Abstract
Increasing evidence suggests that endothelial cytotoxicity from reactive oxygen species (ROS) contributes to the pathogenesis of acute lung injury. Treatments designed to increase intracellular cGMP attenuate ROS-mediated apoptosis and necrosis in several cell types, but the mechanisms are not understood, and the effect of cGMP on pulmonary endothelial cell death remains controversial. In the current study, increasing intracellular cGMP by either 8pCPT-cGMP (50 μM) or atrial natriuretic peptide (10 nM) significantly attenuated cell death in H2O2-challenged mouse lung microvascular (MLMVEC) monolayers. 8pCPT-cGMP also decreased perfusate LDH release in isolated mouse lungs exposed to H2O2 or ischemia-reperfusion. The protective effect of increasing cGMP in MLMVECs was accompanied by enhanced endothelial H2O2 scavenging (measured by H2O2 electrode) and decreased intracellular ROS concentration (measured by 2′,7′-dichlorofluorescin fluorescence) as well as decreased phosphorylation of p38 MAPK and Akt. The cGMP-mediated cytoprotection and increased H2O2 scavenging required >2 h of 8pCPT-cGMP incubation in wild-type MLMVEC and were absent in MLMVEC from protein kinase G (PKGI)−/− mice suggesting a PKGI-mediated effect on gene regulation. Catalase and glutathione peroxidase 1 (Gpx-1) protein were increased by cGMP in wild-type but not PKGI−/− MLMVEC monolayers. Both the cGMP-mediated increases in antioxidant proteins and H2O2 scavenging were prevented by inhibition of translation with cycloheximide. 8pCPT-cGMP had minimal effects on catalase and Gpx-1 mRNA. We conclude that cGMP, through PKGI, attenuated H2O2-induced cytotoxicity in MLMVEC by increasing catalase and Gpx-1 expression through an unknown posttranscriptional effect.
Keywords: apoptosis, necrosis, hydrogen peroxide, catalase, glutathione peroxidase
increased endothelial cell death from reactive oxygen species (ROS) contributes to the pathogenesis of acute lung injury (ALI) (56). Based on this concept, inhibition of apoptosis has been proposed as a potential ALI therapy (32). Unfortunately, the complex spectrum of cell death from the carefully orchestrated process of apoptosis to necrosis can result in unpredictable effects when specific cell death pathways are blocked (56). The pharmacological inhibition of ROS production (18) or the administration of endothelial-targeted antioxidant enzymes (53) may prove to be safer approaches to this problem, but these strategies have not yet translated into clinical therapies.
In some clinical situations, an oxidant endothelial insult can be predicted in advance, allowing the potential for a preconditioning treatment to bolster endogenous antioxidant pathways (36). For example, a reperfusion injury occurs in the majority of lung transplant recipients (34). Animal models of ischemia-reperfusion (IR) lung injury suggest that oxidant-mediated endothelial cytotoxicity plays a significant role (59). This clinical scenario could allow a preconditioning treatment to be administered to the donor patient or lungs hours before the reperfusion injury (34). For example, treatments that increase pulmonary endothelial cGMP in the donor lung have been shown to decrease reperfusion injury in animal models of lung transplantation (27, 31, 47).
cGMP is produced by NO stimulation of soluble guanylyl cyclase (sGC) or natriuretic peptide stimulation of particulate guanylyl cyclase (pGC). cGMP has multiple intracellular targets including ion channels, cyclic nucleotide-sensitive phosphodiesterases (PDE), and the serine/threonine kinase PKGI (40). We have previously shown that increased cGMP blocked H2O2-induced endothelial barrier dysfunction in vitro by a PKGI-dependent mechanism (39, 44). Others have reported that cGMP was capable of attenuating oxidant-mediated cell death in several cell types (15, 46). In cardiomyocytes, protection was attributed to an increase in the antiapoptotic protein Bcl-2 (15), whereas in neuroblastoma cells, cGMP/PKGI activation inhibited apoptosis through upregulation of the antioxidant proteins thioredoxin (Trx) and thioredoxin-peroxidase 1 (Tpx-1) (3).
Less is known about cGMP-mediated effects in ROS-induced pulmonary capillary endothelial cell cytotoxicity. Recently, Hemnes et al. (26) demonstrated that increased lung PKGI activity from administration of the PDE5 inhibitor sildenafil attenuated a bleomycin-induced increase in lung ROS concentration and fibrosis. These data suggested that activation of the cGMP pathway either blocked ROS formation or enhanced ROS scavenging.
The goal of the present study was to determine the effect of the cGMP/PKGI signaling pathway on ROS-induced lung endothelial cytotoxicity. Our results demonstrate that increasing intracellular cGMP by either 8pCPT-cGMP or atrial natriuretic peptide (ANP) administration attenuated cell death and evidence of cytotoxicity in H2O2-challenged mouse lung microvascular (MLMVEC) monolayers and ROS-injured isolated perfused mouse lungs, respectively. The protective effect of increasing cGMP in MLMVECs was accompanied by enhanced H2O2 scavenging; both effects were absent in MLMVEC from PKGI−/− mice suggesting a PKGI-mediated effect on gene regulation. Consistent with this result, catalase and glutathione peroxidase 1 (Gpx-1) protein were increased in wild-type but not PKGI−/− MLMVEC monolayers exposed to 8pCPT-cGMP. Both the cGMP-mediated increases in antioxidant proteins and H2O2 scavenging were prevented by inhibition of translation with cycloheximide but cGMP had minimal effects on catalase and Gpx-1 mRNA. Our results suggest that cGMP, through PKGI, attenuated H2O2-induced cytotoxicity by increasing catalase and Gpx-1 expression and H2O2 scavenging through an as yet undetermined posttranscriptional effect.
MATERIALS AND METHODS
Antibodies and reagents.
Anti-catalase, anti-vasodilator-stimulated phosphoprotein (VASP), and anti-pVASP (Ser239) antibodies were from CalBiochem (San Diego, CA). The anti-pVASP (Ser239) antibody, which binds to human VASP phosphorylated at Ser239, has significant affinity toward the corresponding Ser235 site in murine VASP (35). Anti-Trx-1, anti-Mn superoxide dismutase (MnSOD), anti-p38, anti-phospho-p38, anti-Akt, and anti-phospho-Akt (Thr308) antibodies, as well as cell lysis buffer concentrate, were from Cell Signaling Technology (Beverly, MA). Anti-glutathione peroxidase-1 was obtained from Abcam. Secondary antibodies and all Western blotting reagents were from Bio-Rad (Hercules, CA). DMEM, Brinster's BMOC-3 media, FBS, and TRIzol reagent were from Invitrogen (Carlsbad, CA). 8pCPT-cGMP was obtained from Axxora (San Diego, CA) and dissolved in serum-free DMEM. ANP (dissolved in sterile 0.9% NaCl), H2O2, BSA, protease inhibitor cocktail, and cycloheximide (CHX) (dissolved in DMSO) were obtained from Sigma (St. Louis, MO). Endothelial cell growth supplement (ECGS) was from Millipore (02-102) (Billerica, MA).
Animals.
All protocols in this study were approved by the Animal Care and Use Committee of the Johns Hopkins Medical Institutions. Wild-type C57Bl/6 mice were obtained at 6–8 wk from Jackson Laboratories (Bar Harbor, ME). PKGI−/− mice were a generous gift from Dr. Alexander Pfeifer (Univ. of Bonn, Bonn, Germany). PKGI−/− mice lacked part of the exon in PKGI that encodes the ATP binding site necessary for catalytic activity (45). Mice were maintained by the Division of Comparative Medicine of the Johns Hopkins University. Mice were provided food and water ad libitum.
Genotyping.
Genomic DNA was extracted from tail snips and subjected to PCR using three primers to identify PKGI−/− mice. The primers for the wild-type allele, 5′-GCTCTACTCGTCCGAAACCT and 5′-CTGCCACTTCTGATAAATACTGAT, were combined with a neomycin-specific primer 5′-GCCTGCTCTTTACTGAAGGCTCT to identify wild-type (500 bp) and targeted (750 bp) fragments. After PCR amplification, the fragments were separated by gel electrophoresis using a 2% agarose gel with ethidium bromide.
MLMVEC isolation and culture.
