Abstract
Aim: Data concerning conversion and reversion rates in the serial testing of healthcare workers (HCWs) is rare. So far, there is no consensus on how to define and interpret interferon-gamma release assays (IGRA) conversions and reversions, or how to deal with such results. We analysed conversion and reversion rates in the serial testing of HCWs using an IGRA.
Methods: The study population comprises 287 HCWs, who participated in routine occupational safety and health screening for latent tuberculosis infection (LTBI) with the QuantiFERON-TB® Gold In-Tube assay (QFT). Four different definitions for conversion and reversion were applied: 1) transgression or regression above/below the cut-off; 2) increase from <0.2 to >0.7 IU/ml or decrease from >0.7 to <0.2 IU/ml; 3) transgression or regression above/below the cut-off plus change of ≥0.50 IU/ml; and 4) transgression or regression above/below the cut-off plus change of ≥0.70 IU/ml.
Results: The highest conversion and reversion rates of 6.1% (95% CI 3.5 to 9.9) and 32.6% (95% CI 19.1 to 48.5) respectively were observed with the least stringent definition of negative to positive. The most stringent definition of an increase of ≥0.7 IU/ml above the cut-point produced the lowest conversion rate of 2.5% (95% CI 0.9 to 5.3). Using an uncertainty zone from 0.2 to 0.7 IU/ml gave low conversion (2.6%) and reversion rates (15.4%).
Conclusion: Our data confirmed the findings of previous studies that suggest that a simplistic dichotomous negative to positive definition of the IGRA might be deceptive because of the high number of spontaneous conversions and reversions. Therefore using an uncertainty zone around the cut-point (e.g. 0.2 to 0.7 IU/ml) could improve the discrimination between unspecific variation around the diagnostic cut-off and true conversion or reversion.
Keywords: serial testing, interferon-gamma release assay, latent TB infection, healthcare workers
Abstract
Hintergrund: Die neuen Richtlinien zur Diagnose einer Latenten Tuberkulose Infektion (LTBI) bei Beschäftigten im Gesundheitswesen empfehlen den Interferon-gamma Release Assays (IGRA) als Diagnoseinstrument. Trotz wachsender Erfahrung mit dem Test liegen bislang nur wenige Daten zum Seriellen Testen vor. Dies gilt vor allem für Länder mit niedriger LTBI- Prävalenz. Bei wiederholten Tests ist bislang unklar, ab wann von wahren „Konversionen und Reversionen“ auszugehen ist, wie diese interpretiert werden sollen und wann eine Chemoprävention indiziert ist. Wir haben in unserer Studie Konversions- und Reversionsraten bei Beschäftigten im Gesundheitsdienst analysiert.
Methode: Insgesamt wurden 287 Beschäftigte im Gesundheitswesen, die routinemäßig arbeitsmedizinisch auf TB untersucht wurden, in die Studie eingeschlossen und zu zwei Zeitpunkten mit dem QuantiFERON-TB Gold In Tube (QFT) untersucht. Vier unterschiedliche Definitionen für Konversionen und Reversionen wurden analysiert. Zusätzlich wurde die Verwendung einer Grauzone um den Grenzwert (0,35 IU/ml) von 0,2–0,7 IU/ml untersucht.
Ergebnisse: Bei 15% der Beschäftigten waren beide Testergebnisse positiv. Die höchsten Konversion (6,1%) und Reversionsraten (32,6%) wurden mit der am wenigsten stringenten Definition von negativ zu positiv beobachtet. Die niedrigsten Raten (2,5%) fanden sich bei der strengsten Definition mit einem Anstieg von ≥0,7 IU/ml über dem Grenzwert von 0,35 IU/ml. Bei der Verwendung einer Grauzone von 0,2–0,7 IU/ml um den Grenzwert lagen die Konversionsrate bei 2,6% und die Reversionsrate bei 15,4%. Bei einem positiven QFT-Test wurde eine Röntgenuntersuchung zum Ausschluss einer aktiven Tuberkulose (TB) durchgeführt. Keiner der Probanden hat bislang eine aktive TB entwickelt.
Schlussfolgerung: Die Konversions- und Reversionsraten variieren in Abhängigkeit der jeweiligen Definition. Unsere Daten bestätigen die Annahme einer Grauzone zwischen 0,2 und 0,7 IU/ml bei wiederholten Routineuntersuchungen von Personen mit einem erhöhten LTBI- Risiko. Dies sollte in einer größeren Studienpopulation evaluiert werden. Zur Progressionsrate eines positiven QFT in Niedrig- Inzidenzländern liegen wenige Daten vor. Deshalb sollten Personen, deren Testergebnisse in diese Grauzone fallen vor Beginn einer Chemoprävention nochmals mit dem QFT untersucht werden.
