Abstract
We have previously implicated transient receptor potential vanilloid 4 (TRPV4) channels and alveolar macrophages in initiating the permeability increase in response to high peak inflation pressure (PIP) ventilation. Alveolar macrophages were harvested from TRPV4−/− and TRPV4+/+ mice and instilled in the lungs of mice of the opposite genotype. Filtration coefficients (Kf) measured in isolated perfused lungs after ventilation with successive 30-min periods of 9, 25, and 35 cmH2O PIP did not significantly increase in lungs from TRPV4−/− mice but increased >2.2-fold in TRPV4+/+ lungs, TRPV4+/+ lungs instilled with TRPV4−/− macrophages, and TRPV4−/− lungs instilled with TRPV4+/+ macrophages after ventilation with 35 cmH2O PIP. Activation of TRPV4 with 4-α-phorbol didecanoate (4αPDD) significantly increased intracellular calcium, superoxide, and nitric oxide production in TRPV4+/+ macrophages but not TRPV4−/− macrophages. Cross-sectional areas increased nearly 3-fold in TRPV4+/+ macrophages compared with TRPV4−/− macrophages after 4αPDD. Immunohistochemistry staining of lung tissue for nitrotyrosine revealed increased amounts in high PIP ventilated TRPV4+/+ lungs compared with low PIP ventilated TRPV4+/+ or high PIP ventilated TRPV4−/− lungs. Thus TRPV4+/+ macrophages restored susceptibility of TRPV4−/− lungs to mechanical injury. A TRPV4 agonist increased intracellular calcium and reactive oxygen and nitrogen species in harvested TRPV4+/+ macrophages but not TRPV4−/− macrophages. Kf increases correlated with tissue nitrotyrosine, a marker of peroxynitrite production.
Keywords: pulmonary edema, filtration coefficient, superoxide, nitric oxide, Ca2+ channels, peroxynitrite, 4-α-phorbol didecanoate
previous experimental studies on ventilator-induced lung injury (VILI) indicate a rapid increase in lung vascular permeability after overdistension with high airway pressures and lung volumes (5, 6, 30). The increased permeability is calcium-dependent because it was attenuated by gadolinium, an inhibitor of stretch-activated, nonselective cation channel activity (31, 34). We (14) recently presented evidence that the transient receptor potential vanilloid-4 (TRPV4) channel was this stretch-activated cation channel. High airway pressure-induced permeability increases were abolished in lungs of TRPV4 knockout (KO) and in wild-type (WT) mice treated with inhibitors of TRPV channels, arachidonic acid production, and P-450 epoxygenases.
Recent studies also suggest that the alveolar macrophage (AM) has a critical role in the rapid initial increase in permeability after lung overdistension because macrophage depletion attenuates VILI in rats (7, 10). The AM is a sentinel cell that can initiate lung injury and inflammation because macrophage depletion attenuates lung injury after challenges with sepsis (9), live bacteria (15), or ischemia-reperfusion (48). The number of AM recovered by bronchoalveolar lavage (BAL) decrease within minutes after initiation of ventilation with high airway pressures, indicating a rapid activation and firm adhesion (7, 10). Pugin et al. (35) compared cultures of various lung cell types during cyclical strain and identified the lung macrophage as the major source of inflammatory cytokines, chemokines, and matrix metalloproteinase-9 (MMP-9) during mechanical stretch. AM are also sources of reactive oxygen and nitrogen species (28). Although TRPV4 has been identified in macrophages by immunohistochemistry (2), the role of the TRPV4 channel in macrophage function and the mechanisms whereby activation of macrophages initiates overdistension-induced lung injury have not been studied.
In the present study, we addressed the hypothesis that TRPV4 expressed in macrophages plays a central role in the initial transduction of VILI. We instilled WT (TRPV4+/+) macrophages into lungs of KO (TRPV4−/−) mice and instilled TRPV4−/− macrophages into lungs of TRPV4+/+ mice. The filtration coefficient (Kf) was measured in lungs isolated from these mice in response to ventilation with low or high peak inflation pressures (PIP). The instilled TRPV4+/+ macrophages restored the Kf increase produced by high PIP in TRPV4−/− mouse lungs. Harvested macrophages were also labeled with specific fluorescent dyes sensitive to levels of intracellular calcium, superoxide, and nitric oxide (NO) and challenged with a TRPV4 channel agonist, 4-α-phorbol didecanoate (4αPDD), and the fluorescence was measured. Cross-sectional areas of the two macrophage genotypes were also measured in response to overnight culture and the TRPV4 agonist. Lung tissue samples from six experimental groups were immunostained for nitrotyrosine, and the color intensity was evaluated.
METHODS
TRPV4 KO Mice
The TRPV4 KO mice were generated by Cre-lox excision of exon 12 of the TRPV4 gene, which codes for the pore-loop region and the adjacent transmembrane domain (21). Mice were genotyped using mouse tail DNA using PCR primers described in our (2) previous online supplement. PCR products resolved on agarose gel indicate a 2.1-kb band for TRPV4+/+ mice and a 1.1-kb band for TRPV4−/− mice, the latter reflecting excision of exon 12. The TRPV4−/− mice have been backcrossed with the C57BL/6 background mice for 8 generations so that C57BL/6 could be used as controls (14). The 8-fold backcross with the C57BL/6 background would lead to retention of only 0.38% of the non-C57BL/6 background, and previous studies on Kf in mouse lungs indicated no significant differences between the α1G KO mice and littermates bred on the C57BL/6 background and littermates of these TRPV4 KO mice relative to baseline lung Kf values or Kf increases after 10 μmol of the TRPV4 agonist, 4αPDD (2, 43).
The TRPV4−/− breeding colony was housed in the University of South Alabama Vivarium and monitored by veterinary personnel. All protocols were approved by the Animal Care and Use Committee of the University of South Alabama College of Medicine and met all National Institutes of Health guidelines.
