Abstract
Action potentials (APs) provide the primary means of rapid information transfer in the nervous system. Where exactly these signals are initiated in neurons has been a basic question in neurobiology and the subject of extensive study. Converging lines of evidence indicate that APs are initiated in a discrete and highly specialized portion of the axon—the axon initial segment (AIS). The authors review key aspects of the organization and function of the AIS and focus on recent work that has provided important insights into its electrical signaling properties. In addition to its main role in AP initiation, the new findings suggest that the AIS is also a site of complex AP modulation by specific types of ion channels localized to this axonal domain.
Keywords: action potential initiation, neocortical neurons, GABA, fast-spiking interneurons, axon initial segment (AIS), Kv1 potassium channels
One of the remarkable specializations of neurons is their exquisite morphology and highly polarized nature. The axon is the main output structure of neurons that allows the activity of single cells to be rapidly conveyed to large numbers of post-synaptic targets. The axon initial segment (AIS) is the proximal portion of the axon beginning at the emergence of the axon from the soma (the axon hillock) and ending at the onset of the myelin sheath. This, of course, is a general definition that must be modified in specific cases (such as for unmyelinated axons and cells in which the axon emerges from a dendrite).
Multiple lines of evidence point to the AIS as the site of initiation of the action potential (AP), the basic unit of information transfer in the nervous system. AP initiation occurs at the AIS because the threshold for AP generation is lowest at this site. This in turn is due to the presence of a high density—and perhaps unique properties—of voltage-gated sodium (Na+) channels at the AIS. Cellular and molecular studies show that the AIS is a highly organized subcellular compartment, or domain, that possesses a number of ultrastructural and biochemical specializations. Moreover, in the cortex, the AIS is the specific and only target of a unique subtype of GABAergic interneuron, the chandelier cell. Finally, the AIS expresses a number of functionally important and disease-related molecules that appear to provide “electrogenic” tuning of neuronal excitability.
Investigation of the axon dates back to some of the earliest anatomical studies of Kölliker, Remak, and Deiters in the 19th century. Following the elucidation of the ionic mechanisms responsible for the generation of the AP, work by several investigators in the 1950s suggested that the AP is initiated at a discrete site, and that this site was in fact the AIS (see Box 1 and Fig. 1). Research in subsequent decades has further refined these initial observations and identified many new aspects of the structure and functional specializations of the axon. It has become clear that the axon contains several specialized domains, each with unique structure and functional properties, including the AIS, the nodes of Ranvier and adjacent paranodes and juxtaparanodes in myelinated axons, the preterminal axon, and finally, the presynaptic terminal itself. The AIS has received increasing attention in recent years, and some aspects of this work have been discussed in further detail in recent accounts (Ogawa and Rasband 2008; Kress and Mennerick 2009, see also Stuart and others 1997b; Salzer 2003).
Box 1. Some History of the Axon Initial Segment.
A full treatment of the history behind the current conceptualization of the axon initial segment (AIS) is beyond the scope of this review, but aspects of this history can be found in the book by Shepherd (1991). We highlight some landmark developments here.
Kölliker (1849) and Remak (1855) were the first to propose that nerve fibers (axons) arise from neurons (although who was actually first is apparently the subject of some debate; Shepherd 1991). It may have been Kölliker who likely made the first description of the axon initial segment per se (Kölliker 1849), whereas Deiters (1865) is often credited with differentiating axon from dendrite. In addition, Deiters described the origin of the axon or “axon cylinder,” which seems to correspond to the modern-day definition of the axon hillock. Deiters also described a thin portion of the axon at the emergence from the cell soma, which in turn may correspond to the AIS (Fig. 1A). Ramón y Cajal, in his theory of dynamic polarization (Ramón y Cajal 1897, 1989), proposed that the axon represented the output structure of the neuron: “the transmission of the nervous impulse is always from the dendritic branches and the cell body to the axon” (Ramón y Cajal 1989). However, it was not until the 1950s, after the introduction of intracellular recording and the elucidation of the mechanism of spike generation, that evidence emerged that APs are initiated at a discrete site. Much initial work at this time suggested that the site of AP initiation was, in fact, the AIS.
The site of action potential initiation was hypothesized to be at the origin of the axon/axon hillock (Bishop 1953), the soma (Fatt 1957), or at the AIS (Araki and Otani 1955; Coombs and others 1957a, 1957b). Araki and Otani (1955) and Coombs and others (1957a, 1957b) showed that the threshold of AP initiation was lower in the axon than in the soma. But, perhaps the first direct evidence that the site of AP initiation was at the AIS was provided by Edwards and Ottoson (1958) in the neuron of the lobster stretch receptor, showing that the origin of the electrical impulse occurred in the axon but at some distance from the soma. Edwards and Otteson (1958) demonstrated that a spike generated first in the AIS would propagate both orthodromically down the axon and antidromically toward the soma and dendrites (Fig. 1B). Such experimental data was supported by early modeling studies (e.g., Dodge and Cooley 1973). These authors also speculated that the lower threshold for AP generation was due to a high density of Na+ channels at the AIS.
With the application of electron microscopy to the study of neurons, Palay and others (1968) and Conradi (1966) provided the first detailed anatomical descriptions of the AIS based on ultrastructural data. These authors noted an electron-dense undercoating at the AIS resembling that seen at the node of Ranvier. This undercoating likely represents the enrichment of proteins involved in anchoring ion channels to the AIS. Palay and others (1968) further concluded that the ultrastructure of the AIS was very similar in myelinated and in unmyelinated axons (Fig. 1C).
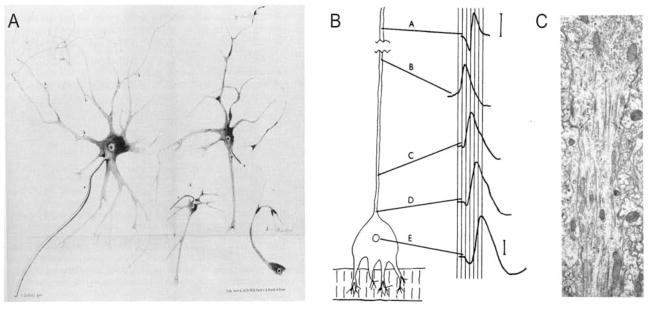
Figure 1.
Early studies of the axon. A, Deiters’ illustration of isolated motor neurons from the anterior horn of the spinal cord. In the large cell at the left, he differentiates (a) the single “main axis cylinder extension” from the (b) multiple “fine axis cylinder extensions coming from the protoplasmic extensions…” (Deiters 1865; reproduced by permission of Oxford University Press, Inc, from Shepherd 1991). Based on these descriptions, Deiters is often credited with first distinguishing the axon from dendrites. B, Edwards and Ottoson’s demonstration of axonal initiation of orthodromic impulses in the giant lobster stretch receptor. Using a preparation in which simultaneous extracellular electrodes could be visually positioned at the soma (E) and at varying distances along the axon (A–D), Edwards and Ottoson showed that in response to activation by stretch, the spike is first recorded at site B, about 500 μm from the cell body (negativity is upward in these extracellular tracings; from Edwards and Ottoson 1958). C, Electron micrograph of a pyramidal cell axon initial segment from rat cerebral cortex. The axon hillock is at the top of the image. Arrows indicate the beginning of the characteristic undercoating and the beginning of the axon initial segment (from Palay and others, copyright 1968. Originally published in The Journal of Cell Biology, 38:193–201).
The present review is focused on our current understanding of the function of the AIS. We provide a broad overview of this axonal compartment and summarize the evidence indicating that the AIS is indeed the site of AP initiation. We also include a more detailed discussion of very recent discoveries of modulation of cortical neuron function by ion channels present at the AIS, focusing on their role in regulating spike timing, spike shape, and spike influence on synaptic transmission.
This review highlights the view that, besides serving as the site of AP initiation, the AIS is also a complex molecular and biophysical subcellular compartment and is capable of exerting powerful and dynamic modulatory effects on neuronal function.
Molecular Organization of the AIS
Although the early anatomists provided the first morphological description of the axon and the AIS, it has been the revolution in molecular biology and insights from cell biology that have led to the current view of the AIS as a specialized subcellular domain with rich molecular organization. In general, the AIS is characterized molecularly as the region of proximal axon enriched in sodium channels; however, the initial segment contains a variety of other proteins, many with as yet ill-defined roles in AIS organization and function.
Multiple Types of Ion Channels Are Enriched at the AIS
Recent study of the AIS has revealed that, in addition to the high density of sodium channels necessary for AP initiation (see section titled “The AIS Is the Site of AP Initiation”), this neuronal compartment possesses a variety of other ion channels suggesting complex modulation of AIS excitability. These channels include voltage-gated potassium channels of the Kv1, Kv2, and Kv7 (KCNQ) subfamilies, and possibly voltage-gated calcium channels. The specific contribution of these channels to spike generation, and their role in regulating repetitive firing, AP shape and propagation, are beginning to be explored in detail.
