Abstract
We have previously demonstrated that intranasal administration of ambient ultrafine particles (UFP) acts as an adjuvant for primary allergic sensitization to ovalbumin (OVA) in Balb/c mice. It is important to find out whether inhaled UFP exert the same effect on the secondary immune response as a way of explaining asthma flares in already-sensitized individuals due to traffic exposure near a freeway. The objective of this study is to determine whether inhalation exposure to ambient UFP near an urban freeway could enhance the secondary immune response to OVA in already-sensitized mice. Prior OVA-sensitized animals were exposed to concentrated ambient UFP at the time of secondary OVA challenge in our mobile animal laboratory in Los Angeles. OVA-specific antibody production, airway morphometry, allergic airway inflammation, cytokine gene expression, and oxidative stress marker were assessed. As few as five ambient UFP exposures were sufficient to promote the OVA recall immune response, including generating allergic airway inflammation in smaller and more distal airways compared with the adjuvant effect of intranasally instilled UFP on the primary immune response. The secondary immune response was characterized by the T helper 2 and IL-17 cytokine gene expression in the lung. In summary, our results demonstrated that inhalation of prooxidative ambient UFP could effectively boost the secondary immune response to an experimental allergen, indicating that vehicular traffic exposure could exacerbate allergic inflammation in already-sensitized subjects.
Keywords: allergic inflammation, oxidative stress, distal lung
ambient particulate matter (PM) exposure is a contributing factor to increased respiratory morbidity and mortality in an urban environment (37, 48, 51). Epidemiological studies have demonstrated that increased asthma prevalence, including the number of patients diagnosed with the disease as well as asthma-related hospital visits, is closely associated with PM levels in the ambient air, the regional motor vehicle traffic density, and residential proximity to freeways (21, 47, 52, 53). Several mechanisms have been proposed to explain PM effects in asthma (44). While acute asthma flares in response to vehicle emission may be caused by an exacerbation of existing airway inflammation or airway hyperreactivity, the possibility also needs to be entertained that the increase in allergic inflammation could originate at the level of boosting of the secondary immune response to common environmental allergens in already-sensitized people (11–13, 30, 45). This would be a logical extension of the idea that ambient PM or diesel exhaust particle (DEP) exposure acts as an adjuvant that can lead to a de novo priming of the immune response to common environmental or experimental allergen (11–13, 30, 36, 45). We have recently confirmed that intranasal instillation of ambient ultrafine particles (UFP) derived from vehicular emissions close to a major Los Angeles freeway is capable of generating a new immune response to ovalbumin (OVA) in Balb/c mice (36). Noteworthy, this effect could be obtained with as little as 500 ng of the ambient nanoparticles delivered on four or fewer occasions intranasally (36). A key question now becomes whether similar sensitization is possible during inhalation of real-life particles near a freeway and whether this adjuvant effect is also relevant to the secondary immune response in already-sensitized subjects. No data exists about the adjuvant effect of ambient UFP on the recall immune response to allergen. This information is critical in understanding one of the possible mechanisms by which ambient PM exposure may induce asthma exacerbation in atopic people. For instance, this could explain the dramatic increase in asthma exacerbations and increased number of emergency room visits that follow a few hours after a sudden surge in ambient PM levels or commuting on a busy freeway (64).
A key objective of this communication is to demonstrate that inhalation exposure to ambient UFP is capable of exerting an adjuvant effect on a recall immune response to OVA in our sensitive mouse model (36). Previous attempts at developing a reproducible model has failed because of uncertainty about the exposure levels that are required for an adjuvant effect as well as overlooking the possibility that an excessive particle dose can actually interfere in antigen-specific immune activation. In this regard, we have shown that primary OVA sensitization can be achieved with nanogram quantities of nasally instilled UFP, and the response declines when using microgram quantities of DEP (36). Since the secondary immune response can theoretically be triggered by very low antigen levels, it is possible that even a limited number of UFP exposures may trigger an adjuvant effect in mice (36). This could explain the epidemiological observations in humans of ambient PM increase leading to increased respiratory morbidity without a clear threshold level (56). We demonstrate that as few as five UFP exposures are capable of boosting eosinophilic airway inflammation, IgE production, T helper 2 (Th2) and IL-17 cytokine gene expression as well as the expression of a sensitive oxidative stress marker in the lungs of prior OVA-sensitized Balb/c mice. Moreover, compared with the intranasal model, aerosolized inhalation leads to allergic inflammation at the level of a more distal retention site in the centriacinar region of the lung.
MATERIALS AND METHODS
Eight- to 10-wk-old female Balb/c mice were obtained from the Jackson Laboratory. Animal housing conditions and all experiments were approved by the Chancellor's Animal Research Committee at University of California, Los Angeles.
Reagents.
The limulus amebocyte lysate assay kit for endotoxin detection and an endotoxin removing gel column were obtained from Lonza (Walkersville, MD) and Pierce (Rockford, IL), respectively. OVA (grade V) and dithiothreitol (DTT) were from Sigma-Aldrich (St. Louis, MO). Ketamine and xylazine were purchased from Phoenix Pharmaceutical (St. Joseph, MO). VectaStain ABCAP kit was obtained from Vector Laboratories (Burlingame, CA). RNeasy mini kit and RNase-Free DNase set were purchased from Qiagen (Valencia, CA). High-capacity cDNA reverse transcription kit and TaqMan gene expression assay reagents were from Applied Biosystems (Foster City, CA). Ym1 antibody was provided by Dr. Shioko Kimura (National Cancer Institute, Bethesda, MD).
Ambient particle collection and endotoxin detection.
