Abstract
AIMS
This study aimed to identify differentially overexpressed membrane-enriched as well as cytosolic proteins in SAG sensitive and resistant clinical strains of L. donovani isolated from VL patients which are involved in the drug resistance mechanism.
METHODS
The proteins in the membrane-enriched as well as cytosolic fractions of drug-sensitive as well as drug-resistant clinical isolates were separated using two-dimensional gel electrophoresis and overexpressed identified protein spots of interest were excised and analysed using MALDI-TOF/TOF.
RESULTS
Six out of 12 overexpressed proteins were identified in the membrane-enriched fraction of the SAG resistant strain of L. donovani whereas 14 out of 18 spots were identified in the cytosolic fraction as compared with the SAG sensitive strain. The major proteins in the membrane-enriched fraction were ABC transporter, HSP-83, GPI protein transamidase, cysteine–leucine rich protein and 60S ribosomal protein L23a whereas in the cytosolic fraction proliferative cell nuclear antigen (PCNA), proteasome alpha 5 subunit, carboxypeptidase, HSP-70, enolase, fructose-1,6-bisphosphate aldolase, tubulin-beta chain have been identified. Most of these proteins have been reported as potential drug targets, except 60S ribosomal protein L23a and PCNA which have not been reported to date for their possible involvement in drug resistance against VL.
CONCLUSION
This study for the first time provided a cumulative proteomic analysis of proteins overexpressed in drug resistant clinical isolates of L. donovani indicating their possible role in antimony resistance of the parasite. Identified proteins provide a vast field to be exploited for novel treatment strategies against VL such as cloning and overexpression of these targets to produce recombinant therapeutic/prophylactic proteins.
Keywords: 2-DGE, Leishmania donovani, MALDI-TOF-MS, membrane enriched and cytosolic proteins, promastigotes, sodium antimony gluconate resistant clinical isolate
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Over 60% of patients with visceral leishmaniasis (VL) in India and Sudan have become unresponsive to treatment with pentavalent antimonials, the first line of drugs for over 60 years. The drug resistance mechanism, studied so far in in vitro selected laboratory strains, has been attributed to various biochemical parameters. The resistance to Sb (V) in Leishmania field isolates is still unexplored.
THIS STUDY ADDS
In order to elucidate for the first time the mechanism of drug resistance in field isolates, this study was done in those clinically relevant field isolates which were either responsive or non responsive to SAG. A comparison of proteome profiles of membrane-enriched as well as cytosolic protein fractions of these isolates has pinpointed the multiple overexpressed proteins in resistant isolates. This study has indicated their possible essential role in antimony resistance of the parasite and provides a vast field to be exploited to find much needed novel treatment strategies against VL.
Introduction
More than 10 million people around the world are currently affected by Leishmania sp [1] with the most frequent prevalence in the tropics and subtropics. Given the difficulties linked to vector (sandfly) control and the lack of an effective vaccine, the control of leishmaniasis relies mostly on chemotherapy. To worsen matters, treatment is increasingly failing due to increasing resistance of the parasites to the most common anti-leishmanial drug SAG, a pentavalent antimony [Sb (V)], in several parts of the world, and most notably in India [2, 3]. New drugs such as liposomal amphotericin B and miltefosine are prohibitively expensive for the most affected populations. It is now well established that Sb (V) is a pro-drug that requires biological reduction to active Sb (III). Although, a single cellular target cannot yet be discounted, it is believed that Sb (V)/Sb (III) may interact with several targets including trypanothione, the main reduced cellular thiol of the parasite [4]. The mechanism by which Leishmania acquires resistance to antimonials has led to contradictory results for several decades. The high level resistance to antimony observed in Leishmania can be due to simultaneous selection of loss in metal reduction, decreased drug uptake, increased glutathione and trypanothione synthesis, and increased transport (sequestration or efflux) of thiol-metal conjugates [5]. The parasite could have other mechanisms that confer metal resistance. In contrast to in vitro selected strains, resistance to Sb (V) in Leishmania field isolates is not well understood. Since, the available treatment for leishmaniasis poses many problems, research needs to be focused on how antimonial drugs work and why they sometimes fail which would be instructive in the development of new therapies. In the present study, we have compared the proteome profile of membrane-enriched as well as cytosolic proteins of Sb (V)-resistant and -sensitive Indian L. donovani field isolates to identify the proteins which are overexpressing in these clinical isolates representing their probable role in drug resistance.
Methods
Leishmania clinical isolates and their maintenance
L. donovani clinical/field isolates SAG sensitive (2001) as well as SAG resistant (2039), procured from patients admitted to the Kala Azar Medical Research Centre, Muzaffarpur, Bihar, were grown/cultured in RPMI-1640 medium supplemented with 10% heat-inactivated foetal bovine serum (Sigma, USA) at 25°C as described previously [6]. The strains have also been maintained in hamsters through serial passage, i.e. from amastigote to amastigote [6]. The sensitivity and resistance of these clinical isolates against SAG have been demonstrated both in vitro and in vivo[6].
