Abstract
A decrease in vascular elasticity and an increase in pulse wave velocity in hyperhomocysteinemic (HHcy) cystathionine-β-synthase heterozygote knockout (CBS−/+) mice has been observed. Nitric oxide (NO) is a potential regulator of matrix metalloproteinase (MMP) activity in MMP-NO-tissue inhibitor of metalloproteinase (TIMP) inhibitory tertiary complex. However, the contribution of the nitric oxide synthase (NOS) isoforms eNOS and iNOS in the activation of latent MMP is unclear. We hypothesize that the differential production of NO contributes to oxidative stress and increased oxidative/nitrative activation of MMP, resulting in vascular remodeling in response to HHcy. The overall goal is to elucidate the contribution of the NOS isoforms, endothelial and inducible, in the collagen/elastin switch. Experiments were performed on six groups of animals [wild-type (WT), eNOS−/−, and iNOS−/− with and without homocysteine (Hcy) treatment (0.67 g/l) for 8–12 wk]. In vivo echograph was performed to assess aortic timed flow velocity for indirect compliance measurement. Histological determination of collagen and elastin with trichrome and van Gieson stains, respectively, was performed. In situ measurement of superoxide generation using dihydroethidium was used. Differential expression of eNOS, iNOS, nitrotyrosine, MMP-2 and -9, and elastin were measured by quantitative PCR and Western blot analyses. The 2% gelatin zymography was used to assess MMP activity. The increase in O2− and robust activity of MMP-9 in eNOS−/−, WT+Hcy, and eNOS−/−+Hcy was accompanied by the gross disorganization and thickening of the ECM along with extensive collagen deposition and elastin degradation (collagen/elastin switch) resulting in a decrease in aortic timed flow velocity. Results show that an increase in iNOS activity is a key contributor to HHcy-mediated collagen/elastin switch and resulting decline in aortic compliance.
Keywords: aorta stiffness, matrix metalloproteinase, tissue inhibitor of metalloproteinase, reactive oxygen species, nitrotyrosine, superoxide dismutase, thioredoxin, NOX4, endothelial function, hypertension
homocysteine (Hcy) is a toxic sulfur-containing, nonprotein amino acid. Excessive circulating Hcy or hyperhomocysteinemia (HHcy) is now considered an independent risk factor for development of cardiovascular diseases including ischemic heart disease, stroke, and peripheral vascular disease. It has been well-documented that Hcy is associated with alterations in both the vascular wall structure and function. Although the exact pathophysiological mechanism(s) linking HHcy to vascular disease remain(s) unclear, numerous studies have suggested that Hcy increases oxidative stress, limits the bioavailability of nitric oxide (NO), and alters the elastic properties of vascular walls by increasing matrix metalloproteinase (MMP) activity (13). Previous studies have also found that HHcy does not greatly affect endothelial-mediated NO production but rather decreases its bioavailability, which contributes to structural and functional changes or vascular dysfunction. In contrast, Hcy has also been shown to cause the upregulation of inducible nitric oxide synthase (iNOS), which may contribute to the inflammatory response that characterizes atherogenesis and may account for the adverse effects of Hcy. Interestingly, endothelial-derived NO has been found to significantly contribute to Hcy-mediated nitrotyrosine generation, MMPs activation, and endothelial dysfunction. It is not completely understood how Hcy instigates changes in redox homeostasis and MMP-mediated vascular remodeling; however, both are mediated by changes in NO. Therefore, recent studies led us to question the actual contribution of NO and more specifically the differential contribution of endothelial nitric oxide synthase (eNOS) or iNOS isoforms in Hcy-mediated vascular dysfunction.
It has been shown that Hcy instigated reactive oxygen species (ROS) production. The data support previous findings that Hcy instigates ROS production by several mechanisms independent of the conventional Hcy autooxidation by the increased expression of NADPH oxidase subunit NOX4. These studies also suggested that, although antioxidant systems remain elevated, the marked increase in ROS generation is overwhelming and difficult to reduce and can lead to alterations in tissue morphology. Also, the increased ROS generation has a direct affect on the levels of eNOS mRNA that suggests an upregulation of the eNOS protein. The involvement of iNOS and its large NO production along with eNOS leads to the increased generation of ONOO−, decreased NO bioavailability, and subsequent elevations in nitrotyrosine.
The vascular remodeling therefore alters vascular function by decreasing the aortic blood flow velocity. In addition to structural alterations, Hcy-generated radicals stimulated the generation of ONOO−. This could directly damage the vascular endothelium, impair NO production, and decrease normal endothelial-mediated vasorelaxation. In isolated aortic rings treated with Hcy, there was an attenuation of aortic relaxation to the endothelium-dependent agonist ACh (4). NO-mediated vasodilation was impaired in a dose-response manner in healthy subjects during short-term HHcy induced by methionine loading (25). In addition, direct Hcy-induced endothelial cell damage or cell death could naturally decrease the ability of the endothelium to produce and release NO. Hcy-mediated endothelium dysfunction has been reported in human and animals models of HHcy and is primarily mediated by oxidative inactivation of NO, further suggesting a role of eNOS and iNOS in vascular damage (4). Also, the increase in collagen deposition and resulting decrease in vascular elasticity may have mediated increased vasoconstrictor sensitivity. These data are supported by previous studies showing that the oxidative environment created by Hcy and the reduction in NO bioavailability both contribute to increased MMP-mediated vascular remodeling and resulting vascular pathologies (19, 22). These results strongly suggest that MMPs are potentially major mediators of arterial remodeling and that NO is an important factor to consider. Furthermore, the process of abnormal vascular remodeling and resulting hypertrophy may be mediated by the actions of iNOS.
MMP-2 is constitutive, and MMP-9 is inducible. Tissue inhibitor of metalloproteinase-3 (TIMP-3) induces apoptosis (1). TIMP-4 regulates MMP-9 (17). In addition to nitrotyrosine, increased oxidative radicals mediated the increased activation of MMP-9 and subsequent degradation of ECM components elastin and collagen. However, because the turnover of collagen is fast, more collagen is laid down in the outer interstitial and inner medial layers of the aorta wall. The Hcy-mediated elevation in MMP expression and activity results in structural remodeling of the vessel wall. Balanced composition of the ECM is necessary to maintain proper structural geometry and function of vessels and tissues (22). MMPs, specifically gelatinases MMP-2 and -9, are involved in maintaining this balance by actively participating in the degradation and synthesis of the ECM components. Previous studies have shown that composition and morphological changes in the vessel wall in atherosclerosis and hypertension are associated with increased MMP activity (1, 12). Hcy is known to increase MMP protein expression as well as the activity of latent inactive MMPs (23). Consequently, increased MMP activity is responsible for excessive degradation and accumulation of matrix components elastin and collagen and resulting pathological remodeling. Abnormal and uncontrolled proteolytic activity is detrimental to vascular tissues and can cause alterations in vessel wall structure and function.
