Abstract
Background
Epidemiologic studies found childhood mumps might protect against ovarian cancer. To explain this association, we investigated whether mumps might engender immunity to ovarian cancer through antibodies against the cancer-associated antigen MUC1 abnormally expressed in the inflamed parotid gland.
Methods
Through various health agencies, we obtained sera from 161 cases with mumps parotitis. Sera were obtained from 194 healthy controls. We used an ELISA to measure anti-MUC1 antibodies and electro-chemiluminescence assays to measure MUC1 and CA 125. Log-transformed measurements were analyzed by t-tests, generalized linear models, and Pearson or Spearman correlations. We also conducted a meta-analysis of all published studies regarding mumps and ovarian cancer.
Results
Adjusting for assay batch, age, and sex, the level of anti-MUC1 antibodies was significantly higher in mumps cases compared to controls (p = 0.002). Free circulating levels of CA 125, but not MUC1, were also higher in cases (p = 0.02). From the meta-analysis, the pooled odds ratio estimate (and 95% CI) for the mumps and ovarian cancer association was 0.81 (0.68–0.96) (p = 0.01).
Conclusion
Mumps parotitis may lead to expression and immune recognition of a tumor-associated form of MUC1 and create effective immune surveillance of ovarian cancer cells that express this form of MUC1.
Keywords: Ovarian cancer, Mumps parotitis, MUC1, CA125
Introduction
In one of the earliest case–control studies of ovarian cancer, West observed that women with the disease were less likely to report having had mumps compared to women with benign ovarian cysts [1], suggesting that childhood mumps might protect against the subsequent development of ovarian cancer. Eight additional observational studies addressing mumps and ovarian cancer were published [2–9], and in all but two [2, 9], controls were more likely to report a history of mumps than cases, suggesting that mumps might be associated with lower ovarian cancer risk. Despite this intriguing observation, biologic mechanisms were not pursued. As time passed and with the introduction of mumps vaccination in the late 1960s, the association between mumps and ovarian cancer was rendered seemingly irrelevant and largely forgotten.
Recently, we proposed and tested a new hypothesis that can unite many apparently unrelated ovarian cancer risk factors [10]. Protective risk factors may work through events that raise immunity against normal cell proteins that may be abnormally overexpressed in injured or inflamed tissues and also later on nascent ovarian cancer cells making them targets of effective immune surveillance. As an example of such molecules, we have studied the cell surface glycoprotein and tumor-associated antigen, mucin 1 (MUC1). MUC1 is a product of the mucin family of genes that also includes MUC16 (CA125). MUC1 is normally expressed at low levels and, in an extensively glycosylated form, on epithelia of the genito-urinary, respiratory, and digestive tracts as well as breast ducts. It is overexpressed in a hypoglycosylated (immunogenic) form in most adenocarcinomas, including breast and ovarian tumors. We previously reported that (acute) inflammatory events that affect tissues that normally express MUC1, like a tubal ligation or a breast mastitis, found to protect against ovarian cancer, might do so by causing overexpression of the hypoglycosylated form of MUC1, leading to an immune response and immune memory for the type of MUC1 later found on ovarian cancer cells [10]. Just as the presence of anti-MUC1 antibodies in patients with cancer at diagnosis may be associated with a more favorable disease prognosis [11], pre-existing antibodies might prevent disease in the first place.
Salivary glands also express a low level of the fully glycosylated form of MUC1 on apical ductal surfaces [12]. We reasoned that abnormal expression of MUC1 during a mumps infection might induce lasting anti-MUC1 immunity (measured by anti-MUC1 antibodies), giving a mechanistic explanation to the phenomenological association between mumps and ovarian cancer. In the current study, we sought to test this hypothesis by comparing levels of anti-MUC1 antibodies (as well as MUC1 and CA 125 antigens) in samples obtained from patients diagnosed with mumps versus healthy controls.
Methods
Mumps cases
We surveyed several Regional and State Health Departments about the availability of specimens for research purposes from cases with a mumps infection. Our final sample included a total of 161 cases with clinically diagnosed mumps parotitis from four different agencies, three contributed 149 paired samples (during the acute and convalescent phase of the infection) and one contributed single samples (Table 1). Thirty-nine paired samples were available from cases with clinically diagnosed mumps parotitis who reported to the Health Protection Agency Centre for Infections (London, UK). Fifty-two paired samples were obtained from the Specialist Virology Centre of Edinburgh collected between fall 2004 and mid 2005. Cases were predominantly young adults and older teenagers born before routine mumps immunization was introduced in the regions. Additionally, 58 paired serum samples collected from cases with confirmed or probable mumps infection during a 2007 outbreak were received from The Queen Elizabeth II Health Sciences Centre (Nova Scotia, Canada). These included 35 paired sera from laboratory-confirmed cases of mumps and 23 paired sera from cases with symptomatic parotitis but low mumps titer. Of these 23 samples, 18 had an epidemiologic link to a laboratory-confirmed case. Finally, 12 one-time samples collected between 2000 and 2008 and obtained some time during mumps parotitis were provided by the North Dakota Department of Public Health. All samples were shipped to our laboratory on dry ice via overnight courier. Institutional Review Boards (IRBs) associated with the local Health departments or hospitals approved release of specimens without identifying information. Only age, sex, and mumps viral titer (where available) were linked to the specimens that were therefore considered anonymized and exempt by IRBs at the Brigham and Women’s Hospital and University of Pittsburgh.
