Abstract
High levels of insulin-like growth factor-1 (IGF-1) have been associated with a significant increase in colon cancer risk. Additionally, IGF-1 inhibits apoptosis and stimulates proliferation of colonic epithelial cells in vitro. Unfortunately, IGF-1 knockout mice have severe developmental abnormalities and most do not survive, making it difficult to study how genetic ablation of IGF-1 affects colon tumorigenesis. To test the hypothesis that inhibition of IGF-1 prevents colon tumorigenesis, we utilized a preexisting mouse model containing a deletion of the igf1 gene in the liver through a Cre/loxP system. These liver-specific IGF-1 deficient (LID) mice display a 50–75% reduction in circulating IGF-1 levels. We conducted a pilot study to assess the impact of liver-specific IGF-1 deficiency on azoxymethane (AOM)-induced colon tumors. LID mice had a significant inhibition of colon tumor multiplicity in the proximal area of the colon compared to their wild-type littermates. We examined markers of proliferation and apoptosis in the colons of the LID and wild-type mice to see if these were consistent with tumorigenesis. We observed a decrease in proliferation in the colons of the LID mice and an increase in apoptosis. Finally, we examined cytokine levels to determine whether IGF-1 interacts with inflammatory pathways to affect colon tumorigenesis. We observed a significant reduction in the levels of 7 out of 10 cytokines that were measured in the LID mice as compared to wild-type littermates. Results from this pilot study support the hypothesis that reductions in circulating IGF-1 levels may prevent colon tumorigenesis and affect both proliferation and apoptosis. Future experiments will investigate downstream genes of the IGF-1 receptor.
Keywords: IGF-1, colon, azoxymethane
Introduction
Insulin-like growth factor-1 (IGF-1) is a member of a family of insulin-related peptides including insulin [1]. IGF-1 plays an important role in growth and development, especially during the prenatal and postnatal periods [2,3]. IGF-1's role in growth and development has been illustrated by IGF-1 null mice that are severely growth restricted and die at high rates soon after birth [4]. Additionally, those IGF-1 null mice that survive continue to be growth restricted, have developmental abnormalities in the brain, muscle, bone, and lung, and are infertile [4,5].
IGF-1 and IGF-1 receptor (IGF-1R), a transmembrane tyrosine kinase receptor, are expressed in all tissues although the liver is the major contributor to circulating IGF-1 levels [6,7]. Bioactivity of IGF-1 in tissues is related to circulating IGF-1 levels, as well as local production [1,8]. Circulating IGF-1 is usually bound by IGF-binding proteins (IGFBPs) so it can be delivered to target tissues, where through activation of IGF-1R it can modulate cell growth [1]. Specifically, IGF-1 plays a role in cell proliferation and inhibition of apoptosis through activation of the mitogen-activated protein kinase (MAPK) and phosphoinositide 3′-kinase (PI3K) pathways [9–11].
Increased levels of IGF-1 have been associated with increased colon cancer risk [12–14]. One of the first suggestions that IGF-1 was correlated with colon cancer risk came from people with the condition acromegaly. Acromegaly, which results in increased expression of IGF-1, has been associated with an elevated risk of developing colorectal tumors [15–17]. Additionally, many studies, including the Physicians' Health Study and the Nurses' Health Study, have illustrated a positive association between circulating IGF-1 levels and colon cancer risk [12–14].
The mechanisms of how increased levels of IGF-1 lead to colon cancer are not well understood. It has been demonstrated that the expression of both IGF-1 and IGF-1R is increased in most cancer cells, and IGF-1 is able to induce cell growth in a variety of cancer cell lines [10,18,19]. Therefore, we used a mouse model of liver-specific deficiency of IGF-1 to investigate how reduction of circulating IGF-1 levels affected colon tumorigenesis and the pathways that may be involved. These mice have been shown to display reductions in mammary tumor development [20] as well as fewer epithelial tumors in response to the two-stage skin carcinogenesis protocol [21]. Furthermore, liver-specific IGF-1 gene deleted (LID) mice had an inhibition of tumor growth and metastasis in a transplantable colon cancer model [22]. Therefore, we used this model system in the azoxymethane (AOM) colon cancer model to determine whether a reduction of circulating IGF-1 could inhibit chemically induced colon tumors.
