Abstract
Macrophage derived-endothelin-1 (ET-1) has been suggested to contribute to a number of chronic lung diseases. Whether the ET-1 cascade from non-vascular sources (inflammatory cells) also contributes to pulmonary artery hypertension (PAH) and in particular to heritable PAH (HPAH) with known bone morphogenetic protein type 2 receptor (BMPR2) mutations is not known. We tested this notion using bone marrow-derived macrophages (BMDM; precursors of tissue macrophages) isolated from ROSA26rtTAXTetO7-tet-BMPR2R899X mice (model of PAH with universal expression of a mutated BMPR2 gene) with and without activation by LPS and in human lung tissue from HPAH with BMPR2 mutations and idiopathic PAH (IPAH). At baseline ETA and ETB receptors and endothelin converting enzyme (ECE) gene expression was reduced in BMPR2 mutant BMDM compared with controls. In control BMDM, LPS resulted in increased ppET-1 gene expression and ET-1 in culture media, whereas ETA and ETB receptor and ECE gene expression was decreased. These findings were more severe in BMPR2 mutant BMDM. Antagonism of the ETB receptor resulted in increased ET-1 in the media, suggesting that decreased ET-1 uptake by the ETB receptor contributes to the elevation. While ET-1 expression was demonstrated in lung macrophages from controls and IPAH and HPAH patients, ETA and ETB expression was decreased in the HPAH, but not IPAH, patients compared with controls. We conclude that reduced expression of macrophage ET-1 receptors in HPAH increases lung ET-1 and may contribute to the pathogenesis and maintenance of HPAH. This is the first description of protein expression that distinguishes HPAH from IPAH in patients.
Keywords: endothelin receptors A and B, endothelin-1, pulmonary artery hypertension, mouse model of heritable pulmonary artery hypertension
pulmonary arterial hypertension (PAH) is a progressive disease in which widespread occlusion of small pulmonary arteries leads to increased pulmonary vascular resistance and subsequent right ventricular failure. PAH can be either heritable (HPAH) or idiopathic (IPAH) in nature. Heterozygous germline mutations in the bone morphogenetic protein type 2 receptor (BMPR2) have been identified as a gene underlying HPAH. BMPR2 mutations have now been identified in ∼70% of families with HPAH and ∼20% in patients with IPAH (21).
Until recently, the clinical course and pathology of HPAH and IPAH have been considered similar (20, 26). However, several papers now point to differences between the heritable and idiopathic forms of the disease. Patients with either HPAH or IPAH with nonsynonymous BMPR2 mutations have been shown to be less vasoreactive than patients without an identifiable BMPR2 mutation (10) and less likely to respond to acute vasodialator testing (24). Furthermore, patients with PAH and a BMPR2 mutation have been reported to present ∼10 years earlier and with more severe disease than patients without a mutation (34). These data suggest that mutations in BMPR2 are associated with distinct disease phenotypes.
The small vasoactive peptide endothelin-1 (ET-1) has been shown to mediate the vascular remodeling of PAH by promoting vasoconstriction, smooth muscle proliferation (43), protein synthesis (5), and production of a variety of cytokines and growth factors (18). ET-1 activates a complex cascade of intracellular events by binding to its specific receptors, endothelin receptor A (ETA) and endothelin receptor B (ETB). These receptors are distributed in a variety of cells and tissues in different proportions, suggesting a potential regulatory mechanism.
In patients with PAH, plasma ET-1 is increased (4) and the elevated levels correlate with increased pulmonary vascular resistance and pulmonary arterial pressure, and decreased cardiac output (25, 33). Furthermore, expression of ET-1 is increased in lung vascular endothelial cells of patients with PAH (15). Increased levels of plasma ET-1 and expression of pulmonary ET-1 have also been described in animal models of PAH (32).
Although vascular endothelial cells express and secrete the majority of ET-1, lung macrophages also show increased expression of the ET-1 cascade under various pathological conditions (1, 9, 22, 37, 39). Infiltration of various inflammatory cells, including macrophages, has been found in the plexiform lesions in IPAH (38) and in CD8+ lymphocytes in the lung periphery (2). Furthermore, we demonstrated in a mouse model (SM22-rtTA x TetO7-BMPR2R899X) of HPAH (a BMPR2 mutation expressed in SMC) that large numbers of macrophages and T cells are present in the adventitial layer of the pulmonary arteries (41). A recent study demonstrated that in normotensive human pulmonary artery segments, BMPR2 ligand inhibits contraction in response to ET-1 and thus regulates the effects of ET-1 in the pulmonary circulation suggesting that mutations in BMPR2 have an ability to alter the regulatory mechanisms governing the ET-1 cascade in pulmonary arteries (6). These findings led us to hypothesize that a BMPR2 mutation results in alterations in the ET-1 cascade in lung macrophages. To test this hypothesis, we examined bone marrow-derived macrophages (BMDM), precursors of tissue macrophages isolated from ROSA 26rtTA X TetO7-tet-BMPR2R899X mice (a model of HPAH with universal expression of a mutated BMPR2 gene) with and without activation by LPS and paraffin-embedded lung tissue from ROSA 26rtTA X TetO7-tet-BMPR2R899X mice and HPAH patients with known BMPR2 mutations and IPAH patients compared with controls. Our results reveal that both ETA and ETB receptor gene expression is reduced in BMDM from mice expressing a BMPR2 mutation, a finding that is recapitulated in lung macrophages from ROSA 26rtTA X TetO7-tet-BMPR2R899X mice and patients with HPAH but not IPAH. We conclude that the reduction in ET-1 receptors in lung macrophages from HPAH patients leads to increased local levels of ET-1, which have a paracrine effect on cells in the microenvironment. These findings may contribute to the clinical differences seen in patients with HPAH and IPAH.
METHODS
Harvesting and maturation of macrophages from mouse bone marrow-derived cells.