Table 1.
Details related to mumps case and control specimens
| Site | Status | Dates of sera collection | Storage temperature (°C) | Female (n) | Male (n) | Median age (min–max) |
|---|---|---|---|---|---|---|
| Edinburgh | Mumps casea | Fall 2004–Summer 2005 | −20 | 22 | 30 | 19 (13–42) |
| London | Mumps caseb | 2005 | −30 | 24 | 15 | 19 (9–56) |
| North Dakota | Mumps casec | 2000–2008 | −70 | 6 | 6 | 22 (5–49) |
| Nova Scotia | Mumps cased | April–August 2007 | −70 | 35 | 23 | 25 (15–63) |
| Boston | Control | February–June 2008 | −80 | 53 | 23 | 21 (19–70) |
| Illinois | Control | February–June 2006 | −20 | 35 | 4 | 44 (7–70) |
| Nova Scotia | Control | April–August 2007 | −70 | 42 | 25 | 26 (16–63) |
| Pittsburgh | Control | October 2006 | −20 | 5 | 7 | 30 (26–42) |
The confirmatory tests for cases were complement fixation test (positive test was fourfold change in titer) and virus isolation from sample (parotid duct swab or urine) taken at same time as acute serum. IgG-seroconversion was positive. Tests used were DiaSorin Mumps IgG EIA until early 2005, then changed to Nova-Tec Mumps IgG EIA positive/negative as directed by the manufacturer. IgM—Microimmune Mumps IgM EIA positive/negative as directed by the manufacturer
All samples were from clinical cases of mumps confirmed by laboratory testing by MicroImmune IgM test (positive in at least one sample, manufacturer’s criteria,>3.5 test to c.o.) and/or a significant increase in Mumps IgG (measured by Behring assay;<0.1 negative;>0.2 positive, 0.1–0.2 equivocal, manufacturer’s criteria)
Positive screening values were IgG > 1:16 and IgM >1:16, and end point values were not determined. All testing was done by indirect immunofluorescence
All samples were from clinical cases of mumps. Confirmed case defined by Public Health Agency of Canada (PHAC) case definition [25]. Laboratory-confirmed cases include those who have mumps identified using a previously described RT-PCR from urine or buccal swabs [26] or detection of mumps-specific IgM antibody in serum
Controls
A total of 194 controls were obtained from four sites (Table 1). Twelve samples were received from the Department of Immunology at the University of Pittsburgh. These were University volunteers who were recruited to provide information on the distribution of “normal” anti-MUC1 antibody levels to compare against response to a MUC1 peptide vaccine in various patients with cancer. Single serum samples from 67 participants of a health screening program (who did not have mumps parotitis) were obtained from the Queen Elizabeth II Health Sciences Centre (Nova Scotia, Canada). In the course of surveying State Laboratories, the Illinois Department of public health indicated they could not provide mumps specimens but could supply 39 specimens from individuals who were asymptomatic but seeking to assess the status of their immunity to mumps. Finally, 76 samples were collected from healthy volunteers in Boston from Research Blood Components, a commercial blood bank in Boston, MA where all blood donors must have been free of any symptoms suggestive of a viral illness. As with the mumps cases, all specimens were de-identified and linked only to age at collection and sex.
Assays for anti-MUC1 antibodies
Anti-MUC1 antibodies were measured against a synthetic 100-mer MUC1 peptide corresponding to five tandem repeats of the MUC1 polypeptide core repeat region [10]. The polypeptide mimics the hypoglycosylated state of MUC1 found in ovarian cancer. Briefly, MUC1-coated Immulon wells (Dynax, Chantilly, VA) and peptide-negative plates were incubated overnight and washed three times with PBS before addition of 100 μl of 2.5% bovine serum albumin in PBS. Serially diluted plasma (1:40–1:80 in PBS) was added to MUC1-coated plates and incubated at room temperature. Plates were washed 5× with 100 μl PBS and 0.1% Tween 20 detergent. Alkaline phosphatase-labeled goat anti-human polyvalent IgM, IgG, IgA (50 μl) (Sigma-Aldrich, St. Louis, MO) diluted 1:1,000 was added before plates were again washed 5× with PBS–Tween. Alkaline phosphatase substrate pNPP (100 μl) (Sigma-Aldrich) was added. Plates were incubated before the stop solution (0.5 mol/l NaOH) was added. We used the MRX Revelation plate reader (Thermo Labsystems, Chantilly, VA) to read absorbance values at 405–410 nm, which were subtracted from absorbance values obtained from antigen-negative plates to account for non-specific binding.