MLMVEC were isolated and purified as previously described (38) with modifications. Briefly, mice were killed by cervical dislocation, a thoracotomy was performed, and the lungs were removed. Large airways were excluded, and the lung was rinsed with DMEM (Invitrogen, Carlsbad, CA), minced, and digested in 1 ml of collagenase (1 mg/ml; Sigma, St. Louis, MO) at 37°C for 20 min. The digest was filtered through sterile mesh and centrifuged (400 g for 7 min). The pellet was resuspended in DMEM supplemented with 20% FBS, 150 μg/ml ECGS, 100 μg/ml penicillin/streptomycin, and 0.25 μg/ml amphotericin B, and placed in a 0.1% gelatin-coated T-25 flask. After reaching confluence, the cells were stained overnight with acetylated LDL/Alexa Fluor 488 conjugate (Molecular Probes/Invitrogen L23380) and sorted into a purified endothelial population using a FACS ARIA (Becton Dickinson, Franklin Lakes, NJ). Endothelial phenotype was confirmed by observing for typical cobblestone morphology and immunostaining for platelet endothelial cell adhesion molecule and von Willebrand factor. All experiments were performed with cells between passages 2 and 10.
Adenoviral infection.
A recombinant adenovirus containing the full-length cDNA for human PKGIβ with a GFP expression cassette (Ad.PKGI) was obtained from Dr. K. D. Bloch (Harvard Univ.) as well as a control adenovirus containing the cDNA for β-galactosidase and GFP (Ad.βGal). Human pulmonary artery endothelial cells (HPAECs) were grown to 80% confluence in six-well plates, the media was removed, and complete media containing 1 μl/ml of adenovirus was added and incubated for 60 min as described (39). An equal volume of complete media without virus was then added, and the cells were incubated overnight at 37°C with 5% CO2.
Cell death assessment.
Cell death was determined after exposure to H2O2 by fluorescence microscopy and evaluation for apoptotic nuclear morphology as previously described (14). Briefly, MLMVEC were treated with 8pCPT-cGMP (50 μM), ANP (10 nM), or diluent for 2 h and then serum-starved for an additional 2 h in the presence of 8pCPT-cGMP or diluent for a total of 4 h of 8pCPT-cGMP treatment. We chose 50 μM 8pCPT-cGMP because this concentration was shown to activate PKGI and not protein kinase A (39). The concentration of ANP (10 nM) was chosen because we previously measured a peak circulating plasma ANP concentration of 4 ± 2 nM in a group of six intact mice following unilateral IR injury (16).
The cells were incubated with H2O2 for 1 h followed by a change to complete media with 8pCPT-cGMP or diluent. After 18 h, cells were stained with Hoechst and propidium iodide (PI) and examined with an Olympus IX 51 inverted fluorescence microscope for condensed and fragmented nuclear morphology consistent with apoptosis. Three fields of ∼500 cells were photographed per experimental condition and counted in a blinded fashion with the outcome expressed as apoptotic cells as a percentage of total cells.
Additional experiments were performed in HPAEC monolayers, which do not express PKGI in culture (39, 49). HPAEC monolayers were infected with Ad.PKGI or Ad.βGal before treatment with 8pCPT-cGMP for 30 min or 48 h. The infected monolayers were then exposed to 100 μM H2O2 for 1 h. After 18 h, the cells were stained with Hoechst and PI and evaluated for condensed nuclei as described above.
H2O2 electrode.
Real-time H2O2 concentrations were measured with an H2O2 electrode system (Apollo 4000 Free Radical Analyzer; World Precision Instruments, Sarasota, FL). The ISO-HPO-2 electrode was mounted in a multiport water-jacketed sample chamber (NOCHM-4 Four-Port Closed Chamber, World Precision Instruments). MLMVEC from one well of a six-well plate were treated with 8pCPT-cGMP (50 μM) or ANP (10 nM) for 2 or 4 h, washed twice with PBS, collected with trypsin, centrifuged, and resuspended in 2 ml of serum-free DMEM. The cell suspension was placed into the sample chamber, which was warmed to 37°C and continuously stirred with a magnetic stir bar. At intervals, H2O2 was added to achieve a specified predicted concentration in the cell suspension while continuously monitoring temperature and H2O2-induced current. The H2O2 signal was allowed to completely decay before the next concentration of H2O2 was added. The data were saved on a personal computer.
Measurement of intracellular ROS.
Intracellular ROS were measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, Molecular Probes D399). MLMVEC monolayers were treated with 50 μM 8pCPT-cGMP or diluent for 4 h. H2DCFDA was dissolved in 100% ethanol, and cells were loaded with 20 μM H2DCFDA for 45 min in the dark at 37°C. After the loading period, cells were washed twice with PBS and placed in serum-free DMEM. H2O2 was added to the desired concentration. Ten minutes after addition of H2O2, the cells were visualized using an Olympus IX 51 inverted fluorescence microscope and photographed using a Cooke digital camera using an exposure time of 254 ms. Quantification of the fluorescence intensity was performed using ImagePro 5.0 software (MediaCybernetics, Silver Spring, MD).
Measurement of cGMP.
MLMVEC monolayers were exposed to ANP (10 nM) for 20 min in serum-free media before adding 1 ml of cold 0.1 M HCl. Both cells and supernatant were harvested and stored at −80°C. Samples were then processed for cGMP determination by ELISA (Assay Designs, Ann Arbor, MI) according to the manufacturer's directions. Total cGMP (pmol) was expressed per mg protein (BCA assay; Pierce Chemical, Rockford, IL).
Gel electrophoresis and immunoblot analysis.
Confluent monolayers of MLMVEC were harvested, and cell lysates were prepared as previously described (39). Densitometric analysis was performed using Un-Scan-It Gel Automated Digitizing System software, version 5.1 (Silk Scientific, Orem, UT).
SuperArray assay.
Confluent monolayers of MLMVEC in six-well plates were treated with 8pCPT-cGMP (50 μM) or diluents for 4 h and harvested into 1 ml of TRIzol reagent. mRNA was then purified by the Johns Hopkins University Lowe Family Genomics Core and assayed using the PAMM-065 Mouse Oxidative Stress and Antioxidant Defense PCR SuperArray (SuperArray Biosciences, Frederick, MD), a targeted cDNA array of 84 oxidative stress genes.
Quantitative real-time RT-PCR.
MLMVEC were rinsed once with ice-cold PBS, treated with TRIzol reagent (Invitrogen, Carlsbad, CA), sonicated, and purified using the RNeasy Mini Kit (QIAGEN, Valencia, CA). RNA yield was calculated using spectrophotometry (NanoDrop, Wilmington, DE) and purity assessed by A260/A280 ratio. Gene-specific primers were designed from mouse catalase mRNA (acc. no. NM_009804), Gpx1 mRNA (NM_008160), and GAPDH mRNA (NM_008084). The primers used for catalase were 5′-TATTGCCGTTCGATTCTCCACAGT-3′ and 5′-TTTCCCACAAGATCCCAGTTACCA-3′. The Gpx-1 primers were 5′-TGCAATCAGTTCGGACACCAG-3′ and 5′-CATTCACTTCGCACTTCTCAAACA-3′. The primers for GAPDH were 5′-CTCATGACCACAGTCCATGC-3′ and 3′-ACATTGGGGTTAGGAACACG-5′.
RNA (0.5 μg) from each sample was converted to cDNA (after genomic DNA wipeout) using QuantiTect Reverse Transcription kit (Qiagen). qPCR reactions were performed with QuantiTect SYBR Green PCR Master Mix (Qiagen) using 1 μl of cDNA as the template in each 25-μl reaction mixture. PCR assays were performed with a Chrom4 thermal cycler system (MJ Research, Waltham, MA). Cycling conditions were: initial enzyme inactivation at 95°C for 15 min, followed by 40 cycles at 95°C for 20 s, 57°C for 30 s, and 72°C for 40 s. Using the same protocol, we generated standard curves from serial dilutions of purified PCR products, which allowed quantification of the specific mRNA of interest. The threshold cycle value for each sample was used to calculate the initial quantity of cDNA template by the standard curve method. We also normalized data from each sample by dividing the quantity of target gene cDNA by the quantity of GAPDH cDNA to correct for variability in individual samples. Parallel reactions were run using RNA sample as template to assess the degree of contaminating genomic DNA. Negative control reactions without template were also performed.
Isolated mouse lungs.
