Abstract
Early-life respiratory infection with Pseudomonas aeruginosa is common in children with cystic fibrosis or immune deficits. Although many of its clinical manifestations involve neural reflexes, little information is available on the peripheral nervous system of infected airways. This study sought to determine whether early-life infection triggers a neurogenic-mediated immunoinflammatory response, the mechanisms of this response, and its relationship with other immunoinflammatory pathways. Weanling and adult rats were inoculated with suspensions containing P. aeruginosa (PAO1) coated on alginate microspheres suspended in Tris-CaCl2 buffer. Five days after infection, rats were injected with capsaicin to stimulate nociceptive nerves in the airway mucosa, and microvascular permeability was measured using Evans blue as a tracer. PAO1 increased neurogenic inflammation in the extra- and intrapulmonary compartments of weanlings but not in adults. The mechanism involves selective overexpression of NGF, which is critical for the local increase in microvascular permeability and for the infiltration of polymorphonuclear leukocytes into infected lung parenchyma. These effects are mediated in part by induction of downstream inflammatory cytokines and chemokines, especially IL-1β, IL-18, and leptin. Our data suggest that neurogenic-mediated immunoinflammatory mechanisms play important roles in airway inflammation and hyperreactivity associated with P. aeruginosa when infection occurs early in life.
Keywords: cystic fibrosis, immunodeficiency, nerve growth factor, neuroimmunomodulation
pseudomonas aeruginosa causes chronic lung infections in patients with immune deficits and cystic fibrosis (CF) (16), resulting in significant morbidity and mortality (36). Once acquired, this infection is difficult to eradicate and leads to more severe respiratory disease and declining lung function (44). Aggressive eradication therapy in early life has been used in some CF centers with good clinical outcomes (12, 13, 20), but the reason for its success is not completely understood. This has led to the hypothesis that CF bronchopulmonary disease is initiated by infections in the infants' lower airways, even in the absence of overt clinical symptoms, and is followed by airway obstruction and destruction as a later and presumably secondary feature (11, 18).
Although many of the clinical manifestations of P. aeruginosa infection involve neural reflexes initiated from the nociceptive innervation of the airways (e.g., cough, bronchospasm, mucus secretion), very little is known about the influence of this gram-negative bacterium on neural development and neuroimmunomodulation. We have shown previously that viral infections, particularly those caused by the respiratory syncytial virus (RSV), can render the airways abnormally susceptible to the proinflammatory and immunomodulatory effects of the peptide neurotransmitter substance P. This innate defense mechanism is the result of increased biosynthesis in vagal ganglionic cells (32), rapidly increased expression of heat-gated ion channels controlling its release from nociceptive vagal axons on stimulation by airborne irritants (50), as well as overexpression of target neurokinin receptors by the airway epithelium, vascular endothelium, and multiple cellular effectors of inflammation and immunity (15, 17, 35).
We have also shown, first in weanling rodents (15) and more recently in human infants (45), that a critical mechanism of virus-induced airway inflammation and hyperreactivity is the upregulation of specific neurotrophic factors and receptors, particularly the prototypical NGF, which direct neural growth and reactivity in the respiratory tract. Therefore, in this study, we first sought to determine the impact of early-life P. aeruginosa respiratory infections on neurotrophic pathways and the consequent local changes in neurogenic-mediated inflammation. These experiments were conducted primarily in weanling rats strain Fischer 344 (F344) because of the large amount of information on their respiratory neurobiology accrued in previous studies (30), and the infection was induced by endotracheal inoculation of P. aeruginosa-coated alginate microspheres as previously described in adult rats (29).
In this model, we found neurogenic-mediated inflammation markedly increased when the infection started in early life but not in adulthood. To define the mechanism of this effect, we then measured in the lungs of these young rat mRNA transcripts and protein expression of the principal neurotrophic factors and receptors as well as other nonneural factors critical for lung growth and development. Finally, to understand the pathophysiological and therapeutic implications of our molecular findings, we inhibited NGF activity in P. aeruginosa-infected weanling rats using selective immunological and chemical blockers and studied their systemic effect on weight gain as well as the local effects on vascular and cellular immunoinflammatory responses and cytokine/chemokine expression profile in the respiratory tract.
METHODS
Animals.
Based on our previous work (17, 32, 35), we used weanling (2-wk-old) F344 rats delivered in our barrier facility from pathogen-free dams. To study age-related differences, we also used pathogen-free adult rats (12-wk-old) purchased from Charles River Laboratories (Wilmington, MA) or from Harlan Laboratories (Indianapolis, IN). To prevent microbial contamination, all animals were maintained under strict barrier conditions, and their cages were serviced by specially trained husbandry personnel. The cages were placed on racks providing positive individual ventilation with class 100 air to each cage at the rate of approximately one cage change of air per minute delivered through filter tops (Maxi-Miser; Thoren Caging Systems, Hazleton, PA). Separate rooms were used to house infected and pathogen-free rats. All manipulations were conducted inside a class 100 laminar flow hood. Bedding and water were autoclaved before use, and food was irradiated before use and unpacked only under laminar flow. Cages and water bottles were run through a tunnel washer after every use and disinfected with both chemicals and heat. All experimental procedures followed in this study were approved by the Institutional Animal Care and Use Committee.
P. aeruginosa preparation and inoculation.
The alginate microspheres were prepared at Florida International University. P. aeruginosa strain PAO1 was inoculated onto lysogeny broth (LB) plates and grown overnight. One colony was taken and inoculated into a sterile flask containing 100 ml of LB medium and incubated for 20 h. After centrifugation of the broth, the pellet was resuspended in 3 ml of LB, and 1 ml of this suspension was mixed with 9 ml of alginate solution. The microspheres were then formed by dripping the solution into 0.1 M CaCl2 in 0.1 M Tris·HCl buffer at pH 7.4. All microspheres were used or discarded within 24 h. Inoculations were carried out using a titer of 1 × 108 colony-forming units (cfu)/ml, which has been shown previously to induce chronic infections in rodent models (29, 41, 42).
To localize the P. aeruginosa infection to the lower respiratory tract, we delivered the inoculum by endotracheal instillation. While under sedation with pentobarbital sodium (50 mg/kg ip), the thorax and anterior cervical area were transilluminated and the tongue manipulated using blunt forceps. After visualization of the cords, a blunt-tip syringe (Hamilton, Reno, NV) was advanced past the cords to deliver a volume of 50 μl for weanlings or 100 μl for adults. Controls were inoculated in the same fashion with sterile alginate microspheres suspended in Tris-CaCl2 buffer or with Tris-CaCl2 buffer alone. After inoculation, the rats were housed in their cage before the terminal experiments.
Nerve stimulation.
Rats were reanesthetized with pentobarbital sodium (50 mg/kg ip), and the femoral vein was exposed. PAO1-infected rats and noninfected controls received a 2-min intravenous infusion of capsaicin (8-methyl-N-vanillyl-6-nonenamide; Sigma, St. Louis, MO) dissolved in a vehicle having a final concentration of 0.75% ethanol, 0.375% Tween 80, and 0.85% NaCl in aqueous solution. This neurotoxin binds selectively the transient receptor potential channel, vanilloid subfamily member 1 (TRPV1) highly expressed on C-type nociceptive vagal neurons, thereby releasing the neurotransmitters stored in their terminal varicosities (14). This pharmacological strategy has been used in a number of animal models to mimic exposure of the airways to airborne irritants (14), and ablation of capsaicin-sensitive nerves has been shown to inhibit the inflammatory response of the airway mucosa to tobacco smoke and mechanical and chemical irritants (38). Furthermore, capsaicin is routinely used in clinical settings to test airway reactivity and cough reflex in respiratory patients.