Introduction
The increased risk of latent tuberculosis infection (LTBI) and active TB as an occupational disease in healthcare workers is well established [1]. Therefore, the screening of healthcare workers (HCW) for LTBI and preventive treatment are fundamental components of prevention programmes [2]. Serial testing either after recent exposure to a confirmed TB source case (contact investigation) or within periodical occupational safety and health (OSH) measures are required for surveillance and with regard to the recognition and compensation of TB as an occupational disease [3].
However, the conventional tuberculin skin test (TST) has known limitations in accuracy and reliability. Furthermore, interpretation of serial TST results is complicated by non-specific variation and because of its intradermal application, by potential boosting by precedent tests [4]. The interferon-gamma (INF-γ) release assays (IGRAs) provide a new tool for LTBI diagnosis and surveillance for new TB infections [5]. The IGRAs allow ex-vivo testing and therefore are not prone to boosting. Furthermore, IGRAs are highly specific, giving them advantages over the TST especially in Bacillus Calmette Guérin (BCG)-vaccinated populations [6], [7]. Although IGRAs have been recommended for serial testing in some countries [2], [8], there is still uncertainty regarding the interpretation of serial IGRA test results [5], [9]. A few studies suggest that conversions, reversions and non-specific variations occur with the IGRA in serial testing just as they do in TST serial testing [10], [11], [12], [13], [14]. So far there is no consensus on how to define and interpret IGRA conversions and reversions and deal with such results [5].
As with the TST, IGRA results are determined by several factors such as precision of measurement technique, intrapersonal biological variation, new infection (conversion), transient infection and transition of Mycobacterium tuberculosis (MTB) from replication to a dormant state no longer stimulating cell-mediated immune response (reversion). MTB cannot be directly observed in the body. Its presence and replication activity can therefore only be measured indirectly by antigen-specific response via a TST or an IGRA [12].
Data on IGRA interpretation in serial testing is scarce. Preliminary data showed that, similar to with a TST, a simplistic dichotomous negative to positive definition may not be appropriate. The few published studies are hampered by small sample sizes and allow limited conclusions [5], [10], [13], [14], [15], [16]. Consequently, different ‘uncertainty zones’ around the manufacturer's predefined cut-points as well as definitions of ‘true’ conversions and reversions have been suggested [5]. Based on Indian data, a person whose IFN-γ result increased from <0.20 and exceeded 0.50 IU/ml on the repeat test was considered to have a ‘true conversion’. Likewise, a person whose IFN-γ result decreased from >0.50 and fell to <0.20 IU/ml was considered to have a ‘true reversion’ [5]. Based on South African data, it was suggested that an increase in IFN-γ response from below 0.35 IU/ml to above 0.70 IU/ml for the QFT assay could be used to define conversions [16]. High spontaneous reversion rates were reported when the first QFT showed INF-γ concentration of between 0.35 and 0.7 IU/ml [15].
In our prospective cohort study we analysed conversion and reversion rates in serial testing of HCWs with QFT depending on baseline concentration of INF-γ as well as for different definitions of conversions and reversions. Assuming that a small variation in baseline INF-γ concentration does not result in high changes to conversion and reversion rates, we attempted to derive an uncertainty zone around the cut-off for the QFT to be used in serial testing.
Materials and methods
Study design and subjects
The population of this prospective cohort study comprises HCWs from 14 different hospitals in Germany, who participated in annual or biennial routine TB screening from January 2006 to October 2009, in accordance with the German guidelines for occupational safety and health (OSH) or TB contact investigations after exposure to culture- confirmed TB-source cases by occupational physicians. We did not apply a recent TST, because in accordance with the national guideline from 2007 the IGRA is recommended for the screening procedures among HCW. All participants were evaluated at baseline using a standardised interview and questionnaire and the QFT. All participants with a positive QFT at baseline or showing a conversion were offered a clinical and radiological examination to rule out active TB. The subsequent offer of preventive chemotherapy with Isoniazid for 9 months according to the current national recommendations was the responsibility of the respective occupational health practitioner and the practice-based pulmonary specialist [8]. No one of the study population with a positive QFT at baseline accepted chemotherapy. The follow-up included a second QFT and a second standardised questionnaire.