Laboratory personnel were blinded to case–control status of the samples. Blood specimens from cases and controls as well as paired samples from the same participants were assayed on the same plate, which also included masked quality control samples (three to five replicates per plate). Samples were assayed in two batches. Batch 1 included samples from London and controls from the University of Pittsburgh, which were assayed in triplicate and in four dilutions (1:40, 1:80, 1:160, and 1:320). Average values across triplicates were reported for each sample. In order to increase the number of specimens per assay plate and thus decrease inter-plate variability, samples in batch 2 (which included all the remaining study specimens) were assayed only in two dilutions (1:40 and 1:80). Triplicates of each sample were assayed on different plates, and average values of triplicates were reported for each sample. The coefficients of variation (CV) for the batch 1 and 2 positive controls were 18 and 4%, respectively. The Spearman rank correlation between the 1:40 and 1:80 dilutions was 0.95. Readings at 1:40 were used for the current analysis.
Assays for CA 125 and MUC1
Serum levels of CA 125 and MUC1 were measured (blinded to case status) using electro-chemiluminescence (ECL) assays and Imager 2400 (Meso Scale Discovery, Gaithersburg, MD, USA). The ECL platform allows assays using very small volumes and has been validated against traditional ELISA [13]. The linearity ranges were 1.2–5,000 U/ml for the CA 125 and 0.98–4,000 mU/ml for the MUC1 assay. The serum samples were tested undiluted for the CA 125 and diluted 1:200 for the MUC1 assay. A positive quality control (QC) sample was run on each plate in duplicate. The QC sample had a mean CA 125 concentration of 450.47 U/ml and a mean MUC1 concentration of 63.73 U/ml. The coefficient of variation was calculated as 100* (SD/average) for each assay plate and between plates. The intra-plate CV% for CA 125 varied from 2.2 to 13.1% with inter-plate CV = 9.1%. The intra-plate CV% for MUC1 varied from 0 to 4.7% with inter-plate CV = 11.0%.
Meta-analysis
We searched the literature for studies that examined the association between history of mumps parotitis and subsequent development of ovarian cancer. On June 2009, we searched MEDLINE (via PubMed), Web of Science and EMBASE using the search terms mumps, parotitis, ovarian neoplasms, ovarian cancer. No limits on publication dates or on language were imposed. This search yielded a total of 34 references, nine of which referred to original contributions. Of these, one [1] did not provide estimates for the association between mumps parotitis and ovarian cancer and, therefore, was not included in the meta-analysis, which included a total of eight case–control studies [2–9]. We estimated a summary odds ratio of ovarian cancer and the associated 95% confidence interval across studies using a random effects model, and statistical heterogeneity was assessed using I2 and Cochran Chi-square statistics [14].
Statistical analysis
The anti-MUC1 antibody, MUC1, and CA 125 distributions were skewed right, so we first log transformed the values to normalize their distributions. We examined geometric mean levels by case–control status and used t-tests to assess differences by case–control status stratified by laboratory batch number, site, age, and sex. We used linear regression to examine the mean difference in log-transformed anti-MUC1 antibody or MUC1 and CA 125 antigen levels, adjusted for age, sex, and laboratory batch. For sites with continuous mumps titer data available (London and Edinburgh), we used Spearman rank correlations to assess the relationship between titer levels and anti-MUC1 antibody levels. Pearson correlations were used to examine the relationship between anti-MUC1 antibody levels and MUC1 antigen levels. The SAS version 9.1 statistical package (SAS Institute, Cary, NC) was used for all analyses. All p values are two-sided with significance levels set at <0.05.
Results
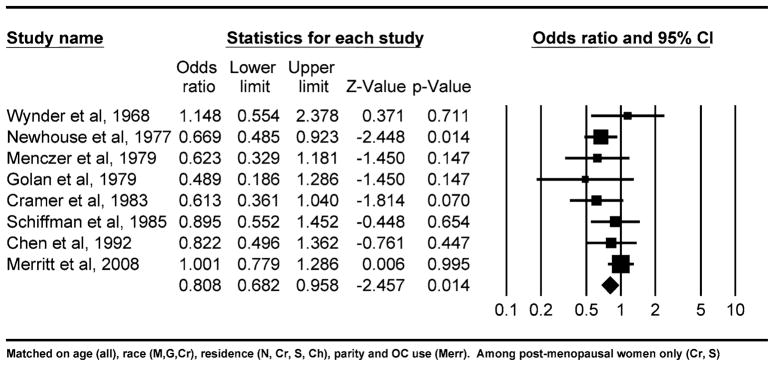
We identified eight case–control studies that provided odds ratios for the association between mumps parotitis and ovarian cancer [2–8]. In all but two of these studies [2], controls were more likely to report a history of mumps than cases. From these studies, using a random effects model, we estimated that the overall risk of ovarian cancer associated with history of mumps parotitis was 0.81 with 95% confidence interval of 0.68–0.96 (p = 0.01), suggesting that mumps is significantly and inversely associated with ovarian cancer risk (Fig. 1). A fixed effects model produced similar study estimates. The I2 test for statistical heterogeneity indicated small variability among studies that could not be explained by chance, 11%, and the Cochran’s Q statistic for heterogeneity was not significant (p = 0.30).