Isolated Lung Preparation
C57BL/6 WT male mice (Charles River Laboratories) and TRPV4−/− mice weighing 19.6–32.0 g (24.1 ± 0.3 g) were anesthetized with an intraperitoneal injection of 100 mg/kg pentobarbital sodium. The trachea was cannulated, and the mice were ventilated with a gas mixture of 20% O2-5% CO2-75% N2 by using a Harvard rodent ventilator (model 683; Harvard, South Natick, MA). The tidal volume was adjusted to obtain a PIP of ∼9 cmH2O at a respiratory rate of 90 breaths/min, with a positive end-expiratory pressure (PEEP) of ∼2.5 cmH2O. The chest was opened, 100 IU of heparin sodium was injected into the right ventricle, and a suture was placed around the pulmonary artery with aorta. Cannulas (0.86-mm inner diameter and 1.27-mm outer diameter) were placed in the pulmonary artery and left atrium, and lung and heart were excised en bloc and suspended from a balance beam attached to a force transducer (model FT03 C; Grass, Quincy, MA). The initial 1–2 ml of perfusate, which contained residual blood cells and plasma, were discarded and not recirculated. All lungs were perfused with 1% bovine serum albumin-3% clinical grade dextran in Earle's balanced salt solution by using a roller pump (MINIPULS 2; Gilson, Middleton, WI) at a constant flow rate of 0.75 ml/min in a recirculating system that had a system volume of 10 ml. The venous outflow was collected in a reservoir, the height of which could be adjusted to increase venous pressure. Pulmonary arterial pressure and pulmonary venous pressure (Ppv) were zeroed at the midlung level, airway pressure was measured by using Cobe pressure transducer (Lakewood, CO), and pressures and lung weight were continuously recorded on a Grass model 7D polygraph (14).
Kf.
After a 30-min equilibration period and attainment of an isogravimetric state, Ppv was increased by 6 cmH2O for 20 min. The change in capillary pressure (Ppc) was determined by double occlusion before and after the Ppv increase. Kf (in ml·min−1·cmH2O−1·100 g−1) was calculated as the rate of lung weight gain between 18 and 20 min divided by the change in Ppc. All Kf values were normalized to 100-g predicted lung weight (PLW) on the basis of the ratio of lung-to-body weight (BW) according to PLW = (0.00452 ± 0.0003) BW (29).
Lung wet-to-dry weight ratios.
After the measurement of final Kf, the lung was harvested, wet weight was determined, and samples were desiccated at 80°C for 1 wk before the dry weight was determined. The wet weight divided by dry weight was calculated for the wet-to-dry lung weight ratio (W/D ratio).
High and low PIP ventilation protocols.
The lungs were ventilated for 30 min with 9 cmH2O PIP and with ∼2.5 cmH2O PEEP at 40 breaths/min followed by an increased venous pressure for 20 min for measurement of Kf. Lungs were then divided into one of two protocols as shown in Fig. 1. The low PIP protocol required two additional 30-min periods of 9 cmH2O PIP ventilation followed by Kf measurements at the end of each 30-min period. In lungs assigned to a high PIP protocol, lungs were sequentially ventilated for 30-min periods at 25 and then 35 cmH2O PIP. Each period was ended with Kf measurements as previously described (14). In all groups, perfused buffer was maintained at 37°C, and Kf was measured at 30, 80, and 130 min, i.e., the venous reservoir was raised at these times for the 20-min Kf measurement period, and the weight gain slope was measured during the last 2 min of the venous pressure increase. The temperature was maintained by direct measurement of the perfusate using a type K thermometer (Extech Instruments, Waltham, MA) inserted into the right ventricle of the isolated heart-lung preparation.
Fig. 1.
Time course for the increases in peak inflation pressure (PIP) and venous pressure for the high PIP (solid line) and low PIP (dashed line) protocols.
Macrophage transfer protocol.
WT (TRPV4+/+) or TRPV4 KO (TRPV4−/−) mice were anesthetized with intraperitoneal injection of 100 mg/kg pentobarbital sodium. To obtain AM, BAL was performed 3 times with 0.5 ml of PBS. Pooled BAL fluid from 2 mice of each genotype was centrifuged at 500 g for 10 min, and the cells were resuspended in 50 μl of PBS for tracheal instillation in each experimental mouse. This resulted in an average of 517,000 ± 74,000 TRPV4+/+ and 627,000 ± 113,000 TRPV4−/− cells per instillation. Differential BAL cell counts indicated 88.7 ± 0.5% macrophages, 10.9 ± 0.6% lymphocytes, and 0.3 ± 0.1% neutrophils in TRPV4+/+ BAL (n = 4) and 87.6 ± 1.3% macrophages and 11.7 ± 1.2% lymphocytes and 0.6 ± 0.2% neutrophils in TRPV4−/− BAL (n = 4). TRPV4−/− mice were anesthetized and intubated. Then, 50 μl of the macrophage suspension was injected into the lung through tracheal tube followed by 15 min of low PIP ventilation with 2 cmH2O PEEP to spread the fluid to the alveoli. The tracheal tube was removed, mice were kept in the cages for 16 h, and then Kf and W/D ratio were measured.
Experimental groups.
Isolated lung experiments were performed on groups (n = 5) ventilated with the low or high PIP protocols with or without the addition of AM from either WT or KO mice. C57BL/6 mice were used as WT controls as the KO breeders were backcrossed some 8 generations against this background. Kf measurements at baseline and after high vascular pressure injury were not statistically different between C57BL/6 mice and true littermates (unpublished data). Using the low PIP (LP) protocol, groups included: 1) LP WT group of WT mouse lungs ventilated with low PIP; 2) LP WT+KO AM group of WT lungs instilled with KO AM ventilated with low PIP; 3) LP KO group of TRPV4 KO lungs ventilated with low PIP; and 4) LP KO+WT AM group of KO mice instilled with WT AM ventilated with low PIP. Groups ventilated with the high PIP (HP) protocol included: 5) HP WT group of WT mouse lungs ventilated with high PIP; 6) HP WT+KO AM group of WT lungs instilled with KO AM ventilated with high PIP; 7) HP KO group of TRPV4 KO lungs ventilated with high PIP; and 8) HP KO+WT AM group of KO mice instilled with WT AM ventilated with high PIP.