Following CDC and national guidelines, HCWs in infection and TB wards are considered to be at high risk, workers with regular patient contact in other wards are considered to be at medium risk and workers with no regular patient contact or no contact to biological material are considered to be at low risk [2], [3]. Upon commencement of employment, all workers are examined. HCWs considered high-risk are evaluated annually. All others are evaluated every other year or after known exposure to patients with active TB.
Questionnaire items
Information on the following variables was collected using a standardised questionnaire: age, gender, reason for testing, occupational exposure to TB, time spent working in healthcare sector, own and family history of TB, place of birth, prior TST results, job title, workplace, chest radiograph findings and BCG vaccination. BCG vaccination history was assessed through the individual’s vaccination record or scars.
Diagnostic methods
For the IGRA, the QuantiFERON-TB® Gold In-Tube assay (Cellestis Limited, Carnegie, Australia) was administrated following the manufacturer’s protocol. The test was considered positive if INF-γ was ≥0.35 IU/ml after correction for the negative control. Concentrations of above 10 IU/ml were set at 10 IU/ml because of imprecision of measurement at these high concentrations [5]. The mean period between the two tests was 12 months (standard deviation 4 months).
Definitions of conversions and reversions
One of our objectives was to assess whether variations in definitions produce different rates of conversion and reversion. Therefore, four different definitions for conversion and reversion were applied: 1) transgression or regression based on the cut-off from 0.35 IU/ml; 2) increase from <0.2 to >0.7 IU/ml or decrease from >0.7 to <0.2 IU/ml; 3) transgression or regression above cut-off (0.35 IU/ml) plus change of ≥0.50 IU/ml; and 4) transgression or regression above cut-off (0.35 IU/ml) plus change ≥0.70 IU/ml.
Uncertainty zone analyses
Previous studies had suggested the use of a grey zone for QFT results. Harada et al. used a grey zone of 0.10 to 0.35 IU/ml and excluded results in this zone from conversion rate calculations. Pai et al. [4] used an approach of drawing a zone uncertainty of 0.20 to 0.50 IU/ml. We explored an alternative grey zone from 0.20 to 0.70 IU/ml and excluded all those who had at least one QFT within this grey zone from calculation.
Statistical analysis
Baseline INF-γ concentration was categorised in small increments in order to observe at which increment the highest change in conversion and reversion rates occurs. A 95% confidence interval (CI) for proportions was calculated. If the 95% CI did not overlap, differences between proportions were considered to be statistically significant. Chi-square tests were used for categorical data.
The study protocol was approved by the ethics committee of the Hamburg Medical Council. All participants gave their written informed consent prior to their inclusion in the study.
Results
Study population and baseline results
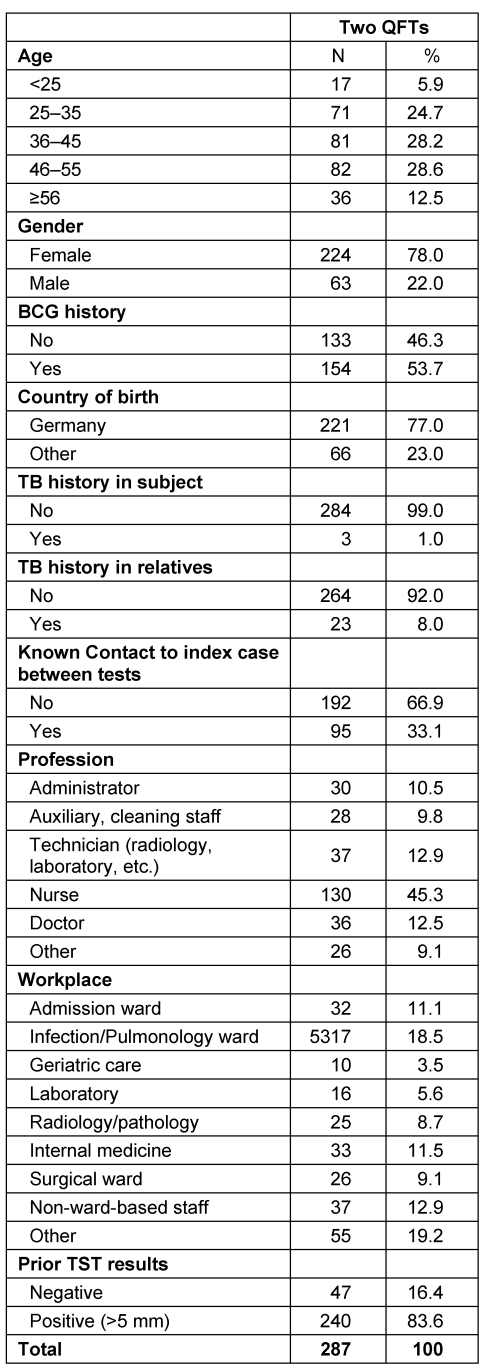
Two hundred and eighty-seven HCWs with two consecutive QFT tests were enrolled in this study. No indeterminate results were observed. The baseline characteristics of the study cohort are shown in Table 1 (Tab. 1). 78% of the cohort was female and the median age was 40 years. Nurses and doctors made up 57.8% of the study population. 79.8% (n=229) of the cohort showed persistently negative QFT results over a mean period of 12 months (standard deviation 4 months) and 10.1% (n=29) was persistently positive in both tests (no table).