The in situ isolated perfused mouse lung preparation was performed as previously described (16, 51). Briefly, mice were anesthetized with intraperitoneal ketamine (150 mg/kg) and acetylpromazine (15 mg/kg), and a tracheostomy was performed. The mouse was ventilated at 6 ml/kg tidal volume at a rate of 120 breaths/min (MiniVent Mouse Ventilator 845, Harvard Apparatus) with 3 mmHg end-expiratory pressure. A sternotomy was performed, and cannuli were inserted into the pulmonary artery and left heart. Inspired gas was changed to 21% O2 and 5% CO2. Lung perfusion was then begun with warmed BMOC-3 supplemented with 3% BSA in an open circuit. Flow was increased to 2.5 ml/min, and the lungs were flushed for 5 min to remove blood before establishing recirculation. Left atrial pressure was maintained at 6 mmHg. Either 8pCPT-cGMP (50 μM) or diluent was added, and the lungs were perfused for 2 h. In the first set of experiments, at 2 h, 5 mM H2O2 was added to the perfusate, and the lungs were perfused for an additional 1 h. Perfusate samples were taken at 0, 15, 30, and 60 min after H2O2 addition for the determination of LDH activity. In a second set of experiments, perfusion was stopped, and the lungs were ventilated for 10 min with 100% N2. Ventilation was then stopped, and the lungs were allowed to deflate. After an additional 50 min, ventilation was resumed with 21% O2, 5% CO2, and reperfusion initiated. Samples of perfusate were taken at 0, 15, 30, and 60 min of reperfusion and assayed for LDH. Vascular and airway pressures were monitored and recorded (PowerLab 4/30 computer system; ADInstruments, Colorado Springs, CO). In a third set of experiments, either 8pCPT-cGMP or diluent was added to the perfusate as described above, and the lungs were perfused for 2 h before rapidly excising and snap-freezing the lungs in liquid nitrogen for Western analysis of catalase and Gpx-1 as described previously (16, 51).
LDH assay.
LDH activity was measured in perfusate samples using the Promega CytoTox96 Non-Radioactivity Cytotoxicity Assay (Promega, Madison, WI) according to the manufacturer's instructions.
Statistics.
All time course measurements or other data incorporating two factors were analyzed using a two-factor ANOVA with either one or two repeated measures. Other data were analyzed using the appropriate Student's t-test for comparisons between two groups or a randomized one-factor ANOVA with least significant difference post hoc testing to compare mean values from multiple treatment groups. Non-normal data were logarithmically converted before analysis. Values presented in text are means ± SE. Differences were considered significant when P ≤ 0.05.
RESULTS
Effect of 8pCPT-cGMP or ANP on H2O2-induced in vitro MLMVEC death and isolated lung perfusate LDH.
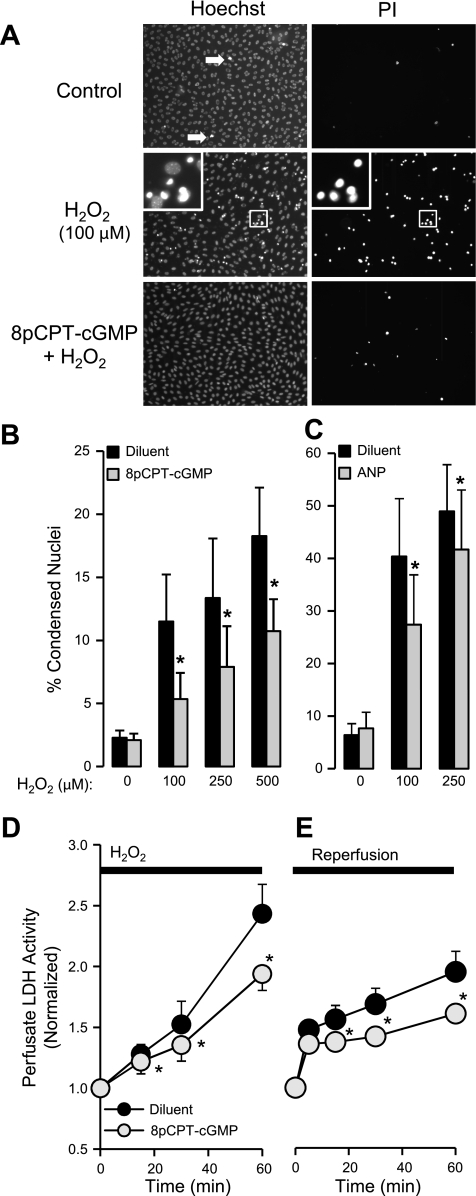
Increasing concentrations of H2O2 significantly increased the percentage of condensed and fragmented nuclei (Fig. 1A) from a basal value of 2.3 ± 0.6% to 18.3 ± 3.9% following exposure to 500 μM H2O2 (Fig. 1B). Pretreatment with 8pCPT-cGMP (50 μM) for either 2 or 4 h significantly (P < 0.05, n = 5–10) attenuated the percentage of cells with condensed nuclei across the whole range of H2O2 concentration by an average of 45%. Because the results from the two 8pCPT-cGMP exposure times were identical, the 8pCPT-cGMP data shown in Fig. 1B are the combined results from 2 and 4 h. Most of the PI-positive cells revealed PI-positive staining of condensed fragmented nuclei suggesting late apoptotic cells. A smaller population of PI-positive cells was enlarged with cytoplasmic staining suggesting necrosis. These cells also appeared to be decreased by 8pCPT-cGMP, but their numbers were too small to quantify. As suggested by Fig. 1A and quantified in separate experiments, 8pCPT-cGMP had no effect on cell proliferation over 4 h, indicating that the results shown in Fig. 1B were not due to a change in total cell numbers (3.2 × 105 vs. 2.9 × 105 cells/ml in diluent and 4 h 8pCPT-cGMP groups, respectively; n = 4).
Fig. 1.
A: mouse lung microvascular (MLMVEC) monolayers stained with Hoechst and propidium iodide (PI) after being exposed to diluent (control), diluent and H2O2 (100 μM), or 8pCPT-cGMP (50 μM) and H2O2. 8pCPT-cGMP or diluent were present for 4 h before the addition of H2O2. The images were obtained 18 h later. White arrows, condensed fragmented nuclei. The magnified image contrasts normal nuclear morphology from the condensed forms. Effect of 8pCPT-cGMP (50 μM; n = 5–10) (B) or ANP (10 nM; n = 4) (C) on the dose-response relationship between increasing concentrations of H2O2 and the percentage of condensed MLMVEC nuclei. Values are means ± SE. *P < 0.05 vs. diluent-treated cells. D: effect of 8pCPT-cGMP (50 μM) on the time course of perfusate LDH activity in isolated mouse lungs subjected to H2O2 (5 mM) or diluent 2 h later (n = 4). Values are means ± SE. *P < 0.005 vs. control lungs. E: effect of 8pCPT-cGMP (50 μM) on the time course of perfusate LDH activity in isolated mouse lungs subjected to 60 min of hypoxic unventilated ischemia followed by 60 min of normoxic reperfusion (n = 6). Values are means ± SE. *P < 0.05 vs. control lungs.
To determine if an increase in endogenous endothelial cGMP would produce a similar effect to 8pCPT-cGMP, we also examined the effect of ANP (10 nM) pretreatment on H2O2 cell death in MLMVEC monolayers. Figure 1C shows that 4 h of ANP pretreatment conferred a significant protective effect decreasing the percentage of condensed fragmented nuclei following 100 μM H2O2 by an average of 39% (P < 0.05 ANOVA interaction, n = 4). Of note, this protective effect of ANP occurred despite the fact that these MLMVEC monolayer experiments exhibited an enhanced sensitivity to H2O2-induced cell death compared with the 8pCPT-cGMP experiments shown in Fig. 1A. We evaluated the effect of ANP on cGMP production in MLMVEC in separate experiments. Specifically, 10 nM ANP increased MLMVEC cGMP by 40% after 20 min compared with diluent-treated cells (0.52 ± 0.07 vs. 0.37 ± 0.05 pmol/mg cGMP, respectively; n = 10, P < 0.05).
We next examined whether pretreatment with 8pCPT-cGMP would protect against oxidant injury in the intact lung. Isolated mouse lungs were perfused with 50 μM 8pCPT-cGMP for 2 h before adding 5 mM H2O2 and continuing for an additional hour. We monitored LDH release into the perfusate as an indication of endothelial cytotoxicity. In preliminary experiments, we determined that 5 mM H2O2 was necessary to observe a significant increase in perfusate LDH (data not shown). As shown in Fig. 1D, significantly less LDH activity was detected in the perfusate in 8pCPT-cGMP-treated lungs at all time points (P < 0.005, n = 4–5), consistent with decreased endothelial cytotoxicity. To determine if 8pCPT-cGMP would also prevent cytotoxicity from endogenous ROS, we examined the effect of 8pCPT-cGMP on lung IR injury. This injury is known to involve the generation of endogenous ROS (1) and significant endothelial cytotoxicity (58). To accentuate ROS production and cytotoxicity, we superimposed hypoxia-reoxygenation (17, 58) on the IR injury (IR/HR). Similar to the H2O2 results, pretreatment with 8pCPT-cGMP significantly decreased perfusate LDH activity from 15–60 min of reperfusion (P < 0.05, n = 6).
Role of PKGI in cGMP-mediated attenuation of H2O2 endothelial cell death.