Macrophage TRPV4 Expression
AM were harvested from either WT (n = 2) or TRPV4−/− (n = 2) mice. Total RNA was extracted from the pooled macrophages in each genotype using a Qiagen RNeasy Plus Mini Kit with on-column removal of genomic DNA. For comparison, we also isolated total RNA from cultured murine lung epithelial (MLE) and alveolar macrophage (RAW) cell lines (n = 2 in each case). After first-strand cDNA synthesis using 1.5 μg of total RNA (SuperScript II; Invitrogen, Eugene, OR), PCR was performed to amplify TRPV4 mRNA. Primers designed to amplify a sequence of the TRPV4 mRNA coding for the pore-loop region of the protein were sense 5′-TCACGAAGAAATGCCCTGGAGTGA-3′ and antisense 5′-ACTGCAACTTCCAGATGTGCTTGC-3′ (612-bp predicted product length), and those for GADPH mRNA as a control were sense 5′-TGTGTCCGTCGTGGATCTGA-3′ and antisense 5′-CCTGCTTCACCACCTTCTTGAT-3′ and were obtained from Integrated DNA Technologies (IDT). PCR reactions (50 μl) included 3-μl cDNA, 2-μl primer mix (5 μM each sense and antisense primers), and 45-μl Platinum Blue PCR SuperMix (Invitrogen). cDNA was amplified in a thermal cycler (Hybaid) using the following conditions: denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, for 35 cycles, with a final extension at 72°C for 10 min. Products were separated on a 1.8% agarose gel and visualized by ethidium bromide staining.
Confocal fluorescence imaging.
Macrophage fluorescence studies were performed using a spinning-disk laser confocal microscope (RS-3 UltraVIEW; PerkinElmer). AM were cultured overnight in coverslip bottom 35-mm petri dishes. During fluorescence measurements, the dishes had a volume of ∼2.5 ml and were continuously perfused at 8 ml/min with 37°C Earle's balanced salt solution bubbled with 5% CO2-balance air. For measurement of intracellular Ca2+, cells were loaded with Ca2+ indicator solution (containing HEPES buffer, 10 μM fluo-4 AM, 0.01% DMSO, 0.02% Pluronic F-127) for 30 min at 37°C in the dark. Ca2+ fluorescence was measured by excitation at 488 nm and emission filtered at 510 nm. To measure intracellular NO production, cells were loaded for 30 min with 10 μM 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) diacetate, fluorescence was measured by excitation at 495 nm, and emission was measured at 515 nm. Superoxide production was measured by loading the cells with 500 nM MitoTracker Red dye for 30 min, fluorescence was measured by excitation at 578 nm, and emission was measured at 599 nm. Fluo-4 AM, DAF-FM, and reduced MitoTracker Red were obtained from Invitrogen. 4αPDD was obtained from Sigma (St. Louis, MO). Drugs were dissolved in DMSO before use. Macrophage cross-sectional areas and diameters were measured by drawing a region of interest around individual cells and calculating mean intensity, cross-sectional areas, and diameters using UltraVIEW software. The system was calibrated using 6-μm calibration beads. Fluorescence was measured before and after challenge with 10 μM 4αPDD, a TRPV channel agonist.
Scanning Electron Microscopy
Macrophages were placed on glass coverslips and fixed with 3% glutaraldehyde in cacodylate buffer. Specimens were rinsed in cacodylate buffer, postfixed with osmium tetroxide for 30 min, dehydrated, and chemically dried with hexamethyldisilazane (HMDS; Ted Pella, Redding, CA). The coverslips were attached to stubs, and the specimens were coated with gold-palladium in a Denton DSM-5A cold sputter module (Denton Vacuum, Moorestown, NJ). Specimens were viewed and photographed in a Philips XL20 scanning electron microscope (FEI, Hillsboro, OR).
Immunohistochemistry Staining for Nitrotyrosine
Sections of mouse lung tissue from LP KO, HP KO, LP WT, HP WT, HP KO+WT AM, and HP WT+KO AM groups were fixed with 4% paraformaldehyde, processed, and embedded in paraffin. Sections (5 μm) were incubated overnight at 4°C with 1:500 primary antibodies to nitrotyrosine (Cell Signaling Technology) followed by incubation for 1 h at room temperature with a horseradish peroxidase-conjugated secondary antibody (1:100; Santa Cruz Biotechnology) as previously described (2). Sections were stained with diaminobenzidine and counterstained with hematoxylin. The presence of nitrotyrosine was indicated by the brown peroxidase reaction product. Light micrographs were obtained, and a semiquantitative analysis was performed using MetaMorph multispectral software (CRi, Woburn, MA). The software allowed selective threshold intensities of red, green, and blue to be adjusted. Blue intensity was adjusted to remove air spaces, and tissue red intensity of tissue areas above threshold were recorded. Taking the difference of these parameters, a staining coefficient was calculated. Although the threshold areas correlated with injury, a better correlation was calculated by multiplying the threshold area by average pixel intensity. The mean values (n = 4) for each group were normalized by dividing by the value of the LP KO group.
Statistical Analysis
All values are expressed as means ± SE. One- or two-way ANOVA with repeated measures followed by a Student-Newman-Keuls posttest or linear regression analysis was used. Significant differences were determined where P < 0.05.
RESULTS
Macrophage Genotype Effects on Kf and Edema
The permeability and edema responses of isolated perfused mouse lungs to PIP and the addition of macrophages with or without functional TRPV4 channels are shown in Fig. 2. In groups ventilated with the low PIP protocol (Fig. 2A), the Kf in the LP WT+KO AM group was significantly (35%) higher than that in the LP KO group at the 130-min time period and its baseline value. There were no other differences between other groups or time periods. Figure 2B shows Kf measured after the high PIP ventilation protocol. Kf in the HP KO group did not change significantly from baseline after ventilation with either 25 or 35 cmH2O PIP. In the HP WT, HP WT+KO AM, and HP KO+WT AM groups, Kf increased significantly after ventilation with 25 cmH2O PIP and showed a further significant increase after the 35 cmH2O PIP ventilation period. The Kf for these 3 groups increased ∼2.2-fold after 35 cmH2O PIP compared with Kf after 9 cmH2O PIP ventilation but were not significantly different from each other at any time period. However, Kf in these 3 groups were significantly greater than the Kf for the HP KO group after both the 25 and 35 cmH2O PIP ventilation periods.
