric patients.
Drug interactions
Absorption of posaconazole is generally favoured by low gastric pH values. As a consequence, drugs that inhibit gastric acid secretion, thereby elevating gastric pH values, may have a pronounced effect on posaconazole bioavailability. Although antacids do not significantly influence the clinical pharmacokinetics of the triazole [48], co-administration of posaconazole (400 mg twice daily) with the H2-antihistaminic agent cimetidine was associated with a reduced posaconazole bioavailability of about 39% [49]. In addition, preliminary results indicate that concomitant administration of a PPI can reduce posaconazole Cmax by about 50% [50, 51]. As a consequence, the concomitant use of PPIs or H2-antihistaminic agents with posaconazole should be avoided whenever possible. Whether an empiric dose escalation of posaconazole would circumvent this interaction remains unclear and may be limited for pharmacoeconomic reasons. Such drug interactions highlight the need for a parenteral formulation of posaconazole for certain patient groups.
A decrease in Cmax and area under the concentration–time curve (AUC) values of about 20% was observed when posaconazole was administered concomitantly with metoclopramide, which can increase gastric emptying therefore allowing the drug to reach the small intestine faster [50]. As a consequence, it can be assumed that more pronounced diarrhoea may impair posaconazole absorption via reduced contact time in the intestine. Indeed, preliminary data in patients with GVHD strengthen this hypothesis: patients with diarrhoea (n = 18) had a lower Cmax than those without diarrhoea (n = 223) (median Cmax, 623 ± 685 ng ml−1vs. 1460 ± 972 ng ml−1, respectively) [10].
Posaconazole is a potent inhibitor of CYP3A4 in the liver, which is especially important when a concomitantly administered CYP3A4 substrate either undergoes an extensive first-pass effect or has a small therapeutic window [52, 53]. For example, when posaconazole 200 mg to 400 mg was administered concomitantly with midazolam 2 mg orally, the Cmax and AUC of the benzodiazepine were increased by two- and five-fold, respectively (Table 6) [54]. The midazolam Cmax concerntration was somewhat lower when the benzodiazepine was given intravenously because of the absence of the first-pass effect. However, while posaconazole can be classified as a strong CYP3A inhibitor (≥five-fold increase in substrate AUC) [55], its inhibition is weaker than that of the structurally related ketoconazole [54].
Table 6.
Increase of midazolam Cmax and AUC after oral or peritoneal administration during concomitant posaconazole or ketoconazole therapy [54]
| Drug regimen | P.o. MDZ (2 mg) | I.v. MDZ (0.4 mg) |
|---|---|---|
| Posaconazole (200–400 mg) | MDZ Cmax: 2-fold increase | MDZ Cmax: 1.3–1.6-fold increase |
| MDZ AUC: 5-fold increase | MDZ AUC: 5–6-fold increase | |
| Ketoconazole (400 mg) | MDZ Cmax: 3-fold increase | MDZ Cmax: 1.6-fold increase |
| MDZ AUC: 8-fold increase | MDZ AUC: 8-fold increase |
AUC, area under the concentration–time curve; Cmax, maximum plasma drug concentration; i.v., intravenously; MDZ, midazolam; p.o., orally.
The more extensive the first-pass effect of the concomitantly administered CYP3A4 substrate, the more pronounced the expected increase of its Cmax and AUC during posaconazole administration. However, drug interaction studies between posaconazole and simvastatin have not been published, even though simvastatin undergoes an extensive first-pass effect [56].
Several immunosuppressants, including ciclosporin, sirolimus and tacrolimus, also undergo an extensive first-pass effect. As a consequence, Cmax and AUC of these drugs can be significantly increased with posaconazole co-administration. The Cmax and AUC of tacrolimus increased by 2.2- and 4.2-fold, respectively, on day 14 during concomitant posaconazole administration; a moderate interaction [57]. As with other triazoles, posaconazole co-administration has a potent effect on the clinical pharmacokinetics of sirolimus; the Cmax and AUC of the immunosuppressive agent were increased by 6.7- and 8.9-fold, respectively, in healthy subjects after co-administration with posaconazole [58]. Because its first-pass effect was comparatively lower, ciclosporin pharmacokinetics appeared to be less affected during posaconazole co-administration, with less-intensive dose reductions (14%–29%) required [57]. However, it is recommended that ciclosporin blood concentrations are monitored more closely during posaconazole co-administration [57]. Further, CYP3A4 substrates whose plasma concentrations can be elevated during posaconazole co-administration include several calcium antagonists (e.g. felodipine, nifedipine, verapamil, diltiazem) and vinca alkaloids [3, 59]. Drug interaction studies with CYP3A4 substrates, such as all-trans retinoic acid, ergot alkaloids, alfentanil and quinidine, are still lacking. However, based on data from itraconazole or voriconazole, clinically relevant interactions with posaconazole are likely to occur (Table 7) [3, 22, 60, 61]. Besides the potential for associated pharmacokinetic drug interactions, co-administration of posaconazole with drugs such as terfenadine or quinidine is contraindicated to avoid critical QTc interval prolongation.