Vascular permeability.
To study neurogenic inflammatory responses in PAO1-infected airways, groups of weanling rats or adult rats were inoculated endotracheally with PAO1-coated alginate microspheres, sterile alginate microspheres, or Tris-CaCl2 buffer. Airway microvascular permeability was assessed at 5 days postinoculation by injecting a tracer (Evans blue dye; 30 mg/kg iv over 5 s) into the femoral vein immediately before nerve stimulation to measure the exudation of macromolecules from the airway tracheobronchial circulation. All chemicals were delivered in a volume of 1 ml/kg body wt.
Five minutes after injection of the tracer, the chest was opened, a cannula was inserted into the ascending aorta through the left ventricle, and the circulation was perfused for 2 min with phosphate-buffered saline with a syringe pump set at a rate of 25 ml/min for weanlings or 50 ml/min for adults (17, 35). The extrapulmonary airways (from the 1st tracheal ring to the end of the main stem bronchi) and the left lung were dissected and prepared for Evans blue extraction. The specimens, free of connective tissue and opened along the ventral midline, were blotted with absorbent paper, weighed, and incubated in 1 ml of formamide (Sigma) at 50°C for 18 h to extract the extravasated Evans blue dye. The exudation of Evans blue-labeled macromolecules from the tracheobronchial microcirculation was quantified by measuring the optical density of formamide extracts at 620-nm wavelength, interpolated from a standard curve of Evans blue concentrations (0.5–10 mg/ml), and expressed in nanograms per milligram wet tissue weight.
Histopathological and morphometric analysis.
The right lung from each animal was fixed in 10% buffered formalin, embedded in paraffin, cut in 3-mm-thick sections, and stained with hematoxylin and eosin for histopathological analysis. All slides were coded and interpreted by two independent pathologists who were blinded to whether the specimens were from an infected or noninfected rat. To quantitate the histopathological changes associated with PAO1 infection, the pathologists counted the maximal number of polymorphonuclear leukocytes (PMNs) and lymphocytes observed per high-power field (HPF) at ×40 magnification.
RT-PCR.
Lung tissues were collected from the rats after euthanasia with a lethal dose of pentobarbital followed by exsanguination, immediately flash-frozen in liquid nitrogen, and stored in a freezer at −80°C until further processing. The frozen specimens were placed in lysis buffer and homogenized using a conventional rotor-stator homogenizer (Brinkmann Instruments, Westbury, NY) for 45–60 s. Total RNA was extracted from lung homogenates using RNeasy Mini Kits (Qiagen, Hilden, Germany) according to the manufacturer's specifications. RNA samples were added to 50 μl of master mix consisting of 400 μM each dNTPs, 10 units of RNase inhibitor, 2 μl of enzyme mix containing Taq DNA Polymerase (1-step RT-PCR; Promega, Madison, WI), and 50 pmol each of primers flanking the nucleotide sequence for the target gene or for the housekeeping gene β-actin (31). The same master mix without the RNA sample was used as a negative control. The primer pairs illustrated in Table 1 were designed on the basis of previously published protocols and were used to differentiate cDNA-generated PCR products from genomic DNA contamination. Amplification was performed using a GeneAmp PCR System 9600 thermal cycler (PerkinElmer, Waltham, MA). The process was started with an initial step at 50°C for 30 min, then 95°C for 15 min followed by 25–35 cycles with a denaturing step followed by an annealing step, an extension step, and then one final extension step at 68–72°C for 10 min. All programs included a 4°C hold step at the end. Amplified PCR products were size-fractionated by electrophoresis through a 2% agarose gel and stained with ethidium bromide. The gels were photographed using an imaging system (FOTO/Analyst Luminary Workstation; Fotodyne, Hartland, WI), and the intensity of DNA bands was analyzed by computerized densitometry (TotalLab TL-101 Image Analysis Software) and expressed as the ratio of the densitometry score measured for each target gene normalized by the β-actin control.
Table 1.
Primers for RT-PCR amplification
| Target | Sense Amino Acids | Antisense Amino Acids | Product Size, bp | Acc. no. | GenInfo Identifier |
|---|---|---|---|---|---|
| NGF | 100–105 | 225–230 | 395 | M36589 | 310738 |
| BDNF | 129–134 | 230–236 | 293 | M61178 | 24225 |
| p75NTR | 60–65 | 274–279 | 663 | M012601 | 24596 |
| TrkA | 138–145 | 354–360 | 690 | M021589 | 59109 |
| TrkB | 42–49 | 251–260 | 664 | M55291 | 25054 |
| EGF | 834–841 | 1,011–1018 | 536 | NM-012842 | 25313 |
| EGFR | 465–472 | 720–727 | 690 | NM-031507 | 24329 |
| TGF-β | 174–182 | 249–256 | 250 | NM-021578 | 59086 |
| VEGF | 207–213 | 266–272 | 199 | NM-031836 | 83785 |
| β-Actin | 216–223 | 369–375 | 285 | C005111.2 | 81822 |
BDNF, brain-derived neurotrophic factor; Trk, tropomyosin-related kinase; EGFR, EGF receptor; TGF, transforming growth factor.
Neurotrophin immunoassays.
NGF and brain-derived neurotrophic factor (BDNF) protein levels in the lungs were measured with a commercial kit (Promega) using the antibody sandwich technique. In brief, lung tissue samples (100 mg) were homogenized in 5 volumes of lysis buffer containing Tris·HCl (20 mM), NaCl (150 mM), Nonidet P-40 (1%), glycerol (10%), phenylmethylsulfonyl fluoride (1 mM), aprotinin (10 μg/ml), leupeptin (1 μg/ml), and sodium vanadate (0.5 mM). The supernatants of homogenized tissue samples were incubated for 18 h at 4°C in 96-well plates coated with 100 μl of specific polyclonal antibody (1.5 μg/ml in 100 mM carbonate coating buffer, pH 9.7) to bind NGF or BDNF from the homogenates. After washing, a specific rat monoclonal antibody was applied (0.6 μg/ml in buffer, 100 μl/well) and incubated for 18 h at 4°C to bind the captured NGF or BDNF. The plates were again thoroughly washed, and horseradish peroxidase-conjugated antibody to rat IgG was added to each well and incubated for 3 h at room temperature to detect the amount of specifically bound monoclonal antibody. After final washing to remove unbound antibody conjugate, the chromogenic substrate was added, and the color change generated by the reaction was read at a wavelength of 450 nm. Test samples and standards (100 μl/well) were measured in duplicate. Using this assay, NGF and BDNF can be quantified with a lower detection limit of 15.6 pg/ml and <2% cross-reactivity with other neurotrophic factors.
NGF inhibition.
To test the effects of selective neurotrophin inhibitors on the vascular and cellular inflammatory response in PAO1-infected airways, we used two different strategies: 1) immunological inhibition with a specific NGF-blocking antibody (15); or 2) chemical inhibition of receptor tyrosine kinase with the specific antagonist K252a (24). The antibody or antagonist was injected subcutaneously 60 min before PAO1 inoculation and again 60 min before nerve stimulation.
