Abstract
The olfactory epithelium constitutes the sole source of regenerating neural cells that can be obtained from a living human. As such, primary cultures derived from olfactory epithelial biopsies can be utilized to study neurobiological characteristics of individuals under different conditions and disease states. Here, using such cultures, we report in vitro generation of cells that exhibit a complex neuronal phenotype, encompassing receptors and signaling pathways pertinent to both olfaction and other aspects of CNS function. Using in situ hybridization, we demonstrate for the first time the native expression of olfactory receptors in cultured cells derived from olfactory epithelial tissue. We further establish the presence and function of olfactory transduction molecules in these cells using immunocytochemistry, calcium imaging and molecular methods. Western blot analysis revealed the expression of neurotransmitter receptors for dopamine (D2R), serotonin (5HT2C) and NMDA subtypes 1 and 2A/2B. Stimulation with dopamine or serotonin enhanced receptor G protein activation in a subtype specific manner, based on 35S-GTP incorporation assay. Functional characteristics of the cultured cells are demonstrated through enhanced tyrosine phosphorylation of NMDAR 2A/2B and recruitment of signaling partners in response to NMDA stimulation. The array of neuronal characteristics observed here establish that proliferating cells derived from the human olfactory epithelium differentiate in vitro to express functional and molecular attributes of mature olfactory neurons. These cultured neural cells exhibit neurotransmitter pathways important in a number of neuropsychiatric disorders. Their ready availability from living humans thus provides a new tool to link functional and molecular features of neural cells with clinical characteristics of individual living patients.
Keywords: neurogenesis, odorant receptor, NMDA receptor, serotonin receptor, dopamine receptor, neuronal cell culture
Introduction
The olfactory epithelium is capable of regeneration throughout life, and this process has been widely studied in a variety of in vivo and in vitro systems. As the only source of regenerating neural cells accessible via biopsy, these cells provide the ability to link clinical/behavioral characteristics of living human subjects with the cellular and molecular functions underlying a diverse range of neuronal processes such as neuronal maturation and survival, odorant detection and transduction and calcium homeostasis. However, it is not known whether these cells contain receptor and transduction machinery for neurotransmitter pathways implicated in neuropsychiatric disorders such as bipolar depression or schizophrenia.
Previous studies have utilized OSNs from biopsies as a model system to examine neurophysiological processes in various psychiatric conditions such as bipolar disorder (Hahn et al., 2005a), schizophrenia (Feron et al., 1999a), and Alzheimer's dementia (Ghanbari et al., 2004). Pharmacologic agents which target various neurotransmitter systems such as dopamine, serotonin and others, have been utilized in the treatment of psychiatric illnesses, although the mechanism (s) of the therapeutic effects of these agents are not yet fully elucidated. Additional neurotransmitters, intracellular signaling mechanisms and gene regulatory pathways are also implicated in the pathophysiology of psychiatric disorders. The expression and function of these receptors and intracellular signaling mechanisms in the human olfactory epithelium have not been examined. In previous work, we reported methods for primary culture of human olfactory sensory neurons (OSNs) in vitro and demonstrated expression of neuronal markers and functional responses to odorant mixtures in these cells (Gomez et al., 2000). Our goal was to establish whether a level of differentiation and maturation occurs in this model system that reflects physiological features of mature neurons, including G protein coupled receptors, associated signaling molecules and functional responses to stimuli relevant to olfaction and neuropsychiatry. We present data demonstrating that cells generated within these primary cultures exhibit many key neuronal and olfactory-specific characteristics in vitro that may be useful for future studies of a variety of neurophysiological processes and pathologies. The ability to generate cultures from live subjects enables the examination of individual variability with respect to an array of neural processes and therapeutic mechanisms relevant to many diseases and disorders that result in or are causally linked to neuronal dysfunction.
Experimental Procedures
Human olfactory epithelium samples
Human olfactory biopsies were obtained by experienced otorhinolaryngologists from five female and three male, non–smoking, healthy volunteers ages from 22 – 56 yrs. Individuals with a current or recent history of substance use, oral or intranasal medication use, history of olfactory impairment or overt sinus pathology upon endoscopic exam were excluded. Volunteers provided informed consent by signing a document describing the nature and possible consequences of participation. Work with human tissue described in this manuscript was conducted in accordance with the Declaration of Helsinki according to protocols approved by the Institutional Review Boards of both Thomas Jefferson University and the University of Pennsylvania. Biopsies comprised of ∼1mm3 tissue were excised from the middle turbinate and opposing septum under local anesthetic as described elsewhere (Lowry and Pribitkin, 1995) and immediately transferred to culture media for transport to the laboratory. The number and subject characteristics for the biopsies used are provided below in reference to each specific assay.
Primary cultures of human olfactory neurons
Human olfactory culture cells (hOE) were derived from healthy, adult subjects as previously described (Gomez et al., 2000). Briefly, biopsies were minced coarsely and incubated in isolation solution (145mM NaCl, 5mM KCl, 2mM EDTA, 1mM Na-Pyruvate, 20mM HEPES, 100 μg/mL gentamycin, 5μl/ml papain, 5mM L-cysteine) for ∼30 min, triturated and centrifuged at 600Xg for 5 minutes. The pellet was resuspended in culture medium and transferred to a 25 cm2 culture flask coated with Cell Tak (20ug/cm2) and allowed to grow for 1 – 2 weeks until cell growth was sufficient for transfer to continuous culture. Cells were maintained in 75 cm2 culture flasks in a humidified incubator (37°C, 5% CO2). Cultured cells established in this fashion grew to confluence in ∼8-10 days. Based on preliminary experiments, cultures could be frozen at passage 2-3, thawed and used to generate new cultures with similar growth rates and proportions of mature cells, and typically became senescent after 6 – 10 passages. For in situ hybridization or immunocytochemistry experiments, freshly plated cells were maintained in 100 cm2 culture dishes in a humidified incubator and allowed to grow on untreated sterile 24 × 60 mm glass coverslips (Clay Adams/Thomas Scientific, Swedesboro, NJ) until the cells achieved approximately 80% confluence, at which point they were used for histological assays described. For functional studies, cells were plated in 96-well plates (Corning Costar). For all experiments, we used cells passaged less than six times, for which 80% confluence was achieved by day 5-6 post-plating.
Animals
Male, 8wk old Sprague-Dawley rats (average wt 200-300g) were euthanized according to protocols approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania and Monell Chemical Senses Center. Olfactory epithelium (turbinates and septum) was immediately excised and homogenized for protein extraction and immunoblotting using standard protocols as described (Gomez et al., 2000).
Cellular extracts
Whole cell extracts from the confluent hOE cultures of three healthy volunteers, a 22 yo old female, a 56 yo female and a 44 yo male, were homogenized in homogenization buffer containing 25mM Tris-HCl, 200mM NaCl (Thermo Fisher Scientific, Waltham, MA), 0.5% digitonin (MP Biomedicals, Irvine, CA), 2mM EDTA, 0.5mM EGTA, protease inhibitor cocktail (P8340), phosphatase inhibitors I and II (P2850 and P5726), 0.5% IGEPAL and 0.2% deoxycholic acid (Sigma-Aldrich Co., St. Louis, MO). Homogenates were extracted at 4°C for 1 hour and cleared by centrifugation at 10,000 × g for 20 minutes. The supernatant was collected and protein concentration was assessed with a modified Lowry assay (Bio-Rad Laboratories, Hercules, CA).
PCR
Olfactory epithelial cells derived from the 44 yo healthy volunteer above, were grown to confluence in DMEM containing 10% fetal bovine serum and harvested into TRIZOL® (Invitrogen Co., Carlsbad, CA). Total RNA was extracted with Quiagen RNeasy kit (Valencia, CA). RNA quality was assessed by Agilent 21000 Bioanalyzer (Foster City, CA) and quantified by Nanodrop (Wilmington, DE). cDNA was synthesized from 2ug of total RNA and PCR performed as described (MacDonald et al., 2006) on Techne “Touchgene Gradient” thermocycler from 40ng of RNA equivalent cDNA, with a blank RT control. Primers were designed with Primer 3 (Rosen and Skaletsky, 2000) for the following genes (Table 1): CNGA2 (OcNc1), ADCY3 (ACIII) and NTRK2 (TrkB). 5uL of each reaction and a 100bp ladder (Invitrogen) were run on a 1.6% agarose gel and stained with ethidium bromide.
Table 1.
