Abstract
The mechanical strength of the periodontal ligament (PDL) was first measured as force required to extract a tooth from its socket using human specimens. Thereafter, tooth-PDL-bone preparations have extensively been used for measurement of the mechanical response of the PDL. In vitro treatments of such specimens with specific enzymes allowed one to investigate into the roles of the structural components in the mechanical support of the PDL. The viscoelastic responses of the PDL may be examined by analysis of the stress-relaxation. Video polarised microscopy suggested that the collagen molecules and fibrils in the stretched fibre bundles progressively align along the deformation direction during the relaxation. The stress-relaxation process of the PDL can be well expressed by a function with three exponential decay terms. Analysis after in vitro digestion of the collagen fibres by collagenase revealed that the collagen fibre components may play an important role in the long-term relaxation component of the stress-relaxation process of the PDL. The dynamic measurements of the viscoelastic properties of the PDL have recently suggested that the PDL can absorb more energy in compression than in shear and tension. These viscoelastic mechanisms of the PDL tissue could reduce the risk of injury to the PDL.
1. Introduction
The periodontal ligament (PDL) is a fibrous connective tissue that firmly binds the tooth to its surrounding bony socket. Its principal function is the mechanical support of the tooth during mastication [1–4]. Previous review studies by Moxham and Berkovitz (1995) [1], Nishihira et al. (2003) [3], and Chiba (2004) [4] have described the mechanical strength and viscoelasticity of this tissue structure. The extracellular compartment of the PDL tissue consists mostly of collagen fibre bundles with just a few oxytalan fibres, the latter running at approximately right angles to the collagen fibre bundles. These fibres are embedded in ground substances together with cells, blood vessels, and nerves [2]. This review article describes the mechanical properties of the PDL with emphasis on the relations between its mechanical function and structural components. Our intention is to explore these relations to improve our understanding of the mechanisms behind the tooth support function of the PDL.
2. The Mechanical Strength of the PDL
2.1. Measurement of the Mechanical Strength of the PDL
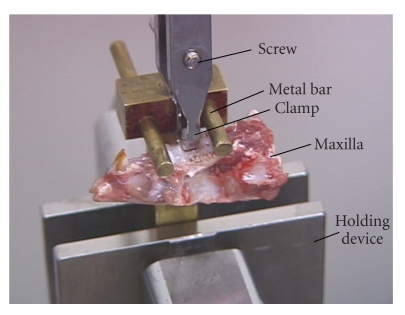
A pioneering study by Yamada's group was the first to measure the mechanical strength of the PDL [5]. They reported the force required to extract a tooth from its socket using specimens from human unembalmed cadavers (Table 1). Using a similar technique (Figure 1), Chiba and his colleagues measured the force required to extract teeth on the normal (Table 1) and experimentally altered PDL in experimental animals [6, 9]. Since these raw values showed a rather simple association with tooth size, standardised values for mechanical strengths (N/mm2) of the PDL per area facing the roots were then calculated [5] (Table 2). Thus, the mechanical strengths for human and mouse PDLs show similar values ranging between 1.2 and 1.7, and 1.8 and 3.0 N/mm2, respectively. Therefore, the application of standardisation principles eliminated this variation due to size differences among species.
Table 1.
Extraction forces of teeth in various species.
| Species | Teeth | Age | Extraction force (N) | Reference |
|---|---|---|---|---|
| Human | Incisors | 20–49 yr | 170–240 | [5] |
| Canines | 20–49 yr | 320–370 | [5] | |
| Premolars | 20–49 yr | 240–300 | [5] | |
| Molars | 20–49 yr | 300–360 | [5] | |
| Rat | Mandibular | |||
| First molar | 6 wk | 43–48 | [6] | |
| 3–32 wk | 16–46 | [7] | ||
| Second molar | 7–10 wk | 18–27 | [8] | |
| Third molar | 7–10 wk | 8–13 | [8] | |
| Incisor | 4–24 wk | 25–74 | [9] | |
| Maxillary | ||||
| First molar | 7–10 wk | 22–34 | [8] | |
| Second molar | 7–10 wk | 20–30 | [8] | |
| Third molar | 7–10 wk | 8–18 | [8] | |
| Mouse | Mandibular | |||
| First molar | 4–8 wk | 6–11 | Shibata et al. (unpublished) | |
| Hamster | Mandibular | |||
| First molar | 4–16 wk | 23–25 | [10] | |
Figure 1.
Picture of the device used to extract a whole tooth, which is similar to that reported by Yoshimatsu et al. (1956) [5] and modified after Chiba and Ohkawa [6]. The crown of the tooth is held by the clamp, which is connected to a load cell of a materials testing machine. The holding device is fixed to a crosshead of the machine, which moves downwards to measure extraction force.
Table 2.
Mechanical strengths of the periodontal ligament in various species and of other connective tissues and artificial materials.
| Species | Teeth | Age | Mechanical strength (N/mm2) | Mode of testing | Reference |
|---|---|---|---|---|---|
| Human | Incisors | 20–49 yr | 1.5–1.6 | extraction of tooth | [5] |
| Canines | 20–49 yr | 1.6–1.7 | extraction of tooth | [5] | |
| Premolars | 20–49 yr | 1.4 | extraction of tooth | [5] | |
| 50 yr | 2.6–3.9 | tension | [11] | ||
| 23–55 yr | 2.4–3.0 | extrusion | [12] | ||
| 78–79 yr | 1–3.5 | extrusion | [13] | ||
| Molars | 20–49 yr | 1.2 | extraction of tooth | [5] | |
| 13–37 yr | 0.3–3.0 | tension | [11] | ||
| 20–65 yr | 1.8–2.7 | intrusion | [14] | ||
| 20–65 yr | 2.1–2.6 | extrusion | [14] | ||
| Monkey | Maxillary | ||||
| Incisor | Young adult | 1.2–1.9 | extrusion | [15] | |
| Rat | Mandibular | ||||
| First molar | 5–6 wk | 1.3–1.6 | extrusion | [16] | |
| 2–24 month | 1.4–0.9 | extrusion | [17] | ||
| Incisor | 5–6 wk | 0.7 | extrusion | [18] | |
| 6 wk | 0.2–1.8 | intrusion | [19] | ||
| 2–24 month | 1–1.6 | extrusion | [20] | ||
| Mouse | Mandibular | ||||
| First molar | 4–8 wk | 1.9–3 | extraction of tooth | Shibata et al. (unpublished) | |
| Incisor | 10 wk | 0.5–2 | extrusion | [21] | |
| Hamster | Mandibular | ||||
| First molar | 8 wk | 3–7 | extrusion | [22] | |
| Incisor | 10 wk | 0.4–2.8 | extrusion | [21] | |
| Rabbit | Mandibular | ||||
| Incisor | 17 wk | 0.5–1.4 | extrusion | [21] | |
| 17 wk | 3.2 | tension | [23] | ||
| Pig | Mandibular | ||||
| Molars | adult | 3.8 | tension | [24] | |
| Bovine | Mandibular | ||||
| Molars | 1–2.5 | tension | [25] | ||
| Third Molar | 3–5 yr | 3.2–4.3 | tension | [26] | |
| First molar | 3–5 yr | 6 | tension | [27] | |
| Other connective tissues | |||||
| Rat skin | 6–12 | tension | [28] | ||
| Rat tail tendon | 10–50 | tension | [28] | ||
| Elastin | 4–6 | tension | [29] | ||
| Bone | 150 | tension | [29] | ||
| Dentine | 40 | tension | [29] | ||
| Silk | 350 | tension | [29] | ||
| Artificial materials | |||||
| Glass fibre | 300–1000 | tension | [30] | ||
| Titanium alloys | 700–1400 | tension | [30] | ||
| Steel | 3000 | tension | [30] | ||
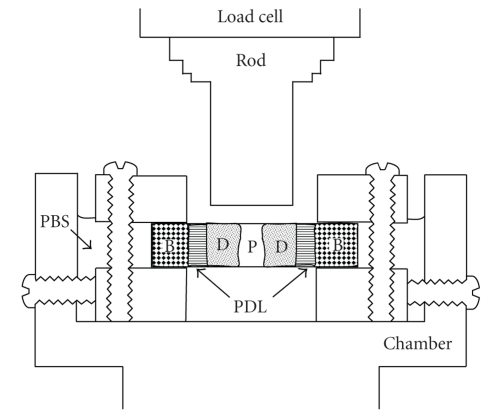
However, it was pointed out [11, 14] that tooth extraction procedure may introduce an artifact, since the fibres would tend to rupture unevenly due to the root curvature and the different vertical alignment of the fibres: the apical fibres in the vertical direction would rupture first, followed by the horizontal fibres. Further, it is difficult to measure the surface area of the PDL facing the tooth roots. To control these artifacts, Atkinson and Ralph (1977) used a tooth-PDL-bone complex for tensional loading of the PDL [11]. Further, Ralph (1982) developed a preparation of a transverse section of a tooth with its surrounding PDL and alveolar bone and a technique for loading the PDL in shear [14]. Mandel et al. (1986) have devised an elaborate technique for shear loading of the human PDL using a similar specimen [12]. Figure 2 presents a diagram showing an apparatus similar to their device. Thus, the artifact due to root curvature can be eliminated and it is now possible to obtain a standardised stress-strain curve of the PDL in shear [12–22] or tension [11, 23–25], allowing for comparisons between different species and between different sizes of teeth [28].
Figure 2.
Diagram of the apparatus redrawn for clarity, which has been originally developed by Ralph (1982) [14] and has been modified by Mandel et al. (1986) [12]. A transverse section of a tooth root fastened between two metal plates is immersed in phosphate-buffered saline (PBS). The plates are fixed by screws within a chamber, which is mounted in a materials testing machine. As the chamber moves upwards at a constant speed, the periodontal ligament (PDL) is loaded by metal rod connected to a load cell of the machine. B: bone; D: dentine; P: dental pulp.