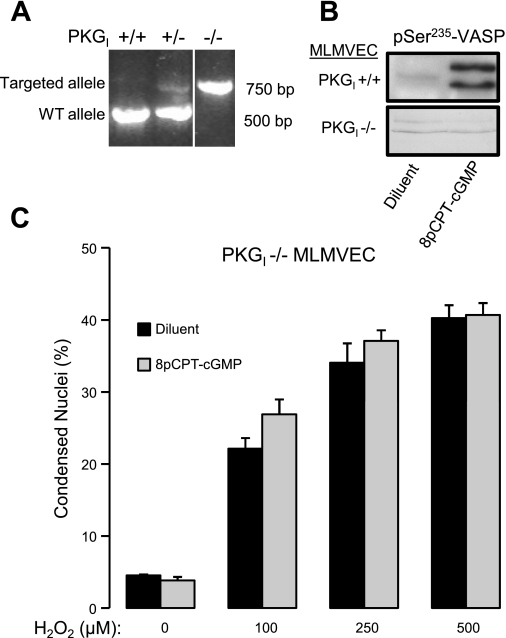
PKGI−/− mice were identified by the presence of a single 750-bp band expected for the targeted PKGI allele (45) (Fig. 2A). The lack of PKGI enzymatic activity in MLMVEC isolated from PKGI−/− mice was confirmed by showing that, unlike wild-type MLMVEC, PKGI−/− MLMVEC demonstrated no phosphorylation of VASP on Ser235 (Fig. 2B), the PKGI preferred phosphorylation site (39). As shown in Fig. 2C, MLMVEC isolated from PKGI−/− did not show protection from H2O2-induced cell death following treatment with 8pCPT-cGMP for 4 h (P = not significant, n = 4).
Fig. 2.
A: ethidium bromide staining of PCR fragments used to identify PKGI−/− mice. The order of lanes from the same gel has been rearranged (indicated by the white line between lanes). B: Western blot of vasodilator-stimulated phosphoprotein (VASP) phosphorylated on Ser235 in PKGI+/+ and PKGI−/− exposed to diluent or 8pCPT-cGMP (50 μM). C: effect of 8pCPT-cGMP (50 μM) on the dose-response relationship between increasing concentrations of H2O2 and the percentage of condensed MLMVEC nuclei (n = 4). Values are means ± SE. P = not significant.
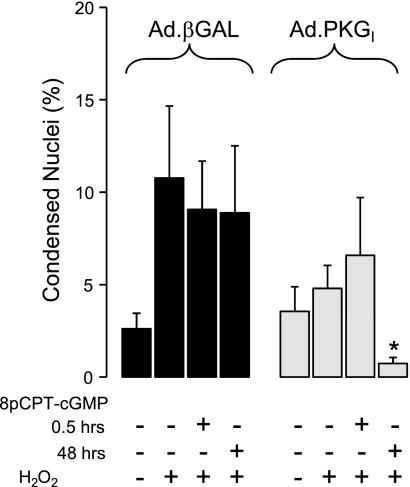
To provide additional confirmation that PKGI is required for cGMP-mediated protection against H2O2-induced cell death, we performed a gain-of-function experiment utilizing cultured HPAEC. We previously demonstrated that HPAEC lack PKGI expression and activity in vitro (39). We also showed in these cells that introduction of wild-type human PKGI with Ad.PKGI infection restored PKGI expression and VASP phosphorylation at Ser239 (39, 49). Figure 3 shows that incubation of Ad.PKGI-infected HPAEC monolayers with 8pCPT-cGMP for 48 h but not 30 min, resulted in significant protection from H2O2-induced cell death assessed 18 h after H2O2 administration (P < 0.05, n = 8). Of note, this PKGI construct has significant constitutive activity (9), likely explaining why the effect of H2O2 on cell death was attenuated in Ad.PKGI-infected cells even in the absence of 8pCPT-cGMP activation. The infection efficiency was >95% as determined by GFP expression (data not shown). 8pCPT-cGMP had no effect at either exposure time in HPAECs infected with the control adenovirus.
Fig. 3.
Effect of Ad.PKGI infection or infection with the control Ad.βGAL virus in HPAEC on the % condensed nuclei resulting from the administration of H2O2 (250 μM). Some monolayers were pretreated with 8pCPT-cGMP (50 μM) for either 0.5 or 48 h before H2O2 administration (n = 8). Values are means ± SE. *P < 0.05 vs. corresponding Ad.βGAL-infected group.
Effect of 8pCPT-cGMP and PKGI on H2O2 scavenging by MLMVECs.
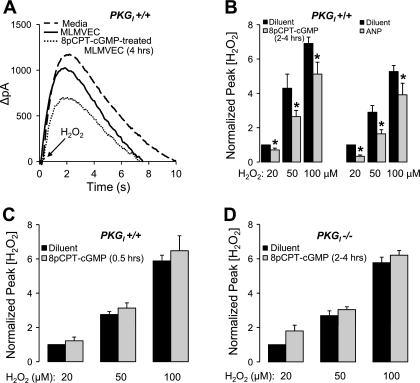
Figure 4A shows the time course of ΔpA generated by the H2O2 electrode from a single experiment in which 100 μM H2O2 was rapidly added to the measurement chamber in the presence of media alone, wild-type diluent-treated MLMVEC, or wild-type MLMVEC pretreated with 8pCPT-cGMP for 4 h. The presence of MLMVEC attenuated the peak ΔpA compared with media alone demonstrating the antioxidant activity of the MLMVECs. Because the downslopes of the curves did not differ as a function of 8pCPT-cGMP treatment, we quantified the effect of 8pCPT-cGMP by the peak pA value. 8pCPT-cGMP pretreatment for 2 or 4 h produced the same average decrease in the peak ΔpA, so the data from these groups were combined (Fig. 4B). 8pCPT-cGMP significantly reduced the measured peak H2O2 concentration across a range of added H2O2 concentrations by an average of 29 ± 6% (P < 0.05, n = 8). Figure 4B also shows a significant inhibition of ΔpA (43 ± 0.07%) following 4 h of pretreatment with ANP (10 nM) before exposure to the same H2O2 concentrations (P < 0.05, n = 5). The effect of 8pCPT-cGMP on ΔpA was lost in wild-type MLMVEC if the incubation period was shortened to 30 min (Fig. 4C). There was also no effect of 8pCPT-cGMP in PKGI −/− MLMVECs (Fig. 4D) despite the same incubation times utilized in Fig. 4B.
Fig. 4.
A: Time course of ΔpA generated by the H2O2 electrode from a single experiment in which 100 μM H2O2 was rapidly added to the measurement chamber in the presence of media alone, wild-type diluent-treated MLMVEC, or wild-type MLMVEC pretreated with 8pCPT-cGMP (50 μM) for 4 h. B: effect of 8pCPT-cGMP (50 μM; n = 8) incubated for 2–4 h or ANP (10 nM; n = 5) incubated for 4 h, on the dose-response relationship between increasing added concentrations of H2O2 and the peak ΔpA normalized to the 20 μM H2O2 control value in MLMVEC. Values are means ± SE. *P < 0.05 vs. diluent-treated cells. C: effect of incubating with 8pCPT-cGMP (50 μM) for only 0.5 h on the dose-response relationship between increasing concentrations of H2O2 and the peak ΔpA normalized to the 20 μM H2O2 control value in MLMVEC (n = 4). Values are means ± SE. P = not significant. D: effect of 8pCPT-cGMP (50 μM) incubation for 2–4 h on the dose-response relationship between increasing added concentrations of H2O2 and the peak ΔpA normalized to the 20 μM H2O2 control value in PKGI−/− MLMVECs (n = 4). Values are means ± SE. P = not significant.
To examine the possibility that the cGMP-treated cells were actively secreting an antioxidant substance, we repeated the protocol using the same volume of cell-free conditioned media from wild-type MLMVEC exposed to 4 h of 8pCPT-cGMP that was present in Fig. 4B and found no effect on the ΔpA resulting from 20, 50, or 100 mM H2O2 (P > 0.05, n = 5; data not shown). We also determined that 8pCPT-cGMP had no effect on cell proliferation over 4 h (3.2 × 105 vs. 2.9 × 105 cells/ml in diluent and 8pCPT-cGMP groups, respectively; n = 4) indicating that the results shown in Fig. 4B were not due to an increase in cell number.