Methods
Animal Study Design
LID mice were used in this study. These mice were engineered using the Cre/loxP system where mice with loxP-flanked IGF-1 gene were mated with transgenic mice expressing Cre recombinase in the liver. A total of 30 LID mice and 11 wild-type control mice were used for this project. All mice were given the colon-cancer-specific carcinogen, AOM via intraperitoneal injections. They were injected once a week for 6 wk with 10 mg/kg AOM.
LID and wild-type mice were sacrificed 20 wk after the final AOM injection. Colons were removed, stretched on filter paper, and placed in 10% buffered formalin overnight. After 24 h, colons were placed in 70% ethanol.
Circulating IGF-1 and cytokine analysis
Total serum IGF-1 concentrations were measured in 25 μL samples using a commercially available radioimmunoassay (RIA) kit (Diagnostics Systems Laboratories, Webster, TX). Serum samples from 24 LID mice and 11 wild-type mice were also sent to Millipore Contract Laboratory Services (St. Charles, MO) for analysis using their mouse cytokine multiplex assay, which analyzed 10 cytokines, including GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, and TNF-α. Each sample was run in duplicate and the average was used. Cytokine levels below 3.2 pg/μL were considered nondetectable.
Tumor visualization
Colons were stained with methylene blue to enumerate the number of tumors. Tumors were counted independently by two blinded investigators. After tumors were counted, colons were embedded in paraffin, and sectioned onto coated slides for immunohistochemical analysis.
Immunohistochemistry
Immunohistochemistry was performed as previously described [23]. Briefly, slides were deparaffinized, rehydrated, and then microwaved-heated for antigen retrieval in Antigen Retrieval Solution (Vector Laboratories, Inc., Burlingame, CA) for 20 min. Endogenous peroxidases were blocked and sections were washed in phosphate-buffered saline containing 0.1% Triton X-100 for 20 min to reduce nonspecific binding. Next, sections were blocked with Vectastain Blocking Serum from the Vectastain Elite ABC Kit (Vector Laboratories) for 20 min, incubated with the primary antibody (1:500, proliferating cell nuclear antigen (PCNA), Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4°C. After several washes, sections were treated with a biotinylated antigoat IgG for PCNA for 30 min at room temperature, treated with an avidin and biotinylated horseradish peroxidase complex, and stained with the chromogen, 3,3′-diaminobenzidine (DAB) (Vector Laboratories) for 1 min. Sections were counterstained with Vector's Hematoxylin QS Nuclear Counterstain for 45 s, dehydrated, and placed in xylene before adding Permount and a coverslip.
TUNEL staining
Sections were used to evaluate apoptosis with the ApopTag Kit (Serologicals Corporation, Norcross, GA) according to the manufacturer's protocol and as previously described [23]. Briefly, sections were deparaffinized, rehydrated, and pretreated with Proteinase K. Next endogenous peroxidases were quenched, sections were equilibrated, and incubated with the terminal deoxynucleotidyl transferase TdT enzyme in order to add digoxigenin-labeled nucleotides to the 3′-OH ends of the DNA. The reaction was stopped and sections were incubated with digoxigenin peroxidase conjugate. The sections were incubated with peroxidase substrate, counterstained with methyl green, washed in 100% butanol, dehydrated, and coverslipped.
Statistical Analyses
Tumor multiplicity and circulating IGF-1 levels were analyzed using a Student's t-test. For immunohistochemical analysis, the staining index was calculated based on the number of positive cells divided by a total number of 1000 cells counted. The staining index was averaged for the LID mice and the wild-type mice and compared using a Student's t-test. Cytokine analysis was analyzed in a similar manner with the values being averaged for the LID mice and compared to the wild-type mice via a Student's t-test. Adjustment for multiple comparisons was done using Bonferroni correction [24]. Because 10 cytokines were analyzed, the P-value for significance would be 0.005 (0.05/10). Using this very conservative manner of adjusting for multiple comparisons, 7 of the 10 cytokines were still significant. Only IL-1β had a P-value that was above 0.005. All other values were reported as significant if the P-value was less than 0.05.