Table 1. Description of the study population.
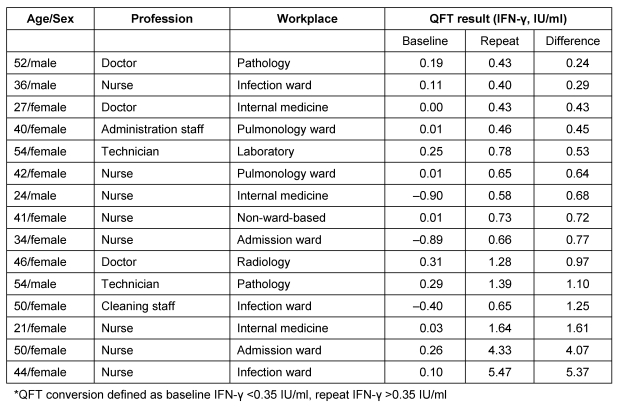
53.3% of the conversion occurred in nurses and 20% in doctors (Table 2 (Tab. 2)). 40% of participants with a conversion worked on wards with a high risk, e.g. infection/admission ward or pulmonology ward, while 26.6% worked in a laboratory, in pathology or on a radiology department.
Table 2. Changes in the IFN-γ values in 15 HCWs who had QFT conversions*.
Incidence of QFT conversions and reversions
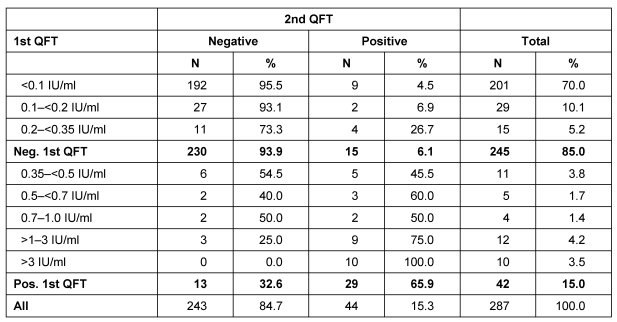
The first and second QFT was positive in 15.0% of the HCWs (Table 3 (Tab. 3)). If a simple dichotomous approach (negative to positive and vice versa) was chosen, a conversion occurred in 6.1% of those negative in the first QFT and a reversion occurred in 32.6% of those positive in the first QFT. Reversion and conversion rates depended on the INF-γ concentration of the first QFT. Conversion occurred in 4.5% of the 201 HCWs with an INF-γ concentration at baseline below 0.1 IU/ml but in 26.7% of the 15 HCWs with an INF-γ concentration of 0.2 to <0.35 IU/ml. In the 12 HCWs with a baseline INF-γ concentration of >1.0 to 3.0 IU/ml tree reversions (25.0%) occurred, while in the 16 (11+5) HCWs with a baseline INF-γ concentration of ≥0.35 to <0.7 IU/ml approximately every second HCW showed a reversion.
Table 3. Results of second QFT depending on INF-γ concentration in first QFT.