Na+ Channels
Consistent with its function of spike initiation, the AIS is characterized by a span of membrane enriched in sodium channels. Although less extensive than K+ channel diversity, there are also multiple types of voltage-gated Na+ channels (Catterall and others 2005). The physiological significance of this diversity is incompletely understood, but recent studies have begun to demonstrate important differences in their properties and functional roles (Rush and others 2007). The molecular identity of the sodium channels at the AIS likely depends on cell type and is subject to developmental changes; however, Nav1.6, the most abundantly expressed Na+ channel isoform in the nervous system, appears to also be one of the most prevalent isoforms at the AIS (Boiko and others 2003; Ogawa and Rasband 2008). It has been suggested that, in cortex, NaV1.1 is expressed mainly at the AIS of fast-spiking (FS) GABAergic inhibitory interneurons (Yu and others 2006; Ogiwara and others 2007), which is interesting because loss of function mutations in the gene encoding this channel protein produce epilepsy (Ragsdale 2008).
Kv1 Potassium Channels
There is a growing list of K+ channels identified at the AIS. Potassium channels are by far the most diverse group of ion channels and have many important roles as regulators of cell excitability, controlling spike threshold, shape, and repetitive firing (Coetzee and others 1999; Rudy and others 2009); hence, the observation of their selective enrichment at the AIS hints at the possibility of complex local modulation of spike generation and spike properties by these channels. Some of this complex regulation is beginning to be unraveled. Members of the Kv1 subfamily of voltage-gated K+ channels (see Box 2) are of particular interest because they activate at subthreshold membrane potentials and thus can influence spike initiation. The voltage- and time-dependent activation and inactivation properties of these channels also introduce interesting dynamics to the electrical properties of the AIS. Several laboratories have recently demonstrated the presence of Kv1 channels at the AIS in a variety of species and cell types, and where examined, they have been shown to have important roles in regulating AP initiation and the shape of the axonal AP (Goldberg and others 2006; Inda and others 2006; Kuba and others 2006; Kole and others 2007; Shu and others 2007; Van Wart and others 2007; Goldberg and others 2008; Lorincz and Nusser 2008).
Box 2. Diversity and Molecular Organization of Kv1 Channels.
The Kv1 subfamily consists of eight genes (Kv1.1 to Kv1.8) in rodent and human, making it the largest subfamily among the voltage-gated K+ channels. Like other Kv channels, four Kv1 pore-forming subunits combine to form a functional channel. This tetrameric organization generates a large diversity of possible subunit combinations, because Kv1 subunits can heteromultimerize with other members of the Kv1 subfamily. Depending on the subunit composition, Kv1 channels expressed in heterologous systems can produce currents ranging from sustained outward currents (sometimes referred to as “delayed rectifying” currents) to fast-transient “A-type” currents. Adding to this diversity of Kv1-mediated currents is their association with auxiliary (β) subunits, known as Kvβs. Association with different Kvβ subunits modifies the properties of Kv1 channels and thus these auxiliary proteins are likely important determinants of native channel properties in neurons. Importantly, association with particular Kvβ subunits (e.g., Kvβ1 and Kvβ3) can confer fast inactivation to otherwise slowly inactivating or noninactivating Kv1 channels. Most Kv1 channels expressed in heterologous cells tend to activate at more negative voltages than most other voltage-gated channels. Therefore Kv1 channels contribute to subthreshold neuronal excitability.
The presence of a single Kv1.1, Kv1.2, and/or Kv1.6 subunit in the channel complex confers sensitivity to the mamba snake toxin, dendrotoxin-I (DTX-I). As these Kv1 subunits are the most prominently expressed in CNS neurons, it is therefore likely that most neuronal Kv1-mediated currents are DTX sensitive. There are also additional, although still limited, pharmacological tools that allow further identification of the channel subunits mediating a Kv1 current. For example, dendrotoxin-K (DTX-K) blocks channels containing at least one Kv1.1 subunit far better than those having other Kv1 proteins. On the other hand, rTityustoxin-Kα toxin preferentially blocks channels containing Kv1.2 subunits (see Harvey 2001).
As Kv1 genes are widely and prominently expressed in brain, it is therefore likely that most if not all neurons in the CNS express several types of Kv1 subunits. In general, Kv1 channels are expressed predominantly in axons, including synaptic terminals, although some neurons have been shown to express Kv1 proteins in somatic and dendritic compartments as well (reviewed in Lai and Jan 2006; Vacher and others 2008; Rudy and others 2009).
Other Ion Channels at the AIS
Several other types of ion channels have also been reported at the AIS of central neurons. In the medial nucleus of the trapezoid body (MNTB), Kv2-mediated currents accounted for a large proportion of the delayed-rectifier current measured at the soma, and immunohistochemical staining for Kv2.2 subunits revealed their enrichment at the AIS, occupying a more proximal location than the Kv1 channels at the AIS of these cells (Johnston and others 2008). Because of the lack of specific blockers of Kv2.2 channels it has not been possible to isolate their functional contribution in these neurons. However, based on modeling, Johnston and others have suggested that the Kv2.2 current facilitates high frequency firing by hyperpolarizing the interspike membrane potential, facilitating the recovery of sodium channels from inactivation. It was also recently suggested that Kv2.1, the other known member of the Kv2 subfamily, is expressed at the AIS of hippocampal and neocortical neurons (Sarmiere and others 2008).
Members of the KCNQ (Kv7) subfamily of potassium channels are also clustered at the AIS of several cell types. KCNQ channels mediate the subthreshold-operating M-type K+ current. These are slow-activating, noninactivating, voltage-dependent currents that are believed to be major modulators of neuronal excitability in many neurons. They contribute to stabilizing the resting potential and limit repetitive firing. They were initially discovered as the currents mediating muscarinic excitation of neuronal activity, and hence called M-currents (Brown and Adams 1980). This subfamily of K+ channels is of great interest because four of its five members have been associated with human disease (reviewed in Rudy and others 2009).
Cooper and colleagues showed that two members of this subfamily, KCNQ2 (Kv7.2) and KCNQ3 (Kv7.3), are enriched at the AIS in several neuronal populations, including hippocampal and neocortical pyramidal cells (Devaux and others 2004; Pan and others 2006). Pan and others (2006) showed that KCNQ2 and 3 channels contain a short motif, nearly identical to one found in all mammalian voltage-gated Na+ channels that mediates interaction with the AIS cytoskeletal scaffold protein ankyrin G.
Recently, Shah and others (2008) used an ankyrin G-binding peptide to dissociate KCNQ channels from ankyrin and concluded that targeting of the channels to the AIS was important for their control of the excitability in CA1 hippocampal pyramidal neurons. Treatment with the competing peptide induced spontaneous firing resembling the effects of pharmacological block of the channels (Shah and others 2008).
Last, using two-photon imaging of interneurons in the dorsal cochlear nucleus, Bender and Trussell (2009) found that calcium transients evoked by repetitive firing were larger in the AIS than at the soma. Pharmacological experiments implicated T- and R-type voltage-gated calcium channel enrichment at the AIS. Similar calcium transients were observed at the AIS of layer 5 pyramidal cells and cerebellar Purkinje neurons (Bender and Trussell 2009). The function of these AIS calcium channels and their precise distribution within this domain remain to be established.
Subdomains within the AIS?
Interestingly, there is some evidence for molecular subdomains of Na+ channel isoforms even within the AIS. In retinal ganglion cells, Van Wart and others found sodium channel isoform Nav1.1 staining was restricted to the proximal portion of the AIS after which Nav1.6 staining intensity increased for the remainder of the initial segment. In addition these authors found that Kv1.2 staining was restricted to the distal portion of the AIS (Van Wart and others 2007). Segregation of sodium channel isoforms was also observed within the AIS of parvalbumin-positive cortical interneurons (Lorincz and Nusser 2008). Preliminary results from our group suggest there may also be a segregation of Kv K+ channels within subregions of the AIS among cortical interneurons. Such discrete molecular organization raises questions as to the mechanisms of assembly of such subdomains and their potential functional significance.
Molecular Machinery Organizing the AIS
Many of the proteins enriched at the AIS are thought to be a part of the molecular machinery involved in organizing this domain. The details of this process and how it differs in distinct neuronal cell types are still poorly understood. These proteins include the scaffolding proteins ankyrin G and βIV spectrin and the cell adhesion molecules neurofascin-186 and NrCam, proteins that are also enriched at nodes of Ranveir (Salzer 2003; Ogawa and Rasband 2008). However, whereas the formation of the nodes of Ranvier requires axoglial interactions, the formation of the AIS appears to be intrinsically specified in an ankyrin G-dependent manner (Zhou and others 1998; Jenkins and Bennett 2001; Dzhashiashvili and others 2007). As mentioned earlier, the clustering of sodium and KCNQ2/3 channels at the AIS is known to require ankyrin G and to depend on a nearly identical targeting motif (Zhou and others 1998; Pan and others 2006). The molecular details of how other ion channels (such as Kv1 channels) are targeted to the AIS are active areas of investigation (Ogawa and others 2008).
A more extensive account of what is known about the mechanisms of assembly and maintenance of the molecular organization at the AIS is beyond the scope of this review. We refer the reader to recent reviews on this topic (Hedstrom and Rasband 2006; Ogawa and Rasband 2008). However, it is important to mention that knockdown of ankyrin G not only affects Na+ channel localization, but entirely disrupts the maintenance of neuronal polarity, with the axon acquiring dendritic properties. This includes the redistribution into the former axon of proteins normally restricted to somatodendritic domains and the assembly of spines and postsynaptic densities (Hedstrom and others 2008). This finding indicates that the AIS not only has critical functions in neuronal excitability but in maintaining neuronal structure itself.