BMDM were cultured from equal numbers of male and female 8-wk-old ROSA 26rtTA X TetO7-tet-BMPR2R899X mice (a model of HPAH; BMPR2 mutant mice) and ROSA 26 rtTA mice (controls). First, the skin around the femur was soaked in 70% ethanol, and the femur was exposed. The ends of the femurs were clipped, and the bone was flushed with 3 ml of PBS per femur. The perfusate was then centrifuged for 5 min at 1,000 rpm. The stem cells were resuspended in BMDM media and incubated for 5 days at 37°C. The BMDM media contained 10% FBS, 1% penicillin/streptomycin, 1% l-glutamine, and 10% LCM (supernatant obtained from L929 cells grown for 1 wk in DMEM with 10% FBS, 1% penicillin/streptomycin, and 1% l-glutamine). After 5 days of incubation (0.5 mg/ml doxycycline was added on day 4), the cells were washed with ice-cold PBS, scraped, centrifuged, and counted. The average cell count obtained was 1,500,000/mouse. BMDM were then plated into six-well plates (2.5 × 105 cells/well) containing DMEM, 10% FBS, 1% penicillin/streptomycin, and 1% l-glutamine and incubated overnight to allow the cells to attach to the plate before experimentation. To confirm proper macrophage differentiation of the BMDM, macrophage markers Itgam, Mrc1, and Sfpi1 were assayed by quantitative RT-PCR. All three were strongly expressed, with no significant differences between control (Rosa26-rtTA) and BMPR2 mutant (Rosa26-rtTAxTetO7-BMPR2R899X) mice (42). The protocol for mice was approved by Vanderbilt University Institutional Animal Care and Use Committee.
Protocols.
Following maturation of the BMDM from mutant and control mice, the cells were treated with LPS (100 ng/ml, 055:B5; Sigma-Aldrich, St. Louis, MO) for either 4 or 24 h. The cell supernatant was collected at the start of the experiment (time 0) and at 4 and 24 h of LPS stimulation and frozen at −70°C for subsequent measurement of ET-1 levels. After aspiration of the medium, the cells at 0 and 24 h of LPS stimulation were washed with PBS and lysed with 100 μl of lysis buffer (Promega, Madison, WI) for Quantitative Real Time PCR (QRT-PCR).
The effect of ETA and/or ETB receptor inhibitor on ET-1 was studied in the culture medium of BMDM obtained only from control mice. Cultured BMDM were incubated in the presence or absence of either 1 μM ETA receptor antagonist (BQ-123; Alexis Biochemicals, San Diego, CA) or 1 μM ETB receptor antagonist (BQ-788; Alexis Biochemicals) or both with or without LPS stimulation for 24 h. The cell supernatant was collected at 0 and 4 h following LPS stimulation and frozen at −70°C for subsequent measurement of ET-1 levels by ELISA (Assay Designs, Ann Arbor, MI).
Purity of BMDM.
The purity of BMDM was determined as previously described (19, 30, 31). Briefly, on day 5, the BMDM were incubated with cell dissociation solution (Sigma C-591A) for 10 min at 37°C and resuspended in PBS containing 3% FBS at a cell density of 107 cells/ml. The cells were then permeabilized in 1 M HEPES, 1 M NaCl, 1 M sucrose, 1 M MgCl2, and 1% Nonidet P-40 for 10 min at room temperature followed by incubation with rat anti-mouse CD206:FITC antibody (BD Pharmingen, San Jose, CA) for 45 min at 4°C.
RNA extraction and cDNA preparation.
Total RNA was extracted from cultured BMDM using RNeasy Mini kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. RNA concentration was determined by standard spectrometric techniques. First-stand cDNA synthesis was carried out using Superscript II Reverse Transcriptase system (Applied Biosystems, Foster City, CA).
Expression of the BMPR2R899X gene in BMDM from ROSA26rtTAxTetO7-tet-BMPR2R899X mice was confirmed by quantitative real-time PCR (mus-F: ATAGGAGGGAACGGCCATTAG and mus-R: GGTCCCCAAACTCACCCTG). Relative quantification was achieved with the comparative 2−ΔCt method by normalization with expression of the HPRT gene (mus-147F TGCTCGAGATGTCATGAAGGAG and mus-249R TTTAATGTAATCCAGCAGGTCAGC).
Quantitative RT-PCR using primers for mouse ET-1, ETA, ETB, and ECE.
Primers for preproET-1 (ppET-1) were designed using the computer program Primer3 (Primer Express Software; Applied Biosystems, Foster City, CA) [preproET-1F (ppET-1F) ACT TCT GCC ACC TGG ACA TC and ppET-1R GTC TTT CAA GGA ACG CTT GG] and synthesized by Integrated DNA Technologies. ETA, ETB, and ECE primers were obtained from SuperArray Bioscience (SABiosciences, Frederick, MD), and β-microglobulin (βMG) primers were obtained from RealTimePrimers.com. All primers were intron-spanning to avoid inappropriate amplification of residual genomic DNA. Quantitative RT-PCR was performed in triplicate on an ABI PRISM 7000 (Applied Biosystems), each tube containing 2.5 μl of SYBR Green PCR Master Mix (Applied Biosystems), 100 nM (each) primer, and 5 μl of diluted template DNA in a total volume of 25 μl. Signal detection and analysis of results were performed with ABI PRISM 7000 sequence detection software (Applied Biosystems). Relative quantification was achieved with the comparative 2−ΔCt method by normalization with expression of the βMG gene.
Measurement of ET-1 in cell culture fluid.
ET-1 levels in the culture supernatant were measured with a commercial ELISA kit (Assay Designs, Ann Arbor, MI). The intra-assay coefficient of variation was 5.8%, with an inter-assay variation of 4.7%. Cross reactivity to ET-2, ET-3, and Big ET was <0.1%. The ET-1 levels measured in experiments fell within the range of the curve generated by the standard concentrations provided with the ELISA kit. The complete medium alone had undetectable levels of ET-1.
Immunohistochemical analysis of ETA, ETB, and ET-1 in human lung.