As neurotrophic factors are highly conserved in different species (37), we blocked endogenous NGF activity using a polyclonal rabbit anti-mouse antibody (1:2,000 dilution, 4 ml/kg; Sigma), which inhibits NGF-induced growth of PC12 cells at 1:10,000 dilution, and used rabbit purified IgG (1:2,000 dilution, 4 ml/kg; Sigma) as an isotype control. A single dose of this anti-NGF antibody has been shown to inhibit sympathetic innervation in a fetal lamb model for at least 55 days (39). Timing and doses of anti-NGF treatment were chosen on the basis of previous studies that confirmed the efficacy of this antibody in blocking NGF biological effects in our rat model (15).
For receptor tyrosine kinase inhibition, rats were injected subcutaneously with 100 μg/kg K252a (Biomol, Plymouth Meeting, PA), a carbazole alkaloid that inhibits the phosphorylation of tropomyosin-related kinase (Trk) receptors (27) and has been shown to prevent NGF-dependent substance P production in vitro (5) and in vivo (47). Controls were given an equal volume of vehicle (2% DMSO in 0.9% NaCl). Biologically active, nontoxic doses of this inhibitor were chosen based on previously published work in our rat model (24).
Bronchoalveolar lavage.
TRPV1 channels were stimulated with capsaicin 5 days after inoculation (2). Bronchoalveolar lavage (BAL) was carried out in rats under pentobarbital anesthesia by infusing 28 ml/kg phosphate-buffered saline via a tracheal cannula secured with a proximal and distal suture. The fluid was instilled and withdrawn 3 times.
Total cell count was measured using a hemocytometer, and, after appropriate dilution to obtain 20,000–30,000 cells/ml, a microscope slide for differential count was prepared using a cytocentrifuge at 9,500 g for 5 min. The slides were fixed by air-drying and then stained with the Giemsa-Wright method. Cells were identified based on size and morphological features, and any cell that had evidence of artifact or lysis was avoided. Differential cell count was performed on 200 cells to determine the number of PMNs, lymphocytes, and macrophages. All slides were coded and reviewed separately by 2 investigators in our laboratory who were blinded with regard to the experimental conditions associated with the cytospin preparations.
Multiplex analysis.
Lung tissue samples (100 mg) were homogenized in 5 volumes of lysis buffer containing Tris·HCl (20 nM), NaCl (150 nM), Nonidet P-40 (1%), glycerol (10%), phenylmethylsulfonyl fluoride (1 mM), aprotinin (10 μg/ml), leupeptin (1 μg/ml), and sodium vanadate (0.5 mM). Multiplex analysis was performed using the Luminex 100 System (Luminex, Austin, TX). Twenty-three cytokines and chemokines [IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p70), IL-13, IL-17, IL-18, inducible protein 10 (IP-10), eotaxin, leptin, granulocyte colony-stimulating factor (G-CSF), granulocyte/macrophage colony-stimulating factor (GM-CSF), growth-related oncogene (GRO)/keratinocyte-derived chemokine (KC), IFN-γ, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), RANTES, TNF-α] were simultaneously measured in lung tissue homogenates using a Milliplex MAP rat cytokine/chemokine panel (Millipore, Billerica, MA) according to the manufacturer's specifications.
Statistical analysis.
Data are expressed as means ± SE. ANOVA was used to analyze differences between groups (49). Multiple comparisons between means (46) were performed using the Fisher protected least significant difference test. Statistical analysis was performed using the software SigmaStat version 3.5 (Systat Software, Point Richmond, CA) for Windows. Differences with P < 0.05 were considered significant.
RESULTS
Histopathology.
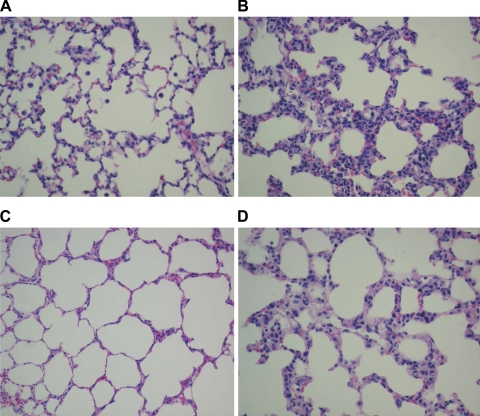
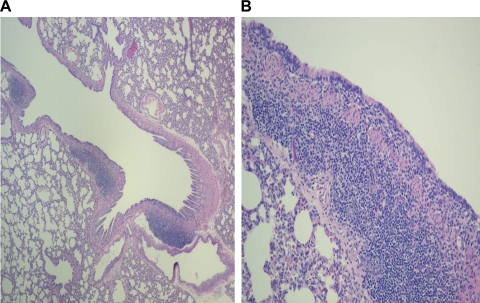
Histopathological changes were observed to determine the effect of the infection on lung morphology. Acute PAO1 infection (5 days postinoculation) in weanling F344 rats caused significant histopathological changes consisting predominantly of lymphomononuclear cellular infiltration of the interstitium and peribronchial areas, whereas the lungs of age-matched, noninfected controls dosed with sterile alginate microspheres or with Tris-CaCl2 buffer were essentially normal with thin alveolar septa and no infiltrates (Fig. 1, A and B). Only sparse PMNs were seen in both infected and noninfected weanlings. The acute changes caused by PAO1 in the alveolar septa of adult rats were morphologically similar, although significantly more PMNs were noted compared with infected weanlings (Fig. 1, C and D). PAO1-infected weanlings also showed marked hyperplasia of the bronchial-associated lymphoid tissue (BALT) accompanied by lymphocytic infiltrate of the bronchial wall (Fig. 2), which was not noted in adults.
Fig. 1.
Histopathology of acute Pseudomonas aeruginosa strain PAO1 infection. The lungs of noninfected weanling (A) and adult (C) strain Fischer 344 (F344) rats were essentially normal with thin alveolar septa and no infiltrates. Interstitial pneumonitis with diffuse widening of alveolar septa due to lymphomononuclear infiltrates was noted in weanlings after 5 days of PAO1 infection (B). The lungs of infected adult rats (D) were almost normal at 5 days, although there was a mild widening of the alveolar septa due to sparse lymphocytic infiltrate. n = 3 rats per group.
Fig. 2.
Bronchial-associated lymphoid tissue (BALT) hyperplasia in weanlings. Compared with noninfected controls (A), PAO1-infected weanling rats also showed marked hyperplasia of the BALT accompanied by lymphocytic infiltrate of the bronchial wall (B), which was not noted in adults. n = 3 rats per group.
Histopathological analysis was also conducted in adult rats infected with PAO1 during early life (at 2 wk of age) and killed 30 days postinoculation. These rats continued to have a predominantly lymphomononuclear infiltrate with few PMNs, a pattern quite similar to that seen during the acute phase. In contrast, rats infected with PAO1 during adulthood (at 12 wk of age) and killed 30 days postinoculation evolved into a severe neutrophilic pneumonia characterized by the formation of multiple microabscesses corresponding to lodged microspheres (data not shown).
Neurogenic inflammation.