RT-PCR primers
| Name | NCBI mRNA LOCUS | Direction | Sequence | Product length | Exon | Species |
|---|---|---|---|---|---|---|
| NTRK2 | NM_006189 | Forward | gtggcggaaaatcttgtagg | 232 | 10 | Homo sapiens |
| Reverse | cgtggtactccgtgtgattg | 11 | ||||
| CNGA2 | NM_005140 | Forward | ggttattcctcccccatcag | 157 | 7 | Homo sapiens |
| Reverse | cagttgccttgctcctcact | 7 | ||||
| ADCY3 | NM_004036 | Forward | tatggcggcttcaggagt | 167 | 18 | Homo sapiens |
| Reverse | tgatgttggtgagcgtatcc | 19 | ||||
Antibodies
The following primary antibodies were used in this study: rabbit polyclonal adenylyl cyclase III (ACIII) (SC-588, Santa Cruz Biotechnology) raised against a peptide corresponding to the C-terminus of ACIII; rabbit polyclonal olfactory cyclic nucleotide gated channel 1 (OcNC1) (APC- 045, Alomone Labs Ltd., Jerusalem, Israel) raised against a peptide KQNH EDDYL SDGIN TPEP, corresponding to residues 643-660 in the C terminal cytoplasmic domain of rat CNGA2; olfactory maker protein (OMP, generous gift from F. Margolis), and rabbit polyconal TrkB (SC-12, Santa Cruz Biotechnology) raised against a peptide corresponding to the C-terminal cytoplasmic domain of mouse TrkB, rabbit polyclonal TrkB (07-225, Upstate) raised against the extracellular domain corresponding to residues 1-429 of the rat TrkB receptor, 5HT2C (BD556335 BD Biosciences), D2R (AB1558 Chemicon), NMDAR1, (SC-1467: western blot, SC-9058: immunopecipitation Santa Cruz), NMDAR2A/2B (SC-9056), PLCγ1 (SC-7290), phosphotyrosine for NR2A/2B (SC-508). Control peptides used in this study were TrkB (SC-12P, Santa Cruz Biotechnology) and OcNc1 (APC-O45, Alomone Labs Ltd).
Immunoblotting
Protein extracts were size fractionated on 4-12% gradient Bis-Tris gels (NuPAGE) and transferred to PVDF membranes. The membranes were washed with phosphate buffered saline (PBS) and blocked with 3% milk in PBS containing 0.3% Tween-20 (PBST) for 30 min. The blots were incubated with antibodies for OcNc1 (1:200), TrkB (1:200), ACIII (1:200), 5HT2C (1:500), D2R (1:200), NMDAR1 (1:500) for 2 hours at room temperature. Peptide blockade was performed by incubating the primary antibody with 5 fold excess peptide by weight, for two hours at room temperature and then diluting the antibody-peptide solution as described for the primary antibody. Membranes were washed twice in PBST and incubated for 1 hour with anti-rabbit, anti-goat or anti-mouse IgG-HRP (1:5,000 dilution). Immunoreactivity was visualized with ECL Western Blotting reagents (Amersham) for 5 to 10 min.
Immunocytochemistry
Dissociated cultured human olfactory cells (passage 5) derived from biopsies from two healthy volunteers, (23yo male and 24yo female) were fixed on day 5 post-plating with 4% paraformaldehyde in PBS (pH 7.2) for 10 min at room temperature and immunocytochemistry performed as previously described (Gomez et al., 2000). Optimal staining conditions were determined by evaluating different primary antibody dilutions, incubation temperatures (4°C vs RT) and primary antibody incubation periods. Frozen sections of rat olfactory epithelial tissue were used as positive controls and the exclusion of the primary antibody and incubation with nonimmune rabbit serum served as negative controls. Briefly, after washing in PBS, fixed cells were blocked with 3% H2O2 for 10 min followed by 3% normal serum, 3% bovine serum albumin and 0.3% Triton X-100 in PBS for 1 hr at room temperature. Cells were then incubated with antibodies for TrkB, ACIII or OcNc1 (1:200) overnight at 4°C. After washing with PBS, cells were then incubated with Alexa Fluor 633 anti-rabbit-IgG (1:500, Molecular Probes Inc.) in blocking buffer for 30 min. After washing in PBS (3×15 min) and distilled water (3×20 min), coverslips were mounted with Vectashield with DAPI (Vector Laboratories). To determine specificity of TrkB and OcNc1 staining, each antibody was pre-incubated with five fold (by weight) of an equal amount of its specific peptide for 3 hours at room temperature and applied in place of primary antibody. Controls for immunofluorescence consisted of omitting the primary antibody (only secondary antibody) and substituting the primary antibody with the host IgG from which the antibody was generated. In all cases these controls revealed no artifactual labeling.
Images were acquired with a Leica TCS SP2 Spectral Confocal Microscope (Leica Microsystems Inc., Mannheim, Germany) using UV, Argon and HeNe lasers. The pinhole diameter was set at the first minimum diameter of the Airy disc for the objective used, giving acceptable resolution of the z-axis for the fluorescent focal plane. The power for the laser beam and gain of the photomultiplier were adjusted to optimize the signal/noise ratio, and held constant for comparison of antibody labeled and control slides. Sequential acquisition of each wavelength was used for some double labeling experiments to prevent crosstalk or bleed through between fluorophores. Leica Scanware software was used to acquire confocal images scanning unidirectionally at a 1024 × 1024 pixel format with 2 lines plus 3 frames averaging. Digital images were arranged and brightfield images were minimally adjusted for contrast and brightness to enhance visibility of cell morphology using Photoshop CS (Adobe Systems Inc., San Jose, CA).
In situ hybridization
In situ hybridization was performed with independent cultures of human olfactory cells derived from biopsies from two female healthy volunteers, (ages 22 and 33 years). Plasmids for probe synthesis were generated by amplification of the indicated sequences from cDNA obtained from human olfactory epithelium and generated as described above. Primers were designed based on published human sequences, with the exception of OcNc1, for which the probe sequence of a rat cDNA, (NM_012928), with 94% homology to the human sequence was used (Table 2). Primers were used at a final concentration of 0.4μM for each primer. Products were generated from human olfactory epithelial cDNA, cloned into the PGem®T-Easy vector (Promega Co.) according to the manufacturer's protocol and sequenced by the University of Pennsylvania Sequencing Center. Sequences were verified by BLAST analysis with all currently available GenBank sequences (Altschul et al., 1990). Sense probes for each sequence were used at equivalent concentrations as controls.
Table 2.
In-Situ hybridization primers
| Name | NCBI mRNA LOCUS | Direction | Sequence | Product length | Exon | Species |
|---|---|---|---|---|---|---|
| OMP | NM_006189 | Forward | cctgacctcaccaacctcat | 223 | 1 | Homo sapiens |
| Reverse | gctggttaaaaaccacggag | 1 | ||||
| OcNc1 | NM_012928 | Forward | agttccgaaaggtcagcaaa | 305 | 7 | Rattus norvegicus |
| (CNGA2) | Reverse | cttgccctccttgatgatgt | 7 | |||
| OR3A1 | NM_002550 | Forward | cagaatctggggccaatg | 308 | 1 | Homo sapiens |
| Reverse | gatggcactctgcacatcag | 1 | ||||
| OR1A1 | NM_014565 | Forward | tgagggaaaataaccagtcc | 249 | 1 | Homo sapiens |
| Reverse | ttgttgaagagtttccgcag | 1 | ||||
Probes were generated from linearized, purified 1μg of plasmid DNA (Sambrook et al., 1989). A mixture of 1μg of linearized plasmid DNA, 1U/μl RNase Inhibitor, 1 × RNA Labeling Mix (DIG RNA Labeling Kit (SP6/T7), Roche, Indianapolis, IN), 1 × Transcription Buffer, and 50U RNA Polymerase (SP6 or T7, depending on the clone orientation) was mixed on ice with nuclease-free water to yield a final volume of 20μl. The mixture was spun and placed in a water bath at 37°C for 2 hours. 2μl 0.2 M EDTA (pH 8.0) was added to the mixture to stop the reaction. The High Pure PCR Purification Kit (Roche) was used to purify labeled RNA according to the manufacturer's instruction. A blot method was used to estimate the yield of labeled RNA.
In situ hybridization of digoxygenin (DIG)-labeled or biotinylated RNA probe was carried out as described in (Rawson et al., 2000). Slides were initially washed with PBS and fixed immediately in 4% paraformaldehyde for 15 min at RT. The slides were prehybridized in a mixture of 50% deionized formamide, 5% dextran sulfate, 1 × Denhardt's solution, 5 × saline/sodium citrate, 0.25mg/ml salmon sperm DNA for at least 30 min at 42°C and then hybridized overnight in 100ul of hybridization buffer with denatured RNA probe at a concentration of 2 – 5ng/μl at 42°C. The hybridized slides were washed 3 × 30 min each in 2 × SSC at 37°C, 1 × SSC at 37°C, 0.2 × SSC at 42°C (the last 1 hour with 0.3% Tween-20), and 1× 5 min in PBS.