Results
Colon Tumor Multiplicity
LID and their wild-type littermates were utilized in an AOM model of colon cancer to determine the effect of decreased levels of circulating IGF-1 on colon carcinogenesis. As has been previously established in other studies, circulating IGF-1 was significantly lower in the LID mice versus the wild-type mice (Table 1). Although LID mice have significantly lower levels of circulating IGF-1, they develop normally. Bodyweight was tracked throughout the study, but no significant differences were observed between the LID and wild-type mice (Figure 1).
Table 1.
IGF-1 and Cytokine Levels in LID Versus Wild-Type Mice 20 wk After the Last AOM Injection
| Cytokine | LID mice (n = 24), mean (SD) |
Wild-type mice (n = 11), mean (SD) |
P-valuea |
|---|---|---|---|
| IGF-1 | 75.37 (4.25) | 163.33 (21.45) | <0.0001 |
| GM-CSF | 218.67 (14.59) | 307.74 (15.35) | 0.0008 |
| IFN-γ | 65.78 (43.86) | 100.45 (3.37) | 0.0001 |
| IL-1β | 18.31 (2.61) | 33.36 (7.58) | 0.0296b |
| IL-2 | 39.69 (11.15) | 56.54 (22.31) | 0.4665 |
| IL-4 | 7.60 (0.42) | 11.75 (0.45) | <0.0001 |
| IL-5 | 16.11 (1.89) | 30.82 (4.12) | 0.0007 |
| IL-6 | 46.68 (8.57) | 56.92 (9.50) | 0.4775 |
| IL-10 | 199.59 (16.99) | 427.38 (52.21) | <0.0001 |
| IL-12 | 401.60 (47.55) | 645.49 (29.15) | 0.0028 |
| TNF-α | 6.31 (0.65) | 11.28 (1.55) | 0.0015 |
P-values were calculated via a Student's t-test.
The P-value for IL-1β does not remain significant after adjustment for multiple comparisons using Bonferroni correction.
Figure 1.
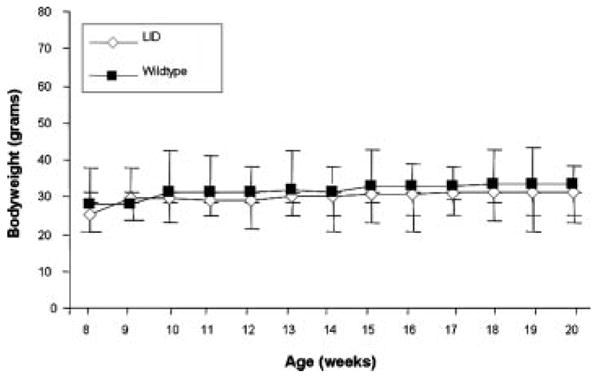
Bodyweights of LID versus wild-type mice. Bodyweight was measured weekly in LID and wild-type mice from 8 wk of age until 20 wk of age. LID mice are represented by the white diamonds, while wild-type mice are represented by the black squares. Each symbol represents the average bodyweight for that week of all LID (n = 30) or wild-type mice (n = 11). There was no statistically significant difference at each time point during the study when analyzed using a Student's t-test.
Colon tumor multiplicity was assessed in LID versus wild-type mice. In addition, colon tumor multiplicity was significantly lower in the LID mice compared to their wild-type littermates 20 wk after the last injection of AOM (t-test, P = 0.0002) (Figure 2A). There was, however, no significant different in tumor incidence between LID and wild-type mice (97% vs. 100%, respectively, data not shown). At the final time point, the LID mice had an average of 8.0 tumors per colon, while the wild-type mice had an average of 13.0 tumors per colon. Additionally, the LID mice had significantly fewer tumors in the proximal end of the colon compared to the wild-type mice (t-test, P < 0.0001) (Table 2). Histological examination of the tumors demonstrated that the majority of the tumors in both the LID and wild-type mice were adenocarcinomas.