Table 2 shows the mechanical strengths of normal PDLs from human and experimental animals. The mechanical strengths of the PDL are of the same order in different sizes of teeth of different species. When compared to other connective tissues [28–30], the mechanical strength of the PDL was found to be most similar to that of skin, which comprises a three-dimensional mesh of collagen fibres. Interestingly, the mechanical strength of the rat tail tendon, which is composed of parallel-fibred collagen, is about 10 times those of the PDL. As cortical bone and tooth dentine containing large amounts of collagens are mineralised, their mechanical strengths are 10- to 40-fold greater than that of the PDL. Artificial materials such as glass fibre and steel show strengths 200- to 600-fold greater [30] than those of the PDL.
Mechanical strength is measured as the load needed to fracture a specimen, which causes breaks in the covalent linkages of the collagen molecular chains and slippages between the unit molecular chains [30]. From the functional point of view, the ratio of the breaking stress to the stress experienced during function may be used to determine the safety margin (safety factor) of each tissue, as pointed out by Alexander (1981) [31]. Engineers design structural elements to have safety margins between 5 and 10 [32, 33]. Komatsu et al. (1998) noted that the safety margin for the PDL ranges between 2 and 5 [21]. Tendons and bones have similar safety margins between 1 and 6 [29, 31]. Biological tissues or structures with this range of safety margins are considered strong enough to successfully fulfill their support function specific to their locations.
2.2. Mechanical Strength in Relation to Structure
In order to elucidate the tooth support mechanisms, Chiba's group systematically investigated the effects, on the mechanical properties of the PDL, of various experimental factors [4] such as periodontal disease [10, 22], hypofunction [18, 35, 36], orthodontic forces [37–39], treatment with inhibitors (lathyrogens) of collagen cross-linking and an inhibitor (hydrocortisone) of collagen degradation or turnover [8, 40–43], age [7, 17, 20, 44, 45], and root levels [19, 21]. They found that the mechanical properties were sensitive to both experimental alterations and to differences in the content, direction, and organisation of the collagen fibre components of the PDL.
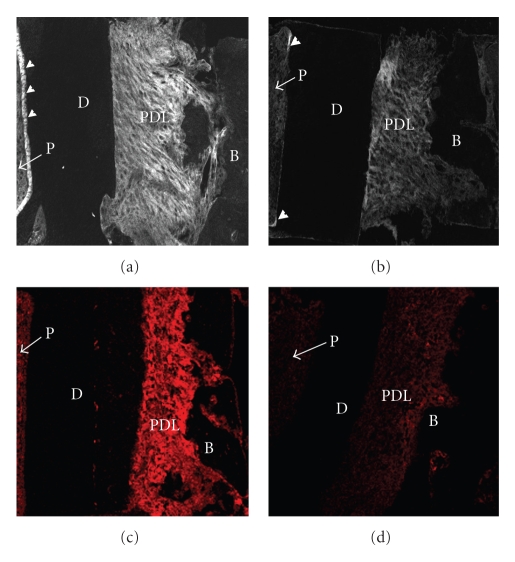
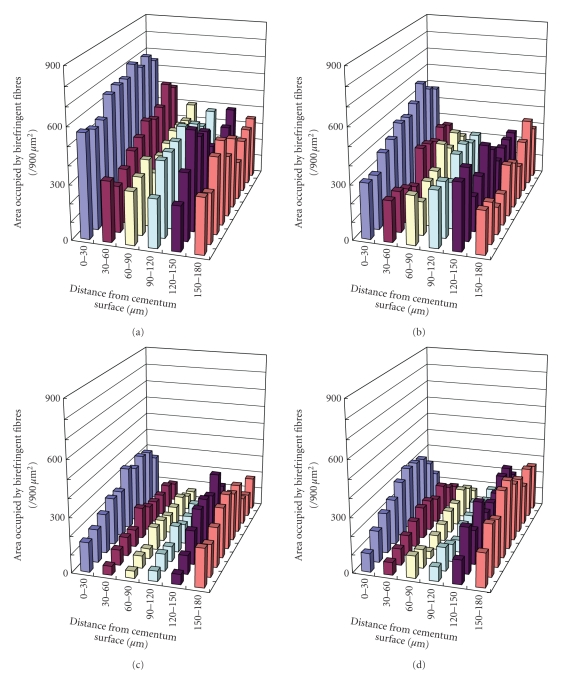
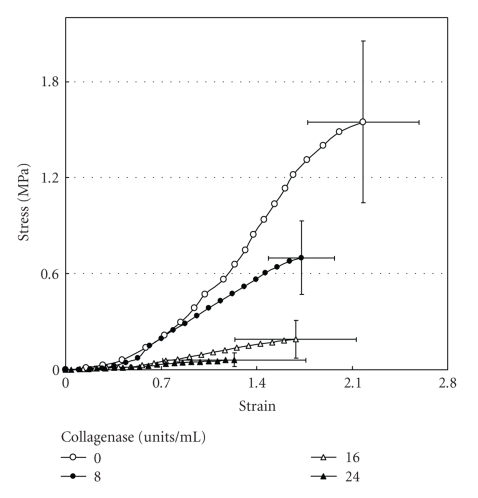
More specific investigations were done by in vitro treatment of mechanical specimens with specific enzymes [46]. The enzyme Clostridium histolyticum collagenase cleaves the peptide bonds in collagen with specificity for the Pro-X-Gly-Pro-Y region and particularly between X and Gly [47]. Immunofluorescent analysis reveals that this enzyme digests types I and III collagens (Figure 3), which are the dominant types of collagen in the PDL [2]. Kawada and Komatsu (2000) have investigated the role of collagen fibres in the mechanical support of the PDL using collagenase [34]. In vitro treatment of PDL specimens with collagenase caused concentration-dependent removals of collagens (Figures 4 and 5) and reductions in mechanical strength of the PDL (Figure 6) [34]. Image analyses were done after collagenase treatment of specimens: the area occupied by birefringent fibres within each square (30 × 30 μm) on each section (Figure 4) was analysed. In control specimens treated with PBS (Figure 5(a)), large regional differences in the area occupied by birefringent fibres were seen across the PDL: birefringent collagen fibres occupy 61%–78% of the squares in the PDL near the cementum surface. Because the region of the PDL near the bone is highly vascular and innervated, the birefringent fibre bundles occupy only 33%–48% of the area. Figures 5(b)–5(d) shows concentration-dependent digestions in the collagen fibres in the PDL specimens. The total areas occupied by birefringent collagen fibres in the regions 0–120 μm from the cementum surface are smaller at the higher concentrations of collagenase. However, since the regions 120–180 μm distant contain blood vessels, nerve fibres, and bones, the reductions are relatively small. Treatments with 8, 16, and 24 units of collagenase reduced the fibre areas by 20%–36%, 50%–75%, and 39–76%, respectively. From the analysis of the shear stress-strain curves (Figure 6), treatments with 8, 16, and 24 units of collagenase were found to reduce the mechanical strengths by 55%, 88%, and 96%, respectively (Figure 6). These data show that the periodontal collagens are load-bearing, key structural components that provide structural and functional integrity to the ligament.
Figure 3.
Immunostaining of sections from PDL specimens treated with PBS (a), (c) or collagenase (24 units/mL) (b), (d) using an antibody against type I collagen (a), (b) or type III collagen (c), (d). Arrowheads indicate predentine. P: dental pulp.
Figure 4.
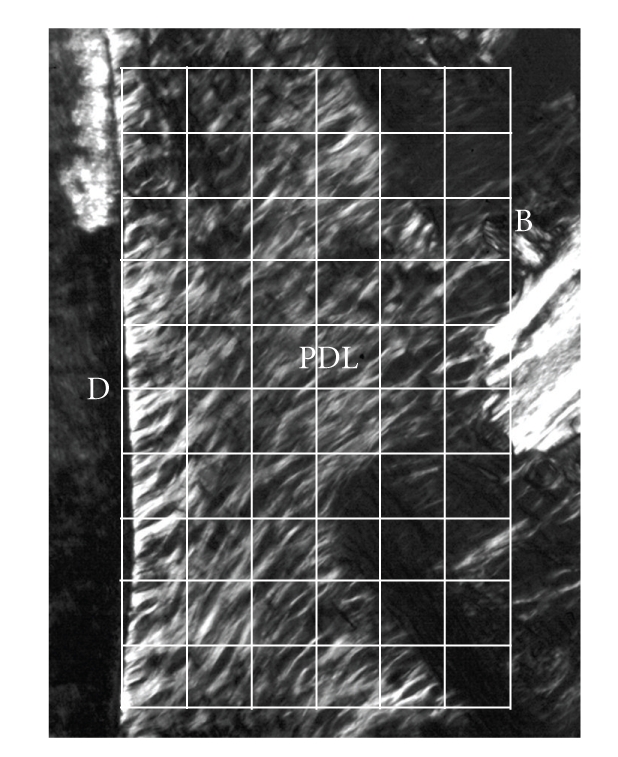
Polarised light micrograph of the periodontal ligament (PDL) on the buccal side in an unstained bucco-lingal section of a mechanical specimen. Image analysis was done for each grid (30 × 30 μm), adapted from Kawada and Komatsu [34].
Figure 5.
Changes in the collagen fibres of PDL specimens after collagenase treatment, as represented by the area occupied by birefringent fibres in each grid (Figure 4). Data were analysed for specimens treated with PBS (a), 8 units/mL collagenase (b), 16 units/mL collagenase (c), or 24 units/mL collagenase (d). Each column represents the mean of six specimens.
Figure 6.
Stress-strain curves of the periodontal ligament obtained from transverse sections of the mesial root of rat mandibular first molars. Sections were incubated in 0, 8, 16, or 24 units of collagnase/mL of PBS at 37°C for 4 hours. Vertical and horizontal bars represent ±1 SD of 6 animals, reproduced from Kawada and Komatsu (2000) [34], by permission.