Fig. 2.
A: filtration coefficient (Kf) after the low PIP ventilation protocol in wild-type lungs (LP WT), WT lungs with knockout (KO) mouse alveolar macrophages added (LP WT+KO AM), KO lungs (LP KO), and KO lungs with WT macrophages added (LP KO+WT AM). *P < 0.05 vs. the same group after 30 min. #P < 0.05 vs. KO group within the same time period. B: filtration coefficient (Kf) after the high PIP ventilation protocol in groups of WT (HP WT) mouse lungs, WT lungs with KO macrophages added (HP WT+KO AM), KO lungs (HP KO), and KO lungs with WT macrophages added (HP KO+WT AM). *P < 0.05 vs. the same group after 30 min. #P < 0.05 vs. KO group within the same time period. C: wet-to-dry lung weight ratios after low and high PIP protocols in all groups. *P < 0.05 vs. same treatment group ventilated with the low PIP protocol. #P < 0.05 vs. KO group within the same time period.
The W/D weight ratios were indicative of the edema gained during the experiments. Figure 2C shows the terminal W/D ratios for the 4 low PIP and 4 high PIP groups. W/D ratios after the low PIP ventilation were 4, 15, and 24% greater in the LP WT, LP WT+KO AM, and LP KO+WT AM groups compared with those of the LP KO group. After low PIP ventilation, the W/D ratios of the LP KO+WT AM group were significantly greater than those for the LP KO group but not different from the other groups. The W/D ratios for all high PIP groups except the HP KO group were significantly greater after high PIP ventilation than the same group after low PIP ventilation. W/D ratios in the HP WT, HP WT+KO AM, and HP KO+WT AM groups were significantly greater than those of the HP KO group by 8, 19, and 21%, respectively. These data indicate that macrophage TRPV4 was required for the permeability increase and increased edema after high PIP ventilation and that the presence of TRPV4 on TRPV4+/+ macrophages restored the pressure-induced permeability increase in TRPV4−/− lungs.
Macrophage TRPV4 and GAPDH Expression
PCR product was measured in WT and KO macrophages obtained by BAL. TRPV4 has previously been identified by immunohistochemistry in mouse lung epithelium, endothelium, and macrophages, but the TRPV4 messenger RNA in AM has not previously been reported. The primers used for the PCR reaction were specific to the pore region of the TRPV4 channel, which we reasoned must be absent in the KO mice. The PCR product indicated that the TRPV4 pore region (612 bp) was intact in the macrophages from WT mice but not the TRPV4 KO mice (data not shown). PCR product for mGADPH (100 bp) used as a control was present in both TRPV4+/+ and TRPV4−/−macrophages.
Effects of TRPV4 Activation on Macrophage Function
Macrophage intracellular calcium.
AM obtained by BAL were labeled with fluo-4 dye as described. Perfusion of the cells with buffer containing 4αPDD produced a heterogeneous increase in fluorescence indicating calcium entry into the TRPV4+/+ macrophages. However, no significant increase was observed in the TRPV4−/− macrophages. Figure 3A shows fluorescence micrographs of fluo-4-labeled TRPV4−/− and TRPV4+/+ macrophages after 4αPDD, whereas Fig. 3B indicates the transient responses of individual TRPV4−/− and TRPV4+/+ macrophages over the first few minutes after adding 4αPDD. Fluorescence intensity increased heterogeneously in TRPV4+/+ macrophages but not in TRPV4−/−macrophages. Figure 3C summarizes the mean calcium responses of TRPV4−/−(n = 67) and TRPV4+/+ (n = 64) macrophages before and after challenge with 4αPDD. Mean fluorescence was not different between TRPV4−/− and TRPV4+/+ macrophages under baseline conditions, but fluorescence intensity was significantly higher in the TRPV4+/+ macrophages after challenge with 4αPDD compared with TRPV4+/+ baseline measurements or TRPV4−/− macrophages at baseline and after 4αPDD treatment. These studies indicate a significant intracellular calcium increase was induced by 4αPDD activation of TRPV4 in the TRPV4+/+ macrophages, but minimal calcium increase was observed in the TRPV4−/− macrophages.
Fig. 3.
Calcium response of transient receptor potential vanilloid 4 TRPV4−/− and TRPV4+/+ macrophages stained with fluo-4 dye treated with 4-α-phorbol didecanoate (4αPDD). A: fluorescence images of TRPV4−/− and TRPV4+/+ macrophages after treatment with 4αPDD. B: time courses of fluorescence intensities of individual TRPV4−/− and TRPV4+/+ macrophages after treatment with 4αPDD. C: mean fluorescence intensities of fluo-4 in TRPV4+/+ and TRPV4−/− macrophages with and without treatment with 4αPDD. *P < 0.05 vs. baseline value in the same genotype. #P < 0.05 vs. TRPV4−/− group after same treatment.
Reactive oxygen species.
AM from TRPV4−/− and TRPV4+/+ mice were labeled with reduced MitoTracker Red dye, which makes a fluorescent product that is trapped in the mitochondria when oxidized by superoxide anions. TRPV4+/+ and TRPV4−/− macrophages stained with MitoTracker Red are shown in Fig. 4A after treatment with 4αPDD. There were no significant differences in fluorescence intensity between macrophage genotypes under baseline conditions, but the fluorescent intensity of the TRPV4+/+ (n = 38) macrophages after 4αPDD treatment was significantly increased compared with baseline values for either untreated TRPV4−/− (n = 55) or TRPV4+/+ macrophages or TRPV4−/− macrophages treated with 4αPDD (Fig. 4B). Although oxidized MitoTracker Red accumulated in the mitochondria, this may also reflect oxygen free radicals produced outside the mitochondria because superoxide and hydrogen peroxide can diffuse into the mitochondria from other cellular sources. However, TRPV4 activation significantly increased production of reactive oxygen species in TRPV4+/+ macrophages.