Table 7.
Major potential and confirmed drug interactions with different co-administered drugs and azole antifungal drugs [3]
| Fluconazole | Itraconazole | Voriconazole | Posaconazole | |
|---|---|---|---|---|
| Ciclosporin | ↑ | ↑ | ↑ | ↑ |
| Everolimus | ↑ | ↑↑ | ↑↑ | ↑↑ |
| Sirolimus | ↑ | ↑↑ | ↑↑ | ↑↑ |
| Tacrolimus | ↑ | ↑ | ↑↑ | ↑↑ |
| Calcium channel blockers | ↑ | ↑ | ↑ | ↑ |
| Antiarrhythmic agents (e.g. quinidine, dofetilide) | ↑ | ↑ | ↑ | ↑ |
| All-trans-retinoic acid | ↑ | ? | ? | ? |
| Busulfan | No | ↑ | ↑ | ? |
| Vinca alkaloids | ? | ↑ | ↑ | ↑ |
| Midazolam | ↑ | ↑ | ↑ | ↑ |
| Simvastatin | ↑ | ↑↑ | ↑↑ | ↑↑ |
| Rifampicin | ↓ FLU | ↓↓ ITR | ↓↓↓ VOR | ↓ POS |
| Phenytoin | ↑ | ↑ | ↑↑ | ↑ |
| ↓↓ FLU | ↓↓ ITR | ↓↓ VOR | ↓ POS | |
| Omeprazole | No | ↓↓ ITR (cap) | ↑ VOR | ↓ POS |
cap, capsule; FLU, fluconazole; ITR, itraconazole; POS, posaconazole; VOR, voriconazole. Table adapted from Lipp HP. Mycoses 2008; 51: 7–18, with permission [3].
Potent enzyme-inducing agents like phenytoin or rifabutin have been shown to increase posaconazole clearance by approximately 90% [62, 63]. Whether these agents interact with posaconazole via an induction of UGT1A4 has not yet been established. Drug–drug interactions with an associated increase of P-glycoprotein as the reason for the observed drug interactions are unlikely because no association of constitutive P-glycoprotein genotype and posaconazole pharmacokinetics has been observed [26].
Discussion
Posaconazole is highly active against an extended spectrum of fungi, including Aspergillus spp., Candida spp., Fusarium spp. and the Zygomycetes. IFIs caused by these pathogens are associated with a high risk of morbidity and mortality, particularly in intensive care unit patients with additional risk factors. The Tarragona strategy is an intensified anti-infective regimen to be instituted at a very early stage of the disease because a delay in instituting such a regimen has been associated with increased mortality in patients with severe infectious diseases, including systemic mycoses [64, 65]. Posaconazole is a promising agent, but a parenteral formulation would be preferable in a very critical target population, to increase the probability of sustaining adequate plasma concentrations. Preliminary data indicate that several factors (e.g. an increase of gastric pH values, administration of the drug on an empty stomach, acute GVHD or more pronounced diarrhoea) may have a significant impact on the absolute bioavailability of posaconazole [10].
Whether TDM should be considered during administration of posaconazole is a matter of debate. Drug variability among individuals was low, according to study results involving healthy volunteers, but was clearly more pronounced in a target population (e.g. patients with GVHD or AML or MDS with neutropenia) [11]. According to post hoc analyses published by Walsh et al. a correlation exists between increased plasma concentrations and increased response rates in patients with invasive aspergillosis [37], which agrees with the results of a study on voriconazole in patients with IFI [66]. However, prospective randomized studies may be needed to define valid trough concentrations that should be exceeded in order to increase the probability of successful treatment in patients with IFI or of successful prophylaxis in those at risk for IFI. Certain questions still remain concerning the recently presented algorithm targeting 700 ng ml−1 posaconazole to be essential during prophylaxis. Are the few patients with IFI enough to define such a threshold value? Is an empirical dosage increase to 400 mg three times daily appropriate, even though this regimen has not been proven in clinical trials? Why do patients with even lower drug concenrations not develop breakthrough infections in a higher percentage than expected?
