Abstract
Small numbers of proangiogenic bone marrow-derived cells (BMDCs) can play pivotal roles in tumor progression. In this issue of Cancer Cell, two papers, utilizing different tumor angiogenesis models, both find that activated MMP-9 delivered by BMDCs modulates neovessel remodeling, thereby promoting tumor growth. The changes in microvascular anatomy induced by MMP-9-expressing BMDCs are strikingly different between the preirradiated tumor vascular bed model employed by Ahn and Brown and the invasive glioblastoma model utilized by Du et al., likely mirroring the complexity of the real tumor microenvironment and the intricacy of roles of different BMDC populations in mediating tumor neoangiogenesis.
The concept that tumor growth mimics reactivation of embryonic development is not new. However, only recently has it come to light that certain stages of tumor blood vessel assembly mimic embryonic vasculogenesis. While tumor blood vessels may develop by co-option or sprouting from pre-existing vessels (adult angiogenesis), similar to that observed in fetal blood vessel formation, circulating vascular progenitor cells can also contribute to neovessel formation (adult vasculogenesis) (Rafii and Lyden, 2008).
Among these modes of tumor vascularization, the contribution of adult vasculogenesis has lately been the subject of intense scrutiny. Following the observation that a subset of hematopoietic and vascular progenitors could be recruited to assemble tumor neovessels (Lyden et al., 2001), many studies have examined these and other subsets of proangiogenic BMDCs that are mobilized in cancer-bearing animals and in humans. The rarity of many of these BMDC subsets in tumors has complicated the analysis of their respective importance and specific functions. Nonetheless, several key concepts have emerged. First, different populations of proangiogenic BMDCs have restricted roles in temporally and spatially supporting vasculogenesis and angiogenesis in specific types of tumors. Second, recruitment of even small numbers of “catalytic” BMDCs to tumors can play a crucial role in tumor progression. Recently, extensive implementation of BM transplantation in conjunction with various genetic reporter systems and immunologic markers has facilitated more detailed investigation of these cells (Rafii and Lyden, 2008).
There are several categories of BMDCs implicated in tumor angiogenesis. One is those capable of contributing structurally to neovessels, such as endothelial progenitor cells (EPCs) and pericyte progenitor cells (PPCs), which incorporate into the vessel wall, thereby supporting the formation of stable tumor vessels. Other subsets of BMDCs are distinct populations of myeloid progenitors, including Gr1+CD11b+ (Yang et al., 2004) and dendritic precursors (Conejo-Garcia et al., 2004) that can differentiate into endothelial-like cells and incorporate lumenally into the tumor neovessels through a poorly characterized process. Yet another important class of BMDCs contributes indirectly to neovascularization by incorporating perivascularly or by delivering cytokines and other key signals that enhance angiogenesis. This includes CXCR4+VEGFR1+ hemangiocytes (Jin et al., 2006), immune accessory cells, and tumor-associated monocytes/macrophages (TAMs) and inflammatory neutrophils (Ardi et al., 2007; Shojaei et al., 2007; Condeelis and Pollard, 2006; Grunewald et al., 2006).
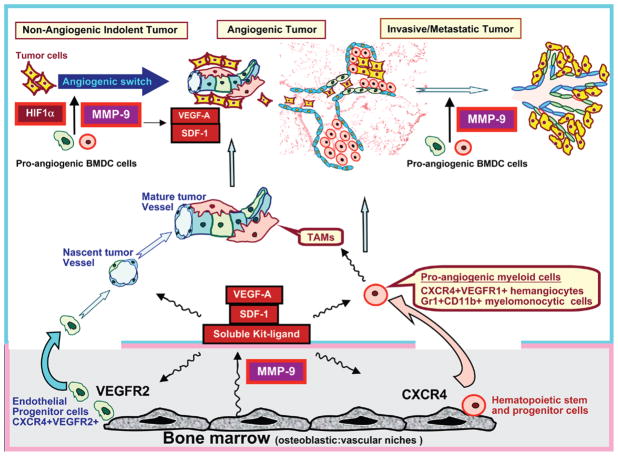
How do BMDCs home to neovascularization sites? One paradigm is provided by the case of CXCR4+VEGFR1+ hemangiocytes (Jin et al., 2006). Under hypoxic conditions, upregulated VEGF-A and SDF-1 (CXCL12), through interaction with their cognate receptors, VEGFR1 and CXCR4, induce the release of activated matrix metalloproteinase-9 (MMP-9) within the BM. Activated MMP-9 liberates soluble kit-ligand, thereby mobilizing CXCR4+ BMDC from the BM into the circulation (Heissig et al., 2002). These cells then home to and are retained within peripheral sites of SDF-1 production, such as the tumor vascular bed (Figure 1).