After washing, the slides were incubated in 1× blocking solution for 1 hour at RT. Incubation of primary antibody (1:200 dilution for anti-DIG-POD; 1:500 dilution for anti-DIG from sheep followed by 1:200 dilution for anti-sheep-POD) was done overnight at 4°C in a humidified chamber. The slides were washed with Washing buffer 3 × 10 min and incubated at RT with either 1U/ml Streptavidin-AP (Boehringer Mannheim, Germany) followed by Vector-Red (Vector-Labs) detection for 10-30 minute or POD conjugated antibody followed by TSA-FITC (Life Science) detection for 7-10 minute according to the manufacturer's instructions.
Slides were viewed on a Nikon Microphot FX/A microscope attached to a Kodak SPOT III digital camera. Images were acquired into ImagePro Plus imaging software. Acquisition parameters (filters, exposure times, microscope settings) were held constant for the, sense and antisense probe and control (no probe) conditions for each experiment. Bright field images were adjusted for brightness and contrast to enhance the visibility of cell morphology using Photoshop CS (Adobe). The entire coverslip was scanned in a grid pattern; cells exhibiting fluorescence above background were counted as labeled cells.
Responses to odorant exposure
Cultures derived from those used for in situ hybridization were used for visualizing changes in intracellular calcium associated with odorant exposure. The culture medium in each well was replaced with warm culture medium supplemented with Fura-2 (5μM) and 20 μg/ml pluronic F-127 (Molecular Probes, Inc.). The 96-well plates were returned to the incubator for 2hrs. The cells were visualized using an inverted fluorescent microscope at excitation wavelengths of 340 and 380nm and an emission wavelength of 510nm. A stimulus delivery head was fitted onto the plate, which allowed for constant flow of fluid into and out of the well, providing a consistent level of fluid over the cells. Stimulus delivery and removal was controlled by a 2 channel peristaltic pump and the delivery head was placed and moved by a robotic plate handler (Hudson Controls). Cells were superfused with MHNK solution (145mM NaCl, 5mM KCl, 1mM CaCl2, 1mM MgCl2, 1mM Na-pyruvate, 20mM HEPES), and tested with odorants via superfusion, with each stimulus delivered for 10 sec. A series of images was acquired for each well at 20X with a CCD camera (Photometrics) as follows: four baseline, 12 post-stimulus, and four baseline, for each of three stimuli. A 2 minute washout followed each post-stimulus block of images. Stimulus selection and delivery, plate movement and focusing, and image acquisition were controlled by custom-designed Discovery-1 software (Molecular Devices). Fluorescence intensity values were recorded, regions of interest defined and ratios computed using Metamorph software (Molecular Devices).
The odor stimuli were the purest available, helional (gift of International Flavors and Fragrances), 2-picoline and pyridine (Sigma), and were prepared on the day of use by dilution in MHNK to a final concentration of 50μM.
Assessment of receptor function
a. G protein activation (for dopamine and 5HT receptors)
As a measure of the functionality of neurotransmitter receptors, we assessed the agonist-induced G-protein activation in hOE culture cells derived from the biopsy of a 32yo female healthy volunteer. To distinguish between specific receptor subtypes, ligand stimulation was performed in conjunction with pretreatment with inhibitors. For dopamine receptors, hOE culture membranes (400 μg) were first pre-incubated with SCH 23390 (D1 dopamine receptor antagonist), 1-sulpiride (D2 dopamine receptor antagonist,) or no antagonist, in the presence of 0.5 nM [35S]GTPαS for 10 min at 37°C. Each sample was then divided equally into two tubes and incubated with or without 1 μM dopamine for 5 min. For 5-HT receptors, membranes (400 μg) were incubated in Krebs-Ringer solution for 10 min at 37°C with 0.5 nM [35S]GTPαS alone or in the presence of specific 5-HT2A receptor antagonist, spiperone, 5-HT2B receptor antagonist, SB 204741 or 5-HT2C receptor antagonist, RS 102221. Each tube of the treated membranes were then divided equally into two tubes and incubated with either Krebs-Ringer solution or 1 μM specific 5-HT2 agonist, DOI for 5 min. The reactions were terminated by addition of MgCl2 (final concentration: 20 mM) and membranes were solubilized in 0.5% digitonin, 0.2% Na cholate and 0.5% NP-40 for 1 hr at 4°C. The samples were diluted and divided equally into 5 tubes each incubated at 4°C for 2 hr with 1 μg normal rabbit IgG (non-specific), anti-Gαs/olf, -Gαi, -Gαo or -Gαq/11 followed by 1 hr incubation with 25 μg protein A/G-conjugated agarose. The levels of [35S]GTPαS bound Gα proteins immunopreciptated (subtracting out non-specific binding) were measured by scintillation spectrometry.
b. co-immunoprecipitation (for NMDA receptors)
HOE culture cells derived from the 32 yo volunteer identified above were incubated in Krebs-Ringer (K-R) solution were incubated with or without 10 μM NMDA (Sigma) +1 μM glycine (Sigma) at 37°C for 15 min. The ligand stimulation was terminated by 0.5mM EGTA and cells were homogenized in the immunoprecipitation buffer containing 25mM Tris, pH 7.5, 200 mM NaCl, 0.5 mM EDTA and 0.5 mM EGTA. The samples were solubilized in 0.5% digitonin, 0.2% Na cholate and 0.5% NP-40 for 1 hr at 4°C. The samples were immunoprecipitated with antibodies for NMDAR1 (NR1) (SC-9058) or NMDAR2A/2B (NR2A/2B) (SC-9056) and the immunoprecipitates were analyzed by Western blotting for signaling partners, PLCγ1 (SC-7290), for NR1 and phosphotyrosine for NR2A/2B (SC-508).
Results
Cultured human olfactory epithelial cells exhibit molecular features of mature olfactory receptor neurons
We have recently characterized the expression of various neuronal markers in human olfactory epithelial tissues (Hahn et al., 2005c). Previously, we and others identified some of these neuronal markers in olfactory epithelial cultures: olfactory marker protein, neuron specific tubulin, neural cell adhesion molecule and adenylate cyclase III (ACIII), an element of the olfactory transduction pathway (Gomez et al., 2000; Ensoli et al., 1998; Vannelli et al., 1995; Wolozin et al., 1992; Margolis, 1980).
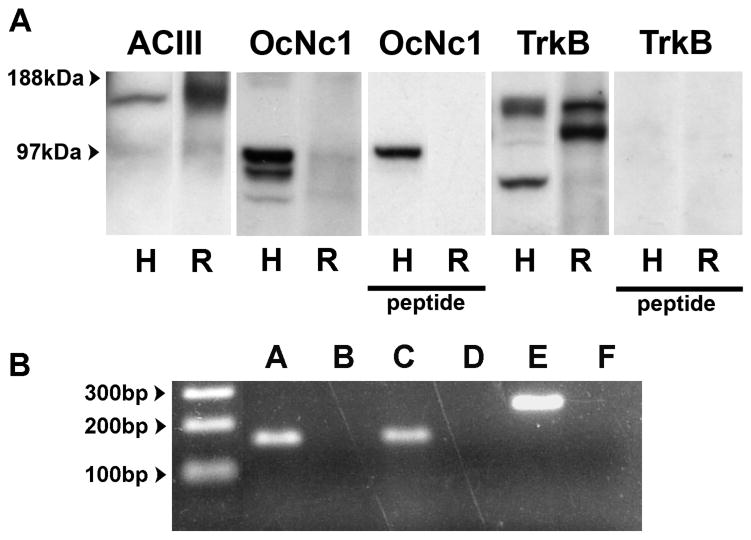
In this study, we evaluated additional molecules relevant to early neuronal differentiation, (tyrosine kinase receptor, TrkB), olfactory transduction (olfactory cyclic nucleotide gated channel, olfactory receptors), and neuromodulation (receptors for dopamine, serotonin and NMDA). Immunoblotting of hOE culture cells was performed with the antibodies against ACIII, OcNc1 and TrkB, in whole cell extracts (Figure 1A). Anti- ACIII detected a previously reported band of 140-150 as well as unidentified lower molecular weight bands. Anti- OcNc1, (against residues 643-660 of rat OcNc1 on the C-terminus in the intracellular domain), detected three bands between 70 and 100kDa and additional lower molecular weight bands. Peptide pre-adsorption eliminated all but the highest molecular weight band. Expected bands were detected for two well published antibodies for TrkB, Upstate 07-225 (against the extracellular domain corresponding to residues 1-429 of the rat TrkB receptor) which detected the full length as well as the truncated form of the molecule as expected by the described peptide sequence (data not shown) and SC-12; Santa Cruz (against the C-terminal cytoplasmic domain). SC-12 detected the full length form (145kDa) of TrkB consistent with other reports (Tucker and Fadool, 2002). These antibodies also detected additional bands in both human OE cell extracts as well as rat OE homogenates (data not shown), likely to be truncated or alternatively spliced forms of the molecule (Ninkina et al., 1997). Pre-adsorption with peptide provided by the manufacturer for SC-12 abolished all bands.