The AIS Is the Site of AP Initiation
The site of AP initiation is an important question to answer definitively, because this has important implications for neuronal function. Moreover, this remains an important issue given our current understanding of the diversity and domain-specific subcellular targeting of ion channels and neurotransmitter receptors (Lai and Jan 2006; Vacher and others 2008).
That the AIS is the likely site of AP initiation has been known since the 1950s (see Box 1). Over the years, many studies questioned this idea, either in general or in terms of its applicability to specific cell types. The issue was reexamined extensively in the 1990s in light of the discovery that dendrites possess active properties and that the AP can back-propagate from the soma into the dendrites (reviewed in Stuart and others 1997b). Together with recent reports providing excellent supporting data, there is now compelling evidence supporting the initial hypothesis that the AIS is the site of AP initiation, which is likely the case for most neurons.
Stuart and Sakmann and Colbert and Johnston demonstrated that the AIS was indeed the site of AP initiation for mammalian cortical principal neurons, based on simultaneous somatic and axonal patch-clamp recordings (Stuart and Sakmann 1994; Colbert and Johnston 1996; Stuart and others 1997a; Fig. 2A). Studies using voltage-sensitive and Na+-sensitive dyes have provided strong additional evidence that the site of AP initiation is at the AIS in layer 5 pyramidal cells in rodent neocortex (Palmer and Stuart 2006; Kole and others 2008a; Fig. 2B and C). Palmer and Stuart further sublocalized the site of AP initiation to the “distal AIS,” approximately 35 μm from the cell soma.
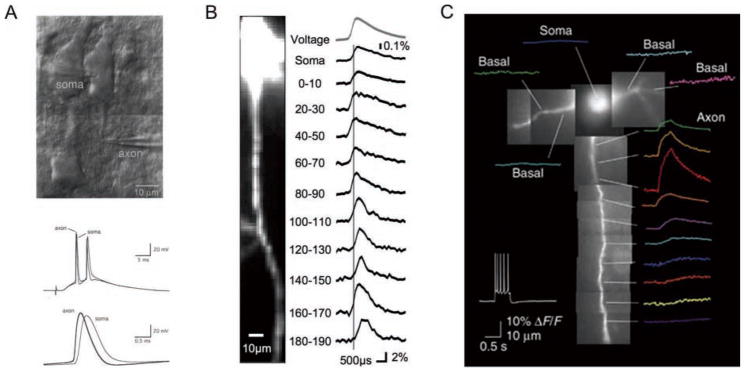
Figure 2.
Electrical and optical recording of spike initiation and Na+ concentration at the axon initial segment (AIS) of cortical layer 5 pyramidal cells. A, top, Infrared-differential interference contrast (IR-DIC) image of simultaneous somatic and axonal whole-cell recording from a layer 5 pyramidal neuron in an acute brain slice. Bottom, Superimposed traces of simultaneous somatic and axonal (thicker trace) recordings in response to extracellular stimulation. The spike recorded at the AIS precedes the spike recorded in the soma (from Stuart and others 1997a). B, Simultaneous imaging of the membrane potential in the soma and proximal axon with voltage-sensitive dye JPW1114. Action potential initiation occurred within the AIS approximately 35 μm from the axon hillock (from Palmer and Stuart 2006). C, Kole and others used the sodium-sensitive dye SBFI to monitor sodium concentration in the axon during a train of action potentials. Consistent with a high local density of sodium channels at the AIS, they found sodium transients were greatest approximately 25 μm from the axon hillock. Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Kole and others, copyright 2008).
AP generation at the AIS was hypothesized to be due to a lower threshold for the generation of APs at the AIS. This, in turn, was thought to be caused by a higher density of Na+ channels at the AIS and/or differences in the electrophysiological properties of the complement of Na+ channels in this compartment. Modeling studies (Dodge and Cooley 1973; Mainen and others 1995) supported the idea that the AIS must contain a higher density of Na+ channels, as did evidence provided by immunohistochemistry (e.g., Wollner and Catterall 1986; Boiko and others 2003). However, there was some ongoing debate of this fact based on electrophysiological data (see Colbert and Johnston 1996; Colbert and Pan 2002), suggesting that the density of Na+ channels at the AIS was the same or only slightly higher than at the soma.
Kole and others (2008a) recently provided strong and elegant evidence that, at least for pyramidal neurons in layer 5 of rodent neocortex, the AIS does indeed contain a much higher density of Na+ channels (approximately 50× the Na+ channel density of that found in the soma and proximal dendrites). This study also supported the existence of a hyperpolarized shift in the voltage dependence of activation and inactivation of Na+ channels at the AIS relative to the soma/dendrite, as suggested previously (Colbert and Pan 2002). Kole and others (2008a) used a clever strategy to resolve the Na+ channel density issue and distinguish somatic/proximal dendritic Na+ currents from those at the AIS. They performed whole-cell voltage-clamp recordings from the soma in a bath solution lacking sodium and then locally applied sodium-rich solution only to the proximal dendrite or the AIS to selectively monitor Na+ currents originating from each compartment. These authors suggested that outside-out patches pulled from the axon or cell-attached patches are largely stripped of Na+ channels because of molecular tethering to the cytoskeleton (Kole and others 2008a), explaining the discrepancy with the studies by Colbert and Johnston (1996) and Colbert and Pan (2002), which had used these methods.
After some ongoing debate (Clark and others 2005), the AIS was also recently shown to be the likely site of AP initiation in cerebellar Purkinje neurons (Khaliq and Raman 2006). However, unlike in other cell types examined, the Purkinje cell soma also appears to be able to support sustained generation of APs after blockade of AIS Na+ channels (Khaliq and Raman 2006). Khaliq and Raman (2006) hypothesize that this may represent a fundamental difference between spontaneously active cells such as Purkinje cells and other cell types. This is an intriguing hypothesis that requires further investigation.
In most cases where it has been investigated, APs are initiated in the proximal axon. It has been shown, for example, that the AIS is the likely site of AP initiation in dopaminergic neurons of the substantia nigra (Hausser and others 1995), granule cells in the dentate gyrus (Schmidt-Hieber and others 2008), mesencephalic trigeminal neurons (Saito and others 2006), hippocampal CA3 pyramidal neurons (Meeks and Mennerick 2007), and rat spinal neurons (Wolff and others 1998). In hippocampal CA1 oriensalveus GABAergic interneurons, AP initiation was shown to be axonal for low-intensity sustained stimuli, but shifted to somatodendritic locations for brief high intensity stimuli (Martina and others 2000).
Why are APs generated at the AIS? The most straightforward answer to this question, supported by new data, is that preferential AP initiation at the AIS is caused by a high local density of Na+ channels at the AIS. Also, the AIS is a smaller electrical compartment, and hence the amount of membrane capacitance that needs to be charged is small compared with that at the soma. This, coupled with the high local density of Na+ channel, produces a lower current threshold for AP generation at the AIS1.
What are the functional implications of the spike being initiated at the AIS? Perhaps with the AP initiated at the AIS, the spike-generating mechanism is electrically isolated from the soma/dendrite, allowing the AIS to serve as the single site of synaptic integration of all the inputs arriving to different parts of the dendrite (Mainen and others 1995). Furthermore, localization of the spike-generating machinery to a small subcellular compartment allows for targeted and potentially powerful modulation of spike initiation by local influences, such as inhibition (e.g., by the AIS-targeting chandelier cell; see Box 3). Finally, the large inward currents produced by the high density of Na+ channels at the AIS can generate sufficient current to charge and depolarize the larger somatic compartment and thereby promote back-propagation of spikes into the dendritic tree (as occurs in cortical pyramidal cells; Stuart and others 1997b). This back- propagation of APs, in turn, has been shown to endow neurons with additional computational abilities and mechanisms for plasticity (Kampa and others 2007).
Box 3. The AIS Is the Specific Target of Chandelier Cells.
Chandelier cells are a morphological subtype of GABAergic interneuron in mammalian cortex that have been the subject of much intrigue since their initial description by Szentágothai and Arbib (1974). Not only do chandelier cells have a fantastic morphology, but these cells exclusively form symmetrical synapses with the axon initial segments of pyramidal cells (Howard and others 2005). Hence, chandelier cells are often referred to as axo-axonic cells, displaying a remarkable specificity in the localization of their synapses (the AIS, but not the soma/dendrite) as well as in the cell types innervated (pyramidal cells, but not other GABAergic cells). It has been hypothesized that these cells might exert a particularly powerful inhibitory control of spike generation by virtue of these GABAergic axo-axonic synapses. Furthermore, alterations in chandelier cell function have been hypothesized to contribute to the pathogenesis of neurological and psychiatric diseases such as epilepsy and schizophrenia (DeFelipe 1999; Lewis and others 2005).