NO is known to be coordinated in the activation site of MMPs, therefore blocking the activation site and having a role in its latency (3, 14). TIMPs specifically interact with pro-MMPs by wrapping themselves around the catalytic domain, holding MMPs in a latent, inactive state. As a result of Hcy-generated ROS, the MMP-TIMP-NO inhibitory tertiary complex is disrupted, leading to the activation of latent MMPs (24). Previously, we (9) have shown in the basement membrane of endothelial cell that NO mediated the latency of MMPs. Interestingly, the MMP-mediated ECM degradation induces cellular proliferation and vascular hypertrophy (20). We hypothesize that Hcy-mediated aortic structural remodeling is caused by differential NO bioavailability that mediates TIMP expression and enhanced MMP activity. Hcy increases peroxynitrite, therefore increasing nitrotyrosine. During this process, Hcy-generated ONOO− activates latent MMPs. Although Hcy attenuates eNOS-NO bioavailability, it induces iNOS. Hcy attenuates NO bioavailability in the vicinity of endothelial, independent of iNOS. The differential expression of each NOS isoform produces differential oxidative stress that causes vascular remodeling. After considering the role of each NOS isoform and their distinctive differential production of NO, we further hypothesize that iNOS is a significant contributor of NO and key stimulus of the deleterious vascular effects initiated by Hcy.
METHODS
Animals.
The following male mouse (8–12 wk) strains were obtained from The Jackson Laboratory (Bar Harbor, ME): C57BL/6J, eNOS knockout (B6.129P2-NOS3tm1Unc/J), iNOS knockout (B6.129P2-NOS2tm1Lau/J), and nNOS knockout (B6.129S4-NOS1tm1Plh/J), including wild-type (WT). The mice were split into two experimental groups: the control group (WT, eNOS−/−, and iNOS−/−), administered normal saline in drinking water; and treatment group (WT, eNOS−/−, and iNOS−/−+Hcy), administered Hcy in drinking water. Each group of mice was fed normal rodent chow (Rodent Laboratory Chow) and had free access to the food and water. Each group was used to access a noninsult model (no direct injury to vascular endothelium or surgical alteration) of vascular remodeling. The genotype and phenotype information was collected from the JAX Mice Database. The care and use of mice in the present study was done in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Louisville School of Medicine. Euthanasia was accomplished by giving an overdose of anesthesia at the end of the experiment.
Hcy supplementation.
To create a condition of mild HHcy, Hcy was supplemented [0.67 g DL-Hcy (Sigma H-4628)/l drinking water] for 8–12 wk. The low Hcy diet was selected because studies in rats had illustrated that this dose induced mild HHcy and was well-tolerated. The dose was calculated by assuming an average water intake of 3–4 ml/day and an average body wt of 30 g. All mice were given standard mouse chow and Hcy water ad libitum. The plasma levels of Hcy were measured by HPLC (16).
Echocardiography.
A Hewlett Packard Sonos 5500 echocardiographic system equipped with a 12-MHz shallow-focus 15-6L (21211A) phased-array transducer was used for the measurement of aortic timed flow velocity. This is the most common noninvasive technique for evaluating cardiovascular physiology and function in anesthetized mice (7). The transducer probe was placed on the left hemithorax of mice in the partial left decubitus position of the shaved chest. Two-dimensional pulsed-wave Doppler echocardiograms were obtained from a long-axis view, directly below the aortic arch. The Doppler probe measured aortic blood timed averaged velocity (centimeters per second) by detecting the difference in frequency between an emitted burst of ultrasound (12 MHz) and the returning echoes from moving blood. Flow velocity can be defined as an average blood volume per second per unit area of the vessel. Doppler flow recordings allow the time-based measurement of acceleration time in addition to the peak aortic blood flow velocity.
Vascular reactivity.
Aortic rings were mounted in a 25-ml tissue myobath containing physiological saline solution (PSS) maintained at 37°C and aerated with a 95% O2-5% CO2 gas mixture at pH 7.4. A total resting tension of 2.0 g was applied stepwise, and each ring was allowed to equilibrate for 60 min before dose-response curves were generated by cumulative addition of α-adrenergic agonist phenylephrine (PE). Aortas were then washed with several changes of PSS and constricted to ∼50% of maximum with PE. Relaxation dose-response curves were generated by cumulative doses of endothelial-dependent ACh (10−9 to 10−6 M). The percent relaxation was calculated based on 100% contraction to 10 nM PE. Changes in isometric tension were recorded using a FORT 10 force transducer and an Acknowledge 3.7.3 MP 100 data acquisition system (World Precision Instruments).
Tissue processing.
At the end of the experiment, the anesthetized mice were prepared for the excision of heart, aorta, left and right kidneys, and liver. Harvested tissue was weighed for comparison between groups. All harvested tissues were washed three to four times with cold PBS. Tissues for molecular analysis (Western blot and zymography) were finely cut and homogenized on ice in 300 μl of indicated ice cold lysis (extraction) buffer and incubated at 4°C with continuous agitation for 24 h without stirring. Western blot lysis buffer contained 25 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 1 mM EDTA, and inhibitor cocktail. Zymographic extraction buffer contained 10 mmol/l cacodylic acid at pH 5.0, 0.15 mol/l NaCl, 1 μmol/l ZnCl2, 20 mmol/l CaCl2, 15 mmol/l NaN3, and 0.01% (vol/vol) Triton X-100. The aortic samples were centrifuged at 14,000 g for 15 min at 4°C. The remaining supernatant fraction containing membrane and soluble proteins was used for further analysis. Protein content was determined by the Bradford method with BSA as the standard. Homogenates were stored at −80°C. A portion of the aortas was embedded in tissue-freezing media for subsequent histological analysis, and another portion was processed for PCR. Whole blood from each animal was also collected in tubes containing a 1:10 ratio to sodium heparin, rapidly mixed, and centrifuged at ∼4,000 g, and the supernatant was transferred to a separate tube and frozen for further analysis.
Histopathology.
To determine whether changes in NOS/NO caused remodeling of vessel wall, descending thoracic aortas were embedded in tissue-freezing medium [Triangle Biomedical Science (TBS), Durham, NC] and snap-frozen in liquid nitrogen. The frozen aortas were sectioned transversely at 8–10 μm with a cryostat (Leica Cryostat 1800; Leica Microsystems), mounted onto Superfrost Plus microscope slides (Fisher Scientific) and coverslipped. The unfixed tissue sections were stained with hematoxylin and eosin (H&E) for general tissue morphology, trichrome for collagen, and van Gieson stain for elastin. The images were taken with a Q-Color 3 digital camera on an IX81 inverted microscope with ×100/1.35 UPlanApo objective (Olympus) and confocal microscope at different magnifications. The aortic medial thickness was measured by a micrometer. Levels of collagen and elastin were measured by colorimetric estimation of isodesmosine and desmosine, respectively, as previously described (5).
In situ determination of ROS generation.