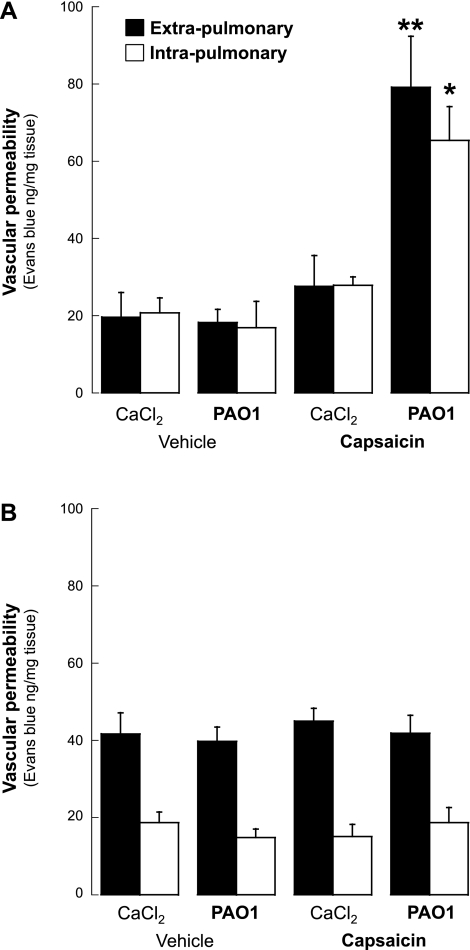
Airway vascular permeability was used to determine the effect of PAO1 infection on neurogenic inflammation. In the absence of nerve stimulation (vehicle of capsaicin), airway vascular permeability after 5 days of PAO1 infection was not different from age-matched noninfected controls, both in weanling (extrapulmonary, P = 0.85; intrapulmonary, P = 0.64; Fig. 3A) and in adult rats (extrapulmonary, P = 0.79; intrapulmonary, P = 0.32; Fig. 3B). Also, confirming our previous studies, vascular permeability in noninfected weanling rats was similar in the extra- and intrapulmonary airways, whereas in adult rats the extrapulmonary circulation was more permeable than the intrapulmonary.
Fig. 3.
Neurogenic inflammation. Following transient receptor potential channel, vanilloid subfamily member 1 (TRPV1) stimulation with capsaicin, the exudation of Evans blue-labeled macromolecules in the airways of weanling rats infected with PAO1 was significantly increased compared with age-matched noninfected controls (A). In contrast, microvascular permeability in adult rats infected with PAO1 was not significantly different from noninfected controls (B). Data are expressed as the means ± SE (n = 5–6 rats per group). *P < 0.05, **P < 0.01 compared with noninfected controls.
However, following TRPV1 stimulation with capsaicin, the exudation of Evans blue-labeled macromolecules in the airways of weanling rats infected with PAO1 was significantly increased compared with age-matched controls that were kept pathogen-free after endotracheal instillation of Tris-CaCl2 buffer (Fig. 3A). Vascular permeability increased by 187% in the extrapulmonary airways (P = 0.003) and by 134% in the intrapulmonary airways (P = 0.02) of infected weanling rats. The instillation of sterile alginate microspheres suspended in Tris-CaCl2 buffer had no significant effect compared with Tris-CaCl2 buffer alone (extrapulmonary, 38.6 ± 2.8 vs. 27.7 ± 8.1, P = 0.2; intrapulmonary, 19.9 ± 3.2 vs. 27.9 ± 2.1, P = 0.1), and therefore we elected to control the subsequent experiments with Tris-CaCl2 buffer alone.
In contrast, vascular permeability in adult rats infected with PAO1 was not significantly different from noninfected controls (Fig. 3B) in either the extrapulmonary (P = 0.6) or the intrapulmonary (P = 0.5) airways. As a result, the response of the airway microvasculature to nociceptive stimulation with capsaicin during PAO1 infection was exquisitely age-dependent both in extra- and intrapulmonary airways, with the permeability in infected weanlings raising 2- to 3.5-fold higher than infected adults (P < 0.001).
Neurotrophins.
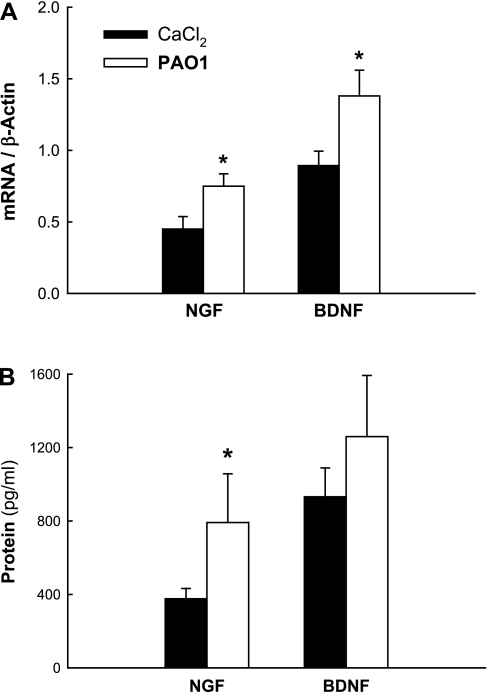
We analyzed the effect of PAO1 infection on the expression of neurotrophic factors and receptors at both the mRNA and protein level. RT-PCR analysis (Fig. 4A) of lung tissues removed from weanling rats after 5 days of PAO1 infection revealed a significant increase in both NGF (P = 0.03) and BDNF (P = 0.04) mRNA expression compared with controls dosed with buffer. Immunoassay analysis (Fig. 4B) of the same groups confirmed that NGF protein concentration was significantly increased in the lung tissues of PAO1-infected weanling rats (P = 0.03). We also found a strong trend of increased BDNF protein expression in weanlings that was, however, not statistically significant (P = 0.37).
Fig. 4.
Neurotrophic factors. RT-PCR analysis of lung tissues from weanling rats infected with PAO1 revealed a significant increase in both NGF and brain-derived neurotrophic factor (BDNF) mRNA expression compared with controls (A). Immunoassay analysis of the same groups confirmed that NGF protein concentration was significantly increased in the lung tissues of PAO1-infected weanling rats (B). Means ± SE (n = 5 rats per group). *P < 0.05 compared with noninfected controls.
RT-PCR analysis was also used to measure expression of the neurotrophin receptors TrkA, TrkB, and p75NTR in lung homogenates of PAO1-infected weanling rats, but no significant change was found (P = 0.95, 0.22, and 0.83, respectively). The effect of PAO1 on lung development was quite selective for the neural component, as gene expression of other major growth factors controlling the development of vascular and epithelial structures remained virtually unchanged (VEGF, P = 0.38; TGF-β, P = 0.07; EGF, P = 0.18; EGFR, P = 0.92).
Vascular effects of NGF inhibition.
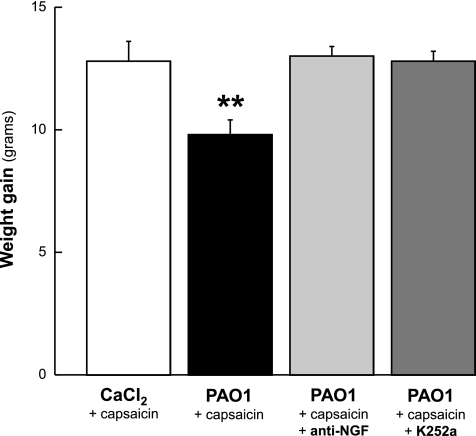
After 5 days of PAO1 infection, average body weight gain was significantly lower in weanling rats compared with noninfected controls (P = 0.004; Fig. 5). In contrast, the weight gain measured in PAO1-infected rats treated with either the NGF-neutralizing antibody (P = 0.7) or the specific receptor tyrosine kinase inhibitor K252a (P = 0.2) was not different from controls.
