Abstract
Inorganic lead compounds are carcinogenic in animals and have carcinogenic potential in humans. In mice, lead (Pb) is a transplacental carcinogen in the kidney. Metallothionein (MT) is a metal-binding protein that can reduce the toxicity of various metals, including Pb, either by direct sequestration or as an antioxidant for metals that generate reactive oxygen species. Although MT appears to reduce Pb carcinogenicity in adult mice it is unknown how MT deficiency may affect Pb carcinogenicity from early life exposure. Thus, groups (n = 10) of pregnant MT-I/II double knockout (MT-null) or 129/SVJ MT wild type (WT) mice were exposed to Pb acetate in the drinking water (0, 2000, 4000 ppm Pb) from gestation day 8 through birth and during lactation. Maternal drinking water Pb exposure continued to weaning at 4 weeks of age and the male offspring were then directly exposed to Pb until 8 weeks of age and observed until 2 years old. High dose (4000 ppm) but not low dose (2000 ppm) Pb reduced survival in the latter part of the study in both MT-null and WT mice. In MT-null mice, but not WT, early life Pb exposure caused a dose-related increase in testicular teratomas, to a maximum incidence of 28% compared to control (4%). Pb-induced renal cystic hyperplasia, considered preneoplastic, were a prominent occurrence in MT-null mice but nearly absent in WT mice. Pb dose-related increases in renal cystic hyperplasia occurred in adult MT-null with early life exposure with maximal incidence of 52%. Pb-treated MT-null mice also showed dose-related increases in urinary bladder hyperplasia with occasional papilloma that were absent in WT mice. Thus, MT deficiency made mice more sensitive to early life Pb exposure with regard to testes tumors, and renal and urinary bladder preneoplastic lesions.
Keywords: lead, carcinogenesis, mice, metallothionein, testes
INTRODUCTION
Metallic agents are an important class of known or suspected human carcinogens (Tokar et al., 2011). Inorganic Pb is considered a probable human carcinogen and is clearly carcinogenic in rodents (IARC 2006). It is thought that the free, ionic species of Pb is critical to the oncogenic response to this metal (IARC 2006). In rodents, inorganic Pb causes tumors in various target sites, but most commonly is associated with kidney cancers (IARC 2006). Brain gliomas have been induced by oral exposure to inorganic Pb compounds in at least three separate studies in rats, an interesting finding given the neurotoxic potential of Pb (IARC 2006). In addition, endocrine-sensitive target sites, such as the testes, adrenals, prostate, pituitary, and mammary glands have shown tumor formation after oral exposure to inorganic Pb compounds in one or more studies (IARC 2006).
Clearly, the single most frequently observed tumors after inorganic Pb exposure in rodents occur in the kidneys in rats (IARC 2006). When chronically exposed as adults to oral inorganic Pb compounds, rats frequently develop renal adenoma and/or adenocarcinoma (IARC 2006). Although mice in general are less sensitive to renal carcinogenesis, kidney cancers and preneoplasia can also be induced in genetically altered MT-I/II knockout (MT-null) mice when chronically (~96 weeks) exposed to inorganic Pb in their drinking water during adulthood (Waalkes et al., 2004). However, a much shorter period (~6 weeks) of indirect early life inorganic Pb exposure that first passes through the maternal system (transplacental/translactational) is carcinogenic in the kidneys of B6C3F1 mouse progeny when they reach adulthood (Waalkes et al., 1995). There is a growing concern about the developmental basis of adult disease (Gluckman and Hanson, 2007), and this concern certainly extends to cancer. In the early life Pb exposure mouse study in question, the pregnant dams were exposed to Pb in the drinking water during gestation and lactation with exposure ending at weaning (Waalkes et al., 1995). The resulting offspring, which only had Pb exposure through the mother, showed marked increases in renal proliferative lesions, including cancers, when they became adults (Waalkes et al., 1995). Unlike tumors in the kidneys generated by long term, continuous Pb exposure in adult mice (IARC 2006; Waalkes et al, 2004), early life Pb exposure induced tumors long after Pb exposure ended and the tumors occurred in the absence of chronic nephropathy (Waalkes et al., 1995), indicating that a cycle of repeated proliferative repair (regenerative hyperplasia) was not necessarily required for renal tumor formation (IARC 2006; Waalkes et al., 1995), a theory of general mode of action for Pb-induced renal carcinogenesis in rodents.
Metallothionein (MT) is a soluble, low-molecular-weight protein that binds metals via numerous cysteines (Klaassen et al. 2009; Vasák 2005). MT is a key factor in the ability of cells to limit the toxicity from exposure to a variety of metals (Klaassen et al. 2009; Vasák 2005), including Pb (Qu et al., 2002; Zuo et al., 2009). The synthesis of MT is markedly increased by exposure to many metals and it is suspected that the increased levels of MT protein then can sequester the metal of concern in a non-toxic form (Klaassen et al. 2009; Qu et al., 2002; Vasák 2005; Zuo et al., 2009). MT exists in multiple forms but for most tissues, like kidney, MT-I and MT-II are clearly the major forms (Klaassen et al., 2009; Qu et al., 2002; Vasak 2005; Zuo et al., 2009). MT avidly binds a variety of metals, so such sequestration is clearly a mechanism by which the protein detoxicates elemental inorganics (Klaassen et al. 2009; Vasák 2005). Pb likely is associated with MT in the inclusion bodies that are typically formed in tissues with high Pb burden and acts to limit the metal’s toxicity (Qu et al., 2002; Zuo et al., 2009). Long-term storage has its drawbacks, as it leaves the metal in place, and, presumably, can eventually be overwhelmed and allow the metal to be mobilized. It also appears that MT can scavenge reactive oxygen species (ROS), either generated by toxic metals, by other metals released by the toxic metals, or by other chemicals, and thus reduce tissue damage from oxidant stress (Klaassen et al. 2009; Sato and Kondoh 2002; Vasák 2005). Indeed, cells deficient in MT are hypersensitive to injury by oxidants (Lazo et al. 1995; Suzuki et al. 2000). In this regard, generation of ROS may be a factor in the carcinogenic mechanism of action of Pb (IARC 2006). In fact, when chronically exposed to oral inorganic Pb as adults, whatever the mechanism, MT-null mice are much more sensitive to the induction of proliferative renal lesions, including tumors, compared with wild-type (WT) mice normally expressing MT (Waalkes et al., 1995).