The ambient UFP (<0.18 μm) for nasal allergic sensitization were collected, through impaction, using an impinger and the Versatile Aerosol Concentration Enrichment System (VACES) in downtown Los Angeles as previously described by us (34, 35, 58). Because the particle growth process is based on supersaturation, the VACES concentrates all UFP (hydrophobic and hydrophilic particles) equally, irrespective of their chemical composition (17, 27–29, 59, 66). The water-soluble fraction of the UFP then becomes part of the solution, leaving the solid non-soluble core of UFP in suspension. The entire mixture was administered to the animals. Endotoxin level was measured as previously described (34, 35, 58).
Ambient UFP inhalation in the mobile laboratory.
Whole body exposures to filtered air (FA) or concentrated ambient UFP (<0.18 μm), the most abundant particles by number in urban Los Angeles, were performed using the VACES in our mobile laboratory near the Los Angeles 110 freeway as previously described (3, 36). Briefly, the ambient air was drawn through an aluminum duct into the VACES and delivered to whole body exposure chambers after a 13.3 ± 2-fold enrichment (Supplemental Table S1; Supplemental Material for this article is available online at the Journal website). Temperature and airflow were controlled to ensure adequate ventilation and minimize buildup of animal-generated dander, ammonia, CO2, and thermal stress. Mobilization of mice between the Hazelton housing chamber and the exposure chambers was performed over the shortest time period possible to limit the exposure to ambient air PM in the trailer. Particle number concentrations were measured with a TSI3022 Condensation Particle Counter, and particle mass concentration was assessed with a DataRAM model DK-2000 (Supplemental Table S1). All animal groups were simultaneously exposed to atmospheres containing FA or concentrated UFP for 4 h/day for 5 days as shown in Fig. 1. Particle mass concentration and UFP composition were measured by collecting particles on 37-mm Teflon filters (PTFE 2-μm pore; Gelman Science, Ann Arbor, MI) (Supplemental Table S1). Concentrations of inorganic ions (sulfate and nitrate), elemental carbon (EC), organic carbon (OC), polycyclic aromatic hydrocarbon (PAH) content, and particle-bound trace elements and metals were analyzed as previously described (3).
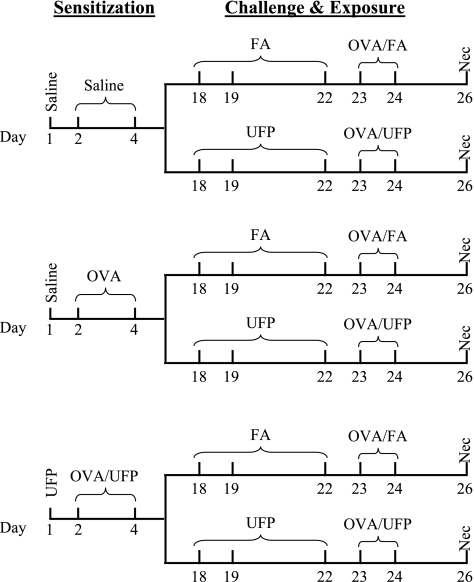
Fig. 1.
Allergic sensitization, ovalbumin (OVA) challenge, and ultrafine particles (UFP) inhalation protocol. Animals were intranasally sensitized with saline, OVA, or OVA + UFP followed by inhalation exposure to filtered air (FA) or concentrated ambient UFP in the presence of 1% aerosolized OVA. Necropsy (Nec) was conducted on day 26.
Allergic sensitization, OVA challenge, and ambient UFP exposure.
Mice were prior sensitized by nasal mucosal endotoxin-free OVA delivery together with a small aliquot of aqueous UFP collected in an impinger (36). The mice were divided into three sensitization groups: saline, OVA only, or OVA plus UFP (OVA/UFP) (Fig. 1). Each sensitization group was further divided into two secondary challenge groups, OVA plus FA (OVA/FA) or OVA plus UFP (OVA/UFP) (Fig. 1). For primary sensitization, mice in the OVA/UFP group received 0.5 μg of the UFP suspension intranasally in a total volume of 50 μl on day 1, whereas the mice in the saline and OVA-alone groups received the same volume of saline. On days 2 and 4, animals in the OVA/UFP group received 0.5 μg of UFP mixed with 10 μg of OVA in a total volume of 50 μl, whereas those in the OVA-alone and saline groups received the same amounts of OVA and saline in the same volume, respectively. The particles and OVA were freshly mixed on each occasion. On days 18 and 19 and again on days 22–24, 50% of animals in each sensitization group were exposed to FA inhalation, whereas the rest were exposed to the concentrated UFP atmosphere from 10 am to 2 pm as described above. All mice were challenged by 1% OVA aerosol delivered through a Schuco 2000 nebulizer (Allied Health Care Products, St. Louis, MO) for 30 min after FA or UFP exposure on days 23 and 24 (Fig. 1) (18, 36). Necropsy was performed on day 26.
Animal necropsy, sample collection, and analysis.
Animal necropsy and bronchoalveolar lavage (BAL) fluid, blood, and lung tissue collection were conducted as previously described (36). BAL differential cell counts and measurement of plasma OVA-specific IgG1 (OVA-IgG1) and IgE (OVA-IgE) by ELISA were performed as previously described by us (18).
Histopathological analysis of the lung.
Hisptopathological analysis and morphometry of the lung were performed as previously described by us (36). Tissue sections were also immunohistochemically stained for major basic protein (MBP) in eosinophils or chitinase 3-like protein 3 (Ym1) protein in macrophages/giant cells. Hematoxylin was used as a counterstain (36).
Analysis of gene expression in the lung by real-time PCR.