Figure 2.
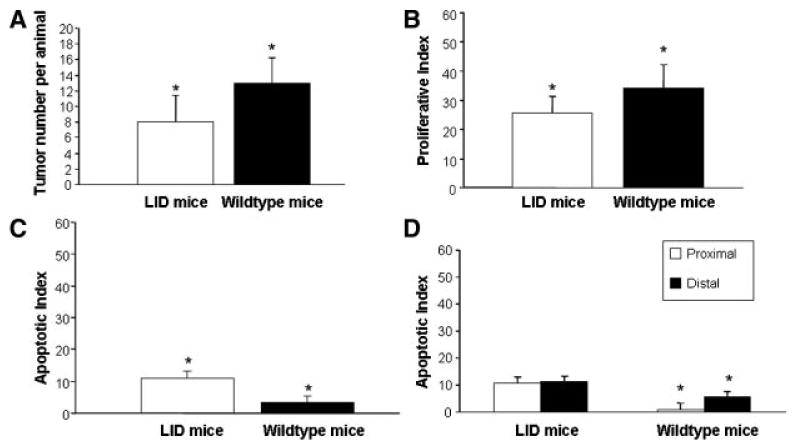
Tumor multiplicity, cellular proliferation, and apoptosis in LID versus wild-type mice 20 wk after the last AOM injection. (A) The total number of tumors was counted along the entire length of the colon after methylene blue staining. Bars represent the average number of tumors per colon in the LID group (n = 30) and the wild-type group (n = 11). *P < 0.0001 via a Student's t-test. (B) Proliferation was assessed via immunohistochemistry for proliferating cell nuclear antigen (PCNA). One thousand tumor cells were counted for each animal and the proliferative index represents the percentage of cells that stained positive for PCNA. The bars represent the average proliferative index for LID mice (n = 29) and wild-type mice (n = 11). *P = 0.0003 via a Student's t-test. (C) Apoptosis in the tumors of LID versus wild-type mice 20 wk after the last AOM injection. Apoptosis was assessed via TUNEL staining. One thousand tumor cells were counted for each animal and the apoptotic index represents the percentage of cells that stained positive and also appeared apoptotic based on cell morphology. The bars represent the average apoptotic index for the LID mice (n = 29) and the wild-type mice (n = 11). *P < 0.0001 via a Student's t-test. (D) Apoptosis in proximal and distal tumors of LID and wild-type mice 20 wk after the last AOM injection. Apoptosis was assessed via TUNEL staining. One thousand tumor cells was counted for both proximal and distal tumors for each animal and the apoptotic index represents the percentage of cells that stained positive and also appeared apoptotic based on cell morphology. The bars represent the average apoptotic index for proximal and distal tumors in the LID (n = 29) and wild-type (n = 11) mice. P < 0.0001 via a Student's t-test.
Table 2.
Mean Number of Tumors in the Proximal and Distal Colon in LID Versus Wild-Type Mice 20 wk After the Last AOM Injection
| Tumor location | Mean number of tumors (SD) in the LID mice (n = 30)a | Mean number of tumors (SD) in the wild-type mice (n = 11) | P-valueb |
|---|---|---|---|
| Proximal | 1.93 (1.53) | 7.64 (1.63) | <0.0001 |
| % of adenocarcinomas | 90 | 94 | |
| Distal | 6.03 (2.86) | 5.36 (2.50) | 0.50 |
| % of adenocarcinomas | 90 | 90 |
One LID mouse out of 30 did not develop tumors.
P-value was calculated via a Student's t-test.
Proliferation and Apoptosis
Proliferation was investigated at 20 wk after the last AOM injection by examining PCNA levels through immunohistochemistry (Figure 2B.) Tumor cells in the LID mice had 25.6% proliferating cells, while tumors in the wild-type mice had 34.4% proliferating cells (t-test, P = 0.0003).