Whether plasma concentrations of posaconazole can accurately reflect drug distribution into deeper compartments remains controversial. However, adequate concentrations in the former may be an important predictor of the latter [67]. Extraordinarily high drug concentrations in pulmonary alveolar macrophages [23] and good activity in CNS infections [24], in spite of limited penetration through the blood–brain barrier, indicate that the correlation of posaconazole clinical pharmacokinetics and its pharmacodynamics should be investigated in more detail.
Competing interests
HPL served as a consultant or has participated as a speaker for Astellas, Gilead, Pfizer, MSD and Schering-Plough.
Dr Lipp would like to thank Schering-Plough Corporation in association with ApotheCom for editorial assistance.
REFERENCES
- 1.Nagappan V, Deresinski S. Posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007;45:1610–7. doi: 10.1086/523576. [DOI] [PubMed] [Google Scholar]
- 2.Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol. 2003;57:218–22. doi: 10.1046/j.1365-2125.2003.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipp HP. Antifungal agents – clinical pharmacokinetics and drug interactions. Mycoses. 2008;51:7–18. doi: 10.1111/j.1439-0507.2008.01523.x. [DOI] [PubMed] [Google Scholar]
- 4.Schiller DS, Fung HB. Posaconazole: an extended-spectrum triazole antifungal agent. Clin Ther. 2007;29:1862–86. doi: 10.1016/j.clinthera.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Keating GM. Posaconazole. Drugs. 2005;65:1553–67. doi: 10.2165/00003495-200565110-00007. [DOI] [PubMed] [Google Scholar]
- 6.Templeton IE, Thummel KE, Kharasch ED, Kunze KL, Hoffer C, Nelson WL, Isoherranen N. Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo. Clin Pharmacol Ther. 2008;83:77–85. doi: 10.1038/sj.clpt.6100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galetin A, Ito K, Hallifax D, Houston JB. CYP3A4 substrate selection and substitution in the prediction of potential drug-drug interactions. J Pharmacol Exp Ther. 2005;314:180–90. doi: 10.1124/jpet.104.082826. [DOI] [PubMed] [Google Scholar]
- 8.Jeong S, Nguyen PD, Desta Z. Comprehensive in vitro inhibition analysis of 8 cytochrome P450 (CYP) enzymes by voriconazole: major effect on CYPs 2B6, 2C9, 2C19 and 3A. Antimicrob Agents Chemother. 2008;53:541–51. doi: 10.1128/AAC.01123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtney R, Pai S, Laughlin M, Lim J, Batra V. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother. 2003;47:2788–95. doi: 10.1128/AAC.47.9.2788-2795.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy. 2007;27:1627–36. doi: 10.1592/phco.27.12.1627. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin ML, Drew RH. Antifungal serum concentration monitoring: an update. J Antimicrob Chemother. 2008;61:17–25. doi: 10.1093/jac/dkm389. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller MA, Messer S, Jones RN. Activity of a new triazole, Sch 56592, compared with those of four other antifungal agents tested against clinical isolates of Candida spp. and Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1997;41:233–5. doi: 10.1128/aac.41.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diekema DJ, Messer SA, Hollis RJ, Jones RN, Pfaller MA. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J Clin Microbiol. 2003;41:3623–6. doi: 10.1128/JCM.41.8.3623-3626.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barchiesi F, Schimizzi AM, Caselli F, Giannini D, Camiletti V, Fileni B, Giacometti A, Di Francesco LF, Scalise G. Activity of the new antifungal triazole, posaconazole, against Cryptococcus neoformans. J Antimicrob Chemother. 2001;48:769–73. doi: 10.1093/jac/48.6.769. [DOI] [PubMed] [Google Scholar]