A recent report questioned this classic conceptualization of chandelier cells as inhibitory, showing that chandelier cell activity can actually drive spikes in target pyramidal cells in layer 2/3 of human and rodent neocortex (Szabadics and others 2006). The basis of this phenomenon was suggested to be due to a relative absence of the chloride transporter KCC2 (which extrudes chloride) at the AIS of these cells, such that the internal chloride concentration at the AIS is high and hence the reversal potential of the GABA-mediated postsynaptic potential produced by chandelier cell activity is depolarizing and actually excitatory. Additional data indicates that a high internal chloride concentration at the AIS is produced by chloride influx by the chloride transporter NKCC1 (Khirug and others 2008). A subsequent study performed in rodent hippocampus has questioned this conclusion (Glickfeld and others 2009). Based on extracellular field recordings, Glickfeld and others (2009) concluded that activation of a chandelier cell produces classical hyperpolarizing inhibitory responses at the majority of their pyramidal cell postsynaptic targets. Final resolution of this issue will require additional study.
Control of Action Potential Timing
As the site of AP initiation, the AIS is uniquely positioned to serve as a key location to regulate when APs are generated. There is a growing appreciation of the complex arrangement of channels clustered at the AIS, yet their individual and concerted contributions to the dynamic regulation of AP threshold remain poorly understood. Several recent studies have specifically investigated the critical role of channel properties and discrete channel localization at the AIS in regulating AP threshold and neuronal responsiveness.
Na+ Channel Localization in the Proximal Axon Regulates the Precise Timing of Action Potential Generation
In what is perhaps one of the most striking examples of the physiological significance of the precise site of AP initiation, Kuba and others, working in the auditory brain stem, provided compelling evidence that the spatial distribution of voltage-gated Na+ channels within the AIS, and hence presumably the precise site of spike initiation, can serve as a fine-tuning mechanism to optimize the neuron’s responsiveness to characteristic auditory frequencies (Kuba and others 2006).
In the nucleus laminaris of birds, the equivalent of the medial superior olive in mammals, neurons are organized in a tonotopic fashion and exhibit a number of specializations depending on the characteristic frequency (CF) of the sounds to which they respond best (Kuba and others 2006). These neurons are sensitive to interaural time differences (ITDs), which are used for sound localization. Kuba and others found that the amplitude of somatically recorded orthodromic and antidromic spikes were much lower in high and middle frequency neurons compared with low frequency neurons. This observation suggested a more distal site of AP initiation in middle and high CF neurons. When the authors explored the distribution of Na+ channels in these neurons they observed a distinct pattern depending on the neuron’s CF (Fig. 3). In agreement with their measurements of spike amplitude and Na+ current at the cell body, they found that Na+ channels were located more distally and along a shorter segment in high CF neurons, whereas in low CF neurons Na+ channels were located closer to the cell body and were enriched in a longer segment in the axon. Na+ channel distribution was intermediate in middle CF neurons. Kuba and others suggested that the site of spike initiation in the axon was arranged so as to produce the most sensitive detection of ITDs at a given CF.
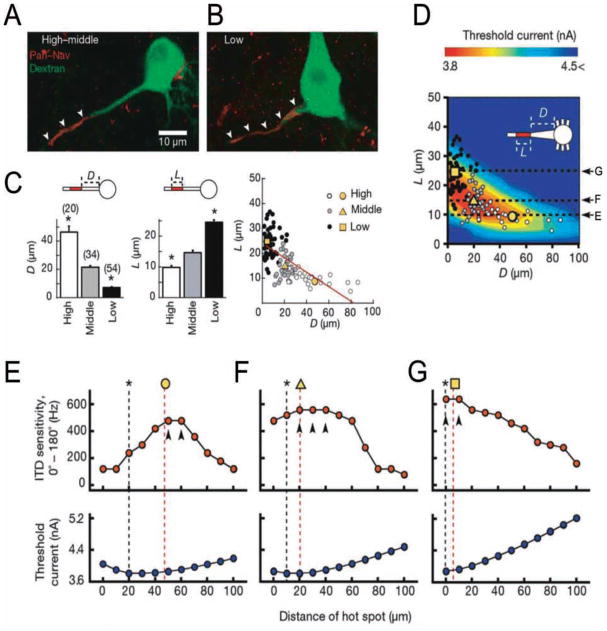
Figure 3.
Precise sodium channel distribution within the axon initial segment (AIS) fine-tunes neuronal responsiveness in the chick nucleus laminaris. The Na+ channel “hot spot” is arranged at greater distances from the cell body as the characteristic frequency (CF) of the neuron increases. A, B, Examples of Na+ channel hot spot localization in high-middle CF neurons (A) and low CF neurons (B). Nav channels = red; retrogradely labeled nucleus laminaris neurons = green. C, Relationship between geometry of axonal Na+ hot spot (Nav immunoreactivity) and CF found in nucleus laminaris (NL) neurons. High CF neurons had shorter hot spots (L) located at greater distance from the cell body (D), whereas low CF neurons hot spots were longer and closer to the cell body (yellow symbols are averages). D, Varying the geometry of the hot spot in multicompartment model simulations of NL neurons showed that the geometry producing the lowest threshold current (color coded; red corresponds to lowest threshold current) resembled the observed distribution of Na+ channels in NL neurons (shown in C). E–G, Dependence of interaural timing difference (ITD) sensitivity and threshold current on distance of Na+ hot spot from soma in model simulations of NL neurons. E, High CF (L = 10 μm). F, Middle CF (L = 15 μm). G, Low CF (L = 25 μm). Asterisks denote minimum threshold from data in panel D. Yellow symbols and red dotted lines indicate the actual observed average distances from data in panel C. Arrowheads show the maximum ITD sensitivity. Adapted by permission from Macmillan Publishers Ltd: Nature Kuba and others, copyright 2006.
Kuba and others (2006) then used a multiple compartment model to investigate how the precise localization and length of the “hot spot” of Na+ channels in the proximal axon influenced AP generation (Fig. 3). Their modeling recapitulated the essential features of the experimental observations and demonstrated that the threshold current for spike generation was critically affected by the geometry of the hot spot of Na+ channels. For example, when the hot spot was short (10 μm), increasing its distance from the soma reduced the threshold current, presumably by isolating the site of spike initiation from the large somatic membrane capacitance, with a minimum threshold (for the detection of an AP at the soma) at a distance of 20 to 30 μm. With a longer hot spot (25 μm) the minimum threshold was found closer to the soma, perhaps because hot spot length increases the current available to charge the somatic capacitance. In addition to the influence of somatic capacitance, the modeling showed that K+ current activation can also significantly affect spike generation. Kuba and others (2006) concluded that electrical isolation of the site of initiation enhances the ITD sensitivity to high frequency inputs.
Why do high CF neurons prefer the strategy of increasing the separation between soma and site of initiation (rather than increasing the length of the Na+ channel hot spot) to maximize ITD sensitivity? Kuba and others argue that, during high frequency stimulation, synaptic potentials temporally summate and depolarize the hot spot, which in turn produces inactivation of Na+ channels. The depolarization, and hence the degree of Na+ channel inactivation, are attenuated as the hot spot is separated farther from the soma. Thus it appears that electrical isolation of the site of AP initiation may carry computational advantages for neurons tuned to respond to high frequency inputs. Furthermore, one of the most intriguing aspects of this study is the demonstration of how AP initiation at the AIS is exquisitely sensitive to what may be considered small changes in the distribution of channels at the AIS, and how this might be used to fine-tune neuronal responsiveness for specific computations.
Voltage-Gated K+ Channels of the Kv1 Subfamily Are Powerful Regulators of Spike Initiation in Neocortical Fast-Spiking Interneurons
An example of how K+ channels present at the AIS can have a major influence on AP generation was recently described in neocortical fast-spiking (FS) GABAergic interneurons. Goldberg and others (2008) found that the threshold for AP generation in neocortical FS cells is dynamically and powerfully regulated by Kv1.1-containing Kv1 channels specifically enriched at the AIS. FS cells are the most numerous class of GABA-releasing (GABAergic) neurons in cortex,2 and provide fast, strong perisomatic inhibition of target cells, effectively suppressing their excitability. This FS cell-mediated inhibition is thought to be important for the maintenance of excitatory-inhibitory balance and the synchronization of pyramidal cells, and is implicated in the generation of fast cortical rhythms (Lawrence and McBain 2003; Traub and others 2004).
FS cells are capable of discharging high frequency trains of APs both in vitro and in vivo (Connors and Gutnick 1990; Swadlow 2003). Goldberg and others (2008) found that channels containing Kv1.1 subunits enriched at the AIS were critical in gating this high frequency discharge, because blockade of Kv1.1 channels at the AIS dramatically lowered AP threshold, resulting in high frequency discharge at what would normally be subthreshold depolarizations (Fig. 4). Furthermore, Kv1.1 channel blockade converted the delay-type discharge pattern of layer 2/3 FS cells to one of continuous fast-spiking without influencing the high frequency firing that defines FS cells (Figs. 4 and 5).
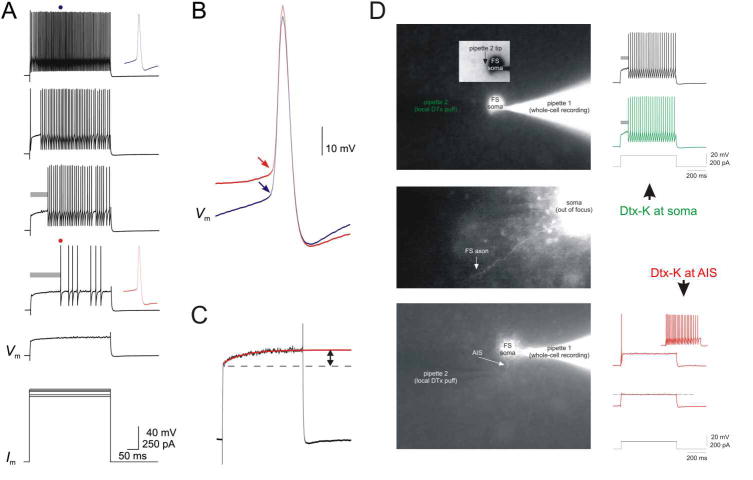
Figure 4.