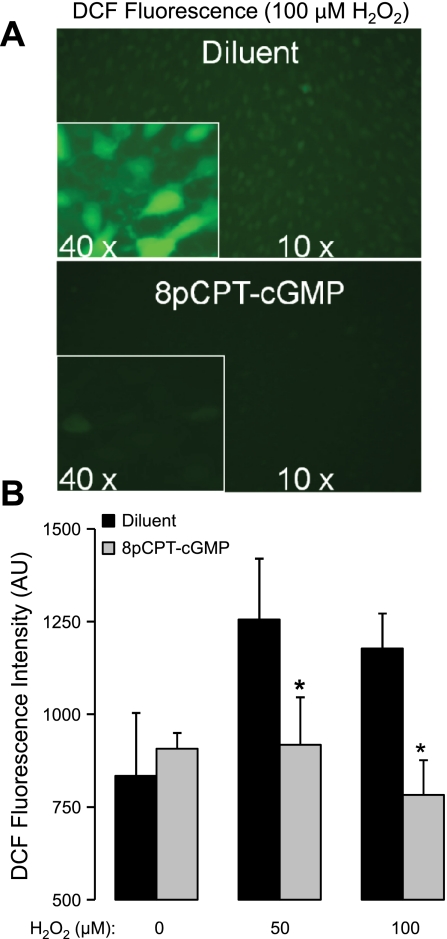
To more directly determine if the cGMP/PKGI-dependent effect on extracellular H2O2 concentration shown in Fig. 4B occurred because of increased intracellular scavenging, we measured MLMVEC DCF fluorescence as an index of intracellular H2O2 concentration. In cellular systems, DCF fluorescence is a sensitive probe for the presence of H2O2 (in the presence of peroxidase activity), peroxynitrite anions, or hydroxyl radicals (22, 41). Although DCF cannot easily distinguish between these species when ROS are endogenously generated, it is reasonable to assume that the fluorescence signal resulting from exogenous H2O2 administration should be dominated by the reaction with H2O2. As shown in Fig. 5, 4 h of 8pCPT-cGMP incubation significantly decreased the DCF fluorescence observed 10 min after adding H2O2 to the MLMVEC monolayers back to basal levels (P < 0.05, n = 5).
Fig. 5.
A: effect of pretreatment with 8pCPT-cGMP (50 μM) 4 h before adding H2O2 (100 μM) on 2′,7′-dichlorofluorescin (DCF) fluorescence in MLMVEC monolayers. Images were obtained 10 min after the addition of H2O2. The exposure time was identical for all images. B: effect of 8pCPT-cGMP (50 μM) incubation for 4 h on the dose-response relationship between increasing added concentrations of H2O2 and the DCF fluorescence intensity (n = 5). Values are means ± SE. *P < 0.05.
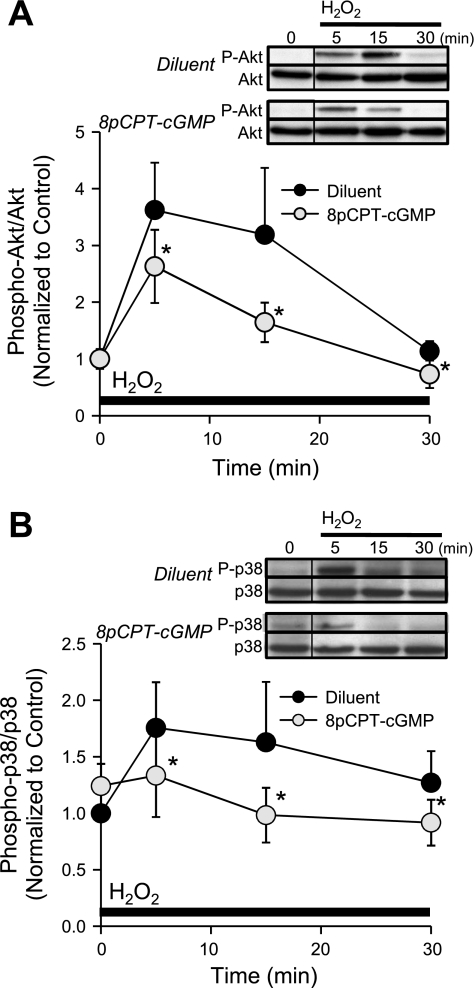
We reasoned that if cGMP signaling resulted in decreased intracellular H2O2 concentration, then intracellular signaling pathways known to be activated by H2O2 administration in endothelium should be attenuated. Figure 6 demonstrates that H2O2 (100 μM) significantly increased phosphorylation of Akt (Fig. 6A) and p38 MAPK (Fig. 6B) in MLMVEC monolayers during the first 30 min of H2O2 exposure. Pretreatment of MLMCEC with 8pCPT-cGMP for 4 h significantly attenuated (P < 0.05, n = 6) H2O2-induced phosphorylation of both kinases.
Fig. 6.
A: time course of phospho-Akt/total Akt (normalized to time 0) Western blot densitometry following the administration of H2O2 (100 μM) in MLMVEC pretreated with 8pCPT-cGMP (50 μM) or diluent 4 h before (n = 6). B: time course of phospho-p38 MAPK/total p38 MAPK (normalized to time 0) Western blot densitometry following the administration of H2O2 (100 μM) in MLMVEC pretreated with 8pCPT-cGMP (50 μM) or diluent 4 h before (n = 6). The order of lanes from the same gel has been rearranged (indicated by the black line between lanes). Values are means ± SE. *P < 0.05 vs. diluent.
Effect of 8pCPT-cGMP on antioxidant enzyme expression.
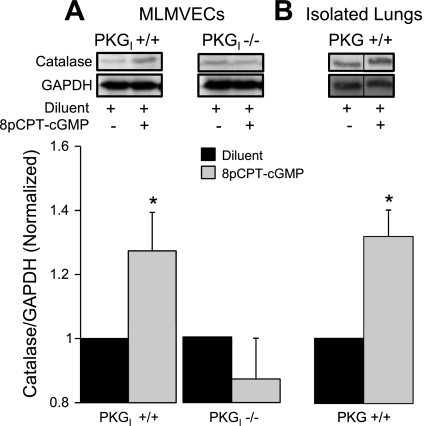
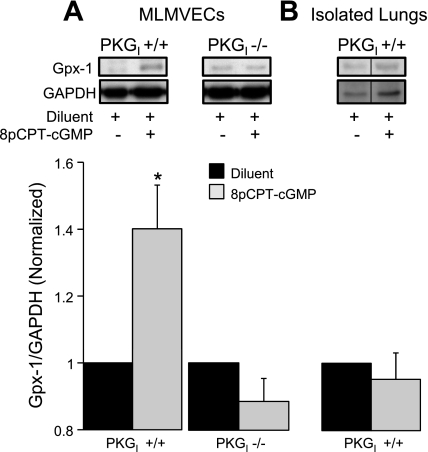
In wild-type MLMVECs, the salutary effect of 8pCPT-cGMP on H2O2 scavenging was accompanied by significant increases (P < 0.05, n = 8) in the expression of both catalase (Fig. 7A) and Gpx-1 (Fig. 8A), the major H2O2 detoxifying enzymes. We found no effect of 8pCPT-cGMP on Trx-1 (P = not significant, n = 15) or MnSOD (P = not significant, n = 12) expression (data not shown). Consistent with the critical role for PKGI in 8pCPT-cGMP-mediated protection from H2O2 cell death and enhanced H2O2 scavenging, 8pCPT-cGMP had no effect on either catalase or Gpx-1 expression in PKGI−/− MLMVECs (Figs. 7A and 8A; P = not significant, n = 7). There was a statistically significant increase (31 ± 8%) in catalase in wild-type mouse lung homogenate following 2 h of 8pCPT-cGMP (50 μM) exposure (Fig. 7B; P < 0.05, n = 3) that was quantitatively similar to the increase in catalase observed in PKGI+/+ MLMVECs following 8pCPT-cGMP treatment. Lung Gpx-1 expression was not altered (Fig. 8B; P = not significant, n = 3).
Fig. 7.
A: effect of 4-h treatment with 8pCPT-cGMP (50 μM) or diluent on catalase protein expression (normalized to GAPDH expression and diluent control) in PKGI+/+ (n = 8) and PKGI−/− (n = 7) MLMVEC monolayers. B: effect of 2-h treatment with 8pCPT-cGMP (50 μM) or diluent on lung catalase protein expression (normalized to GAPDH expression and diluent control) in isolated perfused wild-type mouse lungs (n = 3). The order of lanes from the same gel has been rearranged (indicated by the black line between lanes). Values are means ± SE. *P < 0.05 vs. diluent.
Fig. 8.
A: effect of 4-h treatment with 8pCPT-cGMP (50 μM) or diluent on Gpx-1 protein expression (normalized to GAPDH expression and diluent control) in PKGI+/+ (n = 8) and PKGI−/− (n = 7) MLMVEC monolayers. B: effect of 2-h treatment with 8pCPT-cGMP (50 μM) or diluent on lung Gpx-1 protein expression (normalized to GAPDH expression and diluent control) in isolated perfused wild-type mouse lungs (n = 3). The order of lanes from the same gel has been rearranged (indicated by the black line between lanes). Values are means ± SE. *P < 0.05 vs. diluent.
