Abstract
Many developments have been made in the field of resin composites for dental applications. However, the manifestation of shrinkage due to the polymerization process continues to be a major problem. The material's shrinkage, associated with dynamic development of elastic modulus, creates stresses within the material and its interface with the tooth structure. As a consequence, marginal failure and subsequent secondary caries, marginal staining, restoration displacement, tooth fracture, and/or post-operative sensitivity are clinical drawbacks of resin-composite applications. The aim of the current paper is to present an overview about the shrinkage stresses created during resin-composite applications, consequences, and advances. The paper is based on results of many researches that are available in the literature.
1. Introduction
Since their development in the late 1950s [1, 2], resin composites represent a class of materials widely used in restorative dentistry. Besides acceptable aesthetics properties, resin composites can be directly bonded to tooth structure without removing healthy tissues. Because of its bond ability, by the application of a previous adhesive treatment, the material has found increasing application in modern preventive and conservative dentistry. As a result, resin composites can be used for different purposes such as: anterior and posterior teeth injured or diseased by the caries process, occlusion adjustments, cementation of indirect restorations, bonding orthodontic brackets, and aesthetic teeth transformations.
Traditionally, the adhesive system application consists of the following sequence.
An acid treatment with phosphoric acid that promotes demineralization of inorganic components from the dental structures, which might be enamel, dentin, cementum, or a combination among them, depending on the clinical situation.
The remaining structure is conditioned by a primer solution, usually formulated by hydrophilic monomers and solvents. This step is important to remove water and to infiltrate the spaces, created by the previous demineralization, with resin. Thus, a fluid resin (known as bond) mainly formulated with hydrophobic monomers is applied; the resin is photoactivated, creating an interlocking layer between the polymerized material and the remaining tooth structure. It is important to mention that this two-step procedure might be used as a one-step procedure when simplified adhesives are used.
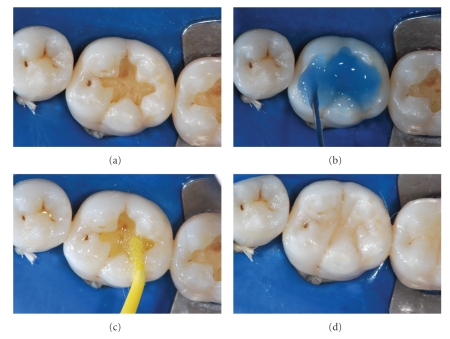
The resin is then photoactivated, creating an interlocking layer between the polymerized material and the remaining tooth structure. After this sequence, the tooth is prepared to receive the resin-composite restoration. Figure 1 shows a clinical sequence of adhesive application and the final restoration.
Figure 1.
(a) After caries removal, the cavity is prepared to receive the resin-composite restoration. (b) Acid etching treatment with phosphoric acid 37%. (c) Application of a simplified (1 step) adhesive system. (d) After the photoactivation procedure, the resin-composite restoration was built. Adhesive system used: Single-Bond (3MESPE). Resin composite: XRV Ultra (Kerr).
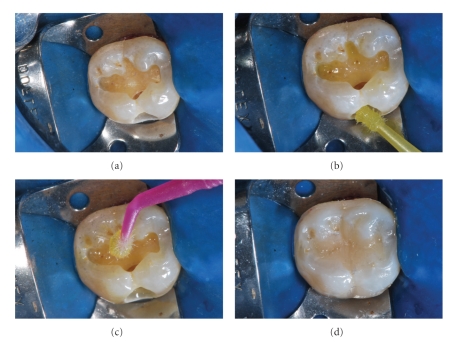
The aforementioned sequence refers to the use of the traditional total-etching technique. On the other hand, there are some dental adhesive systems that do not need the use of previous acid treatment. This class of material is known as the self-etching adhesives, which can be applied in a single or two steps. A clinical case is demonstrated in Figure 2.
Figure 2.
(a) Cavity prepared to receive the resin-composite restoration. (b) Self-etching primer application. (c) Bonding agent application. (d) After the photoactivation procedure, the resin-composite restoration was built. Adhesive system used: Self-etching Silorane (3MESPE). Resin composite: Filtek Silorane (3MESPE).
During the curing process resin composites undergo dimensional shrinkage, inherent manifestation in materials polymerizing through a free-radical mechanism [3]. When a material is cured without bonding to cavity walls, the material is able to shrink and to flow, developing low values of stress. However, in clinical situations when the material has to be placed inside a cavity and should be bonded to the surrounding walls, that is, material deformation is restricted—thereby developing stresses which are also transferred to the bonding region as tensile forces. Consequently, the main outcome is the development of internal contraction stress which can damage the marginal seal of the bonded restorations. These may result in interfacial gap formation and produce postoperative sensitivity, marginal staining, or recurrent caries. In addition, another undesired situation is the cusp displacement, which may result in patient hyper-sensitivity or fracture and crack formation at surrounding walls [4–7]. The correlation between shrinkage of resin composites and gap formation was recently highlighted further with the aid of microtomography [8].
In an attempt to reduce the polymerization shrinkage, researchers have mainly focused in changing either the material's formulation or the mechanism of initiating polymerization. Concerning the formulation, modifications have mainly pertained to filler technology, and recently in the organic matrix through the introduction of alternative matrix such as siloranes and ormocers. Regarding the mechanism of initiating polymerization, studies have shown that a relation exists between polymerization shrinkage stress and light irradiance [3, 9]. Because of this relationship, different light curing protocols have been used aiming to minimize or to control the polymerization shrinkage stress of resin composites.
For a better understanding of the principles related to the shrinkage stress it is necessary to know the basic formulation of resin composite as well the dynamic of polymerization. Thus, the present paper will be focused on the resin-composite basic formulation, polymerization process, measurement of shrinkage stress, and methods to reduce it.
2. Dental Resin Composites: The Basic Formulation and the Polymerization Process
2.1. The Basic Formulation
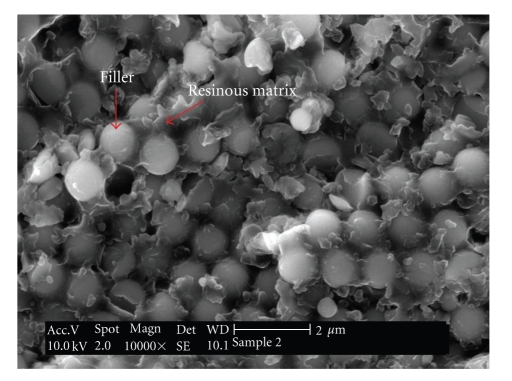
To comprehend the development of shrinkage stresses, it is necessary to understand the basic formulation of resin composites and the polymerization process phenomena. In general terms, resin composites are a combination of inorganic particles surrounded by a coupling agent, dispersed in an organic resinous matrix (Figure 3).
Figure 3.
Scanning electron microscopy (SEM) image of an experimental dental resin composite. It can be easily observed the presence of spherical-shape fillers surrounded by the resin matrix.
2.1.1. The Inorganic Fillers
Particulate inorganic fillers are used in dental resin composites to provide material strengthening and reinforcement [10]. Several types, shapes, sizes, volume fractions, and distributions of filler particles are used in commercial products and all these factors affect the material's properties, such as hardness [11, 12]; thermal stability [13]; radio-opacity [14]; gloss retention and roughness [15]; water sorption; visco-elastic creep and recovery [16]; fracture toughness [17]; fracture behaviour [18, 19]; elastic moduli [20]. The total volume of fillers used in the resin-composite formulations may vary a lot in volume according to the clinical applications, companies' fabrication processes, and the resin matrix viscosity. The composition of fillers may also vary among the different brands in the market and can be quartz, silica, zirconium, strontium, barium, and others. According to Van Noort [21], it is possible to classify dental resin composites according to the following.
Traditional Resin Composites. They are usually formulated with quartz. This class of material shows a mean particle size of 10–20 μm but can present particles up to 40 μm size. This kind of filler was used in the first materials that appeared in the market, but its use decayed due to the low wear resistance and poor aesthetic properties.
Microfilled Resin Composites. They were launched in the market to overcome the problems of poor aesthetic properties. These materials are usually formulated with colloidal silica (around 50% in volume) with an average particle size of 0.02 μm and a range of 0.01–0.05 μm. Unfortunately, the mechanical properties are considered low for application in regions of high occlusal forces.
Hybrid Resin Composites. This kind of material offers intermediate aesthetic properties but excellent mechanical properties by the incorporation of fillers with different average particle sizes (15–20 μm and 0.01–0.05 μm).
Small-Particle Hybrid Dental Composites. They are usually formulated with particles with an average size of less than 1 μm, and a range of 0.1–0.6 μm. These filler distribution ensures that a polished surface can be obtained.
More recently, materials formulated with nanoparticles were introduced in the market. According to the manufacturers, this type of materials is able to ensure good polishing and long-term gloss [22].