Chemicals required
Acrylamide, agarose, bis-acrylamide, biolytes, Coomassie Brilliant Blue (CBB), dithiothreitol (DTT), iodoacetamide, IPG strips, mineral oil and other 2D standards were purchased from Bio-Rad, USA. Trypsin, glycerol, thiourea, protease inhibitor cocktail (PIC), CHAPS, EDTA, TEMED, ammonium persulfate (APS), sodium chloride, mercaptoethanol, glucose, sorbitol, urea, Tris, SDS, TFA, tributyl phosphine (TBP), acetonitrile (AcCN) were obtained from Sigma Chemical Co., USA. α-cyano-4-hydroxycinnamic acid (CHCA), peptide calibration standard mixture (Cal mix.), trifluoro-acetic acid (TFA) and Triton X-100 were purchased from Applied Biosystems, USA.
Isolation of membrane enriched proteins (MEPs) and cytosolic proteins (CPs) of L. donovani
Protein fractions were isolated from promastigotes of L. donovani using the methods described by Molloy et al. [7] and Pavkova et al. [8]. Briefly, late log phase promastigotes (109) of Leishmania parasite culture were disintegrated using repeated cycles of freeze-thawing in liquid nitrogen and the cell pellet was then lyzed by ultrasonication in TNE buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA) with protease inhibitor cocktail (Sigma, USA). The lysate was then incubated with ice-cold 0.1 m sodium carbonate (pH 11) for 30 min to 1 h at 4°C. Finally, MEPs were collected by ultracentrifugation in a Beckman ultracentrifuge (CA, USA) at 120 000 g for 1 h at 4°C in the pellet and with CPs in the supernatant. Such a prefractionation of the proteins reduces the complexity of 2-D maps by removing most non-membrane proteins [9].
Protein sample preparation for two dimensional gel electrophoresis (2-DGE)
MEPs and CPs of L. donovani were precipitated separately in trichloro-acetic acid (TCA) to a final concentration of 10% w/v and washed three times with acetone to eliminate contaminants like nucleic acid and salts (by employing phosphate buffered saline (PBS) or Tris-HCl buffer). The dried sediments were reconstituted in water and stored lyophilized in aliquots after protein estimation. All protein estimations were done by the Lowry method [10]. The dry pellets of MEPs were solubilized in rehydration buffer [7 m urea, 2 m thiourea, 2 mm tributylphosphine (TBP), 40 mm Tris, 4% w/v CHAPS, 1% v/v biolyte pI 3–10, and 1% v/v Triton X-100] and dry pellets of SPs were solubilized in rehydration buffer [7 m urea, 4% w/v CHAPS, 100 mm DTT, 0.5% v/v biolyte pI 3–10, 0.5% v/v Triton X-100 and 40 mm Tris]. Dissolved MEPs and CPs fraction were vortexed and centrifuged at 10 000 g for 10 min to remove insoluble material.
Two dimensional gel electrophoresis (2-DGE) and mass spectrometry
2-DGE was performed as described previously with minor modifications according to the manufacturer's manual (Bio-Rad, USA). Briefly 1.5 mg of MEPs and CPs fractions were solubilized in rehydration buffer, immobilized on dry strips, pI 3–10, 17 cm (Bio-Rad, USA) separately and allowed to rehydrate for 18–22 h. Isoelectric focusing (IEF) was performed at 20°C using the Protean IEF cell (Bio-Rad) according to the manufacturer's instructions. After IEF, the strips were equilibrated in solution A (0.375 m Tris, pH 8.8 containing 6 m urea, 2% SDS, 20% glycerol, 2% w/v DTT) and B (solution A without DTT, but with 2.5% w/v iodoacetamide (IAA) for 20 min at room temperature, the strips were inserted into 12% SDS-PAGE gels (20 × 22 cm), and then sealed with 1% agarose [11]. Electrophoresis was performed initially at 16 mA/gel for 30 min and then at 24 mA/gel at 14°C till the running dye reached the bottom. The gel was stained with colloidal G-250 Coomassie Brilliant Blue (Biosafe; Bio-Rad, CA, USA) and images were acquired by the gel imaging and spot picking system (Investigator™ ProPic, Genomic solution, USA). Gel spots containing the proteins of interest were excised by hand (confirmed by rescanning the gel). The in-gel digestion of proteins and purification of peptides from plugs was carried out according to the manufacturer's manual. Briefly, protein spots were excised, washed with desalted water then with 50% v/v acetonitrile in 25 mm ammonium bicarbonate pH 8.0, shrunk by dehydration in acetonitrile and vacuum dried. Gel pieces were reswollen in 10–20 µl digestion buffer containing sequencing grade modified 10 µg ml−1 trypsin (Promega, Madison, WI, USA). After 15 min, 25 µl of 50 mm ammonium bicarbonate were added to keep the gel pieces wet during tryptic cleavage (37°C, overnight). To extract the peptides, 50% AcCN : 0.3% TFA solution was added, and the samples were incubated for 15 min and vortexed. The separated liquid was dried under vacuum and the peptides were redissolved in 10 µl 0.1% TFA. The peptides were purified with a C18 reversed-phase minicolumn filled in a micropipette tip, ZipTip C18 (Millipore, Bedford, MA, USA), before mass spectrometry. The peptide solution was then mixed with a double volume of matrix, α-cyano-4-hydroxycinnamic acid 10 mg ml−1 (ABI, Farmingham, USA.) in 50% AcCN, 0.1% TFA and spotted onto a MALDI sample plate.