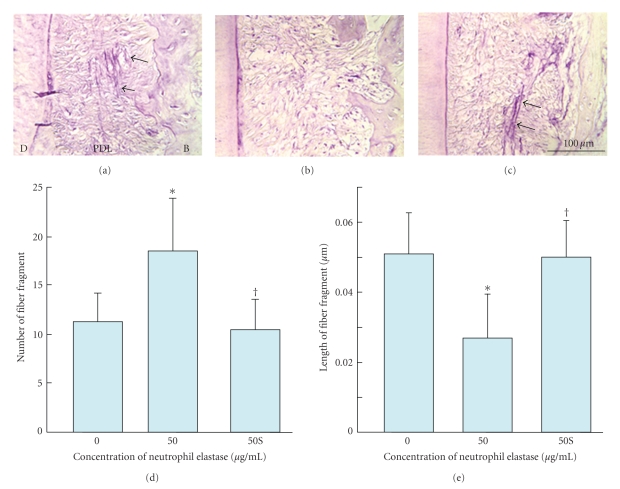
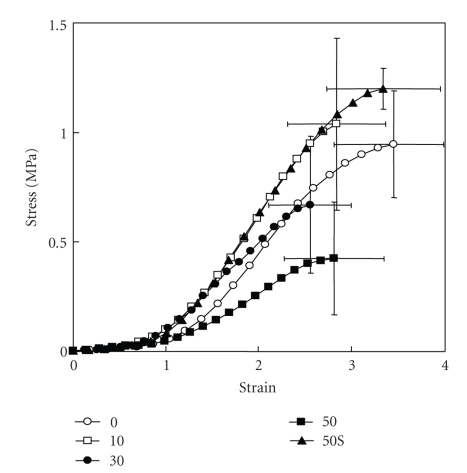
Ujiie et al. (2007) have also recently investigated the effect of treatment of PDL specimens with elastase on the mechanical strength [48]. They found that in vitro treatment with elastase increased fragmentation of the oxytalan fibres (Figure 7) and reduced the mechanical strength of the PDL specimen (Figure 8) in a concentration-dependent manner. The addition of an elastase inhibitor effectively antagonised these effects. This study suggests that the oxytalan fibres may play a role in the mechanical support of the PDL.
Figure 7.
Fragmentation of oxytalan fibres in periodontal ligament (pdl) by neutrophil elastase. (a)–(c) Sagittal sections of the distal side of the distal root of rat mandibular first molars. Histological sections were stained with aldehyde fuchsin-staining. (d) Number of fibre fragments in sections. (e) Length of fibre fragment in sections. Specimens were treated with 0 (A), 50 (B) μg/mL of neutrophil elastase (50), or 50 μg/mL of neutrophil elastase +5 mM sivelestat (elastase inhibitor) (c) (50S). Each column and bar represent the mean + 1 SD of six animals. d: dentine; b: bone. *P < .05 compared with the control; †P < .05 compared with the elastase-treated specimens, reproduced from Ujiie et al. (2008) [48], by permission.
Figure 8.
Stress-strain curves of the periodontal ligament obtained from transverse sections of the mesial root of rat mandibular first molars. Sections were incubated in 0, 10, 30, or 50 mg/mL of neutrophil elastase or with 50 μg/mL of neutrophil elastase +5 mM sivelestat (50S). Vertical and horizontal bars represent ±1 SD of 6 animals, reproduced from Ujiie et al. (2008) [48], by permission.
It has been reported that the interfibrillar ground substances occupies 65% (volume ratio) of the collagen fibre bundles or sheets in the rat PDL [2]. Further studies have investigated the possible roles of interfibrillar ground substances in the mechanical support of the PDL. After an in vitro treatment with α-amylase, scanning electron microscopy (SEM) and histochemistry observed removal of interfibrillar substances and remnant, exposed collagen fibre bundles [49]. The treated PDL specimens had concentration-dependent reduced mechanical strengths. However, as the authors did not quantify the fibre components in the specimens after the treatment, it is difficult to determine the relative contribution of interfibrillar substances to changes in strength. Other studies have used EDTA to remove the interfibrillar substances, and this has been confirmed in PDL specimens by immunohistochemistry with an antibody against chondroitin 6-sulfate and an antibody against chondroitin 4- and dermatan sulfate which are major proteoglycans of the interfibrillar substances in the PDL [50, 51], as well as via SEM. These studies showed that concentration-dependent decreases in mechanical strength. Recent immunohistochemical and ultrastructural studies have shown that proteoglycan filaments run orthogonal and parallel to the collagen fibrils in human periodontal ligament and gingiva [2]. It is assumed that the treatment with α-amylase or EDTA reduced the mechanical strength of the PDL not only by removing the interfibrillar substances but also by reducing the interactions between collagen fibrils and proteoglycans.
3. The Viscoelastic Response of the PDL
From the functional point of view, physiological stress levels are below the mechanical strength of the tissues and physiological responses exhibit time-dependence. Such functional mechanical responses may intimately be related to the viscoelastic time-dependent responses. The viscoelastic responses of the PDL have been examined by hysterysis, creep, and stress-relaxation tests. Kurashima (1963) [52] studied the viscoelastic behaviour of the PDL by measuring stress-relaxation responses in the human PDL and its applicability to the Maxwell model. Bien (1965) described the relaxation of rat teeth using a Maxwell model [53]. Wiils and Picton (1972, 1978) used a Voigt model to describe the creep behaviour of the PDL [54, 55]. Middleton et al. (1990) used a linear viscoelastic model to simulate the behaviour of the PDL [56]. Provatidis (2000) described a viscoelastic behaviour using a finite element technique [57].
The studies by Moxham and Berkovitz are not only a description of the creep behaviour of the PDL of the rabbit incisor (1981) [58] but also an investigation into a role of collagen crosslinks in the creep behaviour (1984) [59]. By administration of an inhibitor of collagen cross-linking (lathyrogens), reductions of collagen cross-linking in collagen fibres occur. As expected, the instantaneous and gradual mobilities of the tooth were greater in the lathyritic than in the pair-fed animals, indicating a role of cross links of the collagen fibres in the viscoelastic response of the PDL.
The viscoelastic response of the PDL has also been demonstrated by varying the velocity of loading, in studies by Chiba and Komatsu (1993), Komatsu and Chiba (1993), and Komatsu and Chiba (1996) [16, 60, 61]. These experiments investigated the shear stress-strain properties of the PDLs in the rat incisor and molar teeth at a wide range of loading velocities using a custom-made testing apparatus. The slowest velocity (1 mm/24 h) imitates the eruption rate of the rat mandibular incisor; the fastest one was 104 mm/24 h, equivalent to 7 mm/min. They found that the linear parts of the stress-strain curves became steeper and the shear mechanical strength increased with increasing velocity, demonstrating typical characteristics of viscoelastic tissues. The mechanisms responsible for decreased mechanical stress at slower strain rates have yet to be determined but may involve the increased time available for stress-relaxation of the tissues at lower velocities [60].
Several methods of measurement and analysis of the stress-relaxation response of the PDL have recently been reported [13, 17, 20, 26, 62–66]. The distinctive property of the stress-relaxation of the PDL is that the amount of stress-relaxation during 10 minutes reaches about 50% of the initially applied stress [17], which is greater than those of other soft connective tissues. These phenomena indicate that the PDL tissue dissipates the strain energy stored in the tissue, during excessive force application, through viscoelastic responses [26, 27, 62]. This mechanism could reduce the risk of injury to the PDL in the case of prolonged static deformation. This sort of viscoelastic response may play an important physiological role in the tooth support mechanisms of the PDL.
4. Analysis of Stress-Relaxation Response of the PDL
In this chapter, I will introduce a typical method for measurement and analysis of stress-relaxation response of the PDL as described by Sanctuary, Komatsu, and Botsis (2003, 2007) [62, 63].
4.1. Measurement of Stress-Relaxation Responses
4.1.1. Preparation of Specimens
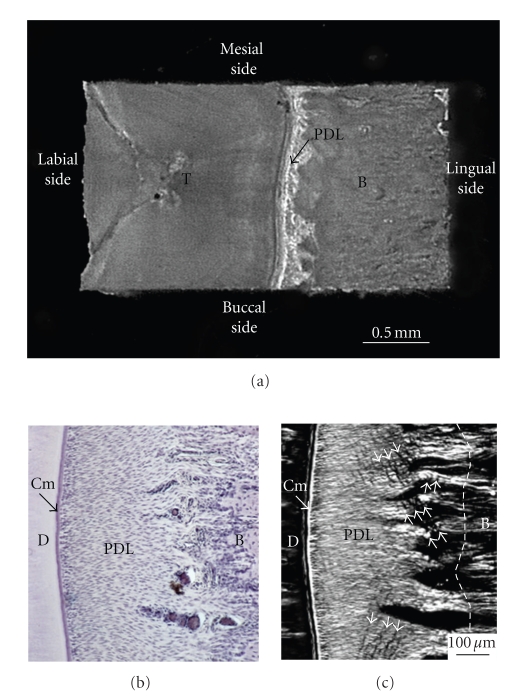
From a rabbit hemimandible, a transverse section (about 0.25 mm in thickness) of the middle region of the incisor tooth with its surrounding PDL and bone is cut. Then, the original width (Wi) of the PDL can be measured with a stereomicroscope as the distance (about 0.45 mm) between the bone and cementum surfaces at the central region on the lingual side of the tooth. Both of the lateral parts of each section are trimmed, and then a final specimen is obtained. The final rectangular specimen consists of tooth, PDL, and bone (Figures 9(a) and 9(b)). The direction of most of the principal collagen fibre bundles in the PDL of the specimen is almost parallel to the direction of testing, as evident under polarised light microscopy (Figure 9(c)). The sectional area (SA, mm2) of the PDL can be calculated as the breadth (mm) × thickness (mm) of the specimen.
Figure 9.