Fig. 4.
Superoxide production in TRPV4−/− and TRPV4+/+ macrophages with and without 4αPDD. A: TRPV4+/+ and TRPV4−/− macrophages stained with MitoTracker Red after treatment with 4αPDD. B: mean fluorescence intensities of MitoTracker Red in TRPV4+/+ and TRPV4−/− macrophages with and without treatment with 4αPDD. *P < 0.05 vs. baseline value in the same genotype. #P < 0.05 vs. TRPV4−/− group after same treatment.
Reactive nitrogen species.
AM from TRPV4−/− and TRPV4+/+ mice were also labeled with DAF-FM, a NO-sensitive dye, and fluorescence intensity was measured. Figure 5A indicates TRPV4+/+ and TRPV4−/− macrophages labeled with DAF-FM dye after 4αPDD, whereas Fig. 5B summarizes the mean fluorescence of TRPV4−/− (n = 19) and TRPV4+/+ (n = 32) macrophages under baseline conditions and 30 min after treatment with 4αPDD. There was no difference in fluorescence intensity between macrophage genotypes at baseline, but the fluorescent intensity of the TRPV4+/+ macrophages after 4αPDD treatment was significantly increased compared with baseline values for TRPV4−/− and TRPV4+/+ macrophages and for 4αPDD-treated TRPV4−/− macrophages. These studies suggest that TRPV4 activation also increased NO production, likely initiated by the increased calcium entry through the TRPV4 channel.
Fig. 5.
Nitric oxide production in TRPV4−/− and TRPV4+/+ macrophages with and without 4αPDD. A: micrographs of TRPV4−/− and TRPV4+/+ macrophages stained with 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) dye after treatment with 4αPDD. B: mean fluorescence intensities of DAF-FM in TRPV4+/+ and TRPV4−/− macrophages with and without treatment with 4αPDD. *P < 0.05 vs. baseline value in the same genotype. #P < 0.05 vs. TRPV4−/− group after same treatment.
Macrophage spreading.
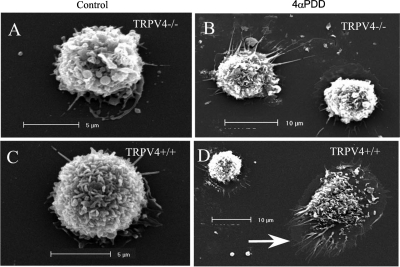
After plating BAL macrophages on glass coverslips, we observed spreading and lamellipodia formation over 24 h in culture, which was greatly increased by treatment with 4αPDD. Figure 6 shows scanning electron micrographs (SEM) of TRPV4−/− (Fig. 6, A and B) and TRPV4+/+ (Fig. 6, C and D) macrophages. Figure 6, A and C, shows freshly harvested TRPV4−/− and TRPV4+/+ macrophages, respectively, obtained by BAL, whereas Fig. 6, B and D, shows TRPV4−/− and TRPV4+/+ macrophages after 24-h incubation in DMEM with 10% FBS and 30-min posttreatment with 4αPDD. The diameters of freshly harvested macrophages of both genotypes were approximately 8–10 μm. Although the diameter of the TRPV4−/− macrophages remained at ∼10 μm with or without 4αPDD challenge, many of the TRPV4+/+ macrophages formed prominent lamellipodia (Fig. 6, arrow) and spread to diameters of ∼27 μm or 3.4-fold that of the freshly harvested macrophages. Figure 7 summarizes mean diameters (Fig. 7A) and cross-sectional areas (Fig. 7B) measured from SEM of TRPV4+/+ and TRPV4−/−macrophages. Each group represents measurements from 7 to 18 cells determined using the UltraVIEW software. Although these data represent cells taken at random, all TRPV4+/+ cells did not spread in a homogeneous manner. Using a diameter threshold of 11.5 μm, none of the TRPV4−/− macrophages exceeded this diameter regardless of treatment. In contrast, 27% of TRPV4+/+ macrophages exceeded this diameter after 24-h incubation in FBS, and 42 and 65% of TRPV4+/+ macrophages exceeded the threshold diameter after incubations with 4αPDD for 30 and 60 min, respectively.
Fig. 6.
Scanning electron micrographs (SEM) of bronchoalveolar lavage macrophages showing freshly harvested TRPV4−/− (A) and TRPV4+/+ (C) macrophages and TRPV4−/− (B) and TRPV4+/+ (D) macrophages after 24-h incubation and treatment with 4αPDD. Note protrusions and spreading (arrow) of TRPV4+/+ macrophage treated with 4αPDD.
Fig. 7.
Bar graphs summarizing mean diameters (A) and mean cross-sectional areas (B) of TRPV4−/− (black bars) and TRPV4+/+ (gray bars) macrophages, freshly harvested (control), after 1 day in culture (baseline) and after challenge with 4αPDD. *P < 0.05 vs. baseline value in the same genotype. **P < 0.05 vs. baseline and 24-h culture groups of the same genotype. #P < 0.05 vs. TRPV4−/− group after same treatment.
Macrophage cross-sectional areas were also measured in fluo-4-labeled macrophages using the UltraVIEW software (data not shown). Although the groups exhibited the same statistical differences as observed for SEM, the magnitude of the differences were not as great. We concluded that the thin lamellipodia could not be visualized as accurately using the fluorescent dye as in the SEM. Whereas the cross-sectional diameter of 4αPDD-treated TRPV4+/+ macrophages was 252% greater than 4αPDD-treated TRPV4−/− macrophages using analysis of SEM, the relative increase between TRPV4+/+ (n = 23) and TRPV4−/− (n = 19) macrophages was only 43% determined using the fluo-4-labeled macrophages. Thus activation of the mechanogated TRPV4 channel initiates signaling pathways for cell adhesion and spreading, but the response is heterogeneous in TRPV4+/+ macrophages.