Figure 1. Multifaceted Role of MMP-9 in Supporting Mobilization and Recruitment of Bone Marrow-Derived Cells in Tumor Vasculogenesis and Neoangiogenesis.
Activated MMP-9, through increasing the bioavailability of Kit-ligand, VEGF-A, and SDF-1, supports the mobilization of the proangiogenic bone marrow-derived cells (BMDCs), including tumor-associated monocytes/macrophages (TAMs), Gr1+CD11b+ myeloid precursors, CXCR4+VEGFR1+ hemangiocytes, and EPCs to the circulation. Irradiation-induced vascular injury or hypoxia-driven upregulation of HIF-1α enhances the release of SDF-1 and VEGF-A, thereby recruiting MMP-9-bearing BMDCs to the neoangiogenic niches, augmenting tumor vasculogenesis, angiogenesis, invasive potential, and metastasis. MMP-9 delivered by BMDCs amplifies tumor neoangiogenesis by increasing the bioavailability of VEGF-A and stem cell active chemocytokines, including SDF-1 and Kit-ligand.
Among the proangiogenic factors that BMDCs manufacture or by which BMDCs themselves are regulated, MMP-9 has emerged as a major player. As an enzyme capable of cleaving many structural extracellular matrix components and other secreted proteins, the proangiogenic functions of MMP-9 are myriad, ranging from directly facilitating endothelial cell invasion to liberating bioactive growth factors (Seandel et al., 2001). Tumor cells, stromal cells (e.g., fibroblasts), and BMDCs serve as the primary sources of MMP-9 that may directly affect distant angiogenic sites and bone marrow (Heissig et al., 2002). However, the clinical significance of MMP-9 delivered by BMDCs locally to the tumor was not previously appreciated. Two new studies in this issue address the underlying problem of why conventional antitumor therapies may ultimately fail, and both point to the role of MMP-9- expressing BMDCs in modulating microvascular anatomy. Remarkably, the specific type of vascular remodeling driven by BMDC-derived MMP-9 appears to be highly dependent on the type of tumor and its defined microenvironment.
Ahn and Brown investigate an important clinical issue of tumor recurrence in the irradiated area after radiotherapy using a model in which the tumor vascular bed has been damaged by irradiation (Ahn and Brown, 2008). Based on previous work, preirradiation has the effect of largely abolishing sprouting angiogenesis and retarding tumor growth. The authors found that CD11b+ cells represent the predominant component of BMDCs in mammary MT1A2 carcinomas. Not only did CD11b+ BMDCs increase in preirradiated MT1A2 tumors, but levels of MMP-9 were also higher, compared to nonpreirradiated control tumors. Correspondingly, when tumors were grown in MMP-9 knockout (KO) mice, those in preirradiated beds were much smaller and had decreased vessel density compared to those in WT control beds. Using transplantation assays, the authors found that the presence of MMP-9 either in the host stroma or in the hematopoietic compartment was sufficient to restore vessel density to control levels. Notably, the tumor vessels in the preirradiated bed tended to be low in pericyte coverage compared to nonirradiated controls in WT mice, attributed to robust production of immature vessels. In contrast, concomitant blockade of angiogenesis (via preirradiation) and of vasculogenesis (in the absence of MMP-9) resulted in small tumors containing only mature-appearing sparse vessels, likely representing co-opted preexisting host vasculature, as a “default” mode of tumor vascularization. Thus, the impaired angiogenesis of the preirradiated tumor bed unmasked the ability of MMP-9+ BMDCs to restore neovascularization.