Fig. 1.
Odds ratios from studies of mumps and ovarian cancer and the summary odds ratio
No significant differences in anti-MUC1 antibody levels were observed between acute and convalescent specimens from those sites providing paired samples (see footnote to Table 2). Therefore, the values were averaged for each subject. Geometric mean anti-MUC1 antibody levels are described in Table 2 by batch and mumps status. There was a clear batch effect for the mumps samples run first with London cases and Pittsburgh controls; both groups having lower mean levels compared to the larger number of specimens run in the second batch. Some variation was noted in anti-MUC1 antibody levels in mumps cases by the source of the specimens contributed to batch 2, but this reflected differences in the composition of the samples by age and sex further illustrated in Table 2. In general, males had lower levels of anti-MUC1 antibodies than females, and antibody levels appeared to decline with age in the male cases and controls. In all age and sex categories from batch 2, the levels of anti-MUC1 antibodies were higher in the mumps cases compared to controls (p = 0.003). As illustrated in Table 3, mumps was a significant predictor of higher anti-MUC1 antibody levels after adjustment for age and sex in generalized linear models either restricted to batch 2 data (p = 0.002) or in a second model which included batch 1 data, as well as a variable for batch in the model (p = 0.002). The models confirmed that age and sex were also significant predictors with higher anti-MUC1 antibody levels in younger individuals and women.
Table 2.
Geometric mean anti-MUC1 antibodies by case–control status
| Mumps cases |
Controls |
t-Test | |||
|---|---|---|---|---|---|
| n (%) | Mean (95% CI) | n (%) | Mean (95% CI) | p Valueb | |
| First batch | |||||
| Sites | |||||
| London (paired)a | 39 | 0.49 (0.41, 0.58) | 0 | – | |
| Pittsburgh (single) | 0 | – | 12 | 0.42 (0.32, 0.55) | 0.37 |
| Second batch | |||||
| Sites | |||||
| Boston (single) | 0 | – | 76 | 0.81 (0.71, 0.92) | |
| Edinburgh (paired)a | 52 | 0.95 (0.82, 1.09) | 0 | – | |
| Illinois (single) | 0 | – | 39 | 0.77 (0.64, 0.93) | |
| North Dakota (single) | 12 | 1.26 (0.87, 1.82) | 0 | – | |
| Nova Scotia (paired)a | 58 | 0.94 (0.81, 1.10) | 67 | 0.80 (0.70, 0.92) | 0.12 |
| Sex and age | |||||
| Males | |||||
| <21 | 31 (52.5) | 0.98 (0.79, 1.22) | 17 (32.7) | 0.86 (0.64, 1.15) | |
| 21–26 | 16 (27.1) | 0.75 (0.56, 1.00) | 19 (36.5) | 0.70 (0.56, 0.89) | |
| >26 | 12 (20.3) | 0.76 (0.56, 1.05) | 16 (30.8) | 0.63 (0.48, 0.83) | |
| All males | 59 | 0.87 (0.75, 1.01) | 52 | 0.73 (0.63, 0.84) | 0.10 |
| Females | |||||
| <21 | 22 (34.9) | 1.09 (0.87, 1.38) | 32 (24.6) | 0.82 (0.65, 1.05) | |
| 21–26 | 20 (31.7) | 0.99 (0.84, 1.18) | 34 (26.2) | 0.87 (0.72, 1.05) | |
| >26 | 21 (33.3) | 1.16 (0.88, 1.53) | 64 (49.2) | 0.81 (0.70, 0.93) | |
| All females | 63 | 1.08 (0.95, 1.23) | 130 | 0.83 (0.75, 0.92) | 0.002 |
| All from second batch | 122 | 0.97 (0.88, 1.07) | 182 | 0.80 (0.73, 0.87) | 0.003 |
The acute and convalescent values were averaged for sites where paired samples were available. The acute and convalescent geometric means (95% CI) for London, Edinburgh, and Nova Scotia were 0.49 (0.42, 0.58), 0.48 (0.40, 0.58), 0.99 (0.85, 1.14), 0.91 (0.78, 1.05), 0.94 (0.80, 1.10), and 0.94 (0.80, 1.10), respectively
t-Test comparisons for the five p values shown are (1) London cases to Pittsburgh controls, (2) Nova Scotia cases to Nova Scotia controls, (3) male cases to male controls, (4) female cases to female controls, and (5) all batch 2 cases to all batch 2 controls
Table 3.