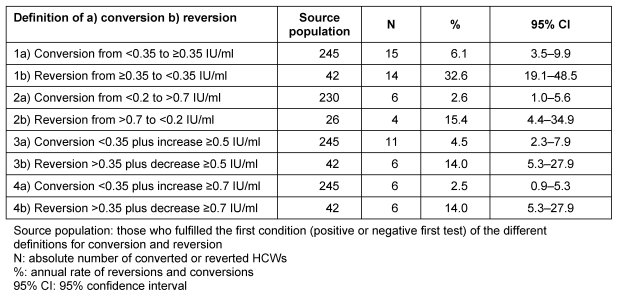
The highest conversion and reversion rates of 6.1% (95% CI 3.5 to 9.9) and 32.6% (95% CI 19.1 to 48.5) were observed with the least stringent definition of negative to positive. The most stringent definition of an increase from ≥0.7 IU/ml above the cut-point produced the lowest conversion rate of 2.5% (95% CI 0.9 to 5.3). Conversion and reversion rates showed differences depending on the definition used (Table 4 (Tab. 4)). The 95% CI of these rates overlapped, indicating no statistically significant difference. There was, however, an almost two-fold difference between the most stringent and least stringent definitions for QFT conversion.
Table 4. Number and rate of conversions and reversions depending on different definitions for ‘true’ conversions and ‘true’ reversions.
For all definitions conversion rates were lower than reversion rates but the difference was statistically significant only for the least stringent definition (1a+b, Table 4 (Tab. 4)).
Uncertainty zone analysis results
Using a grey zone from 0.2 to 0.7 IU/ml and excluding all those who have at least one QFT within this grey zone from calculation gave low conversion (2.6%) and reversion rates (15.4%). Slightly higher rates were obtained when, in addition to a positive to negative approach, a minimal chance of 0.5 IU/ml was requested for conversion (4.5%) and reversion (14.0%).
Clinical outcome at baseline and follow-up
Active TB was ruled out by chest X-rays in all participants with a positive baseline QFT and those with a conversion. None of the 287 participants have developed active TB within the study period from January 2006 to October 2009 so far.
Discussion
The cell-based IGRAs have features that make them ideal for serial testing. However, data on IGRA interpretation in serial testing is rare, especially for low-incidence countries. Our study therefore provides useful data on QFT performance among HCWs in a low-incidence country. The data confirms the findings of previous studies, which suggest that a simplistic dichotomous negative to positive definition of the IGRA may be misleading due to the high number of spontaneous conversions and reversions. Consequently, conversions and reversions were frequent when IFN-γ values were close to the cut-point. Therefore, using an uncertainty zone around the cut-point could be helpful in distinguishing between unimportant variation and true conversion or reversion.
QFT conversions and their interpretation
The observed conversion rate in our study was the highest (6.1%) when the least stringent definition, i.e. transition from negative to positive values, was used, compared to the conversion rate from 2.5% when the most stringent definition was used. Our observed conversion rates from 2.5% up to 6.1% depending on the definition used was higher than the conversion rate of 1.8% as shown in the Japanese HCW study from Yoshiyama and colleagues [15]. In another study from an intermediate-incidence country, where only IGRA-negative subjects were retested, the observed conversion rate was 4.9% [17]. In contrast to our findings, a conversion rate from 11.6% and 24% was reported for Indian HCWs [13].
The prognosis of IGRA conversions and the potential use of IGRAs as a predictive test are important issues. Studies on TB prediction by QFT show promising results in low-incidence countries. So far no study is available that describes the association between changes in IGRA and disease prediction [18]. Pai [19] stated that serial IGRA testing can reveal interesting underlying phenotypes that have different histories and trajectories. Some subjects convert from negative to positive and then revert again. Some converted and remained converted, while others stayed positive for a long time. However, no data exists to link these trajectories of test results with disease progression. It is inconceivable that all subjects will have the same prognosis. Therefore, Pai suggested that those who are persistently negative should have the best prognosis compared to those who recently converted and stayed positive. Nevertheless, without serial testing the underlying phenotypes are not separable and this will undermine the predictive value of a single test result [19].
Until now only a few studies have demonstrated the prognosis of a positive QFT. Diel et al. [18] determined the predictive value of the IGRA for development of active tuberculosis after recent infection. They found a progression rate of 14.6% within the QFT positive subjects who had declined chemoprevention. All of these subjects had highly positive QFT results of >10 IU/ml. These findings led to the hypothesis that individuals with a strongly elevated IFN-γ concentration are most likely to progress to active TB. Consequently, a second cut-off, higher than the diagnostic cut-off, could be used to identify the subgroup that would benefit from preventive chemotherapy [19].