Thus, there is clear evidence that MT can reduce Pb toxicity (Qu et al., 2002; Zuo et al., 2009), including Pb carcinogenicity after exposure in adulthood (Waalkes et al., 2004), either by direct binding or potentially by sequestration of Pb-generated ROS. In this regard, there is growing concern of the developmental basis of adult disease, and Pb is carcinogenic to mice after early life exposure in mice (Waalkes et al., 1995). However, the impact MT deficiency might have on Pb carcinogenicity after early life exposure is unknown. Therefore, the present study tested the hypothesis that poor expression of MT would enhance the carcinogenic effects of early life exposure to inorganic Pb using genetically engineered MT-null mice, which are deficient in the two major forms of MT (MT-I, -II).
MATERIALS AND METHODS
Chemicals
Pb (II) acetate was obtained from Sigma Chemical Co. (St. Louis, MO) and its purity was 99.8%.
Animals and treatment
Animal care was provided in accordance with the U.S. Public Health Policy on the Care and Use of Animals as defined in the Guide to the Care and Use of Animals (NIH Publication 86-23). Homozygous MT-I/II knock-out mice (129-Mt1tm/Bri, Mt2 tm/Bri 129/SvPCJ background) (Masters et al., 1994) and corresponding wild type (WT) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). The homozygous mutants were mated inter se to maintain the line. Mice were housed in a standard barrier facility at a temperature of 68–72°F, with a relative humidity of 50 ± 5%, and a 12 hr light/dark cycle. A basal diet (5L79; Ralston Purina, St. Louis, MO) and acidified water were provided ad libitum. The NCI-Frederick animal facility, where the treatment, holding and biopsy portions of the present study were conducted, and its animal program are accredited by the American Association for Accreditation of Laboratory Animal Care.
A total of 30 WT and 30 MT-null timed primigravid female mice were randomly divided into three groups per phenotype of 10 each and given drinking water with Pb acetate at 0 (control), 2000 ppm Pb (low) or 4000 ppm Pb (high) ad libitum from gestation day 8 on through birth and lactation until the pups were weaned (the maternal animals were then discarded) at 4 weeks –old. After weaning, pups were randomly put into groups (initial n = 25) of males (females were then discarded) based on maternal exposure and strain and Pb exposure continued until 8 weeks of age. Mice were sacrificed when significant clinical signs developed or at 104 weeks of age (birth = time 0). The doses of Pb were selected based on their carcinogenic activity in prior early life exposure work in mice (Waalkes et al., 1995).
Clinical data and pathology
Individual body weights were recorded at intervals of every five weeks. Only terminal body weights are reported for brevity. Clinical signs were checked daily. Mice were killed with CO2 when moribund or at the study termination. A complete necropsy was performed on all moribund animals, animals found dead, or at the terminal sacrifice. The following tissues were taken and processed for histological analysis: brain, testes, liver, kidneys, urinary bladder, lungs, adrenals, spleen, thymus, pituitary, stomach and all grossly abnormal tissues. Four cross sections were taken of each brain. All of the liver and lung tissues from both experimental and control animals were examined for gross pathology. All liver and lung lobes were collected. Longitudinal sections of each kidney were used. For all other tissues, such as stomach, only grossly looking lesions were fixed. No attempts were made to obtain bone marrow and no hematology was performed. All tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin for histological analysis. All pathological assessments were performed such that the pathologist was unaware of the treatment group during assessment. Pb-induced cystic hyperplasia was pathologically defined as tubular cysts lined by one or more layers of abnormal hyperplastic epithelium but not filling the lumen. Pictorial examples of renal cystic hyperplasia in mice are available (Waalkes et al., 1995).
Data analysis
Data are given as lesion incidence (number of affected mice/total mice available for examination) or as mean ± SEM, as appropriate. A probability level of P < 0.05 was considered to indicate a significant difference in all cases. In pair-wise comparison of lesion incidence, a one-sided Fisher’s exact test was used. To analyze dose-related trends in incidence, a two-sided Chi-squared test for trend was used. For multiple comparisons of mean survival data, a two-sided Dunnett’s test was used. Incidence is based on numbers of animals available for pathological analysis, and loss of animals to observation was typically due to autolysis after death that was considered too advanced for appropriate diagnosis.
RESULTS
Pb exposure and survival
Male WT and MT-null mice were exposed to Pb in utero via the maternal system during gestation (gestation day 8 to birth), translactationally from birth to weaning at 4 weeks of age and then directly to drinking water containing Pb (0, 2000, or 4000 ppm Pb) until 8 weeks of age. Tumors and proliferative lesions were then assessed in male mice until the animals reached 2 years of age (see Materials and Methods for details). Table 1 shows long-term survival of WT and MT-null mice exposed to this early life Pb dosing schedule. In both strains, there was a clear reduction at 104 weeks in survival in the group that had received the high Pb dose (4000 ppm Pb). The lower Pb dose did not perturb survival at any time in either strain. Effects on survival did not occur at earlier times, such as 50 weeks, regardless of dose.