2.1.2. The Resinous Matrix
The resin matrix usually consists of organic monomers, photoinitiators, coinitiators, inhibitors of polymerization, UV-stabilizers, and small amounts of additional components that vary according the manufacturer. The organic monomers are added in the fluid state and are converted into rigid polymers through a polymerization process, during the material's clinical application. The polymerization process will be discussed in the next section. At this point, we will discuss the characteristics of the different monomers used by the manufacturers, since they are directly related with the final polymer properties [23].
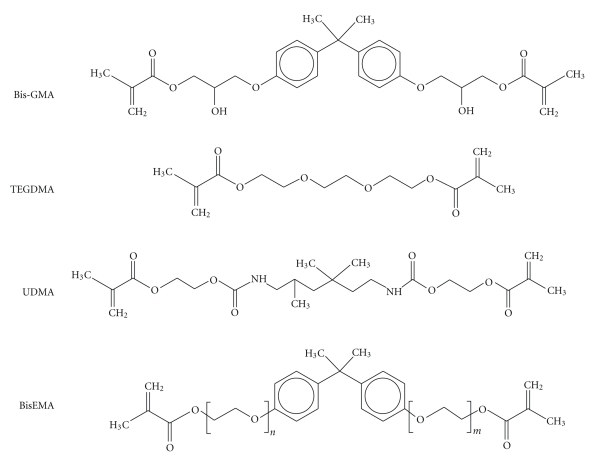
The traditional monomers used in dental composites are shown in Figure 4. Since the introduction of resin composites in the market, the Bis-GMA (2,2 bis[4-2(2-hydroxy-3-methacryloyloxypropoxy)-phenyl] propane) has been widely used in the formulations of dental resin composites [24]. This molecule has a stiff bisphenol A core, that negatively affects the degree of conversion [25], and two pendant hydroxyl groups that are able to form strong hydrogen bonds [26] and, as a consequence, makes the resin viscosity very high—500 000–800 000 mPa.s [27]. Due to the very high molecular weight (512 g/mol), the BisGMA provides lower polymerization shrinkage than other monomers and superior mechanical qualities [28]. Consequently, due to its very high viscosity, the amount of fillers added to the mixture and the handling properties might be affected. Therefore, diluent monomers have to be used, or other ones have to substitute the BisGMA, to make the resin more fluid [28].
Figure 4.
Resin monomers often used in the formulations of dental resin composites.
The triethyleneglycol dimethacrylate (TEGDMA) presents a much lower viscosity (100 mPa.s) [27] than BisGMA and, thus, is frequently used as an efficient diluent monomer in dental resin composites. The high flexibility of TEGDMA is a consequence of its low molecular weight structure (286 g/mol) and also compensates the rigidity of BisGMA and, therefore, the addition of TEGDMA results in resins with higher conversion rate [29]. However, as negative effects, the addition of TEGDMA to the resin formulation is responsible for an increase of the water sorption by the material [30] and shrinkage.
Although BisGMA and TEGDMA are the most traditional monomers used in the formulation of dental resin composites, some others may also be used. The urethane dimethacrylate (UDMA) is a molecule that can be used alone with TEGDMA, or associated with BisGMA and/or some other monomers. Although the molecular weight of this molecule (470 g/mol) [27] is not so far from that of BisGMA, the viscosity is considered quite lower (5000–10 000 mPa.s). It is reported that partial substitution of Bis-GMA by UDMA leads to increased conversion and flexural strength [30, 31]. The explanation for this behavior relies on the greater flexibility and weaker intermolecular bonds promoted by UDMA than BisGMA [30]. Another monomer that is frequently added into resin-composite mixtures is the ethoxylated bis-phenol A methacrylate (BisEMA). Different from BisGMA, the BisEMA does not present the pendant hydroxyl groups that form the hydrogen bonds among molecules and increases viscosity. Therefore, the BisEMA is less viscous than the BisGMA.
The monomers presented until here are considered the most traditional ones used in commercial dental resin composites. The choice of such molecules as well as the ratio and concentration is extremely important to guide the material's application and the final properties [23, 26, 30, 32]. It is important to mention that there are a wide range of other monomers that have also been used and tested.
2.1.3. The Coupling Agent
Since the resin matrix and the inorganic filler do not have chemical affinity, a coupling agent has to be used to bond the inorganic phase (filler particles) with the organic phase (resinous matrix) [33]. The most common agent is γ-methacryloxypropyl-triethoxysilane (γ-MPTS); its use enhances the wettability of the inorganic particles with the organic phase to produce a composite mix. The polymerization process allows the methacrylate groups in the coupling agent to copolymerize with the resin monomers, thus enhancing the interfacial adhesion between the inorganic particles and the organic matrix which may lead to improved properties. This is due to that the enhanced interfacial adhesion between particles and resin decreases debonding of the inorganic particles, thus influencing wear and water uptake ear resistance [21].
2.2. The Polymerization Process
The polymerization reaction is very complex and fast. However, to make the reader understanding easier, the reaction will be discussed as steps.
Resin composites for direct restorative procedures typically employ the camphoroquinone (CQ)/amine as the photoinitiator/coinitiator system [34]. Basically, blue light (400–550 nm) activates CQ and converts it to an excited triplet state. The excited CQ then reacts with a coinitiator to form free radicals, which are molecules with unpaired electrons, starting the polymerization process (activation and initiation stages) [35]. When this reactive radical reacts with a monomer molecule, an active centre is created and propagates the polymerization process.
A second step of the polymerization process is the propagation reaction, which involves the polymer chain growth by rapid sequential addition of monomer to the active centers via covalent bonds until the maximum degree of conversion of C=C double-bonds into C–C bonds is achieved. Before the polymerization process, van der Walls forces act and keep the monomers grouped. At this moment, the distance among the monomers is approximately 4 Å. During the polymerization process, these forces are substituted by covalent bonds, with distances of approximately 1.5 Å. Consequently, volumetric shrinkage occurs [36, 37]. Typical resin composites applied in restorative dentistry exhibit volumetric shrinkage values from less than 1% up to 6%, depending of the formulation and curing conditions [38, 39].
3. Shrinkage-Stress Development
The polymerization shrinkage stress is a very complex phenomenon, since it is dependent on multiple factors. The boundary conditions, the amount of material, the polymerization reaction, the material's formulation, and the resultant properties all play essential roles in stress development and/or transmission to tooth structures [40–46].
3.1. Boundary Conditions and the Amount of Material
As mentioned before, the resin composite undergoes volumetric shrinkage during the polymerization process. At the same time, there is a dynamic increase of the elastic modulus, meaning that the capability of plastic deformation is reduced; that is, the material becomes stiffer. If the material is able to shrink, and enough time is given for material's plastic deformation, relaxation might occur and the final stress magnitude might be low. Unfortunately, regardless of the resin-composite application in Dentistry—cavity restoration, cementation of endodontic posts, cementation of indirect restorations and orthodontic brackets, fixation of dental fragments, and so forth—the material has to be bonded to the tooth structure, reducing the material's chance for plastic deformation. Consequently, within a dental cavity or in situations where the material is constricted within two surfaces (such as cementation procedures), the material's ability of deformation and subsequent stress relaxation is low and the stress level is expected to be high [41].
In dental filling procedures it is important to consider that the cavity configuration varies according to the extent of caries removal, the amount of remaining healthy tissue, the tooth-region and the tooth location (anterior, posterior) and type. Consequently, the level of stress might vary according to the clinical situation. In 1987, Feilzer et al. [41] published a study showing that the expected magnitude of stress might be estimated through the ratio of the bonded to the unbonded areas, also known as the “configuration factor,” or simply “C-factor.” According to these authors, the higher the C-factor (higher amounts of bonded areas), the higher the stress level. On the opposite, a higher ratio of unbonded to bonded walls would be responsible for lower values of stress because shrinkage would freely occur at the unbonded surface areas.
Although it is evident that the C-factor has important role in stress development, it has been suggested that the C-factor approach in isolation may overestimate the effect of the degree of constriction [43]. Two recent studies [47, 48] demonstrate that the C-factor underestimates, or even neglects, the effect of the mass or, equivalently, volume of resin composite applied. Braga et al. [47] verified that shrinkage stress and microleakage were higher in restorations with larger diameters and depths and the authors concluded that microleakage seemed to be related to a restoration's volume, but not to its C-factor.
3.2. The Composition of the Resin Composite
In a general way, it is considered that the magnitude of stress is dependent on the material's volumetric shrinkage strain and its elastic modulus. Therefore, the chemical composition of the resin matrix plays an important role over the magnitude, kinetics of shrinkage strain, and the elastic modulus development. For example, a resin matrix formulated with monomers of high molecular weight (Mw) will result in lower shrinkage values than those formulated with monomers of low Mw. Thus, monomer functionalities, molecular structure, molecular mass and size have major influences upon the amount of shrinkage and also monomer viscosity [49, 50].