Kv1-mediated currents at the axon initial segment (AIS) regulate neocortical fast-spiking (FS) interneuron near-threshold excitability. FS cells in superficial layers of somatosensory cortex respond to near-threshold depolarizing current steps with delayed firing. A, Increasing amounts of depolarizing current injection first produces slow depolarization (bottom Vm trace; expanded in C) followed by delayed firing, and with sufficient current, continuous firing. B, Delayed firing is associated with a positive shift in voltage threshold. Shown are the action potentials (APs) indicated in A; first AP during delayed firing (red trace) and time-matched AP during continuous firing (blue trace). C, Just subthreshold depolarizations produce a slow ramp depolarization in layer 2/3 FS cells (red; single exponential fit, t = 118 ms; arrow, amplitude 4.9 mV). D, Local application of DTX-K at the FS cell AIS abolishes the near-threshold slow ramp depolarization and delayed firing. Modified from Goldberg and others (2008) with permission from Elsevier.
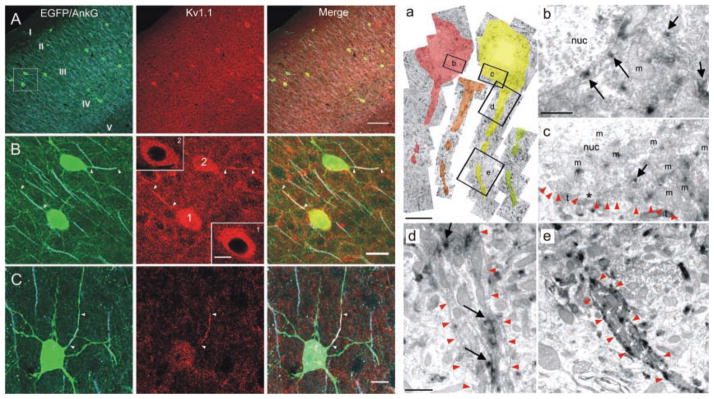
Figure 5.
Kv1.1 subunits are enriched at the axon initial segment (AIS) of neocortical fast-spiking (FS) interneurons. Left panels, A–C, Immunolocalization of Kv1.1 protein in mouse somatosensory cortex. A, Confocal image of double immunolabeling for Kv1.1 and the AIS marker, Ankyrin G (AnkG), in cortex of a transgenic mouse expressing EGFP in FS cells. Left panel, Superimposed EGFP (green) and AnkG (light blue) labeling. Middle panel, Kv1.1 staining (red). Right panel, Merged image showing all three signals. Note the enriched Kv1.1 staining in EGFP-labeled FS cells. Boxed region is magnified in B. B, Higher magnification of FS cells in boxed region in A. Kv1.1 protein colocalizes with AnkG at the AIS of FS cells (arrowheads). Somatic Kv1.1 staining in FS cells surrounded the nucleus, consistent with intracellular protein localization in the soma (insets). C, Additional example of Kv1.1 enrichment at the AIS of EGFP-positive FS cell. Right panels (a–e), Kv1.1 protein is enriched at axonal but not somatic membrane. a, Montage image of successive electron micrographs of Kv1.1-immunopositive nonpyramidal cell. Boxed regions are shown in b to e. Note intracellular Kv1.1 labeling in b and c, the lack of Kv1.1 labeling at the plasma membrane of the soma (c) and the axon hillock (d), and the concentration of Kv1.1 labeling at the membrane of the AIS in e. Red arrowheads outline the somatic plasma membrane in c, and the axonal plasma membrane in d and e. Modified from Goldberg and others (2008) with permission from Elsevier.
In addition to a general dampening of near-threshold excitability, the Kv1-mediated current at the AIS was also required for other dynamic properties of AP threshold in FS cells. The authors found that sufficiently large, fast inputs could “outrun” this dampening influence and efficiently drive FS cells. Last, via accumulation of Kv1 channel inactivation, Goldberg and others found that spike threshold was sensitive to the short-term history of the membrane potential. For example, subthreshold depolarizations applied before a previously subthreshold stimulus could then produce AP discharge, further highlighting the dynamic role of AIS-localized Kv1 channels in the modulation of FS cell AP threshold. These conclusions were further supported by computational modeling, which showed that Kv1 channels must be localized to the spike-generating zone (the AIS) to exert a robust dampening influence on FS cell excitability (Goldberg and others 2008).
Another example of potassium channels at the AIS contributing to modulation of AP generation comes from the brain stem neurons of the medial nucleus of the trapezoid body (MNTB) in the mammalian auditory pathway. These cells link auditory nuclei at the level of the superior olivary complex, and are known to be critical in early auditory processing such as the detection of ITDs, used for sound localization (Schneggenburger and Forsythe 2006).
Like cortical FS cells, these neurons can respond to very high frequency stimuli, a property that largely depends on the presence of K+ channels of the Kv3 subfamily (Rudy and McBain 2001). However, in contrast to FS cells, which respond to suprathreshold current steps with sustained high frequency spike trains, MNTB neurons fire only one AP at the start of the depolarization (Brew and Forsythe 1995). This is mediated by a large Kv1 current that ensures the generation of a single AP per crest of synaptic activity. The Kv1 current, probably mediated by channels containing Kv1.1 and Kv1.2 subunits specifically localized to the AIS, is activated along with the AP and prevents repetitive firing in the face of sustained depolarization (Dodson and others 2002). This provides temporal precision and tight control of spike timing at this auditory nucleus.
In both cell types, cortical FS cells and MNTB neurons, the Kv1 channels are located at the AIS and apparently excluded from somatic membrane, and both seem to contain Kv1.1 and Kv1.2 subunits (Dodson and others 2002; Goldberg and others 2008); however, these channels have different effects on firing in the two types of cell. The basis of these differences remains to be investigated, but may include differences in the kinetics and voltage dependence of the Kv1 current resulting from differences in precise subunit composition (including the types of Kvβ subunits present), differences in the precise localization of the channels within the AIS (given the observations in the nucleus laminaris described earlier), or differences in other membrane properties.
Regulation of AIS Function by GABAA Receptors
A specialized class of cortical GABAergic interneurons, known as chandelier cells, specifically target the AIS of cortical pyramidal cells and are thought to control the timing of their output. This GABAergic control is mediated by postsynaptic GABAA receptors at the AIS of pyramidal cells. The action of GABA at this axo-axonic synapse (whether it produces excitation or inhibition) has been the subject of recent study and controversy (see Box 3). In addition, GABAA receptors containing the α2 subunit are enriched at this axo-axonic synapse in cortex (Nusser and others 1996), which may have important implications for disease (see Box 4) and the actions of benzodiazepines (Rudolph and others 1999; Rudolph and Mohler 2006).
Box 4. The AIS and Disease.
Continued progress in our understanding of the organization and function of the AIS may yield important insights into the link between molecular defects and disease phenotypes. For instance, ion channels now known to be expressed at the AIS of various subtypes of cortical neurons are mutated in human epilepsy syndromes and their presence at the AIS might be a factor contributing to these diseases. One example is the Na+ channel NaV1.1 encoded by the SCN1A gene. Mutations in SCN1A cause a range of epilepsy phenotypes (depending on the exact mutation) that vary in severity and include generalized epilepsy with febrile seizures plus (GEFS+) and severe myoclonic epilepsy of infancy (SMEI; Escayg and others 2000; reviewed in Ragsdale 2008). As we discussed earlier, in cortex, NaV1.1 is expressed at the AIS of parvalbumin expressing GABAergic interneurons (see section titled “Molecular Organization of the AIS”). A recent report demonstrated reduced sodium currents in GABAergic inhibitory interneurons (but not in principal neurons) in mice with partial or total loss of NaV1.1, a mouse model of SMEI (Yu and others 2006; Oakley and others 2009). These data implicate sodium channels at the AIS in the pathogenesis of epilepsy.
K+ channels containing Kv1.1 subunits are another example. Mutations of Kv1.1 (KCNA1) are associated with hyper-excitability phenotypes including episodic ataxia type 1 (EA1) and various forms of epilepsy in humans (Browne and others 1994; Eunson and others 2000; Liguori and others 2001), and genetic deletion of Kv1.1 causes profound epilepsy in mice (Smart and others 1998). As discussed earlier, Kv1.1-containing channels are expressed at the AIS of cortical FS GABAergic interneurons where they have powerful effects on spike initiation. However, Kv1.1 is expressed widely, particularly in axons, juxtaparanodes, and synaptic terminals in several neuronal populations in cortical structures. Therefore it still remains to be shown that channels specifically at the AIS contribute to the epilepsy phenotypes.