TABLE 1.
Survival in male WT or MT-null mice after early life exposure to Pb acetatea
| Phenotype | Pb Dose | Initial Group size | Survival |
Body Weights |
||
|---|---|---|---|---|---|---|
| Average in Weeks | At 50 weeks | At 104 weeks | At time of sacrifice | |||
| WT | 0 (Control) | 25 | 97.5 ± 2.3 | 25 (100%) | 16 (64%) | 31 ± 0.96 g |
| WT | 2000 | 25 | 97.8 ± 2.5 | 25 (100%) | 17 (68%) | 27 ± 0.84 g* |
| WT | 4000 | 25 | 80.6 ± 4.7* | 21 (84%) | 8 (32%)* | 22 ± 0.67 g* |
| MT-null | 0 (Control) | 25 | 101 ± 1.4 | 25 (100%) | 21 (84%) | 30 ± 0.96 g |
| MT-null | 2000 | 25 | 99.2 ± 1.6 | 25 (100%) | 16 (64%) | 28 ± 0.98 g |
| MT-null | 4000 | 25 | 84.2 ± 3.8* | 24 (96%) | 6 (24%)* | 24 ± 0.66 g* |
Doses given as ppm Pb in the drinking water from gestation day 8 through experimental week 8. During gestation and lactation Pb was in maternal water while after weaning (4 weeks of age) Pb was in the water of the offspring. Male offspring were observed for 104 weeks after birth. Sample size (n) equals number of mice available for pathological analysis. Data are expressed as mean +/− SEM or number of animals surviving at a given time (% total animals put on test).
An asterisk indicates a significant difference from phenotype-matched control survival in average weeks or in numbers at a given time point or in body weight at time of sacrifice (P ≤ 0.05).
Body weights
Table 1 shows the effects of early life Pb exposure on body weight at the time of sacrifice. There was a dose-related reduction in both strains of mice which was significant (P < 0.05) at both levels of Pb in the WT mice but only at the high dose level in the MT-null mice. When cross-compared at the same dose of Pb, there were no differences between MT-null and WT mice in body weights.
Testicular tumors
Early life Pb exposure increased the formation of testicular tumors but only in MT-null mice (Table 2). Testicular teratomas were found in excess at both dosages of Pb and showed a trend with dose, although this did not appear particularly strong. The maximal response in MT-null mice was a 28% occurrence of teratomas compared to 4% in control. WT mice did not show any evidence of a Pb-induced increase in testicular tumors of any kind including teratomas (Table 2).
TABLE 2.
Testicular teratoma in male WT or MT-null mice after early life exposure to Pb acetatea
| Phenotype | Pb Dose | n | Testicular Teratoma |
|
|---|---|---|---|---|
| Incidence | Percent | |||
| WT | Control | 25 | 2 | 8% |
| WT | 2000 | 25 | 1 | 4% |
| WT | 4000 | 23 | 3 | 13% |
| trend P = 0.375 | ||||
| MT-null | Control | 25 | 1 | 4% |
| MT-null | 2000 | 24 | 6* | 25% |
| MT-null | 4000 | 25 | 7* | 28% |
| trend P = 0.030* | ||||
Doses given as ppm Pb in the drinking water from gestation day 8 through experimental week 8. During gestation and lactation Pb was in maternal water while after weaning (4 weeks of age) Pb was in the water of the offspring. Male offspring were observed for 104 weeks after birth. Sample size (n) equals number of mice available for pathological analysis.
An asterisk indicates a significant difference from phenotype-matched control incidence or a significant dose-related trend (P ≤ 0.05). Direct comparsions of incidence data were by one-sided Fisher Exact test. If a two-sided test was applied to these same data, MT-null teratoma incidence at both 2000 and 4000 ppm Pb remained significantly greater (P ≤ 0.05) than control.
Figure 1 shows a typical testicular teratoma from an adult mouse exposed to Pb during early life.
Figure 1.
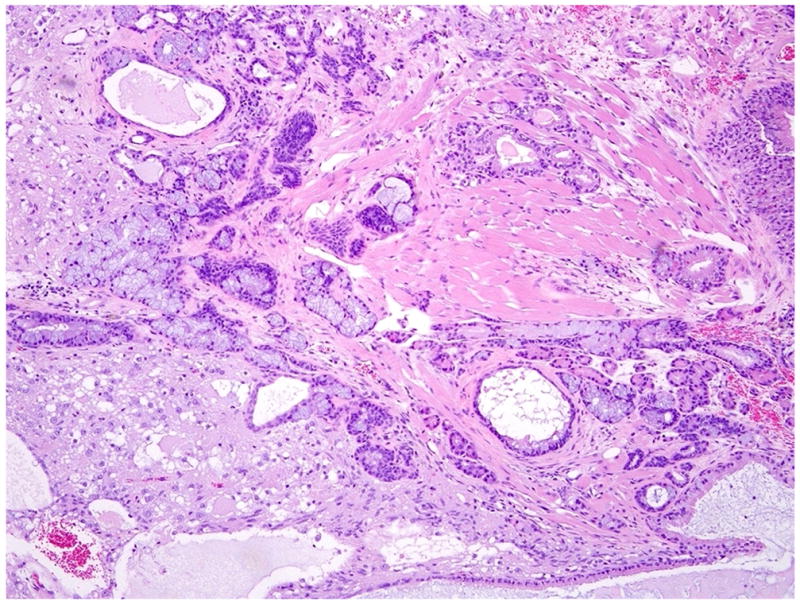
A typical teratoma of the testis from a Pb-exposed mouse. The teratoma is composed predominantly of mature nervous tissue and cysts lined by respiratory epithelium and intestinal epithelium with goblet cells and well differentiated muscular layers. H & E. (300 X).