Due to the fact that inorganic fillers are the stiff component in the resin composite, the higher the filler ration the greater the composite elastic modulus [20]. Consequently, it could be understood that the level of stress developed would also be higher [51]. However this rationale is not so simple, since the resin matrix has much lower elastic modulus than the inorganic phase but shrinks when polymerized. Therefore, the polymer matrix/filler ratio has a dominant effect upon strain and stress developed, and high values of shrinkage, combined with an increasing elastic modulus, produce increased stress within the composite structure and the bonding region [52].
Although the effect of the rate of polymerization over the stress magnitude is not absolutely elucidated, it is considered for some researchers that more time would be available for viscous flow and chain relaxation to occur in a polymer cured at slower rates [53]; that is, the higher the rate of polymerization the higher the magnitude of stress due to relaxation restriction. Since the photoinitiator type and the photoinitiator/resin ratio directly affect the rate of polymerization and degree of conversion [54–56], it could be considered that these two factors would also affect the rate and final magnitude of stress developed [54, 55]. Braga and Ferracane [54] examined experimental materials with different concentrations of inhibitor (2,6-Di-tert-butyl-4-methyl-phenol = BHT) and showed that increased inhibitor concentration reduced the rate of polymerization and the shrinkage stress without significantly compromising the final degree of conversion of monomer to polymer. Moreover, Schneider et al. [55] recently demonstrated that the use of phenyl-propanedione, an alternative photoinitiator, combined with CQ may reduce the rate of stress development without decreasing the final material performance properties.
3.3. Material's Properties
According to the literature, there are three inherent properties of the resin composites that are crucial over the magnitude of stress: the volumetric shrinkage, the material's stiffness (elastic modulus), and the degree of conversion from double carbon bonds into simple carbon bonds. The complexity of polymerization shrinkage stress relies on the fact that these three components are interrelated and it is hard to identify the relative contribution of each individual factor; although some recent studies tried to isolate those [57, 58]. For example, for a certain material, the greater the C=C bond conversion of the monomers, the greater the number of units combining to form the final polymer structure. Consequently, stiffness (elastic modulus) and volumetric shrinkage both increase [3, 51]. Therefore, degree of conversion and stress development are related factors [44, 54, 59].
4. Polymerization Shrinkage: Methods for Evaluation
Since polymerization stress is considered one of the major drawbacks of resin-composite applications, extensive efforts have been made to understand the phenomenon and to devise means for its reduction. Consequently, methods are essential for evaluation of shrinkage strain and shrinkage stress.
4.1. Shrinkage Strain
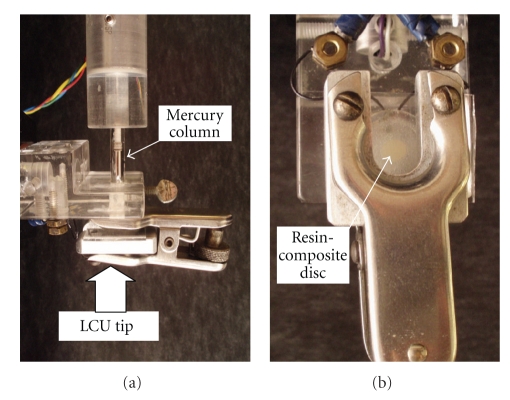
One of the first methods used to measure the polymerization volumetric shrinkage was the mercury dilatometer [60] (Figure 5). This equipment evaluates the volume change of the mercury in a reservoir surrounding the resin-composite specimen trough a thin column and the results are registered according to the amplified linear height variations of this column. Since the temperature of the LCU may affect the results, a thermocouple is attached to the system and volumetric change caused by the temperature from the light source is discounted.
Figure 5.
(a) Mercury dilatometer. It can be observed the mercury column, the clasp that holds the resin composite sample (b), and the place where the LCU is positioned. These pictures were kindly donated by Dr. Carmen Silvia C. Pfeifer. Equipment is from the Division of Biomaterials and Biomechanics, School of Dentistry, Oregon Health & Sciences University (Portland, USA).
In 1991, Watts and Cash [61] described the bonded-disc method to evaluate volumetric shrinkage (Figure 6). For this method, a disc-shaped specimen of uncured resin composite is placed at the centre of a square cross-section brass ring, which is adhesively bonded onto a rigid glass microscope slide. Thus, the top edge of the ring and the disc specimen are covered by a flexible glass microscope coverslip and, over this set, a linear variable differential transformer (LVDT) probe is positioned to measure the plate deflection. The LVDT is connected to a signal conditioning unit and a computer unit that records data over time.
Figure 6.
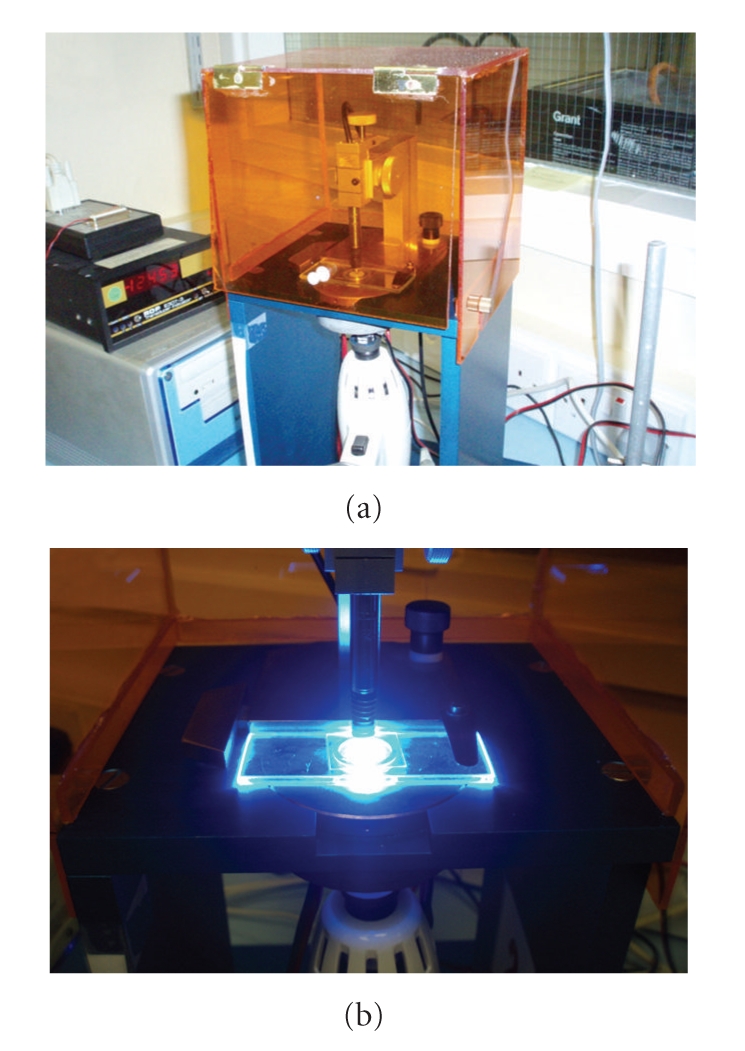
(a) The “Bonded-disc” apparatus. (b) A close view of the LVDT probe in contact with the glass slide during the resin-composite photoactivation. Equipment is from the Biomaterials Research Group, School of Dentistry, University of Manchester (Manchester, UK).
In the same year, Sakaguchi et al. [62] published a work about the use of electrical resistance strain gauges. Many adaptations using the strain gauges were applied latter [63–65]. In 1993, de Gee et al. [66] published a paper describing the linometer, which evaluates the linear displacement of a thin plate positioned on the resin-composite surface during the polymerization process. More recently, complex methods using video images [67, 68], laser speckle contrast analysis [69, 70], and mathematical and computational models [53, 71–73] have also been developed for research applications. Lately, new powerful and promising techniques, such as the X-ray microtomography, have been employed to investigate polymerization shrinkage [74]. Kakaboura et al. used the X-ray microtomography to evaluate the 3D-marginal adaptation to dentine versus shrinkage strain of two light-cured microhybrid resin composites [8]. The authors used sequential sections of restorations to calculate the interfacial microvoid volume fraction and compared the results with the bonded-disc method. As result, the authors found a strong correlation between the microvoid volume fractions with the data from the bonded-disc apparatus.
4.2. Shrinkage Stress
Methods used to evaluate shrinkage strain are important to understand the material's behavior. However, it is important to remember that shrinkage stress, that is not a material property, is a consequence of multiple factors and specific methods have to be used for evaluation. Such methods are described in the literature: ring slitting method [75, 76], photoelastic analysis [77–79], finite element analysis [42, 80–83], mathematical models [84], crack propagation [85], and force transducers [2, 40, 41, 46, 86, 87].
The “ring-slitting method” is a simple and inexpensive way to evaluate residual stress in ring-shape resin composite specimens [75, 76]. In this method, the resin composite is cured and the gap distance previously created in the ring is measured before and after the polymerization process. Photoelastic or finite element analyses (FEAs) are interesting methods to observe the spatial distribution and concentration areas of stress. While photoelastic analysis determines stress distribution through optical fringes created in specific resins [77–79], FEA evaluates stress distributions by computer models. This method requires not only an anatomically accurate geometry but other input data, especially elastic moduli, Poisson's ratios, and shrinkage strain.