Similarly, mutation of the K+ channel subunits KCNQ2 and KCNQ3, which are also expressed at the AIS of cortical neurons (see section titled “Molecular Organization of the AIS”) produces the epilepsy syndrome of benign familial neonatal convulsions (BFNC; reviewed in Jentsch 2000). Retigabine, an activator of the KCNQ channels, was shown in a recent randomized multi-center clinical trial to be effective in otherwise treatment-refractory partial epilepsy (Porter and others 2007). It is not known, however, how KCNQ channels in different subcellular compartments contribute to BNFC or whether the effect of retigabine is via modulation of KCNQ channels localized to the AIS or other locations.
Finally, there are some intriguing findings in research on schizophrenia that may implicate the AIS. GABAA receptors containing the α2 subunit are enriched at the axo-axonic synapses formed by presynaptic chandelier cells onto the post-synaptic pyramidal neuron AIS in cortex (Nusser and others 1996). It has been shown that there is an increase in the density of α2 GABA-R subunits in postmortem tissue from the prefrontal cortex of individuals with schizophrenia, which is accompanied by a decrease in the density of the GABA transporter 1 (GAT-1) at the presynaptic terminals of chandelier cell synapses (Volk and others 2002). Although it is difficult to separate cause from effect, these findings suggest that there is alteration in the inhibitory control of the AIS in the brains of patients with schizophrenia, which might, in turn, directly implicate chandelier cells in the pathogenesis of this disease or reflect a compensatory response by the chandelier cell system to other defects, possibly at the AIS of pyramidal cells.
Although direct evidence that pathology of the AIS compartment is involved in disease is still lacking, this is an intriguing hypothesis, given that neuronal function is highly sensitive to the properties and location of channels present at this site.
Kv1 Channels and Control of the Axonal Spike Waveform in Neocortical Pyramidal Cells
Enrichment of Kv1-Mediated Currents at the AIS: Insights from Direct Axonal Recordings
In the neocortex, Kv1 channels have also been demonstrated at the AIS of neocortical pyramidal neurons, the predominant cell type in the cortex. In these neurons it has been possible to obtain direct electrophysiological recording from the axon, and hence the properties of the Kv1 channels have been well characterized. This was accomplished either by whole-cell recording from the axonal “blebs” formed at the cut ends of axons sectioned during slice preparation (Shu and others 2006) or directly from the axons of particularly large layer 5 pyramidal cells (Kole and others 2007).
Voltage-clamp recordings confirmed the presence at the AIS of a DTX-I-sensitive K+ current that activated rapidly, inactivated slowly, and was first apparent at subthreshold membrane potentials (Kole and others 2007; Shu and others 2007; see box 2). The Kv1 current was also blocked by rTityustoxin-Kα, suggesting that the channels contain Kv1.2 subunits, but not by DTX-K, suggesting a lack of Kv1.1 proteins (Shu and others 2007). Kole and others found that the amplitude of this current was greatest in the distal (35–55 μm from the axon hillock) portion of the AIS as opposed to the proximal AIS (5–30 μm from the axon hillock) and was reduced at the soma. Interestingly, current densities remained relatively high at more distal parts of the axon (Kole and others 2007). These direct measurements support a prior immunohistochemical study that indicated that Kv1.2 subunits are enriched in the distal portion of the AIS in human pyramidal cells, in contrast to the uniform distribution of sodium channels over the first ~40 μm of the axon (Inda and others 2006).
Kv1 Channels in the Proximal Axon of Neocortical Pyramidal Cells Regulate AP Duration in the Axon Independently of the Soma
Because the neuronal cell body has been the site of most intracellular recordings from neurons, electrophysiologists have tended to have a somatocentric view of neurons. However, some of the important consequences of recording an event that originates in the axon and back-propagates into the soma are beginning to be appreciated in light of the recent direct axonal recordings in neocortical pyramidal cells (Yu and others 2008). One such finding is that the duration of the AP waveform is regulated in the axon independently from the soma.
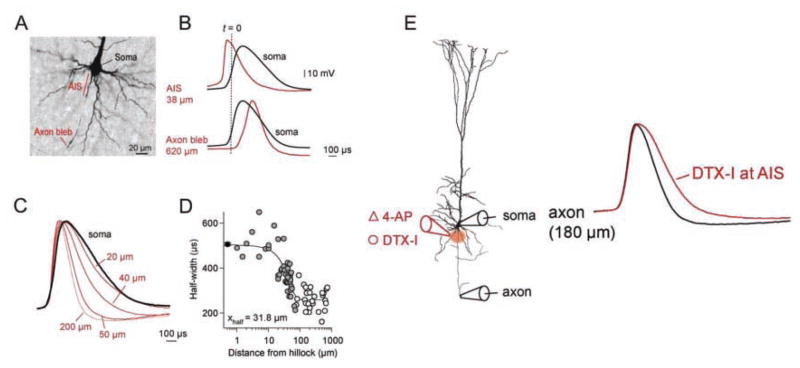
Shu and others (2007) and Kole and others (2007) showed that the Kv1 channels at the AIS of neocortical pyramidal neurons have a major role in regulating spike repolarization at the axon, producing a much briefer spike in the axon than at the soma. Spike duration changed along the axon, matching the observed changes in Kv1 channel density. AP half-width decreased from ~500 μs at the soma to ~250 μs at the distal AIS and beyond. This was accompanied by a concomitant increase in the depth of the after-hyperpolarization (AHP; Kole and others 2007). Application of Kv1 channel blockers produced a large broadening of the axonal but not of the somatically recorded spike (Fig. 6).
Figure 6.
Axonal Kv1 channels selectively control axonal action potential (AP) waveform in neocortical pyramidal cells. A, Image of layer 5 pyramidal cell indicating the AIS and cut axonal bleb. B, Simultaneous whole-cell recordings from the soma (black traces) and the axon initial segment (AIS) or axon bleb (red traces). Dotted line (t = 0) indicates the onset of the somatically recorded AP. Note the AP recorded at the AIS precedes the somatic AP; also note the differences in AP duration between the soma and axonal recordings. C, AP waveform narrows in the axon. Shown are example APs recorded at the soma (black) and at increasing distances along the axon (red traces). D, Plot of axonal AP duration (AP width at half-amplitude: half-width, μs) as a function of recording distance from the axon hillock (μm). E, Local application of Kv1 channel blocker, dendrotoxin-I (DTX-I) at the AIS selectively increases axonal but not somatic AP duration. Shown are the axonal AP before (black) and after (red) DTX-I at the AIS. Modified from (Kole and others 2007) with permission from Elsevier.
After initiation at the AIS, differences in size, and hence the membrane capacitance to be charged, between the soma and the axon will differentially affect the orthodromic and back-propagated spike. Kole and others (2007) observed that local application of Kv1 channel blockers at the AIS broadened the AP at the site of application by 45%, and this increase in spike width propagated down the axon with a length constant of ~230 μm, yet the brief APs recorded at the AIS are not observed at the soma just <50 μm away. Spike width at the soma depends on the repolarizing influence of the channels in that compartment, which do not appear to include appreciable amounts of Kv1 channels. Thus, Kv1 channels are present in high densities at the AIS and with activation kinetics fast enough to play a critical role in repolarization of axonal APs with minimal impact on somatic AP repolarization.
These observations are significant because they demonstrate that spike properties can be independently modulated in the axon and soma by the local constellation of ion channels present in each compartment. As a result, the properties of APs recorded at the soma (as is usually done)—which represent spikes that have back propagated from the site of spike initiation in the axon to the cell body)—may not reflect the properties of the orthodromically propagating axonal spike or the spike waveform at the presynaptic terminal, which is the spike relevant for transmitter release and interneuronal communication.
Kole and others (2007) have suggested that, by shortening the duration of cortical axonal APs as well as by increasing the amplitude of the after-hyperpolarization, the high density of Kv1 channels in the AIS and the axon proper will act to increase the fidelity of axonal AP propagation during high frequency AP bursts. As discussed below, this role of Kv1 channels at the AIS in regulating the duration of the axonal spike may not apply to neocortical FS GABAergic interneurons, which utilize instead Kv3 channels for fast repolarization and facilitation of high frequency sustained repetitive firing (Erisir and others 1999; Lau and others 2000; Rudy and McBain 2001).
The actions of Kv1 channels at the AIS of FS and pyramidal neurons also differ in that, as opposed to the large effects of blocking Kv1 channels at the AIS on AP threshold in FS cells discussed in the previous section, there are less potent effects of DTX-I on the threshold for APs in neocortical pyramidal neurons (Bekkers and Delaney 2001; Guan and others 2007; Goldberg and others 2008). It is not known why Kv1 channels have different effects on spike threshold in these two types of cortical neurons. As discussed earlier for the differences between FS cells and MNTB neurons, possibilities include differences in the kinetics and voltage dependence of the Kv1 current, the precise localization of the channels within the AIS, or differences in other membrane properties.
Kv1 Channels in the Proximal Axon of Pyramidal Neurons Regulate Synaptic Transmission
Direct electrical measurement from axons has also led to the discovery of a new and somewhat unanticipated signaling role played by the AIS and the proximal axon.
In most neurons, graded changes in membrane potential, either synaptic potentials or receptor potentials in sensory neurons, are propagated to the proximal axon where, if they reach threshold, they generate “all-or-none” APs that are propagated to the synaptic terminals to initiate neurotransmitter release. However, in some neurons, graded changes in membrane potential are propagated to the synapse, where they produce graded “analog” transmitter release. It is said that these “graded synapses” use an “analog” code of information transmission, in contrast to information transfer by the discontinuous, impulse-mediated binary or “digital” mechanism (Juusola and others 1996).