Generalized linear models with anti-MUC1 antibody level as the dependent variable
| Coefficienta | p Valuea | |
|---|---|---|
| Model 1: Batch 2 (excluding London and Pittsburgh) | ||
| Age (years) | −0.005 | 0.07 |
| Sex (male vs. female) | −0.19 | 0.005 |
| Mumps (yes vs. no) | 0.21 | 0.002 |
| Model 2: Batch 1 and 2 (including London and Pittsburgh) | ||
| Age (years) | −0.005 | 0.03 |
| Sex (male vs. female) | −0.20 | 0.001 |
| Batch (1 vs. 2) | 0.65 | < 0.0001 |
| Mumps (yes vs. no) | 0.19 | 0.002 |
Each coefficient for models 1 and 2 is adjusted for the other variables in the models
We also examined whether circulating levels of CA125 or MUC1 antigens differed between mumps cases and controls. For many of the samples, including all of those from London, Edinburgh, and Pittsburgh, there was insufficient volume remaining after the anti-MUC1 antibody measurement for the CA125 and MUC1 measurements. In univariate analyses, no significant differences were observed for MUC1 levels but, as shown in Table 4, mumps cases had higher CA125 levels than controls (p = 0.02). In a multivariate linear regression model with log-transformed CA125 as the dependent variable, neither age (coefficient = 0.004, p value = 0.38) nor sex (coefficient = −0.006, p value = 0.95) was significantly associated with CA125, but mumps cases had mean log-transformed CA125 levels that were significantly higher than controls, on average (p = 0.03). These results are similar to the t-tests shown in Table 4. No significant correlations were observed between mumps viral titers supplied for the London and Edinburgh cases and anti-MUC1 antibody levels (data not shown). These were the same cases with insufficient volume to measure MUC1 and CA 125, so the correlations of the antigens with viral titers could not be examined. We examined the correlation between anti-MUC1 antibody levels and MUC1 antigen levels. There was a weak but significant inverse correlation between antigen and antibody levels for mumps cases (r = −0.24, p = 0.05), but not controls (r = −0.09, p = 0.21).
Table 4.
Geometric mean CA 125 and MUC1 by case–control status
| Cases (n) | Controls (n) | CA 125 |
MUC1 |
|||||
|---|---|---|---|---|---|---|---|---|
| Mumps cases | Controls | t-Test | Mumps cases | Controls | t-Test | |||
| Mean (95% CI) | Mean (95% CI) | p Value | Mean (95% CI) | Mean (95% CI) | p Value | |||
| Sites | ||||||||
| Boston (single) | 0 | 76 | – | 15.6 (12.6, 19.3) | – | 33.8 (30.32, 37.7) | ||
| Edinburgh (paired)a | 0 | 0 | – | – | – | – | ||
| Illinois (single) | 0 | 33 | – | 17.9 (13.7, 23.5) | – | 34.6 (29.8, 40.2) | ||
| North Dakota (single) | 9 | 0 | 18.8 (12.5, 28.3) | – | 28.9 (22.3, 37.5) | – | ||
| Nova Scotia (paired)a | 58 | 65 | 23.1 (19.7, 27.1) | 20.0 (16.2, 24.9) | 34.5 (30.9, 38.6) | 36.2 (32.0, 40.9) | ||
| Sex and age | ||||||||
| Males | ||||||||
| <21 | 10 | 17 | 28.6 (23.4, 34.8) | 16.8 (13.0, 21.7) | 33.7 (24.8, 45.8) | 32.7 (24.8, 43.0) | ||
| 21–26 | 9 | 19 | 20.0 (12.3, 32.5) | 15.3 (11.8, 19.9) | 43.5 (30.5, 62.2) | 40.4 (33.0, 49.3) | ||
| >26 | 8 | 14 | 18.1 (13.3, 24.6) | 21.0 (16.1, 27.6) | 39.6 (33.3, 46.9) | 31.7 (24.0, 41.9) | ||
| All males | 27 | 50 | 22.2 (18.4, 26.8) | 17.3 (14.9, 20.0) | 0.04 | 38.5 (32.9, 44.9) | 35.1 (30.6, 40.2) | 0.40 |
| Females | ||||||||
| <21 | 8 | 32 | 18.7 (11.1, 31.5) | 13.2 (7.7, 22.6) | 30.0 (22.3, 40.5) | 34.5 (28.6, 41.7) | ||
| 21–26 | 12 | 32 | 28.7 (19.4, 42.5) | 16.7 (12.6, 22.2) | 31.8 (24.3, 41.7) | 37.7 (32.5, 43.7) | ||
| >26 | 20 | 60 | 21.2 (15.2, 29.6) | 21.4 (17.9, 25.6) | 30.6 (25.0, 37.5) | 33.3 (29.5, 37.7) | ||
| All females | 40 | 124 | 22.6 (18.3, 28.1) | 17.7 (14.9, 21.2) | 0.08 | 30.8 (27.1, 35.1) | 34.7 (31.9, 37.8) | 0.16 |
| All | 67 | 174 | 22.4 (19.4, 26.0) | 17.6 (15.4, 20.1) | 0.02 | 33.7 (30.5, 37.3) | 34.8 (32.5, 37.4) | 0.62 |
The acute and convalescent values were averaged for sites where paired samples were available
Discussion
In this study, we found that sera from individuals during (or just after) symptomatic mumps parotitis have a significantly higher level of anti-MUC1 antibodies than sera from controls without active parotitis. Showing the relevance of this observation to ovarian cancer clearly requires background data that would link mumps, ovarian cancer, and anti-MUC1 antibodies. Concerning the mumps and ovarian cancer association, we performed a meta-analysis of all published original reports to obtain an overall estimate of the effect. In eight observational studies addressing the association, the summary odds ratio was 0.81 with 95% confidence limits of 0.68–0.96 (p = 0.01), suggesting a 19% decrease in risk of ovarian cancer associated with history of mumps parotitis. One of the studies not finding an odds ratio<1 also did not find an association with parity [2], and the second study in which the association was null [9] was the most recent of the studies and would have included many more subjects who had been vaccinated for the mumps. Conversely, several of the key studies (which had looked at other associations besides the mumps) confirmed well-established findings, like protection with pregnancies and oral contraceptive use [3, 6–8]. While prospective studies related to mumps would have been desirable, studies two through eight are likely to constitute the only pre-vaccination era epidemiologic data we will ever have related to this association.