Fig. 5.
Effect of NGF inhibition on weight gain. After 5 days of PAO1 infection, average body weight gain was significantly lower in weanling rats compared with noninfected controls. In contrast, the weight gain measured in PAO1-infected rats treated with either the NGF-neutralizing antibody or with the specific receptor tyrosine kinase inhibitor K252a was not different from controls. Means ± SE (n = 6–8 rats per group). **P < 0.01 compared with noninfected controls.
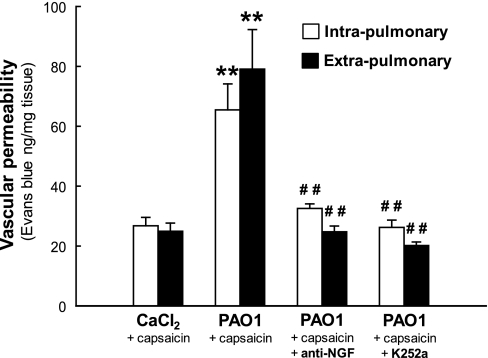
Pretreatment with the anti-NGF antibody markedly reduced neurogenic Evans blue exudation in PAO1-infected weanling rats (Fig. 6), both in the intrapulmonary (−50%; P = 0.004) and extrapulmonary (−69%; P = 0.001) airways, and even stronger inhibition was noted after chemical inhibition of Trk signaling with K252a (intrapulmonary, −60%, P = 0.003; extrapulmonary −75%, P = 0.004). After NGF blockade, Evans blue exudation in the respiratory tract of weanling rats infected with PAO1 was not significantly different from age-matched noninfected controls (P > 0.1). Treatment with isotype control antibody or K252a vehicle had no effect on capsaicin-induced plasma exudation (data not shown).
Fig. 6.
Vascular effects of NGF inhibition. Pretreatment with anti-NGF antibody markedly reduced neurogenic Evans blue exudation in the airways of PAO1-infected weanling rats, and even stronger inhibition was noted after chemical inhibition of tropomyosin-related kinase (Trk) signaling with K252a. After NGF blockade, Evans blue exudation in the respiratory tract of weanling rats infected with PAO1 was not significantly different from age-matched noninfected controls. Means ± SE (n = 6–8 rats per group). **P < 0.01 compared with noninfected controls; ##P < 0.01 compared with infected rats with intact NGF activity.
Cellular effects of NGF inhibition.
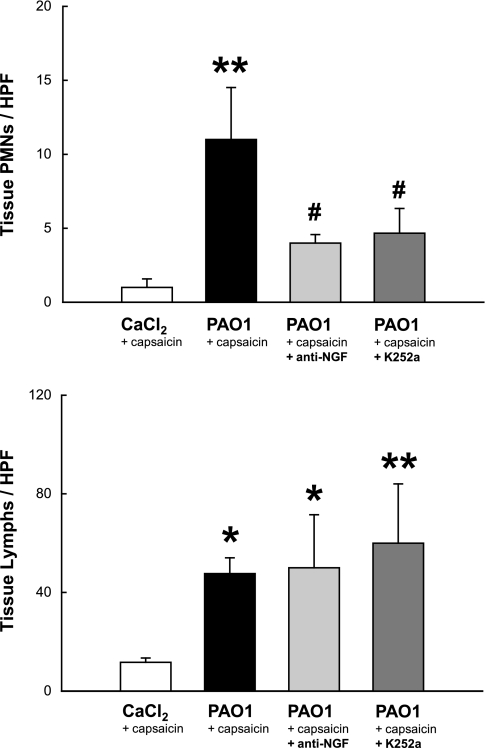
TRPV1 stimulation with capsaicin had negligible effect on the lung parenchyma of noninfected controls but increased >10-fold the influx of PMNs (P = 0.007) and almost 5-fold lymphomononuclear cells (P = 0.01) into PAO1-infected lung tissues (Fig. 7). The effect on PMNs was largely NGF-dependent and strongly inhibited by both anti-NGF and K252a (P = 0.037), whereas the same inhibitors had no effect on lymphomononuclear cells (P = 0.8).
Fig. 7.
Cellular effects of NGF inhibition in lung tissues. TRPV1 stimulation with capsaicin increased >10-fold the influx of polymorphonuclear leukocytes (PMNs) and almost 5-fold lymphomononuclear cells into P. aeruginosa-infected lung tissues. The effect on PMNs was largely NGF-dependent and strongly inhibited by both anti-NGF and K252a, whereas the same inhibitors had no effect on lymphomononuclear cells. Means ± SE (n = 5–6 rats per group). *P < 0.05, **P < 0.01 compared with noninfected controls; #P < 0.05 compared with infected rats with intact NGF activity. HPF, high-power field.
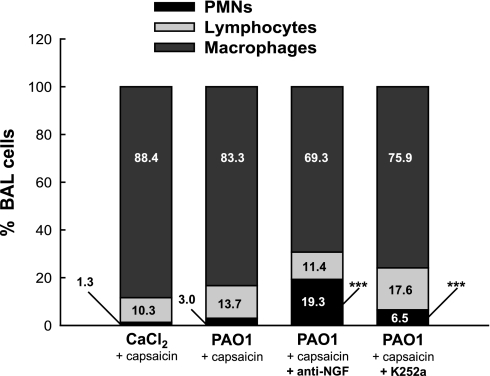
Nerve stimulation during PAO1 infection was also associated with the recruitment of inflammatory cells into the airways. The cellular fraction of the BAL from capsaicin-stimulated noninfected weanling rats was largely of the monocyte/macrophage type, with few lymphocytes and minimal PMNs (Fig. 8). After PAO1 infection, PMNs tripled, without significant changes in the other subpopulations. However, the proportion of PMNs recovered from PAO1-infected airways by BAL increased 15-fold above control after NGF inhibition with the specific blocking antibody (P < 0.001), and a similar, albeit smaller effect was observed after receptor tyrosine kinase inhibition with K252a (P < 0.001). No significant effect was found in rats treated with isotype control antibody or K252a vehicle (data not shown). Also, no significant difference in cell counts was found between infected and noninfected weanling rats in the absence of nerve stimulation (P > 0.3; n = 3 rats per group).
Fig. 8.
Cellular effects of NGF inhibition in airways. The cellular fraction of the bronchoalveolar lavage (BAL) from capsaicin-stimulated noninfected weanling rats was largely of the monocyte/macrophage type, with few lymphocytes and minimal PMNs. After PAO1 infection, PMNs tripled, without significant changes in the other subpopulations. However, the proportion of PMNs recovered from PAO1-infected airways by BAL increased 15-fold above control after NGF inhibition. Means ± SE (n = 5–6 rats per group). ***P < 0.001 compared with noninfected controls.
Cytokine/chemokine effects of NGF inhibition.