Intracortical synaptic transmission has traditionally been presumed to operate in the AP-mediated digital mode of transmission. However, recent studies have overturned this generalization and have revealed that at certain intracortical synapses, namely the hippocampal granule cell (mossy fiber axon)-to-CA3 principal cell synapse and synapses between layer 5 neocortical pyramidal neurons, transmission can occur in a mixed analog-digital fashion. These discoveries have been discussed in detail elsewhere in recent reviews (Clark and Hausser 2006; Alle and Geiger 2008). The main finding is that subthreshold somatodendritic depolarizations can travel significant distances down the axon and modulate AP-dependent transmitter release (Fig. 7).
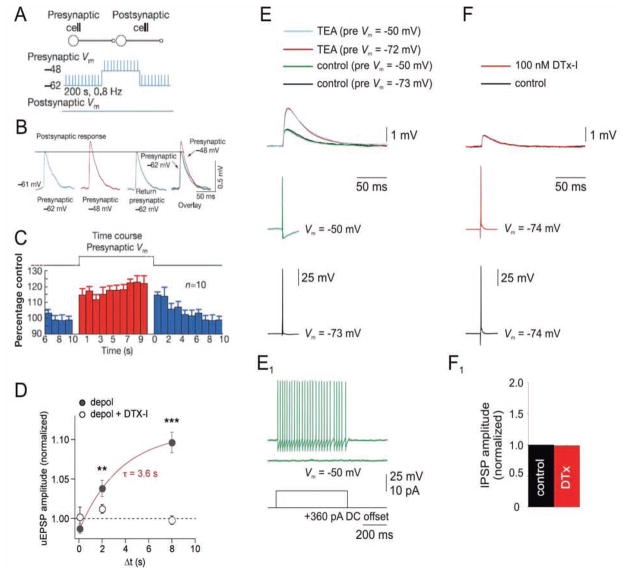
Figure 7.
Presynaptic somatic membrane potential modulates synaptic transmission via the slow inactivation of axonal Kv1 channels in neocortical pyramidal cells. A, Recording protocol from Shu and others (2006) in which the presynaptic layer 5 pyramidal cell membrane potential is alternatively held near rest (−62 mV in this example) or near firing threshold (−48 mV), and brief stimuli produce action potentials (APs; at 0.8 Hz) from each membrane potential. The resulting excitatory post-synaptic potential (EPSPs) in the postsynaptic pyramidal cell are shown in B. B, APs produce facilitated postsynaptic responses when the presynaptic membrane is depolarized (−48 mV; red trace). C, Average time course of facilitation and disfacilitation of EPSPs. Adapted by permission from Macmillan Publishers Ltd: Nature (Shu and others 2006), © 2006. D, Depolarized presynaptic membrane potential-induced facilitation of EPSPs is abolished by Kv1 channel blocker dendrotoxin-I (DTX-I). Plot illustrates the facilitation of EPSPs by increasing durations (Δt) of presynaptic depolarization (solid circles) and block by DTX-I (open circles). Modified from Kole and others (2007) with permission from Elsevier. E, F, Somatic depolarization and DTX-I do not increase unitary AP-evoked GABA postsynaptic potentials (uGPSP) in fast-spiking (FS) interneuron → pyramidal cell (PC) pairs. E, APs evoked from depolarized presynaptic potentials (green traces; −50 mV; sufficient to produce Kv1 channel inactivation based on lack of delayed firing in presynaptic FS cell, E1) or hyperpolarized (~−70 mV) potentials (black traces) produce no change in uGPSP amplitude. However, application of 1 mM TEA, which blocks Kv3 channels, produces an approximately twofold increase in uGPSP amplitude, consistent with previous results (Goldberg and others 2005). F, Bath application of DTX-I has no effect on uGPSP amplitude in FS → PC pairs. F1, Summary data (n = 3). Modified from Goldberg and others (2008) with permission from Elsevier.
We highlight this work here because, in neocortical pyramidal cells (but apparently not in mossy fiber axons), the effect of subthreshold depolarizations on synaptic transmission was found to be mediated by the dendrotoxin-sensitive axonal Kv1 K+ channels described earlier (Kole and others 2007; Shu and others 2007). Subthreshold depolarizations promote inactivation of the Kv1 channels, which in turn produces broadening of the axonal spike. The broadened spike is efficiently propagated to synaptic terminals that are close to the soma (given a length constant of propagation of ~500 μm) producing elevated Ca2+ influx, which in turn increases transmitter release probability (Fig. 7).
In these layer 5 pyramidal neurons, Kv1 channel density remains relatively high throughout the distal axon (Shu and others 2007; Kole and others 2007), and it must be high at the terminal inasmuch as bath applied DTX-I produces a much larger increase in transmitter release than that produced by local application to the AIS or that produced by somatic subthreshold depolarizations (Kole and others 2007). Given this, and the fact that the subthreshold depolarizations themselves propagate down the axon with a similar length constant as the broadened spike, it is not clear if a high density of Kv1 channels specifically at the AIS is necessary for the observed effect of subthreshold depolarizations on spike-evoked transmitter release. This could be tested by investigating the effect of local application of DTX-I at the AIS on the increased transmitter release following subthreshold depolarizations.
In contrast to the pyramidal-to-pyramidal cell synapse, somatic subthreshold depolarizations do not seem to modulate GABA release from cortical FS interneurons onto pyramidal cells (Fig. 7). In addition, in FS cells, DTX-I does not affect the size of the AP-evoked postsynaptic response recorded in pyramidal neurons (Goldberg and others 2005,Goldberg and others 2008).
Why do somatic depolarizations fail to modulate transmitter release from FS neurons? One likely possibility is that Kv1 channels in FS cells do not influence axonal AP repolarization and therefore AP duration. As in the soma of these neurons, fast repolarization of axonal APs is controlled mainly by K+ channels of the Kv3 subfamily, which are highly enriched in the soma, axons, and terminals of FS cells (Goldberg and others 2005; Chow and others 1999). Indeed, blocking Kv3 channels at the FS cell terminals produces enhanced transmitter release, which is likely due to AP broadening as it is associated with an increase in Ca2+ influx (Goldberg and others 2005). Because Kv3 channels do not undergo appreciable voltage- and time-dependent inactivation, subthreshold somatodendritic depolarizations are not expected to modulate transmitter release in a manner paralleling that of Kv1 channels in pyramidal cells.
Conclusions and Future Directions
In conclusion, the AIS is a highly organized axonal domain that appears specialized to function as the site of spike initiation in neurons. Our understanding of the structure and functional roles of the AIS has progressed with the advent of new approaches and new and more refined experimental techniques spanning more than a century of investigation. What has emerged is an appreciation of the molecular complexity of this domain and a better understanding of how some of the ion channels present at the AIS contribute to its electrical signaling properties. It is likely that other ion channels and receptors besides those identified so far are also present at the AIS. We can expect that some of these will be identified soon, as this compartment is the focus of intense study. We also still need to understand how similar channel proteins at the AIS have different effects in different neuronal cell types.
Given that multiple types of ion channels are enriched at the AIS, one key area of future study will be to determine if the AIS is the target of neuromodulatory substances. Ion channels are frequent targets of neuromodulators in other subcellular compartments, and it is intriguing to speculate that key aspects of neuronal function are regulated by modulatory influences at the AIS. Also, synaptic plasticity at the AIS is virtually unexplored. The AIS of cortical pyramidal cells is the specific postsynaptic target of GABAergic chandelier cells, and thus synaptic plasticity at this axo-axonic contact would also be expected to have powerful consequences for AP output. Finally, as the molecular organization and assembly mechanisms of the AIS are better understood, the AIS may prove to be an ideal domain to target the expression of the new generation of genetically encoded activators, inhibitors, and optically gated channels for the control of neuronal output.
Note added in proof: Adding to the evidence that the AIS has functional subdomains, Hu and others (2009) recently showed that distinct Na+ channel isoforms located at the distal and proximal AIS, regulate AP initiation and backpropagation, respectively.
Acknowledgments
The authors thank Barry Connors, Alan Peters, Matthew Rasband, and Edward Zagha for their feedback during the preparation of this review.
Footnotes
Based on simultaneous somatic and axonal patch-clamp recordings, Kole and Stuart (2008b) have shown that because of the activation of AIS localized sodium channels prior to AP generation, the AP voltage threshold is actually more depolarized in the AIS compared to that measured at the soma by conventional methods.
GABA is the main inhibitory neurotransmitter in the CNS.