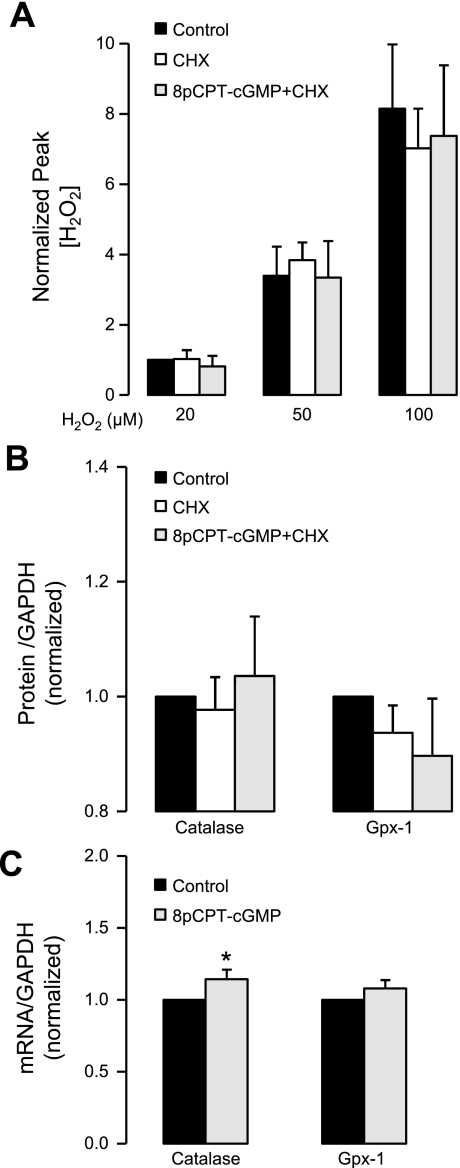
Treatment with cycloheximide to block protein synthesis prevented the increased H2O2 scavenging and antioxidant enzyme expression in MLMVEC monolayers pretreated with 8pCPT-cGMP (Fig. 9, A and B; P > 0.05, n = 4). Real-time RT-PCR revealed a statistically significant increase in catalase (Fig. 9C; P < 0.05) but not Gpx-1 mRNA in MLMVEC (n = 5) following 4 h of exposure to 8pCPT-cGMP, although the magnitude of this effect was less than onefold. Similarly, we found no significant 8pCPT-cGMP-induced changes in any of the antioxidant enzymes assayed in the commercially available PCR array (data not shown).
Fig. 9.
A: effect of diluent, cycloheximide (CHX), and CHX + 8pCPT-cGMP (50 μM) incubated for 4 h on the dose-response relationship between increasing added concentrations of H2O2 and the peak ΔpA (by H2O2 electrode) normalized to the 20 μM H2O2 control value in MLMVEC (n = 4). Values are means ± SE. P = not significant. B: effect of diluent, CHX, and CHX + 8pCPT-cGMP (50 μM) incubated for 4 h on catalase and Gpx-1 protein expression (normalized to GAPDH expression and diluent control) in MLMVEC (n = 4). Values are means ± SE. P = not significant. C: effect of diluent or 8pCPT-cGMP (50 μM) incubated for 4 h on catalase and Gpx-1 mRNA (normalized to GAPDH and diluent control) in MLMVEC (n = 5). Values are means ± SE. *P < 0.05 vs. control.
DISCUSSION
The major finding of this study was that treatments designed to increase intracellular cGMP in MLMVECs attenuated cell death from H2O2. This protective effect was associated with enhanced scavenging of extracellular H2O2 and increased expression of MLMVEC catalase and Gpx-1, the major antioxidant proteins responsible for eliminating H2O2 (29). All of these effects required PKGI expression and at least 2 h of cGMP stimulation suggesting a gene regulatory function rather than a simple PKGI-mediated phosphorylation. This differed from the cGMP/PKGI-mediated endothelial barrier protection that we previously observed in bovine LMVEC (44) and HPAEC (39) in that the barrier protection occurred after <1 h of 8pCPT-cGMP exposure.
The effect of cGMP signaling on ROS-induced endothelial cytotoxicity remains controversial with reports suggesting both pro- and anticytotoxic effects. For example, Polte et al. (48) showed that a cell-permeable cGMP analog blocked cytotoxicity from TNFα in bovine pulmonary artery endothelial cells by an unknown mechanism. Conversely, ANP or 8-bromo-cGMP was found to induce apoptosis in rat systemic endothelial cells in association with an increase in the tumor suppressor gene product p53 (54). In rat LMVEC (RLMVEC), 8-bromo-cGMP failed to attenuate 51Cr release from exogenous H2O2 administration (8), although the cells were exposed to cGMP for only 30 min. We also found no protective effects from 30 min of 8pCPT-cGMP exposure (Figs. 3 and 4C).
More recently, ANP increased apoptosis and inhibited proliferation in RLMVEC in association with a sustained increase in cGMP (60). These results may differ from ours because of species differences between rat and mouse endothelial cells. Alternatively, there could be a difference in PKGI expression in vitro. We previously showed that pulmonary endothelial PKGI expression can be lost in vitro as a function of endothelial type and passage (39). PKGI expression was not determined in the studies demonstrating a lack of cGMP-mediated protection (8, 54, 60). A recent study in retinal cells (52) found that cGMP triggered apoptosis through the direct activation of a cyclic nucleotide-gated cation channel thus demonstrating a PKGI-independent, proapoptotic cGMP effect. In contrast, our results in PKGI−/− MLMVECs (Fig. 2) and HPAECs infected with Ad.PKGI to restore PKGI expression (Fig. 3) demonstrate the absolute requirement of PKGI expression for cGMP-mediated protection and enhanced H2O2 scavenging (Fig. 4D).
Our results are compatible with previous work in other cell types and organs that demonstrated cytoprotective effects of cGMP/PKGI signaling. For example, antiapoptotic effects have been shown in cultured astrocytes (55), cardiomyoctes (15), neurons (12), and neuroblastoma cells (3, 23). The mechanisms for these protective effects remain poorly understood, but cGMP/PKGI signaling was associated with increased Akt activation (15, 23) and Bcl-2 expression (3, 12, 15) as well as inhibition of the mitochondria permeability transition pore and cytochrome c release (23, 55).
The apparent mechanism of cGMP/PKGI protection in MLMVEC in the current study differed from these previous publications, however, because we observed significant H2O2 scavenging (Fig. 4) associated with decreased intracellular ROS levels (Fig. 5) and less Akt phosphorylation (Fig. 6A). These results suggested that we were attenuating the H2O2 signaling cascade upstream, at the level of the intracellular H2O2 concentration through an increase in cellular antioxidant defenses. The elimination of extracellular H2O2 because of intracellular uptake occurred in seconds, whereas the increase in intracellular H2O2 concentration and H2O2-induced signaling were observed over the first 30 min following H2O2 administration. Although we did not quantify the time course of intracellular H2O2 concentration, our data suggest that seconds to minutes of a critical increase in intracellular H2O2 was sufficient to trigger the apoptotic cascade. We cannot completely exclude an additional protective effect by an upregulation of an antiapoptotic protein or a downregulation of a proapoptotic protein, although the PCR array results did not detect cGMP-mediated mRNA changes in any of the major apoptotic regulatory proteins (data not shown). Our results could help to explain the decrease in luminol-enhanced chemiluminescence observed in bleomycin-injured lungs from mice that were pretreated with sildenafil to enhance cGMP/PKGI signaling (26). Although catalase and Gpx-1 were not measured in that study, it was previously demonstrated that bleomycin lung injury is attenuated by liposomal catalase administration suggesting an injurious role for endogenous H2O2 (33). Our conclusion that the cGMP protective effect was upstream of the apoptotic cascade was also consistent with the significant cGMP-mediated decrease in MLMVEC PI uptake (Fig. 1A) and isolated mouse lung LDH release following H2O2 or IR/HR exposure (Fig. 1, D and E).
We have not ruled out an additional protective effect of the inhibition of p38 MAPK phosphorylation (Fig. 6B). The p38 MAPK signaling cascade is known to be activated by ROS and trigger apoptosis (28). Moreover, cGMP/PKGI signaling was shown to inhibit p38 MAPK phosphorylation in cardiac myocytes (21). Based on the similar level of inhibition of Akt activation, however, we suspect that the inhibitory effect on p38 MAPK phosphorylation in our study was the result of a decreased intracellular H2O2 concentration. Additional experiments are planned to determine the mechanism and contribution of p38 inhibition.