MS and MS/MS spectra were acquired in the positive ion mode on MALDI-TOF/TOF Mass Spectrometer, Applied Biosystems 4700 Proteomics Analyzer (Framingham, MA, USA). The instrument was operated in the delayed extraction mode with a delay time of 200 ns. Spectra were obtained by accumulation of 1000 and 4000 consecutive laser shots, respectively, in MS and MS/MS mode and the laser intensities used were in the range of 5000 to 6000. Close external calibration for MS was performed with 4700 Cal Mix (Applied Biosystems, USA) a standard mixture of six peptides des-Arg1-bradykinin (904.4681), angiotensin I (1296.6853), Glu1-Fibrinopeptide B (1570.6774), ACTH [clip 1–17] (2093.0867), ACTH [clip 18–39] (2465.1989) and ACTH [clip 7–38] (3657.9294). Mass calibration for MS/MS spectra was performed by fragment masses of precursor Glu1-Fibrinopeptide B (1570.6774). Peak harvesting was carried out using 4000 Series Explorer™ Software (Applied Biosystems, USA). Only baseline corrections were applied to the raw data.
Database search for protein identification and localization
Database searching for protein identifications was performed with mass spectrometry data (MS or MS/MS) using Global Proteome Server v3.5 software (Applied Biosystems, USA) equipped with MASCOT-Matrix Science search engine (http://www.matrixscience.com/). Only monoisotopic masses were used for searching against the SWISS-PROT (http://www.expasy.ch/sprot/), NCBInr (http://www.protein.sdu.dk/gpmaw/GPMAW/Database/NCBInr/ncbinr.html) and TriTryp (http://tritrypdb.org/tritrypdb/) databases with a minimum number of matched masses set at 4. The maximum peptide precursor tolerance was set at 40 ppm and MS/MS fragment tolerance was defined as 0.2 kDa. At the most one missed cleavage for tryptic peptides was allowed, and the modifications accepted were carbamidomethyl cysteines as fixed modification and methionine oxidation as variable modification. Tandem MS was performed only in the cases where identification appeared ambiguous with MALDI-TOF-MS data. The criteria used to accept the identifications for peptide mass fingerprint included the probabilistic protein score-based confidence interval %, the extent of sequence coverage, the number of peptides matched and whether Leishmania spp. or Trypanosoma spp. protein appeared as top candidates during the first search, when no restriction was applied to the species of origin. Identification criteria with MS/MS data were that peptides count should not be less than 2 and the % confidence interval for the best ion score should be above 95 (significance level P < 0.05). Protein scores greater than 66 were significant (P < 0.05).
Membrane and cytosolic proteins can be regarded according to their name or the name of their homolog protein or prediction of their localization by the programs SOSUI and WoLF PSORT. SOSUI (http://bp.nuap.nagoya-u.ac.jp/sosui) is a very accurate and reliable program for classification and secondary structure prediction of membrane proteins [12]. WoLF PSORT (http://wolfpsort.org) is able to do protein subcellular localization prediction [13].
Results
Analysis of L. donovani membrane enriched sub-proteome
The gels loaded with 1.5 mg MEPs from SAG sensitive and resistant isolates of L. donovani, separated by 2-DGE displayed good resolution as shown in Figure 1. The reproducibility of the 2-D patterns was confirmed by three consecutive runs from the same protein fraction producing identical patterns. In all, a total of 12 well-resolved overexpressed protein spots in the resistant isolates were detected in 2D gel of MEPs (Figure 1) with pI ranging from 3 –10. MALDI analyses identified six protein spots out of 12 which were classified on the basis of function, sub-cellular localization, class/family (Table 1). Minor protein identification failures could be due to sample amount, specific peptide characteristics and extensive post-translational modifications (PTMs) [14]. The database of the L. infantum genome is available and the homology to L. donovani is approximately 99%. Hence reference was made more comprehensively on the basis of L. infantum using MASCOT. In addition, we also observed that the mass and charge of several proteins were different from those predicted by the leishmanial genome, which has been reported to be a common feature of most proteomic analyses, probably reflecting the effect of protein maturation events including PTMs [14].
Figure 1.
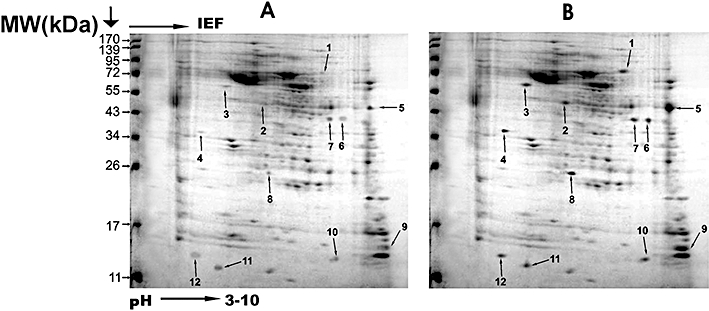
Two-dimensional gel electrophoresis separation of membrane enriched protein (MEP) extracts from SAG sensitive isolate 2001 (A) and SAG resistant isolate 2039 (B) of L. donovani. The numbered spots in (B) represent overexpressed proteins in resistant isolates as compared with sensitive isolates (A) identified by peptide mass fingerprinting (Table 1)
Table 1.