References
- Alle H, Geiger JR. Analog signalling in mammalian cortical axons. Curr Opin Neurobiol. 2008;18:314–20. doi: 10.1016/j.conb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Araki T, Otani T. Response of single motoneurons to direct stimulation in toad’s spinal cord. J Neurophysiol. 1955;18:472–85. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Delaney AJ. Modulation of excitability by alpha-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. J Neurosci. 2001;21:6553–60. doi: 10.1523/JNEUROSCI.21-17-06553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron. 2009;61:259–71. doi: 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop PO. Synaptic transmission; an analysis of the electrical activity of the lateral geniculate nucleus in the cat after optic nerve stimulation. Proc R Soc Lond B Biol Sci. 1953;141:362–92. doi: 10.1098/rspb.1953.0048. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci. 2003;23:2306–13. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. J Neurosci. 1995;15:8011–22. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–6. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8:136–40. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Leyes de la morfologia y dinamismo de las células nerviosas. Rev Trim Micrográf. 1897;2:1–12. [Google Scholar]
- Ramon y Cajal S. Recollections of my life. Cambridge (MA): MIT Press; 1989. [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Chow A, Erisir A, Farb C, Nadal MS, Ozaita A, Lau D, Welker E, Rudy B. K(+) channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J Neurosci. 1999;19:9332–9345. doi: 10.1523/JNEUROSCI.19-21-09332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B, Hausser M. Neural coding: hybrid analog and digital signalling in axons. Curr Biol. 2006;16:R585–8. doi: 10.1016/j.cub.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Clark BA, Monsivais P, Branco T, London M, Hausser M. The site of action potential initiation in cerebellar Purkinje neurons. Nat Neurosci. 2005;8:137–9. doi: 10.1038/nn1390. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–85. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J Neurosci. 1996;16:6676–86. doi: 10.1523/JNEUROSCI.16-21-06676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci. 2002;5:533–8. doi: 10.1038/nn0602-857. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Conradi S. Ultrastructural specialization of the initial axon segment of cat lumbar motoneurons. Preliminary observations. Acta Soc Med Ups. 1966;71:281–4. [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The interpretation of spike potentials of motoneurones. J Physiol. 1957a;139:198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The generation of impulses in motoneurones. J Physiol. 1957b;139:232–49. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122 (Pt 10):1807–22. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–44. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiters OFK. Untersuchungen über Gehirn und Rückenmark des Menschen und der Säugetiere. Braunschweig Vieweg 1865 [Google Scholar]
- Dodge F, Cooley J. Action Potential of the Motorneuron. IBM J Res Dev. 1973;17:219–29. [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci. 2002;22:6953–61. doi: 10.1523/JNEUROSCI.22-16-06953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL. Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J Cell Biol. 2007;177:857–70. doi: 10.1083/jcb.200612012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C, Ottoson D. The site of impulse initiation in a nerve cell of a crustacean stretch receptor. J Physiol. 1958;143:138–48. doi: 10.1113/jphysiol.1958.sp006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol. 1999;82:2476–89. doi: 10.1152/jn.1999.82.5.2476. [DOI] [PubMed] [Google Scholar]
- Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–45. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- Eunson LH, Rea R, Zuberi SM, Youroukos S, Panayiotopoulos CP, Liguori R, et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 2000;48:647–56. [PubMed] [Google Scholar]
- Fatt P. Sequence of events in synaptic activation of a motoneurone. J Neurophysiol. 1957;20:61–80. doi: 10.1152/jn.1957.20.1.61. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12:21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Rudy B. An axonal Dtx sensitive current produces delayed firing and regulates near-threshold excitability of neocortical GABAergic fast- spiking interneurons. Soc Neurosci Abstr. 2006:234.15. [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Watanabe S, Chang SY, Joho RH, Huang ZJ, Leonard CS, et al. Specific functions of synaptically localized potassium channels in synaptic transmission at the neocortical GABAergic fast-spiking cell synapse. J Neurosci. 2005;25:5230–5. doi: 10.1523/JNEUROSCI.0722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, Lee JC, Higgs MH, Spain WJ, Foehring RC. Functional roles of Kv1 channels in neocortical pyramidal neurons. J Neurophysiol. 2007;97:1931–40. doi: 10.1152/jn.00933.2006. [DOI] [PubMed] [Google Scholar]
- Hausser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–47. doi: 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- Harvey AL. Twenty years of dendrotoxins. Toxicon. 2001;39:15–26. doi: 10.1016/s0041-0101(00)00162-8. [DOI] [PubMed] [Google Scholar]
- Hedstrom KL, Rasband MN. Intrinsic and extrinsic determinants of ion channel localization in neurons. J Neurochem. 2006;98:1345–52. doi: 10.1111/j.1471-4159.2006.04001.x. [DOI] [PubMed] [Google Scholar]
- Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–40. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–6. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav1.2 in action potential initiation and backpropagation. 1. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Munoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci U S A. 2006;103:2920–5. doi: 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–46. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Johnston J, Griffin SJ, Baker C, Skrzypiec A, Chernova T, Forsythe ID. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons. J Physiol. 2008;586:3493–509. doi: 10.1113/jphysiol.2008.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M, French AS, Uusitalo RO, Weckstrom M. Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 1996;19:292–7. doi: 10.1016/S0166-2236(96)10028-X. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity. Trends Neurosci. 2007;30:456–63. doi: 10.1016/j.tins.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci. 2006;26:1935–44. doi: 10.1523/JNEUROSCI.4664-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci. 2008;28:4635–9. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–47. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008a;11:178–86. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Is action potential lowest in the axon? Nat Neurosci. 2008b;11:1253–5. doi: 10.1038/nn.2203. [DOI] [PubMed] [Google Scholar]
- Kölliker A. Neurologische Bemerkungen. Z wiss Zool. 1849;1:135–63. [Google Scholar]
- Kress GJ, Mennerick S. Action potential initiation and propagation: upstream influences on neurotransmission. Neuroscience. 2009;158:211–22. doi: 10.1016/j.neuroscience.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–72. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–62. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Lau D, Vega-Saenz de Miera EC, Contreras D, Ozaita A, Harvey M, Chow A, et al. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 2000;20:9071–85. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation--feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–40. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Liguori R, Avoni P, Baruzzi A, Di Stasi V, Montagna P. Familial continuous motor unit activity and epilepsy. Muscle Nerve. 2001;24:630–3. doi: 10.1002/mus.1048. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–40. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Joerges J, Huguenard JR, Sejnowski TJ. A model of spike initiation in neocortical pyramidal neurons. Neuron. 1995;15:1427–39. doi: 10.1016/0896-6273(95)90020-9. [DOI] [PubMed] [Google Scholar]
- Martina M, Vida I, Jonas P. Distal initiation and active propagation of action potentials in interneuron dendrites. Science. 2000;287:295–300. doi: 10.1126/science.287.5451.295. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Action potential initiation and propagation in CA3 pyramidal axons. J Neurophysiol. 2007;97:3460–72. doi: 10.1152/jn.01288.2006. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–44. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Natl Acad Sci U S A. 2009;106:3994–9. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Rasband MN. The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol. 2008;18:307–13. doi: 10.1016/j.conb.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci. 2008;28:5731–9. doi: 10.1523/JNEUROSCI.4431-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. Na(v)1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–14. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Sotelo C, Peters A, Orkand PM. The axon hillock and the initial segment. J Cell Biol. 1968;38:193–201. doi: 10.1083/jcb.38.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–63. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, et al. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Partiot A, Sachdeo R, Nohria V, Alves WM. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS. How do mutant Nav1.1 sodium channels cause epilepsy? Brain Res Rev. 2008;58:149–59. doi: 10.1016/j.brainresrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Remak R. Über den Bau der grauen Säulen im Rückenmark der Säugethiere. Deutsche Klinik. 1855;7:295. [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–26. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Rudy B, Maffie J, Amarillo Y, Clark B, Goldberg EM, Jeong HJ, et al. Voltage gated potassium channels: structure and function of kv1 to kv9 subfamilies. In: Squire L, editor. Encyclopedia of neuroscience. Oxford: Academic Press; 2009. pp. 397–425. [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Murai Y, Sato H, Bae YC, Akaike T, Takada M, et al. Two opposing roles of 4-AP-sensitive K+ current in initiation and invasion of spikes in rat mesencephalic trigeminal neurons. J Neurophysiol. 2006;96:1887–901. doi: 10.1152/jn.00176.2006. [DOI] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Sarmiere PD, Weigle CM, Tamkun MM. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Action potential initiation and propagation in hippocampal mossy fibre axons. J Physiol. 2008;586:1849–57. doi: 10.1113/jphysiol.2007.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell Tissue Res. 2006;326:311–337. doi: 10.1007/s00441-006-0272-7. [DOI] [PubMed] [Google Scholar]
- Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2008;105:7869–74. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. Foundations of the neuron doctrine. New York: Oxford University Press; 1991. [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci U S A. 2007;104:11453–8. doi: 10.1073/pnas.0702041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–5. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, et al. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–19. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol. 1997a;505(Pt 3):617–32. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997b;20:125–31. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–5. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Szentágothai J, Arbib MA. Conceptual models of neural organization. Neurosci Res Program Bull. 1974;12:305–510. [PubMed] [Google Scholar]
- Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–78. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–47. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–52. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–70. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Wolff M, Vogel W, Safronov BV. Uneven distribution of K+ channels in soma, axon and dendrites of rat spinal neurones: functional role of the soma in generation of action potentials. J Physiol. 1998;509(Pt 3):767–76. doi: 10.1111/j.1469-7793.1998.767bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollner DA, Catterall WA. Localization of sodium channels in axon hillocks and initial segments of retinal ganglion cells. Proc Natl Acad Sci U S A. 1986;83:8424–8. doi: 10.1073/pnas.83.21.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–9. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Yu Y, Shu Y, McCormick DA. Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J Neurosci. 2008;28:7260–72. doi: 10.1523/JNEUROSCI.1613-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]