Renal proliferative lesions
The effect of early life exposure on renal preneoplastic lesions is shown in Table 3. In the MT-null mice, renal cystic hyperplasias (see Methods for definition) showed a very clear dose-response relationship with early life Pb exposure and were a common finding at the high dose. Renal cystic hyperplasias are considered preneoplastic lesions in the mouse kidney. The WT mice showed no evidence of Pb-induced cystic hyperplastic lesions. In addition, no chronic nephropathy occurred in any of the Pb-treated groups regardless of strain or Pb dose.
TABLE 3.
Renal cystic lesions in male WT or MT-null mice after early life exposure to Pb acetatea
| Strain | Pb Dose | n | Tumors |
Preneoplasia |
|
|---|---|---|---|---|---|
| Adenoma | Carcinoma | Cystic Hyperplasia | |||
| WT | Control | 25 | 0 | 0 | 0 |
| WT | 2000 | 25 | 0 | 1 | 0 |
| WT | 4000 | 23 | 2 | 0 | 1 |
| trend P = | - | - | - | ||
| MT-null | Control | 25 | 0 | 0 | 0 |
| MT-null | 2000 | 24 | 0 | 0 | 3 |
| MT-null | 4000 | 25 | 0 | 0 | 13* |
| trend P < | - | - | 0.0001* | ||
Doses given as ppm Pb in the drinking water from gestation day 8 through experimental week 8. During gestation and lactation Pb was in maternal water while after weaning (4 weeks of age) Pb was in the water of the offspring. Male offspring were observed for 104 weeks after birth. Sample size (n) equals number of mice available for pathological analysis.
An asterisk indicates a significant difference from phenotype-matched control incidence or a significant dose-related trend (P ≤ 0.05). A dash (−) indicates a trend test would be of little meaning.
Renal tumors were too rare to attribute to any treatment (one renal cell carcinoma occurred at 2000 ppm and 2 adenoma occurred at 4000 ppm) and, interestingly, only occurred in the WT mice despite the lack of any significant preneoplastic lesions (Table 3). No urothelial hyperplasia was seen in the kidney pelvis in any groups regardless of strain or Pb dose.
Urinary bladder proliferative lesions
Pb-treated MT-null mice were also predisposed to proliferative lesions of the urinary bladder in comparison to WT mice which showed no evidence of such Pb-induced lesions (Table 4). In MT-null mice, urinary bladder transitional cell hyperplasia increased from 0% in controls to 36% at the 4000 ppm dose with a clear dose-response relationship (Figure 2). The incidence of urinary bladder hyperplasia (16.7%) in the low Pb dose-treated MT-null mice also approached significance (P = 0.0502). In addition, two urinary bladder papillomas occurred at the high dose in MT-null mice while none occurred in WT animals.
TABLE 4.
Urinary bladder proliferative lesions in male WT or MT-null mice after early life exposure to Pb acetatea
| Strain | Pb Dose | n | Tumors and Preneoplasias |
|
|---|---|---|---|---|
| Papillomas | Hyperplasia | |||
| WT | Control | 25 | 0 | 0 |
| WT | 2000 | 25 | 0 | 2 |
| WT | 4000 | 23 | 0 | 1 |
| trend P = | - | 0.43 | ||
| MT-null | Control | 25 | 0 | 0 |
| MT-null | 2000 | 24 | 0 | 4 |
| MT-null | 4000 | 25 | 2 | 9* |
| trend P = | 0.08 | 0.005* | ||
Doses given as ppm Pb in the drinking water from gestation day 8 through experimental week 8. During gestation and lactation Pb was in maternal water while after weaning (4 weeks of age) Pb was in the water of the offspring. Male offspring were observed for 104 weeks after birth. Sample size (n) equals number of mice available for pathological analysis.
An asterisk indicates a significant difference from phenotype-matched control incidence or a significant dose-related trend (P ≤ 0.05). A dash (−) indicates a trend test would be of little meaning.
Figure 2.
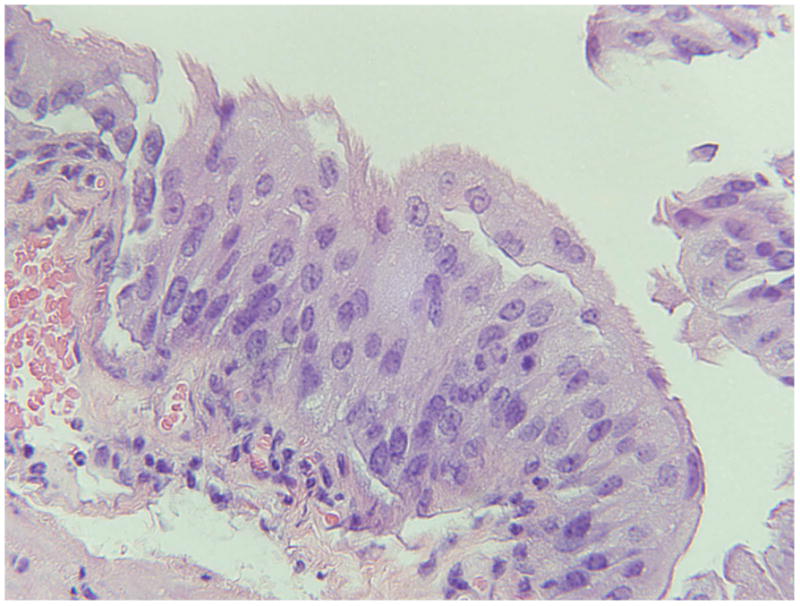
A typical urinary bladder lesion showing hyperplasia (transitional epithelium) from a 4000 ppm lead-treated MT-null mouse. H & E. (100 X).