Figure 1. hOE culture cells express protein markers and mRNA that are characteristic of olfactory sensory neurons.
(A) Protein extracts of dissociated hOE culture cells and of rat olfactory epithelium were immunoblotted with antibodies for ACIII, OcNc1, and TrkB. Anti-ACIII (SC-588) pointed to 110 and 145kDa bands. Anti-OcNc1 (Alomone APC-045) identified three bands between 70kDa and 100kDa in hOE cultures, the lower two of which were suppressed by peptide blockade. Anti-TrkB (SC-12) recognized 80, 110, 145kDa bands, all of which were abolished with peptide adsorption. (B) PCR products of the correct sizes for neuronal mRNAs: OcNc1 (CNGA2), ACIII (ADCY3) and TrkB (NTRK2) were generated from cDNA derived from hOE culture cells. A) CNGA2 (157bp) C) ADCY3 (167bp) E) NTRK2 (232bp) No RT controls: B, D, F. Abbreviations: ACIII; adenylate cyclase type 3, OcNc1; olfactory cyclic nucleotide gated channel 1.
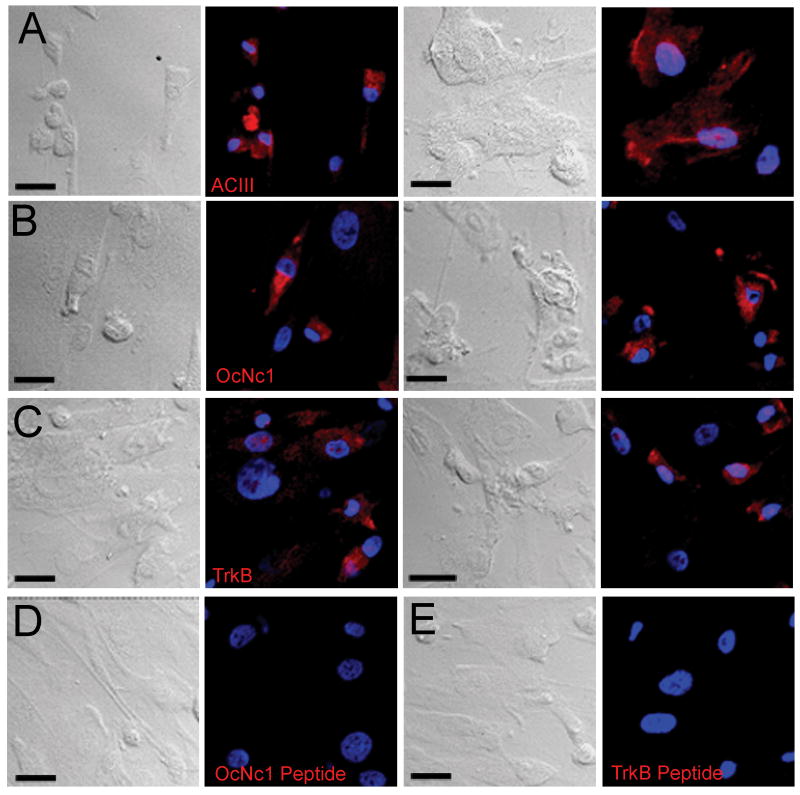
To further verify the expression of these neuronal markers in OE cultures, we examined the expression of OcNc1 (CNGA2), ACIII (ADCY3) and TrkB (NTRK2) with reverse transcriptase PCR (RT-PCR) experiments using mRNAs isolated from the OE cultures of a 44 year old male healthy volunteer. RT-PCR identified amplicons for CNGA2 (157 bp), ADCY3 (167 bp) and NTRK2 (232 bp), while the no RT controls were negative for the expected amplicon (Figure 1B). Cells expressing each of these molecular markers were also identified immunocytochemically (Figure 2, A-C). No immunostaining was detected using antibody pre-absorbed with peptide for OcNc1 and TrkB (Figure 2D, E) or with replacement of antibody with the appropriate IgG for ACIII, (data not shown). While the prevalence of immunoreactive cells varies from coverslip to coverslip, images shown are representative of those obtained from at least four coverslips from each of four independent experiments. Cells from multiple passages (4 – 6) from at least two subjects were included for each antibody. Morphology of the immunolabeled cells varied, and immunolabeling patterns did not overtly correspond to any particular morphology under our culture conditions.
Figure 2. Immunocytochemical detection of ACIII, OcNc1 and TrkB.
HOE culture cells were immunostained as described with the antibodies used for western blotting: (A) anti-ACIII, (B) anti-OcNc1 and (C) anti-TrkB. Images representative of experiments with cultures from two individuals are shown. Specificity of OcNc1 (D) and TrkB (E) antibodies were determined by pre-incubation with specific peptides. Scale bars = 50μm.
OcNc1 and ACIII are essential elements of the primary olfactory transduction cascade (Baker et al., 1999; Wong et al., 2000), and are hallmarks of functionally mature OSNs. In vivo, the TrkB receptor, which binds brain derived neurotrophic factor, is found in cells in the basal – middle regions of the human olfactory epithelium (Nibu et al., 1999) and specifically in immature OSNs in rodent (Roskams et al., 1996). Thus, cells are generated in these primary cultures that exhibit molecular markers of functionally mature and maturing olfactory neurons, even after multiple passages in vitro. These results imply that the signals responsible for induction of key components of the OSN phenotype are retained within the cellular milieu of the peripheral epithelium.
Expression of olfactory specific mRNAs in human olfactory cell cultures
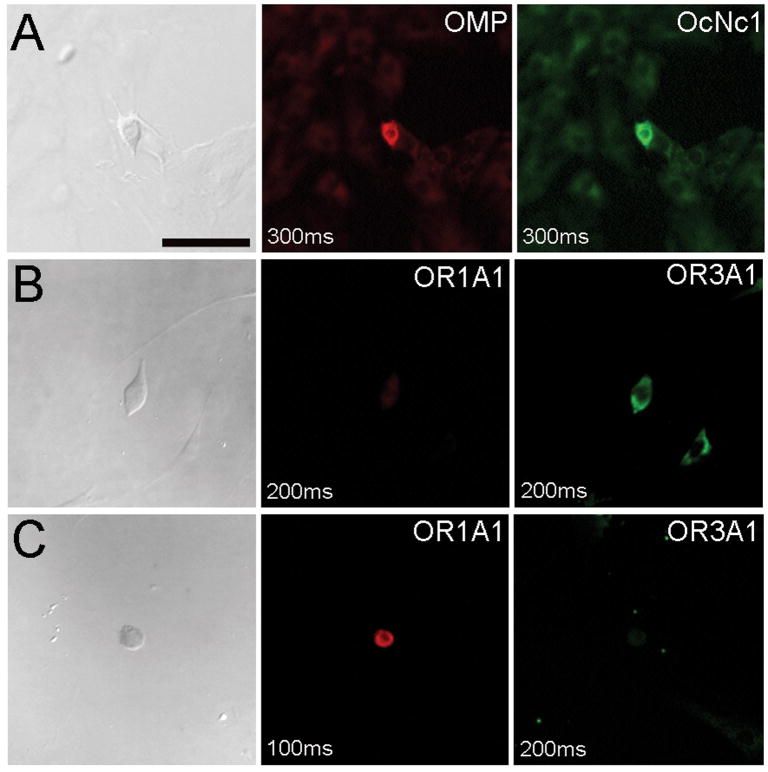
Based on functional and immunocytochemical data obtained previously, we hypothesized that odorant receptors should be present in the cultures. Olfactory marker protein was used as an indicator of a mature neuronal phenotype and OcNc1 was assessed as an indicator of functional status. To determine whether two ORs were present in the cultures, we designed probes specific for these sequences and used these alone or together with an OMP-specific probe, for in situ hybridization experiments. Consistent with the results of immunocytochemical studies (Gomez et al., 2000), approximately 20% of cells were found to react clearly positively with probes for olfactory marker protein (n=822). 73% of OMP labeled cells also reacted with the OcNc1 probe (representative images shown in Figure 3A). A few cells (<5%) labeled with the OcNc probe but not the OMP probe (not shown). In single-labeling experiments, the probe for OR3A1 hybridized strongly with ∼2% of all cells in the culture (n = 143, independent experiments using cultures from two different subjects). Double-label experiments with the two OR probes failed to reveal any cells expressing both of these mRNAs (Figure 3B,C), and the percent of cells expressing either OR was consistent with that found in single probe experiments: 2.2% labeled with the OR3A1 probe only; 2.4% labeled with the OR1A1 probe, and none reacted with both probes (n = 405). No cells were detected when hybridized with sense probes (supplemental data).