A list of over-expressed membrane enriched proteins of SAG resistant L. donovani isolate (2039) identified through two-dimensional gel electrophoresis and MALDI-TOF/TOF mass spectrometry
| Spotnumbera | Protein identifiedb | Speciesc | Accession No.d | Mol. Masse (Pr ed.) | pIf (Pred.) | Pm/% of Sc/Msg | Fold changeh | Functioni | Subcellular Locali zationj | Class/familyk | Remarks/referencel |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cysteine-leucine rich protein | Li | LinJ34.0570 | 155 | 6.1 | 11/9/149 | 1.86 ± 0.66 | UN | MP | Unknown | DR [21, 38] |
| 2 | ABC1 transporter | Lmj | LmjF11.1240 | 200.3 | 5.2 | 9/12/111 | 2.97 ± 0.96 | TP | MP | ABC transporter | DT [22, 23, 39] |
| 3 | ABC transporter | Lmj | LmjF11.0040 | 73.7 | 6.0 | 9/15/99 | 3.17 ± 0.45 | TP | MP | ABC transporter | DT [22, 23, 39] |
| 5 | Heat shock protein 83 (Fragment) | Ld | HSP83_LEIDO | 52.6 | 5.4 | 15/19//125 | 5.3 ± 0.98 | CH | MP | Hydrolase | DR [16] |
| 6 | GPI protein transamidase | Lmx | Q9U5N7_LEIME | 38.8 | 5.5 | 8/11/101 | 3.71 ± 0.38 | PL | MP | Cysteine type endopeptidase | DT [25] |
| 10 | 60S ribosomal protein L23a(L25) | Tb | RL23A_TRYBB | 18.1 | 10.7 | 5/10/87 | 2.03 ± 0.71 | UN | RS | Unknown | DR [40] |
The overexpressed protein spots shown in Figure 1 were identified using peptide mass fingerprinting.
Protein spots number indicated in Figure 1.
Name of identified protein.
Species: Ld: Leishmania donovani; Li: Leishmania infantum; Lmj: Leishmania major; Lmx: Leishmania mexicana; Tb: Trypanosoma brucei.
Accession numbers of protein according to NCBI and Swiss-Prot accession number.
Predicted molecular mass in kDa.
Predicted pI.
Number of peptides matched (Pm)/Percentage of sequence coverage (Sc) /MOWSE score (Ms).
Fold change of overepressed protein in SAG resistant isolate (2039).
Function of identified proteins; CH: Chaperon, PL: Proteolysis, TP: Transport, UN: Unknown.
Sub-cellular localization; MP: Membrane protein, RS: Ribosomal surface.
Class/Family.
Remarks and references; DR: Drug resistance, DT: Drug target. Protein spots analysed but not identified: 4, 7, 8, 9, 11, 12.
Analysis of L. donovani cytosolic sub-proteome
The gels loaded with 1.5 mg of CPs from SAG sensitive and resistant isolates of L. donovani, separated by 2-DE displayed good resolution as shown in Figure 1. The reproducibility of the 2-D patterns was confirmed by three consecutive runs from the same fraction producing identical patterns. A total 18 overexpressed protein spots were detected in CP fractions (Figure 2) of SAG resistant isolates of gels with pI ranging from 3–10. Mass spectrometry analysis identified 14 spots out of 18 in CP fractions as listed in detail in Table 2. Minor protein identification failures could be due to sample amount, specific peptide characteristics and extensive post-translational modification or significant divergence from sequenced strains [14]. Due to the extremely small number of L. donovani protein sequences in databases, reference was made to the more comprehensive sequences for other species of Leishmania using MASCOT. Among these, the major proteins involved in the drug resistance mechanism were proliferative cell nuclear antigen, proteasome alpha 5 subunit, carboxypeptidase, HSP 70 and tubulin beta chain. Some of the other proteins including some enzymes from carbohydrate metabolism and proteolysis have also been reported as potential drug targets viz. fructose-1, 6-bisphosphatealdolase and enolase. In addition, we also observed that the mass and charge of several proteins were different from those predicted by the leishmanial genome, which has been reported to be a common feature of most proteomic analyses, probably reflecting the effect of protein maturation events including co- or post translational modification [14, 15]. The identified proteins were also classified on the basis of their function, sub-cellular localization and class/family (Tables 1 and 2).
Figure 2.
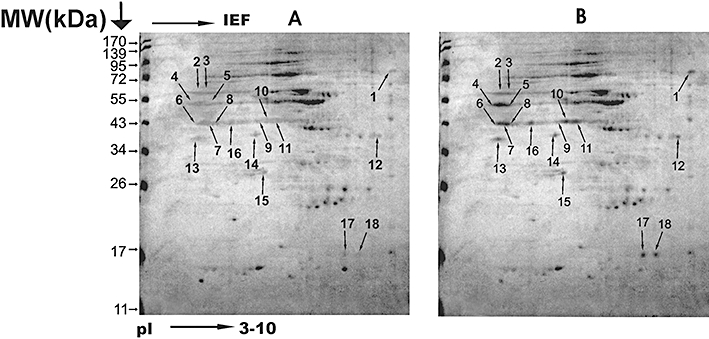
Two-dimensional gel electrophoresis separation of cytosolic protein (CP) from SAG sensitive isolate 2001 (A) and SAG resistant isolate 2039 (B) of L. donovani. The numbered spots in (B) represent overexpressed proteins in resistant isolates as compared with sensitive isolates (A) identified by peptide mass fingerprinting (Table 2)
Table 2.