The expression of proinflammatory cytokine, chemokine, and asthma-related genes was analyzed by real-time PCR. These include keratinocyte-derived chemokine (KC), IFNγ, TNFα, IL-10, monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), IL-6, IL-2, eotaxin, IL-5, IL-13, IL-4, IL-17, mucin 5AC (Muc5AC), resistin-like molecule α (Fizz1), Ym1, chitinase 3-like protein 4 (Ym2), chloride channel calcium-activated 3 (GOB5), and acidic mammalian chitinase (AMCase). Lung samples were harvested and placed in RNA Later (Qiagen, Valencia, CA). Total RNA was isolated using RNeasy Mini Kit according to the manufacturer's instructions. Briefly, tissues were homogenized in lysis buffer (Buffer RLT) containing 2-mercaptoethanol with a 5 mm of rotor-sator Homogenizer (PRO Scientific, Oxford, CT). During RNA purification, DNase digestion was performed on-column using Qiagen RNase-Free DNase Set. Purified RNA was quantified using a GeneQuant Pro spectrophotometer (BioCrom, Cambridge, England). cDNA was generated from 5 μg of total RNA using the High-Capacity cDNA Reverse Transcription Kit. The reaction mixture was incubated at 25°C for 10 min and then 37°C for 2 h. PCR array analysis was performed by pooling aliquots of cDNAs from samples in each experimental group. Quantitative gene expression analysis was performed using TaqMan Gene Expression Assay reagents on the ABI PRISM 7900 HT Sequence Detection System. The PCR cycling parameters were 48°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s followed by 60°C for 1 min. Relative gene expression levels were reported as fold-change (FC) using the ΔΔCt method where FC = 2−ΔΔCt. The mRNA expression of each gene was normalized by subtracting the geometric mean of the Cts from two endogenous controls (Actb and Arbp). Selected genes that had expression levels at least twofold greater in experimental groups relative to the control group were confirmed by relative quantitative real-time RT-PCR using individual animal cDNAs as described above. Statistical differences between ΔΔCt values between groups were statistically determined with two-way ANOVA (SigmaStat, Ashburn, VA; P ≤ 0.05).
DTT assay.
The abiotic assessment of the oxidant potential of UFP was determined by the DTT assay as previously described (10, 35). This assay is premised on the interaction of redox-cycling organic chemicals such as quinones (Q) with DTT as illustrated in the following reactions. In the presence of quinones, 1 mol of DTT plus 2 mol of O2 generates 1 mol of DTT-disulfide plus 2 superoxide (O2·−). The loss of DTT can be followed by its reaction with DTNB.
| (1) |
| (2) |
| (3) |
This assay has been demonstrated as a reliable and rapid method of analyzing the oxidant potential of ambient particulate samples collected in an urban environment (10, 35).
PM composition and chemical analysis.
Quartz and Teflon filters were used for ultrafine ambient concentrated particle (CAP) collection in parallel with CAP inhalation exposure in the mobile laboratory. These filters were used to analyze particle chemical composition and PAH content as previously described (35).
Statistical analysis.
Results were expressed as means ± SE. Differences among groups were evaluated by ANOVA. The Student's t-test was used to distinguish between pairs of groups. P < 0.05 was considered statistically significant.
RESULTS
Inhalation of concentrated ambient UFP boosts the secondary immune response in OVA-sensitized animals.
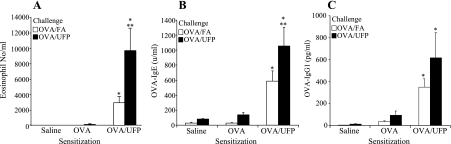
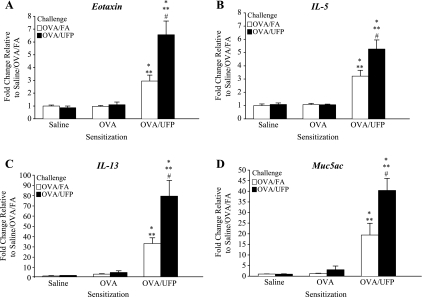
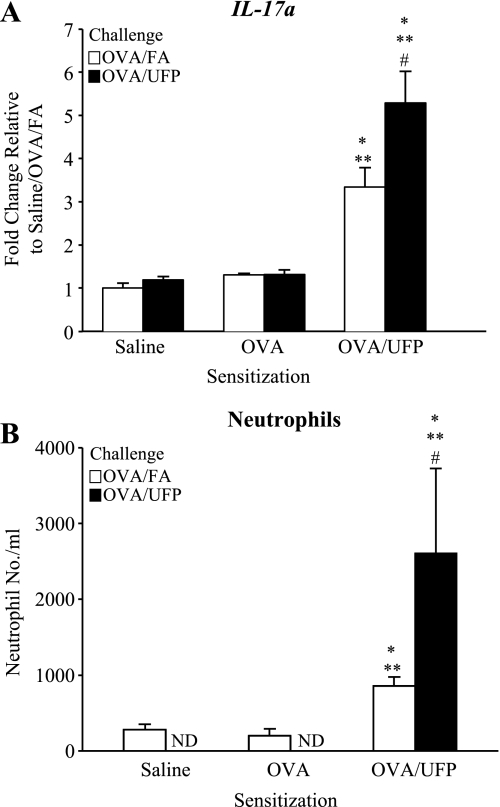
We have previously demonstrated that ambient UFP act as an adjuvant that boosts the primary immune response that is elicited by OVA when intranasally administrated in mice (36). UFP is a potent adjuvant due to its chemical composition, including a high content of redox-cycling organic chemicals (33, 37). In this study, we investigated whether inhalation of ambient UFP could demonstrate an adjuvant effect during the secondary immune response, using the exposure protocol shown in Fig. 1. As shown, this protocol delivers the concentrated UFP as an aerosol that is inhaled before (days 18, 19, and 22) and at the time of OVA inhalation exposure (days 23 and 24). Figure 2 shows that UFP inhalation during secondary OVA challenge (OVA/UFP) significantly enhanced allergic airway inflammation in the mice prior sensitized with OVA plus UFP compared with similarly sensitized animals receiving OVA/FA. The allergic airway response was characterized by the significant increase in the eosinophil count in the BAL (Fig. 2A) together with elevated OVA-IgE and OVA-IgG1 levels in the plasma (Fig. 2, B and C). Lung histology and morphometry demonstrated that UFP exposure during secondary OVA challenge induced a significant eosinophil infiltration and increased mucus production in the lung tissue of sensitized mice compared with animals receiving FA and OVA (Supplemental Fig. S1). Real-time PCR analysis confirmed that compared with OVA/FA challenge, OVA/UFP challenge increased the expression of eotaxin, IL-5, IL-13, TNFα, KC, IL-10, and Muc5ac genes in prior sensitized animals (Fig. 3; Supplemental Table S2). In addition to eliciting a Th2 response, UFP also enhanced IL-17a gene expression (Fig. 4A) together with an increased neutrophil count in the BAL (Fig. 4B). Neither OVA/FA nor OVA/UFP challenge had an impact on allergic inflammation in the animals sensitized by saline or OVA alone (Figs. 2–4; Supplemental Table S2). Together, these data show for the first time that UFP inhalation exposure is quite effective in boosting the secondary immune response after a limited number of exposures.