Lung Tumors
Lung tumors were common in both MT-null and WT mice and were not impacted by early life Pb exposure with the single exception of adenocarcinoma at 2000 ppm in the MT-null (Table 5).
TABLE 5.
Lung tumors in male WT or MT-null mice after early life exposure to Pb acetatea
| Strain | Pb Dose | n | Tumors | ||
|---|---|---|---|---|---|
| Adenoma | Adenocarcinoma | Total | |||
| WT | Control | 25 | 5 | 3 | 8 |
| WT | 2000 | 25 | 7 | 8 | 13 |
| WT | 4000 | 23 | 6 | 5 | 10 |
| MT-null | Control | 25 | 5 | 1 | 7 |
| MT-null | 2000 | 24 | 11 | 6* | 12 |
| MT-null | 4000 | 25 | 6 | 5 | 11 |
Doses given as ppm Pb in the drinking water from gestation day 8 through experimental week 8. During gestation and lactation Pb was in maternal water while after weaning (4 weeks of age) Pb was in the water of the offspring. Male offspring were observed for 104 weeks after birth. Sample size (n) equals number of mice available for pathological analysis.
An asterisk indicates a significant difference from phenotype-matched control incidence. No significant dose-related trends occurred. Total includes animals bearing both separate adenoma and carcinoma.
Spontaneous Tumors
Spontaneous tumors are listed in Table 6. None showed relationships with Pb treatment or clear Pb dose-response relationships.
TABLE 6.
Non-treatment related tumors in male WT or MT-null mice after early life exposure to Pb acetatea
| Strain |
Pb Dose (n) |
Tumors and Preneoplasia |
|---|---|---|
| WT | Control (25) | Harderian gland carcinoma; leg hemangiosarcoma; liver adenoma; liver hemangiosarcoma |
| WT | 2000 (25) | adrenal cortical adenoma; adrenal pheocromocytoma; Harderian gland carcinoma (2); leukemia; spleen histiocytic sarcoma; stomach squamous cell carcinoma; stomach hyperplasia |
| WT | 4000 (23) | adrenal cortical adenoma; liver adenoma |
| MT-null | Control (25) | leukemia; liver adenoma (2); liver hemangioma; lymphoma |
| MT-null | 2000 (24) | adrenal adenoma (3); gall bladder adenoma (1) and hyperplasia (3); Harderian gland carcinoma; leukemia; liver adenoma (2); hepatocellular carcinoma; mesenteric fibrosarcoma; preputial gland adenoma; stomach hyperplasia. |
| MT-null | 4000 (25) | adrenal fibrosarcoma; esophagus hyperplasia; liver adenoma; pituitary adenoma; stomach papilloma (2) and hyperplasia (1); |
Doses given as ppm Pb in the drinking water from gestation day 8 through experimental week 8. During gestation and lactation Pb was in maternal water while after weaning (4 weeks of age) Pb was in the water of the offspring. Male offspring were observed for 104 weeks after birth. Sample size (n) equals number of mice available for pathological analysis. Assessment of combined gall bladder proliferative lesions in MT-null mice treated with 2000 ppm Pb (tumors plus hyperplasia, n = 4 cases) approached significance (P = 0.0502) when compared to control (n = 0 cases).
DISCUSSION
To test our hypothesis that MT deficiency exacerbates early life inorganic Pb-induced carcinogenesis, WT and MT-null mice were exposed to Pb first in utero, then during lactation via breast milk, and then directly as weanlings via their own drinking water until 8 weeks of age. Thus, any oncogenic responses would have occurred long after all experimental Pb exposure had ended. The results show that MT-null mice are more susceptible to development of inorganic Pb-induced testicular tumors, and renal and bladder preneoplastic, proliferative lesions after early life exposure. MT mitigates various aspects of Pb toxicity (Qu et al., 2002; Zuo et al., 2009), and can reduce carcinogenicity of chronic oral inorganic Pb exposure when given throughout adulthood in mice (Waalkes et al., 2004). Furthermore, MT-competent mice only exposed to inorganic Pb in utero and translactationally via maternal drinking water developed renal proliferative lesions, including tumors, indicating early life Pb exposure can be carcinogenic in adulthood (Waalkes et al., 1995). Thus, this present study provides evidence of an enhanced proliferative response in MT-null mice after early life inorganic Pb exposure. Although the doses used in this present work are higher than what humans would encounter, they are within the range for what has often been used in prior work to induce tumors in rodents (IARC 2006).