List of over expressed cytosolic proteins identified by two-dimensional gel electrophoresis and MALDI-TOF/TOF mass spectrometery in SAG resistant clinical isolate (2039) of L. donovani
| Spotnumbera | Protein identifiedb | Speciesc | Accession no.d | Mol. masse (Pr ed.) | pIf (Pred.) | Pm/% of Sc/Msg | Fold changeh | Functioni | Sub-cellular localiz-ationj | Class/familyk | Remarks/referencel |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Heat shock 70 kDa protein (Fragment) | Lmj | HSP70_LEIMA | 56.7 | 6.4 | 9/17/148 | 2 ± 0.51 | CH | MT | Hydrolase | VC [27] |
| 2 | Tubulin beta chain | Lmx | TBB_LEIME | 50.6 | 4.7 | 9/11/113 | 1.81 ± 0.22 | CO | Tubulin | DM [29] | |
| 3 | Tubulin beta chain | Lmx | TBB_LEIME | 50.6 | 4.7 | 9/13/106 | 1.87 ± 0.44 | CO | CP | Tubulin | MM [29] |
| 4 | Carboxypeptidase | Lmj | LmjF14.0180 | 57.0 | 6.0 | 14/17/129 | 2.27 ± 0.4 | PR | CP | Metallo-peptidase | DT [30] |
| 5 | Enolase | Li | LinJ14.1250 | 46.0 | 5.1 | 7/9/102 | 1.9 ± 0.98 | CM | CP | Enolase | DT, VC [33] |
| 6 | Enolase | Li | LinJ14.1250 | 46.0 | 5.1 | 8/9/80 | 2.04 ± 0.72 | CM | CP | Enolase | DT, VC [33] |
| 7 | Fructose-1,6-bisphosphate aldolase | Li | LinJ36.1370 | 40.7 | 8.7 | 10/13/123 | 2.5 ± 0.29 | CM | CP | Lyase | DT, VC [32] |
| 8 | Fructose-1,6-bisphosphate aldolase | Li | LinJ36.1370 | 40.7 | 8.7 | 6/10/75 | 4.33 ± 0 | CM | CP | Lyase | DT, VC [32] |
| 9 | Fructose-1,6-bisphosphate aldolase | Li | LinJ36.1370 | 40.7 | 8.7 | 6/9/77 | 2.72 ± 0.58 | CM | CP | Lyase | DT, VC [32] |
| 10 | Fructose-1,6-bisphosphate aldolase | Li | LinJ36.1370 | 40.7 | 8.7 | 5/7/71 | 2.2 ± 0.77 | CM | CP | Lyase | DT, VC [32] |
| 11 | Fructose-1,6-bisphosphate aldolase | Li | LinJ36.1370 | 40.7 | 8.7 | 6/9/81 | 2.16 ± 0.45 | CM | CP | Lyase | DT, VC [32] |
| 13 | Proliferative cell nuclear antigen | Lmj | LmjF15.1450 | 32.3 | 4.5 | 12/14/109 | 2.17 ± 0.06 | DR | NU | PCNA family | DM [34] |
| 14 | Proteasome alpha 5 subunit | Li | LinJ21.1590 | 26.8 | 4.9 | 10/17/117 | 2.11 ± 0.85 | PR | CP/NU | Peptidase T1A family | DR [36] |
| 16 | Fructose-1,6-bisphosphate aldolase | Li | LinJ36.1370 | 40.7 | 8.7 | 7/12/79 | 2.37 ± 0.96 | CM | CP | Lyase | DT, VC [32] |
The differentially expressed protein spots shown in Figure 2 were identified using peptide mass fingerprinting.
Differentially overexpressed protein spots number indicated in Figure 2.
Name of identified protein.
Species: Li: Leishmania infantum; Lmj: Leishmania major; Lmx: Leishmania mexicana.
Accession numbers of protein according to NCBI and Swiss-Prot accession number.
Predicted Molecular mass.
Predicted pI.
Number of peptides matched (Pm)/Percentage of sequence coverage (Sc) /MOWSE score (Ms).
Fold change of overepressed protein in SAG resistant isolate (2039).
Function of identified proteins; CH: Chaperon, CO: Cytoskeletal organization, PR: Proteolysis, CM: Carbohydrate metabolism, DR: DNA replication.
Sub cellular localization; Cytoplasm: CP, Mitochondria: MT, Nucleus: NU.
Class/Family of identified proteins.
Remarks and references; DM: Disease marker, DR: Drug resistance, DT: Drug target, VC: Vaccine candidate. Protein spots analysed but not identified: 12,15,17,18.