Fig. 2.
Inhalation of UFP during OVA challenge enhanced eosinophilic airway inflammation and OVA-specific antibody production in OVA/UFP-sensitized mice compared with those in the same sensitization group receiving FA instead of UFP. A: bronchoalveolar lavage (BAL) eosinophil count; *P < 0.05 compared with saline and OVA sensitization groups; **P < 0.05 compared with OVA/FA challenge from the corresponding sensitization group. B: OVA-IgE; *P < 0.05 compared with saline and OVA sensitization groups; **P < 0.05 compared with OVA/FA challenge from the corresponding sensitization group. C: OVA-IgG1; *P < 0.05 compared with saline and OVA sensitization groups. Challenge with OVA/FA or OVA/UFP led to similar level of increase in BAL lymphocyte count in OVA/UFP-sensitized animals, but had no effect on monocyte count (data not shown).
Fig. 3.
RT-PCR showing that ambient UFP exposure during OVA challenge enhanced Th2 and Muc5ac gene expression. A: eotaxin; B: IL-5; C: IL-13; D: Muc5ac. *P < 0.05 compared with respective saline sensitization; **P < 0.05 compared with respective OVA sensitization; #P < 0.05 compared with respective OVA/FA.
Fig. 4.
Inhalation of ambient UFP during OVA challenge enhanced IL-17a gene expression and neutrophil influx in the lungs of OVA/UFP-sensitized mice. A: RT-PCR analysis of IL-17a expression in the lung. B: BAL neutrophil count. *P < 0.05 compared with respective saline sensitization; **P < 0.05 compared with respective OVA sensitization; #P < 0.05 compared with respective OVA/FA.
Adjuvant effect of UFP during inhalation exposure targets small airways.
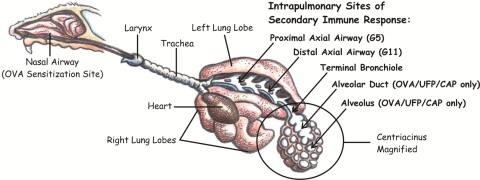
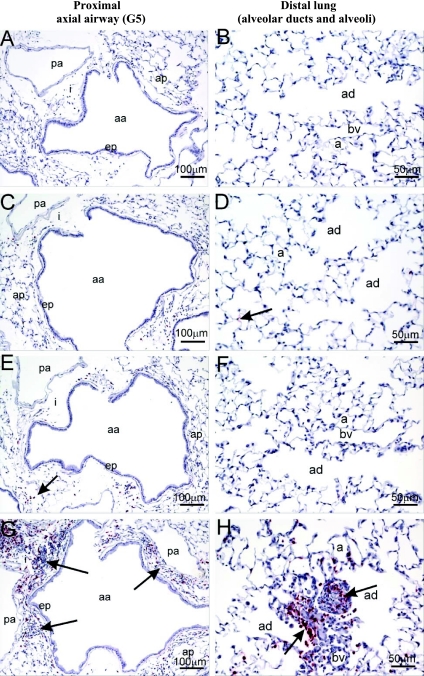
One of the unique physical properties of UFP is their small size which allows them to evade phagocytosis and to deposit in the deep lung due to diffusion as well as sticking to the airway walls due to Van der Waals' forces (7, 25, 31, 55). We performed lung morphometry to delineate the airway generations impacted by the adjuvant effect of UFP in sensitized animals (see Fig. 5 for airway anatomy). Interestingly, although UFP boosted mild allergic inflammation in the proximal (G5) airway, the most profound effects were observed in the centracinar area of distal lung (Fig. 6). This stands in contrast to the intranasal administration effects, which elicit extensive inflammation in the nose and proximal and distal axial airways (36). Figure 6 demonstrates the distribution of the eosinophil and other inflammatory cell infiltrates in the proximal (A, C, E, G) compared with the distal (B, D, F, H) airways. Only OVA/UFP-sensitized mice developed conspicuous histopathological changes at the G5 level, including eosinophil infiltration (Fig. 6E) as well as mucoid metaplasia and metaplasia (Supplemental Fig. S2). UFP inhalation markedly enhanced the peribronchiolar and perivascular inflammation that followed the secondary OVA challenge (Fig. 6G). In contrast to the changes in the proximal airway, the pathological changes were more profound in the distal lung of sensitized animals (Fig. 6H). UFP inhalation induced allergic inflammation that extended as far as preterminal and terminal bronchioli as well as impacting the proximal alveolar duct and adjacent alveolar parenchyma (Figs. 5, 6H, and 7A). This effect on alveolar inflammation is further reflected by the significant increase in BAL eosinophil count in animals exposed to UFP (Fig. 2A).
Fig. 5.
Schematic highlighting of the intrapulmonary impact sites during UFP inhalation. Arrows indicate the potential target site of UFP during the secondary immune response with alveolar duct and alveolus being the major targets. OVA/UFP/CAP only: OVA/UFP sensitization followed by challenge with OVA/ultrafine concentrated ambient particle inhalation.