The induction of testicular teratomas by early life inorganic Pb exposure seen only in MT-null mice is of interest on several levels. The deficiency of MT is consistent with a predisposition to Pb toxicity and carcinogenicity (Qu et al., 2002; Waalkes et al., 2004; Zuo et al., 2009). Further, historical data from MT-null mice obtained and housed in the same fashion as mice in the present study show that testicular teratomas in control mice occurred at a similarly low spontaneous rate (MT-null 2 cases/74 control mice, 3%; Waalkes et al., 2004, 2005, 2006a). Hence, the early life Pb-induced increase in testicular teratoma in the current work is fortified by confidence in control rates from multiple long-term studies in this strain (present study; Waalkes et al., 2004, 2005, 2006a). In addition, testicular teratomas are thought to originate from pluripotent, embryonic stem cells (primordial germ cells; Kimura et al., 2003; Wang and Enders, 1996). Indeed, inoculation of isolated embryonic stem cells into mice will result in the formation of teratomas (Langa et al., 2000; Taylor et al., 2006). Teratomas come from germ cell lesions in situ which are thought to be formed by a disruption of the embryonic, fetal or early postnatal life stem cell environment (Kristensen et al., 2008). Stem cells rapidly differentiate during development. It is thought that differentiating cells in early life are very sensitive to damage from environmental stress, including chemical stress, since development is a highly orchestrated process of differentiation together with extensive proliferation shaped by apoptosis (Birnbaum and Fenton, 2003). These processes (proliferation, differentiation, etc.) provide ample “opportunities” for events leading to chemical carcinogenesis to occur (Birnbaum and Fenton, 2003) and frequently involve stem cells. In general, it is suspected that transplacental carcinogenesis may involve fetal stem cells (Anderson et al., 2000; Waalkes et al., 2007). In fact, we now have solid evidence that other inorganic transplacental carcinogens likely act through stem cell disruption (Tokar et al., 2010a; Tokar et al., 2010b; Waalkes et al., 2008).
The association with MT deficiency, early life Pb exposure and induction of renal preneoplasias was clear in the present study and WT mice were equally insensitive, although they showed a few renal tumors. In prior work, renal proliferative lesions were not observed in MT-null mice treated as adults with other inorganics, including injected nickel or cisplatin (Waalkes et al., 2005; 2006a). MT-null mice, but not WT mice, do show renal cancers and proliferative lesions when treated chronically as adults with oral inorganic Pb (Waalkes et al., 2004). However, similar to the present study, when exposed in utero and translactationally, B6C3F1 mice show renal proliferative lesions, including tumors, as adults (Waalkes et al., 1995). Likewise, SENCAR mice exposed in utero to cisplatin via maternal injection develop renal proliferative lesions in excess (Diwan et al., 1993) while F344 rats exposed to cisplatin in utero readily develop renal cancers with barbital promotion after birth, indicating cisplatin initiation within the kidney (Diwan et al., 1995). In the sites where Pb is found in abundance during exposure, such as the kidney, MT is often associated with inclusion bodies, potentially as a response to sequester and detoxicate Pb (Qu et al., 2002; Zuo et al., 2009). In this regard, though the kidney of WT mice or WT cells in vitro can make Pb-inclusion bodies in abundance when exposed to inorganic Pb, neither the kidney of MT-null mice nor MT-null cells in vitro can make these inclusion bodies (Qu et al., 2002; Zuo et al., 2009). Furthermore, the inability to make MT-containing Pb-storage inclusion bodies causes a clear hypersensitivity to Pb-toxicity at the cellular and organismal levels (Qu et al., 2002; Waalkes et al., 2004; Zuo et al., 2009). This hypersensitivity in MT-null animals includes cancer causation with chronic exposure in adults (Waalkes et al., 2004). In the present study we did not observe Pb inclusion bodies in WT mice after the early life exposures, likely because most of the mice lived long after Pb exposure ended. None-the-less, the inability to form inclusion bodies containing Pb, as seen in MT-null mice from various prior studies (Qu et al., 2002; Waalkes et al., 2004; Zuo et al., 2009) very likely contributed to the outcome of enhanced renal proliferative lesions. In this regard, in rodents after translactational Pb exposure, biokinetic data indicate tissue levels return to normal after a relatively short period of time (4 months; Hejtmancik et al., 1982). Thus, the time lag between Pb exposure and the finding of renal lesions indicates that the genesis of these lesions was set into motion by early events that then manifested in adulthood and did not require the actual presence of excess Pb. It is also noteworthy that additional adulthood exposures to Pb or other renal toxicants, as might be expected in a less well-controlled environment, could very well drive such lesions into overt neoplasia.
The occurrence of proliferative lesions in the urinary bladder, including two early tumors, in MT-null animals after early life Pb exposure was unexpected. Urinary bladder proliferative lesions have not been seen in MT-null mice in association with inorganic Pb or other inorganic carcinogens given in adulthood (Waalkes et al., 2004, 2005, 2006a), or with inorganic Pb exposure during early life in B6C3F1 mice (Waalkes et al., 1995). Similarly, in transplacental studies with inorganic arsenic exposure in CD1 mice, arsenic alone does not induce bladder proliferative lesions but does predispose mice to estrogen-induced stimulation of such lesions, including urinary bladder papilloma and transitional cell carcinoma (Waalkes et al., 2006b, 2006c). Thus, there is the precedent for early life exposure to an inorganic carcinogen and induction of bladder proliferative lesions, although clearly more work is needed to define mechanisms.
Although there is nothing comparable to a MT-null mouse in human populations, MT expression shows a rather high rate of variability in humans (Allan et al., 2000; Bem et al., 1988; Onosaka et al., 1986; Sillevis Smitt et al., 1992; Wu et al., 2000) for reasons that are not clear. In this regard, it appears that persons who poorly express MT tend to be prone to chronic arsenicosis (Liu et al., 2007). A clear role for the natural variability in MT expression in human Pb toxicity has not been defined, and often people are exposed to complex mixtures of inorganics (Wang and Fowler, 2008). Further study is required to define if a poor ability to synthesize MT would predispose humans to Pb toxicity, and perhaps carcinogenicity, as appears to be the case with MT-null mice (Qu et al., 2002; Waalkes et al., 2004; Zuo et al., 2009; present study).