Frozen aorta cross-sections from control and Hcy-treated WT, eNOS−/−, and iNOS−/− were incubated with the nonfluorescent probe 2,7′-dichlorofluorescein (DCFH) to determine ROS generation. To assess ROS formation in response to HHcy, oxidative stress was assessed by incubating aortic homogenates with a nonfluorescent probe, DCFH. DCFH acquires fluorescence properties on reaction with ROS and yields the fluorescent product dichlorofluorescein (DCF). This product can be detected at an emission wavelength of 530 nm (excitation wavelength of 485 nm). Briefly, aortic tissue samples were harvested, placed in cold PBS, and embedded/snap-frozen in tissue-freezing medium (TBS) for cryosectioning. Unfixed aortic segments were cut into 30-μm thick sections using a Reichert-Jung Cryocut 1800 cryostat and thaw-mounted on Superfrost Plus (Fisher Scientific) microscope slides. DCFH (10 μmol/l) was topically applied, and slides were incubated for 30 min in a light-protected humidified chamber at 37°C. DCFH was removed, and sections were coverslipped. Images were obtained in a darkened microscopy room with a laser scanning confocal fluorescence microscope using a long-pass filter (DCFH excitation at 485 nm and detection of fluorescence emission at 530 nm). Study groups and control were processed and imaged in parallel. All images were acquired using identical confocal settings for a given magnification (laser power, iris, gain, and black level). Fluorescence was detected by confocal microscopy (model FV-1000; Olympus). Images were analyzed using Matrox Inspector and presented as mean pixel intensity.
Western blot analysis.
To assess the effects of HHcy on the expression of nitrotyrosine, TIMP-3 and -4, MMP-2 and -9, and β-actin, aortic tissue homogenates were prepared. To the sample, a 5- to 15-μl volume of 2× reducing buffer [1 ml of 0.5 mM Tris·HCl (pH 6.8), 800 μl of glycerol, 400 μl of 2-mercaptoethanol, 1.6 ml of 10% (wt/vol) SDS, and 400 μl of 0.05% (wt/vol) bromophenol blue] was added. Samples were boiled at 95–100°C for 5–10 min. Approximately 15–30 μl of each sample (15 μg of total protein in each sample) and molecular mass marker were loaded and electrophoresed for 120 min at 120 mV on 8–15% SDS-PAGE gel, dependent on molecular mass of protein of interest. Proteins were then transferred overnight to polyvinylidene difluoride membranes in transfer buffer at 4°C and 90 mA. Nonspecific binding sites on the membranes were blocked for 1 h with 5% (wt/vol) nonfat dry milk in Tris-buffered saline with 0.05% Tween 20 (TBS-T). The membranes were incubated with indicated primary antibodies [appropriate dilutions in 5% (wt/vol) nonfat dry milk in TBS-T] for 2 h followed by five washes with TBS-T and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody [appropriate dilution in 5% (wt/vol) nonfat dry milk in TBS-T]. Proteins of interest were detected using a standard enhanced chemiluminescence (ECL) system (Amersham, Arlington Heights, IL) that specifically reacts with the alkaline phosphatase-conjugated secondary antibody. Proteins corresponding to a particular molecular mass were visualized by exposure of the membrane to Kodak chemiluminescence film. Actin blots were used as a loading control. The film bands were scanned using UN-SCAN-IT software and digitized, and densitometry was performed. To verify equal loading, the membranes were stripped with 0.1 N NaOH, blocked, incubated overnight with mouse monoclonal anti-β-actin primary antibody (Sigma) and an HRP-conjugated goat anti-mouse antibody (Santa Cruz Biotechnology), and developed by ECL. Band densities of the control were averaged, and treatment densities were presented as a fold change of the control average. Gels were stained with Coomassie blue to ensure transfer of protein.
Trx-1, Cu/Zn SOD, and nitrotyrosine.
Aortic homogenates were analyzed on different percent gels based on molecular mass of protein and transferred to polyvinylidene difluoride membranes. Membranes were incubated with primary antibody, rabbit polyclonal thioredoxin (Trx; diluted 1:1,000), rabbit polyclonal SOD (diluted 1:1,000), and mouse monoclonal nitrotyrosine (diluted 1:1,000). After a series of washing, membranes were incubated with either goat anti-mouse or goat anti-rabbit secondary antibody conjugated to HRP (diluted 1:3,000).
Zymography.
To determine whether HHcy induces MMP-2 and -9 activity and subsequent vascular remodeling in the aorta, SDS-PAGE gelatin zymography was performed as previously described. Zymography is an electrophoretic technique used to identify proteolytic activity in enzymes separated in polyacrylamide gels under nonreducing conditions. To the aortic homogenates, 5–15 μl of nonreducing buffer was added, and 15–30 μl of sample was loaded with identical amounts of total protein (30 μg) and separated in 8% polyacrylamide gels containing 2% gelatin (2 mg/ml; Sigma). After electrophoresis, gels were washed two to three times for 20 min each in 2.5% Triton-X 100 for the removal of SDS and renaturation of MMPs. The gel was then incubated for 24 h at 37°C in activation buffer [5 mmol/l Tris·HCl (pH 7.4), 0.005% (vol/vol) Brij 35, and 1 mmol/l CaCl2] to allow proteases to digest the surrounding substrate. Gels were stained with Coomassie blue stain (stain adheres to gelatin) until proteolytic activity was identified as clear bands on a blue background, sites of protease digestion of the gelatin substrate. The lytic bands were scanned using Gel Logic 200 image station. Band densities were normalized to control and presented as fold change. Identity of MMPs is based on their molecular mass and confirmed by Western blot analysis.
RT-PCR.
PCR is a method for generating unlimited copies of specific DNA or RNA fragments. Expression of GAPDH (housekeeping gene), MMP-2 and -9, Trx, and NOX4 mRNA were determined with RT-PCR. DNA-free total RNA was isolated from thoracic aortas with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Quantification and purity of the RNA was assessed by A260/A280 absorption (1 A260 unit equals 50 μg of double-stranded DNA/ml). Aliquots (2 μg) of total RNA were reverse-transcribed into cDNA for 5 min at 70°C using 1-μl oligo(dT15) primers (Invitrogen) in a final volume of 5 μl. To this mixture, 4-μl 5× PCR reaction buffer, 4-μl 5 mM MgCl2, 1-μl 10 mM dNTPs, 0.5-μl reaction Im-Prom-II RT, and 20 units of 0.5-μl rRNasin were added for a total volume of 20-μl sample. The mixture was incubated for 60 min at 42°C, heated to 70°C for 15 min, and cooled to 4°C. Sequence-specific oligonucleotide primers were prepared commercially (Invitrogen; Table 1). Each PCR was performed in a final reaction volume of 20 μl containing 2 μl of the cDNA, 10 μl of master mix (Promega), 1.5 μl of forward primer, 1.5 μl of reverse primer, and 5 μl of water. PCR amplification reactions were performed with a DNA Engine Peltier Thermal Cycler (Bio-Rad). Amplified products were resolved on a 1% agarose gel in the presence of ethidium bromide and detected under UV transillumination using the Gel Logic 200 imaging system (Kodak). The band intensities of the PCR product were analyzed with scanning densitometer software and normalized to GAPDH band intensity.