Fig. 6.
Immunohistochemical staining of eosinophilic major basic protein (MBP) demonstrating that OVA/UFP challenge induced more severe allergic inflammation in the alveolar ducts (ad) and alveoli (a) than in the proximal axial airway (aa; generation 5) in OVA/UFP-sensitized mice. A and B: OVA sensitization followed by OVA/FA challenge. C and D: OVA sensitization followed by OVA/UFP challenge. E and F: OVA/UFP sensitization followed by OVA/FA challenge. G and H: OVA/UFP sensitization followed by OVA/UFP challenge. Respiratory epithelium lining axial airway, ep; pulmonary artery, pa; blood vessel, bv. Arrows indicate positive staining of MBP in eosinophils.
Fig. 7.
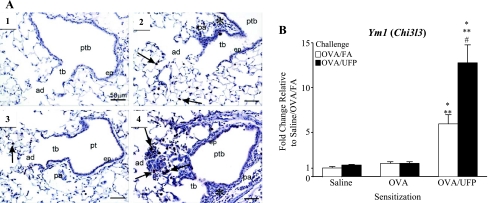
OVA/UFP-enhanced Ym1 expression in the lung. A: immunohistochemistry showing Ym1 expression in alveolar macrophages/giant cells in centriacini, along with inflammation in preterminal (ptb), terminal bronchioles (tb), and alveolar ducts (ad). Arrows indicate positive staining of Ym1 protein. 1: OVA sensitization followed by OVA/FA challenge. 2: OVA/UFP sensitization followed by OVA/FA challenge. 3: OVA sensitization followed by OVA/UFP challenge. 4: OVA/UFP sensitization followed by OVA/UFP challenge. Pulmonary arteries, pa; asterisk, peribronchiolar inflammation. B: Ym1 gene expression in the lung. *P < 0.05 compared with respective saline sensitization; **P < 0.05 compared with respective OVA sensitization; #P < 0.05 compared with respective OVA/FA.
In addition to its size properties, UFP express a large surface area that allow these particulates to carry a rich load of redox-active organic chemicals to the particle deposition site in the respiratory tract (25, 35, 37). Indeed, UFP collected in this experiment exhibited high OC content (81.6%) that is typical of the UFP composition in Los Angeles (Supplemental Fig. S3) (3, 35, 36). The high OC content could be significant to the particles' adjuvant effect, particularly the high content of redox-cycling organic compounds in the OC fraction (25, 35, 37). This is reflected by a relatively high oxidant potential as determined by the DTT assay in which the DTT consumption of 0.05 nmol·μg−1·min−1 is much higher than values typically obtained with PM2.5 (Supplemental Table S3). To determine whether the prooxidant effect of the UFP is reflected in oxidant injury in the lung, we assessed the expression of Ym1 protein, which has recently been characterized as a UFP-induced oxidative stress marker by proteome analysis (24, 65). Immunohistochemical analysis showed prominent Ym1 protein expression in alveolar macrophages/giant cells in the centriacinar region of the lung (Fig. 7A). Enhanced Ym1 expression in lung tissue was also confirmed by real-time PCR analysis (Fig. 7B). Together, our data show that the proinflammatory effects of UFP in the distal lung are accompanied by oxidative stress effects.
DISCUSSION
In this study, we have successfully demonstrated for the first time that even brief UFP exposures near a major urban freeway is sufficient to boost the secondary immune response to OVA in prior sensitized animals. The allergic inflammation in the lung was accompanied by a Th2 cytokine response profile, increased IL-17a gene expression, evidence of more severe inflammation in the centriacinar region, and systemic OVA-specific antibody production. This likely reflects the deposition site of UFP in the lung as a result of their small size. These results are of major importance for the effect of air pollution on asthma.
The major advance in this study is the demonstration that inhalation of ambient UFP exerts a potent adjuvant effect on the secondary immune response. While the effects of other particle types (such as DEP and ultrafine carbon black) have been studied on allergic inflammation through inhalation exposure, there has only been one other study that utilized ambient UFP (2, 16, 18, 30, 39, 41, 60). Different from our work, however, the latter study only found increased IL-5 level in the BAL without evidence of eosinophil inflammation or OVA-IgE increase (30). Thus, our study is the first to demonstrate that inhalation exposure of UFP concurrently with OVA is actually capable of inducing a strong allergic response to OVA. This finding along with the demonstration of Th2 and Th17 immunity, as well as distal lung morphometry analysis, is highly relevant to the potential effects of UFP to atopic humans. While a large body of epidemiological evidence shows a close association of asthma exacerbation with ambient PM levels, there is still a lack of strong evidence that air pollution actually leads to an increase in new cases of asthma (20, 46). On the other hand, it is quite possible that ambient PM exposure can lead to asthma flares in already-sensitized atopic subjects by exerting an adjuvant effect. Since UFP counts are highest on and in proximity to freeways, traffic during commuting or residing or working near a freeway could play a significant role in boosting allergic inflammation in atopic subjects also exposed to outdoor allergens (67). This is in keeping with epidemiological reports showing an association between increased asthma exacerbation and proximity to freeways (43, 52).
Another interesting feature of our study is that previous epidemiological studies demonstrated a linear relationship between particulate air pollution and its adverse respiratory effects without a clearly definable threshold (56). This is in keeping with the possibility that relative small exposure amounts may suffice to exert an adjuvant effect in the immune system. In this regard, we have shown that nanogram quantities of UFP can exert an adjuvant effect in the nose (36). It is possible that the animal exposure in our study could achieve this dose quantity. The amount that the animals were exposed to in our experimental atmosphere is two to five times higher than the in-vehicle exposures that commuters are subjected to while traveling on Los Angeles freeways (3). Our finding that UFP boosting effects can be achieved with as few as five inhalation exposures implies that UFP are highly efficient in boosting the secondary immune response to allergen. In fact, in a follow-up study, we demonstrated that as few as two UFP inhalation exposures could significantly boost eosinophilic inflammation in the lung and an OVA-IgG1 response in already-sensitized animals (Supplemental Fig. S4). Moreover, using our intranasal instillation model, it is now possible to show OVA sensitization with a single 500-ng dose of UFP (data not shown). It remains to be demonstrated in humans what is a minimal exposure amount.