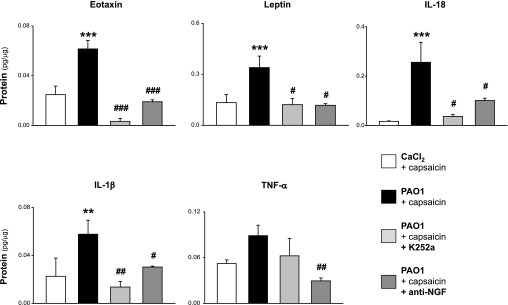
NGF inhibition with the specific blocking antibody or K252a, which blocks Trk signaling, decreased the concentrations of several inflammatory cytokines and chemokines in lung tissues from weanling rats after 5 days of PAO1 infection. In particular, the expression of eotaxin (P < 0.001), leptin (P < 0.05), IL-18 (P < 0.05), and IL-1β (P < 0.05) returned to noninfected control levels with both inhibitors (Fig. 9). A similar trend was also observed for TNF-α, but this effect was statistically significant only with the anti-NGF antibody (P < 0.01). NGF inhibition with either the specific blocking antibody or K252a had no significant effect on IL-2, IL-4, IL-9, IL-10, IL-13, IL-17, GRO/KC, MCP-1, and RANTES (all P > 0.5). IL-1α, IL-5, IL-6, IL-12, IP-10, G-CSF, IFN-γ, and MIP-1α were below the detection threshold of our analysis. No significant difference in cytokine/chemokine expression was found between infected and noninfected weanling rats in the absence of nerve stimulation (P > 0.3).
Fig. 9.
Effect of NGF inhibition on cytokines/chemokines. Neurotrophin inhibition with the specific blocking antibody or with K252a, which blocks Trk signaling, decreased significantly the concentrations of eotaxin, leptin, IL-18, and IL-1β in BAL specimens from PAO1-infected weanling rats, returning their values to noninfected control levels. A similar trend was also observed for TNF-α, but this effect was statistically significant only with the anti-NGF antibody. Means ± SE (n = 4–7 rats per group). **P < 0.01, ***P < 0.001 compared with noninfected controls; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with infected rats with intact NGF activity.
DISCUSSION
This study shows for the first time that lower respiratory tract infections with P. aeruginosa originating early after birth predispose to an array of innate neurogenic-mediated immunoinflammatory responses, as manifested by the local increase in microvascular permeability and PMN infiltration into infected lung parenchyma in response to chemical stimulation of the TRPV1 ion channels expressed on nociceptive vagal afferents. This response differs in many aspects from that shown previously for viruses and for other bacteria. In particular, it is exquisitely age-specific, as it occurs only when the infection originates in early life, but not in adulthood, whereas we have found increased neurogenic inflammation in adult rats infected with RSV (35), adenovirus (34), or parainfluenza virus (33), and others have shown the same with Mycoplasma pulmonis (23). Furthermore, P. aeruginosa affects in a similar fashion the extra- and intrapulmonary microvascular compartments, whereas the effect of viral infection in weanling and adult rats is restricted to the intra- or extrapulmonary airways, respectively (17, 35).
To our knowledge, this is also the first study using an animal model to dissect age-specific differences in the local inflammatory response of the respiratory tract to P. aeruginosa, and its results indicate that such differences are both qualitative and quantitative and highly significant. Only rats infected with PAO1 during adulthood (at 12 wk of age) and killed 30 days postinoculation evolved into a severe suppurative pneumonia consistent with previous description of similar rodent models (29). However, these chronically infected adult rats had no evidence of neurogenic inflammation, suggesting the involvement of different inflammatory pathways depending on the age of the host and time course of the infection (or reinfection), with predominance of neurogenic mechanisms during acute infections or exacerbations occurring in early life. This observation provides a plausible mechanism for the clinical efficacy of aggressive antibiotic therapy started from infancy and maintained throughout childhood even in the absence of overt respiratory disease.
Neurotrophic effects of P. aeruginosa.
The mechanism of P. aeruginosa-induced amplification of the nociceptive networks innervating the respiratory tract is similar to that recently described for RSV and derives from the local overexpression in infected respiratory epithelia (28) of specific neurotrophic genes, particularly NGF. However, differently from RSV, P. aeruginosa does not affect the expression of specific neurotrophic receptors. As we have shown that changes in neurotrophic expression caused by viral infections in rat airways mimic closely the response of infected human airways (45), we speculate that NGF may be involved in the early pathophysiology of airway inflammation and hyperreactivity during P. aeruginosa infections in infants and children and that pharmacological strategies targeting neurotrophic pathways may help delay the clinical manifestations of diseases like CF and bronchiectasis and/or prevent exacerbation episodes. Similar mechanisms have been implicated in other chronic inflammatory conditions, such as inflammatory bowel disease (21, 22) and rheumatoid arthritis (9, 48), supporting the involvement of neurotrophic and neurogenic mechanisms in human diseases characterized by dysregulation of innate immunity.
In addition to studying neurotrophic factors, we also looked at other growth factors involved in critical aspects of lung development like type II cell differentiation, branching morphogenesis, and vascular remodeling (10), but none of these factors changed significantly during P. aeruginosa infection. This observation indicates that neural development is selectively targeted by early-life P. aeruginosa infections.
Neuroimmune effects of P. aeruginosa.
In addition to its critical neurotrophic functions, NGF modulates the activity of multiple nonneural cells involved in inflammatory and immune responses (8), thus functioning as a powerful and eclectic neuroimmunomodulator bridging the gap between innate and adaptive immunity. Although NGF can affect immunity and inflammation both directly and indirectly through changes in sensory innervation, all biological effects described in this study required pharmacological activation of nociceptive vagal afferents, suggesting a predominantly indirect effect.
Thus P. aeruginosa induces a state of neural hyperreactivity in the developing respiratory tract by upregulating NGF expression; this, in turn, rapidly and persistently modifies the phenotype of local C-type nociceptive nerves, lowering their threshold of activation via overexpression of polymodal TRPV1 cation channels (50) and promoting the synthesis of neurotransmitters that will be released synaptically only if the airways are irritated by a second noxious stimulus (32). This “second hit”, provided in our experimental model by the selective TRPV1 agonist capsaicin, can be evoked in the course of natural infections in humans by a variety of physical and chemical irritants that have been shown to activate TRPV1 channels (4), including soluble and particulate environmental pollutants, osmotic changes in airway secretions (as in exercise-induced hyperventilation and in CF), warming or cooling of the airway mucosa (again related to exercise-induced hyperventilation), or acid pH (as in gastroesophageal reflux).
The sensory neurons affected by this “double hit” (i.e., combined or sequential exposure to infectious and irritant agents) have their soma located in the nodose or jugular vagal ganglia and extend their axon processes into the submucosal layer of the airways, reaching even into the single cell layer of ciliated airway epithelial cells. In addition, collateral axons can be found in close proximity to mast cells, capillaries, smooth muscle cells, and exocrine glands. Therefore, exposure of P. aeruginosa-infected airways to physical or chemical irritants can elicit protective reflexes processed via synaptic relay in the brainstem, which leads to coughing, respiratory depression or arrest, mucus hypersecretion, and bronchial hyperresponsiveness. Axonal collaterals can also mediate local protective responses without synaptic relay via secretion of proinflammatory neuropeptides like substance P, a process that is also referred to as neurogenic inflammation and has been implicated in the pathophysiology of CF (7).