Table 1.
Genes, primer sequences, and base pairs used for quantitative RT-PCR analysis
| Gene | Primer Sequence, 5′→3′ | bp |
|---|---|---|
| eNOS | TTCCGGCTGCCACCTGATCCTAA (Forward) | 340 |
| AACATATGTCCTTGCTCAAGGCA (Reverse) | ||
| iNOS | ATGACCAGTATAAGGCAAGC (Forward) | 429 |
| GCTCTGGATGAGCCTATATTG (Reverse) | ||
| NOX4 | CCAGAATGAGGATCCCAGAA (Forward) | 393 |
| TGGAACTTGGGTTCTTCCAG (Reverse) | ||
| Trx | TGGATCCATTTCCATATGGT (Forward) | 210 |
| CCTTGTTAGCACCGGAGAAC (Reverse) | ||
| MMP-2 | ACACTGGGACCTGTCACTCC (Forward) | 599 |
| CCCAGCCAGTCTGATTTGAT (Reverse) | ||
| MMP-9 | GAAGGCAAACCCTGTGTGTT (Forward) | 469 |
| GGATGCCGTCTATGTCGTCT (Reverse) | ||
| Elastin | TGGTGCTACATGTTGGTGCT (Forward) | 645 |
| CAGTGTGAGGAGCCATCTGA (Reverse) | ||
| GAPDH | ACCACAGTCCATGCCATCAC (Forward) | 113 |
| TCCACCACCCTGTTGCTGTA (Reverse) | ||
| G-eNOS | TGGCTACCCGTGATATTGCT | WT, 422 |
| ATTTCCTGTCCCCTGCCTTC | −/−, 500 | |
| GGCCAGTCTCAGAGCCATAC | ||
| G-iNOS | ACATGCAGAATGAGTACCGG | WT, 108 |
| TCAACATCTCCTGGTGGAAC | −/−, 270 | |
| AATATGCGAAGTGGACCTCG |
eNOS and iNOS, endothelial and inducible nitric oxide synthase; NOX4, NADPH oxidase; Trx, thioredoxin; MMP, matrix metalloproteinase; G-eNOS and G-iNOS, genomic eNOS and iNOS; WT, wild-type.
Mouse genotype.
Total DNA was extracted from pooled thoracic aortas using the Gerard Biotech Spin Doctor Genomic DNA Isolation Kit (MidSci-G110L) according to the manufacturer's instructions. To the isolation tube, 500 μl of genomic resuspension buffer was added. Next, 2–3 mm of mouse tails from identified mice was added to the tube and incubated at 57°C overnight. Approximately 800 μl of genomic DNA buffer was added to the tube and then vortexed. To the mixture, 600 μl of isopropanol was added and tube-vortexed. The sample was spun at 14,000 g for 5 min. The supernatant was poured off. The remaining sample was air-dried until alcohol was totally evaporated. To the sample, 250 μl of final resuspension buffer was added, and the sample was vortexed and spun down at 14,000 g for 5 min. The 200-μl supernatant was transferred to a clean tube, and DNA was ready for PCR. Selection of specific primer sequence was done using The Jackson Laboratory genotyping protocol for eNOS and iNOS. The eNOS and iNOS primers (Table 1) were prepared commercially (Invitrogen). PCR amplification reactions were performed with a DNA Engine Peltier Thermal Cycler (Bio-Rad). Amplified products were resolved on a 2% agarose gel in the presence of ethidium bromide and detected under UV transillumination using the Gel Logic 200 imaging system (Kodak). The band intensities of the PCR product was analyzed with scanning densitometer software and normalized to WT band intensity. An aliquot of 20 μl was prepared as follows: eNOS, initiation at 94°C (3 min), followed by 35 cycles consisting of denaturation at 94°C (0.35 s), annealing at 65°C (1 min), and a final extension at 72°C (2 min), analyzed by 2% agarose gel electrophoresis; iNOS, initiation at 94°C (1.5 min), followed by 35 cycles consisting of denaturation at 94°C (0.30 s), annealing at 59°C (0.30 s), and a final extension at 72°C (0.30 s), analyzed by 3% agarose gel electrophoresis (primers: eNOS: WT, 422 bp, and −/−, 500 bp; iNOS: WT, 108 bp, and −/−, 270 bp).
eNOS, iNOS, NOX4, and Trx-1.
RT-PCR was performed on mRNA extracted from mouse aorta extracts. Amplification of mouse GAPDH, eNOS, and iNOS was started with initial denaturation at 94°C (2 min) followed by 29 cycles consisting of denaturation at 94°C (0.30 s), annealing at 57°C (0.30 min), and extension at 72°C (1 min). After the last cycle, a final extension was done at 72°C (2 min). PCR cycling was initiated at 94°C (3 min), followed by 30 cycles consisting of denaturation at 94°C (1 min), annealing at 53°C (1-min NOX4) and 55°C (0.40-s thioredoxin), and a final extension at 72°C (5 min). After amplification, mixtures were analyzed by 1% agarose gel electrophoresis.
Statistical analysis.
Statistical analysis was performed using GraphPad InStat to compare data collected from groups. Differences between groups and controls were determined by one-way ANOVA. If a significant difference was indicated, the Tukey post hoc test was used to identify groups that were significant. A P < 0.05 probability level was considered statistically significant. All values are presented as means ± SE.
RESULTS
The genotype data for eNOS−/− and iNOS−/− mice revealed that mice referenced as eNOS−/− and iNOS−/− are indeed pure homozygote knockout in respective NOS genes (Fig. 1). The Hcy administration caused increase in plasma levels of Hcy to 20–30 μM within 1 day after administration compared with 2–3 μM in untreated mice. The gravimetric data revealed cardiac hypertrophy in WT mice treated with Hcy (Table 2).
Fig. 1.
Genotype of endothelial nitric oxide synthase (eNOS−/−) and inducible nitric oxide synthase (iNOS−/−). Tail was clipped. The DNA was isolated and amplified using eNOS- and iNOS-specific primers. A shift in mobility to higher molecular mass was observed in the disrupted DNA allele in both eNOS−/− and iNOS−/− mouse DNA with respect to their wild-type (WT) allele. PCR was performed using sequence-specific primers for the WT and disrupted mutant allele. The 422-bp band indicates a WT eNOS gene, whereas the 500-bp band indicates a disrupted eNOS gene. The 108-bp band indicates a WT iNOS gene, whereas the 270-bp band indicates a disrupted iNOS gene. Mr, molecular mass marker.
Table 2.
Gravimetric measurements of control WT, eNOS−/−, and iNOS−/− treated with and without homocysteine (Hcy)
| Body Weight (BW), g | Heart Weight (HW), mg | HW/BW, mg/g | |
|---|---|---|---|
| WT | 33 ± 1 | 161 ± 20 | 4.87 ± 0.75 |
| eNOS−/− | 31 ± 1 | 153 ± 21 | 4.93 ± 0.80 |
| iNOS−/− | 29 ± 1 | 160 ± 20 | 5.52 ± 0.65 |
| WT+Hcy | 33 ± 1 | 187 ± 10 | 5.67 ± 0.73* |
| eNOS−/−+Hcy | 31 ± 1 | 158 ± 21 | 5.10 ± 0.85 |
| iNOS−/−+Hcy | 32 ± 0.5 | 136 ± 10 | 4.25 ± 0.90 |
Values are means ± SE of 10 animals for BW and 6 animals for wet HW. BW is in grams, and HW is in milligrams.