- 15.Spreghini E, Maida CM, Milici ME, Scalise G, Barchiesi F. Posaconazole activity against Candida glabrata after exposure to caspofungin or amphotericin B. Antimicrob Agents Chemother. 2008;52:513–7. doi: 10.1128/AAC.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatelli F, Patel R, Mann PA, Mendrick CA, Norris CC, Hare R, Loebenberg D, Black TA, McNicholas PM. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother. 2006;50:2009–15. doi: 10.1128/AAC.00163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cacciapuoti A, Halpern J, Mendrick C, Norris C, Patel R, Loebenberg D. Interaction between posaconazole and caspofungin in concomitant treatment of mice with systemic Aspergillus infection. Antimicrob Agents Chemother. 2006;50:2587–90. doi: 10.1128/AAC.00829-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guembe M, Guinea J, Pelaez T, Torres-Narbona M, Bouza E. Synergistic effect of posaconazole and caspofungin against clinical zygomycetes. Antimicrob Agents Chemother. 2007;51:3457–8. doi: 10.1128/AAC.00595-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuenca-Estrella M. Combinations of antifungal agents in therapy: what value are they? J Antimicrob Chemother. 2004;54:854–69. doi: 10.1093/jac/dkh434. [DOI] [PubMed] [Google Scholar]
- 20.Krishna G, Tarif MA, Xuan F, Martinho M, Angulo D, Cornely OA. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy. 2008;28:1223–32. doi: 10.1592/phco.28.10.1223. [DOI] [PubMed] [Google Scholar]
- 21.Ezzet F, Wexler D, Courtney R, Krishna G, Lim J, Laughlin M. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin Pharmacokinet. 2005;44:211–20. doi: 10.2165/00003088-200544020-00006. [DOI] [PubMed] [Google Scholar]
- 22.Bruggemann RJM, Alffenaar JWC, Blijlevens NMA, Billaud EM, Kosterink JGW, Verweij PE, Burger DM. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009;48:1441–58. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 23.Conte JE, Jr, Golden JA, Krishna G, McIver M, Little E, Zurlinden E. Intrapulmonary pharmacokinetics and pharmacodynamics of posaconazole at steady state in healthy subjects. Antimicrob Agents Chemother. 2008;53:703–7. doi: 10.1128/AAC.00663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitisuttithum P, Negroni R, Graybill JR, Bustamante B, Pappas P, Chapman S, Hare RS, Hardalo CJ. Activity of posaconazole in the treatment of central nervous system fungal infections. J Antimicrob Chemother. 2005;56:745–55. doi: 10.1093/jac/dki288. [DOI] [PubMed] [Google Scholar]
- 25.Ruping MJGT, Albermann N, Ebinger F, Burckhardt I, Beisel C, Muller C, Vehreschild JJ, Kochanek M, Fatkenheuer G, Bangard C, Ullmann AJ, Herr W, Kolbe K, Hallek M, Cornely OA. Posaconazole concentrations in the central nervous system. J Antimicrob Chemother. 2008;62:1468–70. doi: 10.1093/jac/dkn409. [DOI] [PubMed] [Google Scholar]
- 26.Sansone-Parsons A, Krishna G, Simon J, Soni P, Kantesaria B, Herron J, Stoltz R. Effects of age, gender, and race/ethnicity on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob Agents Chemother. 2007;51:495–502. doi: 10.1128/AAC.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosal A, Hapangama N, Yuan Y, Achanfuo-Yeboah J, Iannucci R, Chowdhury S, Alton K, Patrick JE, Zbaida S. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil) Drug Metab Dispos. 2004;32:267–71. doi: 10.1124/dmd.32.2.267. [DOI] [PubMed] [Google Scholar]
- 28.Krieter P, Flannery B, Musick T, Gohdes M, Martinho M, Courtney R. Disposition of posaconazole following single-dose oral administration in healthy subjects. Antimicrob Agents Chemother. 2004;48:3543–51. doi: 10.1128/AAC.48.9.3543-3551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishna G, Sansone-Parsons A, Martinho M, Kantesaria B, Pedicone L. Posaconazole plasma concentrations in juvenile patients with invasive fungal infection. Antimicrob Agents Chemother. 2007;51:812–8. doi: 10.1128/AAC.00454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Courtney R, Sansone A, Smith W, Marbury T, Statkevich P, Martinho M, Laughlin M, Swan S. Posaconazole pharmacokinetics, safety, and tolerability in subjects with varying degrees of chronic renal disease. J Clin Pharmacol. 2005;45:185–92. doi: 10.1177/0091270004271402. [DOI] [PubMed] [Google Scholar]