Immunolocalization of ETA, ETB and ET-1 was performed on archival paraffin-embedded human lung tissue obtained from controls and HPAH and IPAH patients (Table 1). Two of the patients with HPAH received ET receptor antagonist therapy. The study protocol was approved by the institutional research and ethics committees of Vanderbilt University Medical Center. Lung samples were obtained after surgical resection or at autopsy (≤10 h postmortem). Lung sections were deparaffinized, rehydrated, and blocked with 5% normal goat serum, followed by overnight incubation with primary antibody (ETA and ETB from GeneTex, San Antonio, TX; ET-1 from Novus Biologicals, Littleton, CO) at 4°C. The sections were then incubated with biotinylated secondary antibody followed by incubation with HRP-conjugated streptavidin. Diaminobenzidine (DAB) was used as a substrate for HRP. The sections were dehydrated and mounted in Cytoseal XYL (Richard-Allan Scientific, Kalamazoo, MI) for light microscopic examination. Use of tissue samples was approved by the institutional review board at Vanderbilt University Medical Center, and written informed consent was obtained from all living subjects included in the study. Unique identifiers to conceal identity were assigned to all samples.
Table 1.
Characteristics of patients in study group
| Disease | Age | Gender | mPAP | BMPR2 Mutation |
|---|---|---|---|---|
| HPAH | 15 | F | 100 | Exon 3 (T354G) |
| 36 | F | 70 | Exon 12 (1742-1748delA) | |
| 35 | M | 80 | Exon 8 (C994T) | |
| 16 | F | 77 | Exon 3 (T354G) | |
| IPAH | 29 | M | 80 | Negative |
| 37 | F | unknown | Not tested | |
| 32 | F | 66 | Not tested | |
| 34 | M | 80 | Not tested | |
| 24 | M | 74 | Not tested | |
| Controls | 62 | F | ||
| 49 | F | |||
| 73 | M | |||
| 49 | M | |||
| 18 | M |
Colocalization using ETA and ETB in human and mouse lung macrophages.
For colocalization of ETA and ETB in human lung macrophages, lung sections were coincubated with either an ETA or ETB antibody (GeneTex) with a macrophage antibody (25F9; Santa Cruz Biotechnology) overnight at 4°C. The sections were then incubated with an FITC-labeled secondary antibody for macrophages (Sigma) and a Cy3-labeled secondary antibody for either the ETA or ETB receptor (Zymed Laboratories Invitrogen, Carlsbad, CA). The slides were mounted using Vectashield mounting media containing DAPI (Vector Laboratories, Burlingame, CA) for confocal microscopy.
For colocalization of ETA and ETB in mouse lung macrophages, 5-mo-old BMPR2R899X transgenic mice were used. The mice received doxycycline (1 g/kg) in their chow for 8 wk before being euthanized with Beuthanasia-D IP. The lungs were harvested and fixed in 4% paraformaldehyde and embedded in paraffin by standard techniques. Lung sections (5 μm thick) were coincubated either with mouse ETA (Novus Biologicals, Littleton, CO) or mouse ETB antibody (Abcam, Cambridge, MA) and a macrophage antibody (Mac3; BD Pharmingen, San Jose, CA) overnight at 4°C. The sections were then incubated with a Cy3-labeled secondary antibody for macrophages (Zymed) and an FITC-labeled secondary antibody for either ETA (Zymed) or ETB receptor (Sigma). The slides were mounted using Vectashield mounting media containing DAPI (Vector Laboratories) for confocal microscopy.
To preserve the relative fluorescence variation, the microscopy settings were the same for all the samples. The pictures were digitized, and the fluorescence intensity of ET receptors was assessed by comparing the intensity of the labeled ETA and ETB antibodies to the intensity of the labeled macrophage marker using Image J 1.40g software.
Statistical analysis.
Data are expressed as means ± SE. Statistical analyses for quantitative RT-PCR and measurement of ET-1 levels were carried out using a two-way ANOVA with the Bonferroni posttest (GraphPad Prism Software, La Jolla CA). A P < 0.05 was considered significant.
RESULTS
Purity and expression of mutant BMPR2 transgene in BMPR2 mutant BMDM.
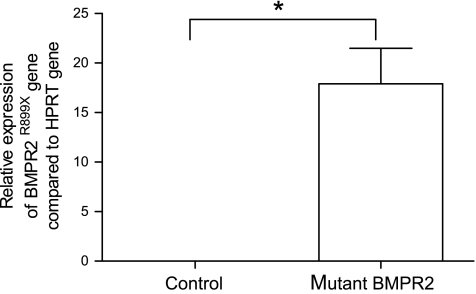
The purity of the BMDM cells as determined by flow cytometry was 90.8 ± 3.9% (Fig. 1). The BMPR2R899X transgene was expressed in BMDM from BMPR2 mutant mice at 17.9 ± 3.6 copies per thousand copies of HPRT when induced with doxycycline, representing an average 37-fold increase in expression compared with those without doxycycline. No expression of the BMPR2R899X transgene was found in control mice.
Fig. 1.
Expression of BMPR2R899X transgene compared with HPRT gene expression in bone marrow-derived macrophages (BMDM) from control and mutant mice determined by quantitative RT-PCR (n = 4); *P < 0.001.
ETA and ETB gene expression in BMDM.
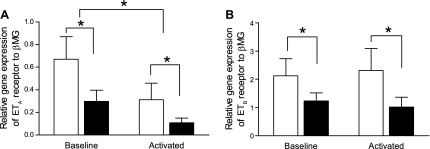
At baseline, ETA and ETB receptor gene expression was significantly greater in control BMDM compared with mutant BMDM. On activation with LPS, expression of the ETA gene was decreased in both groups compared with baseline (time 0) (n = 6; P < 0.05), although the decrease was most striking in mutant BMDM (n = 6; P < 0.05, Fig. 2A). Expression of the ETB gene remained unchanged in both groups with LPS stimulation (Fig. 2B).
Fig. 2.
A and B: quantitative real-time RT-PCR analysis of ETA and ETB receptor gene expression in BMDM from control (open bars) and BMPR2 mutant mice (solid bars). At baseline, ETA and ETB receptor expression was lower in mutant BMDM compared with control cells. In activated BMDM from control and BMPR2 mutant mice, ETA receptor expression was significantly decreased, whereas ETB receptor expression remained unchanged. On activation with LPS, ETA receptor expression was further decreased in BMPR2 mutant BMDM compared with controls. Data are expressed as means ± SE of copy numbers per 200 ng of sample RNA normalized to β-microglobulin (βMG) (n = 6); *P < 0.05.
ppET-1 and ECE gene expression in BMDM.