P < 0.05 compared with WT.
Aortic function.
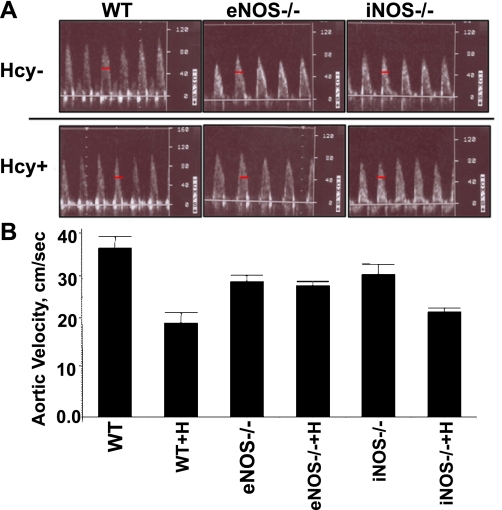
Pulsed wave echocardiography was used to noninvasively determine the aortic flow velocity (centimeters per second), a useful indirect index of arterial stiffness. Aortic blood flow velocity acceleration time measurements were taken from the recorded aortic blood flow velocity waveforms postechocardiography. There was a significant reduction (P < 0.05) in aortic blood flow acceleration time in the WT+Hcy compared with the WT (Fig. 2). To investigate the role of NO in Hcy-mediated changes in aortic blood flow velocity acceleration time, eNOS−/− and iNOS−/− were studied with and without Hcy treatment. Aortic blood velocity acceleration was not significantly different in the eNOS−/− and iNOS−/− control and Hcy-treated mice.
Fig. 2.
Noninvasive echographic measurement of the average aortic velocity acceleration in WT, WT treated with homocysteine (WT+H), eNOS−/−, eNOS−/− treated with homocysteine (eNOS−/−+H), iNOS−/−, and iNOS−/− treated with homocysteine (iNOS−/−+H) mice. A: representative waveforms from WT, eNOS−/−, and iNOS−/− mice treated without homocysteine (Hcy−) and with homocysteine (Hcy+). Velocity acceleration was determined from pulsed Doppler waveforms obtained from aorta in anesthetized mice and measured in centimeters per second as an indicator of change in time (the dash bar is a marker of time). B: bars are means ± SE (n = 4). There was a significant reduction (P < 0.001) in the aortic blood flow velocity in WT+H compared with WT.
Aortic vasoreactivity.
Dose-response curves were generated for aorta from WT and WT+Hcy mice to the contractile agonist PE and the endothelial-dependent vasorelaxant ACh. At higher doses, there was significant difference in the response to PE in aortas from WT+Hcy compared with WT (Fig. 3A). Also, there was rightward shift in ACh dose-response and relaxation in aortas from WT+Hcy compared with WT (Fig. 3B). These data suggested both adrenergic as well as endothelial dysfunction in Hcy-treated WT mice.
Fig. 3.
Response to phenylephrine (PE) and ACh in aorta from WT and WT+H mice. A: dose-response to PE [−log (PE)] was recorded in tension generated in grams. B: ACh relaxation response [−log (ACh)] was measured. Data expressed are percent to maximum PE contraction. Data represent means ± SE (n = 4); *P < 0.05.
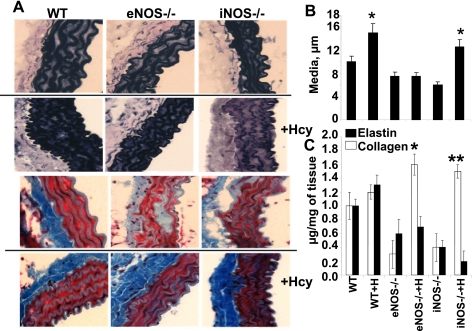
Aortic histology.
The aortic media thickness was significantly higher in WT treated with Hcy (Fig. 4A). In eNOS−/−, Hcy has no effect on medial thickness. Interestingly, the iNOS−/− elicits increase in medial thickness after Hcy challenge (Fig. 4B). These data may suggest a role of eNOS in vascular hypertrophy in WT and iNOS−/− mice treated with Hcy. Figure 4C shows that although elastin is decreased in both knockouts before Hcy treatment, it is not significantly altered and, if anything, is slightly increased in the eNOS knockout with Hcy treatment. These data may suggest a switch in elastin and collagen contents after Hcy treatment.
Fig. 4.
A: histological analysis of elastin (top 6 panels) in frozen aortic sections, stained with van Gieson for elastic fibers in mouse treated without and with Hcy. Images were visualized using optical light microscopy at ×40 magnification. Black staining indicates elastin. Note the thinning of the inner elastic lamina in both the WT and iNOS−/− treated with Hcy. Histological analysis of collagen (bottom 6 panels) in frozen aortic sections, stained with Masson trichrome for collagen fibers in mouse treated without and with Hcy. Images were visualized using optical light microscopy at ×40 magnification. Blue staining indicates sites of collagen deposition or degradation, and red stain is vascular smooth muscle. Note the increase in collagen in all Hcy-treated groups in contrast to the decrease in collagen in eNOS−/− and iNOS−/− controls. B: bar graphs represent aortic wall medial thickness in micrometers. C: elastin collagen contents in aortas from WT, WT+H, eNOS−/−, eNOS−/−+H, iNOS−/−, and iNOS−/−+H mice. Each bar represents mean ± SE (n = 4). *P < 0.05 collagen compared with WT. **P < 0.05 elastin compared with untreated group.
Remodeling.
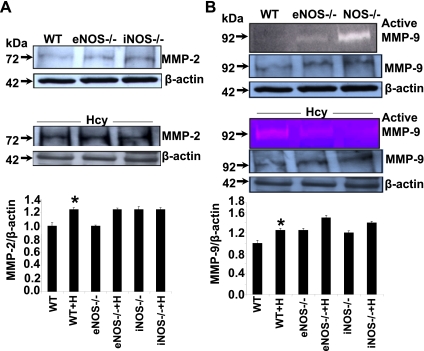
Remodeling by its very nature implies synthesis and degradation of matrix components, so we measured MMP and TIMP levels. Except MMP-2 levels in eNOS−/− (Fig. 5A), the levels of MMP-2 and -9 were increased in WT and knockouts after Hcy treatment (Fig. 5, A and B). These data suggest that eNOS is required for Hcy-mediated MMP-2 induction, suggesting constitutive role of MMP-2 and eNOS in vascular physiology, whereas MMP-9 is induced in pathophysiological condition.
Fig. 5.