In contrast, Du et al. follow up their previous observation that orthotopic HIF-1α KO glioblastoma multiforme (GBM) tumors exhibit relatively dense organized microvessels, compatible with co-option of host vasculature, compared to WT GBM that undergo extensive vascular remodeling. In the current study, the authors use an array of GBM implantation and bone marrow transplantation models to show that HIF-1α-dependent recruitment of proangiogenic MMP-9-bearing BMDCs results in more tumor neovascularization (Du et al., 2008). In the absence of MMP-9, there is less vascular remodeling, and the vasculature resembles that of the normal brain. Their data suggest that MMP-9 liberates matrix-bound VEGF-A, resulting in more VEGF-A/VEGFR2 complex formation, pericyte activation, and vascular remodeling. The authors also show that MMP-9 facilitates the recruitment of EPCs and PPC. In the absence of MMP-9 there is relatively less pericyte investment, and this results in diminished tumor neovascularization.
Although the authors show that PPC and EPC recruitment is MMP-9 dependent, they also show that tumor infiltration of the majority of the BMDC is dependent upon SDF-1 expression levels. Inhibiting the recruitment of BMDC by treating mice carrying WT-GBM with a CXCR4 antagonist, the authors were able to normalize the vascular architecture and block tumor neovascularization.
A key but unexpected finding in this manuscript is the paradoxical ability of VEGF-A to directly regulate GBM invasion. GBM normally invades adjacent parts of the brain by single cells infiltrating into the brain parenchyma. In the absence of VEGF-A or MMP-9, this mode of invasion is blocked and leads to a yet unrecognized mechanism causing deep invasion of the GBM, by using the co-opted blood vessels as a roadmap, in a mode known as perivascular tumor invasion. The data reveal the potential of GBM to adapt to its changing microenvironment and underscore the importance of multimodality and multitargeted treatment of tumors, aimed not only at the microenvironment and the tumor but also on different proangiogenic BMDCs that promote tumor progression.
The alternative modes of GBM invasion have pivotal clinical implications. Many of the newest treatment regimens under clinical investigation for patients with GBM result in either blocking VEGF-A/VEGFR2 interaction or possibly increasing the amount of BM cells circulating in the blood. If these findings are confirmed in humans, then such treatments may inflict unrecognized damage.
Collectively, the data from the two studies suggest that there is significant variation in utilization of BMDCs and bioavailability of MMP-9 between different tumor models. One might expect even greater natural variation in utilization of BM-derived cells and their MMP-9 between human tumor types or perhaps even within a given tumor classification. At an extreme, it is possible that the observed clinical variation in behavior between different metastatic foci within individual patients could be due to local differences in recruitment of BM-derived cells. However, both studies find that a minimum threshold of MMP-9 is required for robust angiogenesis, but the source of the MMP-9 may be less critical than its presence. With the additional layers of complexity revealed in the current studies, it is perhaps not surprising that broad-spectrum MMP inhibitors failed to produce dramatic responses in clinical trials or that antiangiogenic therapy as currently implemented has limited efficacy. In this vein, it should be noted that MMP-9 might have antiangiogenic properties as well, through liberation of matrix-bound inhibitors such as tumstatin (Hamano et al., 2003). Until these subtle features of tumor biology are fully interrogated and placed in context, we must proceed with caution, mindful of both intended and potential unintended consequences of treatment.
References
- Ahn G-O, Brown JM. Cancer Cell. 2008;(this issue) doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Proc Natl Acad Sci USA. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, et al. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, VandenBerg S, Johnson RS, Werb Z, Bergers G. Cancer Cell. 2008;(this issue) doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, et al. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Science. 2008;319:163–164. doi: 10.1126/science.1153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seandel M, Noack-Kunnmann K, Zhu D, Aimes RT, Quigley JP. Blood. 2001;97:2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]