We found increases in both catalase and Gpx-1 protein in 8pCPT-cGMP-treated wild-type but not PKGI−/− MLMVECs (Figs. 7–8). These are the primary enzymes responsible for intracellular H2O2 disposal in mammalian cells (4, 7, 29, 30, 37) and have also been shown to be responsible for most of the clearance of exogenously applied H2O2 (19, 20). In fact, an accepted assay of intracellular catalase activity is to measure the loss of a known concentration of H2O2 added to a cell homogenate utilizing the spectrophotometric absorbance for H2O2 (5, 10). Our method of monitoring exogenous H2O2 by electrode allowed us to use intact endothelial cells thus preserving the normal barriers and compartments between the extracellular H2O2 and intracellular antioxidant enzymes.
When we measured catalase and Gpx-1 protein expression in isolated mouse lungs exposed to either 8pCPT-cGMP or diluent for 2 h, we found a significant increase in catalase (Fig. 7B) but not Gpx-1 (Fig. 8B). There are two potential reasons for the lack of a change in Gpx-1 in the whole lung homogenate. First, the catalase and Gpx-1 expression changes in the cultured cells were measured following 4 h of 8pCPT-cGMP incubation, whereas the isolated lung experiments were performed after 2-h exposures. Thus, it is possible that the cGMP-mediated inhibition of cytotoxicity in both the cells and the intact lungs, which was present at 2 h, was primarily due to an increase in catalase expression. The Gpx-1 expression increase may have required 4 h and contributed less to H2O2 scavenging compared with catalase for the reasons outlined below. Alternatively, the Gpx-1 in other nonendothelial cell populations in the lung may have interfered with the ability to detect a quantitatively small change in endothelial Gpx-1 expression.
Gpx-1 is the major form of Gpx in the lung (6). The relative contributions of Gpx-1 and catalase to H2O2 detoxification in intact cells is poorly understood (29). Studies in erythrocytes suggest that Gpx-1 plays a more significant role at low H2O2 concentrations, whereas catalase becomes more important at higher H2O2 concentrations (13, 29). Additional antioxidant defenses directed toward H2O2 include the thioredoxins (Trx-1 and Trx-2) (24, 57) and Trx-dependent peroxidase (Tpx), which together function as an efficient antioxidant system for lower concentrations of H2O2 (50) and also function as inactivators of apoptosis signal-regulating kinase 1 (42, 57).
The relative importance of catalase, and by inference, H2O2 in ROS-mediated lung injury, was demonstrated by the significant attenuating effect of endothelial-targeted delivery of catalase in animal models of oxidant lung injury (11, 43). To our knowledge, our results are the first to show a cGMP/PKGI-mediated increase in catalase and Gpx-1 protein expression in mammalian cells. Although the absolute magnitude of the cGMP-induced changes in catalase and Gpx-1 protein expression was relatively modest, isolated changes in catalase expression of less than onefold in either direction have been shown to affect H2O2 detoxification and oxidant-mediated cell death (10, 25). Andoh et al. (3) found a cGMP/PKGI-induced increase in Trx-1, Tpx-1, and MnSOD in neuroblastoma cells that was associated with a decrease in lipid peroxidation in serum-starved cells suggesting a functional antioxidant effect upstream of the inhibition of apoptosis. However, there were no measurements of H2O2 detoxification or protein expression of catalase and Gpx-1. We did not find changes in protein expression of either Trx-1 or MnSOD in the current study (data not shown).
Activated PKGI is known to have both transcriptional (2, 3, 12, 15, 46) and posttranscriptional (55) mechanisms of gene regulation. Although inhibition of translation with cycloheximide abrogated both the increases in catalase and Gpx-1 protein and the increased scavenging of extracellular H2O2, we were unable to identify a physiologically significant increase in mRNA for either enzyme following PKGI activation by cGMP. These results suggest a posttranscriptional effect underlying the cGMP/PKGI-induced increase in catalase and Gpx-1 protein. One possibility is that activation of the cGMP/PKGI signaling pathway leads to increased stability of catalase and Gpx-1 protein, increasing cellular levels of protein, independent of mRNA.
Catalase activity and stability are regulated by the c-Abl and Arg tyrosine kinases (50). Increasing H2O2 concentrations activated c-Abl and Arg, resulting in catalase phosphorylation, which increases catalase activity. At more injurious H2O2 levels, c-Abl and Arg caused catalase ubiquitination and cellular apoptosis (50). Thus, cells deficient in c-Abl and Arg demonstrated increased catalase levels, decreased ROS concentrations, and decreased apoptosis. Activated c-Abl and Arg enhance Gpx-1 activity (50), but it is not known if they also regulate Gpx-1 protein stability. It is not known if PKGI has any effect on c-Abl or Arg expression or activation.
Our current and previous data highlight the complex effects of lung endothelial cGMP signaling in ROS-mediated lung injury. We previously found that increases in lung endothelial cGMP for <1 h enhanced basal endothelial barrier function by a PKGI-independent mechanism and then attenuated the barrier dysfunction caused by H2O2 by a mechanism that required PKGI (39, 44). In intact mouse models of ALI, we demonstrated that the timing and mechanism of the increase in cGMP were critical determinates of how the change in cGMP would affect the endothelial barrier. For example, we found that endogenous ANP released during in vivo lung IR contributed to barrier dysfunction in the reperfused lung (16). In a model of ventilator-induced lung injury (51), only cGMP generated by sGC in the midst of the injury proved to be barrier-harmful by a mechanism that appeared to be PKGI independent. Conversely, stimulation of sGC 10 min before the injury was protective, and ANP was always protective regardless of the timing of administration. The injurious effect of the later increase in cGMP from sGC was attributed to a simultaneous stretch-induced increase in phosphodiesterase 2A (PDE2A), a dual-function PDE that preferentially degrades cAMP when cGMP levels are high (51). Our current data add an additional effect of increasing cGMP before ROS-mediated lung injury, namely, a PKGI-dependent increase in endothelial antioxidant enzyme expression.
In conclusion, we found that increased cGMP conferred a cytoprotective effect on MLMVEC monolayers and the pulmonary endothelium of the intact mouse lung in association with 1) enhanced elimination of extracellular H2O2, 2) decreased intracellular ROS concentration, and 3) increased expression of catalase and Gpx-1 by a PKGI-dependent posttranscriptional mechanism. These effects may contribute to the known protection that results from preconditioning with treatments designed to increase intracellular cGMP levels in models of ROS-mediated lung injury (26, 27, 31, 47).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 1F32-HL-090199 (R. S. Stephens) and HL-075236 (D. B. Pearse), a Clinical Innovator Award from the Flight Attendants Medical Research Institute (D. B. Pearse), and a Grant-in-Aid from the Maryland Chapter of the American Heart Association (D. B. Pearse).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Al Mehdi AB, Zhao G, Dodia C, Tozawa K, Costa K, Muzykantov V, Ross C, Blecha F, Dinauer M, Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+. Circ Res 83: 730–737, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Andoh T, Chock PB, Chiueh CC. Preconditioning-mediated neuroprotection: role of nitric oxide, cGMP, and new protein expression. Ann NY Acad Sci 962: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Andoh T, Chiueh CC, Chock PB. Cyclic GMP-dependent protein kinase regulates the expression of thioredoxin and thioredoxin peroxidase-1 during hormesis in response to oxidative stress-induced apoptosis. J Biol Chem 278: 885–890, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Antunes F, Han D, Cadenas E. Relative contributions of heart mitochondria glutathione peroxidase and catalase to H(2)O(2) detoxification in in vivo conditions. Free Radic Biol Med 33: 1260–1267, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195: 133–140, 1952 [PubMed] [Google Scholar]
- 6.Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med 27: 951–965, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J 11: 745–757, 1998 [PubMed] [Google Scholar]
- 8.Chang J, Rao NV, Markewitz BA, Hoidal JR, Michael JR. Nitric oxide donor prevents hydrogen peroxide-mediated endothelial cell injury. Am J Physiol Lung Cell Mol Physiol 270: L931–L940, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Chiche JD, Schlutsmeyer SM, Bloch DB, de la Monte SM, Roberts JD, Jr, Filippov G, Janssens SP, Rosenzweig A, Bloch KD. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J Biol Chem 273: 34263–34271, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Choi SI, Kim TI, Kim KS, Kim BY, Ahn SY, Cho HJ, Lee HK, Cho HS, Kim EK. Decreased catalase expression and increased susceptibility to oxidative stress in primary cultured corneal fibroblasts from patients with granular corneal dystrophy type II. Am J Pathol 175: 248–261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, Ng K, Sweitzer T, Arguiri E, Shuvaev V, Solomides CC, Albelda SM, Muzykantov VR. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol 285: L283–L292, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Ciani E, Guidi S, Bartesaghi R, Contestabile A. Nitric oxide regulates cGMP-dependent cAMP-responsive element binding protein phosphorylation and Bcl-2 expression in cerebellar neurons: implication for a survival role of nitric oxide. J Neurochem 82: 1282–1289, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Cohen G, Hochstein P. Glutathione peroxidase: the primary agent for the elimination of hydrogen peroxide in erythrocytes. Biochemistry 2: 1420–1428, 1963 [DOI] [PubMed] [Google Scholar]