Overall, MT-null mice were clearly more sensitive to the oncogenic effects of early life inorganic Pb exposure, showing testicular teratomas, and renal and urinary bladder proliferative lesions in excess compared to essentially no response in MT-competent WT mice. This fortifies the data that MT plays an important role in Pb toxicity (Qu et al., 2002; Waalkes et al., 2004; Zuo et al., 2009), including carcinogenesis. The present study also adds to the concern that there may be subpopulations based on genetic make-up that are hypersensitive to inorganic carcinogenesis after early life exposure and indicate this is a time of particular sensitivity.
Acknowledgments
The authors wish to thank Drs. L. Keefer, W. Qu, and Y. Sun for critical evaluation of this manuscript, Dr. J. Liu for assistance with the MT-null mice, and D. Logsdon and the Pathology and Histotechnology Laboratory of SAIC Frederick for expert technical assistance. This research was supported in part by the National Toxicology Program, National Institute of Environmental Health Sciences (NIEHS) and by the Intramural Research program of the NIH, National Cancer Institute, Center for Cancer Research. This article may be the work product of an employee or group of employees of the NIEHS, National Institutes of Health (NIH), however, the statements contained herein do not necessarily represent the statements, opinions or conclusions of the NIEHS, NIH or the United States Government. This project was also supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or the policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- MT
metallothionein
- Pb
lead
- WT
wild type
Footnotes
Conflict of Interest statement
Authors declare not conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan AK, Hawksworth GM, Woodhouse LR, Sutherland B, King JC, Beattie JH. Lymphocyte metallothionein mRNA responds to marginal zinc intake in human volunteers. Br J Nutr. 2000;84:747–756. [PubMed] [Google Scholar]
- Anderson LM, Diwan BA, Fear NT, Roman E. Critical windows of exposure for children’s health: cancer in human epidemiological studies and meoplasms in experimental animal models. Environ Health Perspect. 2000;108:573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bem EM, Piotrowski JK, Sobczak-Kozlowska M, Dmuchowski C. Cadmium, zinc, copper and metallothionein levels in human liver. Int Arch Occup Environ Health. 1988;60:413–417. doi: 10.1007/BF00381388. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–394. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwan BA, Kasprzak KS, Rice JM. Transplacental carcinogenic effects of nickel(II) acetate in the renal cortex, renal pelvis and adenohypophysis in F344/NCr rats. Carcinogenesis. 1992;13:1351–1357. doi: 10.1093/carcin/13.8.1351. [DOI] [PubMed] [Google Scholar]
- Diwan BA, Anderson LM, Rehm S, Rice JM. Transplacental carcinogenicity of cisplatin: Initiation of skin tumors and induction of other preneoplastic and neoplastic lesions in SENCAR mice. Cancer Res. 1993;53:3874–3876. [PubMed] [Google Scholar]
- Diwan BA, Anderson LM, Ward JM, Henneman JR, Rice JM. Transplacental carcinogenesis by cisplatin in F344/NCr rats: Promotion of kidney tumors by postnatal administration of sodium barbital. Toxicol Appl Pharmacol. 1995;132:115–121. doi: 10.1006/taap.1995.1092. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Developmental plasticity and human disease: research directions. J Internal Med. 2007;261:461–471. doi: 10.1111/j.1365-2796.2007.01802.x. [DOI] [PubMed] [Google Scholar]
- Hejtmancik MR, Jr, Dawson EB, Williams BJ. Tissue distribution of lead in rat pups nourished by lead-poisoned mothers. J Toxicol Environ Health. 1982;9:77–86. doi: 10.1080/15287398209530143. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Inorganic and Organic Lead Compounds. Vol. 87. Lyon: IARC; 2006. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development. 2003;130:1691–1700. doi: 10.1242/dev.00392. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Liu J, Diwan BA. Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol. 2009;238:215–220. doi: 10.1016/j.taap.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen DM, Sonne SB, Ottesen AM, Perrett RM, Nielsen JE, Almstrup K, Skakkebaek NE, Leffers H, Rajpert-De Meyts E. Origin of pluripotent germ cell tumours: The role of the microenvironment during embryonic development. Mol Cell Endocrinol. 2008;288:111–118. doi: 10.1016/j.mce.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Langa F, Kress C, Colucci-Guyon E, Khun H, Vandormael-Pourin S, Huerre M, Babinet C. Teratocarcinomas induced by embryonic stem (ES) cells lacking vimentin: an approach to study the role of vimentin in tumorigenesis. J Cell Sci. 2000;113:3463–3472. doi: 10.1242/jcs.113.19.3463. [DOI] [PubMed] [Google Scholar]
- Lazo JS, Kondo Y, Dellapiazza D, Michalska AE, Choo KHA, Pitt BR. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem. 1995;270:5506–5510. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- Liu J, Cheng M-L, Yang Q, Shan K-R, Shen J, Zhou Y, Zhang X, Dill AL, Waalkes MP. Blood metallothionein transcript as a biomarker for metal sensitivity: Low blood metallothionein transcripts in arsenicosis patients from Guizhou, China. Environ Health Perspect. 2007;115:1101–1106. doi: 10.1289/ehp.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci (USA) 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onasaka S, Min KS, Fukuhara C, Tanaka K, Tashiro S, Shimizu I, Furuta M, Yasutomi T, Kobashi K, Yamamoto K. Concentrations of metallothionein and metals in malignant and non-malignant tissues in human liver. Toxicology. 1986;38:261–268. doi: 10.1016/0300-483x(86)90142-3. [DOI] [PubMed] [Google Scholar]