Discussion
Variation in the efficacy of drugs against leishmaniasis is due to the differences in drug sensitivity of Leishmania species, the immune status of the patient and the pharmacokinetic properties of the drug. No molecular markers of resistance are available for currently used antileishmanials [5]. The observation that expression levels of several genes known to be altered in in vitro resistant isolates were unchanged in the selected pair of sensitive and resistant clinical isolates, suggests that the resistance mechanism in field parasites may differ from laboratory resistant mutants [16]. In the light of these findings, we performed comparative membrane as well as cytosolic proteomic studies using recent SAG sensitive and resistant clinical isolates of L. donovani highlighting some of the overexpressed proteins (Tables 1 and 2) which hitherto has not been reported. Earlier, we carried out a genetic differentiation study, using AFLP [17], with some SAG sensitive and resistant strains of L. donovani, where we demonstrated that strain 2039 (SAG resistant) is highly polymorphic and totally distinct from all other isolates/strains of L. donovani that are being maintained in our laboratory. Since, our aim was to identify those proteins which may be responsible or involved in the drug resistance mechanism directly, we focused on MALDI analysis of only overexpressed protein spots of the 2039 strain.
Investigation of MEPs is a very challenging task in modern proteome analysis due to their poor solubility but following the protocols of Molloy et al. [7], and Pavkova et al. [8] we have successfully solubilized the membrane proteins with almost negligible remnants. Using this approach membrane subproteomes of some protozoans such as Trypanosoma sp[18]. and Plasmodium sp[19]. have been analysed recently. Further, since it is well known that MEPs constitute important components of the cells participating in signal transduction and might be important in drug resistance [20], most of the overexpressed proteins that have been identified in the MEPs are of interest. One such cysteine-leucine rich protein, found to be nearly two times overexpressed in resistant strain, appears to be involved in protein–protein interactions, transcription, RNA processing and drug resistance [21]. Other overexpressed proteins observed in resistant isolates are ATP-binding cassette (ABC) transporters, a biggest family of MEPs usually reported to be involved in drug resistance in several parasitic protozoans [22].Trypanothione, which is thought to bind to metals in sensitive isolates, is increased in Sb (III)-resistant cells and these metal-trypanothione conjugates are either sequestered into an intracellular organelle by the ABC transporter [23] or extruded outside the cell by an efflux pump [24]. Another important protein-HSP83, found to be more than five-fold expressed in resistant strains, has been reported to be involved in drug resistance and drug-mediated programmed cell death activation by interfering with the mitochondrial membrane potential of L. donovani[16]. GPI protein transamidase, also identified as a highly expressed (> three fold) protein, is mainly involved in proteolysis and has been reported as drug target[25]. 60S ribosomal protein L23a, overexpressed by about two-fold in membrane fractions of resistant isolates is unexplored in parasite research as yet and may be identified as a novel diagnostic marker for drug resistance in VL [26].
Interestingly, among the overexpressed proteins in CPs (Table 2), most noteworthy was the presence of HSP70 which is known to stimulate a strong immunostimulatory response in mammals [27] and L. infantum[28]. Tubulin beta chain (upregulated approximately two-fold more) has been reported as a molecular marker and it has been shown in ovarian cancer that overexpression of class III β-tubulin is the most prominent mechanism of paclitaxel resistance [29]. As such the overexpression of this protein in resistant strains of L. donovani justifiably indicates towards its involvement in SAG resistance. Another overexpressed protein, carboxypeptidase, is known to be involved in peptide catabolism in L. major[30]. Enolase and fructose-1, 6-bisphosphate aldolase, identified in multiple copies and up-regulated more than two-fold in SAG resistant isolates, are reported to be involved in carbohydrate metabolism [31, 32]. A notable contribution of enolase 2 has been reported in methotrexate (MTX) resistance which is an antifolate drug used in the treatment of cancer and autoimmune diseases [33]. The role of aldolase as well as enolase in drug resistance to SAG is yet to be explored. Overexpression of these genes in resistant L. donovani strains, however, emphasizes their possible role in drug resistance. Proliferative cell nuclear antigen (PCNA), an auxiliary protein of DNA polymerase delta has shown a two-fold increased expression indicating its role in drug resistance mechanisms. It has not previously been investigated in Leishmania. This protein has been reported as a proliferation marker with prognostic significance in cancer [34]. The proteasome, found overexpressed in resistance strains, is a multicatalytic proteinase complex which is characterized by its ability to cleave peptides with Arg, Phe, Tyr, Leu, and Glu adjacent to the leaving group at neutral or slightly basic pH that may be the cause of drug resistance [35]. Its role in drug resistance against SAG is still unknown. However, the evidence of its association with drug resistance in cancer cells supportes its role in drug resistance mechanisms [36].
To date most of the studies carried out to identify proteins involved in resistant mechanisms were confined to one or two proteins. This study on the promastigote form of Leishmania clinical isolates for the first time has pinpointed the possibilty of involvement of various proteins that are overexpressed in MEPs and CPs of L. donovani, indicating that multiple mechanisms are responsible for drug résistance. Though, the amastigote form of the parasite is responsible for clinical manifestations, proteomic studies on the promastigote stage are relevant because about 90% of the proteome remains qualitatively unchanged throughout the life cycle of the parasite [37]. This study provides a vast field to be exploited to find much needed novel treatment strategies against visceral leishmaniasis. For example cloning and overexpression of these identified targets could be done to produce recombinant therapeutic/ prophylactic proteins.