- 14.Damico RL, Chesley A, Johnston L, Bind EP, Amaro E, Nijmeh J, Karakas B, Welsh L, Pearse DB, Garcia JG, Crow MT. Macrophage migration inhibitory factor governs endothelial cell sensitivity to LPS-induced apoptosis. Am J Respir Cell Mol Biol 39: 77–85, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem 281: 38644–38652, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Dodd-o JM, Hristopoulos ML, Kibler K, Gutkowska J, Mukaddam-Daher S, Gonzalez A, Welsh-Servinsky LE, Pearse DB. The role of natriuretic peptide receptor-A signaling in unilateral lung ischemia-reperfusion injury in the intact mouse. Am J Physiol Lung Cell Mol Physiol 294: L714–L723, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Dodd-o JM, Welsh LE, Salazar JD, Walinsky PL, Peck EA, Shake JG, Caparrelli DJ, Ziegelstein RC, Zweier JL, Baumgartner WA, Pearse DB. Effect of NADPH oxidase inhibition on cardiopulmonary bypass-induced lung injury. Am J Physiol Heart Circ Physiol 287: H927–H936, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Dodd-o J, Pearse DB. Effect of the NADPH oxidase inhibitor apocynin on ischemia-reperfusion lung injury. Am J Physiol Heart Circ Physiol 279: H303–H312, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Dringen R, Hamprecht B. Involvement of glutathione peroxidase and catalase in the disposal of exogenous hydrogen peroxide by cultured astroglial cells. Brain Res 759: 67–75, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Dringen R, Kussmaul L, Hamprecht B. Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Protoc 2: 223–228, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Fiedler B, Feil R, Hofmann F, Willenbockel C, Drexler H, Smolenski A, Lohmann SM, Wollert KC. cGMP-dependent protein kinase type I inhibits TAB1-p38 mitogen-activated protein kinase apoptosis signaling in cardiac myocytes. J Biol Chem 281: 32831–32840, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Gomes A, Fernandes E, Lima JLFC. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65: 45–80, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Ha KS, Kim KM, Kwon YG, Bai SK, Nam WD, Yoo YM, Kim PKM, Chung HT, Billiar TR, Kim YM. Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. FASEB J 17: 1036–1047, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Haendeler J, Popp R, Goy C, Tischler V, Zeiher AM, Dimmeler S. Cathepsin D and H2O2 stimulate degradation of thioredoxin-1: implication for endothelial cell apoptosis. J Biol Chem 280: 42945–42951, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K, Itoh H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun 372: 51–56, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol 294: L24–L33, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Hillinger S, Sandera P, Carboni GL, Stammberger U, Zalunardo M, Schoedon G, Schmid RA. Survival and graft function in a large animal lung transplant model after 30 h preservation and substitution of the nitric oxide pathway. Eur J Cardiothorac Surg 20: 508–513, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275: 90–94, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Jones DP, Eklow L, Thor H, Orrenius S. Metabolism of hydrogen peroxide in isolated hepatocytes: relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2. Arch Biochem Biophys 210: 505–516, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Kirkman HN, Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci 32: 44–50, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Korom S, Hillinger S, Cardell M, Zhai W, Tan Q, Dutly A, Leskosek B, Weder W. Sildenafil extends survival and graft function in a large animal lung transplantation model. Eur J Cardiothorac Surg 29: 288–293, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Le A, Damico R, Damarla M, Boueiz A, Pae HH, Skirball J, Hasan E, Peng X, Chesley A, Crow MT, Reddy SP, Tuder RM, Hassoun PM. Alveolar cell apoptosis is dependent on p38 MAP kinase-mediated activation of xanthine oxidoreductase in ventilator-induced lung injury. J Appl Physiol 105: 1282–1290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledwozyw A. Protective effect of liposome-entrapped superoxide dismutase and catalase on bleomycin-induced lung injury in rats. I. Antioxidant enzyme activities and lipid peroxidation. Acta Vet Hung 39: 215–224, 1991 [PubMed] [Google Scholar]
- 34.Lee JC, Christie JD. Primary graft dysfunction. Proc Am Thorac Soc 6: 39–46, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Li Z, Ajdic J, Eigenthaler M, Du X. A predominant role for cAMP-dependent protein kinase in the cGMP-induced phosphorylation of vasodilator-stimulated phosphoprotein and platelet inhibition in humans. Blood 101: 4423–4429, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Luh SP, Yang PC. Organ preconditioning: the past, current status, and related lung studies. J Zhejiang Univ Sci B 7: 331–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marinho HS, Antunes F, Pinto RE. Role of glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase in the reduction of lysophospholipid hydroperoxides. Free Radic Biol Med 22: 871–883, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Moldobaeva A, Wagner EM. Difference in proangiogenic potential of systemic and pulmonary endothelium: role of CXCR2. Am J Physiol Lung Cell Mol Physiol 288: L1117–L1123, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Moldobaeva A, Welsh-Servinsky LE, Shimoda LA, Stephens RS, Verin AD, Tuder RM, Pearse DB. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290: L919–L930, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Munzel T, Feil R, Mulsch A, Lohmann SM, Hofmann F, Walter U. Physiology and pathophysiology of vascular signaling controlled by cyclic guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. Circulation 108: 2172–2183, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65: 1575–1582, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Nakamura H, Hoshino T, Ueda S, Wada H, Yodoi J. Redox regulation of lung inflammation by thioredoxin. Antioxid Redox Signal 7: 60–71, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Nowak K, Weih S, Metzger R, Albrecht RF, Post S, Hohenberger P, Gebhard MM, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol 293: L162–L169, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Pearse DB, Shimoda LA, Verin AD, Bogatcheva N, Moon C, Ronnett GV, Welsh LE, Becker PM. Effect of cGMP on lung microvascular endothelial barrier dysfunction following hydrogen peroxide. Endothelium 10: 309–317, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17: 3045–3051, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res 93: 1034–1046, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Pinsky DJ, Naka Y, Chowdhury NC, Liao H, Oz MC, Michler RE, Kubaszewski E, Malinski T, Stern DM. The nitric oxide/cyclic GMP pathway in organ transplantation: critical role in successful lung preservation. Proc Natl Acad Sci USA 91: 12086–12090, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polte T, Oberle S, Schröder H. Nitric oxide protects endothelial cells from tumor necrosis factor-[alpha]-mediated cytotoxicity: possible involvement of cyclic GMP. FEBS Lett 409: 46–48, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Rentsendorj O, Mirzapoiazova T, Adyshev D, Servinsky LE, Renne T, Verin AD, Pearse DB. Role of vasodilator-stimulated phosphoprotein in cGMP-mediated protection of human pulmonary artery endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 294: L686–L697, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal 7: 619–626, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Schmidt EP, Damarla M, Rentsendorj O, Servinsky LE, Zhu B, Moldobaeva A, Gonzalez A, Hassoun PM, Pearse DB. Soluble guanylyl cyclase contributes to ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 295: L1056–L1065, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma AK, Rohrer B. Sustained elevation of intracellular cGMP causes oxidative stress triggering calpain-mediated apoptosis in photoreceptor degeneration. Curr Eye Res 32: 259–269, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, Muzykantov VR. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther 331: 404–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suenobu N, Shichiri M, Iwashina M, Marumo F, Hirata Y. Natriuretic peptides and nitric oxide induce endothelial apoptosis via a cGMP-dependent mechanism. Arterioscler Thromb Vasc Biol 19: 140–146, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Takuma K, Phuagphong P, Lee E, Mori K, Baba A, Matsuda T. Anti-apoptotic effect of cGMP in cultured astrocytes. Inhibition by cGMP-dependent protein kinase of mitochondrial permeable transition pore. J Biol Chem 276: 48093–48099, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 294: L632–L641, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Zhang R, Al Lamki R, Bai L, Streb JW, Miano JM, Bradley J, Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res 94: 1483–1491, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem 279: 10677–10684, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem 278: 1248–1258, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Zhu B, Strada S, Stevens T. Cyclic GMP-specific phosphodiesterase 5 regulates growth and apoptosis in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 289: L196–L206, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.