Effect of Hcy on matrix metalloproteinases MMP-2 and -9. A: Western blot analysis of tissue lysates from aorta of mice in control and Hcy-treated WT, eNOS−/−, and iNOS−/−. Bands indicate MMP-2 and β-actin to verify equal loading of protein. Bar graph for MMP-2 expression quantified by densitometry and expressed fold change from WT control and fold change from WT+H. Bars are means ± SE (n = 3). B: active MMP-9 was detected by zymography as white bands indicate sites of gelatin digestion for 92-kDa pro-MMP-9. Western blot analysis of tissue lysates from aorta of mice in control and Hcy-treated WT, eNOS−/−, and iNOS−/−. A and B: bands indicate MMP-9 and β-actin to verify equal loading of protein. MMP-9 expression was quantified by densitometry and expressed fold change from WT control and fold change from WT+H. Bars are means ± SE (n = 3). *Significant (P < 0.05) increase compared with WT. Although we used control samples on 1 set and treated samples on a different set, the actin blots show similar loading in respective gels.
The levels of TIMP-3 were robust in Hcy-treated WT mice and stayed elevated in knockouts with or without Hcy treatment (Fig. 6A). Contrarily, the levels of TIMP-4 decreased in WT and iNOS−/− mice treated with Hcy (Fig. 6B). These results collectively suggest that because TIMP-3 causes apoptosis and TIMP-4 regulates MMP-mediated remodeling, Hcy causes differential expression in TIMP-3 vs. TIMP-4 in pathogenesis of vascular disease during HHcy.
Fig. 6.
Effect of Hcy on tissue inhibitor of metalloproteinase-3 (TIMP-3) and TIMP-4 levels. A: Western blot analysis of TIMP-3. Bands indicate TIMP-3 and β-actin. TIMP-3 expression was quantified by densitometry and expressed fold change from WT control and fold change from WT+H. Bars are means ± SE (n = 3). B: Western blot analysis of TIMP-4. Bands indicate TIMP-4 and β-actin. TIMP-4 expression quantified by densitometry and expressed fold change from WT control and fold change from WT+H. Bars are means ± SE, (n = 3). *Significant (P < 0.05) increase compared with WT. Although we used control samples on 1 set and treated samples on a different set, the actin blots show similar loading in respective gels.
Oxidative stress.
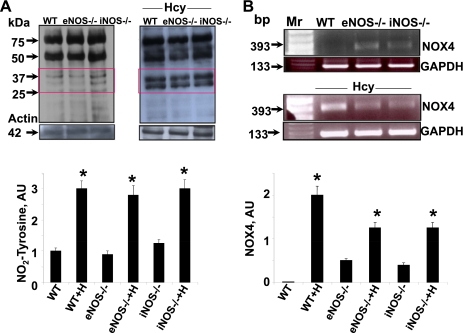
Net oxidative stress is the balance between ROS, reactive nitrogen species (RNS), and reactive thiol species (RTS), so we measured ROS, RNS (nitrotyrosine), and thioredoxin. The levels of ROS were consistently and significantly robust in Hcy-treated WT and knockout mice (Fig. 7). Similarly, the levels of nitrotyrosine were robust in Hcy-treated WT and knockout mice (Fig. 8A). These levels were corroborated with the increase expression of NOX4 in Hcy treatment (Fig. 8B).
Fig. 7.
Hcy-mediated reactive oxygen species (ROS) generation. A: in situ detection of ROS was estimated by 2,7′-dichlorofluorescein (DCFH) in aorta cross-sections from control and Hcy-treated WT, eNOS−/−, and iNOS−/− mice. Note the robust increase in ROS in WT+H. B: bar graphs indicate mean pixel intensity of ROS. The data are means ± SE (n = 2). *P < 0.05 compared with WT.
Fig. 8.
Oxidative stress. A: Hcy-mediated nitrotyrosine generation. Western blot analysis of nitrotyrosine formation as an index of reactive nitrogen species (RNS) in tissue lysates from NOS gene knockout mice with and without Hcy treatment. Bars graphs are nitrosylated proteins at 37 and 25 kDa quantified by densitometry and expressed fold change from WT control and fold change from WT+H. Bars are means ± SE (n = 3). Note the increases in readily visible nitrotyrosine adducts identified by several unidentified bands of 25- to 75-kDa molecular mass. B: effect of Hcy on NADPH oxidase subunit NOX4 mRNA. RT-PCR analysis of tissue mRNA from aorta of mice in WT control, eNOS−/−, and iNOS−/− with and without Hcy. Representative agarose (1%) gel analysis of PCR products of NOX4. Bands indicate NOX4 and GAPDH (a housekeeping gene), which was used as an internal standard to verify equal loading of PCR products. Mr lane with DNA molecular mass marker. NOX4 expression was quantified by densitometry and expressed fold change from WT control and fold change from WT+H. Bars are means ± SE (n = 3). *P < 0.05 compared with WT.
Hcy has no effect on the levels of SOD in WT. However, the SOD in eNOS−/− was lower, and Hcy normalized it. In iNOS, Hcy decreased the levels of SOD compared with untreated (Fig. 9A). The levels of thioredoxin were increased after Hcy treatment in WT and eNOS (Fig. 9B). Interestingly, the levels of thioredoxin were high in iNOS−/− mice, and Hcy treatment normalized the redoxin in iNOS−/− mice (Fig. 9B).
Fig. 9.
Effect of Hcy on SOD1 and thioredoxin. A: Western blot analysis antioxidant SOD1 in tissue lysates from aorta of mice in WT control, eNOS−/−, and iNOS−/−. Bands indicate SOD and β-actin to verify equal loading of protein. SOD expression was quantified by densitometry and expressed fold change from WT control and fold change from WT+H. Bars are means ± SE (n = 3). B: the effect of Hcy on thioredoxin protein expression. Western blot analysis of tissue lysates from aorta of mice in WT control, eNOS−/−, and iNOS−/−. Bands indicate thioredoxin and β-actin (a housekeeping protein), which was used as an internal standard to verify equal loading. Thioredoxin expression was quantified by densitometry and expressed fold change from WT control and fold change from WT+H. Bars are means ± SE (n = 3). *P < 0.05 compared with WT. AU, arbitrary units.
DISCUSSION
Location of the gene products dictate the tissue structure and function (6). We (9) have shown that NO generation in the vicinity of the endothelium regulates MMP activity, vascular remodeling, and function. We (8) have also demonstrated a decrease in elasticity and an increase in pulse wave velocity in HHcy cystathionine-β-synthase heterozygote knockout (CBS−/+) mice.