Apoptosis was also studied in the colon tumors of the LID versus wild-type mice 20 wk after the last AOM injection using a TUNEL assay. The level of apoptosis was significantly higher in the LID animals compared to the wild-type mice (P < 0.001) (Figure 2C). Eleven percent of tumors cells were apoptotic in the LID mice, while only 3.3% of the tumor cells in the wild-type mice were apoptotic. Furthermore, the increased levels of apoptosis observed in the LID mice may help explain the changes seen in tumor multiplicity. We also compared the percentage of apoptotic cells between proximal tumors and normal tumors among both LID and wild-type mice (Figure 2D). The proximal tumors in the wild-type mice had significantly fewer apoptotic cells versus the distal tumors (P < 0.001). However, we observed no significant difference in the percentage of apoptotic cells in the proximal tumors versus the distal tumors among the LID mice (P = 0.11).
Cytokine Expression
Levels of 10 different cytokines were examined in the LID versus wild-type mice. As shown in Table 1, the mean levels of eight cytokines (i.e., GM-CSF, IFN-γ, IL-1β, IL-4, IL-5, IL-10, IL-12, and TNF-α) were significantly lower in the LID versus the wild-type animals. Despite a 20–30% reduction in the mean levels of IL-2 and -6, these differences did not reach statistical significance.
Discussion
We demonstrate for the first time the significant reduction of AOM-induced colon tumors in animals deficient in IGF-1 compared to wild-type animals. Animals were genetically modified to express low levels of circulating IGF-1 through a Cre-lox system. Colon tumorigenesis was induced in these animals using the AOM model, a well-established colon carcinogenesis model that closely mimics the tumorigenic process in humans [25–27]. Twenty weeks after the last injection of AOM, the LID animals, which were deficient in IGF-1, had a significantly lower number of colon tumors compared to their wild-type littermates, which had normal circulating levels of IGF-1. Our results conflict with a recent report by Ealey et al. [28] where they reported no difference in colon tumor number in LID mice versus wild-type control mice. This may be due to a difference in the number of AOM injections. The animals in our study received a total of six weekly injections at 10 mg/kg, while the animals in the Ealey study only received four weekly injections at 10 mg/kg. In addition, the mice were treated with AOM at different ages. Our mice received their first injection of AOM at 8 wk of age, while they were 12 wk in the previous study and there is a study suggesting that susceptibility to AOM differs depending on the age of mice [29].
This decrease in tumor multiplicity supports IGF-1's role in colon carcinogenesis. Furthermore, the fact that the animals deficient in IGF-1 do not have a complete inhibition of colon tumors illustrates that although IGF-1 is involved in colon tumorigenesis, this is not the only active pathway. Reduction of IGF-1 also altered the location of tumors within the colon. Surprisingly, the LID animals had more distal tumors, while the wild-type animals had more tumors in the proximal end of the colon. While the significance of this is unknown, previous literature suggest that in the AOM model, tumors that arise in the proximal area are more aggressive because they have less apoptotic cells and more DNA damage than tumors in the distal end of the colon [30]. In our study, we observed that the wild-type animals did have a lower percentage of apoptotic cells in the proximal tumors versus the distal tumors; however, there was no significant difference in the percentage of apoptotic cells in the proximal versus distal tumors among the LID animals.
The balance between cellular proliferation and apoptosis is vital in tumorigenesis; therefore, we investigated levels of both through immunohistochemistry for PCNA and TUNEL staining in the tumors of the mice. LID mice had significantly fewer proliferating tumor cells and more tumor cells undergoing apoptosis compared to the wild-type mice.
Previous research has suggested a link between IGF-1 and inflammation in colon cancer, although this association has not been well established. Therefore, we compared the levels of 10 cytokines in LID versus wild-type mice to examine the effect of reduced circulating IGF-1 on markers of systemic inflammation. Levels of seven cytokines were significantly lower in the LID mice compared to the wild-type mice, illustrating that these animals have a different inflammatory profile. These seven cytokines included both Th1 (IFN-g, TNF-a, IL-12) and TH2 cytokines (IL-4, IL-5, and IL-10), thus LID mice had a downregulation of proinflammatory and antiinflammatory cytokines. This suggests an overall effect of IGF-1 on inflammation and this may also contribute to the changes observed in tumorigenesis since inflammation has been intimately linked to colon carcinogenesis.