The potency of UFP in terms of their proinflammatory and proallergic effects resides in their unique physical and chemical properties, including their small size, large surface area, and coating with abundant redox-active OC compounds (36, 37, 44). The small particle size allows UFP to penetrate deep into the lung where they are retained with a high rate of efficiency (25, 31, 44). Indeed, morphometry studies confirmed that inhaled UFP elicit profound inflammatory effects at the level of the centriacinar region (Figs. 6 and 7A). The large surface area of the almost-weightless UFP further contribute to making OC compound such as PAH and quinones bioavailable (37). Quinones and PAHs are relevant organic chemical groups that are involved in the oxidative stress effects in the lung (37). In this regard, we have previously demonstrated that there is an excellent correlation between the PAH content of ambient UFP and their ability to act as an adjuvant in the primary immune response (36). Quinones, which can also be formed from the enzymatic conversion of PAH, are capable of generating ROS through redox cycling and electrophilic chemistry that may be relevant to the generation of oxidative stress deep in the lungs of the animals (15, 37, 57). The particle exposure site in this study is generally characterized by a higher content of redox-cycling organic chemicals as also evidenced by the high OC content (81.6%) of the particles that were collected near the 110 Los Angeles Freeway (Supplemental Fig. S3) (3, 35, 36). Previously, we have demonstrated that there is a close correlation between the OC content of the particles and their oxidant potential as determined by the DTT assay as well as particle's ability to activate oxidative stress response pathways in vitro (35). This is in accordance with the relatively high DTT consumption rate of 0.05 nmol·μg−1·min−1 of the UFP collected in this study (Supplemental Table S3). We propose that the high OC content and strong oxidant potential of UFP present on freeways in Los Angeles could be significant in the adjuvant effect of the UFP (25, 35, 37). This notion could also explain the increased Ym1 expression in the centriacinar region of the lung where most of the allergic changes take place (Fig. 7). The alveolar ducts and centriacinar region of the lung represent the actual lung sites where diffusing nano-size particles make contact with the walls of the small airways as result of Van der Waals forces (7, 42). The ability of UFP to evade phagocytosis by macrophages could also mean that these particles could be made available to dendritic cells (DC) that are in close contact with epithelial cells in those areas (4–6, 38).
While the immunological basis for the adjuvant effects of prooxidative PM on the immune system is improperly understood, there is increasing experimental evidence that PM-induced oxidative stress can induce Th2 skewing of the immune response through impacting the antigen presenting function of DC (8, 50, 63). This could impact antigen uptake, antigen presentation, DC costimulatory activity, and cytokine production (8, 32, 50, 63). We have previously demonstrated that organic DEP extracts induce oxidative stress effects in myeloid DC, leading to an inhibition of IL-12 production and subsequent decrease in IFN-γ production in antigen-responsive T cells (8). One proposal is that the overall decrease in Th1 immunity could promote Th2 skewing of the immune response (8). This is compatible with the demonstration that UFP inhalation could promote IL-5, IL-13, and eotaxin production in the lungs of animals secondarily challenged by OVA inhalation, thereby demonstrating that UFP act as an adjuvant in the secondary immune response (Fig. 3). While asthma has traditionally been considered a disease dominated by a Th2 immune response, there is mounting evidence for the involvement of additional pathways such as Th17 immune response (4, 9, 23, 61). This is compatible with the demonstration in our inhalation model of increased IL-17a gene expression in the lung and increased neutrophil levels in the BAL fluid of OVA/UFP-sensitized animals (Fig. 4). Although increased IL-17a gene expression and protein level in response to air pollutants have been reported in animal and human studies, this is the first report showing the effect of UFP inhalation on this pathway (19, 22, 49, 54).
Our study is also important from the perspective of asthma prevention and exacerbation by air pollution particles. Epidemiological studies indicate a close link between surges in ambient PM levels and asthma flares (26, 40). While a host of atmospheric conditions and pollutant sources may contribute to these exacerbations, it is important to consider the role of proximity to freeways in determining UFP concentrations. For example, Zhu et al. (67, 68) have reported that in Los Angeles the UFP level is 7–15 times higher on the freeways than at the urban background sites. It decreases exponentially with downwind distance from the freeways, reaching background levels around 200–300 m from the freeway (67, 68). These findings have been used in California state legislation as a criterion for setting a minimal distance at which schools are allowed to be built from a freeway. In addition to the importance of limiting UFP exposure through distance or time spent on the freeway, our study also has important considerations for asthma treatment. While the most commonly used treatment for asthma flares is the combined use of inhaled corticosteroids and long-acting β2-agonists, we know from nasal challenge studies in humans that intranasal corticosteroids do not effectively suppress DEP-induced inflammatory responses in nose (14). However, a number of new therapies are emerging that target the role of oxidative stress in asthma exacerbation (1, 9). One potential treatment strategy is the use of antioxidants that may interfere in endogenous oxidative stress pathways integral to the disease as well as sources of exogenous oxidative stress such as ambient PM exposure. In this regard, we have previously shown that thiol antioxidants are effective in countering the adjuvant effect of DEP in a mouse allergic inflammation model, including suppressing Ym1 expression as determined by proteome profiling (62, 65).