Preparation of mechanical specimen for uniaxial loading. (a) Mechanical specimen of bone-PDL-tooth complex obtained from a transverse section of the rabbit mandibular incisor. (b) Histological presentation of periodontal ligament. Sections were cut in parallel to the sectional surface of mechanical specimens (Figure 9(a)). Toluidine blue staining. (c) Polarised light microscopic image of the same section. White arrowheads indicate crimps of the collagen fibre bundles. D: dentine; Cm: cementum; PDL: periodontal ligament; B: bone.
4.1.2. Experimental Setup
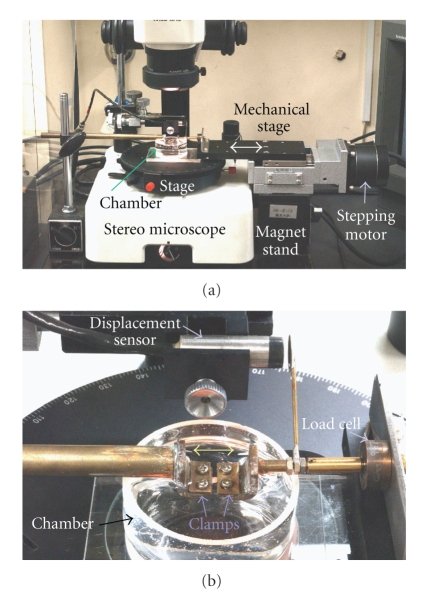
Stress-relaxation tests require precise and quick movements of the actuator and preservation of the initially applied deformations during the relaxation process [67]. A novel uniaxial testing machine was designed by Komatsu and Chiba (2001) [23] (Figure 10). For stress-relaxation tests, this machine was improved; the improvements [62] include, (1) a stepping motor with greater torque, (2) uniaxial movement of the actuator by more precise regulation via a PC-controlled stepping motor, (3) a new load cell with negligible compliance, and (4) improved slippage control of the specimen between clamps during repeated mechanical tests via the use of waterproof sandpaper affixed to the clamps [68]. By using the improved testing machine, initial deformations were able to remain nearly constant during relaxation tests, with mean values for the deformations of 57–56, 78–77, and 99–97 μm over a 300-second period. These changes are negligible compared with the overall deformation levels, indicating a satisfactory regulation during their stress-relaxation tests.
Figure 10.
(a) Experimental setup: horizontal testing apparatus built on a stereomicroscope. (b) A magnified picture of specimen clamps and chamber, adapted from Komatsu and Chiba (2001) [23].
4.1.3. Determination of the Nondestructive Range of Deformation
A standard (mean) load-deformation curve is computed from several specimens to estimate the nondestructive range of deformation for the PDL specimens for stress-relaxation tests. It has been shown that non-destructive range of deformation does not exceed the middle portion of the linear segment of the stress-strain curve for a PDL specimen [68, 69]. Therefore, the functional and non-destructive range of deformation may be determined to be below 100 μm from the mean load-deformation curve [62].
4.1.4. Preconditioning
To ensure the reproducibility of results as pointed out previously [28, 62, 63, 70], each specimen is subjected to three cycles of tensile and compressive loadings between +35 and −35 μm before stress-relaxation tests.
4.1.5. Stress-Relaxation Test
The specimen is then stretched to a deformation level of 35 μm at the fastest speed (63 mm/min) of the testing machine, the movement of the actuator is abruptly stopped, and the deformation is kept constant for 300 seconds to obtain a load-relaxation curve. The specimen is then returned to its original width at the same speed. After a 300-second recovery period, the stress-relaxation tests are repeated sequentially at three different deformation levels (55, 75, and 95 μm) with a recovery period of 300 seconds between each test.
The load-relaxation response after the initial deformation is characterised by a sharp decrease in load, followed by a gradual decrease. Greater load levels are observed at greater deformation levels.
4.1.6. Failure Test
Finally, the specimen is first loaded in compression and then in tension at 1 mm/min until rupture of the PDL. The mechanical homogeneity of the specimen can be assessed by comparing its load-deformation curve with the standard load-deformation curve.
4.1.7. Data Analysis
Load F(t) and deformation D(t) at time (t) are transformed into stress σ(t) and strain ε(t), respectively, using the formulae, F(t)/SA = σ(t), and D(t)/Wi = ε(t) [23].
The relaxation process of a biological specimen may be represented as a relaxation modulus G(t):
| (1) |
G(t) is then normalised with respect to the initial value G(0) to give a normalised relaxation modulus Gr(t) as
| (2) |
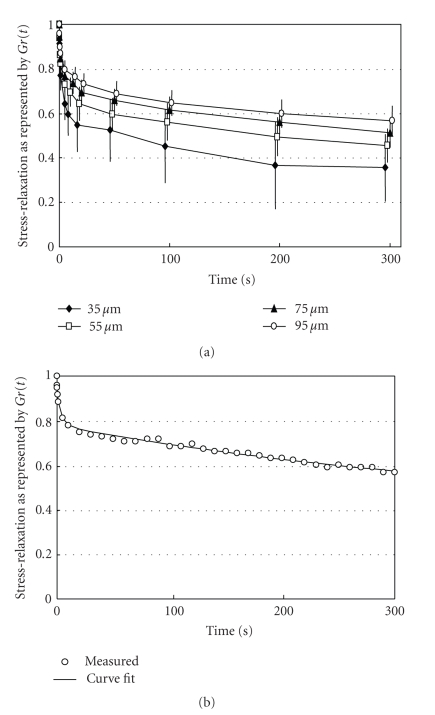
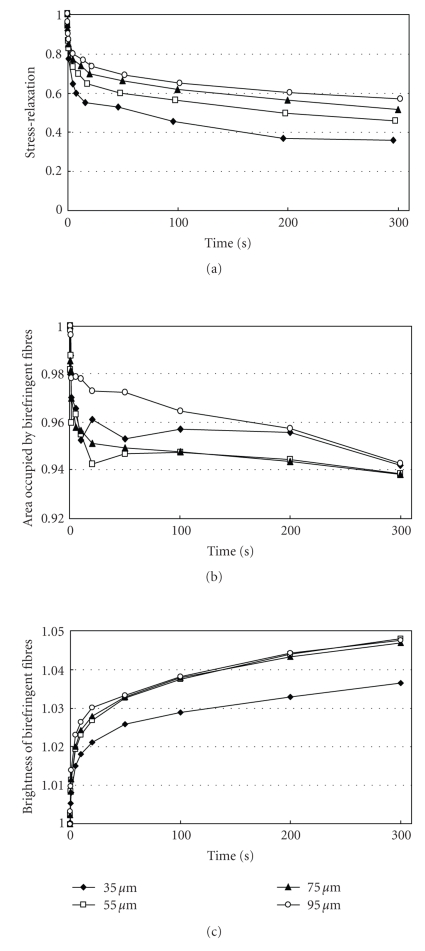
Figure 11(a) shows mean stress-relaxation responses of the PDL at different deformation levels as represented by Gr(t). In this instance, notice that the values for Gr(t) initially decreased rapidly and then did so gradually thereafter. The amounts of the stress-relaxations during 300 seconds are less at greater deformations (ANOVA, P < .001): the mean relaxations in this series were 63%, 53%, 48%, and 44% at 35, 55, 75, and 95 μm, respectively. As the normalised relaxation curves Gr(t) at different strains do not coincide, the behaviour of the PDL can be considered as nonlinear [63, 71]. The nonlinearity of responses implies that several mechanisms are involved in tooth support [53, 62, 63, 71].
Figure 11.
(a) Stress-relaxation as represented by Gr(t) for rabbit periodontal ligament. Each point and bars represent mean ± 1 SD. (b) Typical example of exponential curve fitting. Measured values (open circles) are well expressed by an equation: Gr(t) = 0.09exp (−t/ 0.35) + 0.13exp (−t/ 4.12) + 0.39exp (−t/ 403) + 0.39, reproduced from Komatsu et al. (2007) [62], by permission.
A generalised model for viscoelastic materials can be considered in which several Maxwell elements are assembled in parallel [71]. The relaxation process may be expressed as
| (3) |
where Ai and τi are the ratio and relaxation time (s) of each exponential function, respectively.
The stress-relaxation process of the PDL as represented by Gr(t) is well described by a function with three exponential decay terms and a constant (Figure 11(b)) as
| (4) |
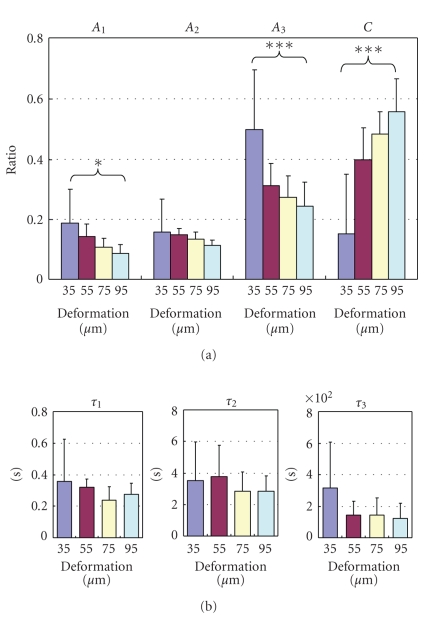
where τ1 < τ2 < τ3, and C is constant, as in the PDLs of rats [17, 20, 26, 62], humans [13], and cows [63]. As τ1, τ2, and τ3 are 0.2–0.4 seconds, 2–4 seconds, and 100–400 seconds, respectively, the stress-relaxation process may consist of short-, medium-, and long-term relaxation components. A1 (ANOVA, P < .02), A2 (ANOVA, P > .05), and A3 (ANOVA, P < .001) are less at greater deformations (Figure 12(a)). In contrast, C is greater at greater deformations (ANOVA, P < .001; Figure 12(a)). The three relaxation times show decreasing tendencies at greater deformations (Figure 12(b)).
Figure 12.
Parameters of stress-relaxation. (a) Ratios (Ai) and (b) relaxation times (τi) of the three exponential decay functions (Figure 11(b)) at the different deformations. Each column and vertical bar represent mean + 1 SD. *P < .05 (ANOVA); ***P < .001 (ANOVA), adapted from Komatsu et al. [62].