Figure 3. hOE culture cells express mRNA for OMP, OcNc1 and odorant receptors.
OMP probe was detected using Vector Red. OcNc1 probe was detected with TSA-FITC. OR3A1 probe was detected with TSA-FITC. The OR1A1 probe was detected with Vector Red when used with the OR3A1 probe. (A) Cell reactive with probes for OMP and OcNc1. (B) Cell hybridized with probes for both OR3A1 and OR1A1, labeling only with OR3A1. (C) Cell hybridized with probes for both OR3A1 and OR1A1, labeling only with OR1A1. Exposure times are noted on each image. Scale bar = 50μm.
Even when hybridized using very low stringency conditions, there was no overlap between expression of the two OR probes we used, and the overall frequency of reactive cells was not altered. This is consistent with the view that each cell expresses only one predominant OR (Mombaerts, 2004) and that this regulation is maintained in vitro.
Cultured cells respond to helional, a ligand for the OR3A1
We next determined whether cells from the same batch used for the molecular studies also responded to helional, an odor shown to activate the OR3A1 receptor in heterologous systems (Wetzel et al., 1999). Due to the low frequency of responses expected to any single odorant (Rawson et al., 1997), we assayed a separate aliquot of cells derived from the same initial cultures in two experiments using a high-throughput imaging system (see Experimental Procedures). Cells were assayed on day 5/6 post-plating with helional, pyridine and 2-picoline, at 50μM each. Helional elicited responses in approximately 5.5% of cells tested at day 5-6 (Figure 4; n = 165), and none of these cells responded to either of the other stimuli applied, although other cells responded selectively to either pyridine (1.8%) or 2-picoline (0.9%). A comparable experiment could not be performed for the OR1A1 ligand(s) as ligands for this receptor have not been reported.
Figure 4. Cultured human olfactory cells respond to helional, a ligand for the OR3A1.
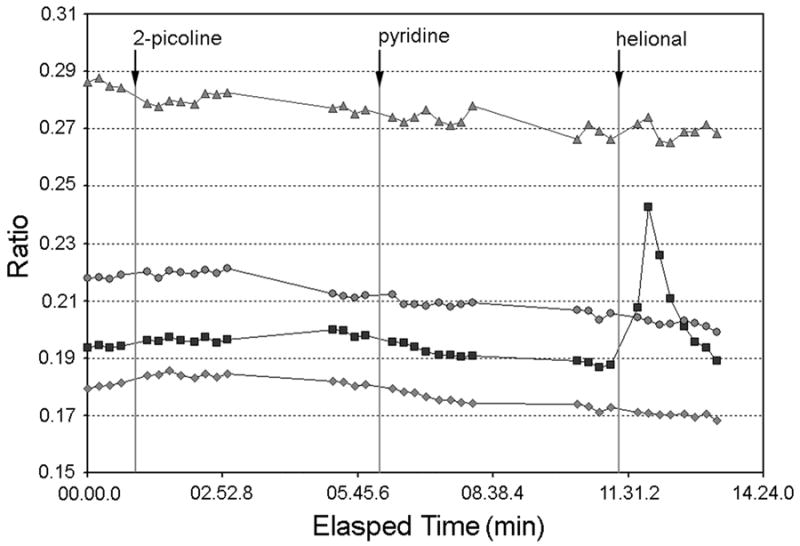
Cultured hOE cells were loaded with Fura-2/AM and imaged as described in Experimental Procedures. Stimuli were added for 10 sec, followed by a 2 minute rinse with Ringer's solution. Figure 4 depicts traces from a subset of 165 cells assayed from two experiments using cells derived from the same source as that used for in situ hybridization. Traces for one helional-responsive and three unresponsive cells are shown. Additional experiments using two independent cultures yielded similar results.
Cultured human olfactory epithelial cells express several neurotransmitter receptors
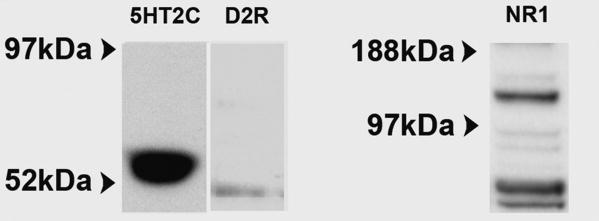
Our data demonstrate that a broad array of phenotypic features characteristic of mature OSNs is recapitulated in these human olfactory cell cultures. We next investigated the presence and functionality of several neurotransmitter receptors not directly linked to odor detection, but which serve as neuromodulators and have been implicated in a variety of neuropsychiatric conditions. First, membrane extracts isolated from hOE cultures were immunoblotted with antibodies for the serotonin receptor subtype, 5HT2C, the dopamine receptor, D2R and NMDA receptors, NMDAR1 (Figure 5) and NMDAR2A/2B (data not shown). Immunoblotting with anti-5HT2C revealed a single strong band at 55kDa along with a non-specific band at 100kDa (data not shown). Likewise anti-D2R detected bands at 47 and 110kDa, representing both the native and denatured forms of D2R which are detected by this antibody. Anti-NMDAR1 detected a band at 110, and an additional double band at ∼90kDa. NMDAR2A detected a band at 180 kDa and NMDAR2B a band at180 kDa (data not shown).
Figure 5. hOE cultures express the neurotransmitter receptors 5HT2C, dopamine D2R, and NMDAR1.
Membrane extracts of hOE cultures were immunoblotted with antibodies for: 5HT2C, D2R and NMDAR1. HOE cultures showed robust expression of 55 kDa bands for 5HT2C receptor, a 48kDa band for D2R and a 110 kDa band for NMDAR1 with an additional double band at approximately 90kDa.
5HT2, D1 and D2 receptors in hOE cultures have functional characteristics
To assess the functional competence of 5HT receptors in hOE cultures, we measured G protein activation in response to ligand stimulation. Membranes of hOE cultured cells were incubated with the 5HT agonist DOI in the presence of 0.5 nM [35S]GTPαS and then the protein extracts were immunoprecipitated with antibodies for Gαs/olf, Gαo, Gαi and Gαq/11. Figure 6A demonstrates a significant enhancement in [35S]GTPαS incorporation for all four isoforms, suggesting that 5HT stimulates more than one subtype of the receptors. To test the role of specific 5HT receptor subtypes, hOE membranes were pre-incubated with spiperone (5-HT2A receptor antagonist), SB 204741 (5-HT2B receptor antagonist) or RS 102221 (5-HT2C receptor antagonist) prior to the stimulation with DOI. Pre-incubation with spiperone and SB 204741 decreased the activation of Gαo and Gαq/11 while pre-treatment with RS 102221 attenuated ligand induced incorporation of [35S]GTPαS for Gαq/11. Together, these suggest that hOE culture cells contain functionally competent 5HT2A, 5HT2B and 5HT2C receptors.
Figure 6. Ligand stimulation of 5-HT and dopamine receptors activate signaling pathways in hOE cultures.
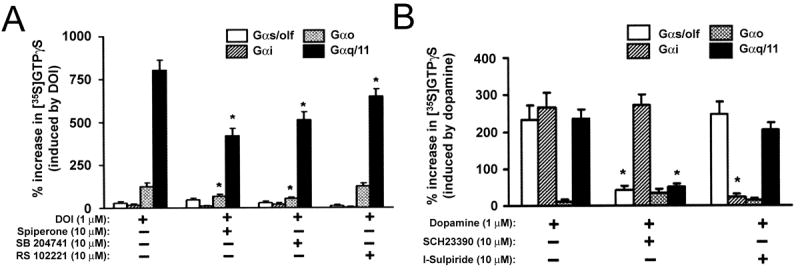
(A) 5HT receptor function. HOE cultures were incubated with DOI, a 5-HT2 agonist, at 37°C for 5 min, in the presence of 0.5 nM [35S]GTPγS. To test the role of specific subtypes in 5-HT mediated activation, the cultured cells were pre-incubated with spiperone (5-HT2A receptor antagonist), SB 204741 (5-HT2B receptor antagonist) or RS 102221 (5-HT2C receptor antagonist) for 10 min. Membrane extracts were immunoprecipitated with antibodies for Gαs/olf, -Gαi, -Gαo or -Gαq/11 and the levels of [35S]GTPγS within the immunoprecipitates were measured by scintillation spectrometry. Statistical differences were analyzed using Newman-Keul's test that followed ANOVA. *p < 0.01. (B) Dopamine receptor function. HOE culture cells were pre-incubated with sch23390 or sulpiride at 37°C for 10 min, which was followed by stimulation with 1 μM dopamine and 1μM ascorbic acid, at 37°C for 5 min in the presence of 0.5 nM [35S]GTPγS. Isoform specific activation of G proteins was assessed for Gαs/olf, -Gαi, -Gαo or -Gαq/11 as described for 5HT receptors in A. Statistical differences were analyzed using Newman-Keul's test that followed ANOVA. *p < 0.01.