IGRA reversions and their interpretation
In line with previous studies among HCWs, we found more reversions (32%) than conversions (6.1%) with the least stringent definition and 2.5% (conversions) and 14.0% (reversions) with the stringent definition of an increase from ≥0.7 IU/ml above the cut-point. In contrast to our findings, in the recent study among Japanese HCWs the conversion rate was much lower (1.8%), while the rate of reversions was higher (41%) [15]. In our data spontaneous reversions were rare (n=3) when the baseline IFN-concentration was >1 IU/ml. No reversion was observed when the baseline IFN-concentration was >3 IU/ml
In order to interpret and use the IGRAs in serial testing, we need evidence on several questions about the reproducibility of T-cell response over time and what threshold should be used to define true reversion [9], [19]. Serial testing studies demonstrated that IGRAs are highly dynamic tests and T-cell responses, especially weak responses tend to fluctuate over time [9]. In the Gambian Study among household contacts a high rate of reversion was revealed within a time span of just three months [10].
One important question is why IGRA reversions occur. As reviewed recently, some reversions may reflect the clearing of the TB infection, some may merely be due to biological variations among the positive IGRAs, and some may be due to variability in laboratory and test procedures [9]. Recently, Hill et al. suggested that the IGRA responses are inherently transient and may require continued exposure to a TB antigen to maintain high frequencies [10]. In line with Nardell [20], they speculated that reversions may simply reflect the life cycle of Mycobacterium tuberculosis, where the Mycobacterium enters a dormant state in which it may not reliably secrete antigens such as ESAT-6 and CFP-10. It is unclear whether an IGRA reversion indicates clearance or resolution of the TB infection. Further research is needed to elucidate the prognosis of IGRA reversions.
Uncertainty zone analysis results
Only a limited number of recent studies focused on the reproducibility of T-cell response over time (within-subject variability) and uncertainty zones analyses in order to improve the interpretation of the IGRA results in serial testing [5], [16], [21], [22], [23]. Using the borderline zone of 0.2 to 0.70 UI/ml in our study and excluding all those who have at least one QFT within this grey zone from calculation gave low conversion (2.6%) and reversion rates (15.4%). However, when using an uncertainty zone of 0.2 to 0.7 IU/ml it should be kept in mind that a QFT result around the cut-off (0.35 IU/ml) does not exclude active TB. Therefore, using a borderline zone in serial testing should always be carefully interpreted with consideration of relevant clinical symptoms [16]. This is because an uncertainty zone means that spontaneous, clinically irrelevant transgressions above the cut-off are probably predominant, but LTBI or even active TB cannot be excluded.
Despite our increasing knowledge, several key questions about latent infection and reactivation of M. tuberculosis remain unanswered. Particularly, it should be noted that both the TST and the IGRA are designed to identify an adaptive immune response against M. tuberculosis, but not necessarily a latent infection. A positive result of currently available diagnostic tests is primarily a measure of an immunological response to stimulation by mycobacterial antigens that should not, therefore, be equated with the presence of live M. tuberculosis in the human host. The proportion of individuals who truly remain infected with M. tuberculosis after IGRA conversion is unknown. It is also uncertain how long adaptive immune responses towards mycobacterial antigens persist in the absence of live mycobacteria. For these reasons, according to the recently published TBNET consensus statement regarding latent TB, based on the informative data currently being produced by IGRA and TST, the term “latent infection” at best implicates “lasting tuberculosis immune responses” and does not necessarily identify a true latent infection with viable microorganisms and the potential risk of developing active disease [24].
Conclusion
The rate of conversions seemed to vary depending on the definitions used. In conclusion, our data suggests the use of an uncertainty zone between 0.2 and 0.7 IU/ml in serial testing with the QFT. Therefore, using a borderline zone in serial testing in HCW should always be carefully interpreted with consideration of relevant clinical symptoms. Using a borderline zone is only recommended for serial testing among adult, healthy and immunocompetent subjects in low incidence countries like HCW. The use of an uncertainty zone is not valid and not recommended for example in pre-treatment screening before anti-TNF-alpha therapy, because of the higher risk for progression from LTBI to active TB in immunocompromised persons. Further research is needed to confirm this grey zone in larger HCW studies. To the best of our knowledge, disease progression in QFT-positive persons is limited in low-incidence countries with little experience in chemoprevention. Persons belonging to this zone should be retested immediately before being offered preventive chemotherapy. Studies on the disease prediction of variations in QFT will answer some of these questions.