Immunohistochemistry Staining for Nitrotyrosine
Samples of lung tissue from the isolated lung experiments were fixed and immunostained for 3-nitrotyrosine. Brown reaction product indicates the presence of reaction product due to nitrotyrosine, and the blue color is a tissue counterstain. The presence of nitrotyrosine is generally accepted as a marker of peroxynitrite activity. Figure 8 shows examples of nitrotyrosine staining in lung tissue samples from the LP KO (Fig. 8A), LP WT (Fig. 8B), HP KO (Fig. 8C), HP WT (Fig. 8D), KO+WT AM (Fig. 8E), and WT+KO AM (Fig. 8F) groups. A significantly greater amount of brown staining was apparent in the high PIP ventilated lungs (Fig. 8, D–F) compared with low PIP ventilated lungs (Fig. 8, A and B) and high PIP TRPV4−/− lungs (Fig. 8C). Thus an increase in nitrotyrosine reaction product was present in all high PIP ventilated groups except HP KO, as indicated in the HP WT, WT+KO AM, and KO+WT AM lungs.
Fig. 8.
Immunohistochemistry staining of mouse lungs for nitrotyrosine (brown color) in lung tissue counterstained with hematoxylin. A, TRPV4−/− lungs ventilated at low PIP; B, TRPV4+/+ lungs ventilated at low PIP; C, TRPV4−/− lungs ventilated at high PIP; D, TRPV4+/+ lungs ventilated with high PIP; E, high PIP ventilated TRPV4−/− lungs with TRPV4+/+ macrophages added; F, high PIP ventilated TRPV4+/+ lungs with TRPV4−/− macrophages added. Brown color indicates nitrotyrosine staining.
The brown immunostaining of nitrotyrosine was quantitated using MetaMorph software to threshold for the red color spectrum. The percent area stained for nitrotyrosine was then multiplied by the average pixel density to obtain a staining coefficient. Six groups were selected that exhibited differences in Kf. Figure 9 shows the relationship of lung section nitrotyrosine immunostaining (n = 4) for each group compared by linear regression with mean Kf values for that group. A significant correlation (r2 = 0.87) was observed with the relationship, Kf = 0.133 + 0.185 nitrotyrosine. This indicates a strong correlation of tissue nitrotyrosine to lung injury.
Fig. 9.
Regression analysis of group Kf values on values of nitrotyrosine staining (area × intensity) relative to the LP KO value in LP KO, HP KO, LP WT, HP WT, HP WT+KO AM, and HP KO+WT AM groups (r2 = 0.87).
DISCUSSION
VILI is a complex syndrome induced by tensile failure and a cellular response to mechanical stress (5, 6, 30) and significantly contributes to mortality during ventilatory support for acute respiratory distress syndrome (ARDS) (4). The rapid onset of the increase in vascular permeability following overdistension of the lungs suggests a rapid intracellular cascade of signaling events that is amplified by increases in cytokine production and neutrophil infiltration some hours later (36, 46). The acute endothelial permeability increase induced by low or high PIP ventilation was evaluated using the gravimetric Kf, the product of endothelial hydraulic conductivity and vascular surface area. Kf is a sensitive and repeatable measure of vascular permeability to fluid in the fully recruited lung (29, 32, 33). The rapid permeability response to lung overdistension has been blocked by gadolinium, an inhibitor of stretch-activated and store-activated cation channels (31, 34) indicating that an increase in endothelial intracellular calcium was a necessary event for the increase in permeability (23). Calcium entry is also essential for numerous receptor ligand-mediated vascular permeability responses (22, 23, 39). Recently, we (14) have established the molecular identity of the stretch-activated cation channel responsible for the high PIP ventilation-induced permeability increase in mouse lungs as the TRPV4 cation channel. Kf increases in isolated mouse lungs induced by high PIP ventilation were abolished in TRPV4 null mouse lungs and in WT lungs after treatment with ruthenium red, a TRPV inhibitor; methanandamide, an arachidonic acid inhibitor; and miconazole, a P-450 inhibitor. The increases in lung intracellular calcium induced by lung overdistension were also attenuated in the TRPV4 null mice and ruthenium red-treated mouse lungs.
We used C57BL/6 mice as WT controls (14) because previous studies on Kf in mouse lungs by us (2) and Wu et al. (43) indicated no significant differences between the α1G KO mice or their littermates bred on the C57BL/6 background or littermates of TRPV4 KO mice relative to either baseline lung Kf values or the Kf increases after 10 μmol of the TRPV4 agonist, 4αPDD. Further comparisons of high vascular pressure injury using 30 cmH2O venous pressure by us (16) on Kf in lungs of TRPV4 littermates with the same venous pressure injury using C57BL/6 mice (unpublished data) also indicated no statistical difference between either baseline or postinjury Kf values. Therefore, we believe there was justification for using C57BL/6 mice as WT controls for the responses studied in these experiments.
An essential role of AM in VILI has also recently been demonstrated. We (7) and Frank et al. (10) depleted macrophages in rat lungs using clodronate-filled liposomes and reported an attenuation of VILI. High volume and pressure ventilation caused rapid adhesion of macrophages after only a few minutes as indicated by a reduced macrophage recovery in lavage fluid. The increased alveolar protein leak, increased lung edema, decreased lung compliance, and reduced gas exchange were attenuated by depletion of macrophages. Macrophage depletion also decreased plasma chemokine expression and alveolar lavage neutrophil counts induced by injurious ventilation.