In summary, we have successfully demonstrated that “real-life” exposures to ambient prooxidative UFP could effectively boost the secondary immune response leading to the exacerbation of existing allergic airway inflammation characterized by Th2 and Th17 cytokine profiles in already-sensitized animals. Inhaled UFP target the distal lung including the alveolar duct and alveolar parenchyma where the generation of oxidative stress may play a role in enhancing the secondary immune response. Although there are some logistic limitations in conducting “real-life” exposures, such as the number of control groups that can be accommodated in the exposure chamber, this work provides an explanation for increased asthma flares after a sudden surge of ambient PM level. While it would have been optimal to compare other adjuvants such as alum for primary sensitization or use of a UFP-only exposure during the secondary exposure, historical control experiments using UFP pharyngeal aspiration have addressed concerns such as whether the particles alone can induce allergic inflammation or whether UFP together with OVA can induce a boosting effect in alum-sensitized animals.
GRANTS
This study was supported by NIH Grants U19 AI-070453, RC2 ES-018766, and RO1-ES-016746, as well as U.S. EPA STAR Award RD-83241301 (to the Southern California Particle Center). This work has not been subjected to the EPA for peer and policy review.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. James Schauer at the University of Wisconsin-Madison for chemical analysis, Dr. Arthur Cho and Debra Schmitz at the Southern California Particle Center for the DTT assay, Dr. James Wagner at Michigan State University for assisting the necropsy, and Dr. Dianne Meacher from University of California, Irvine for coordinating animal exposures.
REFERENCES
- 1.Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. Lancet 372: 1073–1087, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Alessandrini F, Schulz H, Takenaka S, Lentner B, Karg E, Behrendt H, Jakob T. Effects of ultrafine carbon particle inhalation on allergic inflammation of the lung. J Allergy Clin Immunol 117: 824–830, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res 102: 589–596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity 31: 425–437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleck B, Tse DB, Curotto de Lafaille MA, Zhang F, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation and polarization via thymic stromal lymphopoietin. J Clin Immunol 28: 147–156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol 176: 7431–7437, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chalupa DC, Morrow PE, Oberdorster G, Utell MJ, Frampton MW. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect 112: 879–882, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan RC, Wang M, Li N, Yanagawa Y, Onoe K, Lee JJ, Nel AE. Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol 118: 455–465, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Chatila TA, Li N, Garcia-Lloret M, Kim HJ, Nel AE. T-cell effector pathways in allergic diseases: transcriptional mechanisms and therapeutic targets. J Allergy Clin Immunol. 121: 812–823; quiz 824–815, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, Eiguren-Fernandez A, Froines JR. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res 99: 40–47, 2005 [DOI] [PubMed] [Google Scholar]
- 11.de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy 36: 1469–1479, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Sanchez D, Garcia MP, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol 104: 1183–1188, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol 158: 2406–2413, 1997 [PubMed] [Google Scholar]
- 14.Diaz-Sanchez D, Tsien A, Fleming J, Saxon A. Effect of topical fluticasone propionate on the mucosal allergic response induced by ragweed allergen and diesel exhaust particle challenge. Clin Immunol 90: 313–322, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Eiguren-Fernandez A, Shinyashiki M, Schmitz DA, Distefano E, Hinds W, Kumagai Y, Cho AK, Froines JR. Redox and electrophilic properties of vapor- and particle-phase components of ambient aerosols. Environ Res 110: 207–212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimaki H, Ui N, Endo T. Induction of inflammatory response of mice exposed to diesel exhaust is modulated by CD4(+) and CD8(+) T cells. Am J Respir Crit Care Med 164: 1867–1873, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Geller MD, Biswas S, Fine PM, Sioutas C. A compact aerosol concentrator for use in conjunction with low flow-rate continuous aerosol instrumentation. J Aerosol Sci 36: 1006–1022, 2005 [Google Scholar]
- 18.Hao M, Comier S, Wang M, Lee JJ, Nel A. Diesel exhaust particles exert acute effects on airway inflammation and function in murine allergen provocation models. J Allergy Clin Immunol 112: 905–914, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Harrison OJ, Foley J, Bolognese BJ, Long E, 3rd, Podolin PL, Walsh PT. Airway infiltration of CD4+ CCR6+ Th17 type cells associated with chronic cigarette smoke induced airspace enlargement. Immunol Lett 121: 13–21, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Heinrich J, Wichmann HE. Traffic related pollutants in Europe and their effect on allergic disease. Curr Opin Allergy Clin Immunol 4: 341–348, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Holguin F. Traffic, outdoor air pollution, and asthma. Immunol Allergy Clin North Am 28: 577–588, viii–ix, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Ivanov S, Palmberg L, Venge P, Larsson K, Linden A. Interleukin-17A mRNA and protein expression within cells from the human bronchoalveolar space after exposure to organic dust. Respir Res 6: 44, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology 123: 326–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang X, Li N, Wang M, Boontheung P, Sioutas C, Harkema JR, Bramble LA, Nel AE, Loo JA. Adjuvant effects of ambient particulate matter monitored by proteomics of bronchoalveolar lavage fluid. Proteomics 10: 520–531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawanaka Y, Tsuchiya Y, Yun SJ, Sakamoto K. Size distributions of polycyclic aromatic hydrocarbons in the atmosphere and estimation of the contribution of ultrafine particles to their lung deposition. Environ Sci Technol 43: 6851–6856, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kim JJ, Huen K, Adams S, Smorodinsky S, Hoats A, Malig B, Lipsett M, Ostro B. Residential traffic and children's respiratory health. Environ Health Perspect 116: 1274–1279, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Chang MC, Froines JR, Sioutas C. A versatile aerosol concentrator for simultaneous in vivo and in vitro evaluation of toxic effects of coarse, fine and ultrafine particles: Part I: Laboratory evaluation. J Aerosol Sci 11: 1281–1297, 2001 [Google Scholar]
- 28.Kim S, Chang MC, Sioutas C. A new generation of portable coarse, fine and ultrafine particle concentrators for use in inhalation toxicology. Inhal Toxicol 12: 121–137, 2000 [PubMed] [Google Scholar]