We also tested the functionality of dopamine receptors in hOE cultures using the same method. HOE culture membranes were incubated with 1 μM dopamine for 5 min in the presence of 0.5 nM [35S]GTPαS and the protein extracts were immunoprecipitated with antibodies for Gαs/olf, Gαo, Gαi and Gαq/11 (Figure 6B). We found that dopamine stimulation robustly enhanced [35S]GTPαS incorporation for Gαs/olf, Gαi and Gαq/11. The D1 receptor antagonist sch23390, attenuated the activation of Gαs/olf and Gαq/11 whereas sulpiride, a D2 receptor antagonist, decreased the activation of Gαi. These data suggest that ligand binding with D1 and D2 receptors in hOE cultures activates the expected intracellular signaling pathways.
NMDA receptors in hOE cultures demonstrate functional activity
Human olfactory cultured cells were incubated with or without 10 uM NMDA and 1 μM glycine at 37°C for 15 min and the protein extracts of these tissues were immunoprecipitated with antibodies for NMDAR1 or NMDAR2A/2B. The immunoprecipitates were then analyzed for the expression of phospho-tyrosine and signaling partners of NMDAR by immunoblotting. Figure 7 shows that tyrosine phosphorylation of NMDAR and recruitment of their signaling partners was clearly enhanced in the stimulated cells (Figure 7A). Excitotoxic and plasticity-related effects of NMDA are mediated in part by a host of calcium-dependent processes initiated by calcium influx through the NMDAR (Halabisky et al., 2000). Consistent with this calcium permeability, NMDA stimulation of hOE culture cells elicits transient (12%) or graded (35%) calcium responses in a subset of cells expressing voltage-gated calcium channels activated by depolarization with a high KCl solution (Figure. 7B, n=78). Cells imaged similarly in the absence of NMDA stimulation maintain more stable baseline calcium levels over a comparable period (n=74).
Figure 7. Functional integrity of NMDAR signaling in hOE cultures.
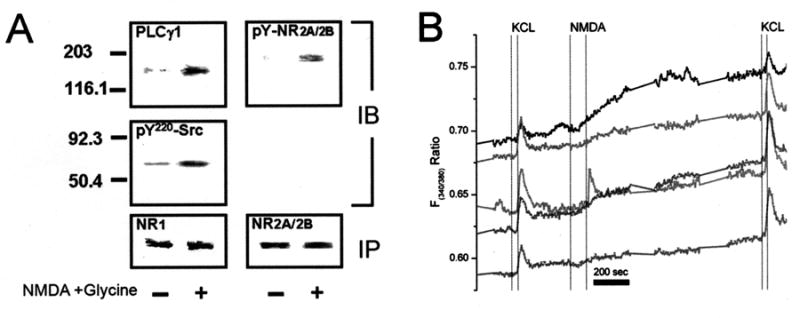
(A) HOE culture cells were incubated with or without 10μM NMDA +1μM glycine. Protein extracts were immunoprecipitated with antibodies for NMDAR1 (NR1) or NMDAR2A/2B (NR2A/2B) and the immunoprecipitates were analyzed by Western blotting for signaling partners, PLCγ1 and pY220-Src, for NR1 and phosphotyrosine for NR2A/2B. (B) Representative traces from fura-2 loaded hOE culture cells stimulated with 10μM NMDA + 1μM glycine. Of 78 cells imaged in three independent experiments, ∼80% responded to depolarization with high potassium (KCl, 50 mM) with a transient increase in intracellular calcium, indicating the presence of voltage-sensitive calcium channels. Of these, 12% exhibited a transient increase in calcium following 60-90 sec exposure to NMDA (10μM + 1μM Glycine) and ∼35% exhibited gradual increases in calcium which were more accelerated than what is typically seen during a similar time period in the absence of NMDA stimulation (n= 74). Abbreviations; PLCγ1: phospholipase C-γ1, pY-NR2A/2B: phosphotyrosine-NMDAR1A/2B.
Discussion
Several groups have examined neuronal characteristics of primary cultures derived from olfactory epithelial biopsy or cadaver tissue (Feron et al., 1999a; Othman et al., 2005; Wolozin et al., 1992; Zhang et al., 2006; Zhang et al., 2004) but the degree to which these cells attain neuronal maturity has not been fully elucidated. The results of our study demonstrate that a subgroup of these cells mature to express odorant receptors, providing a physiological basis for odorant responsiveness of these cells and further validating their functional maturity. In addition, the expression of neurotransmitter receptors and their active signaling mechanisms qualify these cells as a model system to study dysregulated receptor signaling in specific individuals, as might occur in neuropsychiatric illnesses.
Our results, generated from multiple experimental approaches, establish the presence of each component of the odorant detection cascade – from receptor to calcium signal – in cultured cells derived from the adult human olfactory epithelium. The morphology of immunocytochemically labeled cells varied from rounded cells with one or more processes to larger cells with a more varied shape. Cells in culture may vary in shape depending on their age, substrate, proximity to other cells, media and other culture conditions (Wolozin et al 1992., Vawter et al 1996, MacDonald et al 1996, Gomez et al 2000). The single cell functional assays enable comparison of morphology with response to odor or other stimuli. Our experiments and others with these cells indicate no consistent correlation between functional characteristics and a specific morphological cell type in these cell cultures (Gomez et al., 2000; Bryant B., unpublished observations). The observed low frequency of responses to single odorants is consistent with previous studies of freshly dissociated OSNs (Rawson et al., 1997), and with the notion that human OSNs appear to be relatively narrowly tuned. These cultures provide a tool for further studies of selectivity and response profiles where the availability of freshly dissociated OSNs is limited.
Our functional and molecular data suggest that the selectivity in odor responsiveness reported in ex vivo human olfactory neurons (Rawson et al., 1997) is retained in vitro. The in situ hybridization data supports the expression of these two ORs in these cultured cells. The frequency of cells expressing each OR is not unexpected in view of studies of OR expression in rodents suggesting that each OR is expressed in approximately 1% of rodent OSNs in vivo (Buck and Axel, 1991; Buck, 2004). Given the roughly 3-fold fewer number of functional OR genes in human compared to rat, one might predict a somewhat higher frequency of OSNs expressing any given OR. We cannot rule out the possibility that our probes cross-hybridized with other OR mRNAs that have very similar sequences differing only in a few nucleotides. In this context, the OR3A1 probe sequence is 90% identical to a region of the OR3A3 sequence (ACC no. NM_012373) and 89% identical to the OR3A2 sequence (ACC no. NM_002551), and the OR1A1 probe was 83.2% identical to the OR1A2 sequence (ACC no. NM_012352). It is possible that these particular ORs are both represented in the two cultures we examined because of the location of the biopsy from which they were derived, or because of some aspect of the cell culture process. The frequency of cells responsive to helional in a comparable culture is consistent with the OR expression frequency observed in the in situ hybridization experiments. Some difference would be expected as the cells assayed functionally, although from the same parent culture, were not the identical cells used for in situ hybridization. These data suggest a number of issues warranting further study, such as the number of different ORs expressed in cultures, their stability over multiple passages, and consistency among cultures derived from the same or different subjects.
Taken together, the results of the present study demonstrate that in our system, a substantial degree of differentiation, including OR expression, can occur in the absence of olfactory bulb connectivity. These hOE cultures thereby provide an in vitro system to study such fundamental questions as how OR gene expression is regulated in living OSNs, how the odorant response pathways develop during functional maturation of the cell, and how these processes may be modulated by the environment.
Our results also demonstrate the presence and function of a number of neurotransmitter pathways important for modulation of sensitivity and/or maturation of OSNs and other neuronal types. Neurotransmitter receptors for dopamine have been reported previously on rodent OSNs and their nerve terminals (Feron et al., 1999b; Koster et al., 1999). In the epithelium, dopamine receptors are present on both neuronal and non-neuronal cell populations, where dopamine is thought to trigger terminal differentiation and cell death (Feron et al., 1999b). In addition, the D2R agonist bromocriptine inhibits adenylyl cyclase in rat OE (Mania-Farnell et al., 1993), suppresses a hyperpolarization activated cation conductance, modulating OSN activity in short-term cultures (Vargas and Lucero, 1999), and reduces L-type Ca++ channel activity in ORNs (Okada et al., 2003). The expression of D2R was also reported in hOE explant cultures where it triggered neuronal maturation (Feron et al., 1999b). Consistent with these reports, we have identified the presence of D2R by immunoblotting (Figure 5) as well as by radioligand binding assay (data not shown). Further, dopamine induced G protein activation was inhibited either by SCH23390 or sulpiride in an isoform specific manner, which supports the presence of both D1R- like and D2R- like receptor signaling pathways in these cultures. The expression and functionality of dopaminergic receptors are of particular interest in these cells since dopaminergic receptor signaling is thought to be dysregulated in schizophrenia, Parkinson's disease and other neuropsychiatric illnesses (Bonci and Hopf, 2005).