In the present study, we hypothesized that the TRPV4 channel in macrophages contributes to macrophage activation and the increase in vascular permeability after ventilator-induced injury. The high PIP ventilation protocol did not increase Kf in the TRPV4 null mouse lungs. In contrast, instillation of TRPV4+/+ macrophages in TRPV4 null mice 24 h before performing the isolated lung experiments restored the increased Kf response to high PIP ventilation. Ventilation with the low PIP protocol resulted in a small (30%) but significant increase in Kf compared with baseline after 130-min ventilation with 9 cmH2O PIP in TRPV4+/+ lungs supplemented with TRPV4−/− macrophages, but Kf did not change over time in TRPV4−/−lungs with added TRPV4+/+ macrophages. However, after high PIP ventilation (35 cmH2O PIP), Kf increased by 115% in the HP WT+KO AM group and by 100% in the HP KO+WT AM group compared with baseline. These increases were not significantly different from the increase in the HP WT lungs (125%) relative to baseline. The edema accumulation indicated by the W/D ratios followed the general trend of the Kf changes but were determined by multiple factors in addition to vascular permeability. Lungs in all groups accumulated some edema due to the Kf measurements as indicated by experimental W/D ratios that exceeded previously determined baseline lung W/D ratios of 4.47 ± 0.19 in TRPV4+/+ mice and 4.21 ± 0.13 in TRPV4−/− mice of unventilated lungs (47). Thus TRPV4+/+ macrophages were sufficient to increase permeability in TRPV4 null mouse lungs in response to high PIP ventilation. Although we did not deplete the resident macrophages before adding macrophages of the opposite genotype, the fact that addition of TRPV4+/+ macrophages in TRPV4−/− lungs did not produce an increase in permeability after ventilation with the low PIP protocol indicates that the macrophage transfer protocol did not activate the TRPV4+/+ macrophages sufficiently to produce significant injury. Rather, mechanical stress was necessary to activate the TRPV4+/+ macrophages sufficiently to result in injury.
The TRPV channels are cation channels with six transmembrane-spanning segments, a pore-loop region between transmembrane domains 5 and 6, and a greater permeability to calcium than sodium (27). TRPV4 was initially identified as a channel activated by membrane stretch induced by osmotic stimuli (20). Further study demonstrated that TRPV4 is also activated by heat (12, 41), mechanical stimuli (27), the synthetic phorbol ester, 4αPDD (2, 40, 41), and cytochrome P-450 epoxygenase-dependent formation of epoxyeicosatrienoic acids from arachidonic acid (2, 42, 44). Peak gating of TRPV4 with mechanical stretch occurs at core body temperature, with little response at room temperature (26). Phospholipase A2 mediates the formation of arachidonic acid, which can activate TRPV4 via cytochrome P-450 epoxygenase products (19, 40) such as the epoxyeicosatrienoic acids (8). TRPV4-mediated calcium currents are self-limiting through a negative feedback of calcium concentration and can be augmented by tyrosine phosphorylation of the channel protein (45). In previous mouse lung studies, TRPV4 protein was found in lung endothelial cells, epithelial cells, and macrophages using immunohistochemistry (16). Although and TRPV2 and TRPV4 mRNA was observed in lung tissue using PCR (17), TRPV4 mRNA in isolated macrophages has not been reported, and the function of TRPV4 in macrophages has not been previously studied (25). Although immunohistochemistry antibodies recognize the TRPV4 protein in both genotypes, primers were designed specifically for the pore-loop region of the TRPV4 channel to differentiate between TRPV4+/+ and TRPV4−/− macrophages.
AM serve as sentinel cells for phagocytosis of inhaled bacteria and can initiate and coordinate the inflammatory response (18). Activation of AM can initiate the vascular permeability response in lungs after challenge with bacteria, bacterial toxins, and ischemia-reperfusion (9, 15, 48). In addition to phagocytic activity, activated macrophages may produce cytokines IL-1, IL-6, and TNF-α, the chemokines IL-8 and keratinocyte-derived chemokine (KC), as well as MMP, NO, and superoxide (38). Macrophages can produce NO through the eNOS pathway and in greater amounts by the iNOS pathway (9, 24), whereas increased superoxide production occurs largely through the NADPH oxidase pathway (3). Equimolar combination of NO with O2− produces the powerful oxidant peroxynitrite (28). Peroxynitrite can diffuse greater distances than either NO or O2−, and excessive amounts can increase vascular permeability and produce cell injury (28). In the present study, we demonstrated an increased intracellular calcium, an increased NO production, and an increased superoxide production after challenge with 4αPDD in WT macrophages but not in TRPV4 KO macrophages. The high levels of 3-nitrotyrosine in lung tissue after high ventilation in the WT lungs, WT lungs after KO macrophage transfer, and KO lungs after WT macrophage transfer, but not in KO lungs alone, suggests that mechanical activation of the WT macrophages by lung overdistension produced peroxynitrite. Tissue nitrotyrosine has previously been correlated with peroxynitrite production, and in the present study it was correlated with the increase in Kf in the mouse lungs (28). Nitrotyrosine has been shown to be elevated in ARDS, and nitration of surfactant proteins and an increased protein nitrotyrosine were inversely correlated with survival in ARDS (13, 18).
The importance of TRPV4 channels in mechanical activation of macrophages was also demonstrated by the increased cell spreading of WT as opposed to KO macrophages on contact with the glass surface of the coverslip culture dishes. Increased spreading of TRPV4+/+ macrophages occurred after overnight culture in media with 10% FBS, which suggests that firm attachment to the dish surface initiated a signal cascade for cell spreading that was mediated by cell membrane distortion and activation of TRPV4 channels. Further activation of the TRPV4 channels with 4αPDD caused a significant additional spreading of TRPV4+/+ macrophages compared with TRPV4 KO macrophages. Although firm adhesion of macrophages to the culture dish surface implies an adhesion process that does not normally occur in uninjured lungs, the rapid disappearance of lavage macrophages in lungs ventilated with high airway pressures suggests that mechanical activation of macrophages induces a similar firm adhesion in vivo that could be associated with macrophage activation and lung injury (1, 10). ICAM-1 has been shown to be upregulated in various types of lung injury and is an important adhesion molecule for lung epithelial cells and macrophages that may induce this firm adhesion in vivo (11, 37).