- 29.Kim S, Jaques P, Chang MC, Xiong C, Friedlander SK, Sioutas C. A versatile aerosol concentrator for simultaneous in vivo and in vitro evaluation of toxic effects of coarse, fine and ultrafine particles: Part II: Filed evaluation. J Aerosol Sci 11: 1299–1314, 2001 [Google Scholar]
- 30.Kleinman MT, Sioutas C, Froines JR, Fanning E, Hamade A, Mendez L, Meacher D, Oldham M. Inhalation of concentrated ambient particulate matter near a heavily trafficked road stimulates antigen-induced airway responses in mice. Inhal Toxicol 19, Suppl 1: 117–126, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Kreyling WG, Semmler-Behnke M, Moller W. Ultrafine particle-lung interactions: does size matter? J Aerosol Med 19: 74–83, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity 31: 412–424, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol 109: 250–265, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Li N, Kim S, Wang M, Froines J, Sioutas C, Nel A. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal Toxicol 14: 459–486, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111: 455–460, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Wang M, Bramble LA, Schmitz DA, Schauer JJ, Sioutas C, Harkema JR, Nel AE. The adjuvant effect of ambient particulate matter is closely reflected by the particulate oxidant potential. Environ Health Perspect 117: 1116–1123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med 44: 1689–1699, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YJ. TSLP in epithelial cell and dendritic cell cross talk. Adv Immunol 101: 1–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto A, Hiramatsu K, Li Y, Azuma A, Kudoh S, Takizawa H, Sugawara I. Repeated exposure to low-dose diesel exhaust after allergen challenge exaggerates asthmatic responses in mice. Clin Immunol 121: 227–235, 2006 [DOI] [PubMed] [Google Scholar]
- 40.McCreanor J, Cullinan P, Nieuwenhuijsen MJ, Stewart-Evans J, Malliarou E, Jarup L, Harrington R, Svartengren M, Han IK, Ohman-Strickland P, Chung KF, Zhang J. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med 357: 2348–2358, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Miyabara Y, Ichinose T, Takano H, Lim HB, Sagai M. Effects of diesel exhaust on allergic airway inflammation in mice. J Allergy Clin Immunol 102: 805–812, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Moller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Haussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am J Respir Crit Care Med 177: 426–432, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, Wichmann HE, Heinrich J. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med 177: 1331–1337, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science 311: 622–627, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol 102: 539–554, 1998. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor GT, Neas L, Vaughn B, Kattan M, Mitchell H, Crain EF, Evans R, 3rd, Gruchalla R, Morgan W, Stout J, Adams GK, Lippmann M. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J Allergy Clin Immunol 121: 1133–1139 e1131, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Patel MM, Miller RL. Air pollution and childhood asthma: recent advances and future directions. Curr Opin Pediatr 21: 235–242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol 115: 213–219; quiz 220, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med 205: 385–393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, Breysse P, Georas SN, Williams MA. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol 37: 706–719, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riedl MA. The effect of air pollution on asthma and allergy. Curr Allergy Asthma Rep 8: 139–146, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Salam MT, Islam T, Gilliland FD. Recent evidence for adverse effects of residential proximity to traffic sources on asthma. Curr Opin Pulm Med 14: 3–8, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. N Engl J Med 343: 1742–1749, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect 118: 640–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmid O, Moller W, Semmler-Behnke M, Ferron GA, Karg E, Lipka J, Schulz H, Kreyling WG, Stoeger T. Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers 14, Suppl 1: 67–73, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Schwartz J, Ballester F, Saez M, Perez-Hoyos S, Bellido J, Cambra K, Arribas F, Canada A, Perez-Boillos MJ, Sunyer J. The concentration-response relation between air pollution and daily deaths. Environ Health Perspect 109: 1001–1006, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shinyashiki M, Eiguren-Fernandez A, Schmitz DA, Di Stefano E, Li N, Linak WP, Cho SH, Froines JR, Cho AK. Electrophilic and redox properties of diesel exhaust particles. Environ Res 109: 239–244, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ Health Perspect 113: 947–955, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sioutas C, Kim S, Chang M. Development and evaluation of a prototype ultrafine particle concentrator. J Aerosol Sci 30: 1001–1012, 1999 [Google Scholar]
- 60.Takano H, Ichinose T, Miyabara Y, Shibuya T, Lim HB, Yoshikawa T, Sagai M. Inhalation of diesel exhaust enhances allergen-related eosinophil recruitment and airway hyperresponsiveness in mice. Toxicol Appl Pharmacol 150: 328–337, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Wang YH, Liu YJ. The IL-17 cytokine family and their role in allergic inflammation. Curr Opin Immunol 20: 697–702, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitekus MJ, Li N, Zhang M, Wang M, Horwitz MA, Nelson SK, Horwitz LD, Brechun N, Diaz-Sanchez D, Nel AE. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol 168: 2560–2567, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, Yamamoto M, Breysse P, Biswal S, Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol 181: 4545–4559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson AM, Salloway JC, Wake CP, Kelly T. Air pollution and the demand for hospital services: a review. Environ Int 30: 1109–1118, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Wang M, Kang X, Boontheung P, Li N, Nel AE, Loo JA. Oxidative stress and asthma: proteome analysis of chitinase-like proteins and FIZZ1 in lung tissue and bronchoalveolar lavage fluid. J Proteome Res 8: 1631–1638, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhi N, Moore KF, Polidori A, Sioutas C. Field validation of the new miniature versatile aerosol concentration enrichment system (mVACES). Aerosol Sci Tech 40: 1098–1110, 2006 [Google Scholar]
- 67.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc 52: 1032–1042, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y, Hinds WC, Kim S, Shen S, Sioutas C. Study on ultrafine particles and other vehicular pollutants near a busy highway. Atmospher Environ 36: 4375–4383, 2002 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.