The lower stress-relaxation values during 300 seconds at greater deformations (Figure 11(a)) could be attributable to decreases in the ratios of the short- and long-term relaxation components (Figure 12(a)) and, to a lesser extent, to the changes in the relaxation times (Figure 12(b)). In other words, the viscous components may contribute relatively less than do the elastic components at greater deformations. It has been suggested that the decrease in the relaxation rate with increasing strain could reflect larger strains causing greater water loss, which causes the tissues to be more elastic (less viscous) than tissues subjected to lower strains [72].
4.2. Stress-Relaxation Response in Relation to Structure
Although the viscoelastic response of any biological tissue is likely related to its structure, this relation has not been adequately investigated in the PDL [20].
4.2.1. Video-Microscopic Recording of the PDL Structure During Stress-Relaxation Test
Komatsu and Chiba [23] have shown by simultaneous recordings of the mechanical responses coupled with polarised-light video-microscopy that the area and brightness of the birefringent collagen fibre bundles in the PDL specimens are intimately related to its stress-strain behaviour. Quantitative polarised-light microscopy has shown to be useful for investigating the macromolecular orientation and organisation of collagen fibres in living connective tissues without the need for staining [73, 74].
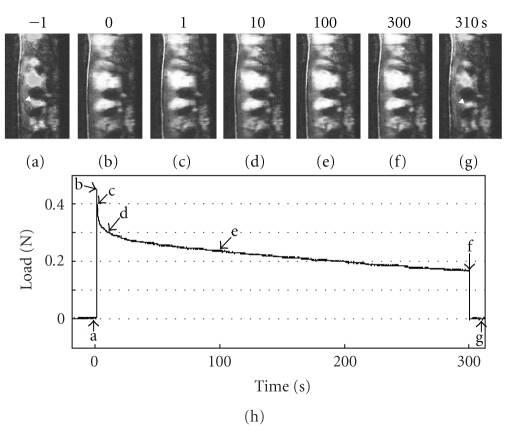
Komatsu, Sanctuary, and Botsis (2007a) [62] further applied the simultaneous recording system to observe the structural basis for the mechanisms of viscoelastic response of the PDL. Before deformation application (Figure 13(a)), most of the birefringent PDL collagen fibre bundles run parallel to the deformation direction. Immediately after applying of a deformation of 95 μm (Figure 13(b)), the birefringent fibres are stretched towards the direction of uniaxial testing. During the load-relaxation for 300 seconds (Figures 13(b)–13(f), the stretched bundles show indistinct changes in their arrangement, although there are gradual increases in the brightness of the birefringent fibres during the relaxation. After the specimen is returned to its original width, it takes several seconds for the PDL fibres to recover their original waviness and birefringence (Figure 13(g)).
Figure 13.
Simultaneous recording of video microscopic images and load values during stress-relaxation under a constant deformation of 75 μm. The video images (a)–(g) correspond to each point (a)–(g) on the load-relaxation curve (h).
During stress-relaxation under each deformation, the area occupied by the birefringent fibres initially decreases rapidly and then gradually (Figure 14). In this series, the amount of the decrease under 95 μm of deformation was less than those under other deformations. In contrast, during stress-relaxation under each deformation, the brightness of the birefringent fibres initially increases rapidly and then only gradually thereafter (Figure 14). In this series, the mean values of the brightness at 55, 75, and 95 μm were greater than those at 35 μm. This image analysis reveals the increasing tendency of the brightness of the birefringent stretched collagen fibres of the PDL during the stress-relaxation: the brightness correlates with progress of stress-relaxation under the four different deformations. The increased brightness may reflect that the collagen molecules and fibrils within the stretched collagen fibre bundles progressively align along the direction of the initial strain application during the relaxation [62]. Such internal rearrangement of collagen could be driven by the strain energy imparted to the specimen on initial stretching, which gradually diminishes as the stress relaxes, as suggested by X-ray diffraction studies [75, 76].
Figure 14.
Changes in relaxation modulus Gr(t), area, and brightness of the birefringent fibres in the periodontal ligament under four different deformations. Each point represents the mean of six specimens, adapted from Komatsu et al. [62].
4.2.2. Stress-Relaxation Responses after Structural Modifications
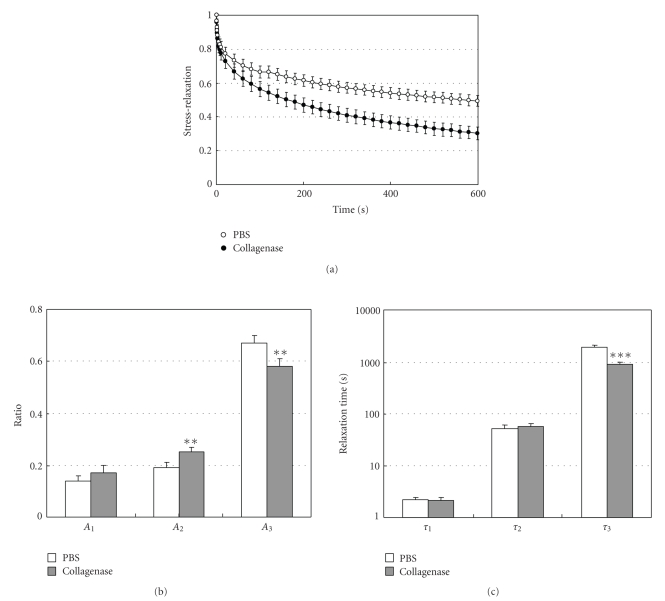
Komatsu et al. (2007b) investigated into the effect of in vitro digestion of collagen with collagenase on the stress-relaxation process of PDL specimens [77]. The collagenase (8 units/mL)-treated specimens relaxed more rapidly than the control specimens over a 600-second period (Figure 15(a)). The amount of relaxation of the collagenase group was 37% greater than that of the PBS group (P < .001). The stress-relaxation processes, as represented by Gr(t), are well described by a function with three exponential decay terms. The ratio (A3) and relaxation time (τ3) of the third exponential term for the collagenase group (Figures 15(b) and 15(c)) were significantly less than those for the PBS group. These results show that the partial removal (20%–36%, see Section 2) of the collagen fibres from the PDL specimens mainly causes decreases of the ratio and relaxation time for the long-term relaxation component. The decrease in the ratio may correspond to the decrease in the quantity of the collagen fibres, while the decrease in the relaxation time may be related to the decreased organisation of the collagen fibres. Therefore, it is likely that the viscoelastic properties of the collagen fibres may play an important role in the long-term relaxation component (τ = 1900 seconds) of the stress-relaxation process in the PDL. In the KWW relaxation component (τ = 1820 seconds) of bone collagen prepared by demineralization of bone, the viscoelastic properties of the collagen fibres are also likely to play a role [78].
Figure 15.
(a) Stress-relaxation curves as represented by Gr(t) for specimens treated with PBS or 8 units/mL collagenase, reproduced from Komatsu et al. [77], by permission. Measured values Gr(t) are well expressed by an equation: Gr(t) = A1exp (−t/τ1) + A2exp (−t/τ2) + A3exp (−t/τ3). (b) Ratios (Ai) and (c) relaxation times (τi) of the three exponential decay functions. Each column and vertical bar represent the mean + 1 SD. **P < .01 and ***P < .001 compared with the PBS group.
5. Dynamic Measurements of Viscoelastic Responses of the PDL
The viscoelastic responses of the PDL such as stress-relaxation to a load and creep to a strain, as mentioned in the previous chapters so far, are static properties. In contrast, the mechanical responses to oscillations are dynamic properties. With dynamic tests, the frequency dependent viscoelastic responses can be examined.
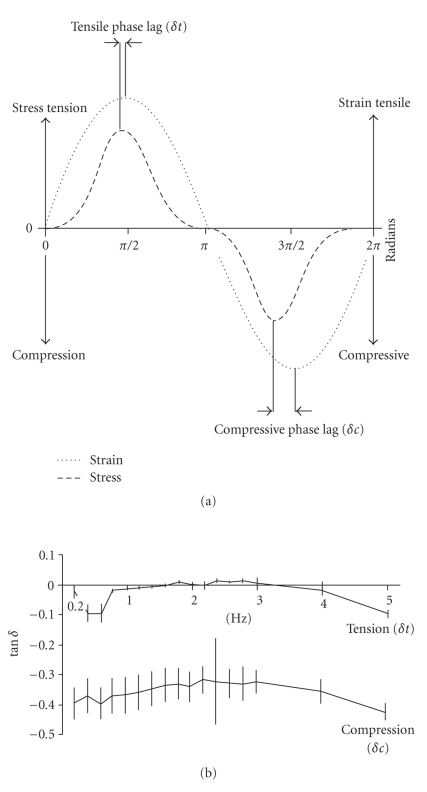
Sanctuary et al. (2005) have subjected bovine PDL specimens (preparations similar to those in Figure 9) to tension-compression harmonic oscillations under physiological conditions, that is, at strain amplitudes between −0.3 and +0.5 with frequencies between 0.2 and 5 Hz (Figure 16(a)) [26, 63]. The difference between the peak strain and the peak resulting stress is called phase lag and is expressed as an angle (δ). For viscoelastic systems, 0 < δ < 90°. The tangent δ is proportional to the ratio between the energy that is dissipated (loss modulus G′′) and the energy that is stored (storage modulus G′) in the system. The phase lag (tan δ) was found to be greater in compression than in tension, but independent of this range of frequency (Figure 16(b)) in line with other biological tissues. These results suggest that the dissipated energy was higher in compression than in tension in the PDL tissue.
Figure 16.
(a) Phase lag (δ) is the angular difference between peak strains and peak stresses. Values for tensile and compressive oscillations may be determined. (b) Phase lag δ, plotted as tan δ, for the tensile (δt) and compressive (δc) half cycle obtained from bovine PDL specimens. Vertical bars indicate ±1 SD, reproduced from Sanctuary et al. [26], by permission.