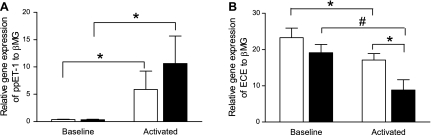
At baseline, preproET-1 (ppET-1) gene expression was low in control and mutant BMDM. Following LPS stimulation, expression of ppET-1 gene increased 6-fold and 10-fold over baseline in both control and mutant BMDM (n = 6; P < 0.05), respectively (Fig. 3A).
Fig. 3.
Quantitative real-time RT-PCR analysis of ppET-1 and ECE gene expression in BMDM from control (open bars) and BMPR2 mutant mice (solid bars). A: at baseline, ppET-1 expression is negligible in control and BMPR2 mutant BMDM. Following activation with LPS, ppET-1 expression was significantly increased in both groups. B: at baseline, ECE gene was expressed in control and BMPR2 mutant BMDM. Following activation with LPS, ECE expression was significantly decreased in both groups. The decrease was most striking in the BMPR2 mutant mice. Data are expressed as means ± SE of copy numbers per 200 ng of sample RNA normalized to β-MG (n = 6); *P < 0.05; #P < 0.01.
At baseline, relative expression of the ECE gene was similar in control and mutant BMDM. Following LPS stimulation, ECE gene expression decreased significantly in both groups (n = 6; P < 0.05); the decrease was most marked in the mutant BMDM reaching significance compared with control cells (P < 0.01) (Fig. 3B).
Secreted ET-1 levels in BMDM cells.
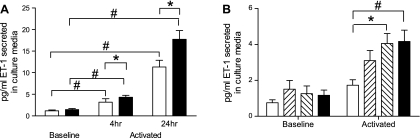
At baseline, ET-1 levels in the culture medium from BMDM were negligible from both the control (n = 6) and BMPR2 mutant (n = 12) cells. Following activation with LPS for 4 h, levels of ET-1 in the culture media were significantly increased in BMDM from both groups and continued to increase at 24 h (P < 0.001). The increase in secreted ET-1 in the BMPR2 mutant group was significantly greater than the controls at both time points (P < 0.05) (Fig. 4A).
Fig. 4.
A: levels of ET-1 in culture media of control (open bars) and BMPR2 mutant BMDM (solid bars) following LPS stimulation for 4 and 24 h. At baseline, low levels of ET-1 were present in culture media of control and BMPR2 mutant BMDM. These levels increased significantly on activation with LPS (#P < 0.001). Levels of ET-1 were significantly greater in culture media of activated mutant BMDM compared with controls (*P < 0.05). Data are expressed as means ± SE; n = 12. B: levels of ET-1 in culture media of control BMDM in the presence of ETA and ETB antagonist (BQ-123 and BQ-788, respectively) at baseline and following LPS stimulation for 4 h. Open bars, no ET-1 receptor antagonists; rightward diagonal bars, presence of ETA antagonist; leftward diagonal bars, presence of ETB antagonist; solid bars, both ET-1 receptor antagonists. At baseline, ETA and ETB receptor antagonists did not affect levels of ET-1 in culture media. On activation with LPS, levels of ET-1 were significantly higher in culture media of BMDM treated with ETB receptor antagonist (*P < 0.05) as well as with both ETA and ETB receptor antagonists (#P < 0.001). Data are expressed as means ± SE, n = 9.
Effect of ETA and ETB receptors on secreted ET-1 levels.
To examine whether the increase in ET-1 was the result of an increase in secretion or reduced clearance, we carried out experiments with ETA (BQ-123) or ETB (BQ-788) receptor antagonists in BMDM from control mice (n = 9). At baseline, ETA and ETB receptor antagonists did not affect levels of ET-1 in culture media. On activation with LPS, levels of ET-1 were significantly higher in culture media of BMDM treated with ETB receptor antagonist (P < 0.05) as well as with both ETB and ETA receptor antagonists (P < 0.001) (Fig. 4B). The effect with the ETA receptor did not reach significance. The increase in ET-1 in the presence of the ETB receptor antagonist suggests that this receptor is responsible for the increased ET-1 in the culture media.
ET-1 localizes in lung macrophages from control, IPAH, and HPAH lungs.
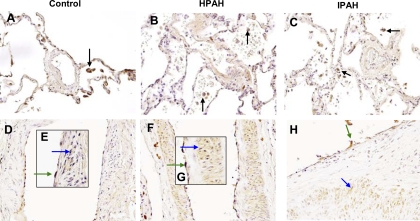
Using immunocytochemical techniques, we demonstrated that ET-1 localized in alveolar macrophages from control, IPAH, and HPAH lungs (Fig. 5, A–C) and that expression of ET-1 was similar in each group; lung tissue macrophages showed similar results (Fig. 5F). ET-1 was also expressed in pulmonary vascular endothelial cells (ECs) and smooth muscle cells (SMCs) from control, IPAH, and HPAH lungs (Fig. 5, D–H).
Fig. 5.
Immunolocalization of ET-1 in control, heritable pulmonary artery hypertension (HPAH), and idiopathic PAH (IPAH) (as a disease control) lungs. ET-1 localizes in alveolar macrophages in control (A), HPAH (B), and IPAH (C) as denoted by arrows. ET-1 also localizes in ECs and SMCs from pulmonary arteries in control (D and E), HPAH with BMPR2 mutation (F and G), and IPAH (H) as denoted by arrows. Magnification, ×400.