Our present data suggest that similar neurogenic mechanisms are responsible for the increase in microvascular permeability and for the infiltration of PMNs into infected lung parenchyma, with the latter effect possibly mediated through induction of proinflammatory cytokines like IL-18 and IL-1β as discussed below. Thus selective immunological or chemical inhibition of this pathway may have profound anti-inflammatory effects especially at the parenchymal level. Interestingly, the decrease in tissue PMNs after NGF inhibition is compartmentalized and mirrored by a comparable increase in the BAL fluid. This suggests that the complex modifications induced by NGF in the cytokine/chemokine milieu, not only favor the recruitment of circulating PMNs from the bloodstream into the lung parenchyma, but also promote the buildup of these inflammatory cells inside the tissues preventing their escape toward the airways lumen.
Notably, the effect of NGF inhibitors on the PMN population collected by BAL was very large, highly statistically significant, and fully reproducible with both immune and chemical NGF inhibition. This finding not only has great pathophysiological importance because of the central role played by neutrophil proteases in the tissue damage that characterizes diseases like CF, but also implies that the information derived from BAL specimens obtained from these patients may not accurately reflect the parenchymal inflammation that essentially determines the clinical evolution and severity of the disease. In contrast, no significant numerical changes were measured in macrophages and lymphocytes after NGF inhibition, both at the lung parenchyma and airway level. Additional studies are necessary to dissect the mechanisms and pathophysiological relevance of this and other aspects of the local immune response to P. aeruginosa, such as the contribution of different T cell subpopulations that may respond differently to NGF inhibition.
An important neuroimmunomodulatory effect exerted by NGF during P. aeruginosa infection was the regulation of selected cytokines and chemokines, particularly eotaxin, leptin, IL-18, IL-1β, and TNF-α. All these molecules are exquisitely proinflammatory (1, 3, 25) and overexpressed in CF lungs (6, 26, 40, 43), and their cross talk with neurotrophic pathways uncovers important pathophysiological and potentially therapeutic implications. IL-1β and IL-18 are closely related structurally and functionally. Both are synthesized as precursor molecules cleaved by the enzyme caspase-1 and play important roles in both innate and acquired immune regulation and inflammation; therefore, they have been identified as therapeutic targets for a variety of inflammatory disorders. If it is confirmed in humans that these critical cytokines and chemokines operate downstream from the rapid NGF response of infected epithelial cells (28), then selective inhibitors of neurotrophic factors and receptors may benefit patients with CF, bronchiectasis, and immunocompromised hosts.
Leptin is involved in the regulation of energy homeostasis, and its expression is strongly associated with body mass. In our model, P. aeruginosa infection was associated with reduced weight gain and leptin overexpression, as in CF patients (43), and both were abolished by selective NGF inhibition. Leptin also exerts peripheral proinflammatory effects as a function of its cytokine-like structure, and its receptors belong to the cytokine class I receptor family. Therefore, leptin may play an important role in CF-related inflammation and the associated failure to thrive, and both effects may be NGF-dependent.
Neurotrophic modulation of the eosinophilotropic chemokine eotaxin is especially intriguing in the setting of allergic bronchopulmonary aspergillosis (ABPA), which is a major complication of persistent asthma and CF characterized by marked local and systemic eosinophilia in response to elevated levels of Aspergillus fumigatus-specific antibodies (25). It is not surprising that our F344 rats did not exhibit a significant eosinophilic response, as no antigen challenge was provided; also, this strain is intrinsically nonatopic and typically has very few circulating and tissue eosinophils. However, it is quite possible that similar levels of eotaxin expression, if confirmed in humans, could amplify eosinophilic-driven allergic reactions to fungal antigens.
As with any other animal model of P. aeruginosa infection (19), our model has several limitations. In particular, it does not have the genetic and phenotypic features of CF airways, but P. aeruginosa attacks also non-CF airways, and even using CF transmembrane conductance regulator (CFTR)-deficient rodents would not solve this problem. Second, our study reflects a single infection with P. aeruginosa, and it cannot reproduce exactly the consequences of the repeated exposures and long-term bacterial colonization typical of CF and immune deficits; furthermore, chronic studies of neurotrophic pathways in rodents are particularly difficult because the expression pattern of the genes involved changes rapidly with age (15). Third, the specific expression profile of cytokines and chemokines may be species-specific and may not reflect exactly the pathophysiology of human disease. Despite these limitations, however, we feel our findings open the way to new translational and clinical research targeting the role of neurotrophic and neuroimmune pathways in CF and other P. aeruginosa-associated human diseases.
Conclusions.
This study shows that lower respiratory tract infection with P. aeruginosa potentiates neurogenic-mediated immunoinflammatory responses in young rats infected early after birth but not in adults. The mechanism involves local overexpression in developing lungs of the gene encoding the prototypical neurotrophic factor NGF, which is selectively targeted, whereas other critical growth factors remain unchanged. NGF overexpression in early life is critical for the local increase in microvascular permeability and for the infiltration of PMNs into infected lung parenchyma. It also exerts systemic metabolic effects, negatively affecting body mass. These multiple activities are mediated, at least in part, by the induction of downstream inflammatory cytokines and chemokines, especially IL-1β and IL-18. Based on this information, we speculate that selective immunological or chemical inhibition of neurotrophic pathways during infancy and childhood may have clinical anti-inflammatory and therapeutic activity in the setting of chronic respiratory diseases characterized by P. aeruginosa infection, such as CF, bronchiectasis, and opportunistic bronchopneumonias.
GRANTS
This research was supported in part by grants from the National Institutes of Health to M. Doud (National Institute of General Medical Sciences Grant GM-061347) and to G. Piedimonte (National Heart, Lung, and Blood Institute Grant HL-61007 and National Institute of Child Health and Human Development Grant NCS-07-11).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Cheryl Walton and Debbie Piktel (West Virginia University) for their invaluable technical assistance and for running multiplex analysis, quantitative PCR, and ELISA, and we thank Dr. Lisa Schneper (Florida International University) for helping with the preparation of alginate microspheres.