Remodeling implies alterations in the content of the ECM. The alterations are partly due to an imbalance in the degradation and synthesis of matrix components, particularly elastin and collagen, that is mediated by the activity of MMPs. Studies have implicated Hcy as a risk factor for vascular disease partly due its instigation of vascular remodeling. These studies clearly establish that Hcy alters ECM composition. Histological analysis of aortas suggested significant hypertrophy and revealed an increase in elastin deposition and degradation (10). Under physiological conditions, elastin turnover is slow, and constant synthesis is not necessary. It has been suggested that Hcy leads to the excessive degradation of elastin in arterials (11). However, under some pathological circumstances, elastin is synthesized with little degradation in a compensatory response to other physiological modifications such as hemodynamic changes (21). It is known that Hcy is involved in excessive matrix deposition that results in hypertension in response to increased blood flow and alterations in sheer stress (11). Other factors including growth factor-mediated induction of smooth muscle cells (SMC) and fibroblasts may be involved in potentiating increased elastin synthesis (21). Therefore, the increase in elastin deposition may be modulated by alternate vascular mediators in response to Hcy as suggested by the alterations in vasoreactivity. The observed elastin deposition also may be an important contributor to the observed medial thickening and marked increase in vessel wall diameter in WT treated with Hcy.
There was also an increase in collagen deposition in the inner media and outer adventitial layers of the vessel wall of all Hcy-treated mice. Atherosclerosis is characterized by an extensive thickening of the arterial intima that is associated with both SMC proliferation and the excessive deposition of ECM protein by intimal SMC (15). Hcy has been shown to induce collagen expression, and the increased deposition of collagen is partly due to paradoxically increased synthesis following excessive degradation (10). The increase in SMC collagen synthesis by Hcy was accompanied by an excessive accumulation of insoluble collagen, demonstrating that the excess collagen produced was not simply degraded.
The mechanism of pathological vascular remodeling in Hcy is poorly understood. NO is known to have important vasculoprotective actions (2). A reduction in its action and/or production predisposes the blood vessels to arteriosclerosis. The contrasting production of NO by eNOS (small basal amounts) and iNOS (large inducible amounts) may cause differential vascular effects. Compared with WT+Hcy, there was an increase in collagen content in eNOS−/−+Hcy, however, the largest collagen deposition was observed in the iNOS−/−+Hcy. A previous study using NOS−/− mice found that eNOS inhibits neointimal formation, iNOS suppresses the development of constrictive remodeling, and NO derived from iNOS exerts vascular inhibitory effects. Thus the excessive collagen accumulation in Hcy-treated iNOS−/− suggests inhibition of collagen deposition may be mediated by iNOS and the absence of iNOS increased collagen synthesis and deposition. Therefore, these data indicate that the minimal collagen deposition seen in WT+Hcy compared with eNOS−/− and iNOS−/− treated with Hcy is mediated by the presence of iNOS.
In the WT mice, Hcy induced increased levels of MMP-2 and -9 and TIMP-3 but decreased TIMP-4, which we (9) previously reported as indicative of collagen remodeling in Hcy. The eNOS −/− mouse showed a similar response to Hcy in regard to MMP-2 and -9 but had a higher basal level of TIMP-3, which decreased following Hcy treatment. TIMP-4 basal levels were decreased in the eNOS knockout and decreased even further following Hcy treatment. This would imply that eNOS was not involved in the regulation of the MMPs but may be a negative regulator of TIMP-3 and is also a regulator of TIMP-4. The role of MMPs must be included as a possible mediator of vascular structural changes. Several lines of evidence suggest that Hcy potentiates increased medial thickening in response to elevated MMP activity in atherosclerosis and hypertension in HHcy (11). We (9) have previously demonstrated that Hcy induced cardiovascular MMP. There was no significant changes in MMP mRNA levels in the Hcy-treated animals. There was an increase in MMP-2 expression and a significant increase in MMP-9 expression in WT+Hcy. It has been demonstrated that targeted MMP-2 deletion attenuated collagen accumulation and remodeling in HHcy. The increase in MMP-9 expression was accompanied by a significant increase in activity. The current studies found that levels of MMP expression and activation corresponded with ECM accumulation and medial thickening in Hcy-treated WT. Therefore, the data suggest that Hcy does not have an effect on the regulation of MMPs at the gene or protein level as shown by the lack of coordination between MMP mRNA and protein expression in both groups with and without Hcy. Rather, MMP regulation is mediated by other mechanisms such as cytokine-mediated and inflammatory processes. The presented data compliment a previous study that increase in MMP-9 activity and TIMP-3 expression decreased TIMP-4 and constrictive collagen remodeling in Hcy (17). Another study found that the ECM accumulation and arterial remodeling observed in an animal model of HHcy-induced arterial hypertension is in part due to increased MMP-2 and -9 activation (10).
The iNOS knockout mouse showed elevated basal levels of MMP-2, which did not alter following Hcy treatment (Fig. 5); MMP-9 levels and Hcy response were unaffected. TIMP-3 basal levels were also elevated in the iNOS−/− mouse and decreased slightly following Hcy treatment, whereas the TIMP-4 response was unaltered. However, the activity assay appears to show that basal MMP-9 is greatly increased in the iNOS knockout mouse and is decreased following Hcy treatment (Fig. 5). Therefore, the Western blot showing an increase in expression does not necessarily translate into increased activity. The activity assay is much more informative than the total levels of MMPs. Since MMP-2 is constitutive, we measured MMP-9 activity by zymography. Although we used control samples on one set and treated samples on another set, the actin blots were similar on respective blots. In addition, the use of a colorimetric or fluorometric kit to directly measure the enzyme activity would be more accurate in quantification of actual MMP activity and may give a clearer picture of the involvement of eNOS and iNOS in MMP regulation and their role in vascular remodeling. Many studies have shown that Hcy reduces the bioavailability of NO and therefore increases the activity of MMPs. The results of this study suggest that differential sources of NO may be a regulator of this effect. There was a significant increase in MMP-9 and an increase in MMP-2 activity in iNOS−/− animals without Hcy. This may suggest that the iNOS, due to its substantial NO production, may potentially be the primary source of NO involved in the MMP-TIMP-NO tertiary complex. It is known that the production of ONOO− can activate MMPs by nitrating tyrosine residues (13). However, when these animals were treated with Hcy, the increase in MMP-9 activity and the generation of ROS was greatly increased in WT+Hcy mice. The increased MMP-9 activity observed in WT+Hcy could be due to the oxidative inhibition of NOS, the oxidative activation of MMPs, in addition to the robust increase in nitrotyrosine generation.
ECM turnover is controlled by the complex balance in levels and activity of MMPs and TIMPs. During normal physiological remodeling, MMPs and TIMPs are in tight coordination to maintain optimal vessel wall composition, structure, and function. However, during injury, TIMP expression increases simultaneously with MMPs. Eventually, TIMP expression declines, and MMP remains elevated. Hcy is known to inactivate TIMP, therefore adding to enhanced MMP activity (14). There was an increase in TIMP-3 and -4 expression in WT+Hcy compared with control. This suggests an increased need to inhibit increasing levels of MMP-2. NO is also known to be involved in MMP inhibition (13).
Under pathological conditions, the inhibitory MMP-TIMP-NO interaction is disrupted, and MMP expression and activity are reduced as TIMP expression is induced (9). The disruption in the MMP-TIMP-NO interaction is partly responsible for adverse ECM accumulation and remodeling in the vessel wall and can result in alterations in vascular function (Fig. 10). These data confirm our hypothesis that Hcy leads to increased MMP activity, decreased TIMP activity, and subsequent morphological alterations. Interestingly, these observations mediated by the differential need of eNOS and iNOS can be both protective and detrimental.