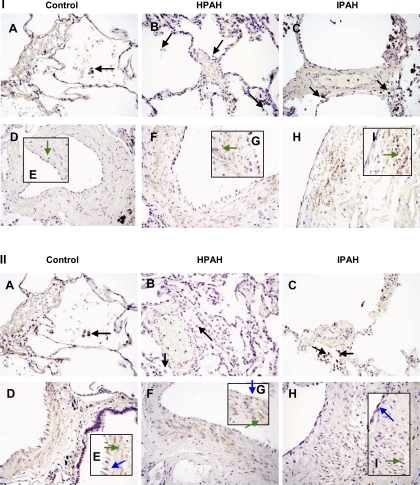
Localization of ETA and ETB receptors is decreased in alveolar macrophages from HPAH.
In control and IPAH lungs, immunocytochemistry revealed that the ETA (Fig. 6, IA and IC) and ETB receptors (Fig. 6, IIA and IIC) were similar in concentration and localized in alveolar macrophages. In contrast, expression of ETA and ETB receptor was decreased in HPAH (n = 4) alveolar macrophages compared with both control (n = 5) and IPAH (n = 5) lungs (Fig. 6, IB and IIB). The ETA receptor also localized in SMCs from control, IPAH, and HPAH lungs (Fig. 6, ID-IH). ETB was localized in ECs in control, IPAH, and HPAH lungs and in SMCs from IPAH and HPAH lung (Fig. 6, IID-IIH). Localization of ETB in SMCs from control was undetectable.
Fig. 6.
Immunolocalization of ETA and ETB receptors in control, HPAH, and IPAH lungs. I: ETA receptors localize in alveolar macrophages in control (A) and IPAH (C). In lung, macrophages from HPAH localization of ETA receptor is decreased (B). Expression of ETA is denoted by arrows in macrophages. ETA receptor also localizes in SMCs from pulmonary arteries in control (D and E), HPAH (F and G), and IPAH (H and I) as denoted by arrows. II: ETB receptor localizes in alveolar macrophages in control (A) and IPAH (C). In macrophages from HPAH, localization of ETB receptor is decreased (B). Expression of ETB receptor is denoted by arrows in macrophages. ETB receptor was also localized in pulmonary artery ECs in control (D and E), HPAH (F and G), and IPAH (H and I) as denoted by arrows and in pulmonary artery SMCs from control (D and E), HPAH (F and G), and IPAH (H and I) as denoted by arrows. Magnification, ×400.
Localization of ETA and ETB receptors in macrophages in human and mouse lung by confocal microscopy.
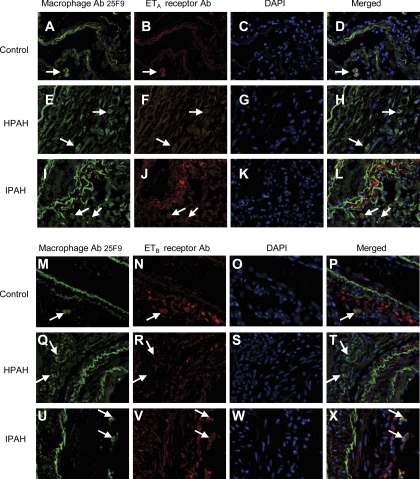
Confocal microscopy was carried out to confirm the reduction of ETA and ETB receptors in macrophages from HPAH, IPAH, and control lungs. As shown in Fig. 7, the macrophages in the adventitial layer of large arteries of control and IPAH lungs colocalize both the ETA receptor (A–D and I–L, respectively) and the ETB receptor (M–P and U–X, respectively). Macrophages from HPAH cases show little, if any, colocalization of either the ETA or ETB receptor (E–H and Q–T, respectively).
Fig. 7.
Confocal microscopy. ETA and ETB receptor localization is reduced in the adventitial macrophages surrounding the large blood vessels in HPAH lung compared with controls and IPAH lungs. Macrophages were identified in the adventitia using the macrophage antibody (Ab) 25F9 (FITC labeled), and localization of ETA and ETB receptors was identified using either ETA or ETB receptors Ab (Cy3 labeled). DAPI was used as the nuclear stain. ETA and ETB receptor in adventitial macrophages in control (A–D and M–P) and IPAH (I–L and U–X) lung tissue, respectively. In contrast, ETA and ETB receptor localization was reduced in adventitial macrophages in HPAH lung tissue (E–H and Q–T), respectively. Magnification, ×400.
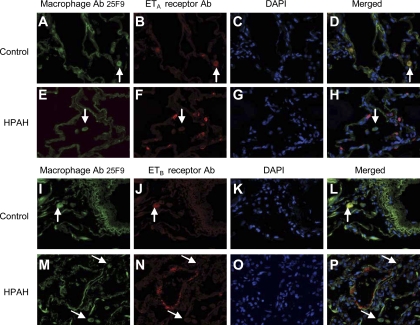
Similarly, the alveolar macrophages and those in close proximity to small arteries of control (A–D and I–L) and IPAH (data not shown) cases demonstrated colocalization of both the ETA and ETB receptors, whereas those from HPAH lungs showed little colocalization of either receptor (Fig. 8, E–H and M–P, respectively).
Fig. 8.
Localization of ETA and ETB receptors in alveolar macrophages and macrophages in close proximity to small blood vessels in HPAH and control lungs. Lung macrophages were identified using the macrophage antibody (Ab) 25F9 (FITC labeled), and localization of ETA and ETB receptors was identified using either ETA or ETB receptor Ab (Cy3 labeled). DAPI was used as the nuclear stain. In control lungs, ETA receptors localize in alveolar macrophages close to small vessels (A–D). In contrast, ETA receptor localization is reduced in lung macrophages surrounding the small vessels in HPAH lung tissue (E–H). Similarly, ETB receptors localized to lung macrophages in close proximity to small pulmonary arteries in control lung (I–L), and ETB expression was reduced in HPAH lungs (M–P). Magnification, ×400.
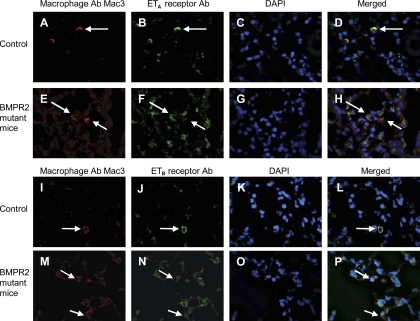
Confocal microscopy in mouse lungs from BMPR2 mutant mice reiterated the reduction of ETa and ETB receptors in lung macrophages compared with control mice (Fig. 9).