Although the previous methods brought important contributions for the current knowledge, it has to be stated that force transducers are the most widely used and versatile methods for analyses of stress development. The wide application of such equipment relies on the fact that it is possible to analyze the influence of important factors, like C-factor and mass of material, by simple variations in cylinder/disk size and aspect ratio. Although the basic principle is the same for all force transducers, there are different measurement approaches for each system, being the instrument compliance the most significant one. Unfortunately, outcomes seem to be dependent upon system compliance, which varies among different studies [52].
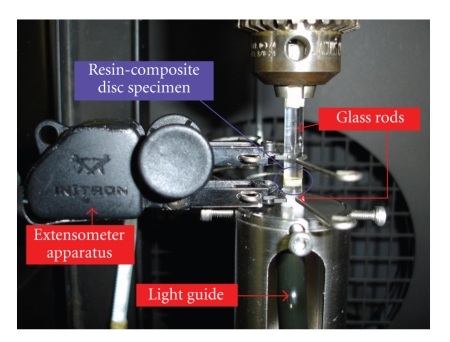
Universal testing machines modified with extensometers connected to a computer servo-control unit are very precise and can identify movement of extension caused by the polymerization shrinkage. As a feedback response, the system compensates deformations and the sample remains constant. Thus, this kind of system presents very low compliance and, consequently, the registered values of stress tend to be higher than those by more compliant methods [83]. Some variations may exist within this method, and a significant one is the kind of substrate to which the resin-composite sample is attached [57]. Figure 7 shows a picture from an extensometer apparatus used to analyze deformations from the resin-composite specimen.
Figure 7.
Extensometer apparatus that is connected to a universal testing machine. As a feedback response, the system compensates deformations and the sample remains constant. Consequently, this kind of method is known as a “low-compliant method.” Pictures kindly donated by Dr. Carmen Silvia C. Pfeifer. Equipment is from the School of Dentistry, University of São Paulo (São Paulo, Brazil).
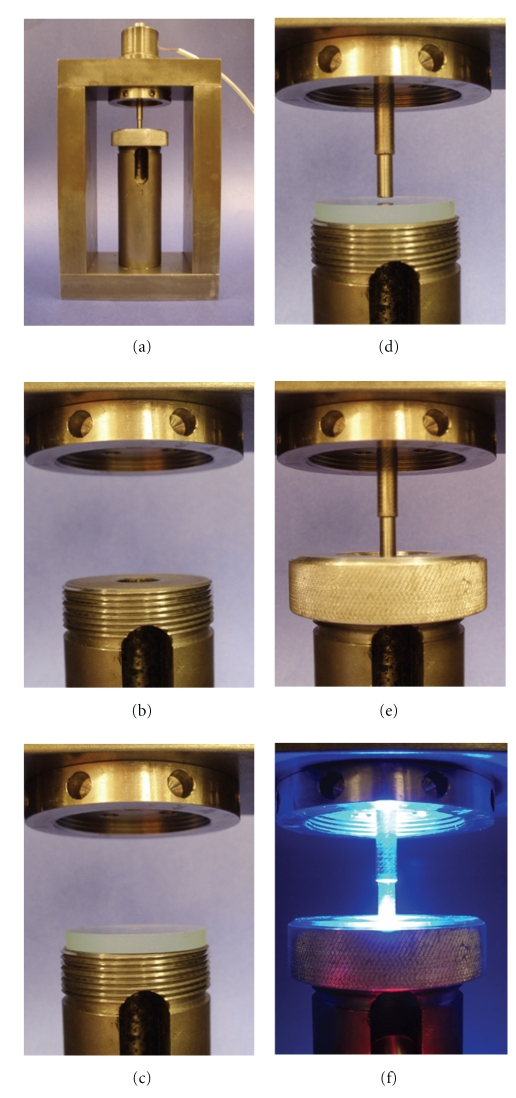
There are also force transducers adapted to systems with unknown or calculated compliance [43, 46, 53, 86, 88]. Figure 8 shows a controlled-compliance apparatus for contraction stress test developed by Sakaguchi et al. (2004) [88]. The apparatus consists of a steel frame and a washer-type load cell through which a steel piston is inserted. The lower part of the frame held a circumferential glass plate that supports the resin-composite specimen. The surfaces of the piston and the glass plate are usually sandblasted and coated with a silane coupling agent to improve the adhesion between the apparatus and the resin-composite specimen. The resin composite is then inserted between the glass and the steel piston and the material is photoactivated through the glass plate. As the materials shrinks, force is recorded and converted to nominal stress by dividing it by the cross-sectional area of the specimen.
Figure 8.
Controlled compliance apparatus for contraction stress test. (a) The entire apparatus with a view of the steel frame and the upper load cell holder; (b) slot for light guide; (c) glass plate positioned; (d) steel piston in position and the space where the resin-composite specimen is positioned; (e) equipment ready for use; (f) light curing procedure during the experiment. These pictures were kindly donated by Dr. Carmen Silvia C. Pfeifer. Equipment is from Division of Biomaterials and Biomechanics, the School of Dentistry, Oregon Health & Sciences University (Portland, USA).
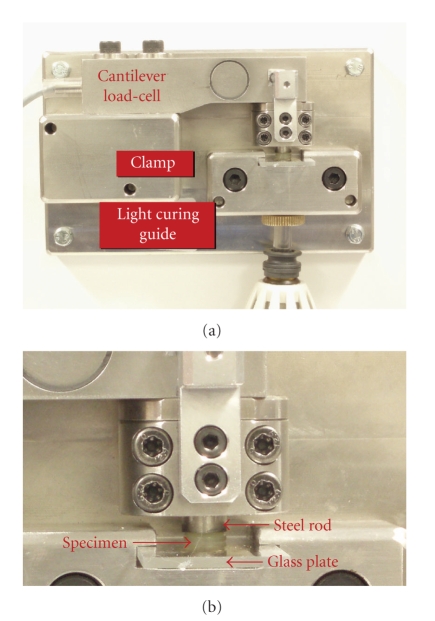
Another apparatus developed for contraction stress test is the Bioman and was designed by Watts et al. (2003) [46] (Figure 9). The system is based on a cantilever load-cell fitted with a rigid integral clamp. The compliant end of the cantilever held a circular steel rod. The counter-face consisted of a removable rigid glass plate that is held rigidly relative to the base plate in a special clamp during measurement. The resin composite is then introduced between the treated (sandblast + silane) plate and vertical rod to form an uncured specimen disk. The resin composite is irradiated through its thickness dimension from below. The load-signal from the cantilever cell is amplified and the signal is acquired by a standard computer. The registered load is then divided by the disk area in order to obtain the stress values in MPa.
Figure 9.
(a) The Bioman stress measurement device. (b) A close view of the resin-composite specimen. Equipment is from the Biomaterials Research Group, School of Dentistry, University of Manchester (Manchester, UK).
Unfortunately, besides variations in the final stress values, the comparisons among different materials can also be affected [52] and different interpretations about a given aspect may also vary when all these methods are used. Therefore, it must be clear that care should be taken when analyzing stress data and phenomena interpretations, since the system compliance has also to be considered. Since the final objective of the in vitro research is to provide valid data that simulate the clinical situations, instrument compliance should be similar to that of the prepared tooth [48, 67].
5. Strategies to Reduce Shrinkage Stress in Clinical Procedures
Many clinical methods have been proposed to reduce shrinkage stress, such as the control of curing light irradiance [9, 89], flowable resin liner application [90], and incremental layering techniques [5, 91, 92]. However, no method has been shown to be totally effective in abating the effects of polymerization shrinkage.
5.1. Incremental Layering Technique
Since difficulties imposed by the cavity configuration (C-factor) play an important role in stress development, many researchers have suggested the use of “incremental layering techniques” for resin-composite restoration to reduce the polymerization shrinkage stress and cusp deflection [91, 93–96]. The rationale is that shrinkage may be less detrimental when there are fewer bonded cavity walls involved at each stage of the restoration procedures. Incremental curing also enhances the degree of cure as thin sections undergo higher degree of cure due to lower light attenuation, thus the net degree of conversion is greater. This yields better mechanical properties but higher shrinkage as well; however, the C-factor changes as well.
In class I cavity, for example, by using a single increment, the resin composite would polymerize within five bonding surfaces (one base and four surrounding walls) while free shrinkage would only occur at the upper surface, producing a very high level of stress between the bonded surfaces. However, by using an incremental technique, the bonded/unbonded ratio would be reduced and, consequently, the stress level within the cavity might be lower, preserving the bonded area.
According to Park et al. [95] the bulk filling technique yielded significantly more cuspal deflection than the incremental filling techniques, concluding that cuspal deflection resulting from polymerization shrinkage can be reduced by incremental filling techniques to obtain optimal outcomes in clinical situations. Lee et al. [91] observed that cusp deflection increased with increasing cavity dimension and C-factor, thus the use of an incremental filling technique or an indirect composite inlay restoration could reduce the cuspal strain. C-factor was shown to be an influencing factor for dentin adhesion [97]; however, using an appropriate layering technique, high bond strengths to deep cavity floors can be achieved.