Despite the epidemiologic evidence that mumps might reduce the risk of ovarian cancer, this association has largely been ignored, probably due to the lack of a plausible biologic explanation. We have presented data here in support of an immunological basis for the association. A protective effect of mumps parotitis on ovarian cancer risk can be explained under a model related to immunity against the surface glycoprotein, MUC1. Events affecting tissues that normally express MUC1 might confer protection because of injury to the tissue causing expression and presentation of a tumor-like (less glycosylated) form of MUC1 to the immune system [10]. This would allow immune recognition of the protein core of MUC1 and generation of an immune response similar to that which occurs with cancer [15, 16]. Since MUC1 is expressed in normal salivary glands, acute inflammation of this tissue with mumps would be expected to induce changes in MUC1 expression and glycosylation similar to that which has been reported for other tissues undergoing inflammation [17–21]. Thus, our observation that anti-MUC1 antibodies are elevated in individuals with mumps is consistent with the interpretation that mumps infection could elicit an immune response to later protect against ovarian cancer.
We began this study with the expectation that, in mumps cases with paired sera, we would find higher MUC1 levels in the acute specimen and higher anti-MUC1 antibody levels in the convalescent specimen. The fact that neither was observed may reflect lack of detail on the timing of blood collections in relation to onset of symptoms. Alternatively, the fact that anti-MUC1 antibody levels were similar (but higher than controls) in both the acute and convalescent sera could be explained if this were not the first time that the mumps cases had seen an inflammatory type of MUC1. In this circumstance, a much more rapid immune response would be expected and might have occurred because of prior inflammatory conditions involving the genito-urinary, respiratory, digestive tract, or breast ducts—tissues which all express MUC1. MUC1 antigen itself was not elevated in cases compared to controls in either the acute or convalescent specimens. Saliva, if it had been available, might have been a better body fluid to look for MUC1, but it is also possible that the presence of anti-MUC1 antibodies interfered with measurement of MUC1 since immune complexes involving MUC1 and antibodies against it have been described [22]. Supporting this possibility was an inverse correlation between anti-MUC1 antibody levels and MUC1 antigen levels in cases (but not controls) noted in our presentation of the results of Table 4. Interestingly, CA 125 was significantly elevated in sera from the mumps cases compared to controls. CA 125 is expressed in salivary gland tumors [23], but we could find no reports of its expression in normal salivary glands. The low volume of mumps specimens precluded measurement of immune complexes or anti-CA125 antibody levels.
There are many limitations of this study, not the least of which was the difficulty of obtaining specimens from individuals with mumps parotitis which limited the size of this study. The samples we obtained were collected between 2000 and 2008 in various public health agencies and were stored under variable conditions (Table 1). Although mumps is now reportable in many regions, we have no way of knowing whether the cases that come to public health attention are representative of mumps infection in the community or a more selected group. Information on precisely when during the course of the infection the samples were collected was limited, and titer data were available from only two sites (London and Edinburgh). Because of the anonymized nature of the case and control specimens, we were unable to correct for potential confounders, other than age and gender. Age could be a key confounder, since we previously reported that anti-MUC1 antibody levels may decline with age [10]. While cases were more likely than controls to be younger and to be male, adjustment by these variables revealed that confounding by age and sex did not account for the relationships between mumps and anti-MUC1 antibodies, CA125, or MUC1 levels. The small size of this study should not have produced an artifactual association between mumps and anti-MUC1 antibody (or CA125 levels) but certainly could have affected the precision of our estimate of the effect.