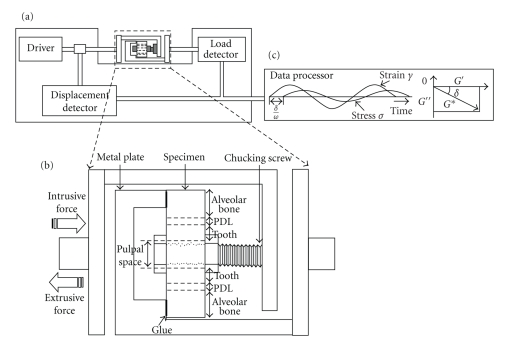
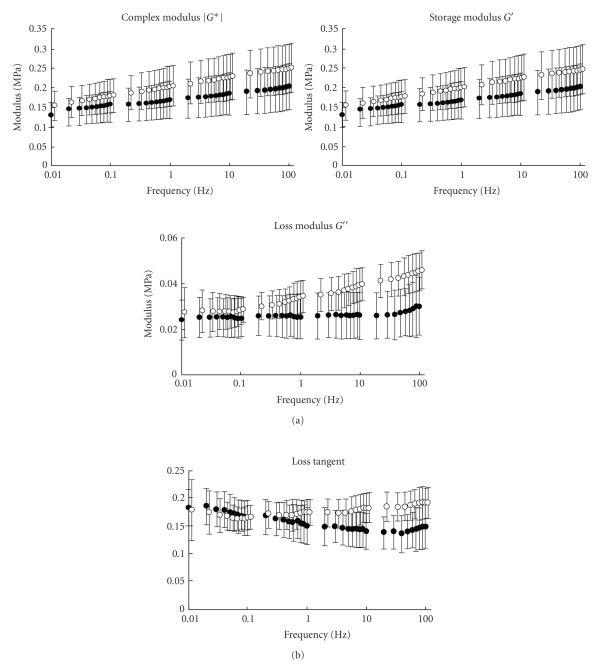
Tanaka et al. (2006) [79] have measured dynamic shear properties of the porcine incisor and molar PDLs at an oscillation amplitude (Figure 17) of Δγ = 1% with a wide range of frequencies (0.01–100 Hz). They found that storage modulus G′ increased with frequency for both PDLs while loss modulus G′′ did so only for the incisor PDL (Figure 18(a)). Tan δ ( = G′′/G′) ranged between 0.1 and 0.2, which was greater in the incisor than in the molar PDL with frequencies between 1 and 100 Hz (Figure 18(b)). The incisor PDL may be considered to have better shock-absorbing characteristics for sudden loading [79].
Figure 17.
(a) Diagram of the dynamic viscoelastometer. (b) Chucking device with a transverse section of a tooth root. (c) A schematic record of dynamic stress and strain during a sinusoidal oscillating strain, reproduced from Tanaka et al. [79], by permission.
Figure 18.
Dynamic shear properties of porcine periodontal ligament. (a) Complex modulus G*, storage modulus G′, and loss modulus G′′ as a function of frequency. (b) Tangent δ ( = G′′/G′) as a function of frequency. Data were obtained from incisor (white circle) and molar (black circle) periodontal ligaments. Vertical bars indicate 1 SD, reproduced from Tanaka et al. [79], by permission.
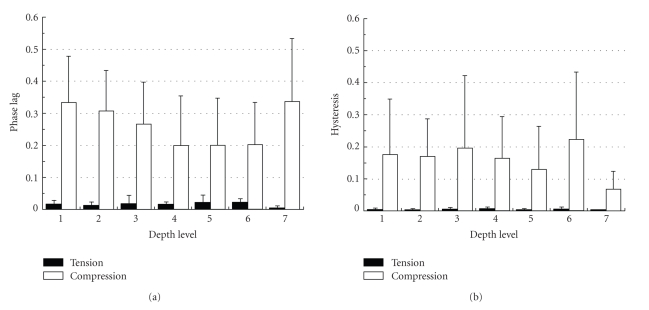
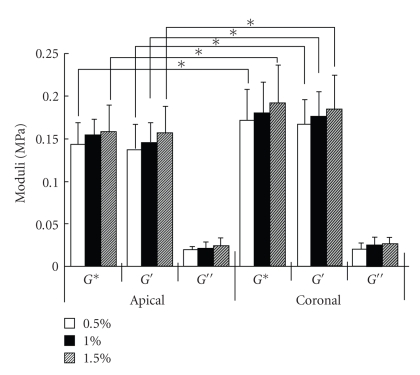
Shibata et al. (2006) [27] have investigated the mechanical response of the bovine PDL at ±35% of the PDL width with a frequency of 1 Hz at different root levels and different locations around the roots. It was found that the hysterisis and phase lag in compression were much higher than in tension, but independent of root levels (Figure 19) and locations around the root. Tanaka et al. (2007) [80] have also investigated dynamic shear properties of the porcine molar PDL at an oscillation amplitudes of Δγ = 0.5, 1, and 1.5% with a wide range of frequencies (0.01–100 Hz). They found that at 2 Hz the coronal PDL had a slight, but significantly, greater storage modulus G′ than the apical PDL while loss modulus G′′ were similar between the two regions (Figure 20). The discrepancy between the former and latter results remains unknown but might be due to the different modes of loading (tension-compression and shear) and/or the different spices.
Figure 19.
Phase lags (a) and hysterisis (b) in tension and compression obtained from bovine periodontal ligament specimens at different root levels, reproduced from Shibata et al. [27], by permission.
Figure 20.
Dynamic shear properties of complex G*, storage G′, and loss G′′ moduli for the apical and coronal regions of porcine PDL. Vertical bar indicates 1 SD. *P < .05, reproduced from Tanaka et al. [80], by permission.
In the bovine PDL as mentioned above [26, 27], the phase lag values in tension and compression ranged between 0 and 0.1, and 0.2 and 0.4, respectively. The dynamic measurements for bovine and porcine PDLs suggest that the PDL can absorb more energy in compression than in shear and tension.
6. Concluding Remarks
Recently, gene-targeted and mutant animals have been used as experimental models to explore the role of individual constituents in the biomechanical behaviour of connective tissues. Investigations into stress-relaxation and strain rate sensitivity of tendons from genetically engineered mice have shown greater and faster stress-relaxation among decorin knockout animals and the elastic parameter was less among those with reduced type I collagen [81]. Histological findings in knockout mice suggest that decorin, fibromodulin, and lumican regulate the fibrillar and suprafibrillar organisation of collagen in the PDL [82, 83].
There may be further mechanisms of mechanical adaptation to functional stress including molecular adaptation to mechanical stress, which is now under investigation in the field of the biomechanics. Further, there have recently been an increasing number of reports on altered gene expression and protein changes in fibrillar and net-forming collagens triggered by the sensing and responding of connective tissue cells to mechanical loads [84].
Future studies using such approaches should further elucidate the relations between viscoelastic behaviour and structure of the PDL.
Acknowledgments
The author would like to thank one of the Chief Editors, Dr. Theodore Eliades, for providing him the opportunity to write this review article. This manuscript is the result of extensive and constructive discussion with many people. Among these, the author would like to especially thank Mototsugu Chiba, Professor Emeritus of Tsurumi University; Andrus Viidik, Professor Emeritus of Aarhus University; and Professor John Botsis, Ecole Polytechnique Federale de Lausanne. He also wishes to express his thanks to many authors and publishers who permitted him to reproduce their figures in this review. The preparation of this manuscript was partially supported by Japanese MEXT.HAITEC (2005–2009).