- 31.Hachem RY, Langston AA, Graybill JR, Perfect JR, Pedicone LD, Patino H, Raad II. Posaconazole as salvage treatment of invasive fungal infections in patients with underlying renal impairment. J Antimicrob Chemother. 2008;62:1386–91. doi: 10.1093/jac/dkn401. doi: 10.1093/jac/dkn401. [DOI] [PubMed] [Google Scholar]
- 32.Andes D, Pascual A, Marchetti O. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother. 2009;53:24–34. doi: 10.1128/AAC.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moton A, Krishna G, Ma L, O'Mara E, Prasad P, McLeod J, Preston RA. Pharmacokinetics of a single dose of the antifungal posaconazole as oral suspension in subjects with hepatic impairment. Curr Med Res Opin. 2009;26:1–7. doi: 10.1185/03007990903364657. doi: 10.1185/03007990903364657. [DOI] [PubMed] [Google Scholar]
- 34.Walsh TJ, Hiemenz JW, Seibel NL, Perfect JR, Horwith G, Lee L, Silber JL, DiNubile MJ, Reboli A, Bow E, Lister J, Anaissie EJ. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998;26:1383–96. doi: 10.1086/516353. [DOI] [PubMed] [Google Scholar]
- 35.Moton A, Krishna G, Wang Z. Tolerability and safety profile of posaconazole: evaluation of 18 controlled studies in healthy volunteers. J Clin Pharm Ther. 2009;34:301–11. doi: 10.1111/j.1365-2710.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 36.Ullmann AJ, Cornely OA, Burchardt A, Hachem R, Kontoyiannis DP, Topelt K, Courtney R, Wexler D, Krishna G, Martinho M, Corcoran G, Raad I. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother. 2006;50:658–66. doi: 10.1128/AAC.50.2.658-666.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik J-AH, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44:2–12. doi: 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 38.Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, Haas A, Ruhnke M, Lode H. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34:563–71. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 39.Kartsonis NA, Saah AJ, Joy Lipka C, Taylor AF, Sable CA. Salvage therapy with caspofungin for invasive aspergillosis: results from the caspofungin compassionate use study. J Infect. 2005;50:196–205. doi: 10.1016/j.jinf.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 41.Cornely OA, Ullmann AJ. Numbers needed to treat with posaconazole prophylaxis to prevent invasive fungal infection and death. Clin Infect Dis. 2008;46:1626–7. doi: 10.1086/587177. [DOI] [PubMed] [Google Scholar]
- 42.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh Y-T, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 43.van Burik J-AH, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61–e65. doi: 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 44.Vazquez JA, Skiest DJ, Nieto L, Northland R, Sanne I, Gogate J, Greaves W, Isaacs R. A multicenter, randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS. Clin Infect Dis. 2006;42:1179–86. doi: 10.1086/501457. [DOI] [PubMed] [Google Scholar]
- 45.Skiest DJ, Vazquez JA, Anstead GM, Graybill JR, Reynes J, Ward D, Hare R, Boparai N, Isaacs R. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin Infect Dis. 2007;44:607–14. doi: 10.1086/511039. [DOI] [PubMed] [Google Scholar]
- 46.Groll AH. Slavage treatment with posaconazole in pediatric patients. Mycoses. 2009:52. [Google Scholar]
- 47.Raad II, Graybill JR, Bustamante AB, Cornely OA, Gaona-flores V, Afif C, Graham DR, Greenberg RN, Hadley S, Langston A, Negroni R, Perfect JR, Pitisuttithum P, Restrepo A, Schiller G, Pedicone L, Ullmann AJ. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006;42:1726–34. doi: 10.1086/504328. [DOI] [PubMed] [Google Scholar]
- 48.Courtney R, Radwanski E, Lim J, Laughlin M. Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men. Antimicrob Agents Chemother. 2004;48:804–8. doi: 10.1128/AAC.48.3.804-808.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courtney R, Wexler D, Statkevich P, Lim J, Batra V, Laughlin M. Effect of cimetidine on the pharmacokinetics of posaconazole in healthy volunteers [abstract]. 9-27-2002. Thesis/Dissertation. Poster presented at: 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 27–30, 2002; San Diego, CA.