Regarding controls, we are certain they did not have an active mumps infection, but it was a diverse group which included university volunteers for the London cases in batch 1 and, in batch 2, individuals without mumps in Illinois who were tested for immunity and blood bank controls from Boston. Community-matched controls were only available for the Nova Scotia subjects in batch 2. Despite this, the levels of anti-MUC1 antibodies in all control groups from batch 2 were very similar and nearly identical to the Nova Scotia controls.
Another limitation was variability in the assay from batch 1 to batch 2. To decrease inter-plate variability after running batch 1, we increased the number of unique specimens per plate for batch 2, which may have contributed to the batch differences. However, despite batch variation, both batches suggested higher antibody levels in mumps cases compared to controls. Moreover, analyses including and excluding batch 1 yielded a similar result.
The epidemiology of mumps parotitis has obviously changed dramatically in the last 40 years. Mumps parotitis was a very common illness in infants and children prior to 1970. With now close to universal vaccination except in the third world, mumps has become a disease of adults who were either born too early for routine vaccination or who have lost immunity after vaccination. For this reason, inferences about the consequences of parotitis on MUC1 immunity based on observations from the specimens tested here may not be generalizable to what might have occurred with childhood infection before vaccination programs began. We point out, however, that the anti-MUC1 antibody response was most robust in younger women suggesting that childhood infection, as would have occurred in the past, might have been the optimum time for engendering immunity against ovarian cancer.
Clearly, mumps vaccination only creates anti-viral antibodies and would not lead to anti-MUC1 antibodies, which we show here require an active parotitis. If it is true that symptomatic mumps protected against ovarian cancer through an immune reaction, a logical consequence is that we might expect an increased incidence of ovarian cancer as symptomatic mumps parotitis infections have decreased through vaccination. In a paper examining incidence patterns for ovarian cancer from 1978 to 1998, rates of invasive serous, endometrioid, and clear cell tumor increased over this time period among white females [24]. Endometrioid and clear cell cancers are the types of ovarian cancer that we found were most strongly linked to the conditions we proposed might be mediated through anti-MUC1 antibodies [10]. The above incidence data may be confounded by diagnostic trends, but re-examination of these disease rates with more recent data and a focus on the birth cohorts most likely to have been vaccinated should be undertaken.
Prior to vaccination, mumps was generally a mild illness but could have serious sequelae including orchitis and sterility, meningitis and deafness, and pancreatitis. Nevertheless, our study suggests there could also have been unanticipated long-term anticancer benefits of a mumps infection, such as we have described in this paper. Understanding the scope of and basis for the potential benefits of childhood infections may allow immunologists to duplicate the beneficial effects at the same time that vaccination provides the means for avoiding a natural infection and its possible immediate consequences. Further study of individuals going through a mumps infection, especially with a focus on mucin immunity, may provide clues to mechanisms for duplicating the beneficial effects of mumps parotitis suggested by this study.
Acknowledgments
We thank Claudia Krause, Bernard Johnson, and Michael Trythall for helping us assemble the specimen sets and for their thoughtful comments on the manuscript and Huaiping Yan and Vanessa Tang-Fernandez for laboratory assistance. Grants: US Department of Defense #W81XWH-07-1-0292 and NIH Grant R01CA123170.
Contributor Information
Daniel W. Cramer, Email: dwcramer@partners.org, Department of Obstetrics and Gynecology Epidemiology Center, Obstetrics and Gynecology Epidemiology Center, Brigham and Women’s Hospital (BWH), Harvard Medical School, 221 Longwood Ave, RFB 366, Boston, MA 02115, USA
Allison F. Vitonis, Department of Obstetrics and Gynecology Epidemiology Center, Obstetrics and Gynecology Epidemiology Center, Brigham and Women’s Hospital (BWH), Harvard Medical School, 221 Longwood Ave, RFB 366, Boston, MA 02115, USA
Simone P. Pinheiro, US Food and Drug Administration, Silver Spring, MD, USA
John R. McKolanis, Department of Immunology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Raina N. Fichorova, Laboratory of Genital Tract Biology, Department of Obstetrics, Gynecology and Reproductive Biology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Kevin E. Brown, Centre for Infections, Health Protection Agency, London, UK
Todd F. Hatchette, Division of Microbiology, Department of Pathology and Laboratory Medicine, Queen Elizabeth II Health Sciences Centre, Halifax, NS, Canada. Department of Pathology, Dalhousie University, Halifax, NS, Canada
Olivera J. Finn, Department of Immunology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