One limitation of this study is that it is a pilot study and therefore has a small number of animals. In particular, when the animals were genotyped, there were not many wild-type mice. Therefore, although the results of this study are intriguing, the results should be interpreted cautiously until replication in a larger study. A second limitation is that although we observed a significant difference in colon tumor multiplicity between LID and wild-type mice, we did not observe a difference in tumor incidence (97% vs. 100%, respectively). The study by Ealey et al. [28] also observed no difference in tumor incidence between wild-type and LID mice (50% vs. 48.7%, respectively). The LID mice are on a mixed background; therefore, part of the pilot study was conducted to see if they would respond to AOM because some strains of mice are not as susceptible to the effects of AOM [31]. It is likely that six weekly injections resulted in the high colon tumor incidence compared to the Ealey study and that future studies should lower the dose to four weekly injections.
In summary, we have demonstrated that reduction of IGF-1 can inhibit colon tumorigenesis in LID mice using the AOM model. This inhibition is associated with a decrease in proliferation, an increase in apoptosis, and an alteration in the inflammatory profile. Future studies will be conducted to further examine the pathways that interact with the IGF-1 pathway in colon tumorigenesis.
Acknowledgments
We thank Lisa Riffle and the Animal Care Facility in Frederick, Maryland, for their support and work on this study.
Abbreviations
- IGF-1
insulin-like growth factor-1
- IGF-1R
IGF-1 receptor
- LID
liver-specific IGF-1 gene deleted
- AOM
azoxymethane
- PCNA
proliferating cell nuclear antigen
References
- 1.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: Biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu JL, LeRoith D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology. 1999;140:5178–5184. doi: 10.1210/endo.140.11.7151. [DOI] [PubMed] [Google Scholar]
- 3.Liu JL, Grinberg A, Westphal H, et al. Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: Manipulation using the Cre/loxP system in transgenic mice. Mol Endocrinol. 1998;12:1452–1462. doi: 10.1210/mend.12.9.0162. [DOI] [PubMed] [Google Scholar]
- 4.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 5.Bartlett WP, Li XS, Williams M, Benkovic S. Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev Biol. 1991;147:239–250. doi: 10.1016/s0012-1606(05)80021-1. [DOI] [PubMed] [Google Scholar]
- 6.Laron Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol Pathol. 2001;54:311–316. doi: 10.1136/mp.54.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeRoith D, Baserga R, Helman L, Roberts CT., Jr Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–59. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 8.Butler AA, Yakar S, Gewolb IH, Karas M, Okubo Y, LeRoith D. Insulin-like growth factor-I receptor signal transduction: At the interface between physiology and cell biology. Comp Biochem Physiol B Biochem Mol Biol. 1998;121:19–26. doi: 10.1016/s0305-0491(98)10106-2. [DOI] [PubMed] [Google Scholar]
- 9.Allen RT, Krueger KD, Dhume A, Agrawal DK. Sustained Akt/PKB activation and transient attenuation of c-jun N-terminal kinase in the inhibition of apoptosis by IGF-1 in vascular smooth muscle cells. Apoptosis. 2005;10:525–535. doi: 10.1007/s10495-005-1882-3. [DOI] [PubMed] [Google Scholar]
- 10.Kundu AK, Nagaoka M, Chowdhury EH, Hirose S, Sasagawa T, Akaike T. IGF-1 induces growth, survival and morphological change of primary hepatocytes on a galactose-bared polymer through both MAPK and beta-catenin pathways. Cell Struct Funct. 2003;28:255–263. doi: 10.1247/csf.28.255. [DOI] [PubMed] [Google Scholar]