Nevertheless the literature is not conclusive concerning the advantages promoted by the incremental layering technique over the effects of resin-composite polymerization shrinkage. Versluis et al. [81] assessed the developing stress fields for different incremental filling techniques by using a theoretical study with Finite Element Analysis (FEA) methods. It was concluded that the incremental filling technique increased the deformation of the restored tooth and could produce higher polymerization stresses at the restoration interface compared with bulk filling. Multiple increments showed to induce greater cuspal movement than a bulk increment in cuspal deflection measurements of premolars [98]. According to Loguercio et al. [99], some evaluated effects of polymerization shrinkage such as gap width, adhesive bond, strength and the cohesive strength of the resin composite were not reduced by the filling technique under the different C-factor cavities.
Despite the controversy over the advantages of incremental build-up of resin composites, this technique has been broadly recommended in direct resin-composite restoration, because it is expected to decrease the C-factor, allowing a certain amount of flow to partially dissipate the shrinkage stress.
5.2. Stress Absorbing Layers with Low Elastic Modulus Liners
Flowable composites are low viscosity resin-based restorative materials, which differ from conventional resin composites in their filler load [100, 101] and resin content. These materials are less rigid and could have a modulus of elasticity 20–30% lower than conventional hybrid composites [42]. The use of a flowable resin composite as an intermediate thin layer has been suggested as a mean of overcoming polymerization shrinkage stress based on the concept of an “elastic cavity wall” suggested for filled adhesives [102–105]. According to the “elastic cavity wall concept” the shrinkage stress generated by a subsequent layer of higher modulus resin composite can be absorbed by an elastic intermediary layer, thereby reducing the stress at the tooth-restoration interface [106] manifested clinically as a reduction in cuspal deflection [90, 107].
However, actual implementation of such a “stress absorbing” material is problematic. Restorative materials encompass a wide variety of shrinkage and elastic modulus values. Consequently, some combinations might give reduced performance compared with the common restorative material applied alone. Flowable resin composites have shown shrinkage stress comparable to conventional resin composites, supporting the hypothesis that the use of flowable materials does not lead to marked stress reduction and the risk of debonding at the adhesive interface as a result of polymerization contraction is similar for both type of materials [108].
5.3. Light Curing Procedures
Diverse photoactivation protocols have been advocated to reduce the polymerization stress [3, 109, 110]. In theory, stress release by viscous flow before the vitrification stage would be allowed to occur without compromising the final polymer properties [14, 15]. Therefore, initial light exposure at lower irradiance values might lead to the formation of a reduced number of polymer growth centers, reducing the reaction rate and decreasing stress development due to the increased opportunity for resin flow before the vitrification stage [89, 111].
There are many types of alternative light-curing methods. The “soft-start” protocol consists of initial light exposure with reduced irradiance for a certain period of time, followed by full irradiance. Another protocol is “pulse-delay” method, where the clinician may apply the initial exposure with reduced light irradiance for a very short period of time of a few seconds and follows a waiting period without irradiance (seconds or even minutes) and fully irradiate later. One important consideration is that some different outcomes may appear among different studies, and these differences may be related with the light curing type used, the irradiance used at the beginning of the light curing procedure, and/or the period without irradiance.
Although the alternative light-curing protocols may not significantly affect final properties of the hardened material, some considerations should be noted. (i) The flowability of a material, during an extended preset stage, may have minimal consequences, because most shrinkage stress is developed during and after the vitrification stage [112]. Therefore, opportunities for polymer relaxation would be restricted during the short period of light activation [113]. (ii) Concurrent experiments on degree of C=C conversion (DC) and stress development show that soft-start irradiation procedures give somewhat lower DC levels, associated with reduced stress [114];. (iii) A reduced polymerization rate is associated with decreased cross-link density (CLD), manifest as greater solvent-softening and/or lower final elastic modulus [115].
5.4. Preheating
Recently, preheating resin composites have been advocated as a method to increase composite flow, improve marginal adaptation and monomer conversion. The benefits of preheating composites may have an impact on daily restorative procedures as well, with the application of shorter light exposure to provide conversion values similar to those seen in unheated conditions [116].
The reasons for increased conversion are based on many factors. Increased temperature decreases system viscosity and enhances radical mobility, resulting in additional polymerization and higher conversion. The collision frequency of unreacted active groups and radicals could increases with elevated curing temperature when below the glass transition temperature [117]. Therefore, at raised temperatures, in theory, it would be possible to obtain higher degree of conversion before the vitrification point, decreasing the magnitude of stress. However, real benefits were not fully demonstrated and, until now, there are no published studies showing stress reduction by warming resin composites.
5.5. Novel Formulations for Reducing Shrinkage Stress
The development of resin composite has mainly focused on filler technology, while the composition of the polymer matrix remained principally unchanged since the introduction of Bis-GMA resin by Bowen in the early 1960s [1]. Shrinkage is an inherent property of dimethacrylate-based formulations. However, recently, novel monomer combinations and alterations of the resin-composite formulation have been developed and evaluated with the goal of decreasing polymerization shrinkage stress.
The most recent modification on the polymer matrix is based on using ring opening polymerization of the silorane molecules, instead of free radical polymerization of dimethacrylate monomers [118]. Silorane resin reveals lower polymerization shrinkage compared to the dimethacrylates. These “cyclic” monomers have provided particularly interesting and commercially viable results. Such monomers “open” their molecular structures with local volumetric expansion and this may partly or totally compensate for volumetric shrinkage from C=C or similar polymerization [118–120].
Changes in the photoinitiator systems and polymerization inhibitors have also been reported. Braga and Ferracane [54] tested experimental materials with different concentrations of inhibitor (2,6-Di-tert-butyl-4-methyl-phenol = BHT) and showed that increased inhibitor concentrations reduced the rate of polymerization and the shrinkage stress without significantly compromising the final degree of conversion. Schneider et al. [55] found that phenyl-propanedione, substituting for part of the camphorquinone content, reduced the stress development rate without compromising the final degree of conversion and degradation resistance of the composite.
Besides change in the resin matrix composition, studies have demonstrated reduced shrinkage stress through alterations in filler content. Condon and Ferracane [121] suggested that addition of nonbonded 40 nm colloidal silica might act as stress-relieving sites through plastic deformation. They also verified that composites with nanofiller particles treated with a nonfunctional silane developed 50% less stress than composites fully treated with the functional coupling agent. Another possible approach is inclusion of a component readily allowing plastic deformation during stress development, such as ultrahigh molecular weight polyethylene (UHMWPE) fibres [122].
6. Conclusions
The current dental resin composites based on dimethacrylates are inevitably linked with shrinkage that can compromise the success and longevity of the restoration and, consequently, clinicians have to coexist with the polymerization shrinkage-stress phenomena. On the other hand, methods for shrinkage stress evaluation are bringing important contributions and the outcomes are being applied to novel formulations and clinical techniques.