In summary, we present for the first time evidence for a functional role of TRPV4 channels in mechanical activation of macrophages and subsequent lung injury. The increased cell spreading of WT macrophages compared with TRPV4 KO macrophages even in the absence of the TRPV4 agonist indicates that the mechanogated TRPV4 channels are important for macrophage spreading and possibly other mechanical functions such as phagocytosis, adhesion, and motility. The increase in calcium entry, NO, and O2− production in WT but not TRPV4−/−macrophages after the TRPV4 agonist indicates that TRPV4 channels are capable of macrophage activation. The presence of increased nitrotyrosine in lungs after high PIP ventilation also suggests a mechanism for the increases in Kf observed. A correlation of the nitrotyrosine staining and Kf suggests that TRPV4 activation of the reactive nitrogen and oxygen pathways forms peroxynitrite, which could account for the rapid increase in vascular permeability in lungs after high pressure and volume ventilation. Restoration of high PIP-induced injury to TRPV4 null lungs by instillation of WT macrophages supports the critical role of mechanical activation of macrophage TRPV4 in initiating this injury.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants P01-HL-66299, HL-084159, and HL-092992.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge Sue Barnes for technical assistance.
REFERENCES
- 1.Akei H, Whitsett JA, Buroker M, Ninomiya T, Tatsumi H, Weaver TE, Ikegami M. Surface tension influences cell shape and phagocytosis in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 291: L572–L579, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broeke RT, Leusink-Muis T, Hilberdink R, Van Ark I, van den Worm E, Villain M, De Clerck F, Blalock JE, Nijkamp FP, Folkerts G. Specific modulation of calmodulin activity induces a dramatic production of superoxide by alveolar macrophages. Lab Invest 84: 29–40, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol 89: 1645–1655, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Dreyfuss D, Saumon G. Ventilator induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157: 294–323, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Eyal FG, Hamm CR, Parker JC. Reduction in alveolar macrophages attenuates acute ventilator induced lung injury in rats. Intensive Care Med 33: 1212–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem 276: 14867–14874, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Farley KS, Wang LF, Razavi HM, Law C, Rohan M, McCormack DG, Mehta S. Effects of macrophage inducible nitric oxide synthase in murine septic lung injury. Am J Physiol Lung Cell Mol Physiol 290: L1164–L1172, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291: L1191–L1198, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Grau GE, Mili N, Lou JN, Morel DR, Ricou B, Lucas R, Suter PM. Phenotypic and functional analysis of pulmonary microvascular endothelial cells from patients with acute respiratory distress syndrome. Lab Invest 74: 761–770, 1996 [PubMed] [Google Scholar]
- 12.Guler A. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22: 6408–6414, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest 94: 2407–2413, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L923–L932, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto S, Pittet JF, Hong K, Folkesson H, Bagby G, Kobzik L, Frevert C, Watanabe K, Tsurufuji S, Wiener-Kronish J. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am J Physiol Lung Cell Mol Physiol 270: L819–L828, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Jian MY, King JA, Al Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol 38: 386–392, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunert-Keil C, Bisping F, Krüger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7: 159, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest 122: 314S–320S, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Liedtke W. TRPV channels' function in osmo- and mechanotransduction. In: TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, edited by Liedtke W, Heller S. Boca Raton, FL: CRC (Chemical Rubber Company Press)-Taylor & Francis, 2006, p. 303–318. [Google Scholar]
- 20.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA 100: 13698–13703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Miles PR, Bowman L, Rengasamy A, Huffman L. Constitutive nitric oxide production by rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 274: L360–L368, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Nagasawa M, Nakagawa Y, Tanaka S, Kojima I. Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J Cell Physiol 210: 692–702, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol 286: C195–C205, 2004 [DOI] [PubMed] [Google Scholar]
- 27.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflügers Arch 451: 193–203, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker JC, Gillespie MN, Taylor AE, Martin SL. Capillary filtration coefficient, vascular resistance and compliance in isolated mouse lungs. J Appl Physiol 87: 1421–1427, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Parker JC, Hernandez LA, Peevy K. Mechanisms of ventilator induced injury. Crit Care Med 21: 131–143, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Parker JC, Ivey C, Tucker A. Gadolinium prevents high airway pressure induced permeability increases in isolated rat lungs. J Appl Physiol 84: 1113–1118, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 286: L231–L246, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Parker JC, Townsley MI. Physiological determinants of the pulmonary filtration coefficient. Am J Physiol Lung Cell Mol Physiol 295: L235–L237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker JC, Yoshikawa S. Vascular segmental permeabilities at high peak inflation pressure in isolated rat lungs. Am J Physiol Lung Cell Mol Physiol 283: L1203–L1209, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol Lung Cell Mol Physiol 275: L1040–L1050, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Quinn DA, Moufarrej RK, Volokhov A, Hales CA. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J Appl Physiol 93: 517–525, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Schmal H, Czermak BJ, Lentsch AB, Bless NM, Beck-Schimmer B, Friedl HP, Ward PA. Soluble ICAM-1 activates lung macrophages and enhances lung injury. J Immunol 161: 3685–3693, 1998 [PubMed] [Google Scholar]
- 38.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis 141: 471–501, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Usatyuk PV, Fomin VP, Shi S, Garcia JG, Schaphorst K, Natarajan V. Role of Ca2+ in diperoxovanadate-induced cytoskeletal remodeling and endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol 285: L1006–L1017, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101: 396–401, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe H. Heat-evoked activation of TRPV4 channels in an HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Jian MY, Xu YC, Zhou C, Al Mehdi AB, Liedtke W, Shin HS, Townsley MI. Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 297: L650–L657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc 4: 77–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem 278: 11520–11527, 1928 [DOI] [PubMed] [Google Scholar]
- 46.Yoshikawa S, King JA, Lausch RN, Penton AM, Eyal FG, Parker JC. Acute ventilator-induced vascular permeability and cytokine responses in isolated and in situ mouse lungs. J Appl Physiol 97: 2190–2199, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Yoshikawa S, King JA, Reynolds SD, Stripp BR, Parker JC. Time and pressure dependence of transvascular Clara cell protein, albumin, and IgG transport during ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 286: L604–L612, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, Kron IL, Laubach VE. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 291: L1018–L1026, 2006. [DOI] [PubMed] [Google Scholar]