References
- 1.Seidler A, Nienhaus A, Diel R. Review of epidemiological studies on the occupational risk of tuberculosis in low-incidence areas. Respiration. 2005;72(4):431–446. doi: 10.1159/000086261. Available from: http://dx.doi.org/10.1159/000086261. [DOI] [PubMed] [Google Scholar]
- 2.Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005;54(RR-15):49–55. [PubMed] [Google Scholar]
- 3.Deutsche Gesetzliche Unfallversicherung. Guidelines for Occupational Medical Examination - Prophylaxis in Occupational Medicine. 4 ed. Stuttgart: Gentner Verlag; 2007. [Google Scholar]
- 4.Pai M, Dheda K, Cunningham J, Scano F, O'Brien R. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis. 2007;7(6):428–438. doi: 10.1016/S1473-3099(07)70086-5. Available from: http://dx.doi.org/10.1016/S1473-3099(07)70086-5. [DOI] [PubMed] [Google Scholar]
- 5.Pai M, Joshi R, Dogra S, Zwerling AA, Gajalakshmi D, Goswami K, Reddy MV, Kalantri A, Hill PC, Menzies D, Hopewell PC. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int J Tuberc Lung Dis. 2009;13(1):84–92. [PMC free article] [PubMed] [Google Scholar]
- 6.Diel R, Ernst M, Döscher G, Visuri-Karbe L, Greinert U, Niemann S, Nienhaus A, Lange C. Avoiding the effect of BCG vaccination in detecting Mycobacterium tuberculosis infection with a blood test. Eur Respir J. 2006;28(1):16–23. doi: 10.1183/09031936.06.00107005. Available from: http://dx.doi.org/10.1183/09031936.06.00107005. [DOI] [PubMed] [Google Scholar]
- 7.Nienhaus A, Schablon A, Diel R. Interferon-Gamma Release Assay for the Diagnosis of Latent TB Infection - Analysis of Discordant Results, when Compared to the Tuberculin Skin Test. PLoS ONE. 2008;3(7):e2665. doi: 10.1371/journal.pone.0002665. Available from: http://dx.doi.org/10.1371/journal.pone.0002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diel R, Forssbohm M, Loytved G, Haas W, Hauer B, Maffei D, Magdorf K, Nienhaus A, Rieder HL, Schaberg T, Zellweger JP, Loddenkemper R German Central Committee against Tuberculosis. Empfehlungen fur die Umgebungsuntersuchungen bei Tuberkulose. Deutsches Zentralkomitee zur Bekampfung der Tuberkulose. [Recommendations for environmental contact tracing in tuberculosis. German Central Committee against Tuberculosis]. Gesundheitswesen. 2007;69(8-9):488–503. doi: 10.1055/s-2007-980089. (Ger). Available from: http://dx.doi.org/10.1055/s-2007-980089. [DOI] [PubMed] [Google Scholar]
- 9.Pai M, O'Brien R. Serial testing for tuberculosis: can we make sense of T cell assay conversions and reversions? PLoS Med. 2007;4(6):e208. doi: 10.1371/journal.pmed.0040208. Available from: http://dx.doi.org/10.1371/journal.pmed.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill PC, Brookes RH, Fox A, Jackson-Sillah D, Jeffries DJ, Lugos MD, Donkor SA, Adetifa IM, de Jong BC, Aiken AM, Adegbola RA, McAdam KP. Longitudinal assessment of an ELISPOT test for Mycobacterium tuberculosis infection. PLoS Med. 2007;4(6):e192. doi: 10.1371/journal.pmed.0040192. Available from: http://dx.doi.org/10.1371/journal.pmed.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill PC, Jeffries DJ, Brookes RH, Fox A, Jackson-Sillah D, Lugos MD, Donkor SA, de Jong BC, Corrah T, Adegbola RA, McAdam KP. Using ELISPOT to expose false positive skin test conversion in tuberculosis contacts. PLoS One. 2007;2(1):e183. doi: 10.1371/journal.pone.0000183. Available from: http://dx.doi.org/10.1371/journal.pone.0000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2006;174(7):831–839. doi: 10.1164/rccm.200511-1783OC. Available from: http://dx.doi.org/10.1164/rccm.200511-1783OC. [DOI] [PubMed] [Google Scholar]