Competing interests
There are no competing interests to declare.
Our grateful acknowledgements are due to the Directors of CDRI/CSIR and CIMAP/CSIR, Lucknow for providing the facilities to make this study possible. Financial assistance to AK from the University Grants Commission (UGC), New Delhi for a Senior Research fellowship and to PM by CSIR, New Delhi is gratefully acknowledged. This has CDRI/CSIR research communication no. 7601.
REFERENCES
- 1.Gazzinelli RT, Ropert C, Campos MA. Role of the Toll/interleukin-1 receptor signaling pathway in host resistance and pathogenesis during infection with protozoan parasites. Immunol Rev. 2004;201:9–25. doi: 10.1111/j.0105-2896.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, More DK, Singh MK, Singh VP, Sharma S, Makharia A, Kumar PCK, Murray HW. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin Infect Dis. 2000;31:1104–7. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- 3.Thakur CP, Narayan S, Ranjan A. Epidemiological, clinical and pharmacological study of antimony-resistant visceral leishmaniasis in Bihar, India. Indian J Med Res. 2004;120:166–72. [PubMed] [Google Scholar]
- 4.Wyllie S, Cunningham ML, Fairlamb AH. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J Biol Chem. 2004;279:39925–32. doi: 10.1074/jbc.M405635200. [DOI] [PubMed] [Google Scholar]
- 5.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–26. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dube A, Singh N, Sundar S, Singh N. Refractoriness to the treatment of sodium stibogluconate in Indian Kala-Azar field isolates persist in in vitro and in vivo experimental models. Parasitol Res. 2005;96:216–23. doi: 10.1007/s00436-005-1339-1. [DOI] [PubMed] [Google Scholar]
- 7.Molloy MP, Herbert BR, Walsh BJ, Tyler MI, Traini M, Sanchez JC, Hochstrasser DF, Williams KL, Gooley AA. Extraction of membrane proteins by differential solubilization for separation using two-dimensional gel electrophoresis. Electrophoresis. 1998;19:837–44. doi: 10.1002/elps.1150190539. [DOI] [PubMed] [Google Scholar]
- 8.Pávková I, Hubálek M, Zechovská J, Lenco J, Stulík J. Francisella tularensis live vaccine strain: proteomic analysis of membrane proteins enriched fraction. Proteomics. 2005;5:2460–7. doi: 10.1002/pmic.200401213. [DOI] [PubMed] [Google Scholar]
- 9.Santoni V, Molloy M, Rabilloud T. Membrane proteins and proteomics: un amour impossible? Electrophoresis. 2000;21:1054–70. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 11.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14:378–9. doi: 10.1093/bioinformatics/14.4.378. [DOI] [PubMed] [Google Scholar]
- 13.Gardy JL, Spencer C, Wang K, Ester M, Tusnady GE, Simon I, Hua S, deFays K, Lambert C, Nakai K, Brinkman FS. PSORT-B: improving protein subcellular localization prediction for Gram-negative bacteria. Nucleic Acids Res. 2003;31:3613–17. doi: 10.1093/nar/gkg602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNicoll F, Drummelsmith J, Muller M, Madore E, Boilard N, Ouellette M, Papadopoulou B. A combined proteomic and transcriptomic approach to the study of stage differentiation in Leishmania infantum. Proteomics. 2006;6:3567–81. doi: 10.1002/pmic.200500853. [DOI] [PubMed] [Google Scholar]
- 15.Sinha S, Arora S, Kosalai K, Namane A, Pym AS, Cole ST. Proteome analysis of the plasma membrane of Mycobacterium tuberculosis. Comp Funct Genomics. 2002;3:470–83. doi: 10.1002/cfg.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergnes B, Gourbal B, Girard I, Sundar S, Drummelsmith J, Ouellette M. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol Cell Proteomics. 2007;6:88–101. doi: 10.1074/mcp.M600319-MCP200. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Boggula VR, Sundar S, Shasany AK, Dube A. Identification of genetic markers in sodium antimony gluconate (SAG) sensitive and resistant Indian clinical isolates of Leishmania donovani through amplified fragment length polymorphism (AFLP) Acta Trop. 2009;110:80–5. doi: 10.1016/j.actatropica.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AP, Vaughan S, Gull K. Evolution of tubulin gene arrays in Trypanosomatid parasites: genomic restructuring in Leishmania. BMC Genomics. 2006;7:261. doi: 10.1186/1471-2164-7-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santoni V, Kieffer S, Desclaux D, Masson F, Rabilloud T. Membrane proteomics: use of additive main effects with multiplicative interaction model to classify plasma membrane proteins according to their solubility and electrophoretic properties. Electrophoresis. 2000;21:3329–44. doi: 10.1002/1522-2683(20001001)21:16<3329::AID-ELPS3329>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Helling S, Schmitt E, Joppich C, Schulenborg T, Mullner S, Felske-Muller S, Wiebringhaus T, Becker G, Linsenmann G, Sitek B, Lutter P, Meyer HE, Marcus K. 2-D differential membrane proteome analysis of scarce protein samples. Proteomics. 2006;6:4506–13. doi: 10.1002/pmic.200600169. [DOI] [PubMed] [Google Scholar]