Serotonin plays a role in early development of the olfactory pathway (Vitalis et al., 2003) and is a well-established neuromodulator in the olfactory systems of invertebrates (Piomelli and Tota, 1983) and sea lamprey (Zielinski et al., 2000). Modulation of forskolin-stimulated electrical activity by serotonin has been observed in frog OSNs (Frings, 1993), and the serotonin-reuptake blocker amitryptyline reduced adenylyl cyclase activity in olfactory neurons and inhibits olfactory neurite outgrowth (Mania-Farnell et al., 1993). These data suggest that 5HT receptors may be present on OSNs, but serotonin receptors have not previously been reported on mature mammalian OSNs. Our study demonstrates that hOE cultures robustly express 5HTR2C and possess subtype specific coupling of 5HTR2A, 5HTR2B and 5HTR2C with G proteins. Although in vivo expression of these receptors in OSNs and other cell types in human olfactory epithelium is unknown, the presence and activity of these receptors in hOE culture cells further position these cells as viable tools to investigate 5HT receptors of specific individuals in the context of neuropsychiatric research.
The excitatory NMDA receptor plays key roles in neuroplasticity, modulation of synaptic transmission and apoptosis (Sheng and Kim, 2002; Lynch and Guttmann, 2001). NMDA receptors have not previously been reported on cells within the olfactory epithelium of any species, although their presence and function in the olfactory bulb is well-established (Friedman and Strowbridge, 2000). Our results, however, indicate that hOE cultures robustly express NMDAR1 (Figure 7A), 2A and 2B (data not shown). Furthermore, functional assays demonstrate that ligand stimulation induced recruitment of NMDAR-linked signaling molecules and enhancement of intracellular calcium influx. Based on the co-expression of voltage-gated calcium channels in cells responding to NMDA (Figure 7B), some of these NMDA-responsive cells appear to be neurons. The presence of these functionally active NMDARs does not appear to be a tissue culture artifact. When freshly obtained human olfactory biopsy tissues were incubated with NMDA, tyrosine phosphorylation of NMDAR1 and recruitment of signaling partners, PLC-γ and src kinase, were also found to increase (Figure 7A). The current data do not address whether these receptors are co-expressed with the other receptors identified, or whether the culture comprises several subpopulations with different neurophysiological characteristics.
The data we present confirm earlier studies demonstrating that OSNs can achieve a high degree of functional and molecular maturity in model systems in which their in vivo target is absent (Rawson et al., 1995; Gomez et al., 2000; Farbman, 1977; Chuah et al., 1991; Wolozin et al., 1992; DeHamer et al., 1994; Vannelli et al., 1995; Pixley, 1996). Importantly, we extend these earlier studies to demonstrate that primary cultures derived from adult human olfactory biopsies could be used to study the regulation of hOR gene expression and single odorant response mechanisms in human OSNs.
Neuronal characteristics of these culture cells, particularly the functionality of neurotransmitter receptors, offer an opportunity to monitor such parameters as biological characteristics of the donor. It will be tempting to define OE biopsy tissues and these culture cells as a cellular system to identify biomarkers associated with diagnosis, prognostic indicators, or responsiveness to therapeutics. In such approaches, however, it will be critical to establish the stability or consistency of each biological parameter of interest, by assessing intra- and inter- subject variability.
We have assessed the expression of D2R, NMDAR1 and CamKII normalized with respect to β-actin in nine randomly selected olfactory cell lines. Expression levels of these proteins varied between subjects with variability (SEM/mean) ranging from 30% (NR1)-45%(D2R) (data not shown). Such variability is most likely to arise from the heterogeneity of cell types, neuronal and non-neuronal, and from varied cellular composition of each cell line. In this regard, single cell based assay strategies, in conjunction with cell type identification, such as electrophysiological studies or single cell based RNA amplification techniques are promising. For future studies, intra- or inter-subject variability must be assumed and the stability of each measure should be tested.
Neurotransmitter receptors appear to be prevalent among these culture cells, suggesting that they are expressed across a large population of mature neurons and/or may be expressed in other cell types. The cell type specific expression of these neurotransmitter receptors needs to be further studied, although at least a subset of NMDA-responsive cells also exhibited voltage-sensitive calcium influx, suggesting a neuronal phenotype. The robust presence and signaling activity of neurotransmitter receptors demonstrates that these cells exhibit greater similarity to their in vivo counterparts, and to CNS neuronal populations relevant to neuropsychiatric illnesses, than previously known.
Supplementary Material
Cell hybridized with sense probes (A) OMP, (B) OcNc1, (C) OR1A1 and (D) OR3A1 showed no labeling. Note the exposure times for OR3A1 sense (D) was shorter than that used in Figure 3B, which were obtained from different experiments. Therefore the corresponding anti-sense OR3A1 image (E) is also included. Exposure times are noted on each image.
Acknowledgments
The authors acknowledge the expert technical assistance of V. Audige, L. Dankulich, C. Klock, A. Vainius and R. Peoples, and comments on the manuscript from J. Reisert and G. Beauchamp.
Supported by the National Institute on Deafness and Other Communication Disorders DC02876, DC006760 and DC00214 (N.E.R.), National Science Foundation DBI-0216310 (N.E.R.), National Alliance for Schizophrenia and Depression (NARSAD) (K.E. B-W.) and National Institute of Mental Health, MH-63946, MH-080194 and NARSAD (C-G. H.).
Abbreviations
- ACIII
adenylate cyclase type 3
- GTP
Guanosine triphosphate
- hOE
human olfactory culture cells
- NR1
NMDAR1
- NR2A/2B
NMDAR2A/2B
- OcNc1
olfactory cyclic nucleotide gated channel 1
- OMP
olfactory marker protein
- OSNs
olfactory sensory neurons
- PBS
phosphate buffered saline
- PLCγ1
phospholipase C-γ1
- pY-NR2A/2B
phosphotyrosine-NMDAR1A/2B
- 5HT
serotonin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baker H, Cummings DM, Munger SD, Margolis JW, Franzen L, Reed RR, Margolis FL. Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): biochemical and morphological consequences in adult mice. J Neurosci. 1999;19(21):9313–9321. doi: 10.1523/JNEUROSCI.19-21-09313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Hopf FW. The dopamine D2 receptor: new surprises from an old friend. Neuron. 2005;47(3):335–338. doi: 10.1016/j.neuron.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;42765:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Buck LB. Olfactory receptors and odor coding in mammals. Nutr Rev. 2004;62(11 Pt 2):S184–S188. doi: 10.1111/j.1753-4887.2004.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Chuah MI, David S, Blaschuk O. Differentiation and survival of rat olfactory epithelial neurons in dissociated cell culture. Brain Res Dev Brain Res. 1991;60(2):123–132. doi: 10.1016/0165-3806(91)90040-p. [DOI] [PubMed] [Google Scholar]
- DeHamer MK, Guevara JL, Hannon K, Olwin BB, Calof AL. Genesis of olfactory receptor neurons in vitro: regulation of progenitor cell divisions by fibroblast growth factors. Neuron. 1994;13(5):1083–1097. doi: 10.1016/0896-6273(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Ensoli F, Fiorelli V, Vannelli B, Barni T, De Cristofaro M, Ensoli B, Thiele CJ. Basic fibroblast growth factor supports human olfactory neurogenesis by autocrine/paracrine mechanisms. Neuroscience. 1998;86(3):881–893. doi: 10.1016/s0306-4522(98)00104-3. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Differentiation of olfactory receptor cells in organ culture. Anat Rec. 1977;189(2):187–199. doi: 10.1002/ar.1091890206. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A. Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res. 1999a;40(3):211–218. doi: 10.1016/s0920-9964(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Feron F, Vincent A, Mackay-Sim A. Dopamine promotes differentiation of olfactory neuron in vitro. Brain Res. 1999b;845(2):252–259. doi: 10.1016/s0006-8993(99)01959-9. [DOI] [PubMed] [Google Scholar]