- 11.Nitta A, Zheng WH, Quirion R. Insulin-like growth factor 1 prevents neuronal cell death induced by corticosterone through activation of the PI3k/Akt pathway. J Neurosci Res. 2004;76:98–103. doi: 10.1002/jnr.20057. [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E, Pollak M, Platz EA, et al. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and the risk of colorectal adenoma and cancer in the Nurses' Health Study. Growth Horm IGF Res. 2000;10:S30–S31. doi: 10.1016/s1096-6374(00)90014-5. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Pollak MN, Platz EA, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9:345–349. [PubMed] [Google Scholar]
- 14.Ma J, Pollak M, Giovannucci E, et al. A prospective study of plasma levels of insulin-like growth factor I (IGF-I) and IGF-binding protein-3, and colorectal cancer risk among men. Growth Horm IGF Res. 2000;10:S28–S29. doi: 10.1016/s1096-6374(00)90013-3. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins PJ. Acromegaly and colon cancer. Growth Horm IGF Res. 2000;10:S35–S36. doi: 10.1016/s1096-6374(00)90017-0. [DOI] [PubMed] [Google Scholar]
- 16.Ritter MM, Richter WO, Schwandt P. Acromegaly and colon cancer. Ann Intern Med. 1987;106:636–637. doi: 10.7326/0003-4819-106-4-636. [DOI] [PubMed] [Google Scholar]
- 17.Ituarte EA, Petrini J, Hershman JM. Acromegaly and colon cancer. Ann Intern Med. 1984;101:627–628. doi: 10.7326/0003-4819-101-5-627. [DOI] [PubMed] [Google Scholar]
- 18.Guo YS, Narayan S, Yallampalli C, Singh P. Characterization of insulin-like growth factor I receptors in human colon cancer. Gastroenterology. 1992;102:1101–1108. [PubMed] [Google Scholar]
- 19.Koenuma M, Yamori T, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res. 1989;80:51–58. doi: 10.1111/j.1349-7006.1989.tb02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Cui K, Miyoshi K, et al. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–4388. [PubMed] [Google Scholar]
- 21.Moore T, Carbajal S, Beltran L, et al. Reduced susceptibility to two-stage skin carcinogenesis in mice with low circulating insulin-like growth factor I levels. Cancer Res. 2008;68:3680–3688. doi: 10.1158/0008-5472.CAN-07-6271. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 23.Olivo SE, Hilakivi-Clarke L. Opposing effects of prepubertal low- and high-fat n-3 polyunsaturated fatty acid diets on rat mammary tumorigenesis. Carcinogenesis. 2005;26:1563–1572. doi: 10.1093/carcin/bgi118. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Multiple significance tests: The Bonferroni method. Br Med J. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy BS. Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ Mol Mutagen. 2004;44:26–35. doi: 10.1002/em.20026. [DOI] [PubMed] [Google Scholar]
- 26.Raju J. Azoxymethane-induced rat aberrant crypt foci: Relevance in studying chemoprevention of colon cancer. World J Gastroenterol. 2008;14:6632–6635. doi: 10.3748/wjg.14.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papanikolaou A, Wang QS, Delker DA, Rosenberg DW. Azoxymethane-induced colon tumors and aberrant crypt foci in mice of different genetic susceptibility. Cancer Lett. 1998;130:29–34. doi: 10.1016/s0304-3835(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 28.Ealey KN, Xuan W, Lu S, Archer MC. Colon carcinogenesis in liver-specific IGF-I-deficient (LID) mice. Int J Cancer. 2008;122:472–476. doi: 10.1002/ijc.23102. [DOI] [PubMed] [Google Scholar]
- 29.Chung H, Wu D, Gay R, et al. Effect of age on susceptibility to azoxymethane-induced colonic aberrant crypt foci formation in C57BL/6JNIA mice. J Gerontol A Biol Sci Med Sci. 2003;58:B400–B405. doi: 10.1093/gerona/58.5.b400. [DOI] [PubMed] [Google Scholar]
- 30.Hong MY, Chapkin RS, Morris JS, et al. Anatomical site-specific response to DNA damage is related to later tumor development in the rat azoxymethane colon carcinogenesis model. Carcinogenesis. 2001;22:1831–1835. doi: 10.1093/carcin/22.11.1831. [DOI] [PubMed] [Google Scholar]
- 31.Nambiar PR, Girnun G, Lillo NA, Guda K, Whiteley HE, Rosenberg DW. Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int J Oncol. 2003;22:145–150. [PubMed] [Google Scholar]