References
- 1.Bowen RL. Properties of a silica-reinforced polymer for dental restorations. The Journal of the American Dental Association. 1963;66:57–64. doi: 10.14219/jada.archive.1963.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bowen RL. Adhesive bonding of various materials to hard tooth tissues—VI: forces developing in direct-filling materials during hardening. The Journal of the American Dental Association. 1967;74(3):439–445. doi: 10.14219/jada.archive.1967.0078. [DOI] [PubMed] [Google Scholar]
- 3.Silikas N, Eliades G, Watts DC. Light intensity effects on resin-composite degree of conversion and shrinkage strain. Dental Materials. 2000;16(4):292–296. doi: 10.1016/s0109-5641(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 4.Causton BE, Miller B, Sefton J. The deformation of cusps by bonded posterior composite restorations: an in vitro study. British Dental Journal. 1985;159(12):397–400. doi: 10.1038/sj.bdj.4805746. [DOI] [PubMed] [Google Scholar]
- 5.McCullock AJ, Smith BG. In vitro studies of cuspal movement produced by adhesive restorative materials. British Dental Journal. 1986;161(11):405–409. doi: 10.1038/sj.bdj.4805990. [DOI] [PubMed] [Google Scholar]
- 6.Meredith N, Setchell DJ. In vitro measurement of cuspal strain and displacement in composite restored teeth. Journal of Dentistry. 1997;25(3-4):331–337. doi: 10.1016/s0300-5712(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 7.Pearson GJ, Hegarty SM. Cusp movement in molar teeth using dentine adhesives and composite filling materials. Biomaterials. 1987;8(6):473–476. doi: 10.1016/0142-9612(87)90084-6. [DOI] [PubMed] [Google Scholar]
- 8.Kakaboura A, Rahiotis C, Watts D, Silikas N, Eliades G. 3D-marginal adaptation versus setting shrinkage in light-cured microhybrid resin composites. Dental Materials. 2007;23(3):272–278. doi: 10.1016/j.dental.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Feilzer AJ, Dooren LH, de Gee AJ, Davidson CL. Influence of light intensity on polymerization shrinkage and integrity of restoration-cavity interface. European Journal of Oral Sciences. 1995;103(5):322–326. doi: 10.1111/j.1600-0722.1995.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferracane JL, Antonio RC, Matsumoto H. Variables affecting the fracture toughness of dental composites. Journal of Dental Research. 1987;66(6):1140–1145. doi: 10.1177/00220345870660060901. [DOI] [PubMed] [Google Scholar]
- 11.Braem M, Finger W, Van Doren VE, Lambrechts P, Vanherle G. Mechanical properties and filler fraction of dental composites. Dental Materials. 1989;5(5):346–349. doi: 10.1016/0109-5641(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Park JH, Imai Y, Kishi T. Microfracture mechanisms of dental resin composites containing spherically-shaped filler particles. Journal of Dental Research. 1994;73(2):499–504. doi: 10.1177/00220345940730020301. [DOI] [PubMed] [Google Scholar]
- 13.Söderholm K-JM. Influence of silane treatment and filler fraction on thermal expansion of composite resins. Journal of Dental Research. 1984;63(11):1321–1326. doi: 10.1177/00220345840630111401. [DOI] [PubMed] [Google Scholar]
- 14.van Dijken JWV, Wing KR, Ruyter IE. An evaluation of the radiopacity of composite restorative materials used in Class I and Class II cavities. Acta Odontologica Scandinavica. 1989;47(6):401–407. doi: 10.3109/00016358909004809. [DOI] [PubMed] [Google Scholar]
- 15.Cavalcante LM, Masouras K, Watts DC, Pimenta LA, Silikas N. Effect of nanofillers’ size on surface properties after toothbrush abrasion. American Journal of Dentistry. 2009;22(1):60–64. [PubMed] [Google Scholar]
- 16.Baroudi K, Silikas N, Watts DC. Time-dependent visco-elastic creep and recovery of flowable composites. European Journal of Oral Sciences. 2007;115(6):517–521. doi: 10.1111/j.1600-0722.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim K-H, Ong JL, Okuno O. The effect of filler loading and morphology on the mechanical properties of contemporary composites. Journal of Prosthetic Dentistry. 2002;87(6):642–649. doi: 10.1067/mpr.2002.125179. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues SA, Jr., Ferracane JL, Della Bona A. Flexural strength and Weibull analysis of a microhybrid and a nanofill composite evaluated by 3- and 4-point bending tests. Dental Materials. 2008;24(3):426–431. doi: 10.1016/j.dental.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues SA, Jr., Scherrer SS, Ferracane JL, Della Bona A. Microstructural characterization and fracture behavior of a microhybrid and a nanofill composite. Dental Materials. 2008;24(9):1281–1288. doi: 10.1016/j.dental.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Masouras K, Silikas N, Watts DC. Correlation of filler content and elastic properties of resin-composites. Dental Materials. 2008;24(7):932–939. doi: 10.1016/j.dental.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Van Noort R. Introduction to Dental Materials. 3rd edition. London, UK: Elsevier; 2007. [Google Scholar]
- 22.Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. Journal of the American Dental Association. 2003;134(10):1382–1390. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 23.Asmussen E, Peutzfeldt A. Influence of UEDMA, BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dental Materials. 1998;14(1):51–56. doi: 10.1016/s0109-5641(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 24.Bowen R. Inventor dental filling material comprising vinyl-silane treated fused silica and a binder consisting of the reaction product of bisphenol and glycidyl methacrylate
- 25.Ferracane JL, Greener EH. The effect of resin formulation on the degree of conversion and mechanical properties of dental restorative resins. Journal of Biomedical Materials Research. 1986;20(1):121–131. doi: 10.1002/jbm.820200111. [DOI] [PubMed] [Google Scholar]
- 26.Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23(8):1819–1829. doi: 10.1016/s0142-9612(01)00308-8. [DOI] [PubMed] [Google Scholar]
- 27.Moszner N, Salz U. New developments of polymeric dental composites. Progress in Polymer Science. 2001;26(4):535–576. [Google Scholar]
- 28.Peutzfeldt A. Resin composites in dentistry: the monomer systems. European Journal of Oral Sciences. 1997;105(2):97–116. doi: 10.1111/j.1600-0722.1997.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 29.Asmussen E, Peutzfeldt A. Influence of selected components on crosslink density in polymer structures. European Journal of Oral Sciences. 2001;109(4):282–285. doi: 10.1034/j.1600-0722.2001.00057.x. [DOI] [PubMed] [Google Scholar]
- 30.Sideridou I, Tserki V, Papanastasiou G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials. 2003;24(4):655–665. doi: 10.1016/s0142-9612(02)00380-0. [DOI] [PubMed] [Google Scholar]
- 31.Floyd CJE, Dickens SH. Network structure of Bis-GMA- and UDMA-based resin systems. Dental Materials. 2006;22(12):1143–1149. doi: 10.1016/j.dental.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Pfeifer CS, Silva LR, Kawano Y, Braga RR. Bis-GMA co-polymerizations: influence on conversion, flexural properties, fracture toughness and susceptibility to ethanol degradation of experimental composites. Dental Materials. 2009;25(9):1136–1141. doi: 10.1016/j.dental.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Mohsen NM, Craig RG. Effect of silanation of fillers on their dispersability by monomer systems. Journal of Oral Rehabilitation. 1995;22(3):183–189. doi: 10.1111/j.1365-2842.1995.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 34.Watts DC, Silikas N. In situ photo-polymerisation and polymerisation-shrinkage phenomena. In: Eliades G, Watts DC, Eliades T, editors. Dental Hard Tissues and Bonding Interfacial Phenomena and Related Properties. Berlin, Germany: Springer; 2005. pp. 123–154. [Google Scholar]
- 35.Stansbury JW. Curing dental resins and composites by photopolymerization. Journal of Esthetic Dentistry. 2000;12(6):300–308. doi: 10.1111/j.1708-8240.2000.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 36.Loshaek S, Fox TG. Cross-linked polymers. I. Factors influencing the efficiency of cross-linking in copolymers of methyl methacrylate and glycol dimethacrylates. Journal of the American Chemical Society. 1953;75(14):3544–3550. [Google Scholar]
- 37.Tobolsky AV, Leonard F, Roeser GP. Use of polymerizable ring compounds in constant volume polymerizations. Journal of Polymer Science. 1948;3:604–606. [Google Scholar]
- 38.Kleverlaan CJ, Feilzer AJ. Polymerization shrinkage and contraction stress of dental resin composites. Dental Materials. 2005;21(12):1150–1157. doi: 10.1016/j.dental.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dental Materials. 2005;21(1):68–74. doi: 10.1016/j.dental.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Davidson CL, de Gee AJ. Relaxation of polymerization contraction stresses by flow in dental composites. Journal of Dental Research. 1984;63(2):146–148. doi: 10.1177/00220345840630021001. [DOI] [PubMed] [Google Scholar]
- 41.Feilzer AJ, de Gee AJ, Davidson CL. Setting stress in composite resin in relation to configuration of the restoration. Journal of Dental Research. 1987;66(11):1636–1639. doi: 10.1177/00220345870660110601. [DOI] [PubMed] [Google Scholar]
- 42.Laughlin GA, Williams JL, Eick JD. The influence of system compliance and sample geometry on composite polymerization shrinkage stress. Journal of Biomedical Materials Research. 2002;63(5):671–678. doi: 10.1002/jbm.10386. [DOI] [PubMed] [Google Scholar]