- 50.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. The pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53:958–66. doi: 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alffenaar JW, van Assen S, van der Werf TS, Kosterink JG, Uges DR. Omeprazole significantly reduces posaconazole serum trough level. Clin Infect Dis. 2009;48:839. doi: 10.1086/597110. [DOI] [PubMed] [Google Scholar]
- 52.Wexler D, Courtney R, Richards W, Banfield C, Lim J, Laughlin M. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. Eur J Pharm Sci. 2004;21:645–53. doi: 10.1016/j.ejps.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Galetin A, Hinton LK, Burt H, Obach RS, Houston JB. Maximal inhibition of intestinal first-pass metabolism as a pragmatic indicator of intestinal contribution to the drug-drug interactions for CYP3A4 cleared drugs. Curr Drug Metab. 2007;8:685–93. doi: 10.2174/138920007782109805. [DOI] [PubMed] [Google Scholar]
- 54.Krishna G, Moton A, Ma L, Savant I, Martinho M, Seiberling M, McLeod J. Effects of oral posaconazole on the pharmacokinetic properties of oral and intravenous midazolam: a phase I, randomized, open-label, crossover study in healthy volunteers. Clin Ther. 2009;31:286–98. doi: 10.1016/j.clinthera.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Huang SM, Temple R, Throckmorton DC, Lesko LJ. Drug interaction studies: study design, data analysis, and implications for dosing and labeling. Clin Pharmacol Ther. 2007;81:298–304. doi: 10.1038/sj.clpt.6100054. [DOI] [PubMed] [Google Scholar]
- 56.Merck & Co., Inc. Zocor® (Simvastatin) Tablets. Whitehouse Station, NJ: Merck & Co., Inc; 2005. [Google Scholar]
- 57.Sansone-Parsons A, Krishna G, Martinho M, Kantesaria B, Gelone S, Mant TG. Effect of oral posaconazole on the pharmacokinetics of cyclosporine and tacrolimus. Pharmacotherapy. 2007;27:825–34. doi: 10.1592/phco.27.6.825. [DOI] [PubMed] [Google Scholar]
- 58.Moton A, Ma L, Krishna G, Martinho M, Seiberling M, McLeod J. Effects of oral posaconazole on the pharmacokinetics of sirolimus. Curr Med Res Opin. 2009;25:701–7. doi: 10.1185/03007990802644209. [DOI] [PubMed] [Google Scholar]
- 59.Drew RH. Posaconazole in the treatment of selected IFIs. Hospital Pharmacy. 2006:34–9. [Google Scholar]
- 60.SP Europe. Noxafil 40 mg/Ml Oral Suspension [Summary of Product Characteristics] Brussels, Belgium: SP Europe; 2007. [Google Scholar]
- 61.Spriet I, Meersseman W, Wilmer WS, Willems HJ, Krishna A. Mini-series: II. Clinical aspects. Clinically relevant CYP450-mediated drug interactions in the ICU. Intensive Care Med. 2009;35:603–12. doi: 10.1007/s00134-008-1383-2. [DOI] [PubMed] [Google Scholar]
- 62.Krishma G, Sansone-Parsons A, Kantesaria B. Drug interaction assessment following concomitant administration of posaconazole and phenytoin in healthy men. Curr Med Res Opin. 2007;23:1415–22. doi: 10.1185/030079907X187937. [DOI] [PubMed] [Google Scholar]
- 63.Krishna G, Parsons A, Kantesaria B, Mant T. Evaluation of the pharmacokinetics of posaconazole and rifabutin following co-administration to healthy men. Curr Med Res Opin. 2007;23:545–52. doi: 10.1185/030079906X167507. [DOI] [PubMed] [Google Scholar]
- 64.Sandiumenge A, Diaz E, Bodi M, Rello J. Therapy of ventilator-associated pneumonia. A patient-based approach based on the ten rules of ‘The Tarragona Strategy’. Intensive Care Med. 2003;29:876–83. doi: 10.1007/s00134-003-1715-1. [DOI] [PubMed] [Google Scholar]
- 65.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–9. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 66.Trifilio S, Singhal S, Williams S, Frankfurt O, Gordon L, Evens A, Winter J, Tallman M, Pi J, Mehta J. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 2007;40:451–6. doi: 10.1038/sj.bmt.1705754. [DOI] [PubMed] [Google Scholar]
- 67.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother. 2008;61:235–7. doi: 10.1093/jac/dkm476. [DOI] [PubMed] [Google Scholar]