- Friedman D, Strowbridge BW. Functional role of NMDA autoreceptors in olfactory mitral cells. J Neurophysiol. 2000;84(1):39–50. doi: 10.1152/jn.2000.84.1.39. [DOI] [PubMed] [Google Scholar]
- Ghanbari HA, Ghanbari K, Harris PL, Jones PK, Kubat Z, Castellani RJ, Wolozin BL, Smith MA, Perry G. Oxidative damage in cultured human olfactory neurons from Alzheimer's disease patients. Aging Cell. 2004;3(1):41–44. doi: 10.1111/j.1474-9728.2004.00083.x. [DOI] [PubMed] [Google Scholar]
- Gomez G, Rawson NE, Hahn CG, Michaels R, Restrepo D. Characteristics of odorant elicited calcium changes in cultured human olfactory neurons. J Neurosci Res. 2000;62(5):737–749. doi: 10.1002/1097-4547(20001201)62:5<737::AID-JNR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Gomez G, Restrepo D, Friedman E, Josiassen R, Pribitkin EA, Lowry LD, Gallop RJ, Rawson NE. Aberrant intracellular calcium signaling in olfactory neurons from patients with bipolar disorder. Am J Psychiatry. 2005a;162(3):616–618. doi: 10.1176/appi.ajp.162.3.616. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Han LY, Rawson NE, Mirza N, Borgmann-Winter K, Lenox RH, Arnold SE. In vivo and in vitro neurogenesis in human olfactory epithelium. Journal of Comparative Neurology. 2005b;483:154–163. doi: 10.1002/cne.20424. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Han LY, Rawson NE, Mirza N, Borgmann-Winter K, Lenox RH, Arnold SE. In vivo and in vitro neurogenesis in human olfactory epithelium. J Comp Neurol. 2005c;483(2):154–163. doi: 10.1002/cne.20424. [DOI] [PubMed] [Google Scholar]
- Halabisky B, Friedman D, Radojicic M, Strowbridge BW. Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J Neurosci. 2000;20(13):5124–5134. doi: 10.1523/JNEUROSCI.20-13-05124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster NL, Norman AB, Richtand NM, Nickell WT, Puche AC, Pixley SK, Shipley MT. Olfactory receptor neurons express D2 dopamine receptors. J Comp Neurol. 1999;411(4):666–673. doi: 10.1002/(sici)1096-9861(19990906)411:4<666::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Lowry LD, Pribitkin EA. Collection of human olfactory tissue. In: Spielman AI, editor. Experimental cell biology of taste and olfaction. CRC Press; Boca Raton: 1995. pp. 47–48. [Google Scholar]
- Lynch DR, Guttmann RP. NMDA receptor pharmacology: perspectives from molecular biology. Curr Drug Targets. 2001;2(3):215–231. doi: 10.2174/1389450013348434. [DOI] [PubMed] [Google Scholar]
- MacDonald ML, Naydenov A, Chu M, Matzilevich D, Konradi C. Decrease in creatine kinase messenger RNA expression in the hippocampus and dorsolateral prefrontal cortex in bipolar disorder. Bipolar Disord. 2006;8(3):255–264. doi: 10.1111/j.1399-5618.2006.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mania-Farnell BL, Farbman AI, Bruch RC. Bromocriptine, a dopamine D2 receptor agonist, inhibits adenylyl cyclase activity in rat olfactory epithelium. Neuroscience. 1993;57(1):173–180. doi: 10.1016/0306-4522(93)90119-z. [DOI] [PubMed] [Google Scholar]
- Margolis FL. A marker protein for the olfactory chemoreceptor neuron. In: Bradshaw RA, editor. Proteins of the nervous system. Raven Press; New York: 1980. pp. 59–84. [Google Scholar]
- Mombaerts P. Odorant receptor gene choice in olfactory sensory neurons: the one receptor-one neuron hypothesis revisited. Curr Opin Neurobiol. 2004;14(1):31–36. doi: 10.1016/j.conb.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Nibu K, Li G, Zhang X, Rawson NE, Restrepo D, Kaga K, Lowry LD, Keane WM, Rothstein JL. Olfactory neuron-specific expression of NeuroD in mouse and human nasal mucosa. Cell Tissue Res. 1999;298(3):405–414. doi: 10.1007/s004419900098. [DOI] [PubMed] [Google Scholar]
- Ninkina N, Grashchuck M, Buchman VL, Davies AM. TrkB variants with deletions in the leucine-rich motifs of the extracellular domain. J Biol Chem. 1997;272(20):13019–13025. doi: 10.1074/jbc.272.20.13019. [DOI] [PubMed] [Google Scholar]
- Okada Y, Miyamoto T, Toda K. Dopamine modulates a voltage-gated calcium channel in rat olfactory receptor neurons. Brain Res. 2003;968(2):248–255. doi: 10.1016/s0006-8993(03)02267-4. [DOI] [PubMed] [Google Scholar]
- Pixley SK. Characterization of olfactory receptor neurons and other cell types in dissociated rat olfactory cell cultures. Int J Dev Neurosci. 1996;14(7-8):823–839. doi: 10.1016/s0736-5748(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Brand JG, Cowart BJ, Lowry LD, Pribitkin EA, Rao VM, Restrepo D. Functionally mature olfactory neurons from two anosmic patients with Kallmann syndrome. Brain Res. 1995;681(1-2):58–64. doi: 10.1016/0006-8993(95)00283-v. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Eberwine J, Dotson R, Jackson J, Ulrich P, Restrepo D. Expression of mRNAs encoding for two different olfactory receptors in a subset of olfactory receptor neurons. J Neurochem. 2000;75(1):185–195. doi: 10.1046/j.1471-4159.2000.0750185.x. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Gomez G, Cowart B, Brand JG, Lowry LD, Pribitkin EA, Restrepo D. Selectivity and response characteristics of human olfactory neurons. J Neurophysiol. 1997;77(3):1606–1613. doi: 10.1152/jn.1997.77.3.1606. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. 365–386. Source code available at http://primer3.sourceforge.net/ [DOI] [PubMed] [Google Scholar]
- Roskams AJ, Bethel MA, Hurt KJ, Ronnett GV. Sequential expression of Trks A, B, and C in the regenerating olfactory neuroepithelium. J Neurosci. 1996;16(4):1294–1307. doi: 10.1523/JNEUROSCI.16-04-01294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning A Laboratory Manual. Cold Spring Harbor Laboratory Press; Plainview: 1989. [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298(5594):776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Tucker K, Fadool DA. Neurotrophin modulation of voltage-gated potassium channels in rat through TrkB receptors is time and sensory experience dependent. J Physiol. 2002;542(Pt 2):413–429. doi: 10.1113/jphysiol.2002.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannelli GB, Ensoli F, Zonefrati R, Kubota Y, Arcangeli A, Becchetti A, Camici G, Barni T, Thiele CJ, Balboni GC. Neuroblast long-term cell cultures from human fetal olfactory epithelium respond to odors. J Neurosci. 1995;15(6):4382–4394. doi: 10.1523/JNEUROSCI.15-06-04382.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas G, Lucero MT. Dopamine modulates inwardly rectifying hyperpolarization-activated current (Ih) in cultured rat olfactory receptor neurons. J Neurophysiol. 1999;81(1):149–158. doi: 10.1152/jn.1999.81.1.149. [DOI] [PubMed] [Google Scholar]
- Wetzel CH, Oles M, Wellerdieck C, Kuczkowiak M, Gisselmann G, Hatt H. Specificity and sensitivity of a human olfactory receptor functionally expressed in human embryonic kidney 293 cells and Xenopus Laevis oocytes. J Neurosci. 1999;19(17):7426–7433. doi: 10.1523/JNEUROSCI.19-17-07426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Sunderland T, Zheng BB, Resau J, Dufy B, Barker J, Swerdlow R, Coon H. Continuous culture of neuronal cells from adult human olfactory epithelium. J Mol Neurosci. 1992;3(3):137–146. doi: 10.1007/BF02919405. [DOI] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27(3):487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Klueber KM, Guo Z, Lu C, Roisen FJ. Adult human olfactory neural progenitors cultured in defined medium. Exp Neurol. 2004;186(2):112–123. doi: 10.1016/j.expneurol.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Zielinski BS, Moretti N, Hua HN, Zaidi AU, Bisaillon AD. Serotonergic nerve fibers in the primary olfactory pathway of the larval sea lamprey, Petromyzon marinus. J Comp Neurol. 2000;420(3):324–334. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell hybridized with sense probes (A) OMP, (B) OcNc1, (C) OR1A1 and (D) OR3A1 showed no labeling. Note the exposure times for OR3A1 sense (D) was shorter than that used in Figure 3B, which were obtained from different experiments. Therefore the corresponding anti-sense OR3A1 image (E) is also included. Exposure times are noted on each image.