- 13.Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Kalantri S, Reingold AL, Colford JM, Jr, Riley LW, Menzies D. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am J Respir Crit Care Med. 2006;174(3):349–355. doi: 10.1164/rccm.200604-472OC. Available from: http://dx.doi.org/10.1164/rccm.200604-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franken WP, Koster BF, Bossink AW, Thijsen SF, Bouwman JJ, van Dissel JT, Arend SM. Follow-up study of tuberculosis-exposed supermarket customers with negative tuberculin skin test results in association with positive gamma interferon release assay results. Clin Vaccine Immunol. 2007;14(9):1239–1241. doi: 10.1128/CVI.00185-07. Available from: http://dx.doi.org/10.1128/CVI.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshiyama T, Harada N, Higuchi K, Nakajima Y, Ogata H. Estimation of incidence of tuberculosis infection in health-care workers using repeated interferon-gamma assays. Epidemiol Infect. 2009;137(12):1691–1698. doi: 10.1017/S0950268809002751. Available from: http://dx.doi.org/10.1017/S0950268809002751. [DOI] [PubMed] [Google Scholar]
- 16.van Zyl-Smit RN, Pai M, Peprah K, Meldau R, Kieck J, Juritz J, Badri M, Zumla A, Sechi LA, Bateman ED, Dheda K. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am J Respir Crit Care Med. 2009;180(1):49–58. doi: 10.1164/rccm.200811-1704OC. Available from: http://dx.doi.org/10.1164/rccm.200811-1704OC. [DOI] [PubMed] [Google Scholar]
- 17.Chee CB, Lim LK, Barkham TM, Koh DR, Lam SO, Shen L, Wang YT. Use of a T cell interferon-gamma release assay to evaluate tuberculosis risk in newly qualified physicians in Singapore healthcare institutions. Infect Control Hosp Epidemiol. 2009;30(9):870–875. doi: 10.1086/599284. Available from: http://dx.doi.org/10.1086/599284. [DOI] [PubMed] [Google Scholar]
- 18.Diel R, Loddenkemper R, Meywald-Walter K, Niemann S, Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2008;177:1164–1170. doi: 10.1164/rccm.200711-1613OC. Available from: http://dx.doi.org/10.1164/rccm.200711-1613OC. [DOI] [PubMed] [Google Scholar]
- 19.Pai M. Spectrum of latent tuberculosis - existing tests cannot resolve the underlying phenotypes. Nat Rev Microbiol. 2010;8(3):242. doi: 10.1038/nrmicro2236-c1. Available from: http://dx.doi.org/10.1038/nrmicro2236-c1. [DOI] [PubMed] [Google Scholar]
- 20.Nardell EA, Wallis RS. Here today--gone tomorrow: the case for transient acute tuberculosis infection. Am J Respir Crit Care Med. 2006;174(7):734–735. doi: 10.1164/rccm.200607-923ED. Available from: http://dx.doi.org/10.1164/rccm.200607-923ED. [DOI] [PubMed] [Google Scholar]
- 21.Perry S, Sanchez L, Yang S, Agarwal Z, Hurst P, Parsonnet J. Reproducibility of QuantiFERON-TB gold in-tube assay. Clin Vaccine Immunol. 2008;15(3):425–432. doi: 10.1128/CVI.00398-07. Available from: http://dx.doi.org/10.1128/CVI.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veerapathran A, Joshi R, Goswami K, Dogra S, Moodie EE, Reddy MV, Kalantri S, Schwartzman K, Behr MA, Menzies D, Pai M. T-cell assays for tuberculosis infection: deriving cut-offs for conversions using reproducibility data. PloS One. 2008;3(3):e1850. doi: 10.1371/journal.pone.0001850. Available from: http://dx.doi.org/10.1371/journal.pone.0001850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Detjen AK, Loebenberg L, Grewal HM, Stanley K, Gutschmidt A, Kruger C, Du Plessis N, Kidd M, Beyers N, Walzl G, Hesseling AC. Short-term reproducibility of a commercial interferon gamma release assay. Clin Vaccine Immunol. 2009;16(8):1170–1175. doi: 10.1128/CVI.00168-09. Available from: http://dx.doi.org/10.1128/CVI.00168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, Bossink A, Magdorf K, Hölscher C, Kampmann B, Arend SM, Detjen A, Bothamley G, Zellweger JP, Milburn H, Diel R, Ravn P, Cobelens F, Cardona PJ, Kan B, Solovic I, Duarte R, Cirillo DM, C Lange TBNET. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33(5):956–973. doi: 10.1183/09031936.00120908. Available from: http://dx.doi.org/10.1183/09031936.00120908. [DOI] [PubMed] [Google Scholar]