Fig. 9.
Localization of ETA and ETB receptors in alveolar macrophages and macrophages in close proximity to small blood vessels in BMPR2 mutant and control mouse lungs. Lung macrophages were identified using the macrophage antibody (Ab) Mac3 (Cy3 labeled), and ETA and ETB receptors were localized using either FITC-labeled ETA and ETB receptor Ab. DAPI was used as the nuclear stain. In control lungs, ETA receptors localize in alveolar macrophages close to small vessels (A–D). In contrast, ETA receptor localization in BMPR2 mutant mice is reduced (E–H). Similarly, ETB receptor localization was reduced in lung macrophages in close proximity to small pulmonary arteries in BMPR2 mutant mice (M–P) compared with controls (I–L). Magnification, ×600.
Quantitation of the ETA and ETB receptors.
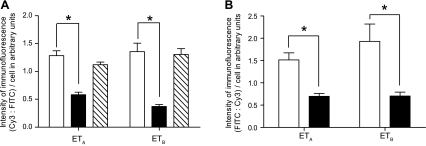
Quantitation of the intensity of the immunofluorescence of the ETA and ETB receptors confirmed a significant reduction in expression in the HPAH cases compared with both the control lungs and those from the IPAH cases (Fig. 10A). The reduction in ETa and ETb receptor expression was recapitulated in lung macrophages from the BMPR2 mutant mice compared with controls (Fig. 10B).
Fig. 10.
A: quantitation of the immunofluorescence intensity of ETA and ETB (CY3-labeled) receptors to FITC-labeled macrophages in control (open bars), HPAH (solid bars), and IPAH (diagonal bars) lungs. Intensity of immunofluorescence for ETA and ETB receptor antibody is significantly reduced in macrophages from HPAH lungs compared with controls and IPAH cases. BMPR2 mutant mice compared with controls. B: ETA (FITC-labeled) and ETB (FITC-labeled) receptors in macrophages (Cy3-labeled) from control (open bar) and BMPR2 mutant (solid bar) mice. Intensity of immunofluorescence for ETA and ETB receptor antibody is significantly reduced in macrophages from BMPR2 mutant mice compared with controls. *P < 0.001.
DISCUSSION
Our data demonstrate that ET-1 and the ETA and ETB receptors are expressed in BMDM (precursors of tissue macrophages) from BMPR2 mutant (ROSA 26-rtTA X TetO7-BMPR2R899X mouse model of HPAH) and control (ROSA26-rtTA) mice. On activation with LPS, ppET-1 gene expression and ET-1 secretion into the culture media is increased, whereas ECE gene expression is decreased in control and mutant BMDM. These findings are more severe in the cells from the BMPR2 mutant mice than in controls. The LPS-induced increase in ET-1 in the culture media was further increased in the presence of an ETB antagonist alone or in conjunction with an ETA antagonist, suggesting that the additional increase is the result of decreased ETB receptor expression. Under baseline conditions, levels of ETA and ETB receptor gene expression was reduced in BMPR2 mutant BMDM, a change that was confirmed directly in the lungs of these mutant mice. Expression of ET-1 was also found in human lung macrophages from control lungs and those from patients with either IPAH or HPAH. Furthermore, a reduction in ETA and ETB receptors was noted in the lungs of patients with HPAH but not those with IPAH compared with controls.
Our current understanding of the role of BMPR2 in adult animals or humans suggests participation in a pathway(s) responsible for resolution of inflammation. BMPR2 regulates cell differentiation and some cytokines through SMAD, glucocorticoid receptor sensitivity through LIMK (7), and ROS and actin organization through an as yet poorly defined mechanism (40). Microarray analysis of whole lung from a transgenic mouse model expressing a dominant negative form of BMPR2 in smooth muscle (SM22-rtTA x TetO7-BMPR2delx4+) demonstrated an increase in cytokines and markers of immune response with increased right ventricular systolic pressure (RVSP) (35). Similarly, the transgenic mouse model expressing a smooth muscle-specific BMPR2R899X transgene demonstrated accumulation of macrophages and T cells in the adventitial layer of pulmonary arteries with an increased RVSP (41). While we believe that complex lesions are a late event in the development of PAH in BMPR2 mutant mouse models, time course studies in Rosa26-rtTA x TetO7-BMPR2R899X show early and persistent recruitment of monocytes to the lungs, both by expression studies in whole lung and by histology (unpublished observations). Universal expression of the BMPR2R899X mutation results in earlier and stronger penetrance than expression in smooth muscle alone, suggesting a contributory role to the mutation in other cell types, including cells of the monocyte lineage whose recruitment is one of the first events in this model. Several lines of evidence thus suggest that suppression of BMPR2 function is proinflammatory through multiple mechanisms, with specific effects depending on cell type (16, 23). Our prior studies thus led us to question the effect of macrophage recruitment within BMPR2 mutants. In the current paper, we chose to focus on the ET-1 cascade in lung macrophages because 1) ET-1 is associated with the pathogenesis of PAH (25) and 2) other lung diseases have highlighted alterations in this pathway in lung macrophages.
A delicate balance between vasoconstrictor and vasodilator agents exists in the small pulmonary arteries and is responsible for normal homeostasis. ET-1 acts as a local autocrine or paracrine agent rather than as a circulating hormone (12, 28). In PAH, it is well established that the endothelial cell is a source of ET-1, but few, if any, studies have addressed the possible contribution of other cells to ET-1 synthesis in the lung. The present study demonstrates that the BMDM from control and BMPR2 mutant mice express ETA, ETB, and ECE genes and minimal levels of ppET-1. These results confirm the notion that ET-1 is expressed by macrophages in culture (9), and our immunocytochemical studies confirm that this is also true in control human lung.