- 21.Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci. 1994;19:415–21. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Castanys-Munoz E, Perez-Victoria JM, Gamarro F, Castanys S. Characterization of an ABCG-like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob Agents Chemother. 2008;52:3573–9. doi: 10.1128/AAC.00587-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouellette M, Legare D, Haimeur A, Grondin K, Roy G, Brochu C, Papadopoulou B. ABC transporters in Leishmania and their role in drug resistance. Drug Resist Updat. 1998;1:43–8. doi: 10.1016/s1368-7646(98)80213-6. [DOI] [PubMed] [Google Scholar]
- 24.El Fadili K, Messier N, Leprohon P, Roy G, Guimond C, Trudel N, Saravia NG, Papadopoulou B, Légaré D, Ouellette M. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob Agents Chemother. 2005;49:1988–93. doi: 10.1128/AAC.49.5.1988-1993.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagamune K, Ohishi K, Ashida H, Hong Y, Hino J, Kangawa K, Inoue N, Maeda Y, Kinoshita T. GPI transamidase of Trypanosoma brucei has two previously uncharacterized (trypanosomatid transamidase 1 and 2) and three common subunits. Proc Natl Acad Sci USA. 2003;100:10682–7. doi: 10.1073/pnas.1833260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez AC, Thomas MC, Martinez-Carretero E, Carmelo E, Lopez MC, Valladares B. Molecular and immunological characterization of L14 ribosomal protein from Leishmania braziliensis. Parasitology. 2004;128(Pt 2):139–47. doi: 10.1017/s0031182003004499. [DOI] [PubMed] [Google Scholar]
- 27.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–22. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rico AI, Del Real G, Soto M, Quijada L, Martinez AC, Alonso C, Requena JM. Characterization of the immunostimulatory properties of Leishmania infantum HSP70 by fusion to the Escherichia coli maltose-binding protein in normal and nu/nu BALB/c mice. Infect Immun. 1998;66:347–52. doi: 10.1128/iai.66.1.347-352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 30.Isaza CE, Zhong X, Rosas LE, White JD, Chen RP, Liang GF, Chan SI, Satoskar AR, Chan MK. A proposed role for Leishmania major carboxypeptidase in peptide catabolism. Biochem Biophys Res Commun. 2008;373:25–9. doi: 10.1016/j.bbrc.2008.05.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundstrom P, Aliaga GR. A subset of proteins found in culture supernatants of Candida albicans includes the abundant, immunodominant, glycolytic enzyme enolase. J Infect Dis. 1994;169:452–6. doi: 10.1093/infdis/169.2.452. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy JS, Wieseman M, Tropea J, Kaslow D, Abraham D, Lustigman S, Tuan R, Guderian RH, Nutman TB. Onchocerca volvulus glycolytic enzyme fructose-1,6-bisphosphate aldolase as a target for a protective immune response in humans. Infect Immun. 2002;70:851–8. doi: 10.1128/IAI.70.2.851-858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selga E, Morales C, Noé V, Peinado MA, Ciudad CJ. Role of Caveolin 1, E-Cadherin, Enolase 2 and PKCalpha on resistance to methotrexate in human HT29 colon cancer cells. BMC Medical Genomics. 2008;1:35. doi: 10.1186/1755-8794-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer ATM, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71:2454–60. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang CC, Bozdech Z, Liu CL, Shipway A, Backes BJ, Harris JL, Bogyo M. Biochemical analysis of the 20 S proteasome of Trypanosoma brucei. J Biol Chem. 2003;278:15800–8. doi: 10.1074/jbc.M300195200. [DOI] [PubMed] [Google Scholar]
- 36.McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008;11:164–79. doi: 10.1016/j.drup.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Cuervo P, de Jesus JB, Junqueira M, Mendonca-Lima L, Gonzalez LJ, Betancourt L, Grimaldi G, Jr, Domonte GB, Fernandes O, Cupolillo E. Proteome analysis of Leishmania (Viannia) braziliensis by two-dimensional gel electrophoresis and mass spectrometry. Mol Biochem Parasitol. 2007;154:6–21. doi: 10.1016/j.molbiopara.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Kobe B, Deisenhofer J. Proteins with leucine-rich repeats. Curr Opin Struct Biol. 1995;5:409–16. doi: 10.1016/0959-440x(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 39.BoseDasgupta S, Ganguly A, Roy A, Mukherjee T, Majumder HK. A novel ATP-binding cassette transporter, ABCG6 is involved in chemoresistance of Leishmania. Mol Biochem Parasitol. 2008;158:176–88. doi: 10.1016/j.molbiopara.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez AC, Martinez E, Carmelo E, Pinero JE, Alonso V, Del Castillo A, Valladares B. Analysis of NLS and rRNA binding motifs in the L25 ribosomal protein from Leishmania (viannia) braziliensis: investigation of its diagnostic capabilities. Parasitology. 2002;125(Pt 1):51–7. doi: 10.1017/s0031182002001804. [DOI] [PubMed] [Google Scholar]