- 43.Miguel A, de la Macorra JC. A predictive formula of the contraction stress in restorative and luting materials attending to free and adhered surfaces, volume and deformation. Dental Materials. 2001;17(3):241–246. doi: 10.1016/s0109-5641(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 44.Stansbury JW, Trujillo-Lemon M, Lu H, Ding X, Lin Y, Ge J. Conversion-dependent shrinkage stress and strain in dental resins and composites. Dental Materials. 2005;21(1):56–67. doi: 10.1016/j.dental.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Watts DC. Reaction kinetics and mechanics in photo-polymerised networks. Dental Materials. 2005;21(1):27–35. doi: 10.1016/j.dental.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dental Materials. 2003;19(1):1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 47.Braga RR, Boaro LCC, Kuroe T, Azevedo CLN, Singer JM. Influence of cavity dimensions and their derivatives (volume and ‘C’ factor) on shrinkage stress development and microleakage of composite restorations. Dental Materials. 2006;22(9):818–823. doi: 10.1016/j.dental.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Watts DC, Satterthwaite JD. Axial shrinkage-stress depends upon both C-factor and composite mass. Dental Materials. 2008;24(1):1–8. doi: 10.1016/j.dental.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Charton C, Falk V, Marchal P, Pla F, Colon P. Influence of Tg, viscosity and chemical structure of monomers on shrinkage stress in light-cured dimethacrylate-based dental resins. Dental Materials. 2007;23(11):1447–1459. doi: 10.1016/j.dental.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Feilzer AJ, Dauvillier BS. Effect of TEGDMA/BisGMA ratio on stress development and viscoelastic properties of experimental two-paste composites. Journal of Dental Research. 2003;82(10):824–828. doi: 10.1177/154405910308201012. [DOI] [PubMed] [Google Scholar]
- 51.Condon JR, Ferracane JL. Assessing the effect of composite formulation on polymerization stress. Journal of the American Dental Association. 2000;131(4):497–503. doi: 10.14219/jada.archive.2000.0207. [DOI] [PubMed] [Google Scholar]
- 52.Ferracane JL. Buonocore Lecture. Placing dental composites—a stressful experience. Operative Dentistry. 2008;33(3):247–257. doi: 10.2341/07-BL2. [DOI] [PubMed] [Google Scholar]
- 53.Sakaguchi RL, Wiltbank BD, Murchison CF. Prediction of composite elastic modulus and polymerization shrinkage by computational micromechanics. Dental Materials. 2004;20(4):397–401. doi: 10.1016/j.dental.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Braga RR, Ferracane JL. Contraction stress related to degree of conversion and reaction kinetics. Journal of Dental Research. 2002;81(2):114–118. [PubMed] [Google Scholar]
- 55.Schneider LFJ, Pfeifer CSC, Consani S, Prahl SA, Ferracane JL. Influence of photoinitiator type on the rate of polymerization, degree of conversion, hardness and yellowing of dental resin composites. Dental Materials. 2008;24(9):1169–1177. doi: 10.1016/j.dental.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Venhoven BAM, de Gee AJ, Davidson CL. Light initiation of dental resins: dynamics of the polymerization. Biomaterials. 1996;17(24):2313–2318. doi: 10.1016/s0142-9612(96)00074-9. [DOI] [PubMed] [Google Scholar]
- 57.Gonçalves F, Pfeifer CS, Ferracane JL, Braga RR. Contraction stress determinants in dimethacrylate composites. Journal of Dental Research. 2008;87(4):367–371. doi: 10.1177/154405910808700404. [DOI] [PubMed] [Google Scholar]
- 58.Pfeifer CS, Ferracane JL, Sakaguchi RL, Braga RR. Factors affecting photopolymerization stress in dental composites. Journal of Dental Research. 2008;87(11):1043–1047. doi: 10.1177/154405910808701114. [DOI] [PubMed] [Google Scholar]
- 59.Calheiros FC, Braga RR, Kawano Y, Ballester RY. Relationship between contraction stress and degree of conversion in restorative composites. Dental Materials. 2004;20(10):939–946. doi: 10.1016/j.dental.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 60.de Gee AJ, Davidson CL, Smith A. A modified dilatometer for continuous recording of volumetric polymerization shrinkage of composite restorative materials. Journal of Dentistry. 1981;9(1):36–42. doi: 10.1016/0300-5712(81)90033-6. [DOI] [PubMed] [Google Scholar]
- 61.Watts DC, Cash AJ. Determination of polymerization shrinkage kinetics in visible-light-cured materials: methods development. Dental Materials. 1991;7(4):281–287. doi: 10.1016/S0109-5641(05)80030-2. [DOI] [PubMed] [Google Scholar]
- 62.Sakaguchi RL, Sasik CT, Bunczak MA, Douglas WH. Strain gauge method for measuring polymerization contraction of composite restoratives. Journal of Dentistry. 1991;19(5):312–316. doi: 10.1016/0300-5712(91)90081-9. [DOI] [PubMed] [Google Scholar]
- 63.Sakaguchi RL, Ferracane JL. Stress transfer from polymerization shrinkage of a chemical-cured composite bonded to a pre-cast composite substrate. Dental Materials. 1998;14(2):106–111. doi: 10.1016/s0109-5641(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 64.Sakaguchi RL, Peters MCRB, Nelson SR, Douglas WH, Poort HW. Effects of polymerization contraction in composite restorations. Journal of Dentistry. 1992;20(3):178–182. doi: 10.1016/0300-5712(92)90133-w. [DOI] [PubMed] [Google Scholar]
- 65.Sakaguchi RL, Versluis A, Douglas WH. Analysis of strain gage method for measurement of post-gel shrinkage in resin composites. Dental Materials. 1997;13(4):233–239. doi: 10.1016/S0109-5641(97)80034-6. [DOI] [PubMed] [Google Scholar]
- 66.de Gee AJ, Feilzer AJ, Davidson CL. True linear polymerization shrinkage of unfilled resins and composites determined with a linometer. Dental Materials. 1993;9(1):11–14. doi: 10.1016/0109-5641(93)90097-a. [DOI] [PubMed] [Google Scholar]
- 67.Lee SH, Chang J, Ferracane J, Lee IB. Influence of instrument compliance and specimen thickness on the polymerization shrinkage stress measurement of light-cured composites. Dental Materials. 2007;23(9):1093–1100. doi: 10.1016/j.dental.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Li J, Fok ASL, Satterthwaite J, Watts DC. Measurement of the full-field polymerization shrinkage and depth of cure of dental composites using digital image correlation. Dental Materials. 2009;25(5):582–588. doi: 10.1016/j.dental.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Sato T, Miyazaki M, Rikuta A. Real-time dimensional change in light-cured composites at various depths using laser speckle contrast analysis. European Journal of Oral Sciences. 2004;112(6):538–544. doi: 10.1111/j.1600-0722.2004.00179.x. [DOI] [PubMed] [Google Scholar]
- 70.Sato T, Miyazaki M, Rikuta A, Kobayashi K. Application of the laser speckle-correlation method for determining the shrinkage vector of a light-cured resin. Dental Materials Journal. 2004;23(3):284–290. doi: 10.4012/dmj.23.284. [DOI] [PubMed] [Google Scholar]
- 71.Atai M, Watts DC. A new kinetic model for the photopolymerization shrinkage-strain of dental composites and resin-monomers. Dental Materials. 2006;22(8):785–791. doi: 10.1016/j.dental.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Koplin C, Jaeger R, Hahn P. Kinetic model for the coupled volumetric and thermal behavior of dental composites. Dental Materials. 2008;24(8):1017–1024. doi: 10.1016/j.dental.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 73.Petrovic LM, Atanackovic TM. A model for shrinkage strain in photo polymerization of dental composites. Dental Materials. 2008;24(4):556–560. doi: 10.1016/j.dental.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 74.Sun J, Lin-Gibson S. X-ray microcomputed tomography for measuring polymerization shrinkage of polymeric dental composites. Dental Materials. 2008;24(2):228–234. doi: 10.1016/j.dental.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Park JW, Ferracane JL. Measuring the residual stress in dental composites using a ring slitting method. Dental Materials. 2005;21(9):882–889. doi: 10.1016/j.dental.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Park JW, Ferracane JL. Residual stress in composites with the thin-ring-slitting approach. Journal of Dental Research. 2006;85(10):945–949. doi: 10.1177/154405910608501015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ernst C-P, Meyer GR, Klöcker K, Willershausen B. Determination of polymerization shrinkage stress by means of a photoelastic investigation. Dental Materials. 2004;20(4):313–321. doi: 10.1016/S0109-5641(03)00109-X. [DOI] [PubMed] [Google Scholar]
- 78.Kinomoto Y, Torii M. Photoelastic analysis of polymerization contraction stresses in resin composite restorations. Journal of Dentistry. 1998;26(2):165–171. doi: 10.1016/s0300-5712(96)00083-8. [DOI] [PubMed] [Google Scholar]
- 79.Kinomoto Y, Torii M, Takeshige F, Ebisu S. Comparison of polymerization contraction stresses between self- and light-curing composites. Journal of Dentistry. 1999;27(5):383–389. doi: 10.1016/s0300-5712(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 80.Ausiello P, Apicella A, Davidson CL, Rengo S. 3D-finite element analyses of cusp movements in a human upper premolar, restored with adhesive resin-based composites. Journal of Biomechanics. 2001;34(10):1269–1277. doi: 10.1016/s0021-9290(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 81.Versluis A, Douglas WH, Cross M, Sakaguchi RL. Does an incremental filling technique reduce polymerization shrinkage stresses? Journal of Dental Research. 1996;75(3):871–878. doi: 10.1177/00220345960750030301. [DOI] [PubMed] [Google Scholar]
- 82.Versluis A, Tantbirojn D, Douglas WH. Do dental composites always shrink toward the light? Journal of Dental Research. 1998;77(6):1435–1445. doi: 10.1177/00220345980770060801. [DOI] [PubMed] [Google Scholar]