- Qu W, Diwan BA, Liu J, Goyer RA, Dawson T, Horton JL, Cherian MG, Waalkes MP. The metallothionein-null phenotype is associated with heightened sensitivity to lead toxicity and an inability to form inclusion bodies. Am J Pathol. 2002;160:1047–1056. doi: 10.1016/S0002-9440(10)64925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kondoh M. Recent studies on metallothionein: Protection against toxicity of heavy metals and oxygen free radicals. Tohoku J Exp Med. 2002;196:9–22. doi: 10.1620/tjem.196.9. [DOI] [PubMed] [Google Scholar]
- Sillevis Smith PA, van Beek H, Baars AJ, Troost D, Louwerse ES, Krops-Hermus AC, de Wolff FA, de Jong JM. Increased metallothionein in the liver and kidney of patients with amyotrophic lateral sclerosis. Arch Neurol. 1992;49:721–724. doi: 10.1001/archneur.1992.00530310063013. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Apostolova MD, Cherian MG. Astrocyte cultures from transgenic mice to study the role of metallothionein in cytotoxicity of tert-butyl hydroperoxide. Toxicology. 2000;145:51–62. doi: 10.1016/s0300-483x(99)00220-6. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Cowin PA, Cunha GR, Pera M, Trounson AO, Pedersen J, Risbridger GP. Formation of human prostate tissue from embryonic stem cells. Nat Methods. 2006;3:179–181. doi: 10.1038/nmeth855. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Waalkes MP. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ Health Perspect. 2010a;118:108–115. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Qu W, Liu J, Liu W, Webber MM, Phang JM, Waalkes MP. Arsenic-specific stem cell selection during malignant transformation. J Natl Cancer Inst. 2010b doi: 10.1093/jnci/djq093. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Benbrahim-Tallaa L, Waalkes MP. Metal Ions in Human Cancer Development. In: Sigel A, Sigel H, Sigel RKO, editors. Metal Ions in Life Sciences. Vol. 8. Metal Ions in Toxicology; London: Royal Society of Chemistry; Cambridge, UK: 2011. Chapter 14. (in press) [PubMed] [Google Scholar]
- Vasak M. Advances in metallothionein structure and functions. J Trace Elem Med Biol. 2005;19:13–17. doi: 10.1016/j.jtemb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Diwan BA, Ward JM, Devor DE, Goyer RA. Renal tubular tumors and atypical hyperplasias in B6C3F1 mice exposed to lead acetate during gestation and lactation occur with minimal chronic nephropathy. Cancer Res. 1995;55:5265–5271. [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Goyer RA, Diwan BA. Metallothionein-I/II double knockout mice are hypersensitive to lead-induced kidney carcinogenesis: Role of inclusion body formation. Cancer Res. 2004;64:7766–7772. doi: 10.1158/0008-5472.CAN-04-2220. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Kasprzak KS, Diwan BA. Metallothionein-I/II double knockout mice are no more sensitive to the carcinogenic effects of nickel subsulfide than wild-type mice. Int J Toxicol. 2005;24:215–220. doi: 10.1080/10915810591000668. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Kasprzak KS, Diwan BA. Hypersusceptibility to cisplatin carcinogenicity in metallothionein-I/II double knockout mice: Production of hepatocellular carcinoma at clinically relevant doses. Int J Cancer. 2006a;119:28–32. doi: 10.1002/ijc.21245. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Enhanced urinary bladder and liver carcinogenesis in male CD1 mice exposed to transplacental inorganic arsenic and postnatal diethylstilbestrol or tamoxifen. Toxicol Appl Pharmacol. 2006b;215:295–305. doi: 10.1016/j.taap.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Powell DA, Diwan BA. Urogenital carcinogenesis in female CD1 mice induced by in utero arsenic exposure is exacerbated by postnatal diethylstilbestrol treatment. Cancer Res. 2006c;66:1337–1345. doi: 10.1158/0008-5472.CAN-05-3530. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol. 2007;222:271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Germolec DR, Trempus CS, Cannon RE, Tokar EJ, Tennant RW, Ward JM, Diwan BA. Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer Res. 2008;68:8278–8285. doi: 10.1158/0008-5472.CAN-08-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Enders GC. Expression of a specific mouse germ cells nuclear antigen (GCNA1) by early embryonic testicular teratoma cells in 129/Sv-Sl/+ mice. Cancer Lett. 1996;100:31–36. doi: 10.1016/0304-3835(95)04068-4. [DOI] [PubMed] [Google Scholar]
- Wang G, Fowler BA. Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol Appl Pharmacol. 2008;233:92–99. doi: 10.1016/j.taap.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Wu MT, Demple B, Bennett RA, Christiani DC, Fan R, Hu H. Individual variability in the zinc inducibility of metallothionein-IIA mRNA in human lymphocytes. J Toxicol Environ Health. 2000;61:553–567. doi: 10.1080/00984100050194081. [DOI] [PubMed] [Google Scholar]
- Zuo P, Qu W, Cooper RN, Goyer RA, Diwan BA, Waalkes MP. Potential role of α-synuclein and metallothionein in lead-induced inclusion body formation. Toxicol Sci. 2009;111:100–108. doi: 10.1093/toxsci/kfp132. [DOI] [PMC free article] [PubMed] [Google Scholar]