REFERENCES
- 1.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 223: 20–38, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Auais A, Adkins B, Napchan G, Piedimonte G. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol 285: L105–L113, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17: 4–12, 2006 [PubMed] [Google Scholar]
- 4.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23: 360–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck H, Winter J. K252a modulates the expression of nerve growth factor-dependent capsaicin sensitivity and substance P levels in cultured adult rat dorsal root ganglion neurones. J Neurochem 67: 345–351, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Chan ED, Choi HS, Cool C, Accurso FJ, Fantuzzi G. Interleukin-18 expression in cystic fibrosis lungs. Chest 121: 84S–85S, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Choi JY, Khansaheb M, Joo NS, Krouse ME, Robbins RC, Weill D, Wine JJ. Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process. J Clin Invest 119: 1189–1200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppola V, Barrick CA, Southon EA, Celeste A, Wang K, Chen B, Haddad el B, Yin J, Nussenzweig A, Subramaniam A, Tessarollo L. Ablation of TrkA function in the immune system causes B cell abnormalities. Development 131: 5185–5195, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Covas MJ, Pinto LA, Pereira Da Silva JA, Victorino RM. Effects of the neuropeptide, substance P, on lymphocyte proliferation in rheumatoid arthritis. J Int Med Res 23: 431–438, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Desai TJ, Cardoso WV. Growth factors in lung development and disease: friends or foe? Respir Res 3: 2, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrell PM, Li Z, Kosorok MR, Laxova A, Green CG, Collins J, Lai HC, Makholm LM, Rock MJ, Splaingard ML. Longitudinal evaluation of bronchopulmonary disease in children with cystic fibrosis. Pediatr Pulmonol 36: 230–240, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Frederiksen B, Koch C, Hoiby N. Changing epidemiology of Pseudomonas aeruginosa infection in Danish cystic fibrosis patients (1974–1995). Pediatr Pulmonol 28: 159–166, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection. J Cyst Fibros 4: 49–54, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43: 143–201, 1991 [PubMed] [Google Scholar]
- 15.Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol 283: L494–L502, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Johansen HK, Hoiby N. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax 47: 109–111, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King KA, Hu C, Rodriguez MM, Romaguera R, Jiang X, Piedimonte G. Exaggerated neurogenic inflammation and substance P receptor upregulation in RSV-infected weanling rats. Am J Respir Cell Mol Biol 24: 101–107, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Koch C. Early infection and progression of cystic fibrosis lung disease. Pediatr Pulmonol 34: 232–236, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kukavica-Ibrulj I, Levesque RC. Animal models of chronic lung infection with Pseudomonas aeruginosa: useful tools for cystic fibrosis studies. Lab Anim 42: 389–412, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Lee TW, Brownlee KG, Denton M, Littlewood JM, Conway SP. Reduction in prevalence of chronic Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatr Pulmonol 37: 104–110, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Mantyh CR, Gates TS, Zimmerman RP, Welton ML, Passaro EP, Jr, Vigna SR, Maggio JE, Kruger L, Mantyh PW. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci USA 85: 3235–3239, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantyh PW, Catton MD, Boehmer CG, Welton ML, Passaro EP, Jr, Maggio JE, Vigna SR. Receptors for sensory neuropeptides in human inflammatory diseases: implications for the effector role of sensory neurons. Peptides 10: 627–645, 1989 [DOI] [PubMed] [Google Scholar]
- 23.McDonald DM, Schoeb TR, Lindsey JR. Mycoplasma pulmonis infections cause long-lasting potentiation of neurogenic inflammation in the respiratory tract of the rat. J Clin Invest 87: 787–799, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohtasham L, Auais A, Piedimonte G. Nerve growth factor mediates steroid-resistant inflammation in respiratory syncytial virus infection. Pediatr Pulmonol 42: 496–504, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Moss RB. Pathophysiology and immunology of allergic bronchopulmonary aspergillosis. Med Mycol 43, Suppl 1: S203–S206, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol 34: 146–162, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Ohmichi M, Decker SJ, Pang L, Saltiel AR. Inhibition of the cellular actions of nerve growth factor by staurosporine and K252A results from the attenuation of the activity of the trk tyrosine kinase. Biochemistry 31: 4034–4039, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Othumpangat S, Gibson LF, Samsell L, Piedimonte G. NGF is an essential survival factor for bronchial epithelial cells during respiratory syncytial virus infection. PLoS One 4: e6444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedersen SS, Shand GH, Hansen BL, Hansen GN. Induction of experimental chronic Pseudomonas aeruginosa lung infection with P. aeruginosa entrapped in alginate microspheres. APMIS 98: 203–211, 1990 [PubMed] [Google Scholar]
- 30.Piedimonte G. Neural mechanisms of respiratory syncytial virus-induced inflammation and prevention of respiratory syncytial virus sequelae. Am J Respir Crit Care Med 163: S18–S21, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Piedimonte G. Pathophysiological mechanisms for the respiratory syncytial virus-reactive airway disease link. Respir Res 3, Suppl 1: S21–S25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piedimonte G, Hegele RG, Auais A. Persistent airway inflammation after resolution of respiratory syncytial virus infection in rats. Pediatr Res 55: 657–665, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Piedimonte G, Nadel JA, Umeno E, McDonald DM. Sendai virus infection potentiates neurogenic inflammation in the rat trachea. J Appl Physiol 68: 754–760, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Piedimonte G, Pickles RJ, Lehmann JR, McCarty D, Costa DL, Boucher RC. Replication-deficient adenoviral vector for gene transfer potentiates airway neurogenic inflammation. Am J Respir Cell Mol Biol 16: 250–258, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Piedimonte G, Rodriguez MM, King KA, McLean S, Jiang X. Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs. Am J Physiol Lung Cell Mol Physiol 277: L831–L840, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Rabin HR, Butler SM, Wohl ME, Geller DE, Colin AA, Schidlow DV, Johnson CA, Konstan MW, Regelmann WE. Pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol 37: 400–406, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Rubin JS, Bradshaw R. Isolation and partial amino acid sequence analysis of nerve growth factor from guinea pig prostate. J Neurosci Res 6: 451–464, 1981 [DOI] [PubMed] [Google Scholar]
- 38.Saria A, Lundberg JM, Skofitsch G, Lembeck F. Vascular protein leakage in various tissue induced by substance P, capsaicin, bradykinin, serotonin, histamine and by antigen challenge. Naunyn Schmiedebergs Arch Pharmacol 324: 212–218, 1983 [DOI] [PubMed] [Google Scholar]
- 39.Schuijers JA, Walker DW, Browne CA, Thorburn GD. Peripheral and brain tissue catecholamine content in intact and anti-NGF-treated fetal sheep. Am J Physiol Regul Integr Comp Physiol 252: R7–R12, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Skinner ML, Schlosser RJ, Lathers D, Neal JG, Woodworth BA, Hall J, Newton DA, Baatz JE. Innate and adaptive mediators in cystic fibrosis and allergic fungal rhinosinusitis. Am J Rhinol 21: 538–541, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Song Z, Moser C, Wu H, Faber V, Kharazmi A, Hoiby N. Cytokine modulating effect of ginseng treatment in a mouse model of Pseudomonas aeruginosa lung infection. J Cyst Fibros 2: 112–119, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Song Z, Wu H, Mathee K, Hoiby N, Kharazmi A. Gerimax ginseng regulates both humoral and cellular immunity during chronic Pseudomonas aeruginosa lung infection. J Altern Complement Med 8: 459–466, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Stylianou C, Galli-Tsinopoulou A, Koliakos G, Fotoulaki M, Nousia-Arvanitakis S. Ghrelin and leptin levels in young adults with cystic fibrosis: relationship with body fat. J Cyst Fibros 6: 293–296, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Taccetti G, Campana S, Festini F, Mascherini M, Doring G. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J 26: 458–461, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med 172: 233–237, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res 47: 1–9, 1980 [DOI] [PubMed] [Google Scholar]
- 47.Wilfong ER, Dey RD. Nerve growth factor and substance P regulation in nasal sensory neurons after toluene diisocyanate exposure. Am J Respir Cell Mol Biol 30: 793–800, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Yokoyama MM, Fujimoto K. Role of lymphocyte activation by substance P in rheumatoid arthritis. Int J Tissue React 12: 1–9, 1990 [PubMed] [Google Scholar]
- 49.Zar JH. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice-Hall, 1984 [Google Scholar]
- 50.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 24: 4211–4223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]