Alterations in the ET-1 cascade are described in several lung diseases. For example, ET-1 levels are increased in the plasma of patients with idiopathic pulmonary fibrosis (39) and in bronchoalveolar lavage in pulmonary sarcoidosis (37). In scleroderma-associated fibrotic lung disease, ET-1 immunoreactivity is increased, and a reduction in ETA with a slight increase in ETB expression has been noted in the diseased lung (1). Furthermore, in systemic sclerosis, alveolar macrophage ET-1 expression is increased upon stimulation with LPS (22). Thus, it is known that alveolar macrophages from diseased lungs demonstrate alterations in the ET-1 cascade. The current study demonstrates that while ET-1 expression is similar to controls in HPAH and IPAH lung macrophages, ETA and ETB receptor expression is reduced in lung macrophages from HPAH but not IPAH. While two of the patients with HPAH received endothelin receptor antagonist therapy, two did not: all four HPAH patients exhibited the reduction in ETA and ETB receptor expression. Furthermore, the one IPAH patient receiving the endothelin receptor antagonist did not show this reduction. Thus, it is unlikely that this therapy contributed to this change. This is the first demonstration of a distinct difference in protein expression between lungs of patients with HPAH and those with IPAH and controls.
ET-1 binds to its G-coupled receptors, ETA and ETB (3). In control lung, the ETA receptor is present on vascular SMCs and is responsible for vasoconstriction, whereas ETB is present on ECs and SMCs and involved in clearance and degradation of ET-1 (13). Our study shows significantly lower expression levels of ETA and ETB in the BMDM from BMPR2 mutant mice than in control mice, and our findings following stimulation with LPS show a further reduction in ETA and ETB receptor gene expression. These findings suggest a link between a BMPR2 mutation and ETA and ETB gene expression. Furthermore, our studies in human lung show a reduction in ETA and ETB expression in alveolar macrophages in HPAH but not IPAH, confirming the notion that a BMPR2 mutation modulates gene expression of the ETA and ETB receptors.
In humans and rodents, two distinct isozymes of ECE, ECE1 (29) and ECE2 (11), catalyze the rate-limiting step in ET-1 synthesis. In IPAH patients, expression of ECE-1 is augmented in the endothelium of diseased pulmonary arteries (14). Similarly, in rats the immunoreactivity for ECE is increased in the vascular wall of pulmonary veins after exposure to hypoxia (36). In BMDM from control mice, expression of the ECE-1 gene was decreased upon activation with LPS and was further decreased in cells with a mutated BMPR2 phenotype. The decreased level of ECE gene expression in LPS-stimulated BMDM might be expected to result in decreased ET-1 expression. However, ET-1 levels in culture medium were increased in LPS-stimulated BMDM from both control and BMPR2 mutant mice.
In pulmonary hypertension, the increased levels of ET-1 in the pulmonary circulation are due to reduced pulmonary clearance of ET-1 (8). The ETB receptors play an important role in the clearance of ET-1 from circulation (13). Blockade of the ETB receptor in rat increases the level of ET-1 in circulation (27), and rats deficient in the ETB receptor show higher levels of plasma ET-1 (17). Thus, the increased ET-1 in the media of BMDM from BMPR2 mutant mice is likely the result of the decrease in ETB receptor expression. The striking reduction in ECE gene expression in the hypertensive cells confirms this idea.
In summary, our findings confirm that ET-1 and its receptors are present in lung macrophages. Furthermore, ETA and ETB receptor expression is decreased in BMDM from a BMPR2 mutant mouse model of HPAH compared with controls. The increased release of ET-1 into the culture media likely reflects the decrease in ETb receptor expression. The decreased expression of ETA and ETB expression is recapitulated in human lung macrophages from patients with HPAH but not IPAH, suggesting that this change is specific for the hereditary form of the disease. We conclude that local increases in lung macrophage ET-1 may contribute to pulmonary arterial hypertension in HPAH through a paracrine effect. The mechanism through which this effect is mediated is not known and is a fruitful avenue for further research. Whether this effect contributes to the clinical differences reported in patients with HPAH and IPAH requires further study.
GRANTS
This work was supported by grants from Actelion, the Pulmonary Hypertension Breakthrough Initiative (funded by the Cardiovascular Medical Research and Education Fund), and National Heart, Lung, and Blood Institute Grant HL-82694-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abraham DJ, Vancheeswaran R, Dashwood MR, Rajkumar VS, Pantelides P, Xu SW, du Bois RM, Black CM. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am J Pathol 151: 831–841, 1997 [PMC free article] [PubMed] [Google Scholar]
- 2.Austin ED, Rock MT, Mosse CA, Vnencak-Jones CL, Yoder SM, Robbins IM, Loyd JE, Meyrick BO. T lymphocyte subset abnormalities in the blood and lung in pulmonary arterial hypertension. Respir Med 104: 454–462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balyakina EV, Chen D, Lawrence ML, Manning S, Parker RE, Shappell SB, Meyrick B. ET-1 receptor gene expression and distribution in L1 and L2 cells from hypertensive sheep pulmonary artery. Am J Physiol Lung Cell Mol Physiol 283: L42–L51, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cacoub P, Dorent R, Nataf P, Carayon A, Riquet M, Noe E, Piette JC, Godeau P, Gandjbakhch I. Endothelin-1 in the lungs of patients with pulmonary hypertension. Cardiovasc Res 33: 196–200, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Balyakina EV, Lawrence M, Christman BW, Meyrick B. Cyclooxygenase is regulated by ET-1 and MAPKs in peripheral lung microvascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 284: L614–L621, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Din S, Sarathchandra P, Yacoub MH, Chester AH. Interaction between bone morphogenetic proteins and endothelin-1 in human pulmonary artery smooth muscle. Vascul Pharmacol 51: 344–349, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Donn R, Payne D, Ray D. Glucocorticoid receptor gene polymorphisms and susceptibility to rheumatoid arthritis. Clin Endocrinol (Oxf) 67: 342–345, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupuis J, Rouleau JL, Cernacek P. Reduced pulmonary clearance of endothelin-1 contributes to the increase of circulating levels in heart failure secondary to myocardial infarction. Circulation 98: 1684–1687, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Ehrenreich H, Anderson RW, Fox CH, Rieckmann P, Hoffman GS, Travis WD, Coligan JE, Kehrl JH, Fauci AS. Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J Exp Med 172: 1741–1748, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott CG, Glissmeyer EW, Havlena GT, Carlquist J, McKinney JT, Rich S, McGoon MD, Scholand MB, Kim M, Jensen RL, Schmidt JW, Ward K. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation 113: 2509–2515, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem 270: 15262–15268, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Frelin C, Guedin D. Why are circulating concentrations of endothelin-1 so low? Cardiovasc Res 28: 1613–1622, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun 199: 1461–1465, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Giaid A. Nitric oxide and endothelin-1 in pulmonary hypertension. Chest 114: 208S–212S, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328: 1732–1739, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM, West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 292: L1473–L1479, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ivy DD, Yanagisawa M, Gariepy CE, Gebb SA, Colvin KL, McMurtry IF. Exaggerated hypoxic pulmonary hypertension in endothelin B receptor-deficient rats. Am J Physiol Lung Cell Mol Physiol 282: L703–L712, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Juergens UR, Racke K, Uen S, Haag S, Lamyel F, Stober M, Gillissen A, Novak N, Vetter H. Inflammatory responses after endothelin B (ETB) receptor activation in human monocytes: new evidence for beneficial anti-inflammatory potency of ETB-receptor antagonism. Pulm Pharmacol Ther 21: 533–539, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Lane KB, Egan B, Vick S, Abdolrasulnia R, Shepherd VL. Characterization of a rat alveolar macrophage cell line that expresses a functional mannose receptor. J Leukoc Biol 64: 345–350, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Loyd JE, Atkinson JB, Pietra GG, Virmani R, Newman JH. Heterogeneity of pathologic lesions in familial primary pulmonary hypertension. Am Rev Respir Dis 138: 952–957, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, Newman JH, Phillips JA, III, Soubrier F, Trembath RC, Chung WK. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 54: S32–S42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odoux C, Crestani B, Lebrun G, Rolland C, Aubin P, Seta N, Kahn MF, Fiet J, Aubier M. Endothelin-1 secretion by alveolar macrophages in systemic sclerosis. Am J Respir Crit Care Med 156: 1429–1435, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol 298: H1235–H1248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenzweig EB, Morse JH, Knowles JA, Chada KK, Khan AM, Roberts KE, McElroy JJ, Juskiw NK, Mallory NC, Rich S, Diamond B, Barst RJ. Clinical implications of determining BMPR2 mutation status in a large cohort of children and adults with pulmonary arterial hypertension. J Heart Lung Transplant 27: 668–674, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, Hoeffken G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest 120: 1562–1569, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Rubin LJ. Primary pulmonary hypertension. N Engl J Med 336: 111–117, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Sato K, Oka M, Hasunuma K, Ohnishi M, Sato K, Kira S. Effects of separate and combined ETA and ETB blockade on ET-1-induced constriction in perfused rat lungs. Am J Physiol Lung Cell Mol Physiol 269: L668–L672, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Schiffrin EL. Endothelin and endothelin antagonists in hypertension. J Hypertens 16: 1891–1895, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M, Kroger B, Jacob E, Seulberger H, Subkowski T, Otter R, Meyer T, Schmalzing G, Hillen H. Molecular characterization of human and bovine endothelin converting enzyme (ECE-1). FEBS Lett 356: 238–243, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Shepherd VL, Abdolrasulnia R, Garrett M, Cowan HB. Down-regulation of mannose receptor activity in macrophages after treatment with lipopolysaccharide and phorbol esters. J Immunol 145: 1530–1536, 1990 [PubMed] [Google Scholar]
- 31.Shepherd VL, Lane KB, Abdolrasulnia R. Ingestion of Candida albicans down-regulates mannose receptor expression on rat macrophages. Arch Biochem Biophys 344: 350–356, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Stelzner TJ, O'Brien RF, Yanagisawa M, Sakurai T, Sato K, Webb S, Zamora M, McMurtry IF, Fisher JH. Increased lung endothelin-1 production in rats with idiopathic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 262: L614–L620, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 114: 464–469, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Sztrymf B, Coulet F, Girerd B, Yaici A, Jais X, Sitbon O, Montani D, Souza R, Simonneau G, Soubrier F, Humbert M. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med 177: 1377–1383, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Tada Y, Majka S, Carr M, Harral J, Crona D, Kuriyama T, West J. Molecular effects of loss of BMPR2 signaling in smooth muscle in a transgenic mouse model of PAH. Am J Physiol Lung Cell Mol Physiol 292: L1556–L1563, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H, Soma S, Muramatsu M, Oka M, Fukuchi Y. Upregulation of ET-1 and its receptors and remodeling in small pulmonary veins under hypoxic conditions. Am J Physiol Lung Cell Mol Physiol 280: L1104–L1114, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Terashita K, Kato S, Sata M, Inoue S, Nakamura H, Tomoike H. Increased endothelin-1 levels of BAL fluid in patients with pulmonary sarcoidosis. Respirology 11: 145–151, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 144: 275–285, 1994 [PMC free article] [PubMed] [Google Scholar]
- 39.Uguccioni M, Pulsatelli L, Grigolo B, Facchini A, Fasano L, Cinti C, Fabbri M, Gasbarrini G, Meliconi R. Endothelin-1 in idiopathic pulmonary fibrosis. J Clin Pathol 48: 330–334, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West J. Cross talk between smad, MAPK, and actin in the etiology of pulmonary arterial hypertension. Adv Exp Med Biol 661: 265–278, 2010 [DOI] [PubMed] [Google Scholar]
- 41.West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D, Fagan K. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol 295: L744–L755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park IH, Sero JE, Zhu H, Perez-Atayde A, Frazier AL, Surani MA, Daley GQ. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 460: 909–913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamora MR, Stelzner TJ, Webb S, Panos RJ, Ruff LJ, Dempsey EC. Overexpression of endothelin-1 and enhanced growth of pulmonary artery smooth muscle cells from fawn-hooded rats. Am J Physiol Lung Cell Mol Physiol 270: L101–L109, 1996 [DOI] [PubMed] [Google Scholar]