References
- 1.Moxham BJ, Berkovitz BKB. The effects of external forces on the periodontal ligament. In: Berkovitz BKB, Moxham BJ, Newman HN, editors. The Periodontal Ligament in Health and Disease. 2nd edition. London, UK: Mosby-Wolfe; 1995. pp. 215–241. [Google Scholar]
- 2.Sloan P, Carter DH. Structural organisation of the fibres of the periodontal ligament. In: Berkovitz BKB, Moxham BJ, Newman HN, editors. The Periodontal Ligament in Health and Disease. 2nd edition. London, UK: Mosby-Wolfe; 1995. pp. 35–53. [Google Scholar]
- 3.Nishihira M, Yamamoto K, Sato Y, Ishikawa H, Natali AN. Mechanics of periodontal ligament. In: Natali AN, editor. Dental Biomechanics. London, UK: Taylor & Francis; 2003. pp. 20–34. [Google Scholar]
- 4.Chiba M. Mechanical properties of the periodontal ligament. Tsurumi University Dental Journal. 2004;30:201–213. [Google Scholar]
- 5.Yoshimatsu N, Hazama H, Bando T. Extractive properties of human teeth. Journal of Kyoto Prefectural University of Medicine. 1956;60:297–300. [Google Scholar]
- 6.Chiba M, Ohkawa S. Measurement of the tensile strength of the periodontium in the rat mandibular first molar. Archives of Oral Biology. 1980;25(8-9):569–572. doi: 10.1016/0003-9969(80)90069-2. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu K, Ohshima S, Chiba M. Measurement of the force required to extract the mandibular first molar from its socket in the dissected jaw of growing young rats. Gerodontology. 1990;9(1):3–7. doi: 10.1111/j.1741-2358.1990.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi T. The effect of oral administration of β-aminopropionitrile (BAPN) on the mechanical strength of the periodontal ligament in the rat mandibular and maxillary molars. Japanese Journal of Oral Biology. 1986;28:590–604. [Google Scholar]
- 9.Chiba M, Ohshima S, Takizawa K. Measurement of the force required to extract the mandibular incisor of rats of various ages. Archives of Oral Biology. 1980;25(10):683–687. doi: 10.1016/0003-9969(80)90101-6. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki Y. Effects of destructive periodontitis, induced by diet, on the mechanical properties of the periodontal ligament of the mandibular first molar in golden hamsters. Journal of Periodontal Research. 1992;27(2):149–158. doi: 10.1111/j.1600-0765.1992.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson HF, Ralph WJ. In vitro strength of the human periodontal ligament. Journal of Dental Research. 1977;56(1):48–52. doi: 10.1177/00220345770560011001. [DOI] [PubMed] [Google Scholar]
- 12.Mandel U, Dalgaard P, Viidik A. A biomechanical study of the human periodontal ligament. Journal of Biomechanics. 1986;19(8):637–639. doi: 10.1016/0021-9290(86)90169-7. [DOI] [PubMed] [Google Scholar]
- 13.Toms SR, Dakin GJ, Lemons JE, Eberhardt AW. Quasi-linear viscoelastic behavior of the human periodontal ligament. Journal of Biomechanics. 2002;35(10):1411–1415. doi: 10.1016/s0021-9290(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 14.Ralph WJ. Tensile behaviour of the periodontal ligament. Journal of Periodontal Research. 1982;17(4):423–426. doi: 10.1111/j.1600-0765.1982.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 15.Mandel U, Viidik A. Effect of splinting on the mechanical and histological properties of the healing periodontal ligament in the vervet monkey (Cercopithecus aethiops) Archives of Oral Biology. 1989;34(3):209–217. doi: 10.1016/0003-9969(89)90010-1. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu K, Chiba M. The effect of velocity of loading on the biomechanical responses of the periodontal ligament in transverse sections of the rat molar in vitro. Archives of Oral Biology. 1993;38(5):369–375. doi: 10.1016/0003-9969(93)90207-3. [DOI] [PubMed] [Google Scholar]
- 17.Komatsu K, Kanazashi M, Shimada A, Shibata T, Viidik A, Chiba M. Effects of age on the stress-strain and stress-relaxation properties of the rat molar periodontal ligament. Archives of Oral Biology. 2004;49(10):817–824. doi: 10.1016/j.archoralbio.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu K. In vitro mechanics of the periodontal ligament in impeded and unimpeded rat mandibular incisors. Archives of Oral Biology. 1988;33(11):783–791. doi: 10.1016/0003-9969(88)90102-1. [DOI] [PubMed] [Google Scholar]
- 19.Chiba M, Yamane A, Ohshima S, Komatsu K. In vitro measurement of regional differences in the mechanical properties of the periodontal ligament in the rat mandibular incisor. Archives of Oral Biology. 1990;35(2):153–161. doi: 10.1016/0003-9969(90)90177-c. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu K, Shibata T, Shimada A, Viidik A, Chiba M. Age-related and regional differences in the stress-strain and stress-relaxation behaviours of the rat incisor periodontal ligament. Journal of Biomechanics. 2004;37(7):1097–1106. doi: 10.1016/j.jbiomech.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Komatsu K, Yamazaki Y, Yamaguchi S, Chiba M. Comparison of biomechanical properties of the incisor periodontal ligament among different species. Anatomical Record. 1998;250(4):408–417. doi: 10.1002/(SICI)1097-0185(199804)250:4<408::AID-AR3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki Y, Komatsu K, Arai T, Chiba M. The effects of a high-carbohydrate diet on the stress-strain behavior of the periodontal ligament of the distal root of the mandibular first molar in hamsters. Journal of Periodontal Research. 2001;36(5):301–308. doi: 10.1034/j.1600-0765.2001.360505.x. [DOI] [PubMed] [Google Scholar]
- 23.Komatsu K, Chiba M. Synchronous recording of load-deformation behaviour and polarized light-microscopic images of the rabbit incisor periodontal ligament during tensile loading. Archives of Oral Biology. 2001;46(10):929–937. doi: 10.1016/s0003-9969(01)00054-1. [DOI] [PubMed] [Google Scholar]
- 24.Dorow C, Krstin N, Sander F-G. Determination of the mechanical properties of the periodontal ligament in a uniaxial tensional experiment. Journal of Orofacial Orthopedics. 2003;64(2):100–107. doi: 10.1007/s00056-003-0225-7. [DOI] [PubMed] [Google Scholar]
- 25.Pini M, Wiskott HWA, Scherrer SS, Botsis J, Belser UC. Mechanical characterization of bovine periodontal ligament. Journal of Periodontal Research. 2002;37(4):237–244. doi: 10.1034/j.1600-0765.2002.00344.x. [DOI] [PubMed] [Google Scholar]
- 26.Sanctuary CS, Wiskott HWA, Justiz J, Botsis J, Belser UC. In vitro time-dependent response of periodontal ligament to mechanical loading. Journal of Applied Physiology. 2005;99(6):2369–2378. doi: 10.1152/japplphysiol.00486.2005. [DOI] [PubMed] [Google Scholar]
- 27.Shibata T, Botsis J, Bergomi M, Mellal A, Komatsu K. Mechanical behavior of bovine periodontal ligament under tension-compression cyclic displacements. European Journal of Oral Sciences. 2006;114(1):74–82. doi: 10.1111/j.1600-0722.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 28.Viidik A. Mechanical properties of parallel-fibred collagenous tissues. In: Viidik A, Vuust J, editors. Biology of Collagen. London, UK: Academic Press; 1980. pp. 237–255. [Google Scholar]
- 29.Currey J. The Mechanical Adaptations of Bones. Princeton, NJ, USA: Princeton University Press; 1984. [Google Scholar]
- 30.Gordon JE. The New Science of Strong Materials or Why You Don’t Fall through the Floor. 2nd edition. London, UK: Penguin Books; 1976. [Google Scholar]
- 31.Alexander RM. Factors of safety in the structure of animals. Science Progress. 1981;67(265):109–130. [PubMed] [Google Scholar]
- 32.Biewener AA. Overview of structural mechanics. In: Biewener AA, editor. Biomechanics: Structures and Systems. Oxford, UK: Oxford University Press; 1992. pp. 1–20. [Google Scholar]
- 33.Swartz SM, Biewener AA. Shape and scaling. In: Biewener AA, editor. Biomechanics: A Practical Approach. Oxford, UK: Oxford University Press; 1992. pp. 21–43. [Google Scholar]
- 34.Kawada J, Komatsu K. In vitro effects of collagenase on biomechanical and morphological features of the rat molar periodontal ligament. Japanese Journal of Oral Biology. 2000;42:193–205. [Google Scholar]
- 35.Chiba M, Ohshima S, Kuroda T, Ohkawa S. Effects of repeated shortenings and of artificial restraint on the tensile strength of the periodontium of the rat mandibular incisor. Archives of Oral Biology. 1981;26(2):135–141. doi: 10.1016/0003-9969(81)90084-4. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita Y, Tonooka K, Chiba M. The effect of hypofunction on the mechanical properties of the periodontium in the rat mandibular first molar. Archives of Oral Biology. 1982;27(10):881–885. doi: 10.1016/0003-9969(82)90045-0. [DOI] [PubMed] [Google Scholar]
- 37.Tsuruta M, Ohkawa S, Nakatani Y, Kuwahara Y, Chiba M. Effect of experimental tooth movement on the mechanical strength of the periodontium in the rat mandibular first molar. Archives of Oral Biology. 1982;27(10):875–879. doi: 10.1016/0003-9969(82)90044-9. [DOI] [PubMed] [Google Scholar]
- 38.Hong RK, Yamane A, Kuwahara Y, Chiba M. The effect of orthodontic retention on the mechanical properties of the periodontal ligament in the rat maxillary first molar. Journal of Dental Research. 1992;71(7):1350–1354. doi: 10.1177/00220345920710070101. [DOI] [PubMed] [Google Scholar]
- 39.Fukui T. Analysis of stress-strain curves in the rat molar periodontal ligament after application of orthodontic force. American Journal of Orthodontics and Dentofacial Orthopedics. 1993;104(1):27–35. doi: 10.1016/0889-5406(93)70024-I. [DOI] [PubMed] [Google Scholar]
- 40.Chiba M, Kuroda T, Ohshima S. Effects of adrenocorticoids on impeded and unimpeded eruption rates and on the mechanical properties of the periodontium in the rat mandibular incisor. Archives of Oral Biology. 1981;26(7):577–583. doi: 10.1016/0003-9969(81)90019-4. [DOI] [PubMed] [Google Scholar]
- 41.Ohkawa S. Effects of orthodontic forces and anti-inflammatory drugs on the mechanical strength of the periodontium in the rat mandibular first molar. American Journal of Orthodontics. 1982;81(6):498–502. doi: 10.1016/0002-9416(82)90429-8. [DOI] [PubMed] [Google Scholar]