References
- 1.West RO. Epidemiology study of malignancies of the ovaries. Cancer. 1966;19:1001–1007. doi: 10.1002/1097-0142(196607)19:7<1001::aid-cncr2820190714>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Wynder EL, Dodo H, Barber HR. Epidemiology of cancer of the ovary. Cancer. 1969;23(2):352–370. doi: 10.1002/1097-0142(196902)23:2<352::aid-cncr2820230212>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Newhouse ML, Pearson RM, Fullerton JM, Boesen EA, Shannon HS. A case control study of carcinoma of the ovary. Br J Prev Soc Med. 1977;31(3):148–153. doi: 10.1136/jech.31.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menczer J, Modan M, Ranon L, Golan A. Possible role of mumps virus in the etiology of ovarian cancer. Cancer. 1979;43(4):1375–1379. doi: 10.1002/1097-0142(197904)43:4<1375::aid-cncr2820430427>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Golan A, Joosting AC, Orchard ME. Mumps virus and ovarian cancer. S Afr Med J. 1979;56(1):18–20. [PubMed] [Google Scholar]
- 6.Cramer DW, Welch WR, Cassells S, Scully RE. Mumps, menarche, menopause, and ovarian cancer. Am J Obstet Gynecol. 1983;147(1):1–6. doi: 10.1016/0002-9378(83)90073-x. [DOI] [PubMed] [Google Scholar]
- 7.Schiffman MH, Hartge P, Lesher LP, McGowan L. Mumps and postmenopausal ovarian cancer. Am J Obstet Gynecol. 1985;152(1):116–118. doi: 10.1016/s0002-9378(85)80198-8. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Wu PC, Lang JH, et al. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol. 1992;21(1):23–29. doi: 10.1093/ije/21.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122(1):170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 10.Cramer DW, Titus-Ernstoff L, McKolanis JR, et al. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1125–1131. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 11.Hamanaka Y, Suehiro Y, Fukui M, et al. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103(1):97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 12.Alos L, Lujan B, Castillo M, et al. Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am J Surg Pathol. 2005;29(6):806–813. doi: 10.1097/01.pas.0000155856.84553.c9. [DOI] [PubMed] [Google Scholar]
- 13.Fichorova RN, Richardson-Harman N, Alfano M, et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem. 2008;80(12):4741–4751. doi: 10.1021/ac702628q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Smith G, Altman D. Systematic reviews in health care: meta-analysis in context. 2. BMJ Publishing Group; London: 2001. [Google Scholar]
- 15.Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci. 2004;41(2):189–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- 16.Vlad AM, Finn OJ. Glycoprotein tumor antigens for immunotherapy of breast cancer. Breast Dis. 2004;20:73–79. doi: 10.3233/bd-2004-20109. [DOI] [PubMed] [Google Scholar]
- 17.Campbell BJ, Yu LG, Rhodes JM. Altered glycosylation in inflammatory bowel disease: a possible role in cancer development. Glycoconj J. 2001;18(11–12):851–858. doi: 10.1023/a:1022240107040. [DOI] [PubMed] [Google Scholar]
- 18.Jerome KR, Kirk AD, Pecher G, Ferguson WW, Finn OJ. A survivor of breast cancer with immunity to MUC-1 mucin, and lactational mastitis. Cancer Immunol Immunother. 1997;43(6):355–360. doi: 10.1007/s002620050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakajima M, Manabe T, Niki Y, Matsushima T. Serum KL-6 level as a monitoring marker in a patient with pulmonary alveolar proteinosis. Thorax. 1998;53(9):809–811. doi: 10.1136/thx.53.9.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takaishi H, Ohara S, Hotta K, et al. Circulating autoantibodies against purified colonic mucin in ulcerative colitis. J Gastroenterol. 2000;35(1):20–27. doi: 10.1007/pl00009971. [DOI] [PubMed] [Google Scholar]
- 21.Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest. 1999;46:151–158. [PubMed] [Google Scholar]
- 22.Vlad AM, Muller S, Cudic M, et al. Complex carbohydrates are not removed during processing of glycoproteins by dendritic cells: processing of tumor antigen MUC1 glycopeptides for presentation to major histocompatibility complex class II-restricted T cells. J Exp Med. 2002;196(11):1435–1446. doi: 10.1084/jem.20020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamura K, Kiyoshima T, Shima K, et al. Immunohistochemical expression of CA19-9 and CA125 in mucoepidermoid and adenoid cystic carcinomas of the salivary gland. Oral Oncol. 2002;38(3):244–250. doi: 10.1016/s1368-8375(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 24.Mink PJ, Sherman ME, Devesa SS. Incidence patterns of invasive and borderline ovarian tumors among white women and black women in the United States. Results from the SEER Program, 1978–1998. Cancer. 2002;95(11):2380–2389. doi: 10.1002/cncr.10935. [DOI] [PubMed] [Google Scholar]
- 25.Tipples G, Beirnes J, Hiebert J, et al. [Accessed 14 July 2009];Laboratory guidelines for the diagnosis of mumps v.3. 2008 April 3; http://www.nml-lnm.gc.ca/guide/docs/Mumps_Lab_guide.pdf.
- 26.Jin L, Beard S, Brown DW. Genetic heterogeneity of mumps virus in the United Kingdom: identification of two new genotypes. J Infect Dis. 1999;180(3):829–833. doi: 10.1086/314957. [DOI] [PubMed] [Google Scholar]