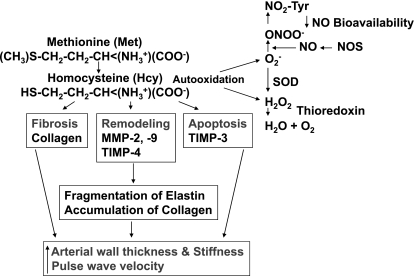
Fig. 10.
Hypothetical presentation of the conclusion. Hcy is generated from methionine. Hcy by autooxidation and by direct activation of MMP, apoptosis, and fibrosis cause elastin degradation and collagen accumulation. This leads to increased aortic wall stress, stiffness, and pulse wave velocity.
To determine the implications of iNOS and eNOS in regulating the redox balance and remodeling, we measured ROS generation in control and Hcy-treated animals and found that ROS production was not significantly effected in either of the knockout animals. We also did not observe any significant change in protein nitrosylation or NOX4 induction between WT and knockout Hcy-treated animals. However, we observed changes in SOD1 and thioredoxin expression. Animal studies have demonstrated that HHcy induces remodeling (increased collagen and decreased elastin) of the ECM of the arterial wall by induction of MMPs by several mechanisms in addition to oxidative activation (22). Hcy-mediated redox changes may induce the accumulation of endothelial cells and SMC in the ECM (18). The O2− mediates the oxidation of those cysteine residues activating latent MMPs and TIMPs (14, 23). Hcy-generated O2− quenches the coordinated NO-generating ONOO−, which exposes the active site and increases the activity of MMPs (13). The Hcy generated ONOO− nitrate tyrosine residues (nitrotyrosine) within MMPs, thus contributing to MMP activation (9).
Limitations.
Collagen is significantly increased in both knockouts following Hcy treatment. If iNOS is responsible for reducing collagen deposition, then the eNOS−/− animals treated with Hcy should be more similar to WT but are not, as we show a significant increase in collagen deposition. Therefore, it appears that both NOS isoforms are important in regulating collagen deposition. In this case, it may be useful to look at the levels of iNOS in the eNOS−/− animals both with and without Hcy treatment (and vice versa for the iNOS−/−); this could reveal whether there was a compensatory increase in the other NOS isoforms in the knockouts. Also, as NO is an important regulator of TIMP activity and nitrosylation and is known to have vasoprotective functions, it is important to measure directly the NO content of the tissue and/or serum in these animals. This would be an important indicator of actual NOX activity and further have implications for TIMP activation/inactivation levels. These studies are in progress.
GRANTS
This work was supported in part by NIH Grants HL-71010 and NS-51568 and an NIH Supplement Award to M. M. Steed as part of a PhD thesis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
A part of this study was presented at the Annual Meeting of Experimental Biology, April 5–9, 2008, San Diego, CA.
REFERENCES
- 1.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest 101: 1478–1487, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87: 682–685, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477: 267–283, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, Heydrick S, Stark R, Klings ES, Moldovan NI, Yaghoubi M, Goldschmidt P, Clermont PJ, Farber HW, Cohen R, Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocysteinemia. J Clin Invest 106: 483–491, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodgkin DD, Gilbert RD, Roos PJ, Sandberg LB, Boucek RJ. Dietary lipid modulation of connective tissue matrix in rat abdominal aorta. Am J Physiol Regul Integr Comp Physiol 262: R389–R394, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Hurtley S. Spatial cell biology. Location, location, location. Introduction. Science 326: 1205, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Kiatchoosakun S, Kirkpatrick D, Hoit BD. Effects of tribromoethanol anesthesia on echocardiographic assessment of left ventricular function in mice. Comp Med 51: 26–29, 2001 [PubMed] [Google Scholar]
- 8.Kumar M, Tyagi N, Moshal KS, Sen U, Kundu S, Mishra PK, Givvimani S, Tyagi SC. Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochem Int 53: 214–219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundu S, Kumar M, Sen U, Mishra PK, Tyagi N, Metreveli N, Lominadze D, Rodriguez W, Tyagi SC. Nitrotyrosinylation, remodeling and endothelial-myocyte uncoupling in iNOS, cystathionine beta synthase (CBS) knockouts and iNOS/CBS double knockout mice. J Cell Biochem 106: 119–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller A, Mujumdar V, Shek E, Guillot J, Angelo M, Pakmer L, Tyagi SC. Hyperhomocysteinemia induces multiorgan damage. Heart Vessels 15: 135–143, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Mujumdar VS, Aru GM, Tyagi SC. Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J Cell Biochem 82: 491–500, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev 85: 1–31, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Radomski A, Sawicki G, Olson DM, Radomski MW. The role of nitric oxide and metalloproteinases in the pathogenesis of hyperoxia-induced lung injury in newborn rats. Br J Pharmacol 125: 1455–1462, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98: 2572–2579, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross R. The pathogenesis of atherosclerosis: a perpective of the 1990s. Nature 362: 801–809, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Sen U, Rodriguez WE, Tyagi N, Kumar M, Kundu S, Tyagi SC. Ciglitazone, a PPARγ agonist ameliorates diabetic nephropathy, in part, through homocysteine clearance. Am J Physiol Endocrinol Metab 295: E1205–E1212, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shastry S, Tyagi N, Moshal KS, Lominadze D, Hayden MR, Tyagi SC. GABA receptors ameliorate Hcy-mediated integrin shedding and constrictive collagen. Remodeling in microvascular endothelial cells. Cell Biochem Biophys 45: 157–165, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 280: C53–C60, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Springman EB, Angleton EL, Birkedal-Hansen H, Van Wart HE. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci USA 87: 364–368, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tummalapalli CM, Tyagi SC. Response of vascular smooth muscle cells to extracellular matrix degradation. J Cell Biochem 75: 515–527, 1999 [PubMed] [Google Scholar]
- 21.Tyagi N, Moshal KS, Ovechkin AV, Rodriguez W, Steed M, Henderson B, Roberts AM, Joshua IG, Tyagi SC. Mitochondrial mechanism of oxidative stress and systemic hypertension in hyperhomocysteine. J Cell Biochem 96: 665–671, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Tyagi SC. Physiology and homeostasis of extracellular matrix: cardiovascular adaptation and remodeling. Pathophysiology 7: 177–182, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Tyagi SC, Matsubara L, Weber KT. Direct extraction and estimation of callagenase(s) activity by zymography in microquantities of rat myocardium and uterus. Clin Biochem 26: 191–198, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Tyagi SC, Smiley LM, Mujumdar VS, Clonts B, Parker JL. Reduction-oxidation (redox) and vascular tissue level of homocysteine in human coronary atherosclerotic lesions and role in vascular ECM remodeling and vascular tone. Mol Cell Biochem 181: 107–116, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr 72: 324–332, 2000 [DOI] [PubMed] [Google Scholar]