- 42.Ohshima S, Nakamura G, Chiba M. Effects of lathyrogens on the mechanical strength of the periodontal ligament in the rat mandibular first molar. Journal of Periodontal Research. 1989;24(5):343–350. doi: 10.1111/j.1600-0765.1989.tb00880.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi S. Analysis of stress-strain curves at fast and slow velocities of loading in vitro in the transverse section of the rat incisor periodontal ligament following the administration of β-aminopropionitrile. Archives of Oral Biology. 1992;37(6):439–444. doi: 10.1016/0003-9969(92)90097-r. [DOI] [PubMed] [Google Scholar]
- 44.Yamane A. The effect of age on the mechanical properties of the periodontal ligament in the incisor teeth of growing young rats. Gerodontology. 1990;9(1):9–16. doi: 10.1111/j.1741-2358.1990.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 45.Yamane A, Ohshima S, Komatsu K, Chiba M. Mechanical properties of the periodontal ligament in the incisor teeth of rats from 6 to 24 months of age. Gerodontology. 1990;9(1):17–23. doi: 10.1111/j.1741-2358.1990.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman AS, Daly CH. A commentary on the use of enzyme probes to elucidate the contributions of individual components to soft-tissue biomechanics in vitro. In: Viidik A, Vuust J, editors. Biology of Collagen. London, UK: Academic Press; 1980. pp. 297–312. [Google Scholar]
- 47.Bond MD, van Wart HE. Purification and separation of individual collagenases of Clostridium histolyticum using red dye ligand chromatography. Biochemistry. 1984;23(13):3077–3085. doi: 10.1021/bi00308a035. [DOI] [PubMed] [Google Scholar]
- 48.Ujiie Y, Shimada A, Komatsu K, et al. Degradation of noncollagenous components by neutrophil elastase reduces the mechanical strength of rat periodontal ligament. Journal of Periodontal Research. 2008;43(1):22–31. doi: 10.1111/j.1600-0765.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe Y, Komatsu K. Biomechanical and morphological studies on the periodontal ligament of the rat molar after treatment with α-amylase in vitro. Connective Tissue Research. 1997;36(1):35–49. doi: 10.3109/03008209709160212. [DOI] [PubMed] [Google Scholar]
- 50.Caterson B, Christner JE, Baker JR, Couchman JR. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Federation Proceedings. 1985;44(2):386–393. [PubMed] [Google Scholar]
- 51.Takagi M, Maeno M, Kagami A, Takahashi Y, Otsuka K. Biochemical and immunocytochemical characterization of mineral binding proteoglycans in rat bone. Journal of Histochemistry and Cytochemistry. 1991;39(1):41–50. doi: 10.1177/39.1.1898498. [DOI] [PubMed] [Google Scholar]
- 52.Kurashima K. The viscoelastic properties of the periodontal ligament. Journal of Japan Stomatological Society. 1963;30:361–385. [Google Scholar]
- 53.Bien SM. Fluid dynamic mechanisms which regulate tooth movement. In: Staple PH, editor. Advances in Oral Biology. Vol. 2. London, UK: Academic Press; 1966. pp. 173–201. [DOI] [PubMed] [Google Scholar]
- 54.Wills DJ, Picton DC, Davies WI. An investigation of the viscoelastic properties of the periodontium in monkeys. Journal of Periodontal Research. 1972;7(1):42–51. doi: 10.1111/j.1600-0765.1972.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 55.Picton DCA, Wills DJ. Viscoelastic properties of the periodontal ligament and mucous membrane. The Journal of Prosthetic Dentistry. 1978;40(3):263–272. doi: 10.1016/0022-3913(78)90031-8. [DOI] [PubMed] [Google Scholar]
- 56.Middleton J, Jones AN, Wilson J. Threedimensional analysis of orthodontic tooth movement. Journal of Biomedical Engineering. 1990;23(4):319–327. doi: 10.1016/0141-5425(90)90007-a. [DOI] [PubMed] [Google Scholar]
- 57.Provatidis CG. A comparative FEM-study of tooth mobility using isotropic and anisotropic models of the periodontal ligament. Medical Engineering and Physics. 2000;22(5):359–370. doi: 10.1016/s1350-4533(00)00055-2. [DOI] [PubMed] [Google Scholar]
- 58.Moxham BJ, Berkovitz BKB. A quantitative assessment of the effects of axially directed extrusive loads on displacement of the impeded and unimpeded rabbit mandibular incisor. Archives of Oral Biology. 1981;26(3):209–215. doi: 10.1016/0003-9969(81)90132-1. [DOI] [PubMed] [Google Scholar]
- 59.Moxham BJ, Berkovitz BKB. The mobility of the lathyritic rabbit mandibular incisor in response to axially-directed extrusive loads. Archives of Oral Biology. 1984;29(10):773–778. doi: 10.1016/0003-9969(84)90005-0. [DOI] [PubMed] [Google Scholar]
- 60.Chiba M, Komatsu K. Mechanical responses of the periodontal ligament in the transverse section of the rat mandibular incisor at various velocities of loading in vitro. Journal of Biomechanics. 1993;26(4-5):561–570. doi: 10.1016/0021-9290(93)90017-9. [DOI] [PubMed] [Google Scholar]
- 61.Komatsu K, Chiba M. Analysis of stress-strain curves and histological observations on the periodontal ligament of impeded and unimpeded rat incisors at low velocities of loading. Japanese Journal of Oral Biology. 1996;38:192–202. [Google Scholar]
- 62.Komatsu K, Sanctuary C, Shibata T, Shimada A, Botsis J. Stress-relaxation and microscopic dynamics of rabbit periodontal ligament. Journal of Biomechanics. 2007;40(3):634–644. doi: 10.1016/j.jbiomech.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 63.Sanctuary C. Experimental investigation of the mechanical behaviour and structure of the bovine periodontal ligament. Lausanne, Switzerland: Swiss Federal Institute of Technology; 2003. Ph.D. thesis. [Google Scholar]
- 64.Natali AN, Pavan PG, Carniel EL, Dorow C. Viscoelastic response of the periodontal ligament: an experimental-numerical analysis. Connective Tissue Research. 2004;45(4-5):222–230. doi: 10.1080/03008200490885742. [DOI] [PubMed] [Google Scholar]
- 65.Komatsu K, Watanabe M, Kanazashi M, Chiba M. Effects of age on stress-strain and stress-relaxation behaviours of the rat incisor periodontal ligament. In: Proceedings of 16th Congress of the International Society of Biomechanics; 1997; University of Tokyo, Tokyo, Japan. p. 411. [Google Scholar]
- 66.Komatsu K, Kanazashi M, Arai T, Chiba M. Effects of hydrocortisone and β-aminopropionitrile on stress-strain and stress-relaxation behaviors, and birefringent retardation of collagen fibers in the rat incisor periodontal ligament. Connective Tissue Research. 2002;43(4):581–588. [PubMed] [Google Scholar]
- 67.Shadwick RE. Soft composites. In: Vincent JFV, editor. Biomechanics-Materials. Oxford, UK: IRL Press; 1992. pp. 133–164. [Google Scholar]
- 68.Viidik A. Structure and function of normal and healing tendons and ligaments. In: Mow VC, Ratcliffe A, Woo SL-Y, editors. Biomechanics of Diarthroidal Joints. New York, NY, USA: Springer; 1990. pp. 3–38. [Google Scholar]
- 69.Komatsu K, Viidik A. Changes in the fibre arrangement of the rat incisor periodontal ligament in relation to various loading levels in vitro. Archives of Oral Biology. 1996;41:147–159. doi: 10.1016/0003-9969(95)00114-x. [DOI] [PubMed] [Google Scholar]
- 70.Pini M, Zysset Ph, Botsis J, Contro R. Tensile and compressive behaviour of the bovine periodontal ligament. Journal of Biomechanics. 2004;37:111–119. doi: 10.1016/s0021-9290(03)00234-3. [DOI] [PubMed] [Google Scholar]
- 71.Wineman AS, Rajagopal KR. Mechanical Response of Polymers: An Introduction. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 72.Provenzano P, Lakes R, Keenan T, Vanderby R. Nonlinear ligament viscoelasticity. Annals of Biomedical Engineering. 2001;29:908–914. doi: 10.1114/1.1408926. [DOI] [PubMed] [Google Scholar]
- 73.Whittaker P, Schwab ME, Canham PB. The molecular organization of collagen in saccular aneurysms assessed by polarized light microscopy. Connective Tissue Research. 1988;17:43–54. doi: 10.3109/03008208808992793. [DOI] [PubMed] [Google Scholar]
- 74.Vilarta R, Vidal BC. Anisotropic and biomechanical properties of tendons modified by exercise and denervation: aggregation and macromolecular order in collagen bundles. Matrix. 1989;9(1):55–61. doi: 10.1016/s0934-8832(89)80019-8. [DOI] [PubMed] [Google Scholar]
- 75.Purslow PP, Wess TJ, Hukins DWL. Collagen orientation and molecular spacing during creep and stress-relaxation in soft connective tissues. Journal of Experimental Biology. 1998;201(1):135–142. doi: 10.1242/jeb.201.1.135. [DOI] [PubMed] [Google Scholar]
- 76.Sasaki N, Shukunami N, Matsushima N, Izumi Y. Time-resolved X-ray diffraction from tendon collagen during creep using synchrotron radiation. Journal of Biomechanics. 1999;32(3):285–292. doi: 10.1016/s0021-9290(98)00174-2. [DOI] [PubMed] [Google Scholar]
- 77.Komatsu K, Shibata T, Shimada A. Analysis of contribution of collagen fibre component in viscoelastic behaviour of periodontal ligament using enzyme probe. Journal of Biomechanics. 2007;40(12):2700–2706. doi: 10.1016/j.jbiomech.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Sasaki N, Nakayama Y, Yoshikawa M, Enyo A. Stress relaxation function of bone and bone collagen. Journal of Biomechanics. 1993;26(12):1369–1376. doi: 10.1016/0021-9290(93)90088-v. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka E, Inubushi T, Koolstra JH, et al. Comparison of dynamic shear properties of the porcine molar and incisor periodontal ligament. Annals of Biomedical Engineering. 2006;34(12):1917–1923. doi: 10.1007/s10439-006-9209-2. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka E, Inubushi T, Takahashi K, et al. Dynamic shear properties of the porcine molar periodontal ligament. Journal of Biomechanics. 2007;40(7):1477–1483. doi: 10.1016/j.jbiomech.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 81.Elliott DM, Robinson PS, Gimbel JA, et al. Effect of altered matrix proteins on quasilinear viscoelastic properties in transgenic mouse tail tendons. Annals of Biomedical Engineering. 2003;31(5):599–605. doi: 10.1114/1.1567282. [DOI] [PubMed] [Google Scholar]
- 82.Häkkinen L, Strassburger S, Kähäri V-M, et al. A role for decorin in the structural organization of periodontal ligament. Laboratory Investigation. 2000;80(12):1869–1880. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- 83.Matheson S, Larjava H, Häkkinen L. Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. Journal of Periodontal Research. 2005;40(4):312–324. doi: 10.1111/j.1600-0765.2005.00800.x. [DOI] [PubMed] [Google Scholar]
- 84.Mackey AL, Heinemeier KM, Koskinen SOA, Kjaer M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connective Tissue Research. 2008;49(3-4):165–168. doi: 10.1080/03008200802151672. [DOI] [PubMed] [Google Scholar]