- 83.Witzel MF, Ballester RY, Meira JBC, Lima RG, Braga RR. Composite shrinkage stress as a function of specimen dimensions and compliance of the testing system. Dental Materials. 2007;23(2):204–210. doi: 10.1016/j.dental.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Li J, Li H, Fok SL. A mathematical analysis of shrinkage stress development in dental composite restorations during resin polymerization. Dental Materials. 2008;24(7):923–931. doi: 10.1016/j.dental.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto T, Ferracane JL, Sakaguchi RL, Swain MV. Calculation of contraction stresses in dental composites by analysis of crack propagation in the matrix surrounding a cavity. Dental Materials. 2009;25(4):543–550. doi: 10.1016/j.dental.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Bouschlicher MR, Vargas MA, Boyer DB. Effect of composite type, light intensity, configuration factor and laser polymerization on polymerization contraction forces. American Journal of Dentistry. 1997;10(2):88–96. [PubMed] [Google Scholar]
- 87.Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dental Materials. 2005;21(10):962–970. doi: 10.1016/j.dental.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 88.Sakaguchi RL, Wiltbank BD, Murchison CF. Contraction force rate of polymer composites is linearly correlated with irradiance. Dental Materials. 2004;20(4):402–407. doi: 10.1016/j.dental.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Uno S, Asmussen E. Marginal adaptation of a restorative resin polymerized at reduced rate. Scandinavian Journal of Dental Research. 1991;99(5):440–444. doi: 10.1111/j.1600-0722.1991.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 90.Alomari QD, Reinhardt JW, Boyer DB. Effect of liners on cusp deflection and gap formation in composite restorations. Operative Dentistry. 2001;26(4):406–411. [PubMed] [Google Scholar]
- 91.Lee M-R, Cho B-H, Son H-H, Um C-M, Lee I-B. Influence of cavity dimension and restoration methods on the cusp deflection of premolars in composite restoration. Dental Materials. 2007;23(3):288–295. doi: 10.1016/j.dental.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 92.Segura A, Donly KJ. In vitro posterior composite polymerization recovery following hygroscopic expansion. Journal of Oral Rehabilitation. 1993;20(5):495–499. doi: 10.1111/j.1365-2842.1993.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 93.Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. Journal of Dentistry. 1997;25(6):435–440. doi: 10.1016/s0300-5712(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 94.Lutz E, Krejci I, Oldenburg TR. Elimination of polymerization stresses at the margins of posterior composite resin restorations: a new restorative technique. Quintessence International. 1986;17(12):777–784. [PubMed] [Google Scholar]
- 95.Park J, Chang J, Ferracane J, Lee IB. How should composite be layered to reduce shrinkage stress: incremental or bulk filling? Dental Materials. 2008;24(11):1501–1505. doi: 10.1016/j.dental.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 96.Suliman AA, Boyer DB, Lakes RS. Cusp movement in premolars resulting from composite polymerization shrinkage. Dental Materials. 1993;9(1):6–10. doi: 10.1016/0109-5641(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 97.Nikolaenko SA, Lohbauer U, Roggendorf M, Petschelt A, Dasch W, Frankenberger R. Influence of c-factor and layering technique on microtensile bond strength to dentin. Dental Materials. 2004;20(6):579–585. doi: 10.1016/j.dental.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 98.Abbas G, Fleming GJP, Harrington E, Shortall ACC, Burke FJT. Cuspal movement and microleakage in premolar teeth restored with a packable composite cured in bulk or in increments. Journal of Dentistry. 2003;31(6):437–444. doi: 10.1016/s0300-5712(02)00121-5. [DOI] [PubMed] [Google Scholar]
- 99.Loguercio AD, Reis A, Ballester RY. Polymerization shrinkage: effects of constraint and filling technique in composite restorations. Dental Materials. 2004;20(3):236–243. doi: 10.1016/S0109-5641(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 100.Baroudi K, Saleh AM, Silikas N, Watts DC. Shrinkage behaviour of flowable resin-composites related to conversion and filler-fraction. Journal of Dentistry. 2007;35(8):651–655. doi: 10.1016/j.jdent.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Helvatjoglu-Antoniades M, Papadogiannis Y, Lakes RS, Dionysopoulos P, Papadogiannis D. Dynamic and static elastic moduli of packable and flowable composite resins and their development after initial photo curing. Dental Materials. 2006;22(5):450–459. doi: 10.1016/j.dental.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 102.Braga RR, Hilton TJ, Ferracane JL. Contraction stress of flowable composite materials and their efficacy as stress-relieving layers. Journal of the American Dental Association. 2003;134(6):721–728. doi: 10.14219/jada.archive.2003.0258. [DOI] [PubMed] [Google Scholar]
- 103.Choi KK, Condon JR, Ferracane JL. The effects of adhesive thickness on polymerization contraction stress of composite. Journal of Dental Research. 2000;79(3):812–817. doi: 10.1177/00220345000790030501. [DOI] [PubMed] [Google Scholar]
- 104.Kemp-Scholte CM, Davidson CL. Complete marginal seal of Class V resin composite restorations effected by increased flexibility. Journal of Dental Research. 1990;69(6):1240–1243. doi: 10.1177/00220345900690060301. [DOI] [PubMed] [Google Scholar]
- 105.Van Meerbeek B, Willems G, Celis JP, et al. Assessment by nano-indentation of the hardness and elasticity of the resin-dentin bonding area. Journal of Dental Research. 1993;72(10):1434–1442. doi: 10.1177/00220345930720101401. [DOI] [PubMed] [Google Scholar]
- 106.Unterbrink GL, Liebenberg WH. Flowable resin composites as “filled adhesives”: literature review and clinical recommendations. Quintessence International. 1999;30(4):249–257. [PubMed] [Google Scholar]
- 107.Cara RR, Fleming GJP, Palin WM, Walmsley AD, Burke FJT. Cuspal deflection and microleakage in premolar teeth restored with resin-based composites with and without an intermediary flowable layer. Journal of Dentistry. 2007;35(6):482–489. doi: 10.1016/j.jdent.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 108.Cadenaro M, Marchesi G, Antoniolli F, Davidson C, De Stefano Dorigo E, Breschi L. Flowability of composites is no guarantee for contraction stress reduction. Dental Materials. 2009;25(5):649–654. doi: 10.1016/j.dental.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 109.Pfeifer CSC, Braga RR, Ferracane JL. Pulse-delay curing: influence of initial irradiance and delay time on shrinkage stress and microhardness of restorative composites. Operative Dentistry. 2006;31(5):610–615. doi: 10.2341/05-118. [DOI] [PubMed] [Google Scholar]
- 110.Sakaguchi RL, Berge HX. Reduced light energy density decreases post-gel contraction while maintaining degree of conversion in composites. Journal of Dentistry. 1998;26(8):695–700. doi: 10.1016/s0300-5712(97)00048-1. [DOI] [PubMed] [Google Scholar]
- 111.Lim B-S, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dental Materials. 2002;18(6):436–444. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 112.Charton C, Colon P, Pla F. Shrinkage stress in light-cured composite resins: influence of material and photoactivation mode. Dental Materials. 2007;23(8):911–920. doi: 10.1016/j.dental.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 113.Lu H, Stansbury JW, Bowman CN. Towards the elucidation of shrinkage stress development and relaxation in dental composites. Dental Materials. 2004;20(10):979–986. doi: 10.1016/j.dental.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. Journal of Dental Research. 2005;84(9):822–826. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 115.Feng L, Suh BI. A mechanism on why slower polymerization of a dental composite produces lower contraction stress. Journal of Biomedical Materials Research Part B. 2006;78(1):63–69. doi: 10.1002/jbm.b.30453. [DOI] [PubMed] [Google Scholar]
- 116.Daronch M, Rueggeberg FA, De Goes MF. Monomer conversion of pre-heated composite. Journal of Dental Research. 2005;84(7):663–667. doi: 10.1177/154405910508400716. [DOI] [PubMed] [Google Scholar]
- 117.Bausch JR, de Lange C, Davidson CL. The influence of temperature on some physical properties of dental composites. Journal of Oral Rehabilitation. 1981;8(4):309–317. doi: 10.1111/j.1365-2842.1981.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 118.Guggenberger R, Weinmann W. Exploring beyond methacrylates. American Journal of Dentistry. 2000;13(5):82D–84D. [PubMed] [Google Scholar]
- 119.Eick JD, Kostoryz EL, Rozzi SM, et al. In vitro biocompatibility of oxirane/polyol dental composites with promising physical properties. Dental Materials. 2002;18(5):413–421. doi: 10.1016/s0109-5641(01)00071-9. [DOI] [PubMed] [Google Scholar]
- 120.Eick JD, Kotha SP, Chappelow CC, et al. Properties of silorane-based dental resins and composites containing a stress-reducing monomer. Dental Materials. 2007;23(8):1011–1017. doi: 10.1016/j.dental.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Condon JR, Ferracane JL. Reduction of composite contraction stress through non-bonded microfiller particles. Dental Materials. 1998;14(4):256–260. doi: 10.1016/s0109-5641(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 122.Ferracane JL, Ferracane LL, Braga RR. Effect of admixed high-density polyethylene (HDPE) spheres on contraction stress and properties of experimental composites. Journal of Biomedical Materials Research Part B. 2003;66(1):318–323. doi: 10.1002/jbm.b.10019. [DOI] [PubMed] [Google Scholar]